Abstract
Objective:
The purpose of this review is to stress the complicated interactions between the microbiota and the development of heart failure. Moreover, the feasibility of modulating intestinal microbes and metabolites as novel therapeutic strategies is discussed.
Data sources:
This study was based on data obtained from PubMed up to March 31, 2019. Articles were selected using the following search terms: “gut microbiota,” “heart failure,” “trimethylamine N-oxide (TMAO),” “short-chain fatty acid (SCFA),” “bile acid,” “uremic toxin,” “treatment,” “diet,” “probiotic,” “prebiotic,” “antibiotic,” and “fecal microbiota transplantation.”
Results:
Accumulated evidence has revealed that the composition of the gut microbiota varies obviously in people with heart failure compared to those with healthy status. Altered gut microbial communities contribute to heart failure through bacterial translocation or affecting multiple metabolic pathways, including the trimethylamine/TMAO, SCFA, bile acid, and uremic toxin pathways. Meanwhile, modulation of the gut microbiota through diet, pre/probiotics, fecal transplantation, and microbial enzyme inhibitors has become a potential therapeutic approach for many metabolic disorders. Specifically, a few studies have focused on the cardioprotective effects of probiotics on heart failure.
Conclusions:
The composition of the gut microbiota in people with heart failure is different from those with healthy status. A reduction in SCFA-producing bacteria in patients with heart failure might be a notable characteristic for patients with heart failure. Moreover, an increase in the microbial potential to produce TMAO and lipopolysaccharides is prominent. More researches focused on the mechanisms of microbial metabolites and the clinical application of multiple therapeutic interventions is necessarily required.
Keywords: Heart failure, Gut microbiota, Dysbiosis, Treatment
Introduction
Heart failure is the end stage of various cardiovascular diseases (CVDs). The prevalence of heart failure in adults is 1% to 2% and increases to more than 10% in patients over 70 years old.[1] The lifetime risk of heart failure is 33% for men and 28% for women at 55 years old. Two major components of the pathogenesis of heart failure are pathologic myocardial remodeling and stimulation of the neuroendocrine system, including the renin-angiotensin-aldosterone system and the sympathetic nervous system.[2,3] The typical symptoms of heart failure include dyspnea, fatigue, and edema of the lower extremities. Some heart failure patients may not exhibit early symptoms, which could lead to missed diagnoses.[4] According to the onset severity and course of symptoms and signs, heart failure is classified into chronic heart failure and acute heart failure. Heart failure leads to a poor prognosis by negatively affecting the quality of life and impairing patients’ social functioning. Prevention of heart failure, timely diagnosis, and initiation of early treatment are critical to successfully reducing mortality and improving prognosis.
Although physicians have accumulated considerable experience in treating heart failure during the past 30 years, the effectiveness of the current treatment regimen is still far from satisfactory. The high incidence and mortality of heart failure have imposed heavy burdens on medical spending and have become a major public health problem, hindering the economic development of all countries.[5] The latest European study (previous European Society of Cardiology heart failure study) revealed that the 12-month all-cause mortality rates were 17% for hospitalized patients with heart failure and 7% for outpatients with stable heart failure and that the 12-month readmission rates were 44% and 32%, respectively.[6] One main reason for the poor prognosis of heart failure is the incomplete knowledge we have regarding associated risk factors. Additionally, the available prognostic markers are not sufficiently precise.[7,8] The gut microbiota plays a critical physiologic and metabolic role in the human body. The gut microbiota could be regarded as an endocrine organ because it not only releases its own products but also metabolizes host metabolites and external nutrients into hormone-like signals, which impact both normal physiologic processes and chronic diseases.[9] It is not surprising that significant interest is focused on the roles of the human gut microbiota in CVD and metabolic disorders, including heart failure.
Human Gut Microbiota
The human intestine is a large bacterial reservoir containing on its surface over 2000 species and at least 1014 bacterial organisms, which is 10 times more than the number of human cells.[10–12] The entirety of microorganisms that coexist with their hosts refers to the gut microbiota, which contains at least 100 times more genetic information than the human genome.[11] It is now well established that the human gut microbiome is pre-dominated by phyla such as Bacteroidetes and Firmicutes. Phyla with lower abundances include Proteobacteria, Actinobacteria, and Verrucomicrobia.[10,13]
The gut microbiota interacts with the host through metabolism-independent pathways such as bacterial translocation-associated endotoxemia and metabolism-dependent pathways, such as the trimethylamine (TMA)/trimethylamine N-oxide (TMAO), short-chain fatty acid (SCFA), bile acid (BA), and uremic toxin pathways.[9] To some extent, human beings’ own cells coexist with the gut microbiota to form a “superorganism.” Human genes and the gut microbiota collectively affect metabolism and immune and inflammatory responses.[14–16]
Physiologic Roles of the Gut Microbiota
In the healthy human gut, the overall microbial community structure remains stable over time within an individual but varies greatly across individuals. Environmental factors that contribute most to interindividual diversity include diet, lifestyle, and antibiotic use. Host genotype, age, and sex also contribute to gut microbiota diversity.[11,16,17] The gut microbiota can interact with the host and perform multiple physiologic functions. A principal role of the gut microbiota is participating in food digestion and nutrient uptake. The gut microbiota produces SCFAs by breaking down dietary fibers through mainly the saccharolytic pathway.[13] A major role of SCFAs is serving as energy substrates for epithelial cells of the gut. Binding of SCFAs to G-protein-coupled receptor 41 (Gpr41) can induce expression of the enteroendocrine hormone peptide YY in gut epithelial L cells, which regulates host appetite and helps increase energy harvesting from the diet. The gut microbiota also digests food through the proteolytic pathway, thus contributing to SCFAs production and formation of cometabolites, such as ammonia, various amines, and thiols.[13] In addition, the gut microbiota plays a role in constituting and regulating intestinal barriers and modulating the host immune system to prevent inappropriate inflammation.[13,18] The gut microbiota assists with the maturation of immunologic tissues by stimulating gut lymphatic tissue, which forms an essential mechanism of defense against pathogens.[13] Furthermore, the gut microbiota can participate in neurologic development. The “gut-brain axis” refers to the assembly of the gut microbiota and the central, parasympathetic, and sympathetic nervous systems. Altered gut microbial composition is associated with neurodegeneration and neuroimmune activation. Finally, the gut microbiota is also involved in maintaining host energy homeostasis and vitamin synthesis.[19]
Gut Microbiota and Diseases
Gut dysbiosis refers to quantitative and qualitative alterations in the composition of the gut microbiota, which has been associated with the pathogenesis of a wide spectrum of diseases, including cancer, infectious diseases, inflammatory bowel disease, metabolic diseases such as diabetes and obesity, autoimmune diseases, autism, and CVD.[20–27] In particular, the relationship between gut dysbiosis and CVD, including hypertension, atherosclerosis, thrombosis and heart failure, has been focused on.[28–31] Accumulated evidence suggests a potential role that dysbiosis of the gut microbiota might play in the onset and progression of heart failure. An overview of the interactions between gut microbiota and heart failure is seen in Figure 1.
