Abstract
Introduction
Appropriate health-related quality of life (HRQOL) in children and adolescents with type 1 diabetes constitutes one of the most important factors that determine treatment effectiveness. There are numerous studies which tackle the issue of the relationship between HRQOL and various clinical and demographic factors, including gender. Therefore, the aim of the present study was to assess HRQOL and identify factors by which it may be affected, with particular emphasis on gender.
Material and methods
The study group included 197 girls and boys (13.9±2.33 years old) with a history of type 1 diabetes (>1 year) treated with the use of insulin pumps. PedsQL Diabetes Module 3.0 questionnaire was used in the assessment of HRQOL. Multivariate linear regression with gender as a covariate was used to investigate the relationship between total PedsQL score and selected variables associated with patient characteristics, insulin dosage and the control of glycemia. Moreover, the presence of gender differences was verified in terms of variables which significantly affected HRQOL.
Results
Significantly higher results were observed in boys as regards the total PedsQL score (70.8±11.91 vs 62.4±13.91; P<0.001) and individual subscales of the questionnaire (except “Worry”). Regression analysis demonstrated the presence of a significant negative relationship between HRQOL assessment and HbA1c concentrations, WHtR value and the frequency of hypoglycemic episodes. However, it was noted that better HRQOL was observed in boys than in girls, regardless of the quality of the metabolic control of diabetes, regular pattern of adipose tissue distribution and experiencing hyperglycemic episodes.
Conclusion
Female gender was an independent factor which adversely affected HRQOL. Other factors which negatively influenced HRQOL included poor metabolic control of diabetes, central distribution of adipose tissue and frequent episodes of hyperglycemia. It seems necessary to focus also on other factors that may potentially influence HRQOL of patients with type 1 diabetes.
Keywords: health-related quality of life, type 1 diabetes, gender differences
Introduction
Type 1 diabetes is one of the most common chronic diseases occurring in children and adolescents.1,2 The treatment process involves daily insulin administration, monitoring glycemia and carbohydrate consumption and undertaking regular physical activity.3 It is worth emphasizing that type 1 diabetes affects all aspects of functioning, including the quality of life.4 It is the most visible in case of adolescents who have to face a particularly difficult challenge of a chronic disease which requires numerous modifications in their previous lifestyle. Moreover, changes associated with puberty may intensify the difficulty adapting to a new situation.5 Therefore, the quality of life described as health-related quality of life (HRQOL) is more and more commonly viewed as an important health index in children with chronic diseases.6,7 It is assumed that in case of children and adolescents with type 1 diabetes HRQOL improvement plays an equally important role in preventing complications as appropriate metabolic control. Therefore, the main aim of treatment should involve not only achieving normoglycemia but also the best possible HRQOL.3,8 According to numerous authors, a correlation is present between various factors and HRQOL in children and adolescents with type 1 diabetes. Notably, numerous studies showed HRQOL reduction in girls compared to boys.1,4,7,9–11 Therefore, the aim of the present study was to assess HRQOL and identify factors by which it may be affected, with particular emphasis on gender, in children and adolescents with type 1 diabetes.
Materials and methods
Material
Patients were recruited between October 2017 and June 2018 at the Department of Diabetology of the Independent Public Children’s Teaching Hospital in Warsaw, in which approximately 70% of the children with type 1 diabetes from the Masovian Voivodeship are treated. The following criteria for inclusion into the study group were determined: at least 1-year history of type 1 diabetes, implementation of insulin pump treatment, no concomitant chronic diseases (especially those requiring dietary modifications). Patients with a different type of diabetes or concomitant chronic diseases (eg, celiac disease), the duration of the disease below 1 year or treated with insulin pen injections were excluded from the study.
