Abstract
Objective:
Modern medical research has proven that human diseases are directly or indirectly related to genes. At the same time, genetic research has also brought updates to diagnostic techniques. Olfactomedin-like 3 (OLFML3) gene is a novel and clinically valuable gene. In order to better understand the role of OLFML3 in human diseases, we discuss and analyze the characteristics, function, and regulation mechanism of the OLFML3 gene in this review.
Data sources:
A comprehensive search in PubMed and ScienceDirect database for English up to March 2019, with the keywords of “Olfactomedin-like 3,” “Olfactomedin,” “extracellular matrix,” “Transforming Growth Factor β1,” “anoikis-resistance,” and “microRNA-155.”
Study selection:
Careful review of all relevant literature, the references of the retrieved articles were also screened to search for potentially relevant papers.
Results:
OLFML3 is a secreted glycoprotein with 406 amino acid residues, belonging to the Olfactomedin (OLF) family. Due to the particularity of its structure and differential expression, OLFML3 has unique biological functions that could be distinct from other members in the OLF family. The currently known functions include embryonic development function and tumorigenesis. The regulation mechanism is still under investigation. It is directly related to many human diseases.
Conclusions:
OLFML3 is a multifunctional glycoprotein that is closely involved in embryonic development, tumor invasion, and metastasis. Unfortunately, current research on this important molecule is still very limited. Further investigations on the possible mechanism of OLFML3 biological functions and modulation will help us develop better diagnostics and treatments.
Keywords: Olfactomedin-like 3, Olfactomedin, Extracellular matrix, Transforming growth factor β1, Anoikis-resistance, microRNA-155
Introduction
Olfactomedin-like 3 (OLFML3), also known as hOLF44, is a secreted glycoprotein consisting of 406 amino acid residues. Having a C-terminal olfactomedin-like (OLF) domain, which is highly conserved in the OLF family, OLFML3 belongs a member of the OLF family, a protein family present in all animal kingdoms with important functions in early development of organisms. “OLF” was initially discovered as a contaminant in chemosensory dendritic cilia preparations purified from olfactory epithelium of the bullfrog nearly 30 years ago.[1] Since then more than 100 OLF members have been found in various species ranging from Caenorhabditis elegans to Homo sapiens.[2] Subsequent works have further demonstrated that the members of the OLF family perform different physiological functions in vertebrate embryogenesis.[3] Being the most important members of the OLF family, the OLF-like sub-family contains five different members with 13 isoforms in mammal cells.[2,4] Among them, OLFML1 is associated with cell proliferation and cell autonomous in human cancer cell[5,6]; OLFML2A and OLFML2B act as photomedins; and OLFML4 plays anti-inflammatory and anti-apoptotic roles.[7] However, the precise role of OLFML3 remains elusive. From the perspective of the OLFML3 structure and expression pattern, OLFML3 appears to be a special member of the OLF family. First, the phylogenetic analysis shows that four of the five OLF-like molecules have well-characterized sequences belong to a few sub-families. However, OLFML3 falls into a newly identified OLF sub-family because of its special sequence which is not shared by any other members.[8] In addition, OLFML3 is differentially expressed in a variety of human tissues, while other members in the OLF sub-family usually show selective tissue expression patterns. As such, this distinctive OLF-like member becomes more attractive. Recent studies have shown that embryonic development is one of the most biological functions of OLFML3 involved in.[9] Other important functions of OLFML3 are in proangiogenesis and anoikis-resistance, contributing potential value in the cancer research field. Therapeutic targets against malignant tumor and regulation of embryonic development are two hot fields of science and medicine. Modern medical research proves that many human diseases are directly or indirectly related to gene amplification or mutation/deletion. With the exploration of the function and regulation of some specific genes, scientists have made a great progress in the field of diagnostic and treatment of human diseases. Although the study of the OLFML3 gene is only in its infancy, we have a plenty of reasons to believe that with the exploration of its biological function and therapeutic mechanism, OLFML3 would serve as a potential specific diagnostic marker and/or a therapeutic target in human cancer in future. In this review, we discuss recent advances in understanding the structure, expression, biological function, and regulation of OLFML3, and its related diseases.
