We provide a concise review of the neurodevelopmental consequences of prenatal opioid exposure and an agenda for future research, addressing extant methodologic shortcomings.
Abstract
Neonatal opioid withdrawal syndrome (NOWS) has risen in prevalence from 1.2 per 1000 births in 2000 to 5.8 per 1000 births in 2012. Symptoms in neonates may include high-pitched cry, tremors, feeding difficulty, hypertonia, watery stools, and breathing problems. However, little is known about the neurodevelopmental consequences of prenatal opioid exposure in infancy, early childhood, and middle childhood. Even less is known about the cognitive, behavioral, and academic outcomes of children who develop NOWS. We review the state of the literature on the neurodevelopmental consequences of prenatal opioid exposure with a particular focus on studies in which NOWS outcomes were examined. Aiming to reduce the incidence of prenatal opioid exposure in the near future, we highlight the need for large studies with prospectively recruited participants and longitudinal designs, taking into account confounding factors such as socioeconomic status, institutional variations in care, and maternal use of other substances, to independently assess the full impact of NOWS. As a more immediate solution, we provide an agenda for future research that leverages the National Institutes of Health Environmental Influences on Child Health Outcomes program to address many of the serious methodologic gaps in the literature, and we answer key questions regarding the short- and long-term neurodevelopmental health of children with prenatal opioid exposure.
From 1999 to 2014 the number of infants exposed to opioids in utero increased by 333%, and in 2014 alone ∼25 908 infants in the United States were born with identified prenatal opioid exposure.1 Upwards of 50% to 80% of newborns exposed to opioids develop neonatal opioid withdrawal syndrome (NOWS),2–4 also known as neonatal abstinence syndrome (NAS), a constellation of symptoms that may include inconsolability and high-pitched cry, tremors, feeding difficulty, hypertonia, watery stools, and respiratory problems.5,6 Little is known about the short- and long-term neurodevelopmental consequences of prenatal opioid exposure in general, and NOWS in particular. Clear data on these associations are critical for policy makers to implement informed clinical and social policy decisions.
In this article, we critically review the research on short- and long-term neurodevelopmental outcomes of children with prenatal opioid exposure. We focus only on the human literature to describe how these outcomes may vary as a function of (1) the type of opioid to which the child is exposed, (2) whether the child was diagnosed with NOWS, and (3) whether adverse neurodevelopmental outcomes are present after controlling for confounding variables. In doing so, we leverage what we have learned from related literatures and provide an agenda for future research in this area. Specifically, we focus on how a concrete and existing opportunity (the National Institutes of Health [NIH] Environmental Influences on Child Health Outcomes [ECHO] program, which includes longitudinal data from ∼50 000 children from >350 sites across the United States) might facilitate new, immediate discoveries.
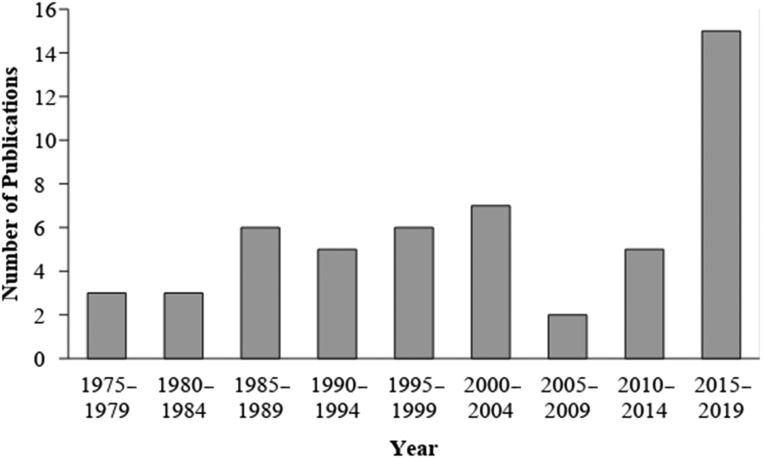
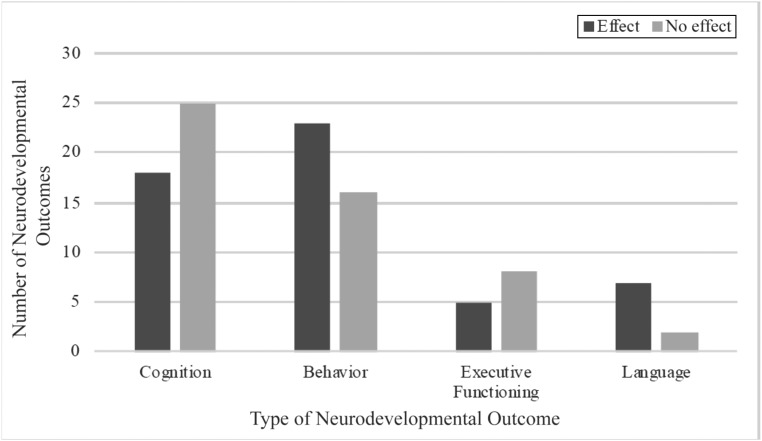
We included literature in which both prenatal opioid exposure and at least 1 neurodevelopmental outcome at birth or later in development were examined. We define neurodevelopment as the unfolding of cognitive, neurologic, behavioral, or emotional functioning from infancy to school-aged years that can be measured behaviorally in a reliable, valid, and standardized manner. Although the number of studies published on prenatal opioid exposure and neurodevelopmental outcomes has increased significantly in recent years, only 18 of the 52 studies were published in the last 5 years, the focus of this state-of-the-art review (since 2013; Fig 1). Figure 2 provides information about the range of neurodevelopmental outcomes included in our review.
FIGURE 1.
Number of publications on the neurodevelopmental outcome of children with prenatal opioid exposure by year (1975–2018; N = 52).
FIGURE 2.
Number and type of neurodevelopmental outcomes examined in studies of children with prenatal opioid exposure.
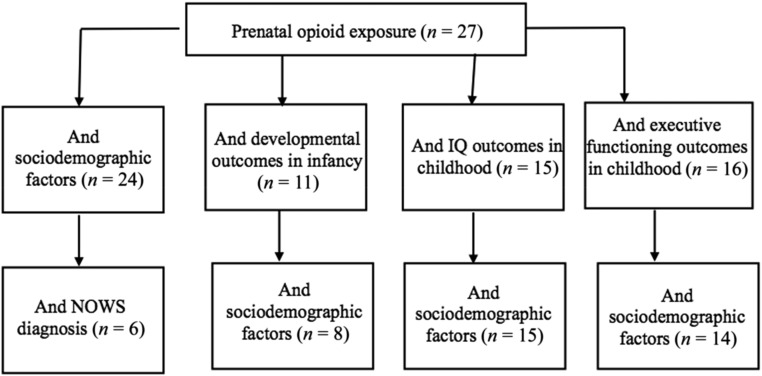
We excluded nonhuman studies, reviews, and qualitative studies (Fig 3). Our search was performed on PubMed and PsycINFO and did not include any publication date restrictions. Search terms were “prenatal opioid exposure,” “prenatal substance exposure,” “development,” “neurodevelopment,” and “cognitive development.” In Table 1, we report the study design, sample size, type of prenatal drug exposure, control group characteristics, sex differences, NOWS outcomes, confounding factors (eg, maternal socioeconomic status; Fig 4), estimates of effect size, and overall findings from the 52 articles reviewed. We highlight in our narrative review research published in the last 5 years (since 2013). However, we include in Table 1 all relevant publications given the small number of studies on neurodevelopmental outcomes published since 2013. We provide a review of the key findings below, separated by developmental period: newborn, infancy, and ≥3 years.
FIGURE 3.
PRISMA flowchart revealing selection of articles included in the review.
TABLE 1.
