Abstract
Background:
Sleep disorders are one of the earliest non-motor symptoms of Parkinson's disease (PD). Sleep disorders could, therefore, have value for recognition and diagnosis in PD. However, no unified classification and diagnostic criteria exist to evaluate sleep disorders by polysomnography (PSG). Utilizing PSG to monitor sleep processes of patients with PD and analyze sleep disorder characteristics and their relationship with demographic parameters could aid in bridging this gap. This preliminary study aimed to evaluate the clinical characteristic of sleep disorders in PD using PSG.
Methods:
PSG was used to evaluate sleep disorders in 27 patients with PD and 20 healthy volunteers between August 2015 and July 2018 in Fujian Medical University Union Hospital. Total sleep time (TST), sleep efficiency (SE), total wake time, and other parameters were compared between the two groups. Finally, the correlation between sleep disorders and age, disease duration, Unified Parkinson's Disease Rating Scale-III scores, Hoehn-Yahr stage, and levodopa dose were analyzed. The main statistical methods included Chi-square test, two independent samples t test, Fisher exact test, and Pearson correlation.
Results:
Sleep fragmentation in the PD group was significantly increased (74.1%) while difficulty falling asleep and early awakening were not, as compared to healthy controls. No significant differences were found in time in bed, sleep latency (SL), non-rapid eye movement (NREM) stage 1 (N1), N1%, N2, N2%, N3%, and NREM% between PD and control groups; but TST (327.96 ± 105.26 min vs. 414.67 ± 78.31 min, P = 0.003), SE (63.26% ± 14.83% vs. 76.8% ± 11.57%, P = 0.001), R N3 (20.00 [39.00] min vs. 61.50 [48.87] min, P = 0.001), NREM (262.59 ± 91.20 min vs. 337.17 ± 63.47 min, P = 0.003), rapid-eye-movement (REM) (32.50 [33.00] min vs. 85.25 [32.12] min, P < 0.001), REM% (9.56 ± 6.01 vs. 15.50 ± 4.81, P = 0.001), REM sleep latency (157.89 ± 99.04 min vs. 103.47 ± 71.70 min, P = 0.034) were significantly reduced in PD group.
Conclusion:
This preliminary study supported that sleep fragmentation was an important clinical characteristic of sleep disorders in PD. Whether sleep fragmentation is a potential quantifiable marker in PD needs to be further investigated in the future study.
Keywords: Parkinson's disease, Sleep fragmentation, Polysomnography, Clinical characteristic
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disease, with clinical manifestations including dyskinesia symptoms, as well as non-motor symptoms such as autonomic and sensory symptoms, olfactory dysfunction, and cognitive impairment and sleep disorders. Researchers and clinicians have been primarily concerned with manifestations of dyskinesia, while non-motor disorders were typically ignored.
Currently, mental, cognitive, and sleep disorders are considered the three most common non-motor dysfunctions that cause morbidity and mortality in patients with PD with sleep disorders being the most common.[1,2] Investigation in a community-based patients with PD sample concluded that sleep disorders were prevalent in patients with PD, with incidence of >82%.[3] Therefore, sleep disorders are not rare in patients with PD, which require increased attention from researchers and clinicians.[4] In recent years, there have been major advances in our understanding of the relationship between sleep disorders and PD, yet many questions remain unanswered.[5,6] Sleep disorder-specific mechanisms in PD are unknown, but may be associated with latent neural degeneration, motor and non-motor dysfunction, and dopamine replacement therapy.[7] Sleep disorders in PD are complex and diverse, and include insomnia, nightmare, rapid-eye-movement (REM) sleep behavior disorder, periodic limb movement disorder, restless legs syndrome, sleep fragmentation (SF), excessive daytime sleepiness, sleep apnea syndrome (SAS), and sleep attacks, all of which seriously impact quality of life in patient with PD.[6]
Nowadays, more and more attention has been paid to the study of sleep disorders in PD. REM sleep behavior disorder (RBD) is a biomarker of the early PD.[5] However, it is not known whether other types of sleep disorders are also sensitive markers of PD.