Structure of OLFML3
OLFML3 is known as HNOEL-iso or HOLF44 in human, ONT1 in Xenopus and chicken, and mNOT3 in mice.[10] In human, the OLFML3 gene is localized on chromosome 1 band P 13.2. It contains a highly conserved OFL domain at the C-terminal, whereas a more variable coiled-coil domain at N-terminal region.[1] [Figure 1] The OLFML3 gene is composed of two introns and three exons. All of the exons and introns junctions satisfy the GT/AG rule. The OLFML3 gene contains a coding DNA sequence of 1221 nucleotides flanked by two untranslated regions (UTRs) and encodes an 1852 nucleotides messenger RNA. The opening reading frame of the OLFML3 gene encodes 406 amino acid residues of protein with both the N-terminal and the C-terminal.[7] A predicted molecular weight is 44,000.[8] Due to its unique attributes, various research has also been carried out in other animals. The OLFML3 gene is located at chromosome 3 and consists of three exons separated by two introns in mouse as well. The protein structure of OLFML3 is highly similar to human's structure. Anti-OLFML3 immune reactivity is found in the perinuclear endoplasmic reticulum and Golgi apparatus and exocytotic vesicles in mice microglial processes.[11] The OLFML3 gene structures in mammalian cells show a remarkably high similarity. The predicted similarity in OLFML3 polypeptide shows 94%, 94%, 92%, 94%, and 65% with human, cattle, mouse, rat, and gallus, respectively. Research shows the OFL domain is highly conserved among human, mouse, and pig, which may be because of the OLF domain involved in intra-cellular folded proteins accumulation.[12]
Figure 1.
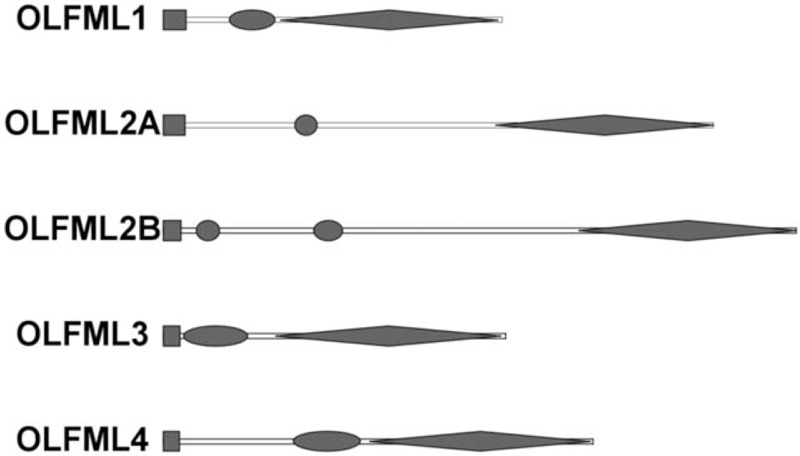
Conserved OLF domain structures of OLFML families. Circle: coiled-coil domain; Rectangle: signal sequence; Rhombus: OLF domain. OLF: Olfactomedin; OLFML: Olfactomedin-like.
Expression of OLFML3
OLFML3 is differentially expressed in various tissues and organs. It has been reported that OLFML3 is particularly abundant expression in placenta and moderate expression in heart and liver, weak in skeletal muscle, small intestine, lung, and kidney, and very weak in colon, spleen, thymus, and brain. In addition, there is a report showing that the expression of OLFML3 in human and baboons ocular tissues including cornea, lens, uvea, and retina.[7] So far, no expression has been detected in peripheral blood leukocytes.[8] It is reported that OLFML3 is only expressed in syncytiotrophoblastic cells on term placenta, but very little expression in the maternal decidua layer.[7] This expression pattern implies that the OLFML3 gene may be associated with fetal development. The OLFML3 gene is one of the most enriched microglia genes which is highly expressed in primary mouse periphery microglia and cytoplasm but is barely detected in macrophage and monocyte populations in the post-natal microglial process.[13] In chick embryos, expression of the OLFML3 is detected at Hensen’ node, axial, and paraxial mesoderm, similar to Xenopus.[14]
At the cell expression level, the OLFML3 gene mainly expresses at an extra-cellular area and likely to participate in the formation of extra-cellular matrix (ECM) structure by interacting with other components.[2] The tumor extra-cellular stroma is mainly composed of fibroblasts, endothelial cells, infiltrating immune cells, and pericytes.[15] The study of Miljkovic-Licina et al showed the expression of OLFML3 is restricted on tumor vascular endothelial cells and vessel-associated pericytes, deposited in the perivascular compartment, while tumor cells themselves do not express OLFML3 mRNA.[10] Furthermore, OLFML3 protein is enriched in the extra-cellular space of vascular-specific endothelial cells and pericytes and its expression level correlate with the activation state of them. Coincidentally, the immunohistochemical staining on human liver sections shows that OLFML3 is also localized extra-cellularly surrounding hepatocytes.[2] This extra-cellular expression pattern implies that the OLFML3 may play an important role in remodeling ECM and promoting tumor angiogenesis. Clinical and experimental data support that extra-cellular stroma cultivates cancer cells and promotes tumor development and invasion.[16]
Bioinformatics analyses of HPA, G-TEX, and CCLE database consistently show that expression of OLFML3 is higher in the female reproductive system. It is preferentially expressed in placenta and broadly expressed in ovary tissue, endometrium. This may imply that there may exist a potential mechanism between OLFML3 and female reproductive system disease. However, no published experimental data is available to prove it, therefore, this inference needs further experimental to confirm.
