Abstract
We decreased antimicrobial drug consumption in an intensive care unit in Lebanon by changing to colistin monotherapy for extensively drug-resistant Acinetobacter baumanii infections. We saw a 78% decrease of A. baumanii in sputum and near-elimination of blaoxa-23-carrying sequence type 2 clone over the 1-year study. Non–A. baumanii multidrug-resistant infections remained stable.
Keywords: XDR, MDR, Acinetobacter baumanii, carbapenem, antimicrobial stewardship, antimicrobial resistance, bacteria, Lebanon, France
The antimicrobial stewardship program (ASP) at Saint Georges Hospital University Medical Center (SGHUMC), a 400-bed tertiary-care center in Beirut, Lebanon, requires an infectious disease (ID) specialist to preauthorize use of restricted broad-spectrum antimicrobial drugs. The ASP regularly monitors the rate of nosocomial infections and the total hospital antimicrobial drug consumption. In the first quarter of 2015, the incidence of extensively drug-resistant (XDR) Acinetobacter baumanii bloodstream infections reached its highest level, 0.47/1,000 patient-days (1). Since 2012, the monthly carbapenem consumption increased steadily, reaching 130 defined daily doses (DDD)/1,000 patient-days in 2015, an absolute increase of 30 DDD/1,000 patient-days during that time. Severely ill patients with predisposing conditions are more likely to develop difficult-to-treat A. baumanii infections. Despite the existing controversy, this patient population routinely is treated with a carbapenem/colistin combination (2–8).
We evaluated 100 nonduplicate XDR A. baumanii isolates at SGHUMC and found no synergy between colistin and carbapenem by the checkerboard technique (9). Consequently, SGHUMC withdrew combination therapy for XDR A. baumanii infections. Our aim was to evaluate the effect of a carbapenem-sparing regimen on ICU antimicrobial consumption, clinical outcome, and microbiological flora.
The Study
The ASP, ID team, and intensive care unit (ICU) physicians approved a plan to reduce use of empiric carbapenems in the ICU and use colistin, tigecycline, or both for patients confirmed with or at high risk for A. baumanii infections. ID physicians evaluated the clinical severity and hemodynamic stability of each patient and had final discretion to prescribe either colistin or tigecycline.
We included all ICU admissions in the study, even recurrent admissions. This ICU has a multidrug-resistant organism surveillance program that collects a sputum sample for culture every third day for intubated patients with abundant secretions. We used these cultures for our evaluation. We considered any culture sample outside this practice a duplicate and excluded it from our analysis. During the study period, we did not modify infection control practices. The study was approved by the institutional review board of SGHUMC.
We retrieved data from the hospital’s computerized ordering system and examined medical records of all ICU admissions during February 1, 2016–January 31, 2017. Clinical data included patient demographics, admission diagnosis, and presence of mechanical ventilation. During February 1–June 30, 2016 (period 1), patients received colistin/carbapenem combination therapy for A. baumanii infections. During July 1, 2016–January 31, 2017 (period 2), we applied our intervention. We recorded the total number of bacterial cultures collected from the ICU and noted the site and date of sampling.
We considered the isolation density the number of clinical isolates/1,000 patient-days and the rate of ventilator-associated pneumonia (VAP) the number of VAP events/1,000 patient-days. We defined variables according to guidelines for XDR A. baumanii from the US Centers for Disease Control and Prevention and World Health Organization (10). We calculated case-fatality and VAP rates following guidelines from the American Thoracic Society and Infectious Diseases Society of America (11).
We grouped antimicrobial drugs into 5 categories: group 1, antimicrobial drugs that do not require ID preapproval, such as third-generation cephalosporins, amoxicillin/clavulanic acid, and quinolones; group 2, oral vancomycin and metronidazole used for Clostridioides difficile therapy; group 3, imipenem and meropenem; group 4, broad-spectrum carbapenem-sparing regimens, including piperacillin/tazobactam, cefepime, ceftazidime, amikacin; and group 5, the XDR A. baumanii–active antimicrobial drugs colistin and tigecycline. We measured antimicrobial drug consumption by DDD per 1,000 patient-days (Table 1).