This study adopted polysomnography (PSG) to monitor sleep structure and processes, analyzed manifestations and related influencing factors of their sleep disorders, and investigated potential other types of sleep disorders in patients with PD, aiming to improve clinicians’ understanding of the clinical characteristics of sleep disorders in patients with PD.
Methods
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Fujian Medical University Union Hospital. Informed written consent was obtained from all participants prior to their enrollment in this study.
Clinical data and grouping
The 27 patients with primary PD (15 males and 12 females; age range: 49–85 years; mean course of disease: 6.8 ± 3.7 years, with range of 0.2–11.9 years) hospitalized in Department of Neurology of the Fujian Medical University Union Hospital between August 2015 and July 2018 were included. Among them, one patient had a Hoehn-Yahr stage of 1, five patients were stage 2, eight patients were stage 2.5, eight patients were stage 3, four patients were stage 4, and one patient was stage 5. One patient received deep brain stimulation surgery. None of the patients received benzodiazepines, hypnotics, or anti-anxiety/anti-depressant medications. In line with the UK PD Society Brain Bank Clinical Diagnostic Criteria,[8] the enrollment criteria were bradykinesia and one of the following: muscle rigidity, resting tremor (4–6 Hz), or postural balance disorder (not caused by visual, vestibular, cerebellar, or proprioceptive sensation). Exclusion criteria included: (1) essential tremor; (2) secondary Parkinson's syndromes and Parkinsonism-plus syndromes; or (3) patients with PD concurrently suffering from other mental disorders and severe cardiopulmonary diseases that influenced sleeping.
For the control group, 20 middle-aged and elderly individuals complaining of abnormal sleep, without obvious central nervous system disorders (as determined through head computed tomography or magnetic resonance imaging), other mental disorders or severe heart and lung diseases that influenced sleep were voluntarily included. Among them, there were 14 males and six females with age ranging from 49 to 74 years (mean age: 64.2 ± 7.7 years). No volunteers received benzodiazepines, hypnotics, or anti-anxiety/anti-depressant medications.
Patients with PD with sleep disorders were divided into early onset (≤50 years old) and late-onset groups (>50 years old) based on onset age; ≤5 years and >5 years groups based on course of disease; tremor dominant group, akinetic-rigid dominant group and postural instability and gait disorder (PIGD) dominant group based on clinical manifestations; and early-stage group and advanced stage group based on Hoehn-Yahr staging.
PSG evaluation
Cadwell Easy II™ digital PSG software (Cadwell Laboratories, Kennewick, WA, USA) was used for sleep scoring.
PSG sleep staging criteria: according to the Manual for the Scoring of Sleep and Associated Events Rules published by the American Academy of Sleep Medicine in 2007, sleep is divided into non-REM (NREM) and REM sleep. NREM includes sleep stages N1, N2, and N3. Stage W (waking period): within the record frame of 30 s, occipital α rhythm (8–13 Hz) accounts for more than 50% of the record frame; or occurrence of one of the following while the recording frame lacks α rhythm: (A) Eye blink frequency of 0.5 to 2.0 Hz; (B) eye movements in reading; (C) irregular conjugate REM accompanied by normal or enhanced sub-mental electroencephalogram (EEG). Sleep stage N1: Patients with α rhythm have a weakened rhythm and visible low-amplitude mixed-frequency EEG activity (>50% of one frame); in subjects without α rhythm, one of the following appears: (A) brain waves showed reduced EEG background frequency of ≥1 Hz compared with stage W; (B) vertex sharp waves; (C) slow eye movement. Sleep stage N2: EEG with sleep spindles and K-complexes; spindle frequency from 11 to 16 Hz, and sleep spindles >0.5 s and in the central area; K-complexes >0.5 s and in the forehead; stage N2 continues until awake, large body movement, stage N3 or REM. Sleep stage N3: δ wave (0.5–2.0 Hz) with high-amplitude (≥75 μV) appears in frontal lead EEG, accounting for at least 20% of one frame, sleep spindles sustainable; stage N3 continues to N2 stage, REM or awakening. REM sleep: visible low-amplitude mixed-frequency EEG activity presenting as low-voltage θ waves (3–7 Hz), “α waves with a low frequency” (1–2 Hz slower than stage W), observable sawtooth waves (2–6 Hz); sub-mental electromyography (EMG) with nearly flat activity and REM; REM stage continues until stage N1 and N2, occurs in K-complexes without eye movement, or continues until awakening.