Characteristics of Studies, Key Findings, and Effect Size Estimates Included in Review
| Publication | Study Design | Sample Size | Type of Drug Exposure | Assessment of Substance Exposure | Age at Examination | Characteristics of Control Group | Examination of Sex Differences? | Examined NOWS Outcomes? | Examined Confounding Factors? | Findings and Effect-Size Estimates |
|---|---|---|---|---|---|---|---|---|---|---|
| Bauman and Levine19 | Cross-sectional | 70 exposed children and 70 matched controls | Methadone | Enrollment in methadone maintenance program | 3+ y | Unexposed, matched to exposed group on maternal race and/or ethnicity, SES, and whether a man was present for the child’s upbringing 70% of the time | No | Yes | Confounding factors thought to be accounted for by matching | Children exposed to methadone had lower scores on intelligence (Cohen’s d = 0.45). Exposed children who developed NOWS were more likely to have lower IQ scores (Cohen’s d = 0.42) and adaptive behavior (Cohen’s d = 0.47) compared with those exposed who did not develop NOWS. |
| Beckwith and Burke20 | Retrospective chart review | 28 exposed and receiving treatment of NOWS; historical control (n = 1700) | Heroin, methadone, other opioid | All exposed infants confirmed via maternal or infant toxicology screen, as found in medical record | Birth | Unexposed infants from the Bayley Scales of Infant Development normative sample | Yes | Yes, all infants in exposed group were treated for NOWS | No | Infants treated with NAS and/or NOWS had lower language and cognition scores when, on average, 55 d old (Cohen’s d ranged from 0.31 to 1.29). |
| Belcher et al21 | Prospective, longitudinal | 157 exposed, historical control | Cocaine, opioids (heroin or methadone) | Urine toxicology screen in mother and newborn | Infancy | Summary scores taken from a previously published article (infants without prenatal drug exposure) | Yes | No | Prenatal tobacco and alcohol exposure and head circumference | Infants exposed to cocaine and opioids were more likely to have abnormal neuromotor examinations compared with infants exposed to cocaine only or opioids only (Cohen’s d ranged from 0.05 to 0.49). |
| Bier et al15 | Retrospective chart review | 84 infants exposed to low-dose methadone, 81 exposed to high-dose methadone, 55 exposed to buprenorphine | Buprenorphine, methadone | Toxicology screen | Birth and infancy | N/A | No | No | Length of hospitalization, infant’s gestational age at time of discharge, No. maternal psychiatric medications taken during pregnancy | Prenatal exposure to high-dose methadone (≥100 mg/d) was related to smaller head circumference compared with prenatal exposure to low-dose methadone or buprenorphine (R2 ranged from 0.05 to 0.13). No significant differences in cognitive outcomes by using the Bayley Scales of Infant Development were found at 4 mo (Cohen’s d = 0.29). |
| Bunikowski et al22 | Prospective, longitudinal | 34 exposed, 42 control | Methadone, nicotine, or heroin | Maternal report or maternal participation in a methadone maintenance program | Infancy | Infants exposed to nicotine who were born at term with no evidence of additional drug exposure | No | No | Maternal age, maternal SES, and exposure to drugs other than nicotine or opioids | Exposure to opioids in utero was related to mild psychomotor developmental impairment in children 12-mo old (Cohen’s d ranged from 0.20 to 0.69). |
| Coyle et al17 | RCT | 39 exposed, no control | Infants randomly assigned to receive buprenorphine or methadone | Maternal participation in an RCT to receive methadone or buprenorphine | Birth | N/A | No | Yes | Average daily morphine dose | Infants exposed to buprenorphine in utero showed fewer stress abstinence signs and hypertonia, required less handling, were less excitable and aroused, and were better at self-regulation compared with infants exposed to methadone (F values ranged from 0.04 to 12.94). Infants who were older when they received treatment of NOWS had higher self-regulation, less excitability, and low hypertonia (correlation coefficients ranged from 0.37 to 0.58). |
| Davis and Templer23 | Cross-sectional | 28 exposed, 28 control | Heroin, methadone | Maternal participation in a methadone maintenance program | 3+ y | Not exposed to substances in utero, but mothers lived with a man addicted to substances at the time of pregnancy | No | No | Child age | Children exposed to opioids in utero had significantly lower cognitive scores on some WISC-R subtests compared with unexposed children (Cohen’s d range from 0.07 to 0.77) and lower neurologic scores on some subtests of the Quick Neurologic Screening Test (Cohen’s d ranged from 0.10 to 0.81). Children exposed to opioids in utero reported significantly higher symptoms of problem behavior on the Burks Behavior Rating Scales compared with their unexposed counterparts (Cohen’s d ranged from 0.28 to 1.63). |
| de Cubas and Field24 | Cross-sectional | 20 exposed, 20 control | Alcohol, cigarettes, methadone | Maternal participation in methadone maintenance program | 3+ y | Children of women who did not use opioids who were matched on age, sex, ethnicity, SES, No. parents, maternal education, alcohol and nicotine use, and perinatal complications | Yes | No | No | There were no differences in IQ between children who were exposed and unexposed. Children exposed to methadone had higher levels of aggression, apprehension, fear, and anxiety and had higher concerns of feeling left out as well as diminished coping skills, as assessed by the Child Behavior Checklist. There was not enough information to provide estimates of effect sizes. |
| Fill et al25 | Retrospective chart review | 1815 children with a history of NAS/NOWS, 5441 control | Not specified | Diagnosis of NAS and/or NOWS in Medicaid insurance claim | 3+ y | Matched 1:3 on the basis of sex, race, region of residence at birth, and Medicaid enrollment status | No | Yes | Maternal education, maternal tobacco use during pregnancy, low birth wt, preterm birth, and NICU admission | Children with a recorded diagnosis of NAS and/or NOWS were more likely to have a recorded referral for evaluation of an educational disability, to meet criteria for an educational disability, and to receive special education therapy or services. Children with a diagnosis of NAS and/or NOWS were significantly more likely to experience developmental delay and speech or language impairment and were also more likely to receive speech therapy services (odds ratios ranged from 1.26 to 1.44). |
| Guo et al26 | Cross-Sectional | 16 who were exposed to opioids in utero and lived with a mother using opioids, 14 who lived with a mother using opioids (no prenatal exposure), 13 boys who were unexposed | Alcohol, cigarettes, heroin, marijuana, and methadone | Self-report from mothers in a drug-treatment program | 3+ y | Women and sons recruited through newspaper advertisements in the Baltimore-Washington metropolitan area | No | No | Maternal nicotine and marijuana use | There were no significant differences in reaction time between the 3 groups (F = 0.7). Boys in both exposure groups had lower memory scores than boys in the control group (F = 4.74). |
| Hans and Jeremy27 | Prospective, longitudinal | 33 exposed, 45 control | Heroin, methadone, and/or pentazocine | Maternal participation in methadone maintenance program, maternal interview, and positive urine toxicology screen result | Infancy | Demographically comparable infants not exposed to opioids | No | No | Use of substance other than opioids, cumulative social-environmental risks, birth wt | There were no differences in cognitive or motor outcomes, as assessed via the Bayley Scales of Infant Development, between infants who were exposed and unexposed once sociodemographic risks were accounted for (F values ranged from 0 to 1.73). |
| Hans and Marcus10 | Prospective, longitudinal | 16 exposed, 23 control | Methadone, alcohol, marijuana, and cocaine | Maternal participation in methadone maintenance program | Infancy | Demographically comparable infants not exposed to opioids | No | No | No | There were no significant differences in mental or psychomotor development, as assessed via the Bayley Scales of Infant Development, between the groups. Infants exposed to opioids had significantly lower motor coordination and higher activity level compared with the unexposed group. There was not enough information to provide estimates of effect size. |
| Hans28 | Prospective, longitudinal | 30 exposed, 44 control | Methadone, alcohol, marijuana, heroin, cocaine, Valium, and/or Talwin | Maternal participation in methadone maintenance program, maternal interview, and positive urine toxicology screen result | Infancy | Demographically comparable infants not exposed to opioids | Yes | No | Maternal SES, maternal IQ, and pregnancy and birth complications | Infants who were exposed had lower psychomotor index scores at 2 y. There were no significant differences between groups on mental development by using the Bayley Scales of Infant Development (η values ranged from 0.15 to 0.28). |
| Heller et al18 | Prospective, longitudinal | 39 exposed, 21 control | Methadone, tobacco | Maternal participation in methadone maintenance program | Birth | Unexposed to substances in utero, demographically similar, and matched for age | Yes | Yes | Maternal tobacco use and infant birth wt | Newborns exposed prenatally to opioids who developed NAS and/or NOWS had poorer regulation and quality of movement compared with the unexposed group. Prenatal opioid exposure (regardless of NAS and/or NOWS diagnosis) was related to higher stress abstinence scores (Cohen’s d ranged from 0 to 1.24). |
| Hickey et al29 | Cross-sectional | 12 exposed in utero, 12 living with a mother using opioids (no prenatal exposure), 12 control | Heroin and/or methadone, alcohol, nicotine, and marijuana | Self-report from mothers in a drug-treatment program | 3+ y | Sons of mothers who reported no drug use in their lifetime or during pregnancy (other than alcohol, tobacco, or marijuana) | No | No | Maternal SES, child age, IQ, anxiety, impulsivity, and visual-motor skills | There were no significant differences in IQ, impulsivity, anxiety, visual-motor skills, or problem behavior. Boys exposed prenatally to opioids and postnatally to opioids were more distractible compared with unexposed controls (Cohen’s d ranged from 0.01 to 1.16). |
| Hunt et al30 | Prospective, longitudinal | 133 exposed, 103 control | Methadone | Enrollment in methadone treatment program, maternal urine toxicology screen | Infancy and 3+ y | Unexposed infants matched for maternal age, height, ethnic background, and previous obstetric history | No | No | No | Infants and preschool-aged children exposed to opioids in utero had poorer cognitive, but not psychomotor, outcomes compared with unexposed infants and preschool-aged children. Preschool-aged children exposed to opioids had significantly lower adaptive behavior and language scores compared with unexposed preschool-aged children (Cohen’s d ranged from 0.16 to 0.84). |
| Huntington et al11 | Prospective, longitudinal | 8 exposed, 12 control | Methadone, alcohol, marijuana, heroin, cocaine, Valium, and/or Talwin | Maternal participation in methadone maintenance program, maternal interview, and positive urine toxicology screen result | Birth | Demographically comparable infants not exposed to opioids | No | No | No | Infants exposed prenatally to methadone had lower mental and psychomotor development scores, as assessed via the Bayley Scales of Infant Development (correlation coefficients ranged from 0.37 to 0.47). |
| Jeremy and Hans7 | Prospective, longitudinal | 29 exposed, 37 control | Methadone, alcohol, marijuana, heroin, cocaine, Valium, and/or Talwin | Maternal participation in methadone maintenance program | Birth | Demographically comparable infants not exposed to opioids | Yes | No | Perinatal complications, birth wt | At birth, newborns with prenatal opioid exposure were jerky, tremulous, tense, and active, as assessed via NBAS. F values ranged from 5.54 to 36.58. At 3.5 wk postpartum, infants with prenatal opioid exposure were more hypertonic. F values ranged from 0 to 3.89. |
| Johnson et al14 | Prospective, longitudinal | 3 exposed | Buprenorphine | Enrollment in treatment program, maternal urine toxicology screen, newborn meconium, and cord plasma | Birth | N/A | No | Yes | No | Prenatal exposure to buprenorphine was related to mild NAS and/or NOWS composed of tremors, hyperactive Moro, and shortened sleep after feeding. There was not enough information to provide estimates of effect size. |
| Jones et al12 | Prospective, longitudinal | 11 exposed to methadone, 10 exposed to buprenorphine | Buprenorphine and methadone | Enrollment in treatment program, toxicology screen | Birth | N/A | Yes | Yes | No | The neurobehavior of infants exposed to buprenorphine versus methadone were similar. Newborns treated for NAS and/or NOWS had increasing quality of movement scores and decreased excitability across the first 2 wk of life. Boys had higher mean scores on habituation compared with girls (Cohen’s d ranged from 0 to 3.86). |