Table 1. Patient demographics, VAP incidence, treatment courses, and antimicrobial drug consumption in study of carbapenem-sparing regimen for XDR Acinetobacter baumannii in an ICU, Beirut, Lebanon* .
| Characteristics | Period 1† | Period 2‡ | p value |
|
|---|---|---|---|---|
| Patient data | ||||
| No. patients | 213 | 324 | NA |
|
| Sex | ||||
| F | 79 | 144 | ||
| M | 134 | 180 | ||
| Mean age | 69 | 68 | ||
| Mean length of hospital stay, d | 6.8 | 6 | ||
| Days in ICU |
1,128 |
1,804 |
||
| Type of admission | ||||
| Medical | 163 | 253 | NA | |
| Surgical | 50 | 71 | ||
| Admitted from home or ED | 73 | 114 | ||
| Transferred from ward | 79 | 141 |
|
|
| Transferred from other hospital | 10 | 16 | ||
| Postoperative |
51 |
57 |
||
| Intubation |
|
|||
| At admission | 64 | 85 | ||
| After admission |
14 |
16 |
||
| Outcome | ||||
| A. baumanii VAP incidence, % | 15 | 3.7 | 0.007 | |
| Discharged | 170 | 259 | ||
| Deceased | 43 | 64 | ||
| Total AB VAP events | 32 | 12 | ||
| Deceased during VAP |
17 |
4 |
|
|
| ICU mean mortality rate/month, % | 20.4 | 19.3 | 0.168 | |
| AB VAP case fatality ratio, % |
7.9 |
1.2 |
0.006 |
|
| No. XDR A. baumanii VAP courses received | ||||
| Colistin and carbapenem | 17 | 2 | ||
| Colistin and tigecycline | 6 | 2 | ||
| Colistin monotherapy | 6 | 6 | ||
| Tigecycline |
3 |
2 |
|
|
| Carbapenem consumption, DDD§ | ||||
| Group 1 | 333 | 320 | 0.465 | |
| Group 2 | 455 | 224 | 0.042 | |
| Group 3 | 541 | 223 | <0.005 | |
| Group 4 | 165 | 145 | 0.808 | |
| Group 5 | ||||
| Colistin | 20 | 9 | <0.019 | |
| Tigecycline |
84 |
62 |
0.570 |
|
| Total restricted antimicrobial drugs, DDD | 1,265 | 663 |
<0.005
|
|
*Bold indicates statistical significance. DDD, defined daily doses; ED, emergency department, ESBL, extended-spectrum β-lactamase; ICU, intensive care unit; NA, not applicable; VAP, ventilator-associated pneumonia; XDR, extensively drug-resistant. †During February 1, 2016–June 30, 2016, ICU patients received colistin/carbapenem therapy for A. baumannii infections. ‡During July 1, 2016–January 31, 2017, ICU implemented carbapenem-sparing regimen for A. baumannii infections. §Group 1, antimicrobial drugs that do not require infectious disease specialist preapproval, such as third-generation cephalosporins, amoxicillin/clavulanic acid, and quinolones; group 2, oral vancomycin and metronidazole used for Clostridioides difficile therapy; group 3, imipenem and meropenem; group 4, broad-spectrum carbapenem-sparing regimens, including piperacillin/tazobactam, cefepime, ceftazidime, amikacin; and group 5, the XDR A. baumanii–active antimicrobial drugs colistin and tigecycline.
We sent 48 laboratory-confirmed A. baumanii isolates, 31 collected during period 1 and 17 during period 2, to IHU-Méditerranée Infection, Aix-Marseille, France, for testing. Samples underwent 4 types of testing: matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Microflex; Bruker Daltonics, https://www.bruker.com); antimicrobial susceptibility testing by disk diffusion method and interpreted according to the European Committee of Antimicrobial Susceptibility Testing 2017; real-time PCR to screen for carbapenemase-encoding genes; and multilocus sequence typing to determine genetic relationships among the isolates.
The ICU admitted 536 patients during the study period; 3 were readmissions. Patient characteristics between the 2 periods were statistically similar (Table 1). Throughout the study, the incidence of A. baumanii VAP decreased from 154.9 to 38/1,000 patient-days (p = 0.007) and the A. baumanii VAP case-fatality ratio dropped from 79 to 12/1,000 patient-days. Non–A. baumanii VAP incidence decreased from 62 to 51/1,000 patient-days. ICU mortality rates from all causes remained unchanged between period 1 and period 2 (Table 1).
