Abstract
Background:
Acute kidney injury (AKI) is a serious and fatal complication of acute myocardial infarction (AMI). It has high short- and long-term mortality rates and a poor prognosis but is potentially preventable. However, the current incidence, risk factors, and outcomes of AKI in the Chinese population are not well understood and would serve the first step to identify high-risk patients who could receive preventative care.
Methods:
The medical data of 1124 hospitalized patients diagnosed with AMI from October 2013 to September 2015 were reviewed. AKI was defined according to the 2012 Kidney Disease Improving Global Outcomes criteria. All the patients were divided into either the AKI group or the non-AKI group. A univariate comparison analysis was performed to identify possible risk factors associated with AKI. A multiple logistic regression analysis was used to identify the independent risk factors for AKI in patients with AMI.
Results:
Overall, the incidence of AKI was 26.0%. The mortality rate of the AKI group was 20.5%, and the mortality rate of the non-AKI group was 0.6% (P < 0.001). Logistic regression analysis showed that the independent risk factors for AKI in patients with AMI included: age (>60 years old) (odds ratio [OR] 1.04, 95% confidence interval [CI] 1.02–1.05, P = 0.000), hypertension (OR 2.51, 95% CI 1.62–3.87, P = 0.000), chronic kidney disease (OR 3.52, 95% CI 2.01–6.16, P = 0.000), Killip class ≥3 (OR 5.22, 95% CI 3.07–8.87, P = 0.000), extensive anterior myocardial infarction (OR 3.02, 95% CI 1.85–4.93, P = 0.000), use of furosemide (OR 1.02, 95% CI 1.02–1.03, P = 0.000), non-use of angiotensin-converting enzyme inhibitors/angiotensin receptor blocker (OR 1.58, 95% CI 1.04–2.40, P = 0.032). These factors provided an accurate tool to identify patients at high risk of developing AKI.
Conclusions:
Approximately 26.0% of patients undergoing AMI developed AKI, and the development of AKI was strongly correlated with in-hospital mortality. The risk factors for AKI in patients with AMI were determined to help identify high-risk patients and make appropriate clinical decisions.
Keywords: Acute myocardial infarction, Acute kidney injury, Risk factor
Introduction
Acute kidney injury (AKI) is a complex disorder that can be triggered by a variety of medical conditions and is generally associated with a poor prognosis. In the past decades, the morbidity of AKI has increased from 3/1000 to 5/1000 in the United States.[1] The situation is equally serious in China; our cross-sectional study indicated that in 2013 alone, there were 1.4 to 2.9 million patients diagnosed with AKI, which result in an estimated medical cost of 13 billion US dollars.[2] In addition, both studies correlated AKI with significant in-hospital mortality.[1,2]
Acute myocardial infarction (AMI) is one of the critical conditions that triggers AKI. This is in part due to the existence of comorbid factors, hemodynamic instability, and the use of medicines with kidney toxicity. Studies indicated that the incidence of AKI ranged from 7.1% to 29.3% during hospitalization in patients with AMI.[3–5] Moreover, when AMI was complicated by cardiogenic shock, the occurrence of AKI reached more than 50%.[6] Among patients with AMI, those with AKI had a 20- to 40-fold higher mortality rate in comparison to the one without AKI. Patients with AKI also had more long-term complications, including recurrent AMI, heart failure, chronic kidney disease progression, and long-term mortality.[2–5] Nevertheless, the morbidity of, the risk factors for, and the consequences of AKI in patients with AMI have not been fully understood.
Moreover, the diagnostic criteria for AKI are still evolving. The current criteria include the Risk Injury Failure Loss End-Stage Renal Disease definition created in 2004 by the Acute Dialysis Quality Initiative group, the Acute Kidney Injury Network (AKIN) criteria devised in 2007, and the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guidelines for AKI developed 2012.[7] Due to the lack of a unified definition, hospitals in various countries define AKI according to different standards, which have led to a lower reproducibility in AMI-induced AKI studies. Current studies have considered several different definitions. Early studies showed that there were three kinds of inclusion criteria for AKI: (1) 2-week serum creatinine (SCr) levels increased >0.3 mg/dL in comparison with the baseline value; (2) the absolute value of SCr during hospitalization increased ≥0.5 mg/dL; and (3) the estimated glomerular filtration rate (eGFR) decreased by more than 25%.[8–10] The different AKI standards have also led to significant heterogeneities in related studies. Several recent studies have used the AKIN standard,[6–10] which does not include the 1.5-fold increase of SCr over baseline, which may underestimate the actual incidence of AKI. Therefore, in our study, the KDIGO definition of AKI was adopted, which includes the increase of SCr as a requirement.