| Kaltenbach et al31 | RCT | 96 infants exposed to methadone or buprenorphine | Buprenorphine, methadone | Enrollment in treatment program, toxicology screen | Infancy | N/A | No | Yes | No | Out of 37 tests used to compare neurodevelopmental differences between groups, 2 were significant. Infants exposed to buprenorphine had lower receptive language scores at 12 mo and less approach behaviors at 3 mo compared with infants exposed to methadone. Out of 37 tests used to compare outcomes of infants who developed NAS and/or NOWS to those who did not, 1 was significant. Infants treated for NAS and/or NOWS showed more frustration at 6 mo compared with infants who did not require treatment (F2 ranged from 0.07 to 0.13). |
| Konijnenberg and Melinder32 | Cross-sectional | 15 exposed, 15 control | Buprenorphine, methadone, and nicotine | Maternal enrollment in opioid maintenance therapy; maternal self-report | 3+ y | Preschool-aged children unexposed to cigarettes, opioids, or any other drugs during pregnancy who were recruited through local health care centers | No | No | Maternal education | Children exposed to opioids made fewer goal-directed eye movements and had poorer fine motor skills compared with an unexposed control. There were no differences in cognitive outcomes or theory of mind between the groups (Cohen’s d ranged from 0.05 to 0.1.64). |
| Konijnenberg and Melinder33 | Cross-sectional | 35 exposed, 31 control | Buprenorphine, methadone, alcohol, tobacco, marijuana, amphetamine, benzodiazepine, other opioids | Maternal enrollment in opioid maintenance therapy; maternal self-report | 3+ y | Preschool-aged children unexposed to cigarettes, opioids, or any other drugs during pregnancy who were recruited through local health care centers | Yes | No | Maternal education and employment | There were no differences in executive functioning or IQ between groups once sociodemographic confounds were included in statistical models (η2 ranged from 0.03 to 0.38). |
| Konijnenberg et al34 | Prospective, longitudinal | 35 exposed, 32 control | Buprenorphine, methadone, benzodiazepine, cannabis, nicotine | Maternal enrollment in opioid maintenance therapy; maternal self-report; maternal urinary screens; infant meconium toxicology | Infancy and 3+ y | Preschool-aged children unexposed to opioids who were recruited through local health care centers and selected on the basis of matched due dates in the exposure group | No | Yes | Birth wt | Preschool-aged children prenatally exposed to opioids had lower scores on inhibitory control, motor imitation, and short-term memory compared with unexposed preschool-aged children (η2 ranged from 0.07 to 0.15). |
| LaGasse et al35 | Cross-sectional | 8 exposed, 10 control | Cocaine, opioids | Meconium assay and maternal report | Infancy | Infants unexposed to drugs | No | No | No | Infants who were exposed reached for objects less often when in the dark. There was not enough information to provide estimates of effect size. |
| Levine and Woodward36 | Prospective, longitudinal | 68 exposed, 88 control | Methadone | Maternal participation in methadone maintenance program; maternal interview and positive urine toxicology screen result; infant meconium | Infancy | Unexposed infants recruited from hospitals at birth | Yes | No | Maternal education, maternal age, family SES, single-parent status, maternal use of substances other than methadone, maternal symptoms of depression, maternal nutrition, and prenatal benzodiazepine use | There were no differences in inhibitory control between the 2 groups after controlling for maternal education and prenatal benzodiazepine use. There were no significant differences in perseverative errors made between the 2 groups (Cohen’s d ranged from 0.14 to 0.70). |
| Lifschitz et al37 | Prospective, longitudinal | 25 children of mothers with untreated opioid addiction, 26 children of mothers receiving methadone therapy, and 41 controls | Heroin, methadone | Maternal participation in methadone maintenance program; maternal interview; medical record review | 3+ y | Unexposed infants matched on maternal age, race, and SES | Yes | Yes | Maternal race, maternal nutritional status, total wt gain during pregnancy, pregnancy and intrapartum high-risk scores, smoking, substance use, parental education and occupation, parental height, No. siblings, Apgar scores, infant’s sex and birth measurements, duration of treatment of NAS and/or NOWS | There were no differences in brain growth or cognitive outcomes between preschool-aged children exposed to opioids and matched unexposed controls (Cohen’s d = 0.30). |
| Melinder et al38 | Cross-sectional | 26 exposed, 23 control | Buprenorphine, methadone, nicotine | Maternal participation in methadone maintenance program | 3+ y | 4-y-old children unexposed to opioids or nicotine matched by child sex and age | Yes | No | Maternal employment, maternal education, and birth wt | There were no differences in eye tracking of slow or fast-moving objects between the groups when controlling for confounding factors. There were no differences in attention performance or cognition between the groups. Children exposed to opioids had more parent-reported attention problems compared with unexposed controls (partial η2 ranged from 0 to 0.30). |
| Merhar et al39 | Retrospective cohort study | 87 children diagnosed with NAS and/or NOWS | Heroin, cocaine, benzodiazepines, marijuana, methadone, buprenorphine, amphetamines, or other opioid | Maternal urine toxicology screen or infant urine, meconium, or umbilical cord toxicology screen | Infancy | N/A | Yes | Yes | No | Infants treated for NOWS had significantly lower cognitive, language, and motor outcomes compared with the Bayley Scales of Infant Development normative sample (Cohen’s d ranged from 0.27 to 0.44). Girls with NAS and/or NOWS scored higher than boys with NAS and/or NOWS on the cognitive and language, but not motor, subscales. |
| Messinger et al40 | Prospective, longitudinal | 474 exposed to cocaine, 50 exposed to opioids, 48 exposed to cocaine and opioids, and 655 unexposed to cocaine or opioids | Cocaine, opioids, alcohol, tobacco, and marijuana | Maternal report, newborn meconium gas chromatography/mass spectroscopy assessment | Infancy | Infants matched on ethnicity, sex, and gestational age who were not exposed to cocaine or opioids | No | No | Maternal use of substances other than cocaine and opioids, maternal education, SES, quality of the home environment, maternal vocabulary, maternal psychological distress, birth wt, gestational age | There were no significant differences in prenatal opioid exposure on cognitive, motor, and behavior outcomes, as assessed by the Bayley Scales of Infant Development, after controlling for relevant covariates (Cohen’s d ranged from 0.02 to 0.42). |
| Moe and Slinning41 | Prospective, longitudinal | 78 exposed, 58 control | Amphetamines, benzodiazepines, cannabis, opiates, tobacco, alcohol | Maternal report; concerns of maternal substance use from medical or social staff; medical record review; urine or blood screening from the mother and newborn was available in some cases | Infancy | Children with no prenatal substance exposure or biomedical risk recruited from maternal and child health centers | Yes | No | SES and prematurity status | Boys, but not girls, with polysubstance exposure had lower mental development scores, as assessed by the Bayley Scales of Infant Development, at 12, 24, and 36 mo. These effects remained after controlling for prematurity and caregiver SES. There were no significant differences in psychomotor development after controlling for prematurity (Cohen’s d ranged from 0.21 to 1.03). |
| Moe42 | Prospective, longitudinal | 64 exposed, 52 control | All reported heroin as the drug of choice, and many used cannabis, benzodiazepines, other opioids, amphetamines, alcohol, nicotine, and antipsychotic medication. | Maternal report; concerns of maternal substance use from medical or social staff; medical record review | 3+ y | Children with no prenatal substance exposure or biomedical risk recruited from maternal and child health centers | Yes | No | Gestational age, parental SES | Children with prenatal opioid exposure had lower perceptual performance outcomes at age 4.5 y by using the McCarthy Scales of Children’s Abilities compared with children who were not exposed and experienced few psychosocial risks. There were no differences in cognitive outcomes between the 2 groups after accounting for covariates (β ranged from 0.17 to 0.23). |
| Nygaard et al43 | Prospective, longitudinal | 72 exposed, 58 control | All reported heroin as the drug of choice, and many used cannabis, benzodiazepines, other opioids, amphetamines, alcohol, nicotine, and antipsychotic medication. | Maternal report; concerns of maternal substance use from medical or social staff; medical record review | Infancy and 3+ y | Children with no prenatal substance exposure or biomedical risk recruited from maternal and child health centers | Yes | No | Parental SES, gestational age, and birth wt | Boys exposed to opioids had lower cognitive scores at 4.5 y compared with unexposed controls. Boys and girls exposed to opioids also had lower IQ at 8.5 y compared with unexposed controls (Cohen’s d ranged from 0.11 to 1.49). |
| Oei et al44 | Cross-sectional | 2234 exposed, 4330 control, 598 265 population comparison | Not available | Medical record review | 3+ y | The first control group includes children without a diagnosis of NAS and/or NOWS matched for gestational age, SES, and sex. The second control group includes an unmatched group of children without a diagnosis of NAS. | Yes | Yes | Prematurity, indigenous status, school remoteness, and parental education level | Children diagnosed with NAS and/or NOWS had significantly lower test scores in grades 3, 5, and 7, and the gap in test scores between groups was larger at grade 7 compared with grade 5 (odds ratios ranged from 2.4 to 3.5). Among children with NAS and/or NOWS, those who were indigenous, who were male, and whose parents did not complete high school were more likely to have poorer academic outcomes. |
| Ornoy et al45 | Prospective, longitudinal | 31 children with mothers addicted to heroin who were living at home, 34 children with mothers addicted to heroin who were adopted, 33 children with fathers addicted to heroin, 32 children who experienced neglect, 30 children reared in moderate to high SES environments | Heroin, psychoactive drugs, methadone, nicotine | Children suspected of being exposed to mothers or fathers who used heroin who were referred to a developmental assessment clinic; maternal report; mothers participating in a methadone maintenance program | 3+ y | There were 2 control groups: children who experienced neglect and children reared in moderate to high SES environments. | Yes | No | No | Children exposed to heroin and children experiencing neglect had significantly lower IQ scores compared with unexposed control children. Children exposed to heroin and children who experienced neglect had significantly lower reading and arithmetic scores compared with unexposed controls and children exposed to heroin who were later adopted. Children exposed to heroin and children who experienced neglect had significantly more symptoms of ADHD and externalizing (but not internalizing) behavior compared with unexposed children (Cohen’s d ranged from 0 to 1.65). |
| Ornoy46 | Prospective, longitudinal | 93 children with mothers addicted to heroin, 85 children with fathers addicted to heroin, 50 children who experienced neglect, 87 children from kindergarten in Jerusalem, 57 children born to mothers with gestational diabetes, and 50 children with birth wt <1501 g | Heroin, psychoactive drugs, methadone | Children suspected of being exposed to mothers or fathers who used heroin who were referred to a developmental assessment clinic; maternal report | 3+ y | There were 4 control groups: children who experienced neglect, children born to mothers with gestational diabetes, children with birth wt <1501 g, and children from kindergarten in Jerusalem. | No | No | No | Children who experienced neglect had significantly lower cognitive and academic scores compared with children with a mother or father addicted to heroin and the control groups. Children exposed to a mother or father dependent on heroin had significantly lower cognitive and academic scores compared with unexposed controls. Children born to a mother dependent on heroin who were adopted had similar cognitive scores as unexposed controls (Cohen’s d ranged from 0.18 to 1.36). Children raised by fathers or mothers dependent on heroin were significantly more likely to have elevated symptoms of ADHD compared with unexposed controls. |
