The development of effective malaria vaccines is hampered by incomplete understanding of the immunological correlates of protective immunity. Recently, the moderate clinical efficacy of the Plasmodium falciparum circumsporozoite protein (CSP)-based RTS,S/AS01E vaccine in phase 3 studies highlighted the urgency to design and test more efficacious next-generation malaria vaccines.
KEYWORDS: antibodies, malaria, protection, vaccine
ABSTRACT
The development of effective malaria vaccines is hampered by incomplete understanding of the immunological correlates of protective immunity. Recently, the moderate clinical efficacy of the Plasmodium falciparum circumsporozoite protein (CSP)-based RTS,S/AS01E vaccine in phase 3 studies highlighted the urgency to design and test more efficacious next-generation malaria vaccines. In this study, we report that immunization with recombinant CSP from Plasmodium yoelii (rPyCSP), when delivered in Montanide ISA 51, induced sterilizing immunity against sporozoite challenge in C57BL/6 and BALB/c strains of mice. This immunity was antibody dependent, as evidenced by the complete loss of immunity in B-cell-knockout (KO) mice and by the ability of immune sera to neutralize sporozoite infectivity in mice. Th2-type isotype IgG1 antibody levels were associated with protective immunity. The fact that immunized gamma interferon (IFN-γ)-KO mice and wild-type (WT) mice have similar levels of protective immunity and the absence of IFN-γ-producing CD4+ and CD8+ T cells in protected mice, as shown by flow cytometry, indicate that the immunity is IFN-γ independent. Protection against sporozoite challenge correlated with higher frequencies of CD4+ T cells that express interleukin-2 (IL-2), IL-4, and tumor necrosis factor alpha (TNF-α). In the RTS,S study, clinical immunity was associated with higher IgG levels and frequencies of IL-2- and TNF-α-producing CD4+ T cells. The other hallmarks of immunity in our study included an increased number of follicular B cells but a loss in follicular T helper cells. These results provide an excellent model system to evaluate the efficacy of novel adjuvants and vaccine dosage and determine the correlates of immunity in the search for superior malaria vaccine candidates.
INTRODUCTION
In the past 8 years, there has been about a 9% decline in malaria cases globally. Although there were an estimated 20 million fewer malaria cases in 2017 than in 2010, data for the period from 2015 to 2017 highlight that no significant progress in reducing global malaria cases was made in this time frame (1). The factors that led to the decline in malaria include insecticide-treated bed nets, vector control, and a policy to diagnose malaria cases and treat then with artemisinin combination therapies; however, the present tools alone are unlikely to eliminate or even control malaria in countries where malaria continues to increase. Therefore, sustainable malaria control and eventual eradication efforts depend on the availability of new tools, including malaria vaccine(s) that would reduce malaria mortality and morbidity and curtail transmission.
Circumsporozoite protein (CSP), a major protein on the sporozoite surface, is also expressed in the early liver stages of the parasite cycle and remains a major malaria vaccine candidate (2, 3). In the past decades, hundreds of millions of dollars have been invested in the development and clinical testing of recombinant vaccines based on Plasmodium falciparum circumsporozoite protein (PfCSP). The most advanced recombinant malaria vaccine, RTS,S/AS01E, consists of a truncated version of PfCSP with 19 NANP repeats and amino acids 207 to 395 from the carboxyl-terminal region of PfCSP of the 3D7 strain of Plasmodium falciparum fused to hepatitis B surface antigen expressed in yeast (4). When delivered in either AS01 or AS02 adjuvant, RTS,S induced partial protection for a short duration in a controlled human malaria infection model (5) and in field efficacy studies (6–8). Recently, the efficacy of RTS,S has been determined in a phase 3 trial in young children and infants in Africa, who are a major target of malaria vaccines. The vaccine’s efficacy over a 1-year follow-up was 56% in children aged 5 to 17 months and 31% in infants aged 6 to 12 weeks at first vaccination (7–9). The immunity waned over time. In a 7-year follow-up substudy, vaccine efficacy dropped from 35.9% in the first year to 3.6% in the seventh year (10). A vaccine of superior efficacy and durability would have a further impact on childhood morbidity and mortality and curtail malaria transmission.
The knowledge of immune mechanisms that confer protection against pre-erythrocytic (sporozoite and liver form)-stage malaria remains incompletely understood. Antibodies are known to render sporozoites noninfectious and prevent their invasion into liver cells in vitro (11) and in experimental challenge studies (12). On the other hand, T cells (CD8+ and CD4+ T cells) and NK T cells have been shown to destroy infected liver cells through cytokine-dependent cellular mechanisms (13, 14). The mechanism by which RTS,S confers protection against clinical malaria has been extensively studied, and yet, a definitive immunological surrogate has yet to be found. Nonetheless, higher antibody levels and polyfunctional CD4+ T cells have been associated with protection in RTS,S-vaccinated individuals (15, 16), and protection from infection and clinical disease is associated with CSP-specific antibodies and CSP-specific CD4+ T cells in naive adult challenge studies (5). Furthermore, White et al. demonstrated that the RTS,S vaccine acts through the induction of high levels of both anti-CSP antibodies and CSP-specific CD4+ T cells, with the antibody response having a greater role (17). An anti-CSP antibody titer of 121 endotoxin units/ml was estimated to prevent about 50% of infections; however, antibody levels wane rapidly, as does the duration of immunity in individuals of all age groups (9).