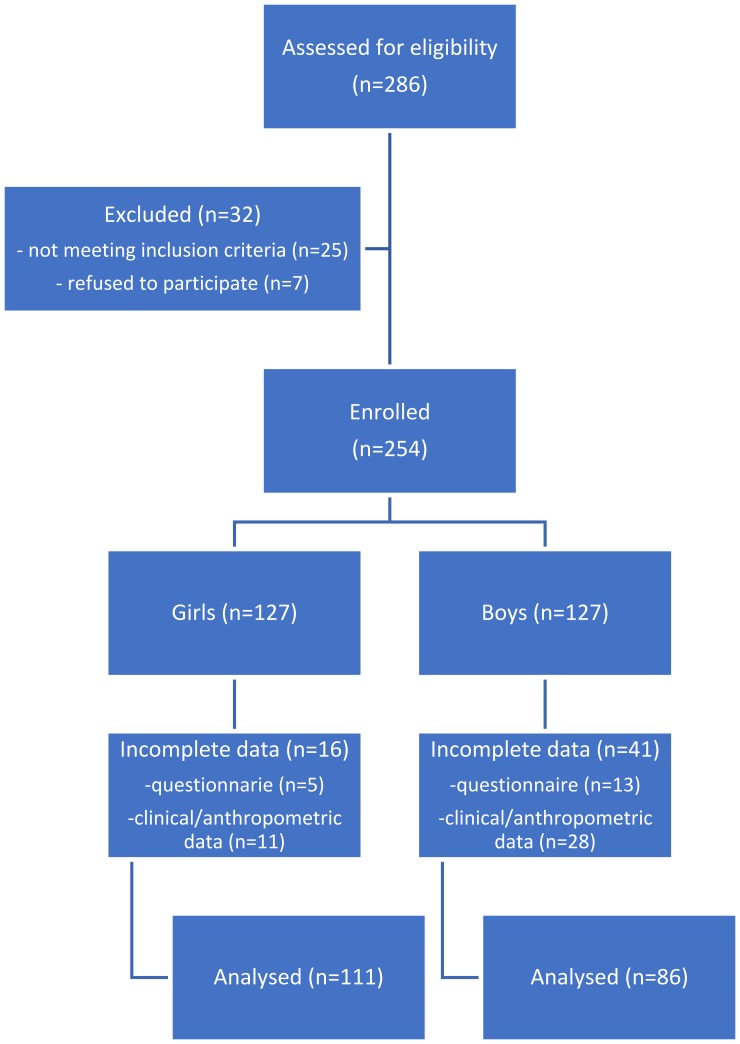
A total of 286 patients were assessed for eligibility of whom 254 patients were enrolled. Due to the incompleteness of data, 57 patients were excluded from the study (Figure 1). The final study group included 197 patients (111 girls and 86 boys) aged 8–18 (13.9±2.33).
Figure 1.
Study flow diagram.
Data were collected on paper by a trained interviewer during an individual interview. A parent or legal guardian was present during interviews with children younger than 12. The data collected were encoded to provide participant anonymity and digitized for future analyses.
Ethical aspects of the study
The non-interventional study design was presented to the Bioethics Committee of the Medical University of Warsaw and accepted without reservations (approval no AKBE/188/17 issued on the 10th of October 2017).
All the participants and their parents or legal guardians expressed oral consent to participate in the study. Before the study, during an individual conversation, each participant was informed about the details concerning the aim and course of the study, anonymity of data and the possibility of resigning at any moment. The Bioethics Committee of the Medical University of Warsaw approved all assumptions of the study.
Methods
All the data were collected throughout patient hospitalizations at the Department of Diabetology. Two versions of a validated Pediatric Questionnaire of the Quality of Life (PedsQL) Diabetes Module 3.0 were used in the study: for children aged 8–12 and adolescents aged 13–18.12 They were completed by the patients during an individual interview. In the case of problems with understanding the questionnaire by patients aged 8–12, the interviewer, not a parent or legal guardian, read the questions. A Polish version of the questionnaire was downloaded after obtaining permission to use via Mapi Research Trust. Wit et al reported that PedsQL questionnaire is one of the most useful tools for monitoring the quality of life of diabetic patients.13
The questionnaire included 28 statements divided into 5 subscales concerning the occurrence of disease-related manifestations (“Diabetes symptoms”), barriers associated with the treatment (“Treatment barriers”) and difficulties related to the adherence to the treatment (“Treatment adherence”), worries connected with the treatment (“Worry”) and problems occurring during communication with medical personnel and other people (“Communication”) over the last month. Each statement was assessed by the patients on a 5-grade scale (0 – never, 1 – almost never, 2 – sometimes, 3 – often, 4 – almost always). According to the procedure proposed by the author of the questionnaire, raw data described on the scale from 0 to 4 were converted into standardized data on the scale from 0 to 100 (0→100, 1→75, 2→50, 3→25, 4→0). Next, mean scores were calculated for the whole questionnaire and for each subscale individually for each patient. Higher scores were correlated with lower severity and should be interpreted as better HRQOL. Authors conducting research in children and adolescents with type 1 diabetes described the details of the structure of PedsQL Diabetes Module and showed that the total score obtained in the questionnaire was characterized by the strongest psychometric properties.9,14
Additionally, sociodemographic data and information about the disease, treatment methods, control of glycemia and the frequency of hypo- and hyperglycemia episodes were collected during individual interviews. According to the recommendations of International Society for Pediatric and Adolescent Diabetes (ISPAD), hypoglycemia was defined as glucose concentrations below 70 mg/dL, while hyperglycemia was defined as glucose concentrations higher than 145 mg/dL (fasting glucose) or above 180 mg/dL (postprandial glucose).15 The remaining clinical and anthropometric data of patients were supplemented based on medical records.