| Ornoy et al47 | Prospective, longitudinal | 83 children with mothers addicted to heroin, 76 children with fathers addicted to heroin, 50 children who experienced neglect, 50 children reared in moderate to high SES environments, 80 children from kindergarten in Jerusalem | Heroin, psychoactive drugs, methadone | Children suspected of being exposed to mothers or fathers who used heroin who were referred to a developmental assessment clinic; maternal report | 3+ y | There were 3 control groups: children who experienced neglect, children reared in moderate to high SES environments, and children from kindergarten in Jerusalem | Yes | Yes | No | Children who experienced neglect had significantly lower cognitive scores compared with children with a mother or father addicted to heroin and the control groups. Children exposed to a father addicted to heroin had significantly lower cognitive scores compared with the unexposed controls (Cohen’s d ranged from 0.32 to 1.21). |
| Rosen and Johnson8 | Prospective, longitudinal | 57 exposed, 31 controls | Methadone, heroin, diazepines, other opioids, cocaine, barbiturates, amitriptyline, amphetamines, alcohol, and nicotine | Maternal enrollment in a methadone maintenance program, maternal interview, and maternal urinary drug screen | Birth and infancy | Infants, some of whom were exposed to nicotine, who were matched on maternal race, SES, infant sex, birth wt, and gestational age | Yes | Yes | Gestational age | Children exposed to methadone in utero had poorer motor tone, poorer fine motor coordination, more abnormal eye findings, and significantly lower scores on the Bayley Scales of Infant Development mental and motor developmental indices at 12 and 18 mo (but not 6 mo) compared with unexposed matched controls (Cohen’s d ranged from 0.25 to 0.97). Withdrawal severity by using the Finnegan scale was not associated with Bayley Scales of Infant Development scores at 18 mo. |
| Salo et al48 | Cross-sectional | 7 children exposed to buprenorphine living with biological mother, 14 exposed to buprenorphine in foster care, 13 unexposed controls | Buprenorphine, benzodiazepine, cannabis, amphetamines, other substances, tobacco, and alcohol | Maternal enrollment in a drug-treatment program; positive urine screening result for buprenorphine in the child at birth | 3+ y | No prenatal substance exposure and living with biological mother since birth | No | No | Gestational age, birth wt, height, No. foster care placements, maternal age, maternal education, maternal caregiving behaviors | There were no significant differences between the groups with respect to cognitive, language, or social and emotional outcomes after controlling for relevant covariates (β ranged from 0.08 to 0.29). |
| Sandtorv et al49 | Cross-sectional | 57 exposed, 171 control | Opioids and other illicit substances | Children referred to a developmental assessment clinic for prenatal opioid exposure; maternal enrollment in an opioid maintenance treatment program or documented NAS and/or NOWS at birth; medical record review; maternal report | 3+ y | Unexposed children from middle to high SES contexts matched on age and sex | Yes | Yes | Child placement in adoptive or foster care | Controlling for covariates, an NAS and/or NOWS diagnosis predicted more inattention symptoms but not hyperactivity and/or impulsivity or symptoms of autism (β ranged from 0.08 to 0.29). |
| Schneider and Hans50 | Cross-sectional | 30 exposed, 44 control | Methadone, heroin, Talwin, alcohol, marijuana, Valium, and cocaine | Enrollment in methadone treatment program, maternal report; maternal urine toxicology screen | Infancy | Demographically comparable infants not exposed to opioids | Yes | No | Prenatal exposure to marijuana, nicotine, and alcohol | No differences in attention between exposure groups were found during free play (Cohen’s d = 0.28). |
| Strauss et al51 | Prospective, longitudinal | 60 exposed, 53 control | Methadone | Maternal enrollment in methadone treatment program | Newborn and infancy | Unexposed infants matched for birth wt, gestational age, Apgar scores, and exposure to anesthesia and analgesia during labor | No | Yes | No | Newborns exposed to opioids were more irritable and tremulous, showed more jerkiness, were less cuddly, were more sensitive to light, were less alert, and were more likely to place their hands to their mouths. Severity of opioid withdrawal was related to lower attention and more tremors. Infants exposed to opioids showed a sharper decline in psychomotor development between 3 and 6 mo compared with unexposed infants. There were no differences in cognitive development between the 2 groups (Cohen’s d ranged from 0.07 to 0.73). |
| Strauss et al52 | Prospective, longitudinal | 31 exposed, 27 control | Methadone | Maternal enrollment in methadone treatment program | 3+ y | Unexposed infants matched for birth wt, gestational age, Apgar scores, and exposure to anesthesia and analgesia during labor | Yes | No | No | There were no differences in cognitive or perceptual performance at 12 mo or 5 y between children exposed to methadone in utero and unexposed controls (Cohen’s d ranged from 0 to 0.25). |
| Uebel et al53 | Retrospective chart review | 3842 diagnosed with NAS and/or NOWS, 1 018 421 without NAS and/or NOWS | Not available | Medical record review | 3+ y | Infants in a birth registry in New South Wales who did not have a diagnosis of NAS and/or NOWS | Yes | Yes | Prematurity, maternal age, maternal smoking, SES, rural status, and maternal indigenous status | Children with a recorded diagnosis of NAS and/or NOWS were more likely to be hospitalized for behavioral disorders (odds ratios ranged from 0.34 to 22.28). |
| van Baar54 | Prospective, longitudinal | 35 exposed, 37 control | Methadone, heroin, cocaine, tranquilizers, and amphetamines | Maternal enrollment in methadone treatment program | Infancy | Term infants unexposed to substances | No | No | Prematurity | Infants who were exposed to opioids in utero were comparable with control infants in nonverbal development and psychomotor development. Infants exposed to opioids had significantly lower cognitive scores at 24 and 30 mo (Cohen’s d ranged from 0.11 to 0.77). |
| van Baar et al13 | Prospective, longitudinal | 35 exposed, 35 control | Methadone, heroin, cocaine, tranquilizers, and amphetamines | Maternal enrollment in methadone treatment program; maternal report, urine toxicology screens from mothers and newborns | 3+ y | Term infants unexposed to substances | No | Yes | Prematurity | The authors reported no differences at birth on the Brazelton Neonatal Behavioral Assessment Scale. At 4 wk, newborns exposed to opioids showed lower orientation scores. There were no differences in motor outcomes from birth to age 5.5 y. The authors reported that children exposed to opioids had lower cognitive and language scores at ages 4.5 and 5.5 y. There were no significant correlations between NOWS severity and developmental outcomes. There was not enough information to report estimates of effect size. |
| Velez et al16 | Cross-sectional | 41 exposed, no control | Buprenorphine, nicotine, SSRIs, amphetamine, barbiturates, benzodiazepine, cocaine, tetrahydrocannabinol, methamphetamine, methadone, morphine, heroin, and oxycodone | Maternal enrollment in medication-assisted treatment program, maternal report, maternal urine toxicology screen | Birth | N/A | No | Yes | Maternal history of drug use, maternal methadone exposure, other substance exposure | Greater maternal buprenorphine dose at delivery was associated with lower quality of movement and self-regulation and greater central nervous system stress abstinence signs. There were no differences in neurobehavior between infants who required pharmacotherapy for NAS and/or NOWS and those who did not. Higher mean Finnegan scores were related to lower self-regulation and alertness and positively correlated with stress abstinence signs and hypertonicity. Correlation coefficients ranged from 0.33 to 0.55. |
| Sundelin Wahlsten and Sarman55 | Cross-sectional | 25 exposed, no control | Buprenorphine, nicotine | Maternal enrollment in medication-assisted treatment program; mothers had to have documented 1 y of opioid dependence | 3+ y | N/A | Yes | Yes | No | There were no differences in verbal IQ scores of children exposed prenatally to buprenorphine compared with the mean scores for the general population. Children exposed to buprenorphine had performance IQ and memory and motor scores on average 1 SD below the mean for the general population (Cohen’s d ranged from 0.18 to 1.82). There were no differences in IQ between children with and without an NAS and/or NOWS diagnosis. There were no significant differences in ADHD symptoms between children exposed prenatally to buprenorphine and children diagnosed with ADHD in the general population. |
| Walhovd et al56 | Cross-sectional | 12 exposed, 12 control | Heroin, sedatives, amphetamine, cannabis, nicotine, alcohol | Maternal enrollment in a residential drug-treatment program undergoing detoxification; maternal report | 3+ y | Pregnant women who reported no illicit substance use; some reported smoking cigarettes and using alcohol during the first trimester | Yes | No | No | There were no differences in IQ scores between the exposed and comparison groups. Visual acuity was significantly lower among infants exposed to opioids compared with unexposed controls (Cohen’s d ranged from 0.01 to 0.59). |
| Wilson et al57 | Cross-sectional | 22 exposed, 55 control | Heroin, methadone, morphine, tranquilizers, sedatives, codeine, barbiturates | Medical record review | 3+ y | Drug environment control: no in utero exposure, but maternal report of living with a partner addicted to opioids or initiating opioids subsequent to child’s birth; high-risk control: identified as high risk on the basis of medical factors at birth; SES control: uneventful pregnancy and comparable SES | No | No | Race, age, sex, SES, and amount of time spent in a school-readiness program | Children (age 3–6 y) exposed prenatally to heroin had significantly lower scores on a test of psycholinguistic abilities and auditory memory compared with children living in low SES environments but not compared with children in the drug environment control group or the high-risk control group. Children exposed to heroin had significantly lower scores on a general cognitive index and showed lower perceptual performance, quantitative performance, and memory performance compared with the control groups. There were no significant differences in mean IQ or in speech and language performance between groups. There was not enough information to provide estimates of effect size. |
| Wilson58 | Prospective, longitudinal | 29 infants exposed to heroin whose mothers were not treated for an OUD, 39 infants exposed to methadone as part of maternal participation in a drug-treatment program, and 57 unexposed controls | Heroin, methadone, tranquilizers, sedatives, other narcotics, marijuana, nicotine, stimulants, alcohol, antidepressants, diazepam, propoxyphene hydrochloride, and glue | Maternal enrollment in medication-assisted treatment program; medical record review; maternal report; maternal urine toxicology screen | 3+ y | Unexposed infants matched for maternal age, race, SES, marital status, and gestational age | No | No | No | At 18 mo, but not at 9 or 24 mo, children exposed to heroin had lower mental development scores compared with unexposed toddlers. There were no significant differences in cognitive outcomes in preschool-aged children exposed to opioids (Cohen’s d ranged from 0.08 to 0.86). |
| Wilson et al9 | Prospective, longitudinal | 29 infants exposed to heroin whose mothers were not treated for an OUD, 39 infants exposed to methadone as part of maternal participation in a drug-treatment program, and 57 unexposed controls | Heroin, methadone, tranquilizers, sedatives, other narcotics, marijuana, nicotine, stimulants, alcohol, antidepressants, diazepam, propoxyphene hydrochloride, and glue | Maternal enrollment in medication-assisted treatment program; medical record review; maternal report; maternal urine toxicology screen | Birth and infancy | Unexposed infants matched for maternal age, race, SES, marital status, and gestational age | No | No | No | Newborns whose mothers were not treated for an OUD had more hypertonicity compared with newborns of mothers treated with methadone and unexposed controls. Infants whose mothers were treated with methadone had significantly lower psychomotor development and fine motor scores compared with unexposed controls. There were no differences in mental development between groups (Cohen’s d ranged from 0 to 0.67). |
ADHD, attention-deficit/hyperactivity disorder; N/A, not applicable; NBAS, neonatal behavioral assessment scale; OUD, opioid use disorder; SES, socioeconomic status; SSRI, selective serotonin reuptake inhibitor; WISC-R, Wechsler Intelligence Scale for Children, Revised.
FIGURE 4.
Covariates examined in the neurodevelopmental literature of children with prenatal opioid exposure.