Depending upon the immunogen and delivery platform, both antibodies and T cells have been shown to mediate immunity against pre-erythrocytic-stage malaria parasites in murine models. Anti-CSP antibodies have been shown to inhibit parasite invasion and are also associated with a reduced risk of clinical malaria (15, 16). It has also been shown that protection from infection and clinical disease is associated with CSP-specific antibodies and CSP-specific CD4+ T cells in naive-adult challenge studies (5). Nonetheless, in spite of decades of efforts, the precise mechanism of CSP-induced immunity against sporozoite challenge remains incompletely defined.
Given the modest efficacy and durability of the RTS,S vaccine, it is imperative that the next-generation malaria vaccines be designed to induce long-lasting immunity in all age groups and undergo clinical testing in areas of endemicity. Designing a superior vaccine would benefit from a better understanding of the mediators of protective immune responses and more potent immunogen-adjuvant formulations that induce higher and more durable protective immune responses.
Here, we report on a recombinant Plasmodium yoelii CSP (rPyCSP)/mouse sporozoite challenge model to define the nature of protective immune responses induced in mice following immunizations with rPyCSP expressed in Saccharomyces cerevisiae and delivered in CpG oligodeoxynucleotide (ODN) plus Montanide ISA 51. We found that the rPyCSP-CpG ODN-ISA51 formulation induced sterilizing immunity in BALB/c and C57BL/6 mice. The results from antibody transfer studies and immunization/challenge in C57BL/6 mice with genetic deletions in B cells, CD4+ T cells, CD8+ T cells, or gamma interferon (IFN-γ) markers indicate that protective immunity is primarily antibody dependent and IFN-γ independent. The rPyCSP-sporozoite challenge model offers a facile approach to finely dissect antigen-to-adjuvant dose relationships and immune correlates of immunity against pre-erythrocytic-stage malaria vaccines.
RESULTS
Recombinant expression of rPyCSP.
Recombinant P. yoelii CSP (rPyCSP) comprising 350 amino acids (P20 to S369) of the full-length molecule was expressed as a secreted His6-tagged protein in S. cerevisiae. The structural integrity of rPyCSP was confirmed by mass spectroscopy and the amino-terminal sequence determination by Edman degradation (data not shown). Antigenic characterization of rPyCSP was determined by migration of the protein band on 4 to 12% PAGE and in enhanced chemiluminescence (ECL)-Western blotting based on its reactivity with the anti-CSP monoclonal antibody (MAb) NYS1. Based on the recombinant PyCSP domain used in the construct, the recombinant plasmid should encode a protein of ∼38.5 kDa. However, the results showed a dominant band at ∼65 kDa, suggesting a discrepancy in the predicted and observed molecular masses of rPyCSP (Fig. 1). Historically, CSP is known to migrate at a higher Mr on SDS-PAGE, and thus, the discrepancy observed here was not surprising (18, 19).
FIG 1.
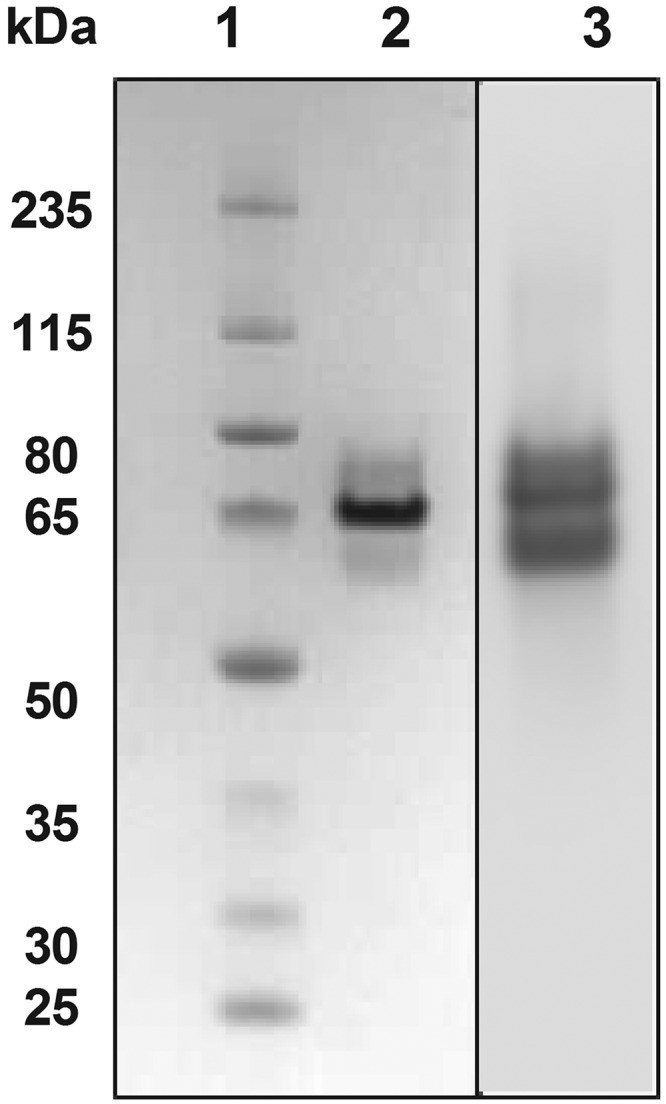
SDS-PAGE and Western blot analysis of purified recombinant circumsporozoite protein (CSP) from Plasmodium yoelii (rPyCSP). Lane 1, molecular weight marker; lane 2, purified rPyCSP; lane 3, ECL-Western blot of rPyCSP using anti-PyCSP MAb NYS1 followed by goat anti-mouse alkaline phosphatase (AP)-conjugated antibody as described in Materials and Methods. rPyCSP was run in duplicate on the same gel and sliced into two portions for use in Coomassie blue staining or Western blot analysis.
