Abstract
Background:
Allogeneic stem-cell transplantation (SCT) is a well-established immunotherapeutic strategy for multiple myeloma (MM) with a potent and often sustained graft-vs.-myeloma effect. This multicenter investigation aimed to analyze the complications and survival of haploidentical SCT in patients with MM, and compare the main outcomes with matched-related donors (MRDs).
Methods:
Haploidentical and MRD SCT was identified from a cohort of 97 patients with MM who received a myeloablative transplantation in 13 hospitals from May 2001 to December 2017. A matched-pair analysis was designed. For each haplo recipient, the recipients were randomly selected from the MRD group and were matched according to the following criteria: year of the hematopoietic SCT (±2 years), disease status at transplantation, and the length of follow-up.
Results:
Seventy cases received MRD and 27 received haploidentical transplantation. The two groups showed no significant differences regarding age, gender, cytogenetic risk, and diagnostic stage. The cumulative incidences of non-relapse mortality (NRM) at 1 and 3 years based on donor type were 20.5% (95% confidence interval [CI], 10.90–30.10%) and 24.2% (95% CI, 13.81–34.59%) for the MRD group and 16.80% (95% CI, 1.71–31.89%) and 28.70% (95% CI, 8.71–48.69%) for the haplo group, respectively. Cumulative incidence of NRM did not differ significantly between the two groups (χ2 = 0.031, P = 0.861). The cumulative incidences of progression-free survival (PFS) and 1 year and 3 years by type of donors were 59.8% (95% CI, 48.24–71.36%) and 45.4% (95% CI, 33.44–57.36%), and 65.6% (95% CI, 47.18–84.02%) and 26.8% (95% CI, 7.59–46. 01%) for MRD and haploidentical donor, respectively. Cumulative incidence of PFS did not differ significantly between the two groups (χ2 = 0.182, P = 0.670). In multivariate analyses, no statistically significant differences were observed between haploidentical and MRD for relapse, NRM, PFS, and overall survival. There were no statistically differences on main outcomes after haploidentical and MRD.
Conclusion:
Haploidentical SCT could be performed safely and feasibly for patients with MM in need.
Keywords: Allogeneic stem-cell transplantation, Multiple myeloma
Introduction
Allogeneic stem-cell transplantation (allo-SCT) is a well-established immunotherapeutic strategy for multiple myeloma (MM) with a potent and often sustained graft-vs.-myeloma effect.[1,2] Therefore, allo-SCT continues to be offered to a significant portion of patients with relapsed/refractory or ultra-high risk MM. During early stage of this treatment, treatment-related mortality (TRM) following allo-SCT for MM has reportedly ranged from 40% to 60%.[3–6] Since then, the TRM after myeloablative and reduced intensity conditioned allo-SCT has decreased with time (48% TRM between 1995 and 2000 vs. 29% TRM between 2001 and 2005) and further improvements are expected owing to improvements in supportive care and new preparative regimens.[7]
Transplantation from a haploidentical family donor has become an established procedure to treat patients with malignant hematologic diseases including relapsed or refractory acute leukemia and lymphoma and serves as a treatment alternative for high-risk hematologic malignant disorders.[8–11] Modified or intensified conditioning regimens and improved supportive care has yielded improved outcomes after haploidentical SCT (haplo-SCT) with decreased treatment-related toxicity and infections, compared to conventional SCT.[12–14] Haplo-SCT has received increasing attention as an alternative to human leukocyte antigen (HLA)-matched SCT in emergent cases. However, limited information is available regarding the use of other alternative donors, such as haploidentical grafts in patients with MM.[15,16] Previously, we reported the safety and efficacy of using grafts from matched sibling donors to treat MM.[17,18] Accordingly, we performed a registry-based study to evaluate the outcomes of transplantation among patients receiving grafts from various donor types including matched siblings and haploidentical-related donors to analyze the role of donor type in MM.
Methods
Ethical approval
The study was conducted in accordance with the tenets of the Declaration of Helsinki. All patients provided informed consent for research. As a retrospective study and data analysis was performed anonymously, this study was exempt from the ethical approval.