Physiological sleep parameters included: (1) Time in bed (TIB; min): total time between turning lights on and off. (2) Total sleep time (TST; min): sum of sleep time in stage N1, N2, N3, and REM. (3) Sleep efficiency (SE; %): Ratio of TST to recording time for sleep in laboratory examination (namely TST × 100/TIB), usually with >85% as normal clinical practice. (4) Total wake time (TWT; min): the number of waking minutes in bed. (5) Sleep latency (SL; min): the time of stage N1 sleep from lights off to 3 min after the first frame, which occurs between 10 and 30 min. (6) REM sleep latency (REM-SL; min): REM sleep time from lights off to the first frame. (7) N1%: percent time in NREM sleep stage N1 in TST; N2%: percent time in NREM sleep stage N2 in TST; N3%: in NREM sleep stage N3 in TST; NREM%: percent time in NREM sleep in TST; REM%: percent time in REM sleep in TST.
SL >30 min in PSG was considered difficulty of falling asleep. More than three arousals exceeding 5 min at night was considered as SF; waking up 1 h before the end of PSG monitoring and failure to fall asleep again after waking up were considered as early awakening
Research methods
Neurologists collected all clinical data via face-to-face interview, examination, and evaluation. All rating scales were completed once and the questionnaire was conducted through patient and family recall. Recorded data included: age, gender, age of onset, disease course, type and dopamine dose, and average daily levodopa equivalent dose (LDE) as calculated by:
LDE = standard levodopa tablets × 1 + levodopa controlled-release tablets × 0.75 + pergolide mesylate tablets × 100 + bromocriptine mesilate tablets × 10 + (standard levodopa tablets × 1 + levodopa controlled-release tablets × 0.75) × 0.33 (entacapone tablets were administrated at the same time) + methanesulfonic acid alpha-dihydroergocryptine tablets × 1.7 + piribedil sustained-release tablets × 1 + pramipexole hydrochloride tablets × 100 + selegiline hydrochloride tablets × 10.[9–11]
The Unified Parkinson's Disease Rating Scale (UPDRS)-III was applied to evaluate dyskinesia in patients with PD. Hoehn-Yahr staging was used to evaluate motor function of patient with PD.
Overnight PSG was conducted, with EEG, electronystagmogram, electrocardiogram, snore, oronasal airflow, sub-mental EMG, blood oxygen saturation, thoracic and abdominal breathing, leg movement, and body position monitored. Sleep behavior disorder and sleep staging were recorded with infrared video to determine the nature. TST, SE, TWT, SL, REM-SL, N1%, N2%, N3%, NREM%, and REM% of the two groups were compared and used to investigate manifestations of sleep disorders. One week before PSG, analeptic and hypnotic drugs were forbidden. Patients were required to go to sleep in the laboratory 1 h earlier than their regular sleep time, for acclimation to sleep monitoring and examination purposes. Upon electrode placement and impedance was detected, PSG monitoring was monitored overnight (minimum 7 h). According to standard methods, EEG signals in F3–A2, F4–Cl, C3–A2, C4–A1, 01–A2, and 02–A1 were synchronously recorded using a surface disk electrode. Sub-mental EMG was recorded using two surface electrodes; eye movement was recorded, by placing an electrode 1 cm superior and inferior outside the right and left outer canthi; respiratory motion of the thorax and abdomen was recorded by impedence-type thoracic and abdominal motion sensor; oronasal airflow and snore were recorded with a pressure-type sensor placed in the nasal vestibule; limb movement was recorded by a displacement transducer fixed on both legs; posture of the body was recorded using a chest-fixed sensor; and blood oxygen saturation of the right index finger was monitored with a blood oxygen saturation sensor.