Biological Function and Regulation of OLFML3
Like Wnt protein, the OLFML3 protein is only identified in multicellular organisms, which demonstrate that they play a role in cell-cell interaction and signal connection.[4] The OLFML3 gene has been documented to have a special physiological function in embryonic development in human.[7] It has also been found that OLFML3 increases growth rate, and modulates cytoskeletal organization, cell adhesion, and migration.[4] In summary, the biological functions of the OLFML3 fall into two categories: the matrix-related embryonic development function and the one associated with cancer [Figure 2].
Figure 2.
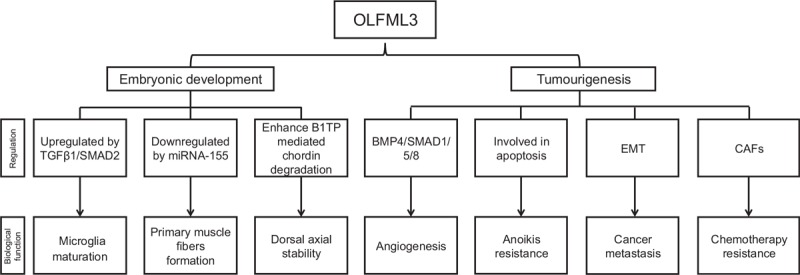
Biological function and regulation of OLFML3. OLFML3: Olfactomedin-like 3; BMP4/SMAD: Bone morphogenetic protein 4/mothers against decapentaplegic homolog, CAFs: Carcinoma-associated fibroblasts, EMT: Epithelial to mesenchymal transition, miRNA-155: microRNA-155, TGFβ1/SMAD2: Transforming growth factorβ1/mothers against decapentaplegic homolog 2.
Embryonic Development Related Function and Regulation
Microglia are important immune cells in the central nervous system (CNS). Maturation of microglia occurs in the early stage of post-natal weeks and is characterized by the establishment of a unique microglia-specific gene expression pattern. OLFML3 as a member of OLF sub-family is involved in the development of the CNS.[1] It plays an important role in microglia process although the expression in brain is very weak. According to the literature, OLFML3 is one of the microglia-specific genes which are not expressed by other macrophage population during embryonic development.[17] In the first post-natal weeks, OLFML3 can discriminate microglia from other macrophage populations and involve in the maturation and functional organization of mice microglia. Nicolas et al demonstrated that OLFML3 is a direct TGFβ1/SMAD2 up-regulated target gene.[11] TGFβ1 as the upstream signal of the OLFML3 gene is activated at post-natal day 7 precedes the establishment of the microglial gene expression pattern.[18] Post-natal microglial TGFβ1/SMAD2 signaling is essential for the induction of immature post-natal microglia but dispensable in maintenance mature adult microglia in vivo. Thus, the OLFML3 gene has an important contribution to the development of the embryonic nervous system.
Another matrix-related embryonic development function of OLFML3 is shown in affecting pre-natal skeletal muscle development. Pre-natal muscle development programmatic determines post-natal muscle status. Formation of primary muscle fibers directly affects the adult total number of muscle fibers. The OLFML3 gene mainly involved in forming pre-natal primary muscle fibers, promoting muscle cell proliferation and affecting post-natal muscle phenotype in porcine. In recent years, miRNAs have emerged as critical regulators of gene expression. miRNA-155 has been proved to be a typical multifunctional regulator associated with cell proliferation and differentiation.[19,20] Zhao et al showed that OLFML3 expression in the porcine pre-natal muscle is down-regulated by miRNA-155 at mRNA level due to the miRNA-dependent target mRNA degradation.[9] The target site of OLFML3 is at 3′-UTR which is specific and unique. Both the sequences of the OLFML3 3′-UTR and the seed sequence of mature miRNA-155 are all conserved in mammals. Therefore, it can be strongly inferred that the target region is probably important in OLFML3 regulation, and the same regulation mechanism may exist in other species.