Study design, inclusion criteria, and data collection
This retrospective registry-based study involved consecutively data on Chinese patients aged over 18 years and diagnosed with MM or plasma cell leukemia (PCL), receiving allogeneic SCT from May 2001 to December 2017. Exclusion criteria were as follows: history of allo-SCT, unrelated donors, non-myeloablative conditioning regimens, received cord blood as a source of stem cells, and unknown donor type. Patients with matched-related donors (MRDs) were assigned to the MRD group; patients with haploidentical-related donors, haplo group. A matched-pair analysis was designed. For each haplo recipient, the recipients were randomly selected from the MRD group and were matched according to the following criteria: year of the hematopoietic SCT (±2 years), disease status at transplantation and the length of follow-up.
Endpoints and definitions
The primary endpoint was progression-free survival (PFS) defined as time from allogeneic SCT to progression, relapse, or death from any cause, whichever occurred first. Secondary endpoints were neutrophil and platelet recovery, acute, and chronic graft-vs.-host disease (GVHD), non-relapse mortality (NRM), relapse incidence, and overall survival (OS). OS was defined as time from transplant to death from any cause.
Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count ≥0.5 × 109/L, without evidence of autologous reconstitution. Platelet engraftment was defined as the first date at which an unsupported platelet count of ≥20 × 109/L for 7 consecutive days was achieved. GVHD was evaluated on the basis of standard criteria.
The myeloablative regimen was defined as a regimen containing total-body irradiation (TBI) with a dose of >6 Gy, >8 mg/kg oral, or >6.4 mg/kg intravenous busulfan (BU) or multiple chemotherapy combinations involving high-dose carmustine, etoposide, cytarabine, and melphalan.[19,20]
Response to treatment was defined in accordance with standard criteria defined previously.[21] Complete response (CR) was defined by negative serum and urine immunofixation and 5% or less plasma cells with normal morphologic features in a bone marrow aspirate. A very good partial response (VGPR) was defined as a 90% reduction in serum paraprotein levels; partial response (PR), a 50% reduction in serum paraprotein levels or a 90% reduction in Bence-Jones protein levels (including patients with Bence-Jones protein alone) or both; stable disease (SD), no change in serum paraprotein levels; progressive disease (PD), a 25% increase in serum paraprotein levels; relapse, resurgence of serum paraprotein, recurrence of bone marrow infiltration, or both in patients displaying a CR and a 50% increase beyond the plateau levels of serum paraprotein in two samples obtained 4 weeks apart from a responder. High-risk cytogenetic abnormalities included del17p, t(4;14), or t(14;16), analyzed via fluorescence in situ hybridization, or conventional metaphase cytogenetics.
Statistical analysis
Categorical variables and quantitative data from the haploidentical and MRD groups were compared using the Chi-squared test and the non-parametric test, respectively. PFS and OS were measured in days and calculated from the date of allo-SCT until the respective events. NRM was defined as death from any cause, which occurred without previous progression or relapse after transplantation. The univariate probabilities of acute GVHD (aGVHD), chronic GVHD (cGVHD), NRM, relapse, OS, and PFS were calculated using cumulative incidence curves to account for competing risks. The effect of donor type on NRM, relapse, OS, and PFS was assessed using the Cox regression analysis. Any covariates with a P value of <0.1 on univariate analysis were included in the multivariate analysis. All P values were based on two-sided hypothesis tests, and the α-value was set at 0.05. The endpoint of the last follow-up for all surviving patients was October 14, 2018. SPSS 19.0 (IBM, Chicago, IL, USA) was used for statistical analysis.