Prenatal Opioid Exposure and Neurodevelopmental Outcomes at Birth
Initial investigations of newborns with prenatal opioid exposure revealed few effects. In 4 studies, newborns exposed to methadone or heroin were more hypertonic, jerky, tremulous, hyperactive; showed poorer attention; and were more irritable but also better able to self soothe compared with a demographically matched control group.7–10 Three studies revealed no differences in neurodevelopmental outcome.11–13 These early studies were limited by low sample sizes and a lack of consideration for confounding factors. In 2002, buprenorphine was approved to treat opioid addiction in pregnant women. Researchers then began comparing the effects of buprenorphine exposure to the standard of care at the time, which was typically methadone. Initial findings revealed that newborns with prenatal buprenorphine exposure had mild symptoms of NOWS composed of tremors, a hyperactive Moro reflex, and shortened sleep after feeding,14 but these symptoms were not as severe as prenatal exposure to high-dose methadone.15 Other studies revealed no significant differences in neurobehavioral outcome between newborns exposed to buprenorphine and those exposed to methadone.12
Among newborns exposed to buprenorphine, a higher dose at delivery predicted lower quality of movement, lower self-regulation, and more stress abstinence signs.16 In a randomized controlled trial (RCT), newborns exposed to buprenorphine, relative to methadone, had fewer stress abstinence signs, were less excitable and aroused, were less hypertonic, required less handling, and were better able to self-regulate.17 Another RCT revealed neurobehavioral differences between newborns exposed to buprenorphine and newborns exposed to methadone, depending on the day on which the neurobehavioral examination was conducted. For example, newborns exposed to buprenorphine showed higher arousal scores on postnatal days 5 and 7 and higher excitability scores on day 7 but lower scores on day 14 compared with newborns exposed to methadone.12
A key question is whether a diagnosis of NOWS portends problematic neurobehavioral outcome in newborns. Newborns exposed to methadone and later diagnosed with NOWS had poorer regulation and quality of movement and higher levels of arousal compared with a demographically similar group of newborns who were not exposed to methadone prenatally18 or who were exposed to opioids but not treated for NOWS.12 Treatment of NOWS by using methadone or buprenorphine seemed to improve quality of movement, with resulting decreases in excitability over time.12 One study revealed no differences in neurobehavioral outcome at postnatal day 3 between newborns who needed pharmacotherapy for NOWS and newborns exposed to opioids who did not require treatment.16
In summary, authors of 5 of 11 studies examine neurobehavioral outcomes in newborns as a consequence of prenatal opioid exposure at birth compared with an unexposed comparison group. Authors of only approximately half of these studies attempted to control for confounding factors such as poverty (Table 1). Published results are inconsistent with respect to whether prenatal opioid exposure results in neurodevelopmental impairments at birth, perhaps reflecting in part the different forms of opioids that were considered as well as the measures used to assess newborn neurobehavior. In the most recent work, researchers examine whether a diagnosis of NOWS predicts impaired neurodevelopmental outcomes (above and beyond exposure to opioids). This limited literature reveals some increased neurodevelopmental risk for newborns who develop NOWS with respect to state regulation, arousal, and quality of movement.
Prenatal Opioid Exposure and Neurodevelopmental Outcomes in Infancy
Of the 21 articles in which neurodevelopmental outcome in infancy was examined, only 6 were published in the last 5 years. In most of these studies, the authors examined the effects of prenatal exposure to opioids (usually heroin, methadone, or buprenorphine) on neurodevelopment in infants compared with unexposed controls (sometimes matched on relevant confounding variables such as maternal age). Early research revealed that prenatal exposure to opioids, such as methadone and heroin, was associated with impaired mental and language development as well as neuromotor and psychomotor development8–11,21,22,28,30,41,51,54 before 24 months, although 6 of 11 of these studies failed to account for important confounders or include a control group of unexposed children.9–11,21,30,51 Other studies in which relevant confounders were examined, such as socioeconomic status, revealed no significant differences in cognitive outcomes between infants exposed to opioids in utero compared with their unexposed peers.27,28,40,50
There were likewise discrepant findings with respect to neurodevelopmental outcomes in more recent studies. The majority of studies in which confounders were controlled for, such as poverty and disruptions in maternal care, revealed no differences in cognitive or executive functioning between infants with and without prenatal opioid exposure.27,36,40 One exception is a study that revealed that boys, but not girls, exposed to opioids and amphetamines, benzodiazepines, cannabis, and tobacco had significantly lower mental development scores by using the Bayley Scales of Infant Development, Second Edition for ages 12 to 36 months compared with unexposed infants matched on age and after controlling for caregiver socioeconomic status.41 Other research revealed that infants exposed to cocaine and opioids reached for objects less often when in the dark compared with unexposed matched controls, indicating poorer motor development.35
The authors of 2 studies attempted to clarify whether exposure to buprenorphine resulted in different neurodevelopmental consequences compared with exposure to methadone. There appears to be little31 to no15 neurodevelopmental differences in exposure to either buprenorphine or methadone at 4 months.15 In an RCT, differences in neurodevelopmental outcome among infants exposed prenatally to methadone compared with buprenorphine (N = 96) were examined at 5 ages: 3, 6, 12, 24, and 36 months.31 Only 2 of 37 outcomes significantly differed between exposure groups. First, infants exposed to buprenorphine had significantly lower expressive and receptive language scores compared with those exposed to methadone at 12 months. Second, infants exposed to buprenorphine were less likely to show positive excitement and approach toward pleasurable activities compared with infants exposed to methadone at 3 months.
The few existing studies in which the authors examine neurodevelopmental differences among infants exposed to opioids who develop NOWS compared with infants exposed to opioids who do not develop NOWS reveal inconsistent findings. In an RCT, only 1 significant difference out of 37 tested revealed that infants treated for NOWS were rated higher by their caregivers on a distress to limitations scale (a component of temperament) at 6 months.31 On the other hand, in a retrospective chart review, infants treated for NOWS with methadone, morphine, or buprenorphine had significantly lower Bayley Scales of Infant Development scores at age 2 years compared with the general population, but on average, they were within 1 SD of the mean for the Bayley Scales of Infant Development.39
Few studies revealed effects of prenatal opioid exposure on neurodevelopmental outcomes in infancy (above and beyond the effects of confounding variables such as socioeconomic status). Studies were limited by small sample sizes, and effects, when found, were small (Table 1). One recently published article with a large (N = 131) sample size of children with prenatal opioid exposure revealed few effects of prenatal opioid exposure, and infants developed within normal limits.31 Comparatively more studies in which authors documented long-term outcomes of prenatal opioid exposure (measured past 2 years of age) were available and are reviewed below.
Prenatal Opioid Exposure: Long-term Neurodevelopmental Consequences
Of the 27 studies of prenatal opioid exposure and outcomes past age 2 years, 8 were published in the last 5 years. In some studies, no differences in cognitive outcomes were documented.24,32–34,37,42,46–48,52,56,58 On the other hand, differences in IQ, neurologic performance,13,23,45,57 and language performance30 were found, although not always when controlling for covariates. Other research revealed that boys (but not girls) exposed to opioids in utero had lower IQ scores, which did not change over time, compared with their unexposed counterparts at 8.5 years of age (above and beyond the effects of adoption, parental socioeconomic status, and earlier cognitive functioning).43 One other study revealed that children exposed to buprenorphine in utero had, on average, performance IQ scores 1 SD below the mean at ages 5 to 6 years as well as memory and motor scores 1 SD below the mean on the basis of national data, although no unexposed control group was examined.55
Although the research on the long-term cognitive outcome of children with prenatal opioid exposure is equivocal, there is more consistency with respect to differences in behavioral outcomes. Children exposed to methadone prenatally had elevated levels of aggression, fear, and anxiety.24 Researchers have found elevated symptoms of attention-deficit/hyperactivity disorder45,46,49 in children who were exposed to opioids in utero compared with children who were not exposed to opioids in utero after controlling for sociodemographic factors and medical history.49 In addition, a diagnosis of NOWS predicted lower attention in children exposed to opioids compared with unexposed controls who were matched on sex and age.49
With respect to executive functioning, children exposed to opioids and other substances (such as alcohol, marijuana, and nicotine) had difficulties with information processing compared with unexposed children, after controlling for demographic factors,26 and boys exposed to methadone or heroin prenatally, and boys who reported only caregiver opioid use during the postnatal period had poorer performance on a vigilance task compared with unexposed controls.29 Four-year-old children exposed to methadone or buprenorphine in utero had lower executive-functioning scores compared with their unexposed peers.34 On the other hand, no differences were found in performance of short-term memory tasks or in inhibition in 4 to 5 year old children exposed to buprenorphine or methadone compared with unexposed children after controlling for maternal education and employment.33
Three studies were focused on visual or visual-motor differences in children exposed to opioids prenatally compared with unexposed children. Children age 4.5 years exposed to opioids prenatally had significantly lower visual-motor and perceptual performance scores compared with unexposed preschool-aged children, after adjustment for the effects of socioeconomic status,42 and poorer lower left eye visual acuity.56 One other study revealed no differences in tests of visual perception, but 4-year-old children exposed to methadone made fewer goal-directed eye movements compared with the mean, controlling for maternal education.32
Children exposed to opioids and diagnosed with NOWS may have poorer neurodevelopmental outcomes compared with their exposed counterparts who were not diagnosed with NOWS. Children with NOWS were more likely to have developmental delays and lower IQ,19 were 2.3 times more likely to be admitted to the hospital for a neuropsychiatric disorder,53 and were more likely to show poorer performance on educational testing,44 meet criteria for a disability, require classroom therapies and services,25 and have lower attention49 compared with children who did not develop NOWS19 and unexposed controls.25,44,49,53 On the other hand, no association was found between a diagnosis of NOWS and visual-motor tasks, attention problems, or general cognitive function among 4-year-old children exposed prenatally to buprenorphine or methadone and nicotine.13,38 The majority of these studies, however, were based on a retrospective chart review. Retrospective chart reviews and medical record evaluations offer the most promise for addressing immediate questions concerning the long-term consequences of prenatal opioid exposure. However, chart reviews have known limitations, including variability in diagnosis and treatment of NAS and insufficient attention paid to confounding variables.59
In summary, there were only 2 prospective studies of the long-term neurodevelopmental consequences of prenatal opioid exposure that were published in the last 5 years. One study revealed that boys exposed to opioids had lower IQ scores at 8.5 years compared with unexposed boys.43 Children with prenatal opioid exposure in the second study had average scores on cognitive measures, but their scores were significantly lower than those of an unexposed comparison group.34 It is therefore too preliminary to draw firm conclusions regarding the long-term neurodevelopmental consequences of prenatal opioid exposure. Retrospective chart reviews revealed that children diagnosed with NOWS are at an increased risk for behavioral and academic problems, although effect sizes in these reviews are variable. These academic challenges may appear as the child reaches school age because of the complexities of cognitive processing that are brought to bear in the classroom, as has been demonstrated in children with prenatal cocaine60 and nicotine61 exposure. The data on long-term cognitive outcomes are unclear, in large part because of a number of methodologic limitations in the literature, which are described below.