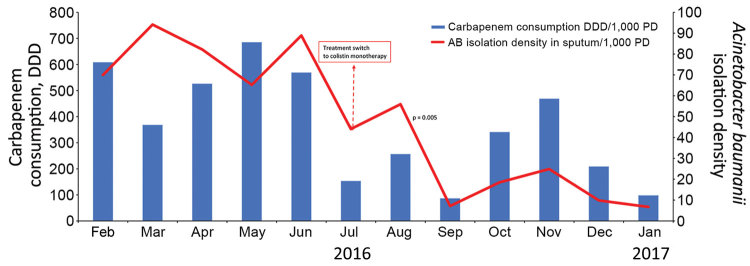
Consumption of group 1 and group 4 antimicrobial drugs was statistically similar during the 2 periods (Table 1). Carbapenem consumption decreased by 59%, a total of 318 DDD/1,000 patient-days, and overall restricted antimicrobial drug consumption dropped 637 DDD/1,000 patient-days (p<0.005). Because isolation of A. baumanii decreased substantially, colistin consumption also decreased by 55%, from 20 DDD/1,000 patient-days in period 1 to 9 DDD/1,000 patient-days in period 2 (p = 0.019) (Figure 1). Tigecycline consumption remained statistically unchanged (84 DDD/1,000 patient-days in period 1, 62 DDD/1,000 patient-days in period 2). Of note, group 2 C. difficile therapy consumption dropped by 231 DDD/1,000 patient-days (p = 0.042), a 51% decrease that likely mirrors reduction in C. difficile infections.
Figure 1.
Isolation density of Acinetobacter baumanii in sputum cultures versus carbapenem consumption in the intensive care unit (ICU) of Saint Georges Hospital University Medical Center, Beirut, Lebanon, during February 1, 2016–January 31, 2017. Rates are measured per 1,000 patient-days. Dashed arrow represents the beginning of period 2 in which we implemented a carbapenem-sparing regimen. DDD, defined daily dose; PD, patient days.
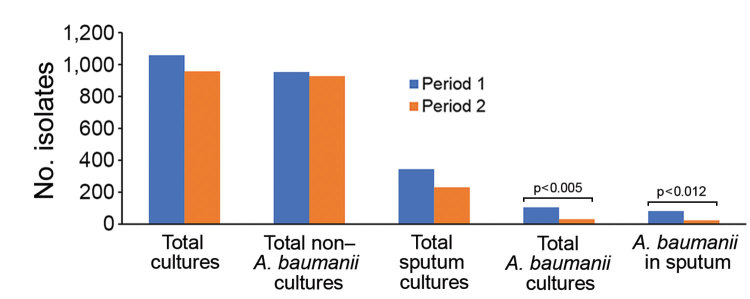
The A. baumanii isolate density in sputum cultures decreased by 70.7%, from 82 to 24/1,000 patient-days, positively correlating with the fall in carbapenem consumption (p = 0.004) (Figure 1). The number of non–A. baumanii multidrug-resistant (MDR) isolates did not increase (Figure 2).
Figure 2.
Isolation density of Acinetobacter baumanii and non–A. baumanii in the intensive care unit (ICU) of Saint Georges Hospital University Medical Center, Beirut, Lebanon, during February 1, 2016–January 31, 2017. Rates are measured in 1,000 patient-days. During period 1, February 1–June 31, 2016, ICU patients received colistin/carbapenem combination therapy for A. baumanii. During period 2, July 1, 2016–January 31, 2017, we implemented a carbapenem-sparing regimen in the ICU.