In the species of Xenopus and chicken, OLFML3 executes the function of reinforcement stability of dorsal axial pattern during the embryonic period.[21] It is expressed in the axial and paraxial mesoderm in the early stage of embryogenesis. OLFML3 enhances BMP1/Tolloid-class proteinases mediated chordin degradation by facilitating enzyme-substrate association.[21] OLFML3 act as a key molecule of a pro-BMP regulator is indispensable for fine-tuning Chordin/BMP signaling in the axial tissue. OLFML3, together with dorsally expressed BMP1, plays an essential role in chordin activity regulation and ensures stable dorsal-ventral patterning in the embryo.
Myocilin and OLFML3 are both members of the OLF family which are expressed in the eye and brain. Similar to myocilin, OLFML3 contains a signal peptide, an N-terminal coiled-coil domain, and a C-terminal OLF domain. It can be observed that the OLFML3 gene is significantly down-regulated (0.72-fold) and is statistically significant (P < 0.05) when elevated amounts of myocilin on aqueous humor of mice.[22] This shows that OLFML3 might be able to interact with other members of this family.
Tumorigenesis, Metastasis, and Regulation
Tumor growth and metastasis depend on angiogenesis and lymphangiogenesis triggered by chemical signals from tumor cells. Angiogenesis refers to the formation of new blood vessels in the body. It is a normal bodily process for healing but also plays an important role in the rapid growth of cancer. It is recently reported that some of the OLF members have the physiological function of angiogenesis in animal models.[23] OLFML3 is considered as a novel promoting pro-angiogenic molecule within the tumor microenvironment that supports proliferation, remodeling, and maturation of tumor vessels. OLFML3 itself or by binding with BMP4 modulators affect angiogenesis during tumor growth and metastasis. BMPs, which act as the largest sub-group of the TGF-β superfamily of signaling molecules, are critical growth factors in the endothelial adaptation associated with tumor progression. BMP2 and BMP4, are pro-angiogenic factors. BMP1 and BMP9 are known for their anti-angiogenic activities.[24,25] OLFML3, which acts as a BMP4 binding protein has two epitopes in the coiled-coil domain and the OLF domain. The two sites of OLFML3 are equally important and functional for angiogenesis when the two domains of OLFML3 interact with BMP4. SMAD1/5/8 is the downstream signaling pathway of OLFML3 that can cause vascular endothelial cell activation.[10] OLFML3 alone or by binding with BMP4 can also enhance the SMAD1/5/8 signaling pathway and lead to SMAD1/5/8 phosphorylation. Not only that, but the combination of OLFML3 and BMP4 will also have an additive effect on lung carcinoma cells. Another experimental result in lung cancer cell lines (A459) showed that OLFML3 is down-regulated by surface protein neuropilin 1 (NRP1) inhibition. NRP1 is a useful biomarker of tumor-initiating cells in the lung cancer. It is a membrane protein that regulates cell migration and proliferation and is closely related to tumor formation and metastasis. NRP1 inhibition is strongly correlated with the OLFML3 down-regulation.[26] Different from the result of Miljkovic-Licina et al, NRP1 inhibition resulted in OLFML3 down-regulation accompanied by increased expression in BMP4 and SMAD. The reason for the differential regulation mechanism may be due to the different characteristics of lung cancer cell lines.
