Abstract
Background:
Depression affects approximately 5% of elderly people and its etiology might be related to chronic stress exposure during neurodevelopmental periods. In this study, we examined the effects of adolescent chronic social stress in aged mice on depressive behaviors and the excitatory-inhibitory (E/I) balance in stress-sensitive regions of the brain.
Methods:
Sixty-four adolescent, male C57BL/6 mice were randomly assigned to either the 7-week (from post-natal days 29 to 77) social instability stress (stress group, n = 32) or normal housing conditions (control group, n = 32). At 15 months of age, 16 mice were randomly selected from each group for a series of behavioral tests, including two depression-related tasks (the sucrose preference test and the tail suspension test). Three days following the last behavioral test, eight mice were randomly selected from each group for immunohistochemical analyses to measure the cell density of parvalbumin (PV+)- and calretinin (CR+)-positive gamma-aminobutyric-acid (GABA)ergic inhibitory inter-neurons, and the expression levels of vesicular transporters of glutamate-1 (VGluT1) and vesicular GABA transporter (VGAT) in three stress-sensitive regions of the brain (the medial pre-frontal cortex [mPFC], hippocampus, and amygdala).
Results:
Behaviorally, compared with the control group, adolescent chronic stress increased depression-like behaviors as shown in decreased sucrose preference (54.96 ± 1.97% vs. 43.11 ± 2.85%, t(22) = 3.417, P = 0.003) and reduced latency to immobility in the tail suspension test (92.77 ± 25.08 s vs. 33.14 ± 5.95 s, t(25) = 2.394, P = 0.025), but did not affect anxiety-like behaviors and pre-pulse inhibition. At the neurobiologic level, adolescent stress down-regulated PV+, not CR+, inter-neuron density in the mPFC (F(1, 39) = 19.30, P < 0.001), and hippocampus (F(1, 42) = 5.823, P = 0.020) and altered the CR+, not PV+, inter-neuron density in the amygdala (F(1, 28) = 23.16, P < 0.001). The VGluT1/VGAT ratio was decreased in all three regions (all F > 10.09, all P < 0.004), which suggests stress-induced hypoexcitability in these regions.
Conclusions:
Chronic stress during adolescence increased depression-like behaviors in aged mice, which may be associated with the E/I imbalance in stress-sensitive brain regions.
Keywords: Adolescence, Aging, Depression, Inter-neuron, Stress, Vesicular glutamate transporter-1
Introduction
Epidemiologic studies suggest that depression affects approximately 5% of elderly people (aged ≥65 years)[1] and its etiology may be related to chronic stress exposure.[2,3] On the contrary, exposure to stressful life events during neurodevelopmental periods is strongly associated with increased risks of psychosis (eg, depression) in later life.[4,5] Therefore, pre-clinical animal studies are warranted to establish the causal link between early-life stress exposure and late-life abnormalities, which may also shed light on understanding the disease pathogenesis and developing putative interventions.
Adolescence is a critical neurodevelopmental period characterized by large-scale, dynamic reorganization of cortical-cortical and cortical-subcortical neural circuits.[6,7] Pre-clinical studies have shown that adolescent chronic stress could increase depression- and anxiety-like behaviors and impair cognitive functions in adult animals, which partially resemble the core symptoms of depression.[8,9] Recently, several studies have examined the behavioral effects of adolescent chronic stress in aged animals[10–13] and found that the elderly, stressed mice exhibited cognitive deficits and a tendency towards increased anxiety-like behaviors.[11,12] However, it remains unclear whether adolescent chronic stress would affect depression-like behaviors in aged animals and, if yes, how the stress-sensitive brain regions are involved.[8,14]
Recent progress on the neurobiologic mechanisms of depression has highlighted the excitatory-inhibitory (E/I) balance involving glutamatergic and gamma-aminobutyric-acid (GABA)ergic neurotransmission in the brain.[15,16] Alterations of glutamatergic and GABAergic neurotransmission have been observed in depressed patients,[17,18] including patients with late-life depression,[19,20] and in animals exposed to chronic stress.[21–25] Considering that adolescence is associated with the pruning of excitatory synapses and the functional maturation of the GABAergic system,[26] stress exposure during adolescence may alter the developmental trajectory of the E/I balance and lead to long-lasting changes in depressive behaviors.
In this study, we examined the effects of adolescent chronic social stress in aged mice on depressive behaviors and E/I balance in stress-sensitive regions of the brain, including the mouse medial pre-frontal cortex (mPFC), hippocampus, and amygdala. We adopted the adolescent social instability stress paradigm, in which cage mates were frequently changed for 7 weeks to create an unstable social environment.[27] The GABAergic inter-neurons can be classified according to the expression of calcium-binding proteins, for example, parvalbumin (PV), calbindin, and calretinin (CR).[28] We examined PV-positive (PV+) and CR-positive (CR+) neurons because these proteins exhibit developmental changes during adolescence.[29] The expression levels of the vesicular transporters of glutamate and GABA, that is, the vesicular glutamate transporter-1 (VGluT1) and vesicular GABA transporter (VGAT), were measured to characterize the E/I balance at the synaptic level.[30,31]
Methods
Ethical approval
The experiments were conducted in compliance with the National Institute of Health Guide for Use and Care of Laboratory Animals and were approved by the Peking University Committee on Animal Care and Use. All efforts were made to minimize animal suffering throughout the experiments.
Animals
Experiments were carried out with the adolescent male C57BL/6N mice (n = 64, aged 21–22 days) from Vital River Laboratories (Beijing, China). All mice were given 7 days for acclimatization before the stress procedure and housed in standard polypropylene cages containing wood shavings (four per cage) under a fixed 12:12 light/dark cycle (lights on 08:00) and with constant temperature (23 ± 1°C). Food and water were provided ad libitum.
Adolescent chronic social instability stress procedure
Adolescent mice were randomly assigned to stress and control groups (n = 32 per group). Mice in the stress group were subjected to the chronic social instability stress procedure during adolescence as previously described.[27] Briefly, rodent composition in each cage was changed twice per week for seven weeks (during post-natal days 29–77). A randomized rotation schedule was used to minimize the possibility of encounter with the same animal during the experiment. Mice in the control group remained housed four in home cages with the same roommates during post-natal days 29 to 77. At the end of the stress procedure, mice in both groups were separated and then single housed until they were 15 months old.