Owing to the sudden on-set and the life-threatening severity of AKI episodes in patients with AMI, early identification of patients with AMI of high risk for AKI is crucial for improving the overall outcome and beneficial for both patient management and therapeutic planning. In the current retrospective study, we investigated the incidence, risk factors, in-hospital complications, and in-hospital mortality related to AKI using the latest definition in a large group of consecutive patients with AMI.
Methods
Ethical approval
This retrospective study was approved by the Ethics Committee of Peking University People's Hospital (No. 2016PHB042-01). Informed consent was obtained from all the patients.
Study population
Clinical data were collected from 1145 consecutive hospitalized patients diagnosed with AMI in Peking University People's Hospital and Beijing Jishuitan Hospital between October 2013 and September 2015. The exclusion criteria included: incomplete baseline clinical information (n = 9), death or discharged within 48 h of admission due to insufficient time for AKI development (n = 4), complication of septic shock (n = 3), and with prior diagnosis of end-stage renal diseases (n = 2). The final study population included 1124 patients.
Protocol
AMI was diagnosed based on classical history of chest pain, diagnostic electrocardiographic changes, the serial elevation of serum cardiac biomarkers, and wall motion abnormalities on an ultrasonic cardiogram. According to the KDIGO criteria, AKI is defined by an increase in SCr of >0.3 mg/dL within 48 h, and ≥1.5-folds increase from the baseline within the first 7 days. AKI stage is defined according to the KDIGO criteria (AKI stage 1: 1.5- to 1.9-fold over baseline or SCr increase ≥0.3 mg/dL; AKI stage 2: 2.0- to 2.9-fold over baseline; AKI stage 3: 3.0-fold baseline or SCr increase ≥4 mg/dL or need of renal replacement therapy).[7] All the patients were evaluated and divided into either the AKI group or the non-AKI group.
Cardiac function of the patients was classified based on the Killip classification. An assessment of the left ventricular ejection fraction (LVEF) was made within the first 24 h of hospitalization by bedside echocardiography. The in-hospital mortality and length of hospital stay were documented for all the patients. In addition, the patients’ records were reviewed to obtain comprehensive baseline demographic information, including age, gender, co-morbidities, and smoking habits; initial vital signs; other complications, including cardiogenic shock and ventricular fibrillation; laboratory tests; the occurrence of emergent percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery; the need for intra-aortic balloon pump (IABP) treatment; brad arrhythmias requiring pacemakers; and the use of mechanical ventilation and medications. The maximum daily doses of intravenous loop diuretics were all converted to furosemide, using following formula: 1 mg bumetanide = 20 mg torsemide = 40 mg furosemide.
Statistical analysis
All descriptive statistics were summarized and displayed as the mean ± standard deviation (SD) or the median with 25% to 75% inter-quartile range. The primary analysis compared the AKI group with the non-AKI group. All the variables were tested for a normal distribution using the Kolmogorov-Smirnov test. Continuous variables and normal distribution data were compared using independent sample t tests. Categorical data were compared using the Chi-square test or Fisher exact test. The independent predictors of AKI were identified using logistic regression and including the factors that were significant in the univariate analysis. A P < 0.05 was considered statistically significant. Multiple logistic regression models were generated using the Enter mode, and the association measures were calculated (adjusted odds ratio) with a confidence interval (CI) of 95%. For development of the logistic regression model, continuous variables were categorized according to the inflection point on the receiver operating characteristic curve (ROC curve). For the investigation of independent risk factors for AKI, stepwise backward elimination multivariate analysis was performed. Variables determined by logistic regression underwent probit regression to calculate the weight of each variable in the predicting score based on the probit coefficient of each variable. The Hosmer-Lemeshow goodness-of-fit test was used to evaluate the agreement between the observed and expected number of survivors and non-survivors according to the model. P > 0.05 would indicate a good fit for the model. All the analyses were performed with SPSS 21.0 software (SPSS Inc., Chicago, IL, USA).