Results
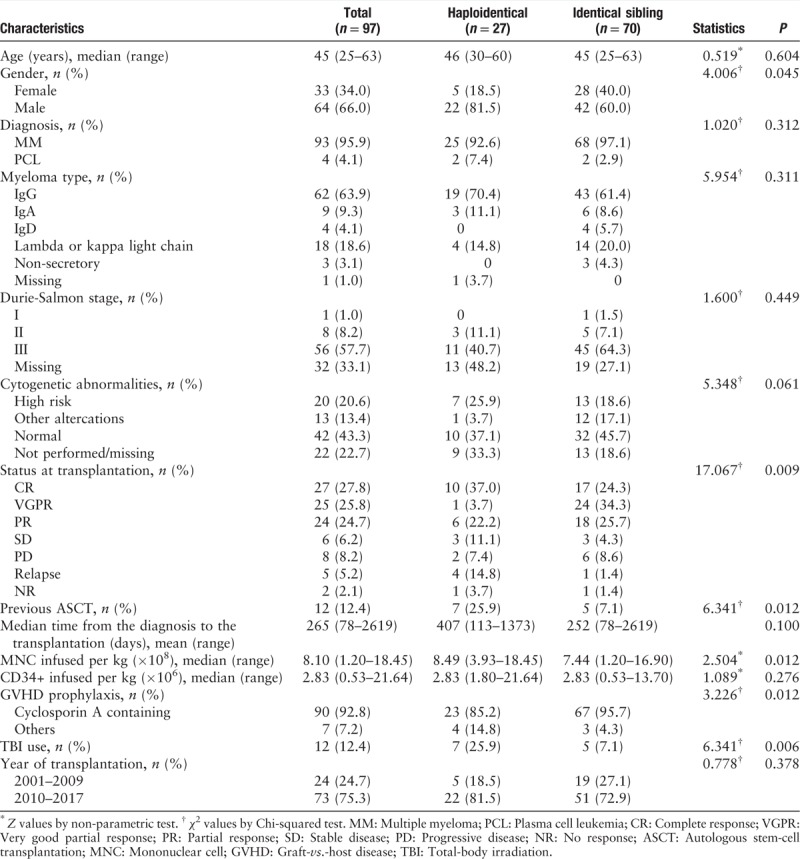
Patient and transplant characteristics are summarized in Table 1. From 2001 to 2017, 97 patients from 13 centers, who fulfilled the eligibility criteria, were included. The median age at transplantation was 45 (range, 25–63) years. The median time from diagnosis to transplantation was 265 (range, 78–2619) days. Diagnosis was MM for 93 (95.9%) patients and PCL for four (4.1%) patients. Only 12 (12.4%) patients received a previous autologous stem cell graft. Data from cytogenetic analysis were available for 75 patients, revealing cytogenetic abnormalities in 33 of 75 (44.0%) patients. High-risk abnormalities (del17p or t[4;14] or t[14;16]) were observed in 20 patients. Thirty-three patients (34.0%) received allo-SCT as first-line therapy, 64 (66.0%) received allo-SCT beyond first-line treatment. Eleven (15.7%) and ten (37.0%) patients in the MRD and haplo groups had a PR status less than that of SD, PD, and NR, respectively (P = 0.009). The most common conditioning regimen was BU and cyclophosphamide (CTX) administered to 62 (63.9%) patients. The combination of cyclosporine A (CsA), mycophenolate mofetil, and a short course of MTX was the most frequent (n = 90, 92.8%) GVHD prophylaxis. Peripheral blood stem cells were the most frequently used as stem cell sources for 56 of 97 (57.7%) transplants, whereas combined bone marrow and peripheral blood stem cells were used in 37 transplants (38.1%).
Table 1.
Characteristics of patients with MM or PCL receiving allogeneic stem-cell transplantation.
Hematologic engraftment
The 21-day cumulative incidences of neutrophil recovery for the MRD and haplo groups were 94.3% (95% confidence interval [CI], 88.8–99.8%) and 92.3% (95% CI, 82.1–100%), respectively (χ2 = 3.791, P = 0.052). The 60-day cumulative incidences of platelet recovery for the MRD and haplo groups were 97.0% (95% CI, 92.9–100%) and 92.3% (95% CI, 82.1–100%), respectively. Platelet engraftment was significantly lower in the haplo group than in the MRD group (χ2 = 3.729, P = 0.030).
Acute and chronic GVHD
Cumulative incidences of grades 2 to 4 acute GVHD at day 100 were 29.2% (95% CI, 18.0–40.4%), and 23.0% (95% CI, 6.9–39.1%) for MRD and haplo groups, while the corresponding probabilities of grade 3 or 4 acute GVHD on day 100 were 7.1% (95% CI, 1.0–13.2%) and 3.7% (95% CI, 0–10.8%), respectively; the risk of acute GVHD being similar between the two groups (χ2 = 0.142, P > 0.05).