Experimental design
Sixteen mice were randomly selected from each group for the behavioral tests. The tests were performed in the following order: the sucrose preference test, the tail suspension test, the open field test, and pre-pulse inhibition (PPI). Three days after the last behavioral test, eight mice randomly selected from each group were sacrificed for immunohistochemical analyses.
Behavioral tests
Sucrose preference test
The sucrose preference test has been widely used to assess anhedonia (a major symptom of depression) by determining animals’ preference for sucrose solution over tap water. Five hours before the test (at 15:00), animals were single housed and water deprived, with free access to food. The test started at 20:00 and animals were provided with two bottles for 1 h, one with 0.2% sucrose solution and the other with tap water. Water and sucrose consumption was measured by weighing the bottles before and after they were given to the mice. The positions of the two bottles were counterbalanced across groups to avoid the potential side-preference bias. Sucrose preference percentage (%) was calculated as (sucrose solution consumption)/(water consumption + sucrose solution consumption) × 100%.
Tail suspension test
The test was conducted following a well-established protocol.[32] The mice were suspended individually by a metal hook 35 cm above the floor using an adhesive tape. The tape was placed approximately 1 cm from the animal's tail tip. Animal tails were crossed through a plastic cylinder before suspension to prevent tail climbing behaviors. The procedure lasted 6 min and the last 4 min was quantified. Mice were considered immobile only when they hung passively and completely motionless. Immobile time and the latency to immobility were used to assess depression-like behaviors (indicative of behavioral despair).
Open field test
The testing arena (50 cm × 50 cm × 50 cm) was made of gray polypropylene and evenly illuminated at 60 lux during testing. The test was performed between 09:00 and 14:00. In the test, animals were individually placed in the corner of the box and allowed to freely explore the environment for 10 min. The apparatus was wiped with 10% ethanol between tests. General locomotor activity was recorded by a video-tracking system. The time spent in the central part and the latency of the first entry to the center were considered as anxiety-related indices, and were scored by ANY-maze 4.98 (Stoelting, Wood Dale, IL, USA).
Pre-pulse inhibition
PPI of the acoustic startle response is an index of sensorimotor gating, expressed as the motor response reduction to a loud acoustic stimulus (pulse, 120 dB) by a preceding weak sound (pre-pulse, 3–12 dB over the background noise).[33] The test was carried out in startle chambers (SR-LAB, San Diego Instruments, San Diego, CA, USA) as described earlier.[34] Briefly, animals were acclimatized to the startle chambers for 15 min on the day before testing. On the testing day, the experiment began with a 5-min acclimatization period, followed by six consecutive pulse stimuli in order to scale down the initial startle response to a stable plateau. The test session then began, consisting of eight trial types: (i) pulse alone: the 120 dB white noise; (ii–iv) pre-pulse plus pulse: a pre-pulse stimulus of 3, 6, or 12 dB above the background noise (68 dB) was presented 100 ms before the pulse; (v–vii) pre-pulse alone: 3, 6, or 12 dB above the background noise; and (viii) no stimulus. Each stimulus was 20 ms and each trial type was presented ten times in a pseudorandom order with the inter-trial interval varying from 8 to 22 s. The startle response was calculated as the average response to pulse-alone trials. PPI was the percent decrease of the startle in pulse-alone compared with startle in pre-pulse-plus-pulse trials: PPI (%) = [1 − (startle response to (pre-pulse-plus-pulse) trial/ (startle response to pulse-alone) trial)] × 100%.
Immunohistochemistry
Mice were anesthetized with sodium pentobarbital (200 mg/kg, intraperitoneally) and transcardially perfused with 0.9% saline followed by 4% buffered paraformaldehyde. Brains were removed, post-fixed for 12 h in the same fixative at 4°C, and dehydrated in 30% sucrose solution at the same temperature. The brains were then quenched in N-hexane at a temperature of –60°C for 20 s and stored in a freezer at –80°C.
Serial coronal sections were prepared through the three stress-sensitive brain regions, that is, the mPFC (Bregma 1.98–1.54 mm) [Figure 1A], the hippocampus (Bregma –1.58 to –3.16 mm) [Figure 1B], and the amygdala (Bregma –1.22 to –1.70 mm), [Figure 1C], at a 30-μm thickness and 180 μm intervals using a cryostat (Leica, Wetzlar, Germany). Free-floating sections were treated with antibodies that recognize PV (1:20,000, Swant, Bellinzona, Switzerland) and CR (1:5000, Swant). Non-specific binding for PV and CR was blocked with 1% donkey serum in 1 × phosphate-buffered saline (PBS). Sections were incubated overnight with primary antibody at 4°C and incubated with secondary antibody and an avidin/biotin complex reagent for 90 min at room temperature (VECTASTAIN Peroxidase ABC Kit rabbit IgG; Vector Laboratories, Burlingame, CA, USA), respectively. The sections were then washed with 1 × PBS and incubated in 0.05% 3,3′-diaminobenzidine.
Figure 1.
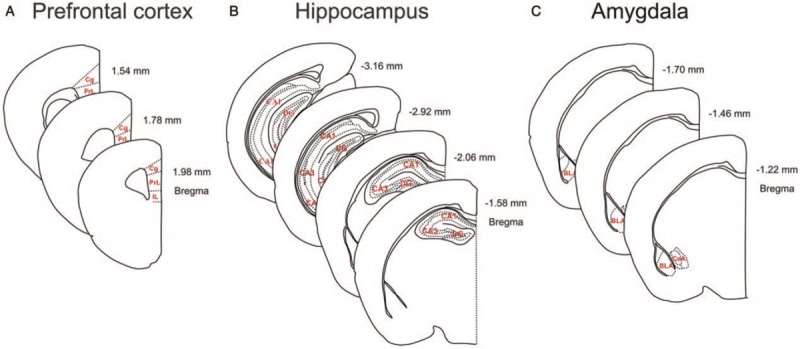
Schematic illustration of sub-regions analyzed in the stress-sensitive areas, including the medial pre-frontal cortex (A), hippocampus (B), and amygdala (C) of aged mice for immunohistochemical analyses.