rPyCSP-induced protective immunity in mice is independent of the host genetic background.
We determined the protective efficacy of rPyCSP and CpG oligodeoxynucleotide 1826 (ODN 1826) (rPyCSP vaccine) emulsified in Montanide ISA in genetically disparate C57BL/6 (H-2b) mice and BALB/c (H-2d) mice following three immunizations delivered by the subcutaneous route. Control mice were given either CpG ODN 1826 emulsified in Montanide ISA (control vaccine) or no immunization. Intravenous injection of naive C57BL/6 mice with 100 P. yoelii sporozoites resulted in infection in 10 of 10 mice by day 6 postchallenge (Table 1). Similarly, 10 of 10 C57BL/6 mice that had received control vaccine developed malaria parasite infection by day 6 following sporozoite challenge. In comparison, 9 of 10 (90%; P < 0.0001, chi square) C57BL/6 mice that received rPyCSP vaccine developed sterilizing immunity against sporozoite challenge and remained free from malaria parasites during the 14-day observation period. The efficacy of rPyCSP vaccine in BALB/c mice was evaluated in five independent experiments. The cumulative data from these experiments showed that 42 of 49 (85.7%; P < 0.0001, chi square) immunized mice developed sterilizing immunity against sporozoite challenge while no protection was observed in naive mice or in BALB/c mice immunized with the control vaccine, as summarized in Table 1. Thus, rPyCSP vaccine was able to induce a high degree of sterilizing immunity against malaria sporozoites in both C57BL/6 and BALB/c strains of mice. These results strongly suggest that a full-length CSP vaccine construct representing the major immunogenic NANP domains and delivered in a strong adjuvant formulation might be able to induce a high degree of protective immunity in genetically disparate HLA populations.
TABLE 1.
Recombinant-PyCSP-induced sterilizing immunity in BALB/c and C57BL/6 micea
| Mouse strain | Vaccine | % protection (no. of mice protected/no. challenged) |
|---|---|---|
| BALB/c | rPyCSP + ISA51 + mouse CpG ODN 1826 | 85.7 (42/49) |
| ISA51 + mouse CpG ODN 1826 | 0 (0/10) | |
| No vaccine | 0 (0/10) | |
| C57BL/6 | rPyCSP + ISA51 + mouse CpG ODN 1826 | 90 (9/10) |
| ISA51 + mouse CpG ODN 1826 | 0 (0/10) | |
| No vaccine | 0 (0/10) | |
Mice were immunized by three subcutaneous injections given at 3-week intervals. Two weeks after the last dose, mice were challenged intravenously with 100 P. yoelii 17XNL sporozoites.
rPyCSP-induced protection in C57BL/6 mice is IFN-γ independent and antibody dependent.
We next wanted to determine the mechanism of immunity induced by rPyCSP. To accomplish this, we immunized CD4, CD8, and IFN-γ knockout (KO) mice from the C57BL/6 genetic background with rPyCSP vaccine and then challenged them with 100 P. yoelii sporozoites. As noted above, 10 of 10 wild-type (WT) C57BL/6 mice developed blood stage parasite infections, but only 3 of 10 (30%) CD4-KO mice and 4 of 9 (44.4%) CD8-KO mice developed immunity (Table 2). The results suggest that while both CD4+ and CD8+ T cells play a prominent role in immunity induced by an rPyCSP-adjuvanted vaccine, protection against pre-erythrocytic-stage malaria can be achieved in the absence of these T cell populations, albeit at a reduced level. In murine malaria parasite models where immunization with radiation-attenuated Plasmodium sporozoites or with CSP delivered as a DNA plasmid was tested, both CD8+ T cells and IFN-γ were found to be necessary to maintain immunity (20–24). To study the role of IFN-γ in rPyCSP vaccine-induced immunity, we immunized IFN-γ-KO mice with rPyCSP and then challenged them with P. yoelii sporozoites. IFN-γ-KO mice have an IFN-γtm1Ts-targeted mutation, and thus, they fail to generate IFN-γ responses (25). We found that 100% of IFN-γ-KO mice (P < 0.0001, chi square) that received rPyCSP vaccine developed sterile immunity, as demonstrated by the absence of asexual blood stage parasites 14 days postchallenge (Table 2), leading to the conclusion that protection induced by rPyCSP in the murine model is IFN-γ independent.