For the entire patient cohort, the 3-year cumulative incidence of chronic GVHD was 28.4% (95% CI, 14.7–42.1%) and 77.9% (95% CI, 52.4–100%) for MRD and haplo groups, respectively. The risk of chronic GVHD was significantly higher in the haplo group than in the MRD group (χ2 = 3.897, P = 0.045).
Other major outcomes
Table 2 summarizes the causes of death in each group. The leading cause of death in the two groups was the recurrence of the primary disease. For the entire patient cohort, the 1- and 3-year cumulative incidences of NRM were 19.5% (95% CI, 11.46–27.54%) and 25.1% (95% CI, 15.89–34.31%), respectively. The cumulative incidences of NRM at 1 and 3 years based on donor type were 20.50% (95% CI, 10.90–30.10%) and 24.20% (95% CI, 13.81–34.59%) for the MRD group and 16.80% (95% CI, 1.71–31.89%) and 28.70% (95% CI, 8.71–48.69%) for the haplo group, respectively [Figure 1A]. Cumulative incidence of NRM did not differ significantly between the two groups (χ2 = 0.031, P = 0.861). A transplantation later than 2010 was an independent significant factor that decreased NRM incidence upon multivariate analysis (hazard ratio [HR], 0.296; 95% CI, 0.132–0.663; P = 0.003).
Table 2.
Causes of death in patients with MM or PCL receiving allogeneic stem-cell transplantation, n (%).
Figure 1.
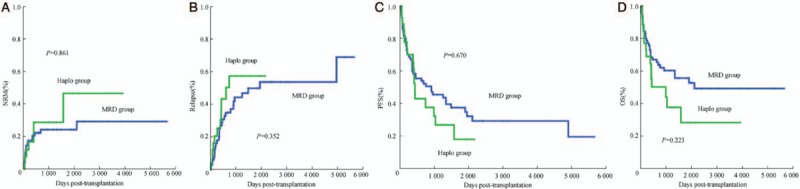
The cumulative incidences of NRM, relapse, PFS, and OS in the MRD group and haplo group. (A) NRM in patients with MM or PCL receiving haploidentical SCT (n = 27) vs. MRD transplantation (n = 70). (B) Relapse in patients with MM or PCL receiving haploidentical SCT (n = 27) vs. MRD transplantation (n = 70). (C) PFS in patients with MM or PCL receiving haploidentical SCT (n = 27) vs. MRD transplantation (n = 70). (D) OS in patients with MM or PCL receiving haploidentical SCT (n = 27) vs. MRD transplantation (n = 70). MM: Multiple myeloma; MRD: Matched-related donor; NRM: Non-relapse mortality; PCL: Plasma cell leukemia; PFS: Progression-free survival; OS: Overall survival; SCT: Stem-cell transplantation.
Overall, at a median follow-up of 1390 days after transplantation, 35.1% patients had CR status. The cumulative incidences of relapse at years 1 and 3 based on donor type were 25.2% (95% CI, 14.03–36.37%) and 44.4% (95% CI, 30.68–58.12%) for the MRD group and 25.5% (95% CI, 7.66–43.34%) and 57.6% (95% CI, 33.69–81.51%) for the haplo group, respectively [Figure 1B]. Cumulative incidence of relapse did not differ significantly between the two groups (χ2 = 0.865, P = 0.352). A status lower than PR at transplantation was an independent risk factor for relapse upon multivariate analysis (HR, 2.483; 95% CI, 1.251–4.925; P = 0.009).