Images were captured at 20× magnification using an Olympus BX51 optical microscope fixed with a charge-coupled device camera (CoolSNAP MP5; Roper Scientific Corporation, Tucson, AZ, USA) and analyzed using NIH ImageJ software (https://imagej.nih.gov/ij/).
Statistical analysis
All data are expressed as the mean ± standard error (SE). Data points that fell beyond two standard deviations from the mean were considered as outliers and excluded from further analysis. SPSS 17.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA) were used to perform the statistical analyses. The Student t test was used to compare pairs of means. Preference scores for sucrose solution vs. tap water in the sucrose preference test were compared using paired t tests in each group. PPI was analyzed by one-way repeated measures analysis of variance (ANOVA) with the pre-pulse intensity as the within-subject factor and group as the between-subject factor. Neurobiologic measures were analyzed using a two-way ANOVA with the group and sub-regions as factors. The significance level for all statistical tests was set at P < 0.05.
Results
Adolescent chronic social stress induced depression-like behaviors in aged mice
Depression-like behaviors
In the sucrose preference test, compared with control mice, stressed mice showed a significant decrease in their preference for sucrose solution (t(22) = 3.417, P = 0.003) [Figure 2A, left]. No difference in the total liquid consumption was found between the two groups (t(22) = 0.210, P = 0.836) [Figure 2A, right]. In the tail suspension test, although there was no significant difference between the two groups in immobility time (t(26) = 0.131, P = 0.897) [Figure 2B, left], stressed mice displayed shorter latencies to immobility (t(25) = 2.394, P = 0.026) [Figure 2B, right]. Taken together, chronic social stress during adolescence increased depression-like behaviors in aged mice.
Figure 2.
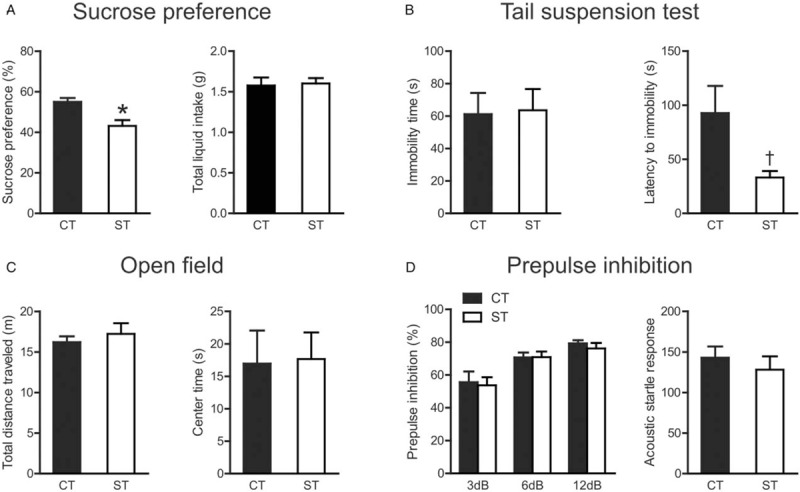
The behavioral effects of adolescent chronic social instability stress in aged mice. (A) Sucrose preference test. (B) Tail suspension test. (C) Open field test. (D) Pre-pulse inhibition test. ∗P < 0.01, †P < 0.05, compared between control and stressed group. Data are presented as mean ± standard error (n = 12–16 per group). CT: Control; ST: Stress.
Anxiety-like behaviors
Chronic social stress in adolescence did not affect the total distance traveled in the open field test (t(29) = 0.691, P = 0.495) [Figure 2C, left], the time spent in the center zone (t(29) = 0.108, P = 0.914) [Figure 2C, right], or the latency to the center zone (t(25) = 0.989, P = 0.332) in aged mice. This indicated that motor activity and anxiety-like behaviors in the open field test in aged mice were unaltered by adolescent chronic stress exposure.
Pre-pulse inhibition
For PPI levels in aged mice [Figure 2D, left], repeated measures ANOVA showed a significant main effect of pre-pulse levels (F(2, 52) = 26.07, P < 0.001), without a significant main effect of group (F(1, 52) = 0.131, P = 0.720) or group × pre-pulse interaction (F(2, 52) = 0.128, P = 0.880). The acoustic startle response in aged mice was not affected by adolescent chronic stress (t(26) = 0.676, P = 0.505) [Figure 2D, right].
Adolescent chronic social stress down-regulated the density of PV+, not CR+, inter-neurons, and altered the excitation-inhibition balance in the mPFC in aged mice
In the mouse mPFC, the numbers of PV+ [Figure 3A] and CR+ [Figure 3B] inter-neurons were counted in three sub-regions. A two-way ANOVA on the PV+ inter-neuron density [Figure 3C] showed a significant main effect for group (F(1, 39) = 19.30, P < 0.001), with a lack of group × sub-region interaction (F(2, 39) = 1.163, P = 0.323), or the main effect of sub-region (F(2, 39) = 0.444, P = 0.644). The main effect of group demonstrated that adolescent chronic stress significantly down-regulated the PV+ inter-neuron density in the mPFC, irrespective of sub-regions. For the CR+ inter-neuron density [Figure 3D], while a significant main effect of sub-region was observed (F(2, 42) = 52.43, P < 0.001), neither the main effect of group (F(1, 42) = 0.040, P = 0.843) nor the group × sub-region interaction (F(2, 42) = 0.115, P = 0.892) approached significance in aged mice.
Figure 3.

Effects of adolescent chronic social instability stress on the density of PV+ and CR+ inter-neurons, the expression levels of VGluT1 and VGAT, and the VGluT1/VGAT ratio in the mPFC in aged mice. (A, B) Representative for immunoreactivity of parvalbumin and calretinin in the control and stressed groups in the mPFC. Immunohistochemical staining. Scale bar = 200 μm. (C, D) The density of PV+ and CR+ inter-neurons in three sub-regions of the mPFC in both groups. (E, F) Representative for immunoreactivity of VGluT1 and VGAT in the mPFC in both groups. Immunohistochemical staining. Scale bar = 200 μm. (G–I) The relative optical density of VGluT1 and VGAT, and their ratio in three sub-regions of the mPFC in both groups. ∗ P < 0.001, indicating the significance of the main effect of group. Data are presented as mean ± standard error (n = 7–8 per group). Cg: Cingulate cortex; CR: Calretinin; CT: Control; IL: Infralimbic cortex; mPFC: Medial pre-frontal cortex; PrL: Pre-limbic cortex; PV: Parvalbumin; ST: Stress; VGAT: Vesicular gamma-aminobutyric acid transporter; VGluT1: Vesicular glutamate transporter-1.