Figure 1.
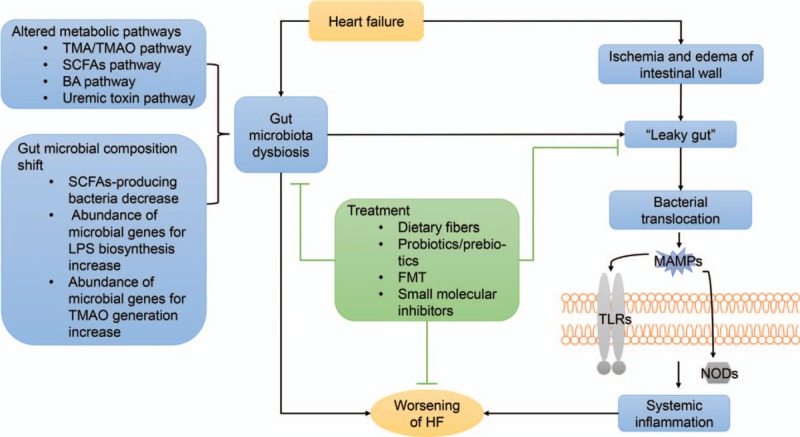
Gut microbiota dysbiosis and bacterial translocation involved in the progression of heart failure. Decreased cardiac output leads to intestinal mucosal ischemia and/or edema, thus leading to a “leaky gut.” Bacterial translocation and systematic inflammation occur subsequently. Moreover, heart failure is accompanied by a shift of the gut microbiota composition as well as varied metabolic pathways, which exacerbate the disease. BA: Bile acid; FMT: Fecal microbiota transplantation; HF: Heart failure LPS: Lipopolysaccharide; MAMPs: Microbe-associated molecular patterns; NOD: Nucleotide oligomerization domain; SCFA: Short-chain fatty acid; TLRs: Toll-like receptors; TMA: Trimethylamine; TMAO: Trimethylamine N-oxide.
Gut Microbiota Dysbiosis in Heart Failure
The objective of microbiome analysis is to detect and characterize the gut microbiota via assessment and classification of its genomes and corresponding metabolites, therefore finding a more comprehensive explanation for the composition and function of the gut microbiota. With the development of sequencing technology, “16S” analysis, which detects the sequence difference of the hypervariable region of the 16S ribosomal ribonucleic acids for taxonomic identification of bacteria, is able to characterize the gut microbiota at a species-level resolution. Furthermore, metagenomics sequencing, which evaluates the composite genetic material present in the microbiome, is capable of characterizing specific taxa of the gut microbiota at a strain-level resolution. With the help of bioinformatics methods, the current technology enables us to study the underlying relationships between exact compositions of the gut microbiome and CVD.[11,19,32,33]
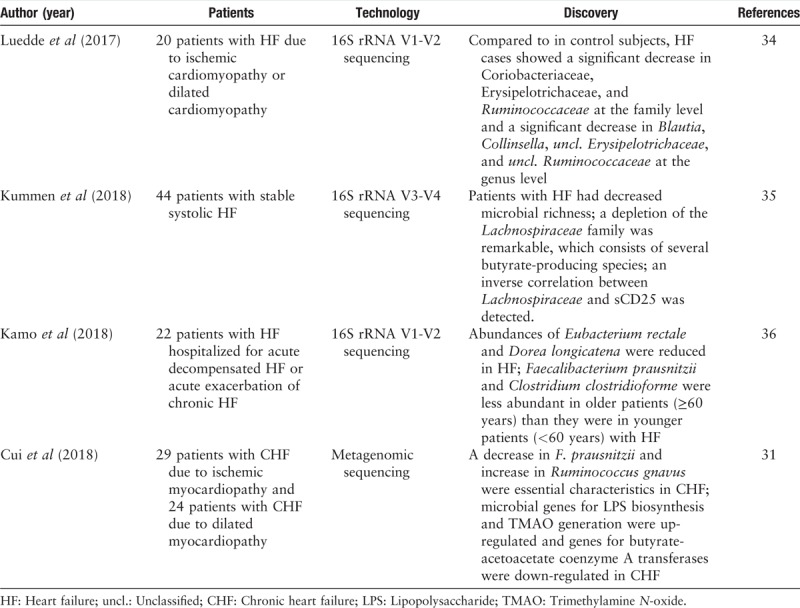
For a healthy individual, anaerobic Bacteroidetes and Firmicutes constitute more than 90% of the total gut bacterial species.[10] Compared to healthy controls, heart failure patients usually have decreased gut microbial richness and a shift in the composition of the gut microbiota. According to Luedde et al's[34] research based on 16S rRNA sequencing, a significant decrease in the abundance of Coriobacteriaceae, Erysipelotrichaceae, and Ruminococcaceae at the family level and a significant decrease in the abundance of Blautia, Collinsella, unclassified Erysipelotrichaceae, and unclassified Ruminococcaceae at the genus level have been shown in the gut of heart failure patients. Moreover, Kummen et al[35] discovered a depletion of the Lachnospiraceae family, which consists of several butyrate-producing species, in heart failure patients through 16S rRNA sequencing technology. In addition, there is an inverse correlation between Lachnospiraceae and sCD25, which is a T-cell activation marker. Such a correlation was even more prominent in patients whose disease was more severe than in those whose disease was less severe. A 16S analysis based on 22 hospitalized patients with heart failure by Kamo et al[36] also reported a reduction in SCFA-producing bacteria such as Eubacterium rectale and Dorea longicatena. In addition, a shift in the gut microbiota compositions in different age groups exists. A major butyrate-producing species (Faecalibacterium prausnitzii) was revealed to be less abundant in old patients with heart failure than in young patients with heart failure. Metagenomics sequencing technology has also been involved in the identification of the gut microbiome in patients with heart failure. Recently, Cui et al[31] discovered decreased enrichment of F. prausnitzii, Oscillibacter sp., and Sutterella wadsworthensis in fecal samples from heart failure patients through metagenomic analyses. For functional analysis, an elevation in the abundance of microbial genes for lipopolysaccharide (LPS) biosynthesis and TMAO generation and a decrease in microbial genes for butyrate-acetoacetate coenzyme A transferases were noted in the gut microbiota of patients with chronic heart failure. Metabolomic and correlation analyses confirmed that the composition of metabolites in fecal and plasma samples from chronic heart failure patients significantly changed compared with those from healthy controls, and the varied metabolic profile was associated with a shift in the gut microbiome. In addition to 16S rRNA or metagenomics sequencing, traditional methods for identifying gut microorganisms such as stool sample collection, bacterial incubation, and isolation have been applied to study the gut flora of patients with heart failure. According to Pasini et al,[37] chronic heart failure patients develop an increased abundance of pathogenic bacterial colonies in their stools. Specifically, patients with relatively more severe disease tend to have a significantly increased ratio of Candida, Campylobacter, and Shigella species.