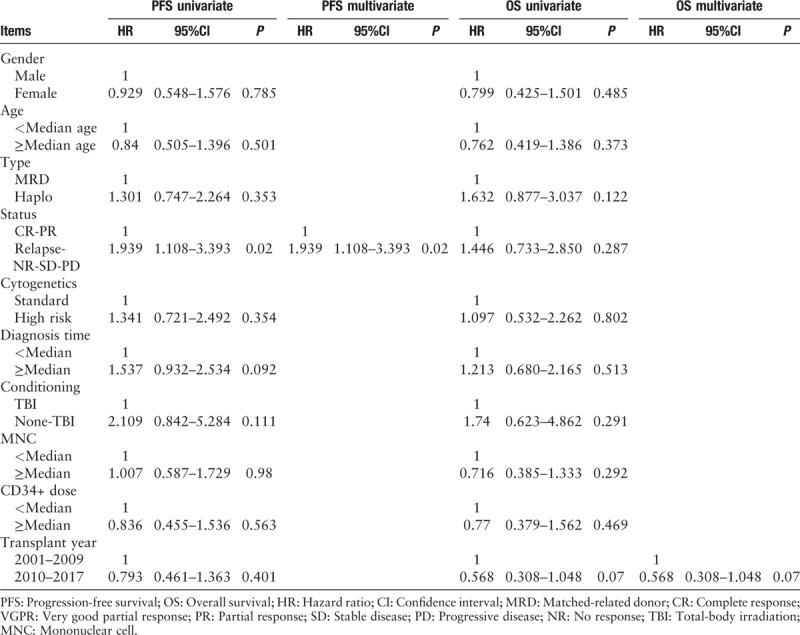
The cumulative incidences of PFS at years 1 and 3 based on donor type were 59.8% (95% CI, 48.24–71.36%) and 45.4% (95% CI, 33.44–57.36%) for the MRD group and 65.6% (95% CI, 47.18–84.02%) and 26.8% (95% CI, 7.59–46.01%) for the haplo group, respectively [Figure 1C]. Cumulative incidence of PFS did not differ significantly between the two groups (χ2 = 0.182, P = 0.670). Furthermore, a status lower than PR was an independent risk factor for relapse upon multivariate analysis (HR, 1.939; 95% CI, 1.108–3.393; P = 0.02).
The cumulative incidences of OS at years 1 and 3 were 72.8% (95% CI, 62.41–83.19%) and 60.1% (95% CI, 48.34–71.86%) for the MRD group and 68.8% (95% CI, 50.77–86.83%) and 37.5% (95% CI, 15.94–59.06%) for the haplo group, respectively [Figure 1D]. The probabilities of OS did not differ significantly between the two groups (χ2 = 1.484, P = 0.223). Furthermore, Tables 3 and 4 show the results of univariate analysis of risk factors regarding their association with clinical outcomes. In summary, the present results indicated that patients with higher tumor burden at transplant had higher relapse rates. Moreover, donor type was not associated with NRM, relapse, DFS, or OS (P > 0.05, Tables 3 and 4).
Table 3.
Univariate and multivariate analyses of factors influencing NRM and relapse for transplantation with donor type.
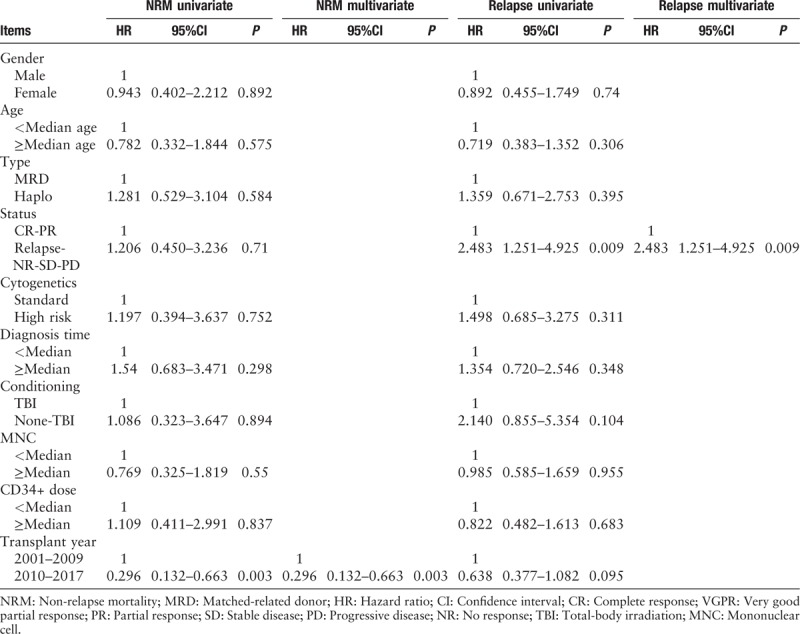
Table 4.
Univariate and multivariate analyses of factors influencing PFS and OS for transplantation with donor type.