All 48 A. baumanii isolates carried extended-spectrum β-lactamase blaTEM-1 genes. The 31 isolates from period 1 were XDR; 30 carried the class D carbapenemase blaoxa-23 gene, and 1 carried the blaoxa-24 gene. Multilocus sequence typing revealed 3 sequence types (STs) in period 1: ST2, 29/31 (93.5%); ST699, 1/31 (3%); and ST627, 1/31 (3%) (Table 2). In period 2, A. baumanii ST2 disappeared; 58.8% (10/17) of isolates belonged to ST25 and 5.9% (1/17) belonged to ST99. The remainder belonged to 6 new STs, assigned ST1200, 1201, 1202, 1203, 1204, and 1205 (35.2%). Of the 17 isolates from period 2, 6 carried the blaoxa-23 gene, 5 the blaoxa-24 gene, and 3 both genes.
Table 2. Specimen type, site of collection, and microbiologic characteristics in study of carbapenem-sparing regimen for extensively drug-resistant Acinetobacter baumannii in an ICU, Beirut, Lebanon* .
| Specimens and testing |
Period 1† |
Period 2‡ |
|---|---|---|
| Specimen type, no. | ||
| Sputum | 31 | 11 |
| Blood | 0 | 3 |
| Wound site or catheter tip |
0 |
3 |
| Site of collection, no. | ||
| Intensive care unit | 21 | 12 |
| Regular floor | 10 | 5 |
| Total no. | 31 | 17 |
| Antimicrobial drug susceptibility testing by disc diffusion, % | ||
|---|---|---|
| Cefepime/ceftazidime | 0 | 64.7 |
| Piperacillin/tazobactam | 0 | 17.65 |
| Imipenem | 0 | 17.65 |
| Colistin |
100 |
100 |
| Total extensively drug-resistant | 100 | 35.3 |
| Carbapenemase genes, no. (%)§ | ||
|---|---|---|
| ESBL blaTEM-1 | 31 (100) | 17 (100) |
| blaoxa-23 | 30 (96.8) | 6 (35.3) |
| blaoxa-24 | 1 (3.2) | 5 (29.4) |
| blaoxa-23 and blaoxa-24 | 0 | 3 (17.6) |
| Sequence type, no. (%)¶ | ||
|---|---|---|
| ST2 | 29 (93.5) | 0 |
| ST699 | 1 (3.25) | 0 |
| ST627 | 1 (3.25) | 0 |
| ST25 | 0 | 10 (58.9) |
| ST99 | 0 | 1 (5.8) |
| New STs, 1200–1206 | 0 | 6 (35.3) |
*Bold indicates statistical significance. ESBL, extended-spectrum beta-lactamase; ICU, intensive care unit; MLST, multilocus sequence typing; PCR, polymerase chain reaction; ST, sequence type. †During February 1, 2016–June 30, 2016, ICU patients received colistin/carbapenem therapy for A. baumannii infections. ‡During July 1, 2016–January 31, 2017, ICU implemented carbapenem-sparing regimen for A. baumannii infections. §Determined by PCR. ¶Determined by multilocus sequence typing.
Overall, XDR A. baumanii isolation decreased by 64.7% from period 1 to period 2. In addition, isolates from period 2 were more antimicrobial-susceptible than in period 1: 64.8% (11/17) sensitive to ceftazidime and cefepime, 17.6% (3/17) to piperacillin/tazobactam, and 17.6% (3/17) to carbapenems (Table 2).
Conclusions
Our prudent use of antimicrobial drugs did not increase mortality rates and had a dramatic effect on antimicrobial consumption and MDR A. baumanii isolate density. A longer study period and larger sample likely would reveal additional effects on XDR infections and outcomes. Many factors could have affected the study results, including patient referrals and seasonality. However, the microbiological findings strongly point to high rates of carbapenem consumption as a sustaining factor in survival of XDR A. baumanii ST2 in our facility. By reducing carbapenem consumption, we broke a vicious cycle.
In the era where clinicians must manage severely ill, MDR-colonized patients, relying on existing guidelines is not enough. A creative, multidisciplinary approach with knowledge of local epidemiology is key to controlling MDR and XDR infections. Investing time in accurate diagnosis and implementing targeted carbapenem-sparing strategies for initial treatment is only possible through trusted collaboration between ID and ICU physicians. The dedication of the ASP and microbiology departments at this facility is an example of a successful active surveillance program for antimicrobial drug consumption and resistance profiles, especially when developing standards of care tailored to meet an institution’s needs.