Statistical analysis
The data with normal distribution were expressed as mean ± standard deviation, and as median (interquartile range) for data with abnormal distribution. Differences in the PSG sleep parameters between PD group and control group were calculated by Chi-square test, Mann-Whitney U test or two independent samples t test. A Fisher exact probability test was applied to compare the incidence of different sleep disorders between PD and control groups. Correlations of partial sleep parameters obtained by PSG monitoring in the PD group were analyzed by Pearson correlation analysis. All data were analyzed with SPSS version 17.0 statistical software (SPSS Inc., Chicago, IL, USA). A P < 0.05 was considered as statistical significance.
Results
General data comparison
Forty-seven subjects were included in this study, including 27 patients with PD and 20 controls. No statistical differences were found in age or gender between the two groups (P > 0.05) [Table 1]. The clinical characteristics of patients with PD with sleep disorders were as follows: early-onset (≤50 years) in five patients (18.5%), and late-onset (>50 years) in 22 patients (81.5%); disease duration ≤5 years in nine patients (33.3%) and >5 years in 18 patients (66.7%); PIGD-dominant in eight patients (29.6%), akinetic-rigid-dominant in 12 patients (44.4%), and tremor-dominant in seven patients (25.9%); Hoehn-Yahr stage ≤2.0 in six patients (22.2%) and >2.0 in 21 patients (77.8%).
Table 1.
Clinical characteristics of the PD group and the control group in this study.

Incidence of different sleep disorders between the PD group and the control group
Incidence of SF in PD group was higher than that of control group (P = 0.019) [Table 2]. However, the incidence of difficulty falling asleep and early awakening between the two groups was not significantly different (P > 0.05). No statistical differences were found in age, gender, disease duration, age onset, UPDRS-III score and LDE between patients with PD with fragmented sleep or not (P > 0.05) [Table 3].
Table 2.
Comparison of the prevalence of sleep disorders with different types between the PD group and the control group.

Table 3.
Clinical characteristics of PD patients with sleep fragmentation or not.

PSG sleep parameters in the PD group and the control group
No significant differences were found in TIB, SL, N1, N1%, N2, N2%, N3%, and NREM% between the two groups (P > 0.05) [Table 4]. Compared with the control group, TST, SE, REM-SL, N3, NREM, REM, and REM% decreased significantly in the PD group (all P < 0.05), while REM-SL and TWT increased significantly (all P < 0.05).
Table 4.
Comparison of sleep parameters by polysomnography between the PD group and the control group.

PSG sleep parameters in PD group with different disease courses
No significant differences in TIB, TST, SE, TWT, REM-SL, N1, N1%, N2, N2%, N3, NREM%, REM, or REM% between the diseases duration ≤5 years and >5 years groups were observed (P > 0.05) [Table 5]. Compared with the duration of disease >5 years group, SL in the duration of disease ≤5 years group was significantly lower (P < 0.05).
Table 5.
Comparison of sleep parameters by polysomnography in the PD group with different durations.