Results
Baseline characterization
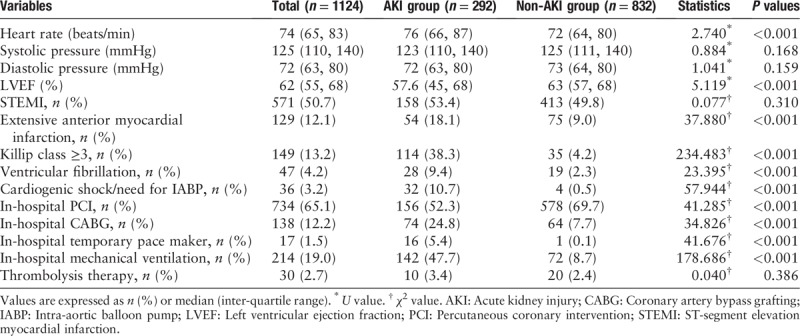
The baseline clinical characteristics of the patients with and without AKI are listed in Table 1. Compared with the non-AKI group, the AKI group was more likely to be older, female, have more co-morbidities, and have a lower baseline eGFR, and higher levels of SCr, troponin I (TNI), brain natriuretic peptide (BNP), C-reactive protein (CRP), and fasting blood glucose. With regard to the use of medications, the AKI group used less angiotensin-converting enzyme inhibitors/angiotensin receptor blocker (ACEI/ARB) and statins but received larger doses of diuretics than the non-AKI group [Table 1]. In addition, compared with the non-AKI group, the AKI group had a higher Killip class and was more likely to have extensive anterior myocardial infarction. Moreover, compared with the non-AKI group, the need of CABG and pacemaker was significantly higher in the AKI group, but in-hospital PCI was relatively lower. In summary, in comparison to the non-AKI group, the AKI group experienced more cardiac complications and associated adverse events [Table 2].
Table 1.
Baseline characteristics in patients with AMI.
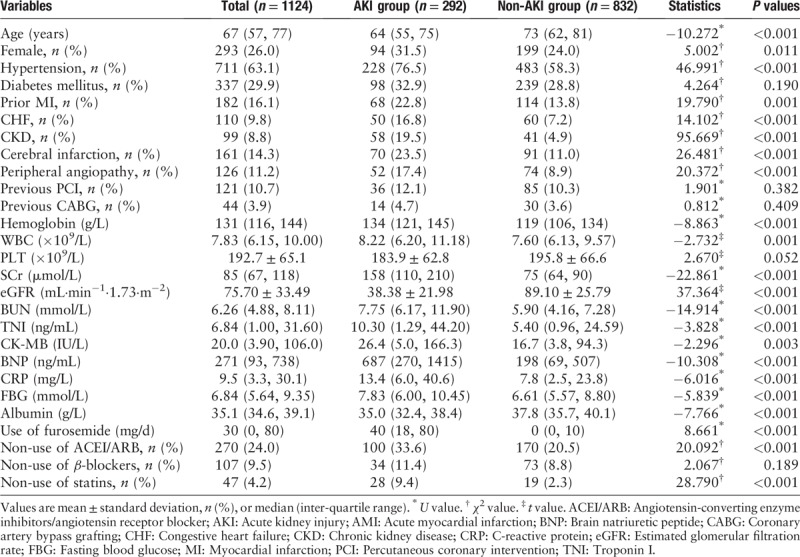
Table 2.
Cardiac-associated data and in-hospital complications.
AKI during hospitalization and its prognosis
In our study, 292 (26.0%) patients developed AKI during hospitalization, including 134 (11.9%) patients with stage 1, 102 (9.1%) patients with stage 2, and 56 (5.0%) patients with stage 3 diseases.
Compared with non-AKI group, the length of hospital stay of the AKI group was longer (20 days vs. 12 days, P < 0.01). Mortality was higher in patients who developed AKI than in those who did not (20.5% vs. 0.6%, P < 0.001). There was a stepwise increase in mortality according to the AKI severity (8.2%, 24.5%, and 42.9% from stages 1, 2 and 3, respectively, P < 0.001) [Figure 1].