A summary of current research on the composition of gut microbiota in heart failure is shown in Table 1. The summary was based on data obtained from PubMed up to March 31, 2019. Articles were selected using the following search terms: “gut microbiota” and “heart failure.” We reviewed the medical literature one by one.
Table 1.
Current research on the composition of gut microbiota in heart failure by high-throughput sequencing technology.
“Leaky Gut” and Bacterial Translocation in Chronic Heart Failure
Under physiologic conditions, the gut microbiota constitutes an essential part of intestinal mucosal barriers that play a necessary role in systemic immunity and metabolism. Healthy gut microbiota is largely responsible for the overall health of the host. However, under pathologic conditions, the gut microbiota may harm the human body by disturbing normal systemic immunity and metabolism through releasing toxic substances into the peripheral circulation. As early as 1999, scientists hypothesized that the intestinal permeability observed in chronic heart failure patients was altered by edema, which in turn led to bacterial translocation as well as endotoxemia. Endotoxemia could trigger systemic inflammatory responses, which aggravate the progression of heart failure.[38] In 2007, Sandek et al[39] proved that compared with control group subjects, patients with chronic heart failure had increased thickness of the intestinal wall, intestinal permeability, and intestinal insufficiency. Moreover, the serum level of anti-Escherichia coli J5 endotoxin IgA was higher in patients with heart failure than that in control subjects. These findings provided evidence for pathologic changes in the gut of patients with heart failure. Based on previous studies, Sandek et al[40] found through in situ fluorescence hybridization that high levels of anaerobic E. rectale in the sigmoid colon mucosa (juxtamucosal bacteria) in patients with heart failure were associated with low perfusion of the mucosa. Decreased blood perfusion resulted in exaggerated hypoxia in intestinal villi, which might account for enrichment of gut-specific anaerobes in patients with heart failure. Increased intestinal mucosal bacteria could trigger inflammation in the body by releasing large quantities of endotoxins into the bloodstream, resulting in cachexia.
Currently, the accumulated literature supports the “gut hypothesis of heart failure,” which affirmed the role of the gut in the pathogenesis of heart failure. The gut hypothesis suggests that decreased cardiac output, aggravating systemic congestion and hypoperfusion, can lead to intestinal mucosal ischemia and/or edema, which creates hypoxia and a hypercapnia status. Subsequently, a decrease in intestinal mucosal pH and diminished passive carrier-mediated transport of D-xylose occurs, leading to a “leaky gut,” which describes increased gut permeability as well as intestinal barrier dysfunction. As a result, increased bacterial translocation occurs, which is accompanied by increased circulating endotoxin release into the peripheral circulatory system. The circulating endotoxins produced by bacteria refer to main structural components of bacteria including LPS, flagellin, peptidoglycans, and formylated peptides, which are recognized as microbe-associated molecular patterns (MAMPs). MAMPs are selectively recognized by pattern recognition receptors (PRRs) such as host Toll-like receptors and nucleotide oligomerization domain-containing receptors. Microbial activation of PRRs could reverse cholesterol transport while promoting insulin resistance and hyperlipidemia.[9] Furthermore, microbial activation of PRRs either on gut epithelial cells or within the vasculature stimulates the host immune response by triggering numerous downstream signaling processes, thus leading to vascular inflammation.[15,41] In addition, LPS could activate systemic inflammation by inducing elevated proinflammatory cytokines such as interleukin-1, interleukin-2, high-sensitivity C-reactive protein, interleukin-6, and tumor necrosis factor-alpha, therefore adversely impacting the disease progression of heart failure.[13,18,19]
Metabolite-Driven Pathways of the Gut Microbiota in Chronic Heart Failure
The gut microbiota can influence host physiologic activities through quantities of processes. In addition to direct translocation of bacteria and releasing gut microbial signals, the gut microbiota can impact hosts through bioactive metabolites that act on distal organs either directly or indirectly.[42] Accumulated evidence suggests that among the numerous metabolites produced by the gut microbiota, a large portion is biologically active and directly absorbed into systemic circulation, whereas others may serve as mediators between microbes and the host after being further metabolized by host enzymes.[13,30,43–46] The gut microbiota interacts with the host through a number of pathways, including the TMA/TMAO, SCFA, BA, and uremic toxin pathways.
TMA/TMAO pathway
Novel technologies herald great advances in gut microbiota studies.[47,48] Wang et al[43] used metabonomics to investigate the relationship between the gut microbiota and CVD. They found that plasma TMA, a metabolite produced by intestinal flora from choline and L-carnitine in food, could be oxidized to TMAO by flavin-containing monooxygenase in the liver. Evidence supports a positive correlation between increased levels of TMAO and the incidence of cardiovascular events in patients with coronary heart disease. Further studies have confirmed that TMAO could promote the process of atherosclerosis.[44,49] Moreover, scientists have pointed out that TMAO is also associated with heart failure. Tang et al[50] found a positive relationship between plasma TMAO levels and 5-year all-cause mortality in 720 patients with stable heart failure. The TMAO level was higher in patients with heart failure than that in control group subjects. The risk of death increased 3 to 4 times in patients with high plasma TMAO levels compared with patients with low plasma TMAO levels. After adjusting for traditional cardiovascular risk factors and B-type natriuretic peptide (BNP) levels, the increased TMAO level still predicted an increased 5-year mortality rate. Suzuki et al[51] also examined plasma TMAO levels in 972 patients with acute heart failure and evaluated the relationship between TMAO levels and in-hospital mortality, all-cause mortality, and the overall incidence of death or readmission due to heart failure within a year. Elevated TMAO levels were correlated with unfavorable outcomes in patients with acute heart failure. In addition, the combination of TMAO levels and N-terminal pro-BNP (NT-proBNP) values could more precisely predict the mortality risk of hospitalized patients with acute heart failure than TMAO levels alone.
Furthermore, Tang et al[52] confirmed that the plasma TMAO levels in patients with chronic heart failure were higher than those in healthy controls. In addition, in patients with heart failure, increased levels of TMAO were associated with relatively poor New York Heart Association (NYHA) grades. Patients with high TMAO levels had worse left ventricular diastolic dysfunction and clinical prognoses than patients with low TMAO levels. In a prospective observational study (155 patients with heart failure, 100 patients with stable coronary heart disease, and 33 healthy controls were included), plasma TMAO levels in patients with heart failure were significantly higher than those in the controls. In addition, TMAO levels were positively associated with increases in NYHA grade and NT-proBNP levels. TMAO levels were not related to the levels of LPS or left ventricular ejection fraction (LVEF), but they were negatively correlated with the non-transplant history of chronic heart failure patients.[53]
Another study in 2016 showed increased incidences of pulmonary edema, higher atrial natriuretic peptide levels, and enhanced left ventricular remodeling, and aortic arch constriction in mice fed with choline or TMAO than those in control mice. A diminished ejection fraction observed in the treated mice also indicated an exacerbation of heart failure.[54] It has been reported that plasma choline, betaine, and TMAO levels are correlated with BNP levels and electrocardiogram indices of diastolic function but not systolic function. TMAO levels were associated with poor prognosis in chronic systolic heart failure after adjustment for cardiorenal indices.[55] The known pathways of TMAO formation and its relationship with heart failure are present in Figure 2.