PSG sleep parameters among PD groups with different types of dyskinesia
According to the UPDRS-III, movement disorders were classified into tremor, akinetic-rigid and PIGD dominant types. Among them, tremor score was the sum of the 20th and 21st item scores; the score of akinetic-rigid score was the sum of 18th, 19th, 22nd to 25th, and 31st item scores; PIGD score was the sum of the 26th to 30th item scores.[12,13]
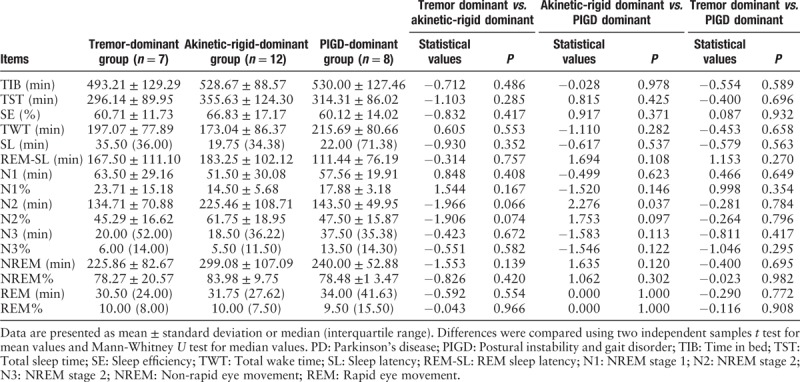
Pairwise comparison among the three-movement disorder groups revealed no significant difference in TIB, TST, SE, TWT, SL, REM-SL, N1, N2, N2%, N3, N3%, NREM, NREM%, REM, or REM% (P > 0.05) [Table 6]. In addition, comparison between the akinetic-rigid dominant and PIGD dominant type groups as well as the tremor dominant and PIGD dominant type groups revealed no significant differences in N2 (P > 0.05), but compared with the akinetic-rigid dominant group, N2 was significantly lower in the PIGD-dominant group (P < 0.05).
Table 6.
Comparison of sleep parameters by polysomnography in the PD group with different types of dyskinesia.
PSG sleep parameters in different staged of PD group
PSG sleep parameters, including TIB, TST, SE, TWT, SL, REM-SL, N1, N1%, N2, N2%, N3, N3%, NREM, NREM%, REM, and REM%, were not significantly different between the early and advanced stage group (P > 0.05) [Table 7].
Table 7.
PSG sleep parameters in the PD patients with different stages in this study.

PD partial sleep parameter correlation
Correlations between PSG-obtained partial sleep parameters (including TST, SE, TWT, REM, and REM%) and factors (including age, course of disease, UPDRS-III score, Hoehn-Yahr stage, and LDE) were analyzed in the PD group, but no apparent correlations were observed [Table 8].
Table 8.
Pearson analysis between the polysomnography-obtained partial parameters and factors in the PD group.

Discussion
Internationally, PSG is recognized as the gold standard for sleep disorder diagnosis and an important means for the differential diagnosis of various sleep disorders. As such, it has been widely applied in clinical and scientific research on the subject. Through monitoring a variety of sleep-associated physiological parameters, PSG has great significance in clinical diagnosis and analysis in various sleep disorders in patients with PD. Relevant studies in recent years suggested sleep disorders are one of the most common non-motor symptoms of PD,[14] and its manifestations in PD are complex and diverse. The prevalence of insomnia in PD is 30.0% to 86.8% in China.[4] Key factors related with insomnia of PD include female gender, disease duration of PD, depression, anxiety, and others, which may lead to SF. More than three arousals exceeding 5 min at night is considered as SF. Main causes related to SF include night motor dysfunction and nocturia.[7] Prior PSG studies have concluded that the characteristics of sleep structure in patients with PD were divergent,[15–17] and most findings suggested that sleep disorders were characterized by prolonged SL, reduced SE, difficult sleep maintenance, decreased slow-wave sleep, and increased limb movement during night-time sleep, etc.