Key Methodologic Shortcomings
The conclusions that can be drawn regarding the neurodevelopmental outcomes of prenatal opioid exposure are limited because of a number of key methodologic shortcomings in the literature.62 One methodologic shortcoming is that the research is plagued with small sample sizes, which makes it difficult to adjust for confounding variables, another limitation of the literature. Important potential confounding variables include prenatal exposure to substances other than opioids, nutrition during pregnancy, inadequate prenatal care, medical complications, socioeconomic status, the quality of the child-rearing environment, maternal mental health, maternal trauma exposure, and partner use of substances, all known to be related to opioid use and known to impact child development. It is also possible that there is confounding by indication (ie, that mothers are using opioids to treat underlying psychiatric illness). Failing to assess for psychotropic medication use, or characterizing opioid exposure as a binary outcome (eg, yes or no), may not capture the true impact of opioid use, which can vary by the type of opioid used, frequency, and trimester.
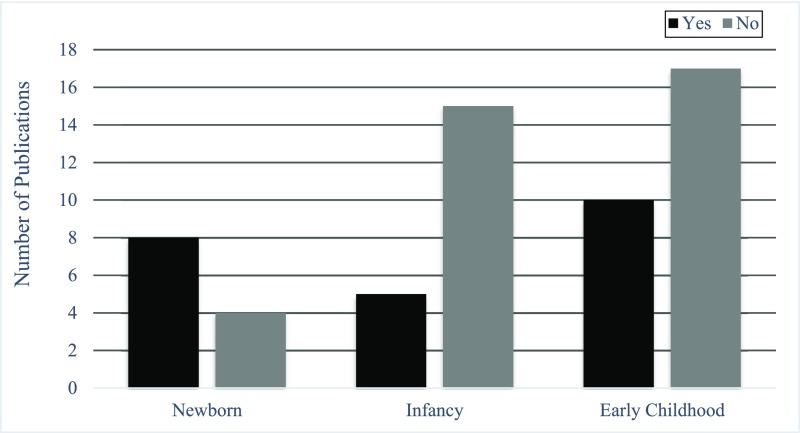
Another limitation is related to the assessment of NOWS. We have attempted to provide a concise review of whether infants exposed to opioids who develop NOWS are at an increased risk for neurodevelopmental impairments compared with their unexposed peers. However, in the vast majority of research, how a NOWS diagnosis may impact neurodevelopmental outcome among children who were exposed to opioids prenatally was not examined. This assessment would require comprehensive identification of infants who were prenatally exposed, independent of whether NOWS was present, which is often not feasible. One difficulty in examining how NOWS affects neurodevelopmental outcomes may be a lack of consensus about the method used to assess and diagnose NOWS. In half of the studies (6 of 12; Fig 5) in which NOWS was examined, the authors relied on whether the child was diagnosed with NOWS on the basis of clinician decision to treat the child without using a screening instrument (n = 2) or on whether a diagnosis was recorded in the medical record (n = 4). The Finnegan Neonatal Abstinence Score Sheet is considered the gold standard measurement of NOWS, but a number of researchers and clinicians have raised concerns about its subjectivity, length, and lack of interrater reliability and validity.63 A related limitation is the lack of widespread use of a neurodevelopmental risk-monitoring marker at birth that can be used to identify newborns at risk for NOWS and track their long-term neurodevelopmental outcomes. The lack of evidence-based assessments in this field makes it difficult for clinicians to improve the management of NOWS.
FIGURE 5.
Number of publications on children with prenatal opioid exposure in which NOWS was examined as an outcome.
In no studies have authors examined how the timing of the opioid exposure during pregnancy can impact neurodevelopmental outcomes, nor have they accounted for potential confounding by maternal genetic or epigenetic factors or addiction liability. Thus, it is unclear if exposure in the first trimester only has differential neurodevelopmental effects from exposure throughout the pregnancy.
A Solution-Oriented Research Agenda
In our view, a solution-oriented research agenda starts by addressing the existing limitations in the literature, which would lead to more rigorous tests of how prenatal opioid exposure could impact neurodevelopmental outcomes across infancy, childhood, and adolescence. First, large prospective studies that account for confounding variables are needed to improve the diagnosis and management of NOWS. To develop these evidence-based assessments, researchers should examine every child with prenatal opioid exposure for NOWS. Researchers should include a short-term neurodevelopmental outcome as a marker of risk for neurodevelopmental impairment in infancy, toddlerhood, or childhood. For example, the NICU Network Neurobehavioral Scale64 can be used to measure neurodevelopment at birth and is predictive of low IQ, adaptive behavior, and problem behavior in 4.5-year-old children.64 Authors of these studies should examine not only neurodevelopment, NOWS, and length-of-stay as outcomes but also length of treatment, length of treatment due to NOWS, and newborn neurobehavior. Outcomes should be assessed beyond early childhood, given that some effects of prenatal opioid exposure may be latent, and not emerge until adolescence when children’s cognitive and executive functioning skills may be challenged in school settings.
These studies should incorporate maternal reports of substance and psychopharmacology use, ideally beginning prenatally, as well as collection of biospecimens so that toxicology screens can be performed on (1) maternal hair, (2) cord blood, and/or (3) meconium. Characteristics of specific populations of women who use opioids while pregnant are needed. For example, researchers could compare street heroin users with women on medically assisted treatment and women on prescription pain medications. Women who use opioids today are different from the population of women who have used opioids in the past because of the emergence of prescription opioids. In short-term follow-up studies, researchers could then test whether a particular kind of opioid exposure is more likely to lead to NOWS. It is also recommended that researchers manage children yearly and, at minimum, include developmental assessments with cognitive, motor, social and emotional, language, and adaptive behavior components. It would also be advantageous to include outcome measures that have received less attention in the literature but that are strong predictors of academic skills, such as executive functioning. There is, however, a disproportionate focus in the literature on adverse outcomes as a consequence of prenatal opioid exposure, such as low IQ or behavior problems. Studies of adaptive outcomes and protective and resilience factors are also needed to inform preventive intervention.
There is a strong heritable risk component to neurodevelopmental disorders, and genetic risk factors continue to emerge through the Psychiatric Genetics Consortium. It will be critical to assess the effects of prenatal opioid exposures in the context of the child’s underlying genetic and epigenetic risk for neurodevelopmental outcomes. An additional promising biomarker is heart rate variability (HRV). HRV provides an index of parasympathetic nervous system functioning and may provide clues about how the NOWS symptom of autonomic instability may develop. Authors of two studies have investigated the HRV of fetuses exposed to opioids in utero.65,66 Fetuses exposed to buprenorphine showed higher HRV compared with fetuses exposed to methadone. To our knowledge, in no studies have researchers examined whether individual differences in fetal HRV predict NOWS onset or severity.
A solution-oriented research agenda, one that is integral to developing methodologically sound tools for monitoring risk for impaired neurodevelopmental outcome, will need to tailor effective treatments to support the healthy development of children with prenatal opioid exposure. Genetic and epigenetic data could be used to inform diagnostic and intervention strategies and personalized medicine approaches to treatment.67,68 In addition to pharmacologic treatment of NOWS, such as buprenorphine, there is growing support for nonpharmacologic treatment approaches.69,70 Rooming-in, breastfeeding, kangaroo care, and swaddling are just some of the hypothesized active ingredients of nonpharmacologic interventions.
How Can the ECHO Program Address These Methodologic Shortcomings?
This future research agenda is ambitious and will require large sample sizes from diverse populations to address the key unanswered research questions in the field. The ECHO program was initiated in September 2016 to examine how environmental exposures early in life can impact health across the life course.71 It is a 7-year initiative that is designed to identify the mechanisms and intervention targets to address 5 pediatric health outcomes: prenatal, perinatal, and early postnatal outcomes; childhood obesity; airways; neurodevelopment; and positive health outcomes.72 A key component of the ECHO program includes a person-reported outcomes core to assess how physical, mental, and social health interact within families across the life span to promote or hinder child health outcomes.73 Existing data are shared across the 71 cohorts, and new data and biospecimens are also collected across sites to generate solution-oriented research that could result in immediate benefits to the health of children.71,74 The strengths of the ECHO program include innovative measures of the postnatal environment, with attention paid to the postnatal rearing environment, that will provide invaluable data about social and emotional, cognitive, motor, and language development. The ECHO program instituted a number of procedures to ensure proper harmonization of data that have already been collected across sites. Scientists at a data analysis center are responsible for identifying common measures across sites and creating data dictionaries so that data can be easily abstracted for cross-cohort data analysis.75 The ECHO program then identified measures to collect as part of the ECHO-wide data collection protocol to address how prenatal exposures can impact the 5 broad outcomes reviewed earlier. The use of an ECHO-wide data collection protocol will reduce variability in the data. The ECHO program has a “train the trainer” model that includes resources for training study staff on the common protocol. The trainers then train their local study staff on the protocol. Regular validity checks, which are managed by the ECHO Coordinating Center, are in place to ensure that consistent data collection procedures occur.72
An advantage of the ECHO program is that ECHO researchers, in collaboration with biostatisticians, are well powered to analyze data from ECHO-wide cohorts to test questions that may not have been feasible with single cohorts. For example, a key concern of ECHO researchers related to NOWS is what the long-term neurodevelopmental outcomes may be for children with prenatal opioid exposure. The goal is for these observational studies to inform clinical trials more broadly as well as clinical trials in the Institutional Development Award States Pediatric Clinical Trials Network.76 This is a network of clinical sites in 17 states that have historically received low rates of NIH funding. The goal is to engage these communities and provide access to clinical trials for children and families in rural and underserved areas.71
There are a number of critical postnatal variables that have been underexamined in the prenatal opioid exposure literature. These include assessment of the quality of the caregiver-child relationship, detailed data about the socioeconomic environment (such as neighborhood quality), and executive functioning. Furthermore, confounding factors, such as maternal prenatal use of substances in addition to opioids, psychopathology, and prenatal care (which also have adverse effects on neurodevelopmental outcomes in children77) can all be considered, which is an advantage given that in most of the studies reviewed, researchers were only able to examine a smaller number of potential confounders. Furthermore, the ECHO program has committed to genotyping all 50 000 children on a common genome-wide association studies platform, which provides a unique opportunity to couple the rich environmental data being collected with genome-wide genetic data.
Another innovative feature of the ECHO program is its focus on understanding positive outcomes.78 Positive health is thought of as biological, functional, behavioral, and experiential assets that strengthen the health of the child.78 As is the case in a risky environment, positive health promoters can have a genetic basis, and they can also be dynamically shaped over time via transactions between the environment and the child.
Attrition is a concern for research with participants who are stressed and using substances. The ECHO program requires that cohorts are only eligible for funding if they can retain 75% of participants. The ECHO program also has regular meetings to discuss best practices for recruitment and retention. For example, the ECHO program conducted a study to examine the factors that motivate individuals to participate in research and to remain in research studies. This information, which includes highlighting how the participants contribute to science, is used to improve recruitment and retention strategies.