Figure 1.
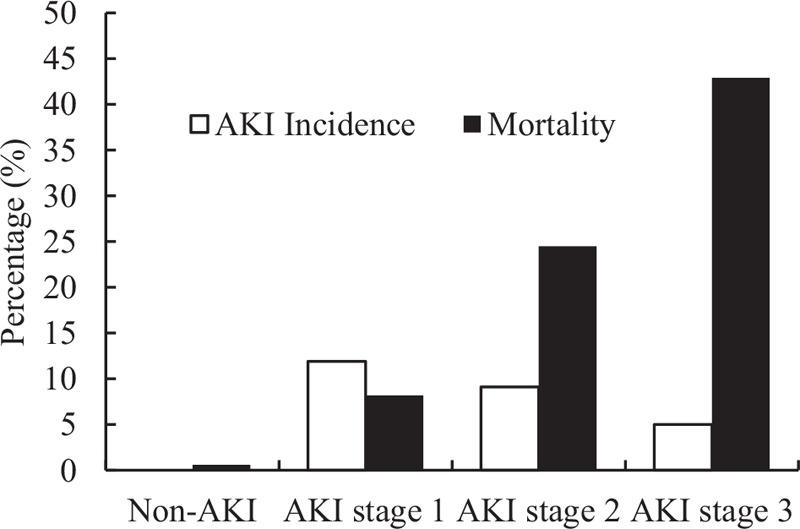
Acute kidney injury incidence and relative mortality among each group by stage. Overall, 26.0% patients developed AKI during the hospitalization, including 11.9% with stage 1, 9.1% with stage 2, and 5.0% with stage 3. Compared with non-AKI group, patients developed AKI have significantly higher in-hospital mortality that raises with stage of AKI (8.2%, 24.5%, and 42.9%, respectively, from stage 1 to 3). AKI: Acute kidney injury.
Risk factors for AKI
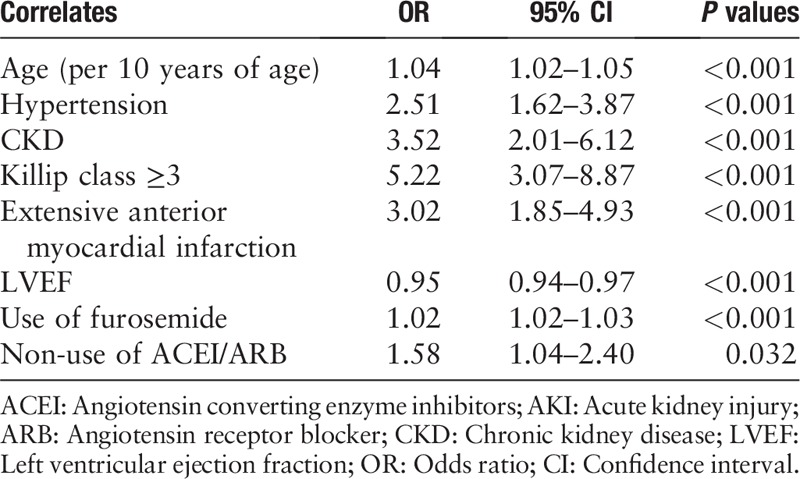
We created a regression model to elucidate the risk factors associated with AKI in patients with AMI. In the final regression model, the following variables were included: age, hypertension, chronic kidney disease (CKD), Killip class ≥3, LVEF, use of furosemide, and non-use of ACEI/ARB.
The major risk factors for AKI were age (>60 years old) (odds ratio [OR] = 1.04, 95% CI 1.02–1.05, P < 0.01), hypertension (OR = 2.51, 95% CI 1.62–3.87, P < 0.01), CKD (OR = 3.52, 95% CI 2.01–6.16, P = 0.000), Killip class ≥3 (OR = 5.22, 95% CI 3.07–8.87, P = 0.000), extensive anterior myocardial infarction (OR = 3.02, 95% CI 1.85–4.93, P = 0.000), use of furosemide (OR = 1.02, 95% CI 1.02–1.03, P = 0.000), and non-use of ACEI/ARB (OR = 1.58, 95% CI 1.04–2.40, P = 0.032) [Table 3]. Moreover, compared with the non-AKI group, patients in AKI group received less statins.