Based on available data, body mass index (BMI) was calculated for each patient according to the formula: BMI = body weight [kg]/height [m]2. The obtained values were interpreted with growth charts for BMI for girls and boys in the Polish population aged 3–18 according to ranges specified by the authors.16 Moreover, the value of waist to height ratio (WHtR) was calculated for each patient expressed as the waist circumference [cm] to body height [cm] ratio. This index is characterized by a universal cutting off value, equaling 0.5. Exceeding this value indicates an increased metabolic risk.17 Additionally, recent glycated hemoglobin (HbA1c) concentrations showed the level of metabolic control in each patient. According to ISPAD standards target HbA1c concentrations should be <7.5% in children and adolescents.15
Statistical analysis
Quantitative variables were described with descriptive statistics. The following measures were determined: central tendency (means, 95% confidence interval, and the median), dispersion (standard deviation, interquartile range), location (upper and lower quartile). Student’s t-test was used to compare the quality of life (total PedsQL score and individual subscales) by gender. The independent variable (factor) was gender and the dependent variable was PedsQL score. The test result was supplemented with the measurement of the unadjusted mean difference with 95% confidence interval (CI). The effect size for the observed difference between means was estimated with the help of Cohen’s d coefficient, whereby 0.2 is considered a “small” effect size, 0.5 represents a “medium” effect size and 0.8 – a “large” effect size.18
In order to determine which potential demographic or clinical characteristics (factors) can significantly affect the total PedsQL score (dependent variable) with moderating influence of gender (covariate), two multivariate linear regression models were tested. It allowed to estimate the combined effect of various factors on the quality of life. The first model included the following factors: HbA1c, age, diabetes duration, place of residence, BMI and WHtR. The second model included factors such as method of controlling glycemia, daily insulin dose, hypoglycemia, hyperglycemia, carbohydrate exchanges (CE) calculation and infections. R-squared value was calculated for each model. Then, separate multiple linear regression models were fit for each demographic and clinical indicator to determine an association with PedsQL total score, after adjustment for gender as a covariate. It was assumed that the minimum clinically important difference (MCID) for the PedsQL total score should be 3.0.12 The verification of the null hypothesis was conducted for each analysis with the a priori assumption of the statistical significance at 0.05. All analyses were performed with STATISTICA version 13.3 (TIBCO Software Inc., Palo Alto, California, United States).
Sample size estimation
Sample size was estimated based on the data collected by Sand et al.11 It was assumed that the difference in means for the dependent variable (total PedsQL score) between girls and boys should be 3.9 (with the expected higher average value in boys, one-tailed hypothesis) with SDgirls=13.1 and SDboys=11.7 (Cohen’s d coefficient 0.31). It was also assumed a priori: α error probability 0.05, power (1-β error probability) 0.80 and allocation ratio 1:1. The assumed total sample size should be N=254.
Results
Analysis of scores obtained in PedsQL questionnaire
The mean score obtained in the questionnaire was 66.1±13.69 (with the maximum value of 100) in the whole study group. Higher results indicated better HRQOL of patients (Table 1).
Table 1.
Total PedsQL score obtained by the patients
| Girls (n=111) | Boys (n=86) | Total (n=197) | |
|---|---|---|---|
| Mean (SD) | 62.4 (13.91) | 70.8 (11.91) | 66.1 (13.69) |
| 95% CI | 59.8, 65.1 | 68.3, 73.4 | 64.2, 68.0 |
| Median (IQR) | 61.6 (22.32) | 72.3 (17.86) | 67.0 (20.54) |
| Q1; Q3 | 51.8, 74.1 | 61.6, 79.5 | 56.3, 76.8 |
Abbreviations: SD, standard deviation; IQR, interquartile range; Q1, lower quartile; Q3, upper quartile; CI, confidence interval.
A significantly higher total score was noted in boys compared to girls (70.8±11.91 vs 62.4±13.91; P<0.001). It was also observed that boys obtained significantly higher results than girls in individual subscales of the questionnaire: “Diabetes symptoms” (65.8±12.85 vs 59.1±14.12; P=0.001), “Treatment barriers” (72.0±19.76 vs 57.5±20.44; P<0.001), “Treatment adherence” (78.9±16.23 vs 68.6±20.06; P<0.001) and “Communication” (78.3±21.44 vs 67.6±24.89; P=0.002). No significant difference between the scores obtained by girls and boys was observed only for “Worry” subscale (Table 2).
Table 2.