TABLE 2.
Recombinant-PyCSP-induced sterilizing immunity in C57BL/6 mice is antibody dependent and IFN-γ independenta
| Mouse strain (description) | Vaccine | % protection (no. of mice protected/no. challenged) | 95% confidence interval |
|---|---|---|---|
| C57BL/6J (wild type) | rPyCSP + ISA51 + mouse CpG ODN 1826 | 90 (9/10) | 55.5–99.7 |
| C57BL/6J (wild type) | ISA51 + mouse CpG ODN 1826 | 0 (0/10) | 0.0–30.8 |
| B6.129S2-Cd4tm1Mak (CD4 KO) | rPyCSP + ISA51 + mouse CpG ODN 1826 | 30 (3/10) | 6.7–65.2 |
| B6.129P2-β2mtm1Unc (CD8 KO) | rPyCSP + ISA51 + mouse CpG ODN 1826 | 44.4 (4/9) | 13.7–78.8 |
| B6.129S7-Ifnγtm1Ts (IFN-γ KO) | rPyCSP + ISA51 + mouse CpG ODN 1826 | 100 (10/10) | 69.2–100 |
| B6.129S2-Igh-6tm1Cgn (B cell KO) | rPyCSP + ISA51 + mouse CpG ODN 1826 | 0 (0/10) | 0.0–30.8 |
Mice were immunized by three subcutaneous injections given at 3-week intervals. Two weeks after the last dose, mice were challenged intravenously with 100 P. yoelii 17XNL sporozoites.
Furthermore, to establish a direct role of antibodies in imparting protection induced by rPyCSP, we performed immunization/challenge studies in B-cell-KO mice with an Igh-6tm1Cgn-targeted mutation, which results in a lack of mature B cells (26). We found that rPyCSP failed to induce any protective immunity in B-cell-KO mice and 100% of the immunized mice developed blood stage parasite infections after the sporozoite challenge, demonstrating that B cells are essential for protection induced by rPyCSP vaccine (Table 2).
Furthermore, since immunity was lost in the majority of CD4- and CD8-KO mice, B cells work in concert with CD4+ and CD8+ T cells in the induction of immunity. These results further corroborate earlier studies in mice that show the role of antibodies, along with help from CD4+ and CD8+ T cells, in sporozoite-induced protection against malaria (27). Similar results have been obtained with the RTS,S vaccine, where anti-CSP antibodies play a greater role than CD4+ T cells (17).
Interestingly, we found that the rPyCSP vaccine-induced protection is IFN-γ independent. IFN-γ-KO mice had sterilizing immunity against sporozoite challenge. While irradiation-attenuated-sporozoite-induced immunity is IFN-γ dependent, earlier studies have demonstrated that IFN-γ secretion by CSP-specific CD8+ T cells is not essential to protect mice against live-sporozoite challenge (28).
Relationship between IgG and IgG isotypes and protective immunity.