Table 3.
The multiple logistic regression model for predicting AKI.
Based on the linear regression analyses, we created a risk score for AKI among patients with AMI. The score considered age, hypertension, CKD, Killip class ≥3, extensive anterior myocardial infarction, use of furosemide and non-use of ACEI/ARB. The weight of each variable was based on multivariate analysis coefficients. The derived risk score for AKI [Table 3] had a good correlation when tested with the Hosmer-Lemeshow method (χ2 = 12.848, P = 0.117) and a discrimination capacity (area under receiving operator characteristics) of 0.907 (0.887–0.926) [Figures 2 and 3].
Figure 2.
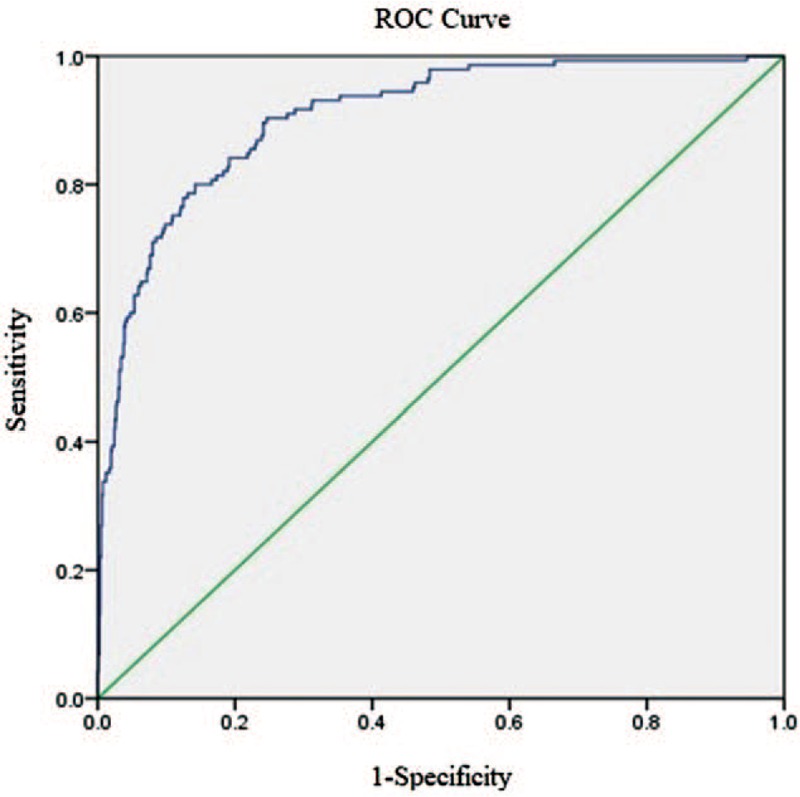
Discrimination of risk scores for developing AKI after AMI (n = 1124). Score considered age, hypertension, CKD, Killip classification, extensive anterior myocardial infarction, use of furosemide, and non-use of ACEI/ARB. The weight of each variable was calculated based on their respective multivariate analysis coefficient. ACEI/ARB: Angiotensin-converting enzyme inhibitors/angiotensin receptor blocker; AKI: Acute kidney injury; AMI: Acute myocardial infarction; CKD: Chronic kidney disease.
Figure 3.