We then examined the VGluT1 [Figure 3E] and VGAT [Figure 3F] immunoreactivity in the three mPFC sub-regions. A two-way ANOVA on VGluT1 immunoreactivity [Figure 3G] showed significant main effects of group (F(1, 39) = 16.45, P < 0.001) and sub-region (F(2, 39) = 5.363, P = 0.009), with a lack of group × sub-region interaction (F(2, 39) = 0.064, P = 0.938). The main effect of group indicated that the expression levels of VGluT1 were decreased by adolescent chronic stress. No significant effects of group or group × sub-region interaction were observed for VGAT immunoreactivity [Figure 3H]. We further analyzed the VGluT1/VGAT ratio to reflect the E/I balance in the mPFC [Figure 3I] and found that the ratio was significantly reduced in aged mice exposed to adolescent chronic social stress (F(1, 36) = 19.59, P < 0.001); furthermore, and the reduction was uniform in the mPFC sub-regions, as suggested by the non-significant group × sub-region interaction (F(2, 36) = 0.429, P = 0.655).
Adolescent chronic social stress decreased the density of PV+, not CR+, inter-neurons, and altered excitation-inhibition balance in the hippocampus in aged mice
We observed stress-induced effects in the hippocampus of aged mice similar to in the mPFC. Two-way ANOVA on PV+ inter-neuron density [Figure 4A and 4C] showed significant main effects of group (F(1, 42) = 5.823, P = 0.020) and sub-region (F(2, 42) = 51.14, P < 0.001), without group × sub-region interaction (F(2, 42) = 2.242, P = 0.119), indicating that adolescent chronic stress down-regulated the density of PV+ inter-neurons in the hippocampus. For CR+ inter-neuron density [Figure 4B and 4D], no significant effects of group (F(1, 42) = 0.531, P = 0.470) or interaction (F(2, 42) = 0.245, P = 0.784) were observed in the hippocampus of aged mice.
Figure 4.
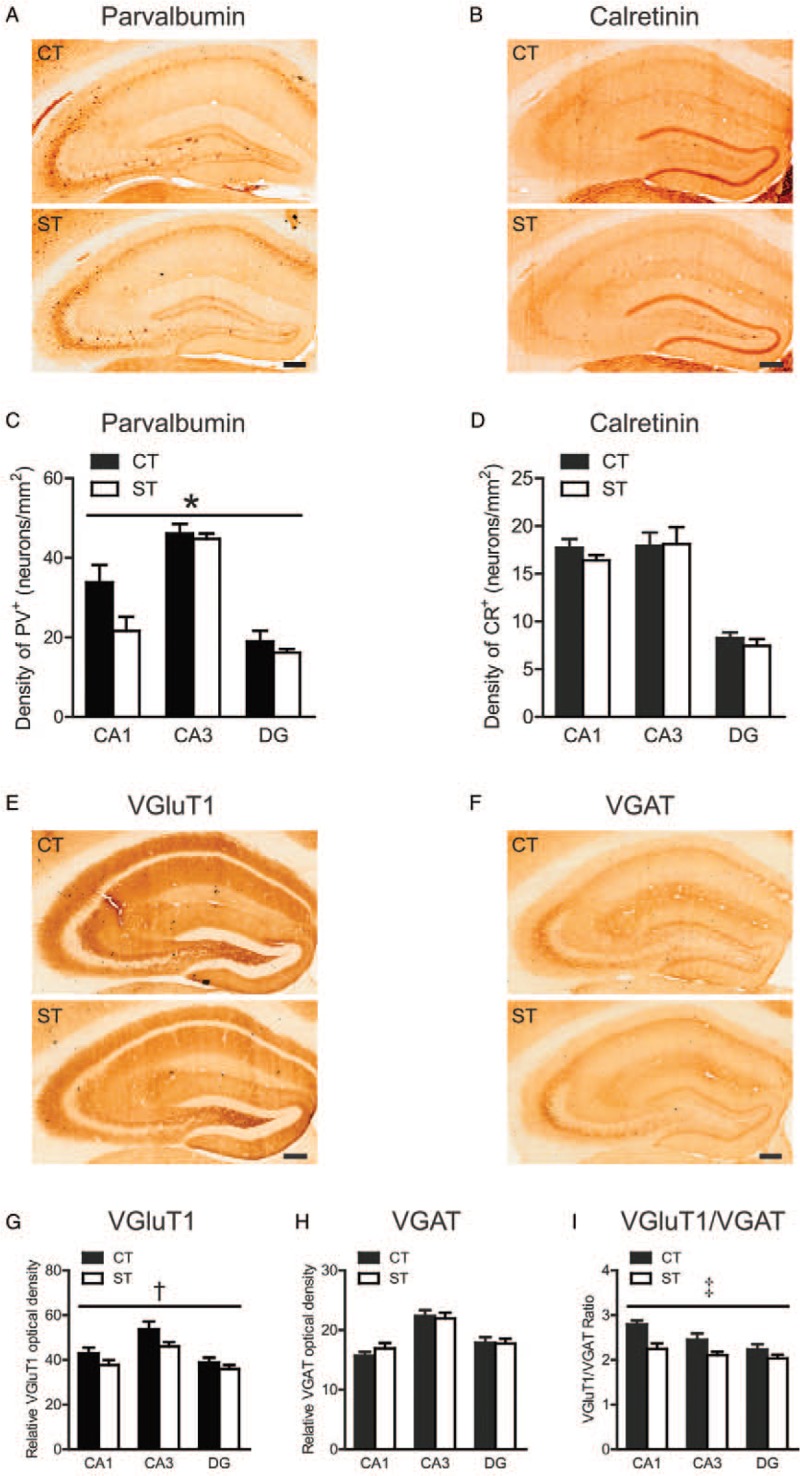
Effects of adolescent chronic social instability stress on the density of PV+ and CR+ inter-neurons, the expression levels of VGluT1 and VGAT, and the VGluT1/VGAT ratio in the hippocampus in aged mice. (A, B) Representative for immunoreactivity of parvalbumin and calretinin in the control and stressed groups in the hippocampus. Immunohistochemical staining. Scale bar = 200 μm. (C, D) The density of PV+ and CR+ inter-neurons in three sub-regions of the hippocampus in both groups. (E, F) Representative for immunoreactivity of VGluT1 and VGAT in the hippocampus in both groups. Immunohistochemical staining. Scale bar = 200 μm. (G–I) The relative optical density of VGluT1 and VGAT, and their ratio in three sub-regions of the hippocampus in both groups. ∗ P < 0.05, †P < 0.01, ‡P < 0.001, indicating the significance of the main effect of group. Data are presented as mean ± standard error (n = 7–8 per group). CA1, Cornu ammonis 1; CA3, Cornu ammonis 3; CT, Control; DG, Dentate gyrus; PV, Parvalbumin; ST, Stress; VGAT, Vesicular gamma-aminobutyric acid transporter; VGluT1, Vesicular glutamate transporter-1.