Scores obtained by the patients in individual subscales of PedsQL questionnaire
| Girls (n=111) | Boys (n=86) | Total (n=197) | Estimate (95% CI) | P-value* | d** | |
|---|---|---|---|---|---|---|
| Diabetes symptoms | 59.1 (14.12) | 65.8 (12.85) | 62.1 (13.95) | −6.7 (−10.5, −2.8) | 0.001 | 0.493 |
| Treatment barriers | 57.5 (20.44) | 72.0 (19.76) | 63.9 (21.34) | −14.5 (−20.2, −8.8) | <0.001 | 0.720 |
| Treatment adherence | 68.6 (20.06) | 78.9 (16.23) | 73.1 (19.14) | −10.3 (−15.6, −5.1) | <0.001 | 0.557 |
| Worry | 61.5 (22.25) | 61.3 (22.43) | 61.4 (22.27) | 0.1 (−6.2, 6.5) | 0.963 | – |
| Communication | 67.6 (24.89) | 78.3 (21.44) | 72.3 (23.98) | −10.7 (−17.3, −4.0) | 0.002 | 0.456 |
| PedsQL total score | 62.4 (13.91) | 70.8 (11.91) | 66.1 (13.69) | −8.4 (−12.1, −4.7) | <0.001 | 0.642 |
Notes: *Student’s t test, **Cohen’s d coefficient.
Abbreviation: CI, confidence interval.
Regression analysis
In the first multivariate linear regression model, it was noted that the combination of the following factors: HbA1c, age, diabetes duration, place of residence, BMI and WHtR in interaction with gender explains over 15% of HRQOL variability (F(17,174)=1.874, P=0.023, R-squared=0.155). In the second model, it was observed that the combination of the following factors: method of controlling glycemia, daily insulin dose, hypoglycemia, hyperglycemia, CE calculation and infections in interaction with gender explains over 12% of HRQOL variability (F(12,162)=1.974, P=0.030, R-squared=0.128).
In the first model of regression analysis, significantly lower HRQOL was reported in patients with unsatisfactory metabolic control (HbA1c≥7.5%) compared to those persons whose glycated hemoglobin values were normal (64.2 [SE 1.3] vs 68.6 [SE 1.4]; P=0.026). Moreover, significantly lower HRQOL was noted in patients with exceeded values of WHtR than in those with normal WHtR (60.5 [SE 2.3] vs 66.5 [SE 1.1]; P=0.034). However, no significant correlations were observed between HRQOL and the age of patients, duration of the disease, place of residence or BMI value (Table 3).
Table 3.
Regression analysis of the relationship between total PedsQL score and patient characteristics
| Explanatory variable | Girls (n) | Boys (n) | All (n) | Adjusted means (SE) | Estimate (95% CI) | P-value |
|---|---|---|---|---|---|---|
| HbA1c (%) | ||||||
| <7.5 (ref.) | 46 | 38 | 84 | 68.6 (1.4) | 0.0 | |
| ≥7.5 | 65 | 48 | 113 | 64.2 (1.3) | −4.4 (−8.2, −0.5) | 0.026 |
| Age (years) | ||||||
| 8–12 (ref.) | 30 | 22 | 52 | 65.3 (2.0) | 0.0 | |
| 13–18 | 81 | 64 | 145 | 66.4 (1.1) | 1.1 (−3.3, 5.5) | 0.619 |
| Diabetes duration (years) | ||||||
| <5 (ref.) | 54 | 36 | 90 | 66.4 (1.4) | 0.0 | |
| ≥5 | 57 | 50 | 107 | 65.9 (1.3) | 1.1 (−3.3, 5.5) | 0.781 |
| Place of residence | ||||||
| Village (ref.) | 20 | 29 | 49 | 68.3 (1.8) | 0.0 | |
| Town | 43 | 29 | 72 | 64.4 (1.6) | −3.9 (−8.8, 1.0) | 0.152 |
| City | 48 | 28 | 76 | 66.3 (1.6) | −2.0 (−6.9, 2.9) | 0.984 |
| BMI | ||||||
| Underweight/normal (ref.) | 80 | 58 | 138 | 66.6 (1.2) | 0.0 | |
| Overweight | 19 | 21 | 40 | 66.4 (1.9) | −0.2 (−5.1, 4.7) | 0.396 |
| Obese | 12 | 7 | 19 | 61.6 (3.0) | −5.1 (−11.9, 1.7) | 0.141 |
| WHtR | ||||||
| Normal (ref.) | 95 | 70 | 165 | 66.5 (1.1) | 0.0 | |
| Above normal | 14 | 13 | 27 | 60.5 (2.3) | −6.0 (−11.5, −0.5) | 0.034 |
Abbreviations: HbA1c, glycated hemoglobin; WHtR, waist to height ratio; BMI, body mass index; CI, confidence interval; ref., reference; SE, standard error.