Our query of the extant ECHO data includes information from 30 different cohorts across the country with data on prenatal opioid exposure and exposure to substances other than opioids, including 10 cohorts with access to information about NOWS. Figure 6 reveals the number of cohorts from whom there is prenatal opioid exposure data in the ECHO program. This figure includes information from a subsample of cohorts in the ECHO program. The number of cohorts in the ECHO program is much larger. There are >50 000 caregiver-child pairs enrolled from 180 sites. In all sites, new data on prenatal opioid exposure will be collected. Figure 6 only includes the existing cohort-level data that are available. Longitudinal follow-up with these infants is ongoing and includes assessments of prenatal opioid exposure and exposure to other substances such as tobacco and psychotropic medications; longitudinal follow-up also includes whether nonpharmacological interventions are being used. Another component of the ECHO program is the Institutional Development Award States Pediatric Clinical Trials Network. The cohort and clinical trial components of the ECHO program can collaborate on translational protocols to evaluate research, treatment, and best practices for treating infants with NOWS.
FIGURE 6.
Number of cohorts in the ECHO program with data on prenatal opioid exposure, NOWS diagnoses, neurodevelopmental outcomes in infancy and childhood, and sociodemographic confounders.
Conclusions
Prenatal opioid exposure is a vexing public health problem that is on the rise. To date, there have been few rigorously designed studies conducted to document the short- and long-term neurodevelopmental consequences of prenatal opioid exposure. The literature on prenatal opioid exposure and short-term impact seems to support no effect.79 Findings from studies in which longer-term neurodevelopmental outcomes are examined, although mixed, provide more support for an association, although the size of the effect varies depending on the outcome studied. Retrospective chart reviews indicate that as children with a previous NOWS diagnosis enter formal schooling, they are at an increased risk for problems with attention, lower IQ, and poorer performance on academic testing compared with children who were exposed to opioids but did not develop NOWS and unexposed controls. Children with prenatal opioid exposure are also more likely to be raised in poverty, and in many studies, exposure to poverty appeared to drive many of the neurodevelopmental outcomes observed. However, the state of the field has serious methodologic shortcomings, and pediatricians and psychologists have scant evidence with which to answer a parent’s question, “How does opioid exposure affect my child?” We provide an agenda for future research in this area that highlights the need for prospective longitudinal data and that takes into account relevant potential confounding factors. We describe how the ECHO initiative, funded by the NIH, can address these shortcomings. The ultimate goal is to document these effects so that we can identify the children most at risk for impairments in neurodevelopmental outcomes.
Acknowledgments
We thank our ECHO colleagues; the medical, nursing, and program staff; and the children and families participating in the ECHO cohorts. See Supplemental Information for full list of collaborators.
Glossary
- ECHO
Environmental Influences on Child Health Outcomes
- HRV
heart rate variability
- NAS
neonatal abstinence syndrome
- NIH
National Institutes of Health
- NOWS
neonatal opioid withdrawal syndrome
- RCT
randomized controlled trial
Footnotes
Dr Conradt, Ms Flannery, and Dr McGrath conceptualized the study, developed the tables and figures, and drafted the manuscript; Drs Lester, Ondersma, Aschner, Annett, Croen, Duarte, Friedman, Guille, Hedderson, Hofheimer, Jones, Ladd-Acosta, Moreland, Neiderhiser, Nguyen, Posner, Ross, and Savitz conceptualized the study and drafted the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Environmental Influences on Child Health Outcomes program, Office of the Director, National Institutes of Health (NIH) under awards U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), UG1OD024942 (Dr Annett), UG3OD023249, UG3OD023289 (Dr Croen, Dr Hedderson), UG3OD023320 (Dr Aschner), UG3OD023328 (Dr Duarte, Dr Posner), UG3OD023347 (Dr Lester, Dr Savitz), and UG3OD023389 (Dr Neiderhiser). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Haight SC, Ko JY, Tong VT, Bohm MK, Callaghan WM. Opioid use disorder documented at delivery hospitalization - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmes AV, Atwood EC, Whalen B, et al. Rooming-in to treat neonatal abstinence syndrome: improved family-centered care at lower cost. Pediatrics. 2016;137(6):e20152929. [DOI] [PubMed] [Google Scholar]
- 3.Ko JY, Patrick SW, Tong VT, et al. Incidence of neonatal abstinence syndrome - 28 states, 1999-2013. MMWR Morb Mortal Wkly Rep. 2016;65(31):799–802 [DOI] [PubMed] [Google Scholar]
- 4.Reddy UM, Davis JM, Ren Z, Greene MF; Opioid Use in Pregnancy, Neonatal Abstinence Syndrome, and Childhood Outcomes Workshop Invited Speakers . Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American College of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstet Gynecol. 2017;130(1):10–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnegan LP, Kaltenbach K. Neonatal Abstinence Syndrome In: Hoekelman RA, Nelson NM, Seidel HM, eds. Primary Pediatric Care, 2nd ed St Louis, MO: Mosby; 1992:pp 1367–1378 [Google Scholar]
- 6.Sutter MB, Leeman L, Hsi A. Neonatal opioid withdrawal syndrome. Obstet Gynecol Clin North Am. 2014;41(2):317–334 [DOI] [PubMed] [Google Scholar]
- 7.Jeremy RJ, Hans SL. Behavior of neonates exposed in utero to methadone as assessed on the Brazelton scale. Infant Behav Dev. 1985;8(3):323–336 [Google Scholar]
- 8.Rosen TS, Johnson HL. Children of methadone-maintained mothers: follow-up to 18 months of age. J Pediatr. 1982;101(2):192–196 [DOI] [PubMed] [Google Scholar]
- 9.Wilson GS, Desmond MM, Wait RB. Follow-up of methadone-treated and untreated narcotic-dependent women and their infants: health, developmental, and social implications. J Pediatr. 1981;98(5):716–722 [DOI] [PubMed] [Google Scholar]
- 10.Hans SL, Marcus J. Motoric and attentional behavior in infants of methadone-maintained women. NIDA Res Monogr. 1983;43:287–293 [PubMed] [Google Scholar]
- 11.Huntington L, Hans SL, Zeskind PS. The relations among cry characteristics, demographic variables, and developmental test scores in infants prenatally exposed to methadone. Infant Behav Dev. 1990;13(4):533–538 [Google Scholar]
- 12.Jones HE, O’Grady KE, Johnson RE, Velez M, Jansson LM. Infant neurobehavior following prenatal exposure to methadone or buprenorphine: results from the neonatal intensive care unit network neurobehavioral scale. Subst Use Misuse. 2010;45(13):2244–2257 [DOI] [PubMed] [Google Scholar]
- 13.van Baar AL, Soepatmi S, Gunning WB, Akkerhuis GW. Development after prenatal exposure to cocaine, heroin and methadone. Acta Paediatr Suppl. 1994;404:40–46 [DOI] [PubMed] [Google Scholar]
- 14.Johnson RE, Jones HE, Jasinski DR, et al. Buprenorphine treatment of pregnant opioid-dependent women: maternal and neonatal outcomes. Drug Alcohol Depend. 2001;63(1):97–103 [DOI] [PubMed] [Google Scholar]
- 15.Bier JB, Finger AS, Bier BA, Johnson TA, Coyle MG. Growth and developmental outcome of infants with in-utero exposure to methadone vs buprenorphine. J Perinatol. 2015;35(8):656–659 [DOI] [PubMed] [Google Scholar]
- 16.Velez ML, McConnell K, Spencer N, et al. Prenatal buprenorphine exposure and neonatal neurobehavioral functioning. Early Hum Dev. 2018;117:7–14 [DOI] [PubMed] [Google Scholar]
- 17.Coyle MG, Salisbury AL, Lester BM, et al. Neonatal neurobehavior effects following buprenorphine versus methadone exposure. Addiction. 2012;107(suppl 1):63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heller NA, Logan BA, Morrison DG, et al. Neonatal abstinence syndrome: neurobehavior at 6 weeks of age in infants with or without pharmacological treatment for withdrawal. Dev Psychobiol. 2017;59(5):574–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauman PS, Levine SA. The development of children of drug addicts. Int J Addict. 1986;21(8):849–863 [DOI] [PubMed] [Google Scholar]
- 20.Beckwith AM, Burke SA. Identification of early developmental deficits in infants with preantal heroin, methadone, and other opioid exposure. Clinical Pediatrics. 2015;54(4):328–335 [DOI] [PubMed] [Google Scholar]
- 21.Belcher HM, Shapiro BK, Leppert M, et al. Sequential neuromotor examination in children with intrauterine cocaine/polydrug exposure. Dev Med Child Neurol. 1999;41(4):240–246 [DOI] [PubMed] [Google Scholar]
- 22.Bunikowski R, Grimmer I, Heiser A, et al. Neurodevelopmental outcome after prenatal exposure to opiates. Eur J Pediatr. 1998;157(9):724–730 [DOI] [PubMed] [Google Scholar]
- 23.Davis DD, Templer DI. Neurobehavioral functioning in children exposed to narcotics in utero. Addict Behav. 1988;13(3):275–283 [DOI] [PubMed] [Google Scholar]
- 24.de Cubas MM, Field T. Children of methadone-dependent women: developmental outcomes. Am J Orthopsychiatry. 1993;63(2):266–276 [DOI] [PubMed] [Google Scholar]
- 25.Fill MA, Miller AM, Wilkinson RH, et al. Educational disabilities among children born with neonatal abstinence syndrome. Pediatrics. 2018;142(3):e20180562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo X, Spencer JW, Suess PE, et al. Cognitive brain potential alterations in boys exposed to opiates: in utero and lifestyle comparisons. Addict Behav. 1994;19(4):429–441 [DOI] [PubMed] [Google Scholar]
- 27.Hans SL, Jeremy RJ. Postneonatal mental and motor development of infants exposed in utero to opioid drugs. Infant Ment Health J. 2001;22(3):300–315 [Google Scholar]
- 28.Hans SL. Developmental consequences of prenatal exposure to methadone. Ann N Y Acad Sci. 1989;562:195–207 [DOI] [PubMed] [Google Scholar]
- 29.Hickey JE, Suess PE, Newlin DB, Spurgeon L, Porges SW. Vagal tone regulation during sustained attention in boys exposed to opiates in utero. Addict Behav. 1995;20(1):43–59 [DOI] [PubMed] [Google Scholar]
- 30.Hunt RW, Tzioumi D, Collins E, Jeffery HE. Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early Hum Dev. 2008;84(1):29–35 [DOI] [PubMed] [Google Scholar]