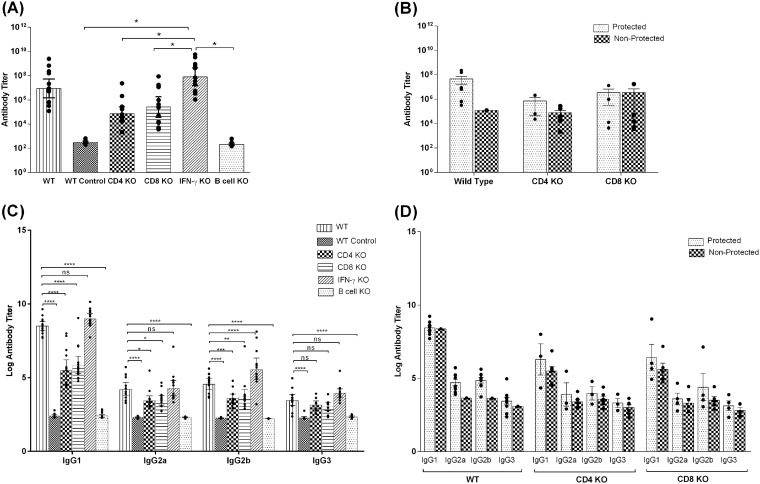
Having established that antibodies are essential for protective immunity, we next wanted to determine if there was any association between anti-CSP-specific IgG, IgG1, IgG2a, IgG2b, and IgG3 levels and protection against malaria sporozoites. The rPyCSP vaccine induced remarkably high IgG titers in the C57BL/6 background, as shown in enzyme-linked immunosorbent assays (ELISAs). The geometric mean (GM) of IgG titers was highest in the IFN-γ-KO mice (GM titer of 4.8 × 107), followed by the GM titer of 3.1 × 106 in the WT mice (Fig. 2A). In comparison to IFN-γ-KO mice, CD4-KO and CD8-KO groups had significantly lower GM titers of 5.1 × 104 and 9.9 × 104 (P = 0.01), respectively. As predicted, the B-cell-KO group had negligible IgG titers. The ELISA titration values shown are calculated values determined as interpolated titers at an optical density at 405 nm of 0.5 (Fig. 2A). We further analyzed the data within the WT, CD4-KO, and CD8-KO immunization groups to determine if the IgG levels differed between the protected and nonprotected mice. In the WT group, the protected mice had a GM titer of 4.5 × 107, whereas the single nonprotected mouse had a titer of 1.2 × 105. However, the sample size of the WT nonprotected group is too small (n = 1) for any statistical analysis. Similarly, nonprotected CD4-KO mice had a GM IgG titer of 7.9 × 104, which was lower than that of the CD4-KO-protected mice, whose GM titer was 7.2 × 105. No significant difference was found in the GM titers of protected versus nonprotected mice in the CD8-KO mouse group (Fig. 2B). The lowest IgG titer in the IFN-γ-KO group, where 10 of 10 mice were protected, was 1.1 × 106. Thus, from these data, we can conclude that while a high IgG titer is associated with protection, especially in mice that have functional CD4 and CD8 T cells, a threshold titer to achieve sterilizing immunity is difficult to define.
FIG 2.
IgG responses in C57BL/6 and genetic knockout (KO) mice immunized with rPyCSP in CpG ODN 1826 and Montanide ISA 51 as determined by ELISA. (A) Total IgG responses in wild-type (WT), unimmunized WT (WT control), CD4-KO, CD8-KO, IFN-γ-KO, and B-cell-KO mice (n = 10). The data were statistically tested by one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test (P = 0.01). (B) IgG responses categorized as values for protected mice versus nonprotected mice in WT, CD4-KO, and CD8-KO groups (n = 10) as determined by ELISA. (C) IgG isotype responses in WT, unimmunized WT (WT control), CD4-KO, CD8-KO, IFN-γ-KO, and B-cell-KO mice (n = 10) as determined by ELISA. The titers were log transformed and plotted as geometric means of log-transformed titers. The data were statistically tested by one-way ANOVA followed by Tukey’s multiple-comparison test. (D) IgG isotypes characterized as protected versus nonprotected mice in WT, CD4-KO and CD8-KO mouse groups as determined by ELISA. The titers were log transformed and plotted as geometric means of log-transformed titers. The ELISA values shown are calculated values determined as interpolated titers at an optical density at 405 nm of 0.5. All error bars show standard errors of the geometric mean values. Dots show the values for individual mice. *, P < 0.01; **, P < 0.001; ***, P < 0.0001; ****, 0.00001; ns, not significant.
We also determined the IgG isotypes in sera of these mice to delineate any Th1 versus Th2 bias in antibody responses and a possible association with protective immunity. The IgG1 titers followed a trend that was similar to that of total IgG responses; the highest titers were obtained in IFN-γ-KO mice, with a GM titer of 1.1 × 109, followed by the WT mice, which had a GM titer of 2.7 × 108. Compared to the IgG1 titers in IFN-γ-KO mice, relatively lower IgG1 titers were observed in the CD4-KO mice (GM titer of 5.3 × 105; P < 0.0001) and CD8-KO mice (GM titer of 8.1 × 105; P < 0.0001). The levels of IgG2a, IgG2b, and IgG3 responses in all immunization groups were significantly lower than the total IgG and IgG1 responses (P < 0.0005). The data were plotted after log transformation of the titers obtained (Fig. 2C). We further analyzed whether the IgG isotype titers were associated with protection in WT, CD4-KO, and CD8-KO immunization groups. In the CD4-KO immunization group, the protected mice had higher IgG1 titers (GM titer of 1.9 × 106) than the nonprotected mice (GM titer of 3.1 × 105). Similarly, the protected mice in the CD8-KO mouse group had high IgG1 titers (GM titer of 2.6 × 106) compared to those of the nonprotected mice (GM titer of 4.1 × 105). There was no significant difference in the IgG2a, IgG2b, and IgG3 titers in both CD4-KO and CD8-KO immunization groups. The data were plotted after log transformation of the titers obtained (Fig. 2D). These data lead us to believe that the loss of IFN-γ and not CD4 and CD8 molecules led to a switch toward an extremely high level of Th2-type antibody response that had a protective effect. Overall, a Th2-associated IgG1 antibody response is associated with PyCSP-induced protective immunity. Scheiblhofer et al. (29) have also shown that a high IgG1/IgG2a ratio translates to protection in mice immunized with a CSP DNA plasmid.