In the second model of regression analysis, significantly lower HRQOL was observed in patients who experienced hyperglycemia daily or several times a week compared to those who experienced no episodes or they occurred only several times a month (65.1 [SE 1.1] vs 70.4 [SE 2.1]; P=0.030). No significant correlations were observed between HRQOL in patients and the method of controlling glycemia, the daily dose of insulin, calculating CE, the frequency of hypoglycemia or infections (Table 4).
Table 4.
Regression analysis of the relationship between total PedsQL score and insulin dosage and control of glycemia
| Explanatory variable | Girls (n) | Boys (n) | All (n) | Adjusted means (SE) | Estimate (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Method of controlling glycemia | ||||||
| BGMS and glucometer/BGMS (ref.) | 38 | 23 | 61 | 66.7 (1.8) | 0.0 | |
| Glucometer | 63 | 63 | 136 | 65.9 (1.2) | −0.8 (−5.0, 3.4) | 0.702 |
| Daily insulin dose (U/kg BW/day) | ||||||
| <1 (ref.) | 86 | 58 | 144 | 67.2 (1.1) | 0.0 | |
| ≥1 | 12 | 19 | 31 | 66.3 (2.6) | −0.8 (−5.9, 4.3) | 0.478 |
| Hypoglycemia | ||||||
| No/several times a month (ref.) | 74 | 57 | 131 | 66.8 (1.2) | 0.0 | |
| Several times a week/every day | 37 | 29 | 66 | 64.8 (1.7) | −2.0 (−6.1, 2.1) | 0.341 |
| Hyperglycemia | ||||||
| No/several times a month (ref.) | 21 | 18 | 39 | 70.4 (2.1) | 0.0 | |
| Several times a week/every day | 90 | 68 | 158 | 65.1 (1.1) | −5.3 (−10.1, −0.5) | 0.030 |
| CE calculation | ||||||
| Yes (ref.) | 97 | 77 | 174 | 66.4 (1.1) | 0.0 | |
| No | 14 | 9 | 23 | 63.7 (2.3) | −2.7 (−8.7, 3.3) | 0.379 |
| Infections | ||||||
| No (ref.) | 68 | 55 | 123 | 66.6 (1.2) | 0.0 | |
| Yes | 43 | 31 | 74 | 65.3 (1.6) | −1.3 (−5.4, 2.6) | 0.495 |
Abbreviations: ref., reference; SE, standard error; CE, carbohydrate exchanges; BGMS, blood glucose monitoring system; CI, confidence interval.
The analysis of the correlation between gender and those variables which significantly influenced the HRQOL of assessed patients showed that HRQOL in boys was higher than in girls regardless of the level of metabolic control. Furthermore, as regards the group of boys with normal and abnormal WHtR, HRQOL was higher than in girls. It was also found that HRQOL was higher in boys than in girls regardless of the frequency of hyperglycemic episodes (Table 5).
Table 5.
Relationship between gender and variables which significantly influence HRQOL
| Variable | Total PedsQL scorea | |
|---|---|---|
| Boys (n=86) | Girls (n=111) | |
| HbA1c (%) | ||
| <7.5 | 71.9 | 65.9 |
| ≥7.5 | 70.0 | 60.0 |
| WHtR | ||
| Normal | 71.2 | 63.0 |
| Above | 65.7 | 55.8 |
| Hyperglycemia | ||
| No/Several times a month | 74.5 | 66.8 |
| Several times a week/every day | 69.9 | 61.4 |
Note: aWeighted mean.
Abbreviations: HbA1c, glycated hemoglobin; WHtR, waist to height ratio.