This study evaluated the types of sleep disorders in patients with PD using PSG, and found that there was no obvious difference in the difficulty of falling asleep or early awakening between the PD group and the control group, while there was a higher incidence of SF in the PD group than the control group [Table 2]. Patients with PD rarely complained of SF, but this symptom was common in PSG. SF can occur in any sleep phase, and it is more common during the shallow sleep (NREM sleep stage 1 and 2), which is in line with a report by Askenasy et al[18] on the treatment of sleep disorders in PD. This study showed that patients with PD suffered from frequent sleep disruption, which affected sleep quality. Norlinah et al[19] found that factors influencing SF at night in patients with PD were numerous, including cramps, night-time limb movement disorder, increased muscle tension and SAS, as well as age and course of disease. These influenced result in prolonged awake time after sleep and consequent daytime fatigue and sleepiness.[14] Concerning sleep structure changes, TST, SE, N3, NREM, REM, and REM% in the PD group were significantly reduced, while TWT was elevated [Table 4]. These findings were consistent with most reports,[20,21] suggesting that patient with PD sleep abnormalities might result from nerve nuclei degeneration and a subsequent imbalance in neurotransmitters, which further disrupts the physiological sleep-wake cycle. Also, decreased slow-wave sleep may be related to reduced high-amplitude low-frequency δ waves in night-time sleep EEGs caused by the disease. Considering the relatively long disease course of patients with PD included in this study, decreased REM and REM% might be related with large doses of dopaminergic drugs. Therefore, attention to the effect of dopaminergic drugs on sleep structure is required to adequately choose therapeutic drugs according to patients’ clinical symptoms. Although no significant differences in SL of REM sleep between the two groups were observed, mean REM-SL in the PD group exceeded normal REM-SL values,[22] and the mean REM-SL in control group was in the normal range. Therefore, prolonged SL in REM sleep is a likely characteristic of PD, and has a certain diagnostic value.
PSG results helped in dividing PD patients with sleep disorders into early and late onset groups, disease course ≤5 years and >5 years groups, different types of dyskinesia, and early and advanced stage groups based on Hoehn-Yahr staging. Sleep parameter analysis revealed that N3% in late-onset group was significantly lower than that of the early onset group, suggesting N3 stage sleep (slow-wave sleep) might be the most affected by age. Moreover, age greatly influences sleep; therefore, reduced N3% in relatively older patients may have little clinical significance. Compared with disease course >5 years group, N3% in the ≤5 years group significantly decreased, while NREM markedly increased. There were no significant differences in PSG sleep parameters between different types of patients with PD in the incidence and manifestation of sleep disorders, which was inconsistent with previous research.[23] This might be due to subject heterogeneity, or grouping methods for dyskinesia and determining methods for sleep disorders in these two studies. Furthermore, there were significant in correlations of REM% with UPDRS-III score and Hoehn-Yahr stage, which supported the findings of prior reports. It might be related to neuropathological changes in PD.[11,24]
It is well known that RBD is a biomarker of PD. However, it is not known whether SF is also a sensitive marker of PD. This research found that SF was an important clinical characteristic of sleep disorders in PD, which could be quantitatively assessed by PSG.
Sleep disturbance including SF is a risk factor for a range of psychiatric conditions,[25–27] including anxiety, depression, cognitive decline, and drug addiction. SF in PD might be associated with anxiety, depression, impulse control disorders, and other psychiatric symptoms. However, the molecular, cellular, and circuit mechanisms of sleep disorder including SF are not well understood. Lower cortical gray matter volume in the lateral orbitofrontal cortex and inferior frontal gyrus pars orbitalis was associated with greater SF in older adults.[28] Ge et al[29] showed medial habenula cholinergic output neurons were sensitive to chronic SF, and also identified a key molecular substrate TWIK-like acid-sensitive K+ channel 3 (TASK-3, also named KCNK9) activity reduced following sleep fragmentation
In conclusions, SF is high rate in patients with PD. This preliminary study supported that SF was an important clinical characteristic of sleep disorders in PD. Sleep fragmentation might be a potential quantifiable marker in PD but still there are issues to be overcome in the future study. As the work here will be used to expand the sample size in multi-center, SF may be a marker in assessment of progression of PD, through the sleep disorders of detection with PSG. Further work is needed to clarify whether this is a consequence of or contributor to SF.
Funding
This work was supported by grants from the National Key Research & Development Program of China (No. 2017YFC1310200), the Fujian Provincial Science and Technology Guiding Project (No. 2016Y0043 and No. 2017Y0041), and the Key Clinical Specialty Discipline Construction Program of Fujian and Nation, China.
Conflicts of interest
None.
Footnotes
How to cite this article: Caia GE, Luob S, Chena LN, Lua JP, Huang YJ, Yea QY. Sleep fragmentation as an important clinical characteristic of sleep disorders in Parkinson's disease: a preliminary study. Chin Med J 2019;132:1788–1795. doi: 10.1097/CM9.0000000000000329
Guo-En Cai and Shan Luo contributed equally to this study.