Antibodies generated following immunization with rPyCSP are protective.
To confirm that the PyCSP-induced protection against the sporozoite challenge is indeed antibody mediated, we incubated P. yoelii sporozoites with the sera acquired from protected mice (immunized wild-type C57BL/6 and IFN-γ-KO mice) and nonprotected mice (immunized B-cell-KO mice or WT mice immunized with control vaccine) for 45 min. The antibody-treated sporozoites were intravenously injected into WT C57BL/6 mice, and the mice were monitored for asexual blood stage parasitemia for 14 days postinfection by counting infected red blood cells on Giemsa-stained blood films (Table 3). The sporozoites treated with sera from protected mice failed to cause any blood stage infection in 100% of the recipient mice, while mice that received sporozoites treated with sera from nonprotected mice developed a blood stage parasite infection (Table 3). These results clearly showed that immunization with rPyCSP vaccine was able to induce neutralizing antibodies that could render infectious sporozoites into noninfectious sporozoites that were unable to cause blood stage parasite infection in malaria-naive mice.
TABLE 3.
Passive transfer of antibodies generated following immunization with rPyCSP confers protection against sporozoite challenge in C57BL/6 micea
| Mouse strain (description) | Vaccine | % protection (no. of mice protected/no. challenged) | 95% confidence interval |
|---|---|---|---|
| C57BL/6J (wild type) | rPyCSP + ISA51 + mouse CpG ODN 1826 | 100 (10/10) | 69.2–100 |
| C57BL/6J (wild type) | ISA51 + mouse CpG ODN 1826 | 0 (0/10) | 0.0–30.8 |
| C57BL/6J (wild type) | No vaccine | 0 (0/10) | 0.0–30.8 |
| B6.129S7-Ifnγtm1Ts (IFN-γ KO) | rPyCSP + ISA51 + mouse CpG ODN 1826 | 100 (10/10) | 69.2–100 |
| B6.129S2-Igh-6tm1Cgn (B-cell KO) | rPyCSP + ISA51 + mouse CpG ODN 1826 | 0 (0/10) | 0.0–30.8 |
Five hundred P. yoelii 17XNL sporozoites were incubated with the pooled sera from protected (PyCSP-immunized wild-type [WT] and IFN-γ knockout [KO] mice on the C57BL/6 background) and nonprotected (PyCSP-immunized B-cell-KO mice and control vaccine-immunized WT mice) groups of mice for 45 min at room temperature. Naive C57BL/6 mice were challenged intravenously with sporozoites that were preincubated with antibody.
Sporozoites neutralized by sera from protected mice fail to invade liver.
We further investigated if the effector function of the anti-CSP antibodies is mediated by rendering sporozoites unable to invade liver cells or by inhibiting the growth of liver form malaria parasites postinvasion. We assessed the sporozoite infectivity in the liver by measuring P. yoelii 18S rRNA copy numbers by reverse transcription-quantitative PCR. The mean rRNA copy number for livers from the nonprotected group of mice was 10,135 (sporozoites incubated with normal sera). The mean rRNA copy number for livers from protected group of mice was 2.93 (sporozoites incubated with sera from immunized wild-type mice). The mean RNA copy number from livers of naive noninfected mice was 0.06 (Table 4). The extremely low RNA copy number from protected mice indicates that the serum from protected mice was able to neutralize the sporozoites and, therefore, the sporozoites failed to invade the liver.
TABLE 4.
Anti-PyCSP antibody-treated sporozoites fail to invade livera
| Mouse group used as serum source | Type of serum used to treat sporozoites | No. of P. yoelii 18S rRNA copies in livers of challenged mice (geometric mean ± SEM) |
|---|---|---|
| Protected | Wild-type immunized mouse sera | 2.935 ± 0.66 |
| Nonprotected | Normal sera | 10,135 ± 389 |
| Uninfected | 0.0665 ± 0.05 |
Naive BALB/c mice were injected with P. yoelii 17XNL sporozoites treated for 45 min with sera from rPyCSP-immunized mice that had sterilizing immunity following sporozoite challenge. Livers were harvested 40 h postchallenge for detection and quantification of the P. yoelii 18S rRNA to measure the developing liver stage parasite burden. RNA was isolated, and reverse transcription-quantitative PCR amplification for 18S RNA was performed.
CD4+ and CD8+ T-cell populations in PyCSP-induced immunity.