Discussion
The analysis of scores obtained in PedsQL questionnaire indicated unsatisfactory HRQOL in the study group of children and adolescents. The mean score obtained by all the patients was 66.1±13.69 (maximum value of 100). As a comparison, Abdul-Rasoul et al conducted a study in 436 patients aged 2–18 with the history of type 1 diabetes longer than 6 months and their parents. The authors also assessed HRQOL with PedsQL questionnaire. The mean score obtained in PedsQL Diabetes Module by children and adolescents aged 5–18 was 70.2±9.8.3 Considerably better results were reported by Sand et al who conducted a study in 130 families of children and adolescents with type 1 diabetes. The mean score obtained in PedsQL Diabetes Module by a group of 108 patients aged 5–18 was 73.8±12.5.11
After dividing the study group by gender we noted significant differences in scores obtained by boys and girls. The total scores obtained by boys were significantly higher (70.8±11.91 vs 62.4±13.91; P<0.001). A similar correlation was reported in a cross-sectional study conducted in a group of 131 patients aged 8–18 with type 1 diabetes. The mean score obtained by boys was also significantly higher compared to girls (74.0±12.0 vs 67.0±14.0; P=0.005).7 Moreover, we observed that boys had markedly higher scores than girls in almost all subscales of the questionnaire: “Diabetes symptoms” (65.8±12.85 vs 59.1±14.12; P=0.001), “Treatment barriers” (72.0±19.76 vs 57.5±20.44; P<0.001), “Treatment adherence” (78.9±16.23 vs 68.6±20.06; P<0.001) and “Communication” (78.3±21.44 vs 67.6±24.89; P=0.002). Similar results were reported in a previously mentioned study by Sand et al in which boys obtained higher scores in all 5 subscales of the questionnaire. However, only in case of “Treatment adherence” subscale, the difference between scores was statistically significant (84.9±13.3 vs 79.0±16.5; P<0.05).11
The discrepancies between the results of boys and girls may be due to different attitude and behavior associated with type 1 diabetes. Seemingly, girls have a greater tendency toward hiding their problems and blaming themselves.19 Moreover, they demand more of themselves than boys at the same age which may negatively influence HRQOL.20 As a comparison, teenage boys most commonly present two extreme types of approach to the disease with the majority coping well with the situation.19 It is corroborated by the results of the analysis of data of 4239 adolescents and young adults with type 1 diabetes with a markedly poorer metabolic control in girls and more common complications in women.2 Notably, adult men with diabetes were more positive and experienced more marked satisfaction in coping with the disease compared to women.19
Based on regression analysis, we observed significantly lower HRQOL in patients with the concentrations of HbA1c≥7.5% compared to individuals with normal metabolic control (64.2 [SE 1.3] vs 68.6 [SE 1.4]; P=0.026). A similar correlation was noted in The Global TEENs Study conducted in 5887 type 1 diabetics aged 8–25 coming from 20 different countries on 5 continents. The highest HRQOL was reported in patients with the concentrations of HbA1c<7.5% (71.5 [SE 0.4]). The lowest HRQOL was observed in individuals with HbA1c≥9.0% (64.8 [SE 0.4]).10 Other authors also documented HRQOL in children and adolescents with type 1 diabetes which was decreasing with an increase of glycated hemoglobin value.1,3,4,7,9 Furthermore, it is highly possible that this correlation is bidirectional.10
Significantly lower HRQOL was also noted in persons whose WHtR value exceeded the reference ranges compared to patients with normal WHtR (60.5 [SE 2.3] vs 66.5 [SE 1.1]; P=0.034). Currently, no studies are available concerning the above correlation in children with type 1 diabetes. However, a cross-sectional study was conducted by Kesztyüs et al in parents of 1888 healthy first- and second-grade children. The authors noted HRQOL reduction in children with central obesity confirmed based on WHtR value.21 Seemingly, this may result from the stigmatization of overweight children by peers which leads to negative emotional and social consequences.21 Notably, no significant discrepancies were observed as regards HRQOL between patients with normal body weight/being underweight, being overweight and obese assessed based on BMI.
Significantly lower HRQOL was observed in patients who experienced hyperglycemia daily or several times a week compared to those who experienced no episodes or if the episodes occurred only several times a month (65.1 [SE 1.1] vs 70.4 [SE 2.1]; P=0.030). It seems understandable because of negative consequences of elevated glycemia. Arif and Roher conducted a study in parents and caregivers of 5530 children and adolescents aged 3–18. The respondents declared HRQOL reduction in case of the occurrence of at least one episode of hyperglycemia.22 Moreover, the Global TEENs Study showed significantly lower HRQOL in patients who experienced an episode of ketoacidosis compared to persons who experienced no such episode (64.0 [SE 0.9] vs 67.3 [SE 0.6]; P<0.001).10 Similar results were also observed in other studies conducted in children and adolescents with type 1 diabetes.1,9
Contrary to the above-described results concerning hyperglycemia, we observed no significant correlation between HRQOL and the frequency of hypoglycemia. Notably, a cross-sectional study conducted in a group of 325 patients aged 2–18 with type 1 diabetes and their parents showed significantly lower HRQOL in a group of children and adolescents aged 8–18 who were the most concerned about the occurrence of hypoglycemia. However, even a history of a severe episode of hypoglycemia was not associated with a significant HRQOL reduction in the same group of patients.23 Furthermore, we did not observe a significant correlation between HRQOL and patient age. Seemingly, this may be due to a marked disproportion between age groups of study participants. It is worth emphasizing that HRQOL reduction with age in children and adolescents with type 1 diabetes was well documented in numerous studies.4,10,11,24
Eventually, it was observed that HRQOL was higher in boys than in girls regardless of metabolic control, WHtR value and the frequency of hyperglycemia episodes. It indicates a strong correlation between gender and HRQOL. It is also confirmed by the results of an analysis conducted by Michel et al in 21590 healthy children and adolescents aged 8–18 from 12 European countries. It was reported that starting from the age of 12 the HRQOL in girls was becoming markedly lower compared to boys.25 It may be associated with puberty and hormonal disturbances and individual skills in coping with stressful situations.25 It may, at least partially, explain poorer HRQOL in girls compared to boys with type 1 diabetes.