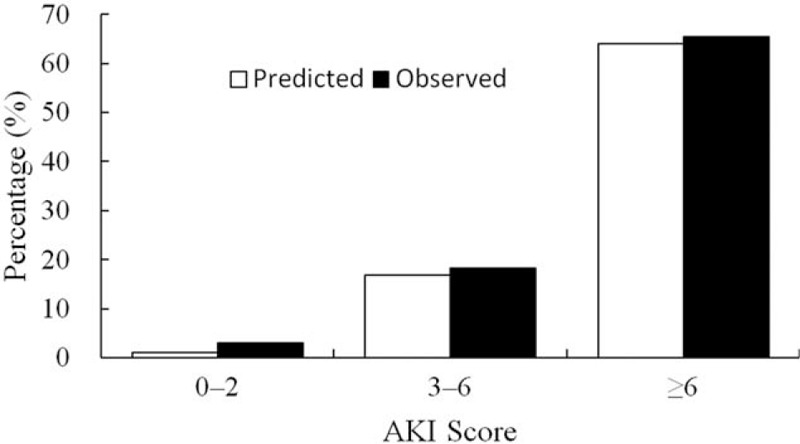
AKI score calibration. Our AKI risk score considers age (>60 years), hypertension, CKD, Killip class ≥3, extensive anterior myocardial infarction, use of furosemide and non-use of ACEI/ARB, one point for each risk factor. It is an accurate tool to identify patients at high risk of developing AKI. ACEI/ARB: Angiotensin-converting enzyme inhibitors/angiotensin receptor blocker; AKI: Acute kidney injury; CKD: Chronic kidney disease.
Discussion
Together with previous studies,[1–5] our study confirmed AKI as a common, serious, and fatal complication of AMI. We found that the incidence of AKI in AMI was as high as 26.0%. Even for patients with stage 1 AKI, the overall mortality during hospitalization was 15 times higher than that of patients without AKI (8.2% vs. 0.6%), and as AKI stage progressed, the mortality increased. Reducing the incidence and mortality rates of AKI after AMI is an urgent issue that needs to be addressed. Identifying the risk factors for AKI among patients with AMI will provide a theoretical basis for future prevention and treatment.
The deterioration of renal function during hospitalization is influenced by many factors, including types of myocardial infarction, patient hemodynamic status, cardiac function, the use of contrast agents and drugs, underlying diseases (hypertension or diabetes mellitus), infection, and the performance of CABG surgery.[3–5,10,11] In our study, we found that age, hypertension, CKD, Killip class ≥3, extensive anterior myocardial infarction, and use of furosemide were the independent risk factors for AMI-related AKI. The use of ACEI/ARB drugs might play a role in preventing AKI. In the retrospective study, the KDIGO AKI definition was adopted. Most previous researches have used only the AKIN criteria that might underestimate the incidence of AKI.
Age (per 10 years) is an independent risk factor for AKI, which may be related to renal atherosclerosis and decreased kidney reserve capacity in elderly patients.[12–14] Almost all related studies concluded that a decrease in basic renal function is an important risk factor for AKI, and our study also verified this conclusion.[9,11,14] A decrease in kidney function was highly associated with AKI. Without sufficient renal reserve, the kidney was more vulnerable when facing acute challenges. A history of CKD was a highly suggestive risk factor in our study.[9,11,13] The incidence of AKI in patients with CKD with AMI was 3.52 times higher than that of patients without CKD, indicating that special attention should be paid to patients with combined AMI and CKD in clinical practice, and careful consideration of their hydration, the dose of contrast agents used may improve outcomes for these patients.
We also found that Killip class ≥3 and extensive anterior myocardial infarction are both risk factors for AKI in patients with AMI. This finding was consistent with the conclusion of prior studies.[14,15] Hemodynamic disorders are more common in patients with Killip class ≥3 and extensive anterior myocardial infarction. They have increased risk for cardiogenic shock, acute heart failure, malignant arrhythmia, and mechanical complications that could lead to a decrease in cardiac output, thus insufficient renal blood flow, which aggravates renal ischemia and inducing AKI.[6] However, we need to note that it was impossible to collect sufficient clinical data related to right-sided heart function due to the nature of retrospective data. Acute right ventricular dysfunction could be combined with acute inferior myocardial infarction. It has been shown that acute right-sided heart failure could also reduce effective renal perfusion through increased central venous pressure and intra-abdominal pressure. Reduced renal perfusion is also a risk factor for AKI.[16]
The use of furosemide is a risk factor for AKI. The use of high dose loop diuretic could cause the agitation of sympathetic nervous system and rennin-angiotensin system (RAS), leading to the increase of peripheral vascular resistance, decrease of LVEF, and eventually renal perfusion, resulting in AKI.[17]
ACEI/ARB is an important first-line antihypertensive drug, which is widely used in the treatment of chronic heart failure and CKD. It has well-defined effects of reducing blood pressure, improving myocardial remodeling, reducing urine protein, and retarding the progression of renal function. In this study, we found ACEI/ARB could be a protective factor against AKI after AMI. However, it is still controversial whether patients with AMI-induced AKI should be treated early with ACEI/ARB. Some studies have shown that the use of ACEI/ARB in patients with insufficient renal perfusion might lead to a decline in the glomerular filtration rate, thereby inducing AKI.[17,18] Conversely, Liu et al[19] found that the expression level of serum angiotensin II in patients with AMI with AKI was significantly increased, indicating the activation of the RAS system in the AKI group and indirectly suggesting the need for the use of ACEI/ARB. Based on the results of the available studies, it is necessary to discuss whether patients with AKI with AMI and CKD should be treated with ACEI/ARB. A study showed that doctors had reduced the use of anti-RAS therapy for patients based on their subjective perception of the side effects of hyperkalemia and elevated SCr.[20] There have been controversial results from recent studies. A clinical study of 6867 cases showed that the application of anti-RAS therapy in patients with CKD with ACS could improve 90-day mortality rates.[21] In contrast, another study suggested no benefit of ACEI/ARB drugs in cardiovascular surgery patients.[22] More studies should be conducted to elucidate the effect of ACEI/ARB on AMI and patients with CKD with regards to the induction of AKI.