Similarly, VGluT1 immunoreactivity [Figure 4E and 4G] was significantly decreased by adolescent chronic social stress (F(1, 39) = 7.702, P = 0.008), regardless of sub-regions (group × sub-region interaction, F(2, 39) = 0.519, P = 0.559). The VGAT immunoreactivity was unaffected by chronic stress exposure [Figure 4F and 4H]. The VGluT1/VGAT ratio was also significantly reduced in aged mice exposed to chronic social instability stress during adolescence (F(1, 39) = 17.51, P < 0.001) [Figure 4I], with no group × sub-region interaction (F(2, 39) = 1.329, P = 0.276).
Chronic social stress during adolescence altered the density of CR+, not PV+, inter-neurons, and the excitation-inhibition balance in the amygdala in aged mice
The effects of adolescent chronic stress on inter-neuron density in the amygdala depended on sub-regions and cell types. The density of PV+ inter-neurons [Figure 5A and 5C] was significantly higher in the basolateral amygdala than in the central amygdala (F(1, 28) = 44.59, P < 0.001) but was not affected by stress exposure (main effect of group: F(1, 28) = 0.025, P = 0.875; group × sub-region interaction: F(1, 28) = 0.235, P = 0.632). A significant group × sub-region interaction was found in the density of CR+ inter-neurons (F(1, 28) = 23.16, P < 0.001) [Figure 5B and 5D], which was driven by a stress-induced increase in the basolateral amygdala and a decrease in the central amygdala (P < 0.01, Bonferroni post hoc test). The main effect of group was not significant (F(1, 28) = 0.005, P = 0.945).
Figure 5.
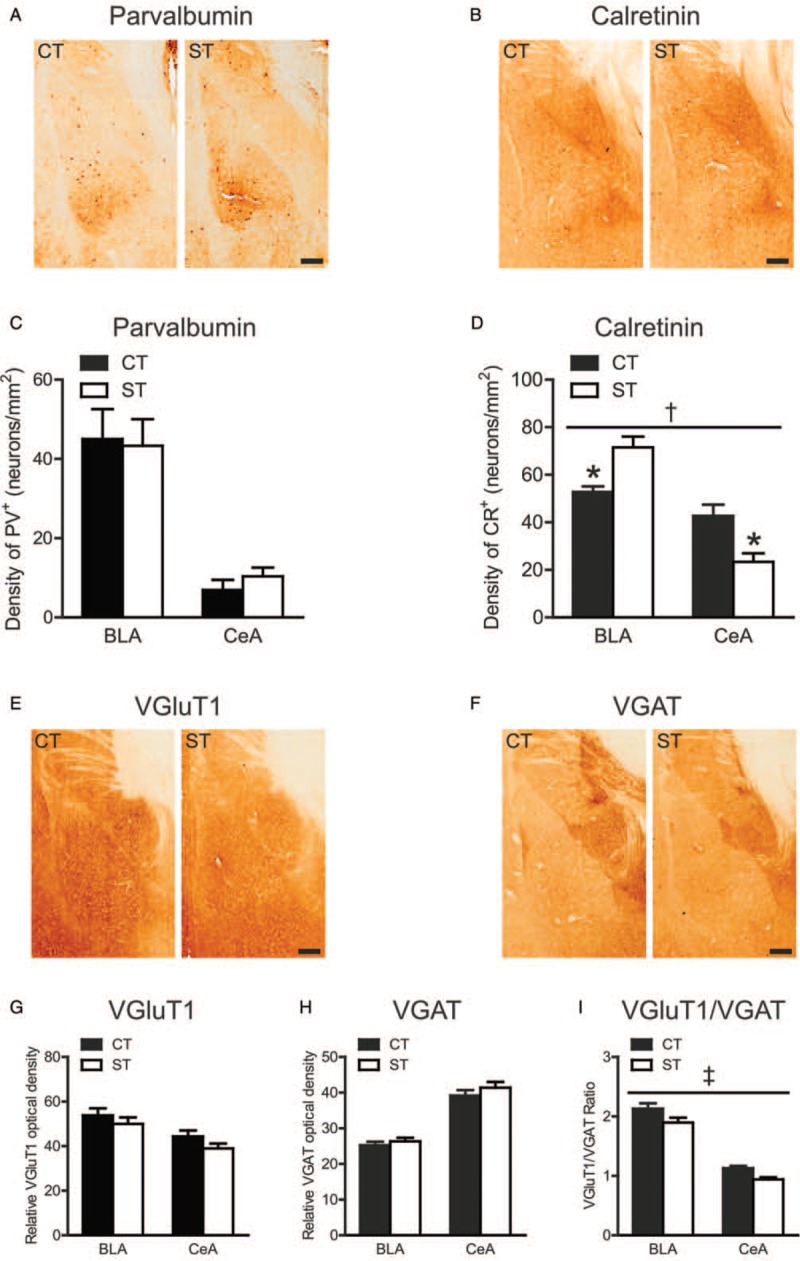
Effects of adolescent chronic social instability stress on density of PV+ and CR+ inter-neurons, the expression levels of VGluT1 and VGAT, and the VGluT1/VGAT ratio in the amygdala in aged mice. (A, B) Representative for immunoreactivity of parvalbumin and calretinin in the control and stressed groups in the amygdala. Immunohistochemical staining. Scale bar = 200 μm. (C, D) The density of PV+ and CR+ inter-neurons in two sub-regions of the amygdala in both groups. (E, F) Representative for immunoreactivity of VGluT1 and VGAT in the amygdala in both groups. Immunohistochemical staining. Scale bar = 200 μm. (G–I) The relative optical density of VGluT1 and VGAT, and their ratio in two sub-regions of the amygdala in both groups. P < 0.01, compared between control and stressed group. †P < 0.001, the group × sub-region interaction. ‡P < 0.01, indicating the significance of the main effect of group. Data are presented as mean ± standard error (n = 7–8 per group). BLA: Basolateral amygdala; CeA: Central amygdala; CR: Calretinin; CT: Control; PV: Parvalbumin; ST: Stress; VGAT: Vesicular gamma-aminobutyric acid transporter; VGluT1: Vesicular glutamate transporter-1.