Discussion
This retrospective study evaluated the feasibility of allogeneic SCT for MM, including grafts from MRD and haploidentical donors. One patient in MRD groups died of graft rejection, and the others achieved successful engraftment. The cumulative incidence of acute GVHD was similar between the MRD and haplo groups. A European Society for Blood and Marrow Transplantation analysis of 4726 patients who underwent allo-SCT reported an NRM of 19% to 30% after 2004.[22] Concurrently, the present study reported that the cumulative incidence of NRM at 1 and 3 years was 19.5% and 25.1%, respectively, in MRD and haplo recipients. Notably, the cumulative incidence of NRM at 1 and 3 years were comparable between the MRD and haplo groups. Twenty-eight-day neutrophil recovery was not significantly different between the haplo and MRD groups. Furthermore, the cumulative incidence of either grade acute GVHD was comparable between the two groups; however, the haplo group displayed a greater frequency of chronic GVHD than the MRD group. Compared with the MRD population, the possibility of those with an increased incidence of chronic GVHD having a relatively beneficial graft-vs.-myeloma effect warrants verification via further case studies.
Better clinical outcomes are probably associated myeloma chemosensitivity during transplantation. Patients with a status lower than PR did not benefit from allo-SCT and had a higher risk of relapse. Considering high-risk features, cytogenetic aberrations are notably associated with an unfavorable prognosis in patients with MM receiving standard therapies. In the current study, high-risk cytogenetic aberrations were not a poor prognostic factor for major outcomes. Concurrent with the observation by Schilling et al and Roos-Weil et al, allo-SCT might overcome the adverse prognosis of high-risk cytogenetic aberrations.[23,24]
Overall, the risk of relapse was still high even after allo-SCT. In our series, the cumulative incidence of relapse at 3 years was as high as 44.4% and 57.6% the for MRD and haplo groups, respectively; however, these rates are comparable to previous reports regarding allo-SCT for MM. Notably, approximately 85% were of a CR/VGPR/PR status before transplantation in the MRD group; however, those in the haplo group were less chemosensitive (63%). Although pre-transplantation remission rates are important, molecular evaluation of minimal-residual disease significantly influences the detection of disease recurrence,[25,26] where immediate therapeutic intervention could extend the duration of remission and survival in a low-risk situation. Therefore, strategies to prevent relapse after allo-SCT may include a series of minimal residual disease monitoring and maintenance with immunomodulatory drugs, proteasome inhibitors, or targeted cell immunotherapy.[27–29]
Naturally, the present study has some limitations inherent to a retrospective analysis. First, conditioning regimens differed and were not standardized. A few regimens included TBI, no significant effect was observed in the outcomes between the TBI and non-TBI groups. Second, information, cytogenetic stratification data, DS stage, and the International Staging System stage were also not available for each patient. Third, maintenance therapy and subsequent salvage regimens after transplantation were unavailable and seemed to vary owing to the long treatment duration from 2001 to 2017. Fourth, this study had a small cohort, thereby precluding definitive conclusions regarding a comparison of clinical outcomes. The limited number of patients in the haplo group was also a strong limitation to analyze outcomes. Hence, comparisons should be explained with caution. And it would be helpful to highlight patients with longer follow-up on survival.
In conclusion, the present results showed no significant differences in the outcomes between patients with MM receiving grafts from MRD and haploidentical donors. Although allo-SCT provided long-term survival for a fraction of patients, post-transplantation maintenance therapy remains a key issue, even in chemosensitive patients treated first via allo-SCT. In addition, strategies to enhance a graft-vs.-myeloma effect after transplantation should be investigated to decrease disease progression.
Funding
This work was supported by grants from Foundation for Innovative Research Groups of the National Natural Science Foundation of China (No. 81621001), and National Natural Science Foundation of China (Nos. 81670167 and 81670166).
Conflicts of interest
None.
Footnotes
How to cite this article: Chen Y, Fu WJ, Xu LP, Ren HY, Lai YR, Liu DH, Liu L, Sun ZM, Wu YB, Wang X, Xia LH, Jiang M, Hu TL, Wan DM, Huang XJ. Comparison of outcomes after human leukocyte antigen-matched and haploidentical hematopoietic stem-cell transplantation for multiple myeloma. Chin Med J 2019;132:1765–1772. doi: 10.1097/CM9.0000000000000341
Yao Chen and Wei-Jun Fu contributed equally to this study.