It should be emphasized that all factors included in the first (HbA1c, age, diabetes duration, place of residence, BMI and WHtR) and second (method of controlling glycemia, daily insulin dose, hypoglycemia, hyperglycemia, CE calculation and infections) regression model in interaction with gender had a relatively small impact on HRQOL: 15% (F(17,174)=1.874, P=0.023, R-squared=0.155) and 12% (F(12,162)=1.974, P=0.030, R-squared=0.128). This indicates that a significant part of HRQOL variability was also affected by other factors. It seems that they can be related to both the patient’s lifestyle (eg, physical activity) and the patient’s family (eg, the level of education of parents or guardians).10
The present results are not free from limitations. One of them is the paucity of data concerning other factors with a potential influence on HRQOL, associated with patients’ lifestyle or the characteristics of their families. The disproportion between age groups of study participants and the patients’ declarations concerning the method of controlling glycemia, calculating CE and the frequency of hypo- and hyperglycemia episodes are also a kind of limitation when interpreting the results.
Conclusion
All in all, the obtained results indicate that the HRQOL scores of the study group including children and adolescents with type 1 diabetes are unsatisfactory. The factors with a markedly negative influence on HRQOL include higher HbA1c concentrations, WHtR value above the reference ranges and frequent episodes of hyperglycemia. Based on the above results it may be concluded that being a female is an independent factor which determines HRQOL deterioration. However, it should be emphasized that the combined impact of the examined factors on HRQOL was relatively small. Therefore, it seems necessary to focus also on other factors that may potentially influence the HRQOL of patients with type 1 diabetes.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Frøisland DH, Graue M, Markestad T, Skrivarhaug T, Wentzel-Larsen T, Dahl-Jørgensen K. Health-related quality of life among Norwegian children and adolescents with type 1 diabetes on intensive insulin treatment: a population-based study. Acta Paediatr. 2013;102(9):889–895. doi: 10.1111/apa.12312 [DOI] [PubMed] [Google Scholar]
- 2.Samuelsson U, Anderzén J, Gudbjörnsdottir S, Steineck I, Åkesson K, Hanberger L. Teenage girls with type 1 diabetes have poorer metabolic control than boys and face more complications in early adulthood. J Diabetes Complications. 2016;30(5):917–922. doi: 10.1016/j.jdiacomp.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 3.Abdul-Rasoul M, AlOtaibi F, Abdulla A, Rahme Z, AlShawaf F. Quality of life of children and adolescents with type 1 diabetes in Kuwait. Med Princ Pract. 2013;22(4):379–384. doi: 10.1159/000347052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murillo M, Bel J, Pérez J, et al. Health-related quality of life (HRQOL) and its associated factors in children with type 1 diabetes mellitus (T1DM). BMC Pediatr. 2017;17(1):16. doi: 10.1186/s12887-017-0969-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faulkner MS. Quality of life for adolescents with type 1 diabetes: parental and youth perspectives. Pediatr Nurs. 2003;29(5):362–368. [PubMed] [Google Scholar]
- 6.Grey M. Quality of life in youth with type 1 diabetes. J Pediatr. 2012;161(2):180–181. doi: 10.1016/j.jpeds.2012.02.050 [DOI] [PubMed] [Google Scholar]
- 7.Petersson C, Huus K, Enskär K, Hanberger L, Samulesson U, Åkesson K. Impact of type 1 diabetes on health-related quality of life among 8–18-year-old children. Compr Child Adolesc Nurs. 2016;39(4):245–255. doi: 10.1080/24694193.2016.1196265 [DOI] [Google Scholar]
- 8.Hoey H, Aanstoot HJ, Chiarelli F, et al. Good metabolic control is associated with better quality of life in 2,101 adolescents with type 1 diabetes. Diabetes Care. 2001;24(11):1923–1928. doi: 10.2337/diacare.24.11.1923 [DOI] [PubMed] [Google Scholar]