Our risk score had good discriminatory capacity to predict AKI risk following the diagnosis of AMI. It can help emergency physicians to identify high-risk patient earlier, therefore helping the patients avoiding exposure to excessive contrast volume, nephrotoxic medicine, and hemodynamic instability, which all can reduce renal injury and improve outcomes.
However, our study had some obvious limitations. First, it was a non-randomized, retrospective, observational study, and may have been subject to bias. Second, we only observed in-hospital outcomes and lacked data on long-term prognoses. In addition, one major limitation was the lack of information regarding the volume of contrast material used, which was only available for a minority of the patients. Contrast-induced nephropathy is a prevalent and preventable complication of coronary angiography and is reported to be the third most common cause of hospital-acquired renal failure, but it was not included in the analysis.
Finally, we conclude that AKI is a perilous and common complication in patients with AMI. Its occurrence is associated with adverse in-hospital outcomes. A simple score was developed to predict AKI risk in patients with AMI.
Conflicts of interest
None.
Footnotes
How to cite this article: Wang C, Pei YY, Ma YH, Ma XL, Liu ZW, Zhu JH, Li CS. Risk factors for acute kidney injury in patients with acute myocardial infarction. Chin Med J 2019;132:1660–1665. doi: 10.1097/CM9.0000000000000293
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005; 16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Xing G, Wang L, Wu Y, Li S, Xu G, et al. Acute kidney injury in China: a cross-sectional survey. Lancet 2015; 386:1465–1471. doi: 10.1016/S0140-6736(15)00344-X. [DOI] [PubMed] [Google Scholar]
- 3.Shacham Y, Leshem-Rubinow E, Steinvil A, Assa EB, Keren G, Roth A, et al. Renal impairment according to acute kidney injury network criteria among ST elevation myocardial infarction patients undergoing primary percutaneous intervention: a retrospective observational study. Clin Res Cardiol 2014; 103:525–532. doi: 10.1007/s00392-014-0680-8. [DOI] [PubMed] [Google Scholar]
- 4.Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv 2014; 7:1–9. doi: 10.1016/j.jcin.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang SH, Jeong MH, Ahmed K, Kim MC, Cho KH, Lee MG, et al. Different clinical outcomes of acute kidney injury according to acute kidney injury network criteria in patients between ST elevation and non-ST elevation myocardial infarction. Int J Cardiol 2011; 150:99–101. doi: 10.1016/j.ijcard.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Marenzi G, Assanelli E, Campodonico J, De Metrio M, Lauri G, Marana I, et al. Acute kidney injury in ST-segment elevation acute myocardial infarction complicated by cardiogenic shock at admission. Crit Care Med 2010; 38:438–444. doi: 10.1097/CCM.0b013e3181b9eb3b. [DOI] [PubMed] [Google Scholar]
- 7.Pickering JW, Endre ZH. The definition and detection of acute kidney injury. J Renal Inj Prev 2014; 3:21–25. doi: 10.12861/jrip.2014.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weisbord SD, Chen H, Stone RA, Kip KE, Fine MJ, Saul MI, et al. Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J Am Soc Nephrol 2006; 17:2871–2877. doi: 10.1681/ASN.2006030301. [DOI] [PubMed] [Google Scholar]