Figure 2.
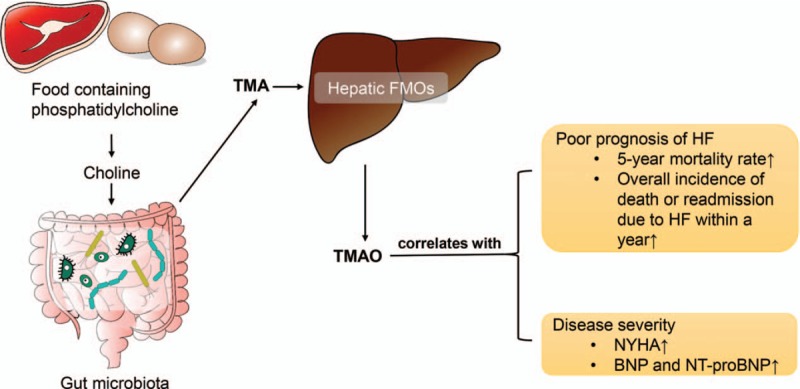
Pathways of trimethylamine N-oxide formation and its relationship with heart failure. TMA is formed through metabolization of choline and choline-containing compounds from diets by gut microbiota in the intestinal lumen. TMA can be absorbed from the intestine and delivered to the liver where FMO convert it to TMAO. It is found that TMAO is correlated with poor prognosis and severity of HF. BNP: B-type natriuretic peptide; FMO: Flavin-containing monooxygenase; HF: Heart failure; NT-proBNP: N-terminal pro-BNP; NYHA: New York Heart Association; TMA: Trimethylamine; TMAO: Trimethylamine N-oxide.
SCFA pathway
SCFAs are major products of microbial fermentation of dietary fibers in the gut. Both the saccharolytic pathway and proteolytic pathway participate in the production of SCFAs, but the former contributes more than the latter. Since several correlation analyses have revealed a remarkable decrease of SCFA-producing bacteria in patients with heart failure, a cardioprotective role of SCFAs seems to exist. SCFAs seem to promote post-infarction cardiac repair through inducing infiltration of CX3CR1+ monocytes in the peri-infarct zone.[56] Accumulated evidence indicates that SCFAs play a role in mediating the host immune system. For example, butyrate plays an anti-inflammatory role through inducing Foxp3+ Treg cell proliferation and suppressing the generation of Th17 cells by activating G protein-coupled receptor 43.[57] Moreover, SCFAs play a gut barrier-protective role. Through activating the hypoxia-inducible factor, butyrates help to maintain the physiologic relative hypoxia state in colon epithelial mucosa, which is essential in maintaining gut barrier function.[58] In addition, SCFAs could modulate host blood pressure. Propionate, which is an SCFA shown to induce vasodilation in vitro, could modulate mouse blood pressure in a mutually antagonistic way. Propionate induces renin secretion and thus elevates blood pressure through binding to Olfr78, which is an olfactory receptor expressed in the renal juxtaglomerular apparatus. However, propionate also presents powerful hypotensive effects by binding to Gpr41, which is another SCFA receptor expressed in smooth muscle cells of small vessels.[59] Considering the roles that SCFAs play in gut barrier protection, blood pressure modulation, and the immune system, SCFAs probably play an essential role in pathways associated with heart failure, which still requires further investigation. A summary of the known cardioprotective roles of SCFAs is present in Figure 3.
Figure 3.
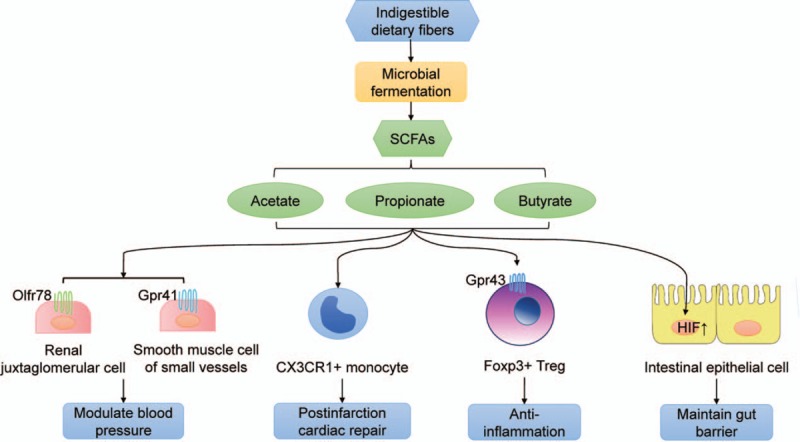
Roles of short-chain fatty acids involved in cardiovascular diseases. SCFAs are major products of microbial fermentation of dietary fibers. SCFAs mainly present cardioprotective effects, including modulating blood pressure, promoting post-infarction cardiac repair, anti-inflammation and maintaining gut barrier. Gpr41: G-protein-coupled receptor 41; Gpr43: G-protein-coupled receptor 43; HIF: Hypoxia-inducible factor; SCFA: Short-chain fatty acid; Treg: Regulatory T cell.
Bile acid pathway
Primary BAs are produced in the liver and secreted into the gut through the biliary system. It is well established that the gut microbiota profoundly impacts BA metabolism by promoting deconjugation, dehydrogenation, and dihydroxylation of primary BAs.[60] The physiologic function of BAs is to facilitate the absorption of dietary fat, fat-soluble molecules and cholesterol.[61] Farnesoid X receptor is highly expressed in the liver and ileum, which negatively regulates BA synthesis by regulating distinct transcriptional networks. However, tauro-beta-muricholic acid (TβMCA), which is an abundant primary BA, could up-regulate the BA pool size and composition by acting as a farnesoid X receptor antagonist. It is suggested that particular microbiota may have the capacity to suppress BA synthesis by reducing the levels of TβMCA.[60] According to a cross-sectional study, an increased ratio of secondary to primary BAs in serum was found in patients with chronic heart failure, and this ratio was revealed to be associated with reduced overall survival in univariate analysis.[55] Considering that the production of secondary BAs depends on the gut microbiota, identifying specific BAs and correlated microbial enzymes as well as host receptors might help for understanding the underlying mechanism through which the gut microbiota plays a role in modifying BA composition and thus impact metabolic disorders involved in the progression of heart failure.