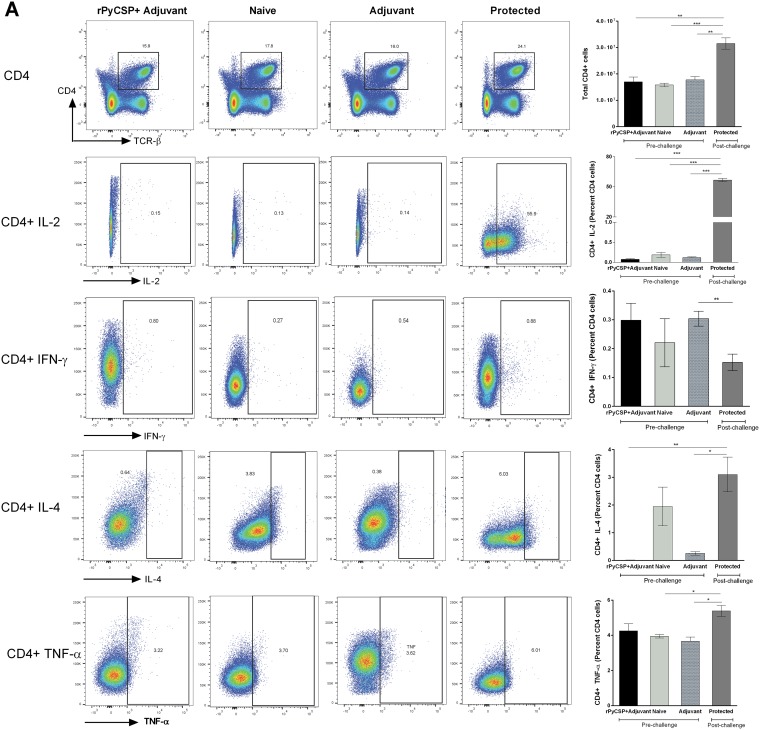
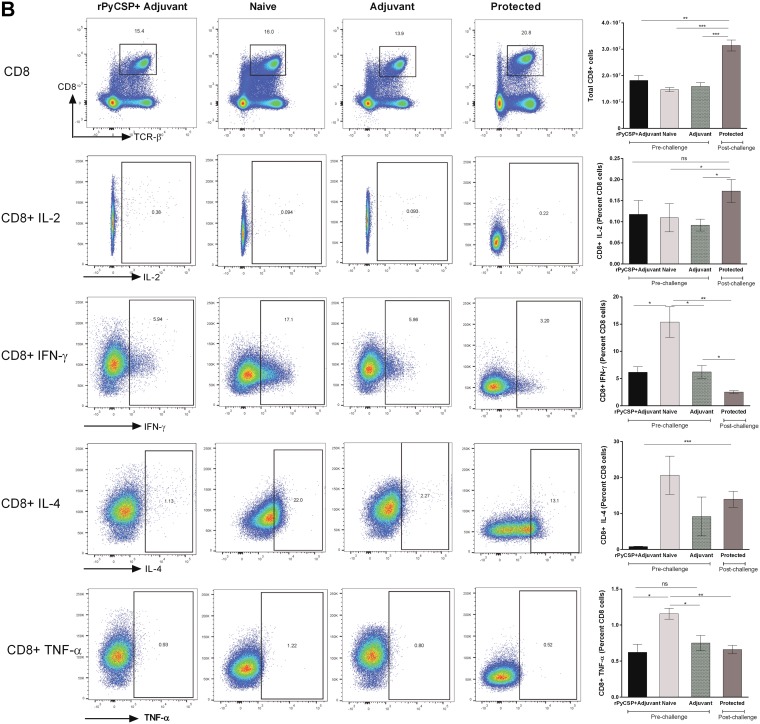
It has previously been shown that RTS,S induces CSP-specific antibodies and CD4+ T cells in African children (5, 17). To determine the contribution of T cells in protective immunity, we quantitated CD4+ and CD8+ T cells in the spleens of immunized mice (rPyCSP in adjuvant) prior to P. yoelii strain 17XNL (Py17XNL) sporozoite challenge (prechallenge immunized mice) and in immunized mice that did not develop blood stage infection post-sporozoite challenge (protected), as well as in mice injected with adjuvant alone and naive mice, by flow cytometric analysis. We found that although the percentage of CD4+ T cells did not increase significantly in the immunized protected mice (data not shown), the total splenic CD4+ T cell population increased significantly compared to those of the prechallenge immunized (P < 0.005), naive (P < 0.0001), and adjuvant-alone (P < 0.005) mouse groups (Fig. 3A). We obtained similar results upon comparison of CD8+ T cells among the four groups. Total splenic CD8+ T cells increased significantly in the protected mice compared to the populations in the unchallenged immunized mice (P < 0.01), naive mice (P < 0.0001), and adjuvant-alone mice (P < 0.001) (Fig. 3B).
FIG 3.
Analysis of CD4+ and CD8+ T cells and their secreted cytokines in C57BL/6 mouse groups by flow cytometry. Groups (n = 6) comprised rPyCSP + adjuvant (prechallenge), naive (unimmunized), adjuvant alone, and protected mice. Protected mice were immunized with rPyCSP and challenged with Py17XNL sporozoites and did not develop blood stage infection. Splenocytes were isolated 14 days after sporozoite challenge, and the expression of IL-2, IFN-γ, IL-4, and TNF-α was measured in CD4+ TCRαβ+ cells (A) and CD8+ TCRαβ+ cells (B). Representative dot plots are shown. All error bars show standard deviations of the mean values. *, P < 0.01; **, P < 0.001; ***, P < 0.0001; ns, not significant by Student’s t test.
rPyCSP-mediated protection is characterized by increased Il-2, Il-4, and TNF-α and absence of IFN-γ in C57BL/6 mice.