- 9.Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Wiviott SD. Short-term outcomes of acute myocardial infarction in patients with acute kidney injury: a report from the national cardiovascular data registry. Circulation 2012; 125:497–504. doi: 10.1161/CIRCULATIONAHA.111.039909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koreny M, Karth GD, Geppert A, Neunteufl T, Priglinger U, Heinz G, et al. Prognosis of patients who develop acute renal failure during the first 24 hours of cardiogenic shock after myocardial infarction. Am J Med 2002; 112:115–119. doi: 10.1016/S0002-9343(01)01070-1. [DOI] [PubMed] [Google Scholar]
- 11.Levin A, Warnock DG, Mehta RL, Kellum JA, Shah SV, Molitoris BA, et al. Improving outcomes from acute kidney injury: report of an initiative. Am J Kidney Dis 2007; 50:1–4. doi: 10.1053/j.ajkd.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Palomba H, de Castro I, Neto AL, Lage S, Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS score. Kidney Int 2007; 72:624–631. doi: 10.1038/sj.ki.5002419. [DOI] [PubMed] [Google Scholar]
- 13.Akgul O, Uyarel H, Pusuroglu H, Isiksacan N, Turen S, Erturk M, et al. High BNP level as risk factor for acute kidney injury and predictor of all-cause mortality in STEMI patients. Herz 2014; 39:507–514. doi: 10.1007/s00059-013-3853-8. [DOI] [PubMed] [Google Scholar]
- 14.Queiroz RE, de Oliveira LS, de Albuquerque CA, Santana Cde A, Brasil PM, Carneiro LL, et al. Acute kidney injury risk in patients with ST-segment elevation myocardial infarction at presentation to the ED. Am J Emerg Med 2012; 30:1921–1927. doi: 10.1016/j.ajem.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Amin AP, Spertus JA, Reid KJ, Lan X, Buchanan DM, Decker C, et al. The prognostic importance of worsening renal function during an acute myocardial infarction on long-term mortality. Am Heart J 2010; 160:1065–1071. doi: 10.1016/j.ahj.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Viswanathan G, Gilbert S. The cardiorenal syndrome: making the connection. Int J Nephrol 2010; 2011:283137.doi: 10.4061/2011/283137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahesh B, Yim B, Robson D, Pillai R, Ratnatunga C, Pigott D. Does furosemide prevent renal dysfunction in high-risk cardiac surgical patients? Results of a double-blinded prospective randomised trial. Eur J Cardiothorac Surg 2008; 33:370–376. doi: 10.1016/j.ejcts.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Mansfield KE, Nitsch D, Smeeth L, Bhaskaran K, Tomlinson LA. Prescription of renin-angiotensin system blockers and risk of acute kidney injury: a population-based cohort study. BMJ Open 2016; 6:e012690.doi: 10.1136/bmjopen-2016-012690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu KL, Lee KT, Chang CH, Chen YC, Lin SM, Chu PH. Elevated plasma thrombomodulin and angiopoietin-2 predict the development of acute kidney injury in patients with acute myocardial infarction. Crit Care 2014; 18:R100.doi: 10.1186/cc13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wetmore JB, Tang F, Sharma A, Jones PG, Spertus JA. The association of chronic kidney disease with the use of renin-angiotensin system inhibitors after acute myocardial infarction. Am Heart J 2015; 170:735–743. doi: 10.1016/j.ahj.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddan DN, Szczech L, Bhapkar MV, Moliterno DJ, Califf RM, Ohman EM, et al. Renal function, concomitant medication use and outcomes following acute coronary syndromes. Nephrol Dial Transplant 2005; 20:2105–2112. doi: 10.1093/ndt/gfh981. [DOI] [PubMed] [Google Scholar]
- 22.Arora P, Rajagopalam S, Ranjan R, Kolli H, Singh M, Venuto R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 2008; 3:1266–1273. doi: 10.2215/CJN.05271107. [DOI] [PMC free article] [PubMed] [Google Scholar]