References
- 1.Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood 1996; 87:1196–1198. doi: 10.1046/j.1439-0353.2002.t01-1-02619.x. [PubMed] [Google Scholar]
- 2.Mohty M, Boiron JM, Damaj G, Michallet AS, Bay JO, Faucher C, et al. Graft-versus-myeloma effect following antithymocyte globulin-based reduced intensity conditioning allogeneic stem cell transplantation. Bone Marrow Transplant 2004; 34:77–84. doi: 10.1038/sj.bmt.1704531. [DOI] [PubMed] [Google Scholar]
- 3.Buckner CD, Fefer A, Bensinger WI, Storb R, Durie BG, Appelbaum FR, et al. Marrow transplantation for malignant plasma cell disorders: summary of the Seattle experience. Eur J Haematol 1989; 43:186–190. doi: 10.1111/j.1600-0609.1989.tb01515.x. [DOI] [PubMed] [Google Scholar]
- 4.Bensinger WI, Buckner CD, Anasetti C, Clift R, Storb R, Barnett T, et al. Allogeneic marrow transplantation for multiple myeloma: an analysis of risk factors on outcome. Blood 1996; 88:2787–2793. doi: 10.1007/s002770050230. [PubMed] [Google Scholar]
- 5.Bjorkstrand BB, Ljungman P, Svensson H, Hermans J, Alegre A, Apperley J, et al. Allogeneic bone marrow transplantation versus autologous stem cell transplantation in multiple myeloma: a retrospective case-matched study from the European Group for Blood and Marrow Transplantation. Blood 1996; 88:4711–4718. doi: 10.1006/bcmd.1996.0112. [PubMed] [Google Scholar]
- 6.Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol 2006; 24:929–936. doi: 10.1200/jco.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Zhang MJ, Peigang L, Dispenzieri A, Milone GA, Lonial S, et al. Trends in allogeneic stem cell transplantation for multiple myeloma: a CIBMTR analysis. Blood 2011; 118:1979–1988. doi: 10.1182/blood-2011-02-337329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant 2012; 18:1859–1866. doi: 10.1016/j.bbmt.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Gorgeis J, Zhang X, Connor K, Brown S, Solomon SR, Morris LE, et al. T cell-replete HLA haploidentical donor transplantation with post-transplant cyclophosphamide is an effective salvage for patients relapsing after an HLA-matched related or matched unrelated donor transplantation. Biol Blood Marrow Transplant 2016; 22:1861–1866. doi: 10.1016/j.bbmt.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical versus matched-sibling transplant in adults with Philadelphia-negative high-risk acute lymphoblastic leukemia: a biologically phase III randomized study. Clin Cancer Res 2016; 22:3467–3476. doi: 10.1158/1078-0432.CCR-15-2335. [DOI] [PubMed] [Google Scholar]
- 11.How J, Slade M, Vu K, DiPersio JF, Westervelt P, Uy GL, et al. T cell-replete peripheral blood haploidentical hematopoietic cell transplantation with post-transplantation cyclophosphamide results in outcomes similar to transplantation from traditionally matched donors in active disease acute myeloid leukemia. Biol Blood Marrow Transplant 2017; 23:648–653. doi: 10.1016/j.bbmt.2017.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol 2013; 31:1310–1316. doi: 10.1200/JCO.2012.44.3523. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood 2015; 125:3956–3562. doi: 10.1182/blood-2015-02-627786. [DOI] [PubMed] [Google Scholar]
- 14.McCurdy SR, Kasamon YL, Kanakry CG, Bolaños-Meade J, Tsai HL, Showel MM, et al. Comparable composite endpoints after HLA-matched and HLA-haploidentical transplantation with post-transplantation cyclophosphamide. Haematologica 2017; 102:391–400. doi: 10.3324/haematol.2016.144139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castagna L, Mussetti A, Devillier R, Dominietto A, Marcatti M, Milone G, et al. Haploidentical allogeneic hematopoietic cell transplantation for multiple myeloma using post transplant cyclophosphamide GVHD prophylaxis. Biol Blood Marrow Transplant 2017; 23:1549–1554. doi: 10.1016/j.bbmt.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Lu J, Xu LP, Chen H, Zhang XH, Wang FR, et al. Safety and efficacy of haploidentical stem cell transplantation for multiple myeloma. Bone Marrow Transplant 2018; 53:507–510. doi: 10.1038/s41409-017-0069-1. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XH, Huang XJ, Liu KY, Xu LP, Liu DH, Chen H, et al. Modified conditioning regimen busulfan-cyclophosphamide followed by allogeneic stem cell transplantation in patients with multiple myeloma. Chin Med J 2007; 120:463–468. doi: 10.1001/jama.297.11.1257. [PubMed] [Google Scholar]