Uremic toxin pathway
It is well accepted that CVD and chronic kidney disease are closely interrelated via the so-called cardiorenal syndrome, which could accelerate the progression of failure in both organs.[18] The microbial urease of the gut microbiota is able to hydrolyze urea to form ammonia, which is later transformed into ammonium hydroxide, leading to the production of uremic toxins such as indoxyl sulfate and p-cresyl sulfate. It was reported that indoxyl sulfate and p-cresyl sulfate were associated with adverse cardiovascular outcomes. A direct effect of indoxyl sulfate on cardiomyocytes is the stimulation of cardiac fibroblasts and collagen synthesis via activation of the p38 mitogen-activated protein kinase, p42/44 mitogen-activated protein kinase, and nuclear factor κB pathways, thus leading to adverse cardiac remodeling.[17,62]
Targeting the Gut Microbiota for Treatment
The already known correlations between altered gut microbial compositions and susceptibility for cardiometabolic disorders remind us of the possibility of the gut microbiome as a potential novel target for therapeutics. Regulating the gut microbiota has shown promising prospects in curing various diseases, including diabetes, cancer, and so on.[63–65] Three major intervention principles have been focused on, namely, targeting microbiota compositions, targeting metabolic pathways, and applying mucosal barrier protectors. We discuss mainly treatment strategies based on modulating gut microbiota compositions and metabolic pathways in this review.
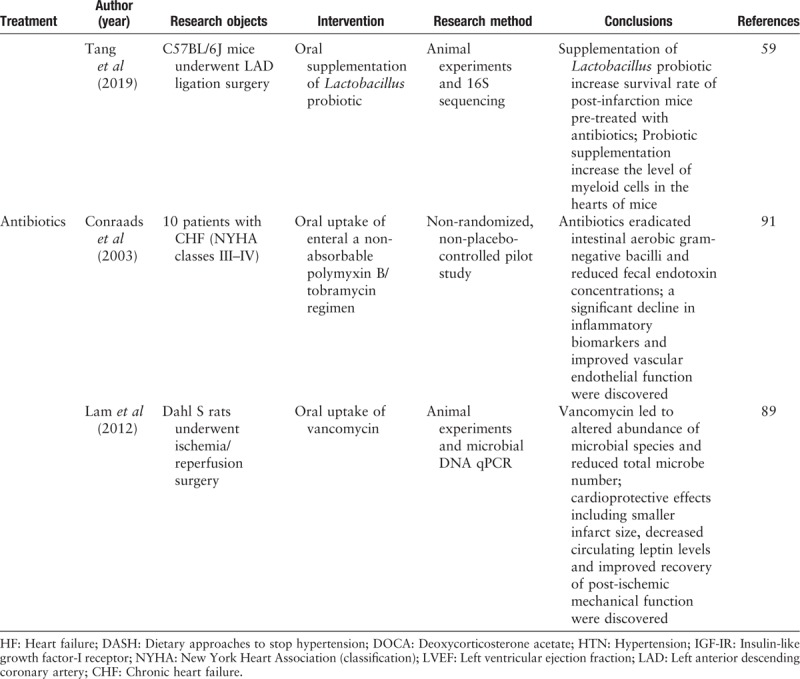
A summary of current research on targeting gut microbiota for the treatment of heart failure is shown in Table 2 . The summary was based on data obtained from PubMed up to March 31, 2019. Articles were selected using the following search terms: “gut microbiota,” “heart failure,” “cardiovascular disease,” “treatment,” “diet,” “probiotic,” “prebiotic,” “antibiotic,” and “fecal microbiota transplantation.” We reviewed the medical literature one by one.
Table 2.
Current research on targeting gut microbiota for the treatment of heart failure.
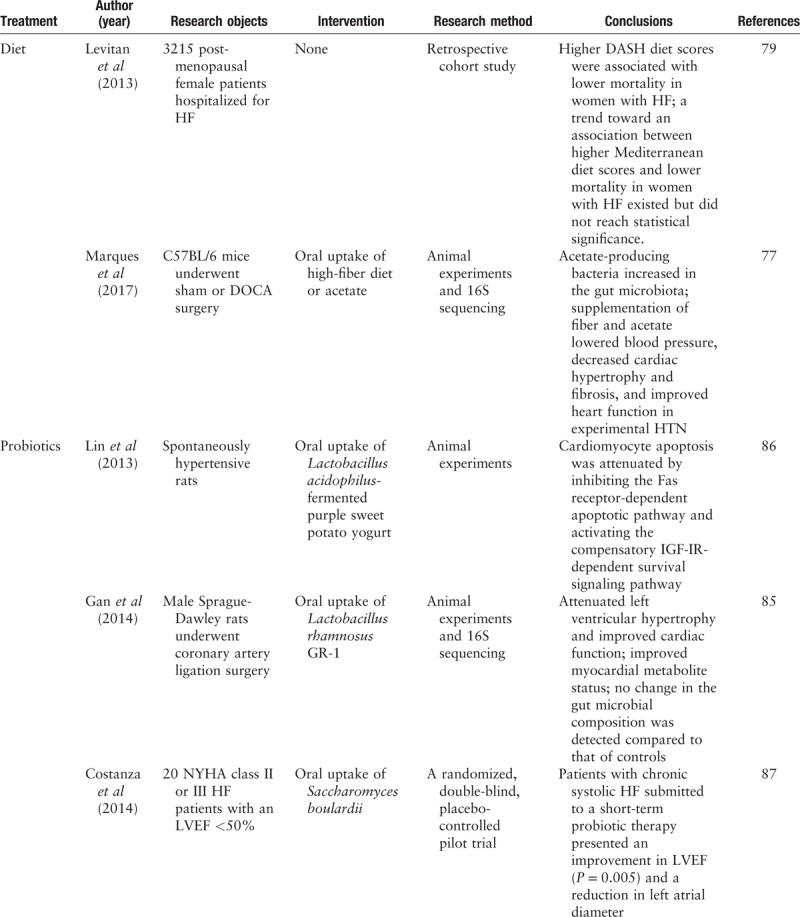
Table 2 (Continued).
Current research on targeting gut microbiota for the treatment of heart failure.
Diet modulation
Currently, diet modulation represents a major therapeutic strategy utilized to treat chronic metabolic diseases in clinical practice.[66] Many clinical studies proved that dietary nutritional intervention was effective in reducing cardiovascular risk.[67–70] A retrospective cohort study based on 3215 post-menopausal female participants revealed that relatively high dietary approaches to stop hypertension diet scores were modestly associated with a low mortality in women with heart failure. The Mediterranean diet presented a trend toward an association with decreased heart failure mortality, although statistical significance was not reached.[71] It has been reported that a high-fiber diet can prevent the development of heart failure and effectively improve myocardial remodeling in hypertensive mice by increasing the abundance of acetate-producing microbiota.[69] However, although many studies confirmed that dietary interaction was associated with improved cardiac function and heart failure biomarkers, few of them focused on the impact that such lifestyle intervention had on the gut microbial community structure and function as well as the underlying mechanistic interplay.[72,73] Studies exploring the impact of dietary interventions on heart failure from the perspective of the gut microbiome in humans are needed.