References
- 1.Pacchetti C, Manni R, Zangaglia R, Glorioso M, Cristina S, Terzaghi M, et al. A questionnaire on sleep and mental disorders in Parkinson's disease (QSMDPD): development and application of a new screening tool. Funct Neurol 2004; 19:83–99. [PubMed] [Google Scholar]
- 2.Gan-Or Z, Alcalay RN, Rouleau GA, Postuma RB. Sleep disorders and Parkinson disease; lessons from genetics. Sleep Med Rev 2018; 41:101–112. doi: 10.1016/j.smrv.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Oerlemans WG, de Weerd AW. The prevalence of sleep disorders in patients with Parkinson's disease. A self-reported, community-based survey. Sleep Med 2002; 3:147–149. doi: 10.1016/S1389-9457(01)00127-7. [DOI] [PubMed] [Google Scholar]
- 4.Liu CF, Wang T, Zhan SQ, Geng DQ, Wang J, Liu J, et al. Management recommendations on sleep disturbance of patients with Parkinson's disease. Chin Med J 2018; 131:2976–2985. doi: 10.4103/0366-6999.247210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen Y, Liu CF. Sleep disorders in Parkinson's disease: present status and future prospects. Chin Med J 2018; 131:883–885. doi: 10.4103/0366-6999.229903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albers JA, Chand P, Anch AM. Multifactorial sleep disturbance in Parkinson's disease. Sleep Med 2017; 35:41–48. doi: 10.1016/j.sleep.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Falup-Pecurariu C, Diaconu Ş. Sleep dysfunction in Parkinson's disease. Int Rev Neurobiol 2017; 133:719–742. doi: 10.1016/bs.irn.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 8.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 1988; 51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta M, Singh G, Khwaja GA, Mehndiratta MM. Hallucinations in Parkinson's disease – a study of forty three patients. J Assoc Physicians India 2004; 52:703–706. [PubMed] [Google Scholar]
- 10.Reichmann H, Herting B, Mïller A, Sommer U. Switching and combining dopamine agonists. J Neural Transm (Vienna) 2003; 110:1393–1400. doi: 10.1007/s00702-003-0081-z. [DOI] [PubMed] [Google Scholar]
- 11.Furumoto H. Excessive daytime somnolence in Japanese patients with Parkinson's disease. Eur J Neurol 2004; 11:535–540. doi: 10.1111/j.1468-1331.2004.00827.x. [DOI] [PubMed] [Google Scholar]
- 12.Baba Y, Putzke JD, Whaley NR, Wszolek ZK, Uitti RJ. Gender and the Parkinson's disease phenotype. J Neurol 2005; 252:1201–1205. doi: 10.1007/s00415-005-0835-7. [DOI] [PubMed] [Google Scholar]
- 13.Liu ZL, Wang LM, Tao EX. Study of the clinical heterogeneity of movement disorder in Parkinson disease (in Chinese). J Southern Med Univ 2008; 28:447–448. doi: 10.1016/S1872-2040(08)60071-7. [PubMed] [Google Scholar]
- 14.Comella CL. Sleep disorders in Parkinson's disease: an overview. Mov Disord 2007; 22:S367–S373. doi: 10.1002/mds.21682. [DOI] [PubMed] [Google Scholar]
- 15.Thorpy MJ, Adler CH. Parkinson's disease sleep. Neurol Clin 2005; 23:1187–1208. doi: 10.1016/j.ncl.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Placidi F, Izzi F, Romigi A, Stanzione P, Marciani MG, Brusa L, et al. Sleep-wake cycle and effects of cabergoline monotherapy in de novo Parkinson's disease patients. An ambulatory polysomnographic study. J Neurol 2008; 255:1032–1037. doi: 10.1007/s00415-008-0836-4. [DOI] [PubMed] [Google Scholar]