A two-way ANOVA uncovered no significant stress effects on the expression levels of VGluT1 (main effect of group: F(1, 26) = 2.856, P = 0.103) [Figure 5E and 5G] or VGAT (main effect of group: F(1, 28) = 1.551, P = 0.223) [Figure 5F and 5H] in aged mice. The VGluT1/VGAT ratio was significantly reduced by adolescent chronic stress (F(1, 26) = 10.09, P = 0.004) [Figure 5I] in aged mice, without a significant group × sub-region interaction (F(1, 26) = 0.136, P = 0.715).
Discussion
The current study examined the long-term effects of chronic social stress exposure during adolescence on depression-like behaviors and the E/I balance in aged mice. Behaviorally, adolescent chronic stress decreased sucrose preference (indicative of anhedonia) and the latency to immobility in the tail suspension test (indicative of behavioral despair), but did not affect anxiety-like behaviors and PPI levels. E/I neurotransmission was assessed by the number of GABAergic inhibitory inter-neurons and the expression levels of VGluT1 and VGAT in stress-sensitive brain regions. We found that the effects of adolescent chronic stress on the inter-neuron density depend on brain regions and inter-neuron types. In the mouse mPFC and hippocampus, adolescent stress decreased the PV+, not CR+, inter-neuron density, whereas in the mouse amygdala, the same stress altered the CR+, not PV+, inter-neuron density. All three regions exhibited a stress-induced reduction in the VGluT1/VGAT ratio. Taken together, these results demonstrate that chronic social stress during adolescence increases depression-like behaviors and alters the E/I balance in aged mice.
The effects of adolescent chronic stress on depressive behaviors in adult animals have been consistently reported, which suggests that adolescent stress exposure may disrupt the neurodevelopmental trajectory, producing long-term adverse effects in adulthood.[9] Our study extends research into depressive behaviors in stressed adult animals to aged mice. Intriguingly, the adolescent chronic social instability stress paradigm that we used did not alter immobility in adult CD1 mice,[13] or it only increased immobility in the adult mice with higher baseline corticosterone levels in the tail suspension test.[35] We also observed comparable immobility durations between stressed and control groups in aged mice, which is partially consistent with previous findings in adults. Nevertheless, the presence of both reduced latency to immobility in the tail suspension test and decreased sucrose preference (indicative of anhedonia) indicates that adolescent social stress increases depressive behaviors in aged mice. Our work and previous studies did not observe increased anxiety-like behaviors (in the open field test or in the acoustic startle response test),[10,12,13] or PPI changes in aged mice.[10] Therefore, it is possible that aging may render previously stressed animals more vulnerable to depressive behaviors.
Inhibitory inter-neurons are integral to the regulation of the E/I balance and comprise many subtypes that vary in morphologic, physiologic, and molecular characteristics.[36] The PV+ inter-neurons primarily innervate the somata or the axon initial segments of pyramidal cells, whereas CR+ inter-neurons are known to innervate deep-layer pyramidal cells, as well as other types of GABAergic inter-neurons.[28,37] Only a few studies have investigated the effects of adolescent stress on inhibitory inter-neurons, focusing on pre-frontal PV+ cells and reporting stress-induced alterations dependent on age and stress paradigms.[23,24] Our study examined the effects of adolescent stress on inhibitory inter-neurons in aged mice and found that adolescent stress exposure selectively decreased the number of PV+, not CR+, inter-neurons in the mPFC and hippocampus. The findings are consistent with the selective modulation of PV+, but not CR+, inter-neurons during early life insults[38] or N-methyl-d-aspartate receptor antagonism.[39] The causal involvement of PV+ inter-neurons in depression-like behaviors has been well established[40] and the decreased PV+ inter-neuron density we observed may underlie the increased depression-like behaviors in aged mice.
Intriguingly, chronic social stress during adolescence modulated the number of CR+, not PV+, inter-neurons in the amygdala in aged mice. The stress-induced CR+ inter-neuron density changes are different in the basolateral and central amygdala, which may be related to different cell types in these two sub-regions. While the basolateral amygdala is a more cortical-like structure,[41] the central amygdala is heavily populated by GABAergic medium spiny neurons.[42] Recent studies have reported increased depression-like behaviors and elevated CR expression levels in the amygdala of mice subjected to maternal separation or bilateral ovariectomy,[43,44] providing a link between depressive behaviors and amygdala CR levels.