- 31.Kaltenbach K, O’Grady KE, Heil SH, et al. Prenatal exposure to methadone or buprenorphine: early childhood developmental outcomes. Drug Alcohol Depend. 2018;185:40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konijnenberg C, Melinder A. Neurodevelopmental investigation of the mirror neurone system in children of women receiving opioid maintenance therapy during pregnancy. Addiction. 2013;108(1):154–160 [DOI] [PubMed] [Google Scholar]
- 33.Konijnenberg C, Melinder A. Visual selective attention is impaired in children prenatally exposed to opioid agonist medication. Eur Addict Res. 2015;21(2):63–70 [DOI] [PubMed] [Google Scholar]
- 34.Konijnenberg C, Sarfi M, Melinder A. Mother-child interaction and cognitive development in children prenatally exposed to methadone or buprenorphine. Early Hum Dev. 2016;101:91–97 [DOI] [PubMed] [Google Scholar]
- 35.LaGasse LL, Van Vorst RF, Brunner SM, Lester BM. Effects of in utero exposure to cocaine and/or opiates on infants’ reaching behavior. Ann N Y Acad Sci. 1998;846(1):405–407 [DOI] [PubMed] [Google Scholar]
- 36.Levine TA, Woodward LJ. Early inhibitory control and working memory abilities of children prenatally exposed to methadone. Early Hum Dev. 2018;116:68–75 [DOI] [PubMed] [Google Scholar]
- 37.Lifschitz MH, Wilson GS, Smith EO, Desmond MM. Factors affecting head growth and intellectual function in children of drug addicts. Pediatrics. 1985;75(2):269–274 [PubMed] [Google Scholar]
- 38.Melinder A, Konijnenberg C, Sarfi M. Deviant smooth pursuit in preschool children exposed prenatally to methadone or buprenorphine and tobacco affects integrative visuomotor capabilities. Addiction. 2013;108(12):2175–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merhar SL, McAllister JM, Wedig-Stevie KE, et al. Retrospective review of neurodevelopmental outcomes in infants treated for neonatal abstinence syndrome. J Perinatol. 2018;38(5):587–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messinger DS, Bauer CR, Das A, et al. The maternal lifestyle study: cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics. 2004;113(6):1677–1685 [DOI] [PubMed] [Google Scholar]
- 41.Moe V, Slinning K. Children prenatally exposed to substances: gender-related differences in outcome from infancy to 3 years of age. Infant Ment Health J. 2001;22(3):334–350 [Google Scholar]
- 42.Moe V. Foster-placed and adopted children exposed in utero to opiates and other substances: prediction and outcome at four and a half years. J Dev Behav Pediatr. 2002;23(5):330–339 [DOI] [PubMed] [Google Scholar]
- 43.Nygaard E, Moe V, Slinning K, Walhovd KB. Longitudinal cognitive development of children born to mothers with opioid and polysubstance use. Pediatr Res. 2015;78(3):330–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oei JL, Melhuish E, Uebel H, et al. Neonatal abstinence syndrome and high school performance. Pediatrics. 2017;139(2):e20162651. [DOI] [PubMed] [Google Scholar]
- 45.Ornoy A, Segal J, Bar-Hamburger R, Greenbaum C. Developmental outcome of school-age children born to mothers with heroin dependency: importance of environmental factors. Dev Med Child Neurol. 2001;43(10):668–675 [DOI] [PubMed] [Google Scholar]
- 46.Ornoy A. The impact of intrauterine exposure versus postnatal environment in neurodevelopmental toxicity: long-term neurobehavioral studies in children at risk for developmental disorders. Toxicol Lett. 2003;140–141:171–181 [DOI] [PubMed] [Google Scholar]
- 47.Ornoy A, Michailevskaya V, Lukashov I, Bar-Hamburger R, Harel S. The developmental outcome of children born to heroin-dependent mothers, raised at home or adopted. Child Abuse Negl. 1996;20(5):385–396 [DOI] [PubMed] [Google Scholar]
- 48.Salo S, Kivistö K, Korja R, et al. Emotional availability, parental self-efficacy beliefs, and child development in caregiver-child relationships with buprenorphine-exposed 3-year-olds. Parenting. 2009;9(3–4):244–259 [Google Scholar]
- 49.Sandtorv LB, Fevang SKE, Nilsen SA, et al. Symptoms associated with attention deficit/hyperactivity disorder and autism spectrum disorders in school-aged children prenatally exposed to substances. Subst Abuse. 2018;12:1178221818765773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider JW, Hans SL. Effects of prenatal exposure to opioids on focused attention in toddlers during free play. J Dev Behav Pediatr. 1996;17(4):240–247 [PubMed] [Google Scholar]
- 51.Strauss ME, Starr RH, Ostrea EM, Chavez CJ, Stryker JC. Behavioural concomitants of prenatal addiction to narcotics. J Pediatr. 1976;89(5):842–846 [DOI] [PubMed] [Google Scholar]
- 52.Strauss ME, Lessen-Firestone JK, Chavez CJ, Stryker JC. Children of methadone-treated women at five years of age. Pharmacol Biochem Behav. 1979;11(suppl):3–6 [PubMed] [Google Scholar]
- 53.Uebel H, Wright IM, Burns L, et al. Reasons for rehospitalization in children who had neonatal abstinence syndrome. Pediatrics. 2015;136(4). Available at: www.pediatrics.org/cgi/content/full/136/4/e811 [DOI] [PubMed] [Google Scholar]
- 54.van Baar A. Development of infants of drug dependent mothers. J Child Psychol Psychiatry. 1990;31(6):911–920 [DOI] [PubMed] [Google Scholar]
- 55.Sundelin Wahlsten V, Sarman I. Neurobehavioural development of preschool-age children born to addicted mothers given opiate maintenance treatment with buprenorphine during pregnancy. Acta Paediatr. 2013;102(5):544–549 [DOI] [PubMed] [Google Scholar]
- 56.Walhovd KB, Bjørnebekk A, Haabrekke K, et al. Child neuroanatomical, neurocognitive, and visual acuity outcomes with maternal opioid and polysubstance detoxification. Pediatr Neurol. 2015;52(3):326–332.e3 [DOI] [PubMed] [Google Scholar]
- 57.Wilson GS, McCreary R, Kean J, Baxter JC. The development of preschool children of heroin-addicted mothers: a controlled study. Pediatrics. 1979;63(1):135–141 [PubMed] [Google Scholar]
- 58.Wilson GS. Clinical studies of infants and children exposed prenatally to heroin. Ann N Y Acad Sci. 1989;562:183–194 [DOI] [PubMed] [Google Scholar]
- 59.Jones HE, O’Grady KE, Kaltenbach K. Reconsidering retrospective review of neurodevelopmental outcomes in infants treated for neonatal abstinence syndrome. J Perinatol. 2018;38(9):1280–1281 [DOI] [PubMed] [Google Scholar]
- 60.Lester BM, LaGasse LL, Seifer R. Cocaine exposure and children: the meaning of subtle effects. Science. 1998;282(5389):633–634 [DOI] [PubMed] [Google Scholar]
- 61.Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2003;25(4):427–436 [DOI] [PubMed] [Google Scholar]
- 62.Konijnenberg C. Methodological issues in assessing the impact of prenatal drug exposure. Subst Abuse. 2015;9(suppl 2):39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez-Pomar E, Christian A, Devlin L, et al. Analysis of the factors that influence the Finnegan Neonatal Abstinence Scoring System. J Perinatol. 2017;37(7):814–817 [DOI] [PubMed] [Google Scholar]
- 64.Liu J, Bann C, Lester B, et al. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics. 2010;125(1). Available at: www.pediatrics.org/cgi/content/full/125/1/e90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jansson LM, Dipietro JA, Velez M, et al. Fetal neurobehavioral effects of exposure to methadone or buprenorphine. Neurotoxicol Teratol. 2011;33(2):240–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jansson LM, Velez M, McConnell K, et al. Maternal buprenorphine treatment and fetal neurobehavioral development. Am J Obstet Gynecol. 2017;216(5):529.e1-529.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wachman EM, Hayes MJ, Lester BM, et al. Epigenetic variation in the mu-opioid receptor gene in infants with neonatal abstinence syndrome. J Pediatr. 2014;165(3):472–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McLaughlin P, Mactier H, Gillis C, et al. Increased DNA methylation of ABCB1, CYP2D6, and OPRM1 genes in newborn infants of methadone-maintained opioid-dependent mothers. J Pediatr. 2017;190:180–184.e1 [DOI] [PubMed] [Google Scholar]
- 69.Wachman EM, Schiff DM, Silverstein M. Neonatal abstinence syndrome: advances in diagnosis and treatment. JAMA. 2018;319(13):1362–1374 [DOI] [PubMed] [Google Scholar]
- 70.Coyle MG, Brogly SB, Ahmed MS, Patrick SW, Jones HE. Neonatal abstinence syndrome. Nat Rev Dis Primers. 2018;4(1):47. [DOI] [PubMed] [Google Scholar]
- 71.Gillman MW, Blaisdell CJ. Environmental influences on child health outcomes, a research program of the National Institutes of Health. Curr Opin Pediatr. 2018;30(2):260–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith B, Knox S, Benjamin DK Jr. Coordination of the Environmental influences on Child Health Outcomes program: so the whole is greater than the sum of its parts. Curr Opin Pediatr. 2018;30(2):263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blackwell CK, Wakschlag LS, Gershon RC, Cella D; ECHO PRO Core . Measurement framework for the Environmental influences on Child Health Outcomes research program. Curr Opin Pediatr. 2018;30(2):276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wright RO, Teitelbaum S, Thompson C, Balshaw D; CHEAR Network . The child health exposure analysis resource as a vehicle to measure environment in the environmental influences on child health outcomes program. Curr Opin Pediatr. 2018;30(2):285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jacobson LP, Lau B, Catellier D, Parker CB. An Environmental influences on Child Health Outcomes viewpoint of data analysis centers for collaborative study designs. Curr Opin Pediatr. 2018;30(2):269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Snowden J, Darden P, Palumbo P, Saul P, Lee J; IDeA States Pediatric Clinical Trials Network . The institutional development award states pediatric clinical trials network: building research capacity among the rural and medically underserved. Curr Opin Pediatr. 2018;30(2):297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Conradt E, Crowell SE, Lester BM. Early life stress and environmental influences on the neurodevelopment of children with prenatal opioid exposure. Neurobiol Stress. 2018;9:48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forrest CB, Blackwell CK, Camargo CA Jr. Advancing the science of children’s positive health in the National Institutes of Health Environmental Influences on Child Health Outcomes (ECHO) research program. J Pediatr. 2018;196:298–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jones HE, Kaltenbach K, Benjamin T, Wachman EM, O’Grady KE. Prenatal opioid exposure, neonatal abstinence syndrome/neonatal opioid withdrawal syndrome, and later child development research: shortcomings and solutions. J Addict Med. 2019;13(2):90–92 [DOI] [PubMed] [Google Scholar]