Probiotics/prebiotics
Probiotics are defined as live “beneficial” bacteria utilized to re-establish an appropriate intestinal balance. Potential mechanisms of probiotics include mainly pH modulation, antibacterial substance production, and competition with pathogens.[74,75] Plasma cytokine levels are generally increased in patients with heart failure, and inflammatory pathways are widely involved in the onset and development of chronic heart failure.[76] Regulating the intestinal microecosystem may be a potential therapeutic strategy to improve cardiac function and clinical prognosis by optimizing gut flora metabolism and reducing inflammation responses in humans. Another approach to achieve similar effects on modulating intestinal microbiota is the use of prebiotics. Prebiotics are defined as “selectively fermented ingredients that result in specific changes, in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon host health.”[77] Typical prebiotics refer to indigestible molecules such as oligosaccharides or complex saccharides. According to Gan et al's[78] research, although no changes in the gut microbial compositions were detected by 16S rRNA sequencing afterwards, oral supplement of Lactobacillus rhamnosus GR-1 can effectively attenuate left ventricular hypertrophy and significantly improve hemodynamic parameters in post-infarction heart failure rat models. This effect may be achieved by improving myocardial metabolite status, such as decreasing the leptin/adiponectin plasma concentration ratio. In another animal experiment, Lin et al[79] reported that probiotic-fermented purple sweet potato yogurt might reverse congestive heart failure induced by hypertension through attenuating cardiomyocyte apoptosis by inhibiting Fas receptor-dependent apoptotic pathways but activating compensatory IGF-IR-dependent pathways in spontaneously hypertensive rats. Similar findings were validated in humans, as Costanza et al[80] conducted a randomized placebo-controlled pilot trial focusing on treating chronic systolic heart failure patients with Saccharomyces boulardii probiotics, which yielded a decrease in cholesterol, uric acid, and left ventricle diameter; an improvement in LVEF; and a reduction in left atrial diameter.
Antibiotics
Scientists tried to cure disease by eliminating the pathogens. It has been reported that oral administration of vancomycin significantly impacts host microbiota diversity by inducing a decrease in gram-positive bacteria and a compensatory increase in gram-negative bacteria. Subsequently, BA dihydroxylation and peripheral insulin sensitivity were suppressed as a result.[81] Lam et al[82,83] suggested that taking vancomycin orally led to a reduced total microbial number in Dahl S rats and presented cardioprotective effects including reduced myocardial infarction size and reduced circulating leptin levels in an ischemia/reperfusion rat model. According to Conraads et al,[84] selective decontamination of the digestive tract (SDD, using an enteral non-absorbable polymyxin B/tobramycin regimen) induced decreased fecal endotoxin concentrations and showed anti-inflammatory effects. In addition, improved vascular endothelial function presented as an increase in flow-mediated dilation. However, the listed variations returned to baseline levels after discontinuation of the SDD. Although antibiotics show cardiovascular protective impacts to some extent, such impacts seem to be limited within the period of medication. In addition, antibiotics usually reduce the total microbiota in the gut, which probably includes beneficial bacteria. Currently, the general consensus for antibiotic intervention is that such non-specific antimicrobial approaches may be more harmful than beneficial.
Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) aims at introducing fecal contents from healthy subjects into the gut of patients, which seems to be effective in the treatment of recurrent or refractory Clostridium difficile infection (CDI).[85] However, for most diseases except CDI, the efficacy of FMT is somehow limited according to current studies.[86] In addition, the potential risk of transferring endotoxins or infectious agents may cause adverse complications.[13] Further studies will be required to optimize factors such as dosing, delivery route, and formulation of FMT to improve the therapeutic efficacy. It is also anticipated that whole-microbiome transplantation will eventually be replaced by transplantation of a defined group of bacteria.[86]
Molecular inhibitors of the TMAO pathway
Recent studies have revealed clear links between the TMAO pathway and poor prognosis of heart failure. The development of a small molecule drug for inhibiting microbial generation of TMA has become a potential therapeutic strategy for CVDs.[29] The 3,3-dimethyl-1-butanol, a choline structural analog, is able to inhibit microbial generation of TMA from quantities of nutrients. Although temporarily there is no evidence supporting that a TMA/TMAO inhibitor can improve heart failure, this molecular therapy has shown great potential in treating heart failure. This potential also reminds us of the possibility of developing other molecular drugs as research on gut microbiomes and metabolomics progresses in the field of heart failure.
Conclusions and Perspectives
Currently, heart failure remains a major health burden. The rapid development of high-throughput sequencing technology enables us to uncover the previously unappreciated complexity of the gut microbiome. Since Wang and Tang et al's impressive research thoroughly revealed the interplay between gut microbes and atherosclerosis through the TMA/TMAO pathway, it has gradually become consensus that the gut microbiota contributes to cardiovascular pathophysiology via multiple metabolic and physiologic pathways. Through the identification of bacterial metabolites, it is possible for us to explore numerous microbial pathways that may be involved in the pathogenesis of cardiometabolic disorders and search for potential biomarkers for diagnosis and treatment. Except for the many clinical studies that have already demonstrated an association between TMA/TMAO and adverse outcomes of patients, a few studies based on 16S or metagenomics sequencing have discovered a reduction in SCFA-producing bacterial species in patients with heart failure, especially some butyrate-producing species such as F. prausnitzii and E. rectale. In addition to being major energy substrates of gut epithelial cells, SCFAs play essential roles in the maintenance of host glucose homeostasis and the immune system. A shift in the gut microbiota into a composition lacking in SCFA-producing bacteria might be a notable characteristic for patients with heart failure. Future studies are needed to explore the deep correlations between microbes, SCFAs and host cardiovascular health.
By modulation of gut microbiota composition and function through diet, pre/probiotics, FMT, and microbial enzyme inhibitors, it may become feasible for us to alter metabolic profiles in a preferred direction that is beneficial for host health in the long term. Transplantation of a defined group of bacteria or utilization of special microbial enzyme inhibitors, such as DMB, can probably adjust blood levels of biologically active microbial-derived metabolites by modulating gut microbial compositions or targeting specific microbial pathways, thus achieving a more personalized and accurate therapeutic intervention. However, neither approach has been studied to date in patients with heart failure. Future research is required to complement this gap. Finally, the use of pre/probiotics has shown great potential in treating heart failure. However, most studies have focused on a correlation between the oral uptake of probiotics and changes in heart failure phenotypes; only a few studies have explored the variations of gut microbial compositions and functions brought about by intervention with pre/probiotics, let alone the underlying metabolic and physiologic mechanisms. Pre/probiotics remain a cost-effective and practical option for intervention that is an area of active investigation, but mechanistic understanding is strongly needed.