Besides the alterations of inter-neuron cell density, we also examined the expression levels of VGluT1 and VGAT to reflect the E/I balance at the synaptic level. The two vesicular transporters are located in the pre-synaptic terminals of excitatory and inhibitory synapses, respectively, and are responsible for loading glutamate and GABA into vesicles for their subsequent release into the synaptic cleft.[45] We found that adolescent stress resulted in a reduced VGluT1/VGAT ratio in the three brain regions, which were primarily driven by VGluT1 down-regulation. Previous studies have shown that VGluT1 is associated with depression-like behaviors[46] and is involved in rapid antidepressant-like effects.[47] It is therefore possible that the depression-like behaviors in aged mice may be attributed to the decrease in VGluT1 levels in stress-sensitive brain regions. The unaffected VGAT levels could be related to compensatory VGAT expression in other types of inter-neurons. The reduced VGluT1/VGAT ratios might indicate hypofunctionality of these brain regions,[8,14,48] which is consistent with previous reports of decreased, long-term potentiation in the hippocampal CA1 following adolescent stress.[11] The relationship between the VGluT1/VGAT ratio and the density of inter-neuron subpopulations remains unclear. One possible link for PV+ inter-neurons is that reduced excitatory input decreases their activities, which may lead to the reduced PV expression, given that PV expression is activity dependent.[49,50]
Our study has several limitations which should be addressed in future studies. First, it is unclear whether changes in inter-neuron density resulted from neuronal loss or altered expression of the corresponding calcium binding proteins. We analyzed the expression levels of PV and CR using immunohistochemistry and found that PV expression was significantly decreased in the mouse mPFC and hippocampus, whereas CR expression was unaltered (data not shown). While this finding may provide initial evidence for altered PV expression by adolescent stress, future studies are warranted to disentangle the two possibilities. Second, stress-induced hypoexcitability in the brain regions is speculation based on the reduced VGluT1/VGAT ratios and needs to be confirmed using more direct measures of neuronal functioning (eg, electrophysiology). Finally, our findings suggest a correlational relationship between the depressive phenotype and neurobiologic changes in the E/I balance. To what extent the observed neural changes might mediate behavioral changes warrants further studies.
In summary, adolescent stress exposure induces depression-like behaviors in aged mice. These behaviors are accompanied by GABAergic dysfunction and an altered E/I balance in the mPFC, hippocampus, and amygdala. Together, these results are consistent with the notion that an E/I imbalance may be involved in the neurobiology of depression. The adverse effects of adolescent stress exposure across the lifespan call for early interventions during this vulnerable period.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81630031, 81401129, 81571321, and 81571312), the Beijing Brain Project (No. Z171100000117016), the National Key Basic Research Program of China (973 program; No. 2015CB856401), and the Peking University Medicine Seed Fund for Interdisciplinary Research (No. BMU2017MX021).
Conflicts of interest
None.
Footnotes
How to cite this article: Wang HL, Sun YX, Liu X, Wang H, Ma YN, Su YA, Li JT, Si TM. Adolescent stress increases depression-like behaviors and alters the excitatory-inhibitory balance in aged mice. Chin Med J 2019;132:1689–1699. doi: 10.1097/CM9.0000000000000313
References
- 1.Byers AL, Yaffe K, Covinsky KE, Friedman MB, Bruce ML. High occurrence of mood and anxiety disorders among older adults: The National Comorbidity Survey Replication. Arch Gen Psychiatry 2010; 67:489–496. doi: 10.1001/archgenpsychiatry.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aziz R, Steffens DC. What are the causes of late-life depression? Psychiatr Clin North Am 2013; 36:497–516. doi: 10.1016/j.psc.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draper B, Pfaff JJ, Pirkis J, Snowdon J, Lautenschlager NT, Wilson I, et al. Long-term effects of childhood abuse on the quality of life and health of older people: results from the Depression and Early Prevention of Suicide in General Practice Project. J Am Geriatr Soc 2008; 56:262–271. doi: 10.1111/j.1532-5415.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 4.Morgan C, Gayer-Anderson C. Childhood adversities and psychosis: evidence, challenges, implications. World Psychiatry 2016; 15:93–102. doi: 10.1002/wps.20330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull 2012; 38:661–671. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 2000; 24:417–463. doi: 10.1016/S0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 7.Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A 2011; 108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green MR, McCormick CM. Effects of stressors in adolescence on learning and memory in rodent models. Horm Behav 2013; 64:364–379. doi: 10.1016/j.yhbeh.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 9.McCormick CM, Green MR. From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience 2013; 249:242–257. doi: 10.1016/j.neuroscience.2012.08.063. [DOI] [PubMed] [Google Scholar]
- 10.Morrison KE, Narasimhan S, Fein E, Bale TL. Peripubertal stress with social support promotes resilience in the face of aging. Endocrinology 2016; 157:2002–2014. doi: 10.1210/en.2015-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterlemann V, Rammes G, Wolf M, Liebl C, Ganea K, Muller MB, et al. Chronic social stress during adolescence induces cognitive impairment in aged mice. Hippocampus 2010; 20:540–549. doi: 10.1002/hipo.20655. [DOI] [PubMed] [Google Scholar]
- 12.Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, et al. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: implications for stress-related disorders. Horm Behav 2008; 53:386–394. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Scharf SH, Sterlemann V, Liebl C, Muller MB, Schmidt MV. Chronic social stress during adolescence: interplay of paroxetine treatment and ageing. Neuropharmacology 2013; 72:38–46. doi: 10.1016/j.neuropharm.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 14.Watt MJ, Weber MA, Davies SR, Forster GL. Impact of juvenile chronic stress on adult cortico-accumbal function: Implications for cognition and addiction. Prog Neuropsychopharmacol Biol Psychiatry 2017; 79:136–154. doi: 10.1016/j.pnpbp.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov 2017; 16:472–486. doi: 10.1038/nrd.2017.16. [DOI] [PubMed] [Google Scholar]
- 16.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 2011; 16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 2007; 32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry 2012; 17:1130–1142. doi: 10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khundakar A, Morris C, Thomas AJ. The immunohistochemical examination of GABAergic inter-neuron markers in the dorsolateral prefrontal cortex of patients with late-life depression. Int Psychogeriatr 2011; 23:644–653. doi: 10.1017/S1041610210001444. [DOI] [PubMed] [Google Scholar]