- 17.Shpirer I, Miniovitz A, Klein C, Goldstein R, Prokhorov T, Theitler J, et al. Excessive daytime sleepiness in patients with Parkinson's disease: a polysomnography study. Mov Disord 2006; 21:1432–1438. doi: 10.1002/mds.21002. [DOI] [PubMed] [Google Scholar]
- 18.Askenasy JJ, Yahr MD. Reversal of sleep disturbance in Parkinson's disease by antiparkinsonian therapy: a preliminary study. Neurology 1985; 35:527–532. doi: 10.1212/WNL.35.4.527. [DOI] [PubMed] [Google Scholar]
- 19.Norlinah MI, Afidah KN, Noradina AT, Shamsul AS, Hamidon BB, Sahathevan R, et al. Sleep disturbances in Malaysian patients with Parkinson's disease using polysomnography and PDSS. Parkinsonism Relat Disord 2009; 15:670–674. doi: 10.1016/j.parkreldis.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Mao ZJ, Liu CC, Ji SQ, Yang QM, Ye HX, Han HY, et al. Clinical characteristics of sleep disorders in patients with Parkinson's disease. J Huazhong Univ Sci Technolog Med Sci 2017; 37:100–104. doi: 10.1007/s11596-017-1701-4. [DOI] [PubMed] [Google Scholar]
- 21.Shen Y, Dai YP, Wang Y, Li J, Xiong KP, Mao CJ, et al. Two polysomnographic features of REM sleep behavior disorder: clinical variations insight for Parkinson's disease. Parkinsonism Relat Disord 2017; 44:66–72. doi: 10.1016/j.parkreldis.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Antczak JM, Rakowicz MJ, Banach M, Derejko M, Sienkiewicz J, Zalewska U, et al. Negative influence of L-dopa on subjectively assessed sleep but not on nocturnal polysomnography in Parkinson's disease. Pharmacol Rep 2013; 65:614–623. doi: 10.1016/S1734-1140(13)71038-7. [DOI] [PubMed] [Google Scholar]
- 23.Lou F, Cao D, Luo XG, Ren Y. Clinical characteristics of sleep disorders in different subtypes of patients with Parkinson disease (in Chinese). Chin J Clinicians (Electronic Edition) 2011; 5:5567–5572. doi: 10.3877/cma.j.issn.1674-0785.2011.19.007. [Google Scholar]
- 24.Rye DB, Bliwise DL, Dihenia B, Gurecki P. FAST TRACK: daytime sleepiness in Parkinson's disease. J Sleep Res 2000; 9:63–69. doi: 10.1046/j.1365-2869.2000.00201.x. [DOI] [PubMed] [Google Scholar]
- 25.Junho BT, Kummer A, Cardoso FE, Teixeira AL, Rocha NP. Sleep quality is associated with the severity of clinical symptoms in Parkinson's disease. Acta Neurol Belg 2018; 118:85–91. doi: 10.1007/s13760-017-0868-6. [DOI] [PubMed] [Google Scholar]
- 26.Kurtis MM, Rodriguez-Blazquez C, Martinez-Martin P, Group ELEP. Relationship between sleep disorders and other non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord 2013; 19:1152–1155. doi: 10.1016/j.parkreldis.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Sobreira E, Sobreira-Neto MA, Pena-Pereira MA, Chagas M, Fernandes R, Eckeli AL, et al. Global cognitive performance is associated with sleep efficiency measured by polysomnography in patients with Parkinson's disease. Psychiatry Clin Neurosci 2019; 73:248–253. doi: 10.1111/pcn.12819. [DOI] [PubMed] [Google Scholar]
- 28.Lim AS, Fleischman DA, Dawe RJ, Yu L, Arfanakis K, Buchman AS, et al. Regional neocortical gray matter structure and sleep fragmentation in older adults. Sleep 2016; 39:227–235. doi: 10.5665/sleep.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ge F, Mu P, Guo R, Cai L, Liu Z, Dong Y, et al. Chronic sleep fragmentation enhances habenula cholinergic neural activity. Mol Psychiatry 2019; Apr 12. [Epub ahead of print]. doi: 10.1038/s41380-019-0419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