Anoikis is a form of programmed cell death that occurs in anchorage-dependent cells when they detach from the surrounding ECM. OLFML3 plays a role in promoting tumor growth in relation to anoikis resistance. OLFML3 is one of the most highly differentially expressed genes in poorly differentiated lung cancer cell line DLKP. Keenan et al showed a positive correlation between the level of OLFML3 expression and anoikis resistance in different invasive ability DLKP sub-populations.[27] Similarly, in the nasal carcinoma and breast cancer cell lines, the anoikis-sensitive cell expresses little or no OLFML3 but the anoikis-resistance cell expresses higher OLFML3. These suggest that OLFML3 expression may have a role in anoikis resistance.[27] The mechanism of OLFML3 in regulation of anoikis is not very clear yet. According to the literature, apoptosis and autophagy are two common cell death pathways of anoikis.[28] It is possible that the property of the OLFML3 in preventing anoikis is related to intra-cellular interaction with apoptotic machinery. Secreted OLFML3 may interact with cell surface receptors or secreted proteins to prevent anoikis signaling.[27]
Epithelial to mesenchymal transition (EMT) is a process in which cancer cells lose adhesion but obtain invasive properties.[29] EMT is considered an important symbol of cancer metastasis.[30,31] OLFML3 is a representative molecule related in the EMT regulation. Breast carcinoma metastasis suppressor gene 1 together with lysine-specific histone demethylase 1 target OLFML3 gene executes transcriptional suppression by occupying co-target gene promoters. Decreased OLFML3 mRNA levels weaken the EMT process, thereby inhibiting breast cancer metastasis.[32] Carcinoma-associated fibroblasts (CAFs) are the major components of mesenchymal cells in the inflammatory tumor microenvironment. They are not only involved in EMT but also participate in chemotherapy resistance.[33] Activated CAFs that have undergone EMT in cancer stroma contribute to tumor progression and metastasis.[34] The important contribution of OLFML3 is to participate in the formation of the ECM and play a role in developmental processes.[5] Stroma CAFs has the functions of promoting immune escape.[35] OLFML3 is one of the up-regulation factors secreted by CAFs that play a role in ECM modifications of the tumor.[36,37] The OLFML3 gene acts as a selective biomarker of cancer stromal, is significantly up-regulated in CAFs of mouse and human colorectal cancer samples.[36] A clinical trial data for head and neck cancer shows that OLFML3 is significantly highly expressed after pre-operation cetuximab treatment. This clear up-regulation of expression of OLFML3 implicated both OLFML3 and CAFs are involved in chemotherapy resistance.[37]
OLFML3-related Diseases
Biomarkers and therapeutic target
Accurate detection of cancer biomarkers is the key to screening, treating, and predicting malignant tumors. OLFML3 is a specific molecule associated with tumorigenesis. As discussed above, the specific expression of OLFML3 is present in human and animal malignant tumor tissues. Its high expression was demonstrated in microglioma, breast, lung, and colon cancer. Usually, high activity of oncogenes is associated with a poor prognosis and worse survival rates. OLFML3 can also act as an indicator of fat metabolism state. A study which describes the pig backfat tissue transcription profile, reports that OLFML3 is significantly under-expressed in fat pig contrasting with lean pig.[38] This might give us a clue indicating whether the OLFML3 gene is involved in regulating adipogenesis and used to predict obesity.
With the increase of malignant tumor incidence, researchers are increasingly concerned about the relationship between genes and cancer. Development of therapeutic targets becomes a hot spot in tumor research because of impossible surgery and insufficient efficiency of current chemotherapy and radiation therapy. Nowadays, anti-angiogenic therapy has been in wide use against numerous cancers. In clinics, single targeted therapy against vascular endothelial growth factor (VEGF) signaling pathway has shown a limited long-term benefit and most patients develop resistance to the therapy. One of resistance mechanisms is the increased pericyte coverage of newly formed vessels. Furthermore, upon cessation of VEGF therapy, pericytes that remain present in the tumor microenvironment provide scaffolds for rapid revascularization. OLFML3 has been found to promote angiogenesis by signaling to both endothelial cells and pericytes. It is well known that tumor angiogenesis is important for tumor growth and metastasis.[39] Targeting OLFML3 therapy may have the potential value because of its angiogenesis function and dual expression in both endothelial and pericytes. Therefore, blocking OLFML3 holds promise to control tumor growth by targeting a single molecule that affects two distinct sites within the tumor microenvironment.