- 20.Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan KR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol Psychiatry 2005; 58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czeh B, Vardya I, Varga Z, Febbraro F, Csabai D, Martis LS, et al. Long-term stress disrupts the structural and functional integrity of GABAergic neuronal networks in the medial prefrontal cortex of rats. Front Cell Neurosci 2018; 12:148.doi: 10.3389/fncel.2018.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czeh B, Varga ZK, Henningsen K, Kovacs GL, Miseta A, Wiborg O. Chronic stress reduces the number of GABAergic interneurons in the adult rat hippocampus, dorsal-ventral and region-specific differences. Hippocampus 2015; 25:393–405. doi: 10.1002/hipo.22382. [DOI] [PubMed] [Google Scholar]
- 23.Mackowiak M, Latusz J, Glowacka U, Bator E, Bilecki W. Adolescent social isolation affects parvalbumin expression in the medial prefrontal cortex in the MAM-E17 model of schizophrenia. Metab Brain Dis 2019; 34:341–352. doi: 10.1007/s11011-018-0359-3. [DOI] [PubMed] [Google Scholar]
- 24.Page CE, Coutellier L. Adolescent stress disrupts the maturation of anxiety-related behaviors and alters the developmental trajectory of the prefrontal cortex in a sex- and age-specific manner. Neuroscience 2018; 390:265–277. doi: 10.1016/j.neuroscience.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 25.Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 2012; 73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilb W. Development of the GABAergic system from birth to adolescence. Neuroscientist 2012; 18:613–630. doi: 10.1177/1073858411422114. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt MV, Sterlemann V, Ganea K, Liebl C, Alam S, Harbich D, et al. Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology 2007; 32:417–429. doi: 10.1016/j.psyneuen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: morphology and quantitative distribution. J Comp Neurol 1997; 377:465–499. [DOI] [PubMed] [Google Scholar]
- 29.Caballero A, Flores-Barrera E, Cass DK, Tseng KY. Differential regulation of parvalbumin and calretinin interneurons in the prefrontal cortex during adolescence. Brain Struct Funct 2014; 219:395–406. doi: 10.1007/s00429-013-0508-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li JT, Zhao YY, Wang HL, Wang XD, Su YA, Si TM. Long-term effects of neonatal exposure to MK-801 on recognition memory and excitatory-inhibitory balance in rat hippocampus. Neuroscience 2015; 308:134–143. doi: 10.1016/j.neuroscience.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Santos M, D’Amico D, Spadoni O, Amador-Arjona A, Stork O, Dierssen M. Hippocampal hyperexcitability underlies enhanced fear memories in TgNTRK3, a panic disorder mouse model. J Neurosci 2013; 33:15259–15271. doi: 10.1523/JNEUROSCI.2161-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, Gould TD. The tail suspension test. J Vis Exp 2012; e3769.doi: 10.3791/3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geyer MA. Assessing prepulse inhibition of startle in wild-type and knockout mice. Psychopharmacology (Berl) 1999; 147:11–13. doi: 10.1007/s002130051130. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Xie X, Li Y, Liu X, Liao X, Su YA, et al. Differential behavioral and neurobiological effects of chronic corticosterone treatment in adolescent and adult rats. Front Mol Neurosci 2017; 10:25.doi: 10.3389/fnmol.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt MV, Scharf SH, Sterlemann V, Ganea K, Liebl C, Holsboer F, et al. High susceptibility to chronic social stress is associated with a depression-like phenotype. Psychoneuroendocrinology 2010; 35:635–643. doi: 10.1016/j.psyneuen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature 2014; 505:318–326. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat 1997; 14:1–19. doi: 10.1016/S0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- 38.Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiatry 2013; 73:574–582. doi: 10.1016/j.biopsych.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Li JT, Su YA, Wang HL, Zhao YY, Liao XM, Wang XD, et al. Repeated blockade of NMDA receptors during adolescence impairs reversal learning and disrupts GABAergic interneurons in rat medial prefrontal cortex. Front Mol Neurosci 2016; 9:17.doi: 10.3389/fnmol.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perova Z, Delevich X, Li B. Depression of excitatory synapses onto parvalbumin interneurons in the medial prefrontal cortex in susceptibility to stress. J Neurosci 2015; 35:3201–3206. doi: 10.1523/JNEUROSCI.2670-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spampanato J, Polepalli J, Sah P. Interneurons in the basolateral amygdala. Neuropharmacology 2011; 60:765–773. doi: 10.1016/j.neuropharm.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol 1993; 330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- 43.Fang YY, Zeng P, Qu N, Ning LN, Chu J, Zhang T, et al. Evidence of altered depression and dementia-related proteins in the brains of young rats after ovariectomy. J Neurochem 2018; 146:703–721. doi: 10.1111/jnc.14537. [DOI] [PubMed] [Google Scholar]
- 44.Lukkes JL, Meda S, Norman KJ, Andersen SL. Anhedonic behavior and gamma-amino butyric acid during a sensitive period in female rats exposed to early adversity. J Psychiatr Res 2018; 100:8–15. doi: 10.1016/j.jpsychires.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eiden LE. The vesicular neurotransmitter transporters: current perspectives and future prospects. FASEB J 2000; 14:2396–2400. doi: 10.1096/fj.00-0817rev. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Garcia AL, Elizalde N, Matrov D, Harro J, Wojcik SM, Venzala E, et al. Increased vulnerability to depressive-like behavior of mice with decreased expression of VGLUT1. Biol Psychiatry 2009; 66:275–282. doi: 10.1016/j.biopsych.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 47.Yu H, Li M, Zhou D, Lv D, Liao Q, Lou Z, et al. Vesicular glutamate transporter 1 (VGLUT1)-mediated glutamate release and membrane GluA1 activation is involved in the rapid antidepressant-like effects of scopolamine in mice. Neuropharmacology 2018; 131:209–222. doi: 10.1016/j.neuropharm.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 48.Lo Iacono L, Carola V. The impact of adolescent stress experiences on neurobiological development. Semin Cell Dev Biol 2018; 77:93–103. doi: 10.1016/j.semcdb.2017.09.040. [DOI] [PubMed] [Google Scholar]
- 49.Shepard R, Coutellier L. Changes in the prefrontal glutamatergic and parvalbumin systems of mice exposed to unpredictable chronic stress. Mol Neurobiol 2018; 55:2591–2602. doi: 10.1007/s12035-017-0528-0. [DOI] [PubMed] [Google Scholar]
- 50.Tropea D, Kreiman G, Lyckman A, Mukherjee S, Yu H, Horng S, et al. Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nat Neurosci 2006; 9:660–668. doi: 10.1038/nn1689. [DOI] [PubMed] [Google Scholar]