Although several studies revealed significant correlations between either the gut microbiota composition or their derived metabolites and the phenotypes of heart failure, the sample size of each study is not large enough. Heart failure is the end stage of cardiogenic diseases and is usually accompanied by multiple complications. Many factors including etiology, complications, drugs, host genetic heterogeneity, and lifestyles may contribute to confounding effects in the clinical study. Therefore, well-designed, prospective, and longitudinal clinical studies based on large cohorts are still needed to reveal the actual transformation of the gut microbiota composition and metabolic profile in heart failure.
Doubtlessly, a striking and intriguing association exists between the gut microbiota and the cardiovascular system that probably plays a large role in CVDs. However, few studies have investigated in depth a direct role of the gut microbiota in heart failure and associated complications at the mechanistic and causal levels. Further investigations are definitely in demand to better understand intermicrobial interactions and microbial-host interactions and how they are related to the underlying molecular entities involved in disease progression.
Cardiac disease has been a burden throughout history. With improved quality of life and prolonged life expectancy, the prevalence of cardiac diseases currently continues to escalate, which means that more people will enter the stage of heart failure. More innovative diagnostic and therapeutic approaches for heart failure are urgently in demand. As we gradually gain a deeper understanding of gut microbiota and heart failure interplay, the question of how to bring microbial information into clinical practice remains a major challenge. Of course, high-throughput technologies including 16S and metagenomics sequencing can provide profound information about a single patient's gut microbiota compositions, but such technologies are quite expensive and no evidence clearly clarified their utility in clinical practice as yet. Instead, studying metabolic profiles in blood and urine may be a practical way to guide personalized interventions. Undoubtedly, further investigations to explore the translational potential of mechanism research and the clinical application values of multiple therapeutic interventions are necessarily required.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81670329) and Chinese Academy of Medical Sciences Initiative for Innovative Medicine (No. 2016-I2M-3-011).
Conflicts of interest
None.
Footnotes
How to cite this article: Chen X, Li HY, Hu XM, Zhang Y, Zhang SY. Current understanding of gut microbiota alterations and related therapeutic intervention strategies in heart failure. Chin Med J 2019;132:1843–1855. doi: 10.1097/CM9.0000000000000330
References
- 1.Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J 2004; 25:1614–1619. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Shah AM, Mann DL. In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet 2011; 378:704–712. doi: 10.1016/S0140-6736(11)60894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature 2008; 451:919–928. doi: 10.1038/nature06798. [DOI] [PubMed] [Google Scholar]
- 4.van Riet EE, Hoes AW, Limburg A, Landman MA, van der Hoeven H, Rutten FH. Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur J Heart Fail 2014; 16:772–777. doi: 10.1002/ejhf.110. [DOI] [PubMed] [Google Scholar]
- 5.Dassanayaka S, Jones SP. Recent developments in heart failure. Circ Res 2015; 117:e58–e63. doi: 10.1161/CIRCRESAHA.115.305765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maggioni AP, Dahlstrom U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, et al. EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail 2013; 15:808–817. doi: 10.1093/eurjhf/hft050. [DOI] [PubMed] [Google Scholar]
- 7.Rahimi K, Bennett D, Conrad N, Williams TM, Basu J, Dwight J, et al. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail 2014; 2:440–446. doi: 10.1016/j.jchf.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Ouwerkerk W, Voors AA, Zwinderman AH. Factors influencing the predictive power of models for predicting mortality and/or heart failure hospitalization in patients with heart failure. JACC Heart Fail 2014; 2:429–436. doi: 10.1016/j.jchf.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Brown JM, Hazen SL. The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu Rev Med 2015; 66:343–359. doi: 10.1146/annurev-med-060513-093205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zabell A, Tang WH. Targeting the microbiome in heart failure. Curr Treat Options Cardiovasc Med 2017; 19:27.doi: 10.1007/s11936-017-0528-4. [DOI] [PubMed] [Google Scholar]
- 12.Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev 2010; 90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 13.Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res 2017; 120:1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007; 449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology 2009; 136:65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A 2008; 105:2117–2122. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagatomo Y, Tang WH. Intersections between microbiome and heart failure: revisiting the gut hypothesis. J Card Fail 2015; 21:973–980. doi: 10.1016/j.cardfail.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitai T, Kirsop J, Tang WH. Exploring the microbiome in heart failure. Curr Heart Fail Rep 2016; 13:103–109. doi: 10.1007/s11897-016-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitai T, Tang WHW. Gut microbiota in cardiovascular disease and heart failure. Clin Sci (Lond) 2018; 132:85–91. doi: 10.1042/CS20171090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrett WS. Cancer and the microbiota. Science 2015; 348:80–86. doi: 10.1126/science.aaa4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao W, Caro F, Robins W, Mekalanos JJ. Antagonism toward the intestinal microbiota and its effect on Vibrio cholerae virulence. Science 2018; 359:210–213. doi: 10.1126/science.aap8775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu H, Khosravi A, Kusumawardhani IP, Kwon AH, Vasconcelos AC, Cunha LD, et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science 2016; 352:1116–1120. doi: 10.1126/science.aad9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012; 489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Yin A, Li H, Wang R, Wu G, Shen J, et al. Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children. EBioMedicine 2015; 2:968–984. doi: 10.1016/j.ebiom.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 2013; 57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med 2015; 21:895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- 27.Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 2017; 5:10.doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017; 5:14.doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 2015; 163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016; 165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui X, Ye L, Li J, Jin L, Wang W, Li S, et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep 2018; 8:635.doi: 10.1038/s41598-017-18756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A 2011; 108 Suppl 1:4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlsson FH, Fak F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 2012; 3:1245.doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luedde M, Winkler T, Heinsen FA, Ruhlemann MC, Spehlmann ME, Bajrovic A, et al. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail 2017; 4:282–290. doi: 10.1002/ehf2.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kummen M, Mayerhofer CCK, Vestad B, Broch K, Awoyemi A, Storm-Larsen C, et al. Gut microbiota signature in heart failure defined from profiling of 2 independent cohorts. J Am Coll Cardiol 2018; 71:1184–1186. doi: 10.1016/j.jacc.2017.12.057. [DOI] [PubMed] [Google Scholar]
- 36.Kamo T, Akazawa H, Suda W, Saga-Kamo A, Shimizu Y, Yagi H, et al. Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS One 2017; 12:e0174099.doi: 10.1371/journal.pone.0174099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail 2016; 4:220–227. doi: 10.1016/j.jchf.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, et al. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet 1999; 353:1838–1842. doi: 10.1016/S0140-6736(98)09286-1. [DOI] [PubMed] [Google Scholar]