Acknowledgments
We thank our partners and microbiologists at Méditerranée Infection in Aix-Marseille, France, for conducting antimicrobial susceptibility testing and molecular methods.
Biography
Dr. Chamieh is an infectious disease specialist and graduate of Saint George Hospital University Medical Center, Beirut, Lebanon, where she is an active member of the Antimicrobial Stewardship and the Infection Control Departments. She is currently pursuing a PhD at Aix-Marseille University, Méditerranée Infection, Marseille, France, in microbiology, with a research focus on antimicrobial resistance mechanisms and surveillance of multidrug resistance.
Footnotes
Suggested citation for this article: Chamieh A, Nawfal TD, Ballouz T, Afif C, Juvelekian G, Hlais S, et al. Control and elimination of extensively drug-resistant Acinetobacter baumanii in an intensive care unit. Emerg Infect Dis. 2019 Sep [date cited]. https://doi.org/10.3201/eid2509.181626
These authors contributed equally to this article.
References
- 1.Ballouz T, Aridi J, Afif C, Irani J, Lakis C, Nasreddine R, et al. Risk factors, clinical presentation, and outcome of Acinetobacter baumannii bacteremia. Front Cell Infect Microbiol. 2017;7:156. 10.3389/fcimb.2017.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falagas ME, Rafailidis PI, Ioannidou E, Alexiou VG, Matthaiou DK, Karageorgopoulos DE, et al. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents. 2010;35:194–9. 10.1016/j.ijantimicag.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 3.Batirel A, Balkan II, Karabay O, Agalar C, Akalin S, Alici O, et al. Comparison of colistin-carbapenem, colistin-sulbactam, and colistin plus other antibacterial agents for the treatment of extremely drug-resistant Acinetobacter baumannii bloodstream infections. Eur J Clin Microbiol Infect Dis. 2014;33:1311–22. 10.1007/s10096-014-2070-6 [DOI] [PubMed] [Google Scholar]
- 4.Cai Y, Chai D, Wang R, Liang B, Bai N. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother. 2012;67:1607–15. 10.1093/jac/dks084 [DOI] [PubMed] [Google Scholar]
- 5.Haddad FA, Van Horn K, Carbonaro C, Aguero-Rosenfeld M, Wormser GP. Evaluation of antibiotic combinations against multidrug-resistant Acinetobacter baumannii using the E-test. Eur J Clin Microbiol Infect Dis. 2005;24:577–9. 10.1007/s10096-005-1366-y [DOI] [PubMed] [Google Scholar]
- 6.Rigatto MH, Vieira FJ, Antochevis LC, Behle TF, Lopes NT, Zavascki AP. Polymyxin B in combination with antimicrobials lacking in vitro activity versus polymyxin B in monotherapy in critically ill patients with Acinetobacter baumannii or Pseudomonas aeruginosa infections. Antimicrob Agents Chemother. 2015;59:6575–80. 10.1128/AAC.00494-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tripodi MF, Durante-Mangoni E, Fortunato R, Utili R, Zarrilli R. Comparative activities of colistin, rifampicin, imipenem and sulbactam/ampicillin alone or in combination against epidemic multidrug-resistant Acinetobacter baumannii isolates producing OXA-58 carbapenemases. Int J Antimicrob Agents. 2007;30:537–40. 10.1016/j.ijantimicag.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Zhao M, Chen Y, Bian X, Li Y, Shi J, et al. Synergistic killing by meropenem and colistin combination of carbapenem-resistant Acinetobacter baumannii isolates from Chinese patients in an in vitro pharmacokinetic/pharmacodynamic model. Int J Antimicrob Agents. 2016;48:559–63. 10.1016/j.ijantimicag.2016.07.018 [DOI] [PubMed] [Google Scholar]
- 9.Hajjar Soudeiha M, Dahdouh E, Daoud Z, Sarkis DK. Phenotypic and genotypic detection of β-lactamases in Acinetobacter spp. isolates recovered from Lebanese patients over a 1-year period. J Glob Antimicrob Resist. 2018;12:107–12. 10.1016/j.jgar.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 10.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 11.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–111. 10.1093/cid/ciw353 [DOI] [PMC free article] [PubMed] [Google Scholar]