- 18.Lan HF, Yuan ZG, Zhang CY, Chen YB, Fu WJ, Jiang H, et al. Allogeneic peripheral blood stem cell transplantation from sibling donors for 10 patients with multiple myeloma [in Chinese]. Chin J Hematol 2012; 33:191–194. doi: 10.3760/cma.j.issn.0253-2727.2012.03.010. [PubMed] [Google Scholar]
- 19.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2009; 15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaussant Y, Daguindau E, Pugin A, Mohty M, Avet-Loiseau H, Roos-Weil D, et al. Hematopoietic stem cell transplantation in multiple myeloma: a retrospective study of the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). Biol Blood Marrow Transplant 2015; 21:1452–1459. doi: 10.1016/j.bbmt.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 22.Sobh M, Michallet M, Gahrton G, Iacobelli S, van Biezen A, Schönland S, et al. Allogeneic hematopoietic cell transplantation for multiple myeloma in Europe: trends and outcomes over 25 years. A study by the EBMT Chronic Malignancies Working Party. Leukemia 2016; 30:2047–2054. doi: 10.1038/leu.2016.101. [DOI] [PubMed] [Google Scholar]
- 23.Schilling G, Hansen T, Shimoni A, Zabelina T, Pérez-Simón JA, Gutierrez NC, et al. Impact of genetic abnormalities on survival after allogeneic hematopoietic stem cell transplantation in multiple myeloma. Leukemia 2008; 22:1250–1255. doi: 10.1038/leu.2008.88. [DOI] [PubMed] [Google Scholar]
- 24.Roos-Weil D, Moreau P, Avet-Loiseau H, Golmard JL, Kuentz M, Vigouroux S, et al. Impact of genetic abnormalities after allogeneic stem cell transplantation in multiple myeloma: a report of the Société Française de Greffe de Moelle et de Thérapie Cellulaire. Haematologica 2011; 96:1504–1511. doi: 10.3324/haematol.2011.042713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corradini P, Cavo M, Lokhorst H, Martinelli G, Terragna C, Majolino I, et al. Molecular remission after myeloablative allogeneic stem cell transplantation predicts a better relapse-free survival in patients with multiple myeloma. Blood 2003; 102:1927–1929. doi: 10.1182/blood-2003-01-0189. [DOI] [PubMed] [Google Scholar]
- 26.Kroger N, Badbaran A, Zabelina T, Ayuk F, Wolschke C, Alchalby H, et al. Impact of high-risk cytogenetics and achievement of molecular remission on long-term freedom from disease after autologous-allogeneic tandem transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant 2013; 19:398–404. doi: 10.1016/j.bbmt.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Ladetto M, Ferrero S, Drandi D, Festuccia M, Patriarca F, Mordini N, et al. Prospective molecular monitoring of minimal residual disease after non-myeloablative allografting in newly diagnosed multiple myeloma. Leukemia 2015; 30:1211–1214. doi: 10.1038/leu.2015.269. [DOI] [PubMed] [Google Scholar]
- 28.Michallet M, Sobh M, El-Cheikh J, Morisset S, Sirvent A, Reman O, et al. Evolving strategies with immunomodulating drugs and tandem autologous/allogeneic hematopoietic stem cell transplantation in first line high risk multiple myeloma patients. Exp Hematol 2013; 41:1008–1015. doi: 10.1016/j.exphem.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Caballero-Velázquez T, López-Corral L, Encinas C, Castilla-Llorente C, Martino R, Rosiñol L, et al. Phase II clinical trial for the evaluation of bortezomib within the reduced intensity conditioning regimen (RIC) and post-allogeneic transplantation for high-risk myeloma patients. Br J Haematol 2013; 162:474–482. doi: 10.1111/bjh.12410. [DOI] [PubMed] [Google Scholar]