- 9.Lawrence JM, Yi-Frazier JP, Black MH, et al. Demographic and clinical correlates of diabetes-related quality of life among youth with type 1 diabetes. J Pediatr. 2012;161(2):201–207. doi: 10.1016/j.jpeds.2012.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson BJ, Laffel LM, Domenger C, et al. Factors associated with diabetes-specific health-related quality of life in youth with type 1 diabetes: the Global TEENs Study. Diabetes Care. 2017;40(8):1002–1009. doi: 10.2337/dc16-1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sand P, Kljajić M, Schaller J, Forsander G. The reliability of the health related quality of life questionnaire PedsQL 3.0 Diabetes ModuleTM for Swedish children with type 1 diabetes. Acta Paediatr. 2012;101(8):344–349. doi: 10.1111/j.1651-2227.2012.02632.x [DOI] [PubMed] [Google Scholar]
- 12.Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL™ in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory™ generic core scales and type 1 diabetes module. Diabetes Care. 2003;26(3):631–637. doi: 10.2337/diacare.26.3.631 [DOI] [PubMed] [Google Scholar]
- 13.de Wit M. Delemarre-van de Waal HA, Pouwer F, RJBJ Gemke, FJ Snoek. Monitoring health related quality of life in adolescents with diabetes: a review of measures. Arch Dis Child. 2007;92(5):434–439. doi: 10.1136/adc.2006.102236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nansel TR, Weisberg-Benchell J, Wysocki T, Laffel L, Anderson B. Quality of life in children with type 1 diabetes: a comparison of general and diabetes-specific measures and support for a unitary diabetes quality-of-life construct. Diabet Med. 2008;25(11):1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rewers MJ, Pillay K, de Beaufort C, et al. ISPAD Clinical Practice Consensus Guidelines 2014 Compedium. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes. 2014;15(Suppl 20):102–114. doi: 10.1111/pedi.12103 [DOI] [PubMed] [Google Scholar]
- 16.Kułaga Z, Różdżyńska-Świątkowska A, Grajda A, et al. Siatki centylowe dla oceny wzrastania i stanu odżywienia polskich dzieci i młodzieży od urodzenia do 18 roku życia [Percentile charts for growth and nutritional status assessment in Polish children and adolescents from birth to 18 year of age] Standardy medyczne/Pediatria. 2015;12:119–135. Polish. [Google Scholar]
- 17.Ashwell M, Hsieh SD. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. 2005;56(5):303–307. doi: 10.1080/09637480500195066 [DOI] [PubMed] [Google Scholar]
- 18.Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141(1):2–18. doi: 10.1037/a0024338 [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui MA, Khan MF, Carline TE. Gender differences in living with diabetes mellitus. Mater Sociomed. 2013;25(2):140–142. doi: 10.5455/msm.2013.25.140-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiebe DJ, Berg CA, Korbel C, et al. Children’s appraisals of maternal involvement in coping with diabetes: enhancing our understanding of adherence, metabolic control, and quality of life across adolescence. J Pediatr Psychol. 2005;30(2):167–178. doi: 10.1093/jpepsy/jsi004 [DOI] [PubMed] [Google Scholar]
- 21.Kesztyüs D, Wirt T, Kobel S, et al. Is central obesity associated with poorer health and health-related quality of life in primary school children? Cross-sectional results from the Baden-Württemberg study. BMC Public Health. 2013;13:260. doi: 10.1186/1471-2458-13-260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arif AA, Rohrer JE. The relationship between obesity, hyperglycemia symptoms, and health-related quality of life among Hispanic and non-Hispanic white children and adolescents. BMC Fam Pract. 2006;7:3. doi: 10.1186/1471-2296-7-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson SR, Cooper MN, Davis EA, Jones TW. Hypoglycaemia, fear of hypoglycaemia and quality of life in children with type 1 diabetes and their parents. Diabet Med. 2013;30(9):1126–1131. doi: 10.1111/dme.12247 [DOI] [PubMed] [Google Scholar]
- 24.Wagner VM, Müller-Godeffroy E, von Sengbusch S, Häger S, Thyen U. Age, metabolic control and type of insulin regime influences health-related quality of life in children and adolescents with type 1 diabetes mellitus. Eur J Pediatr. 2005;164(8):491–496. doi: 10.1007/s00431-005-1681-4 [DOI] [PubMed] [Google Scholar]
- 25.Michel G, Bisegger C, Fuhr DC, Abel T; The KIDSCREEN group. Age and gender differences in health-related quality of life of children and adolescents in Europe: a multilevel analysis. Qual Life Res. 2009;18(9):1147–1157. doi: 10.1007/s11136-009-9478-y [DOI] [PubMed] [Google Scholar]