Eye development and ocular diseases
OLFML3 is strongly expressed in ocular tissue of human and animal embryos and participates in eye development.[40] It plays an angiogenic role in ocular tissue and thus participates in the anterior segment and retinal diseases. Genetic factors are considered to play a key role in all major forms of glaucoma. Although mutations in several genes, including myocilin, optineurin, and CYP1B1, have been reported to cause glaucoma, these genes account for less than 10% of cases worldwide. In recent years, large scale genetic studies that have examined the blood samples of thousands of glaucoma patients have been instrumental in the discovery of more common genetic risk factors for glaucoma. OLFML3 was shown to associate with the development of open-angle glaucoma. Abnormality of OLFML3 in the microglia of eye could interfere with the opening of the iridocorneal angle and disrupt the formation of the proper drainage channels.[40] It has already known that OLFML3 has an angiogenic effect on eye tissues. Some siRNAs or antibodies directly against OLFML3 have been patented for use as mediators of angiogenesis and may be effective in treatment of pathologic vascularity and retinal disease.[41]
Amyotrophic lateral sclerosis (ALS)
OLFML3 is identified as the microglia-specific gene. There is loss expression of the microglial specific OLFML3 in spinal cord tissue of familial and sporadic ALS. Loss expression of OLFML3 suppresses microglia biological functions and leads to ALS.[42] miR-155 acts as the upstream regulator and shows an increased expression in microglia and spinal cord tissue of human ALS. It directly targets and down-regulates the OLFML3 and reduces TGFβ1 by suppressing of SMAD221 and SMAD5.[43,44] Up-regulation of OLFML3 expression by miR-155 ablation can restore the abnormal microglia molecular signatures and increased ALS survival.[42]
Human tissue engineering
Creating an effective tissue graft that can mimic native structure provide a new idea of facilitating wound regeneration, which has certain innovative meaning. Electrospun polymer scaffolds have the substantial potential of mimicking native ECM due to their high surface to volume ratio and the characteristics of their special structure characteristics.[45] But these artificially simulated materials often lack optimal biological activity of acceleration of cell proliferation, neovascularization, and tissue regeneration. OLFML3 acts as an important ECM related protein and has the properties of accelerating neovascularization during tissue regeneration by promoting endothelial cell proliferation and migration. Using this feature of OLFML3, researchers try to innovate plasma-treated electrospun polymer scaffolds combined with OLFML3 to apply in tissue regeneration and wound healing.[46]
Conclusions
Current knowledge of evidence-based research shows that OLFML3 is a secreted matrix-related glycoprotein and differs greatly from any of the other OLF members. OLFML3 is differentially expressed in multiple tissues with main biologic functions in embryonic development and tumor growth. Its regulatory mechanism is closely related to microRNA and transcription factors. Based on the specific biological functions of OLFML3, it will have broad prospects in biomarkers and gene-targeted therapies. Currently available information; however, represents only the tip of a slowly emerging iceberg. Much more investigations are needed to explore the relationship between OLFML3 and human diseases.
Conflicts of interest
None.
Footnotes
How to cite this article: Jin Y, Li JL. Olfactomedin-like 3: possible functions in embryonic development and tumorigenesis. Chin Med J 2019;132:1733–1738. doi: 10.1097/CM9.0000000000000309
References
- 1.Anholt RR. Olfactomedin proteins: central players in development and disease. Front Cell Dev Biol 2014; 26:1–10. doi: 10.3389/fcell.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng LC, Han ZG, Ma WJ. Elucidation of subfamily segregation and intramolecular coevolution of the olfactomedin-like proteins by comprehensive polygenetic analysis and gene expression pattern assessment. FEBS Lett 2005; 579:5443–5453. doi: 10.1016/j.febslet.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 3.Barembaum M, Moreno TA, Labonne C, Sechrist J, Bronner-Fraser M. Noelin-1 is a secreted glycoprotein involved in generation of the neural crest. Nat Cell Biol 2000; 2:219–225. doi: 10.1038/35008643. [DOI] [PubMed] [Google Scholar]
- 4.Tomarev SI, Nakaya N. Olfactomedin domain-containing proteins: possible mechanisms of action and functions in normal development and pathology. Mol Neurobiol 2009; 40:122–138. doi: 10.1007/s12305-009-8076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murakami K, Kikugawa S, Kobayashi Y, Uehara S, Suzuki T, Kato H, et al. Olfactomedin-like protein OLFML1 inhibits Hippo signaling and mineralization in osteoblasts. Biochem Biophys Res Commun 2018; 505:419–425. doi: 10.1016/j.bbrc.2018.09.112. [DOI] [PubMed] [Google Scholar]