- 39.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 2007; 50:1561–1569. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Sandek A, Swidsinski A, Schroedl W, Watson A, Valentova M, Herrmann R, et al. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol 2014; 64:1092–1102. doi: 10.1016/j.jacc.2014.06.1179. [DOI] [PubMed] [Google Scholar]
- 41.Larsson E, Tremaroli V, Lee YS, Koren O, Nookaew I, Fricker A, et al. Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through MyD88. Gut 2012; 61:1124–1131. doi: 10.1136/gutjnl-2011-301104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature 2007; 449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011; 472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013; 19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013; 504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 46.Seldin MM, Meng YH, Qi HX, Zhu WF, Wang ZE, Hazen SL, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappa B. J Am Heart Assoc 2016; 5:e002767.doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franzosa EA, Hsu T, Sirota-Madi A, Shafquat A, Abu-Ali G, Morgan XC, et al. Sequencing and beyond: integrating molecular ’omics’ for microbial community profiling. Nat Rev Microbiol 2015; 13:360–372. doi: 10.1038/nrmicro3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subramaniam S, Fletcher C. Trimethylamine N-oxide: breathe new life. Br J Pharmacol 2018; 175:1344–1353. doi: 10.1111/bph.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013; 368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol 2014; 64:1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki T, Heaney LM, Bhandari SS, Jones DJ, Ng LL. Trimethylamine N-oxide and prognosis in acute heart failure. Heart 2016; 102:841–848. doi: 10.1136/heartjnl-2015-308826. [DOI] [PubMed] [Google Scholar]
- 52.Tang WH, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, et al. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail 2015; 21:91–96. doi: 10.1016/j.cardfail.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Troseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med 2015; 277:717–726. doi: 10.1111/joim.12328. [DOI] [PubMed] [Google Scholar]
- 54.Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, et al. Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide, exacerbate pressure overload-induced heart failure. Circ Heart Fail 2016; 9:e002314.doi: 10.1161/CIRCHEARTFAILURE.115.002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayerhofer CCK, Ueland T, Broch K, Vincent RP, Cross GF, Dahl CP, et al. Increased secondary/primary bile acid ratio in chronic heart failure. J Card Fail 2017; 23:666–671. doi: 10.1016/j.cardfail.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 56.Tang TWH, Chen H-C, Chen C-Y, Yen CYT, Lin C-J, Prajnamitra RP, et al. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation 2019; 139:647–659. doi: 10.1161/circulationaha.118.035235. [DOI] [PubMed] [Google Scholar]
- 57.Sivaprakasam S, Prasad PD, Singh N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol Ther 2016; 164:144–151. doi: 10.1016/j.pharmthera.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015; 17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A 2013; 110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sayin Sama I, Wahlström A, Felin J, Jäntti S, Marschall H-U, Bamberg K, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 2013; 17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 2003; 72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 62.Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J 2010; 31:1771–1779. doi: 10.1093/eurheartj/ehp574. [DOI] [PubMed] [Google Scholar]
- 63.Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and anticancer immunosurveillance. Cell 2016; 165:276–287. doi: 10.1016/j.cell.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Zitvogel L, Daillere R, Roberti MP, Routy B, Kroemer G. Anticancer effects of the microbiome and its products. Nat Rev Microbiol 2017; 15:465–478. doi: 10.1038/nrmicro.2017.44. [DOI] [PubMed] [Google Scholar]
- 65.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Manneras-Holm L, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 2017; 23:850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 66.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997; 336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 68.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013; 368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 69.Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 2017; 135:964–977. doi: 10.1161/Circulationaha.116.024545. [DOI] [PubMed] [Google Scholar]
- 70.Xiao SM, Fei N, Pang XY, Shen J, Wang LH, Zhang BR, et al. A gut microbiota-targeted dietary intervention for amelioration of chronic inflammation underlying metabolic syndrome. Fems Microbiol Ecol 2014; 87:357–367. doi: 10.1111/1574-6941.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levitan EB, Lewis CE, Tinker LF, Eaton CB, Ahmed A, Manson JE, et al. Mediterranean and DASH diet scores and mortality in women with heart failure: The Women's Health Initiative. Circ Heart Fail 2013; 6:1116–1123. doi: 10.1161/CIRCHEARTFAILURE.113.000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hummel SL, Seymour EM, Brook RD, Sheth SS, Ghosh E, Zhu S, et al. Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail 2013; 6:1165–1171. doi: 10.1161/CIRCHEARTFAILURE.113.000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fito M, Estruch R, Salas-Salvado J, Martinez-Gonzalez MA, Aros F, Vila J, et al. Effect of the Mediterranean diet on heart failure biomarkers: a randomized sample from the PREDIMED trial. Eur J Heart Fail 2014; 16:543–550. doi: 10.1002/ejhf.61. [DOI] [PubMed] [Google Scholar]
- 74.Ojetti V, Lauritano EC, Barbaro F, Migneco A, Ainora ME, Fontana L, et al. Rifaximin pharmacology and clinical implications. Expert Opin Drug Met 2009; 5:675–682. doi: 10.1517/17425250902973695. [DOI] [PubMed] [Google Scholar]
- 75.Delzenne NM, Neyrinck AM, Backhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol 2011; 7:639–646. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- 76.Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res 2015; 116:1254–1268. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 2017; 14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 78.Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail 2014; 7:491–499. doi: 10.1161/CIRCHEARTFAILURE.113.000978. [DOI] [PubMed] [Google Scholar]
- 79.Lin PP, Hsieh YM, Kuo WW, Lin YM, Yeh YL, Lin CC, et al. Probiotic-fermented purple sweet potato yogurt activates compensatory IGFIR/PI3K/Akt survival pathways and attenuates cardiac apoptosis in the hearts of spontaneously hypertensive rats. Int J Mol Med 2013; 32:1319–1328. doi: 10.3892/ijmm.2013.1524. [DOI] [PubMed] [Google Scholar]
- 80.Costanza AC, Moscavitch SD, Faria Neto HC, Mesquita ET. Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int J Cardiol 2015; 179:348–350. doi: 10.1016/j.ijcard.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 81.Vrieze A, Out C, Fuentes S, Jonker L, Reuling I, Kootte RS, et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol 2014; 60:824–831. doi: 10.1016/j.jhep.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 82.Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, et al. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J 2012; 26:1727–1735. doi: 10.1096/fj.11-197921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lam V, Su J, Hsu A, Gross GJ, Salzman NH, Baker JE. Intestinal microbial metabolites are linked to severity of myocardial infarction in rats. PLoS One 2016; 11:e0160840–e160850. doi: 10.1371/journal.pone.0160840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Conraads VM, Jorens PG, De Clerck LS, Van Saene HK, Ieven MM, Bosmans JM, et al. Selective intestinal decontamination in advanced chronic heart failure: a pilot trial. Eur J Heart Fail 2004; 6:483–491. doi: 10.1016/j.ejheart.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 85.Quraishi MN, Widlak M, Bhala N, Moore D, Price M, Sharma N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther 2017; 46:479–493. doi: 10.1111/apt.14201. [DOI] [PubMed] [Google Scholar]
- 86.Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med 2016; 22:713–722. doi: 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]