- 6.Wan B, Zhou YB, Zhang X, Zhu H, Huo K, Han ZG. hOLFML1, a novel secreted glycoprotein, enhances the proliferation of human cancer cell lines in vitro. FEBS Lett 2008; 582:3185–3192. doi: 10.1016/j.febslet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Sanchez IP, Garza-Rodriguex ML, Mohamed-Noriega K, Voruganti VS, Tejero ME, Delgado-Enciso I, et al. Olfactomedin-like 3(OLFML3) gene expression in baboon and human ocular tissue: cornea, lens, uvea, and retina. J Med Primatol 2013; 42:105–111. doi: 10.1111/jmp.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng LC, Liu F, Zhang X, Zhu ZD, Wang ZQ, Han ZG, et al. hOLF44, a secreted glycoprotein with distinct expression pattern, belongs to an uncharacterized olfactomedin-like subfamily newly identified by phylogenetic analysis. FEBS Lett 2004; 571:74–80. doi: 10.1016/j.febslet.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 9.Zhao S, Zhang J, Hou X, Zan L, Wang N, Tang Z, et al. OLFML3 expression is decreased during prenatal muscle development and regulated by microRNA-155 in pigs. Int J Biol Sci 2012; 8:459–469. doi: 10.7150/ijbs.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miljkovic-Licina M, Hammel P, Garrido-Urbani S, Lee BP, Meguenani M, Chaabane C, et al. Targeting olfactomedin-like 3 inhibits tumour growth by impairing angiogenesis and pericyte coverage. Mol Cancer Ther 2012; 11:2588–2599. doi: 10.1158/1535-7163. [DOI] [PubMed] [Google Scholar]
- 11.Neidert N, von Ehr A, Zoller T, Spittau B. Microglia-specific expression of OLFML3 is directly regulated by transforming growth factor β 1-induced SMAD2 signaling. Front Immunol 2018; 9:1728.doi: 10.3389/fimmu.2018.01728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caballero M, Borras T. Inefficient processing of an olfactomedin-deficient myocilin mutant: potential physiological relevance to glaucoma. Biochem Biophys Res Commun 2001; 282:662–670. doi: 10.1006/bbrc.2001.4624. [DOI] [PubMed] [Google Scholar]
- 13.Chiu IM, Morimoto ETA, Goodarzi H, Liao JT, O’Keeffe S, Phatnani HP, et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep 2013; 4:385–401. doi: 10.1016/j.celrep.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakuragi M, Sasai N, Ikeya M, Kawada M, Onai T, Katahira T, et al. Functional analysis of chick ONT1 reveals distinguishable activities among olfactomedin-related signaling factors. Mech Dev 2006; 123:114–123. doi: 10.1016/j.mod.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumour microenvironment. Cancer Cell 2012; 21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 16.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer 2008; 123:2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 17.Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A 2016; 113:E1738–E1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attaai A, Neidert N, Von Ehr A, Potru PS, Zöller T, Spittau B. Postnatal maturation of microglia is associated with alternative activation and activated TGF-β signaling. Glia 2018; 66:1695–1708. doi: 10.1002/glia.23332. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Li C, Liang F, Fan Y, Zhang S. MiRNA-155 promotes proliferation by targeting caudal-type homeobox 1 (CDX1) in glioma cells. Biomed Pharmacother 2017; 95:1759–1764. doi: 10.1016/j.biopha.2017.08.088. [DOI] [PubMed] [Google Scholar]
- 20.Seok HY, Tatsuguchi M, Callis TE, Ha A, Pu WT, Wang DZ. miRNA-155 inhibits expression of the MEF2A protein to repress skeletal muscle differentiation. J Biol Chem 2011; 286:35339–35346. doi: 10.1074/jbc.M111.273276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inomata H, Haraguchi T, Sasai Y. Robust stability of the embryonic axial pattern requires a secreted scaffold for Chordin degradation. Cell 2008; 134:854–865. doi: 10.1016/j.cell.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Paper W, Kroeber M, Heersink S, Stephan DA, Fuchshofer R, Russell P, et al. Elevated amounts of myocilin in the aqueous humor of transgenic mice cause significant changes in ocular gene expression. Exp Eye Res 2008; 87:257–267. doi: 10.1016/j.exer.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukhopadhyay A, Talukdar S, Bhattacharjee A, Ray K. Bioinformatic approaches for identification and characterization of olfactomedin related genes with a potential role in pathogenesis of ocular disorders. Mol Vis 2004; 10:304–314. [PubMed] [Google Scholar]
- 24.Ge G, Fernandea CA, Moses MA, Greenspan DS. Bone morphogenetic protein 1 processes prolactin to a 17-kDa antiangiogenic factor. Proc Natl Acad Sci U S A 2007; 104:10010–10015. doi: 10.1073/pnas.0704179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davic L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, et al. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res 2008; 102:914–922. doi: 10.1161/circresaha.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jimenez-Hernandez LE, Vazquez-Santillan K, Castro-Oropeza R, Martinez-Ruiz G, Munoz-Galindo L, Gonzalez-Torres C, et al. NRP1-positive lung cancer cells possess tumour-initiating properties. Oncol Rep 2018; 39:349–357. doi: 10.3892/or.2017.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keenan J, Joyce H, Aherne S, O’Dea S, Doolan P, Lynch V, et al. Olfactomedin III expression contributes to anoikis-resistance in clonal variants of human lung squamous carcinoma cell line. Exp Cell Res 2012; 318:593–602. doi: 10.1016/j.yexcr.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Horbinski C, Mojesky C, Kyprianou N. Live free or die: tales of homeless (cells) in cancer. Am J Pathol 2010; 177:1044–1052. doi: 10.2353/ajpath.2010.091270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson AC, Ansell A, Jerhammar F, Lindh MB, Grenman R, Munck-Wikland E, et al. Cancer-associated fibroblasts induce matrix metalloproteinase-mediated cetuximab resistance in head and neck squamous cell carcinoma cells. Mol Cancer Res 2012; 10:1158–1168. doi: 10.1158/1541-7786MCR-12-0030. [DOI] [PubMed] [Google Scholar]
- 30.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009; 9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 31.Sun S, Zhang G, Sun Q, Wu Z, Shi W, Yang B, et al. Insulin-induced gene 2 expression correlates with colorectal cancer metastasis and disease outcome. IUBMB Life 2016; 68:65–71. doi: 10.1002/iub.1461. [DOI] [PubMed] [Google Scholar]
- 32.Qiu R, Shi H, Wang S, Leng S, Liu R, Zheng Y, et al. BRMS1 coordinates with LSD1 and suppresses breast cancer cell metastasis. Am J Cancer Res 2018; 10:2030–2045. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Zhang F, Cui JY, Chen L, Chen YT, Liu BW. CAFs enhance paclitaxel resistance by inducing EMT through the IL-6/JAK2/STAT3 pathway. Onco Rep 2018; 39:2081–2090. doi: 10.3892/or.2018.6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasaki K, Sugai T, Ishida K, Osakabe M, Amano H, Kimura H, et al. Analysis of cancer-associated fibroblasts and the epithelial-mesenchymal transition in cutaneous basal cell carcinoma, squamous cell carcinoma, and malignant melanoma. Hum Pathol 2018; 79:1–8. doi: 10.1016/j.humpath.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, et al. Tumour microenvironment complexity: emerging roles in cancer therapy. Cancer Res 2012; 72:2473–2480. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres S, Bartolome RA, Mendes M, Barderas R, Fernandez-Acenero MJ, Pelaez-Garcia A, et al. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin Cancer Res 2013; 21:6006–6019. doi: 10.1158/1078-0432.CCR-13-1130. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz S, Bindea G, Albu RI, Mlecnik B, Machiels JP. Cetuximab promotes epithelial to mesenchymal transition and cancer associated fibroblasts in patients with head and neck cancer. Oncotarget 2015; 33:34288–34299. doi: 10.18632/oncotarget.5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zambonelli P, Gaffo E, Zappaterra M, Bortoluzzi S, Davoli R. Transcriptional profiling of subcutaneous adipose tissue in Italian Large White pigs divergent for backfat thickness. Anim Genet 2016; 47:306–323. doi: 10.1111/age.12413. [DOI] [PubMed] [Google Scholar]
- 39.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011; 473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pugh CA, Farrell LL, Carlisle AJ, Bush SJ, Ewing A, Trejo-Reveles V, et al. Arginine to glutamine variant in Olfactomedin like 3 (OLFML3) is a candidate for severe goniodysgenesis and glaucoma in the border collie dog breed. Genes 2019; 3:943–954. doi: 10.1534/g3.118.200944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joe MK, Sohn S, Hur W, Moon Y, Choi YR, Kee C. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem Biophys Res Commun 2003; 312:592–600. doi: 10.1016/J.BBRC.2003.10.162. [DOI] [PubMed] [Google Scholar]
- 42.Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci 2014; 1:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butovsky O, Jedrychowski MP, Cialic R, Krasemann S, Murugaiyan G, Fanek Z, et al. Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann Neural 2015; 1:75–90. doi: 10.1002/ana.24304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rai D, Kim SW, McKeller MR, Dahia PL, Aguiar RC. Targeting of SMAD5 links microRNA-155 to the TGF-beta pathway and lymphomagenesis. Proc Natl Acad Sci U S A 2010; 7:3111–3116. doi: 10.1073/pnas.0910667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hassiba AJ, El Zowalaty ME, Nasrallah GK, Webster TJ, Luyt AS, Abdullah AM, et al. Review of recent research on biomedical applications of electrospun polymer nanofibers for improved wound healing. Nanomedicine (Lond) 2016; 11:715–737. doi: 10.2217/nnm.15.211. [DOI] [PubMed] [Google Scholar]
- 46.Dunn LL, de Valence S, Tille JC, Hammel P, Walpoth BH, Stocker R, et al. Biodegradable and plasma-treated electrospun scaffolds coated with recombinant Olfactomedin-like 3 for accelerating wound healing and tissue regeneration. Wound Rep Reg 2016; 24:1030–1035. doi: 10.1111/wrr.12485. [DOI] [PubMed] [Google Scholar]