We next measured the cytokines produced by CD4+ and CD8+ T cells by flow cytometry. We observed a significant increase in interleukin-2 (IL-2) (∼60-fold) produced by CD4+ T cells in immunized protected mice compared to the level in the prechallenge mice, as well in the naive mice (P < 0.0001) (Fig. 3A). We also observed a significant increase in CD4+ T cells producing IL-4 in the protected mice compared to the levels in the prechallenge immunized and adjuvant-alone mice (Fig. 3A). We also quantitated CD4+ T cells producing tumor necrosis factor alpha (TNF-α) and found a significant increase in the protected mice compared to the levels in the adjuvant-alone and naive mouse groups (P < 0.01) (Fig. 3A).
We also quantitated IFN-γ-producing CD4+ T cells and found no significant change in the protected mice compared to the levels in the prechallenge and naive mouse groups, and in fact, IFN-γ production was decreased in the protected mice compared to the level in the adjuvant-alone group (P < 0.001) (Fig. 3A). These results further illustrate the IFN-γ-independent nature of rPyCSP-induced immunity in mice. Our results are also in line with the studies showing that RTS,S vaccine induces CD4+ T cells producing a mixture of cytokines, such as, IL-2, TNF-α, and IFN-γ, in young children in sub-Saharan Africa (30).
We did not find any differences in the total numbers or percentages of IL-2-secreting CD8+ T cells between the prechallenge immunized (rPyCSP plus adjuvant group) and protected mice. However, there was a significant increase in the percentage of CD8+ T cells secreting IL-2 in protected mice compared to the percentages in the adjuvant-alone and naive groups (P < 0.01) (Fig. 3B). Interestingly, we observed a remarkable decrease in CD8+ T cells secreting IFN-γ in the protected mice, as well as in the immunized unchallenged mice, compared to the levels in the adjuvant-alone (P < 0.01) and naive mice (P < 0.001) (Fig. 3B). There was no significance difference in the percentage of CD8+ T cells secreting IL-4 in protected mice compared to the percentages in the adjuvant-alone and naive mice (P > 0.05). TNF-α-producing CD8+ T cells were found to be dramatically decreased in the mice immunized with rPyCSP (pre- and post-sporozoite challenge) compared to the level in the naive mice (P < 0.01) (Fig. 3B). Some of the earlier studies have also shown that effector CD8+ T cells do not require IFN-γ for antiparasitic activity against malaria liver stages (28). Our data suggest that rPyCSP-induced immunity in mice may not rely on CD8+ T cells and is IFN-γ independent in the present vaccine model system.
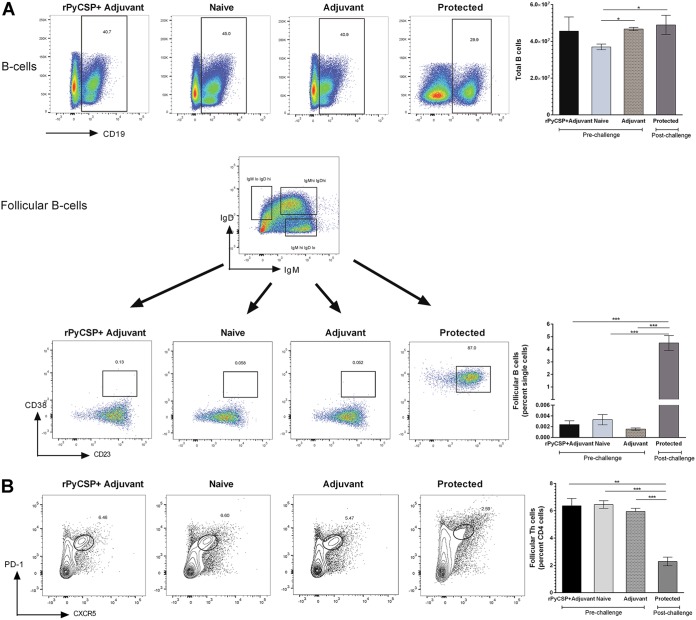
B-cell repertoire and follicular T cells.
To further investigate the role of B cells in PyCSP-induced immunity, we quantified the B-cell repertoire in the spleens of the mice in the four groups (PyCSP in adjuvant pre- and post-sporozoite challenge, adjuvant alone, and no immunization) by flow cytometric analysis. We did not find any significant difference in the B-cell population percentages among the four groups (data not shown). However, we found a significant increase in total splenic B cells in the protected mouse group compared to the amount in the naive mice (P < 0.01) (Fig. 4A, top). We further analyzed the B-cell subpopulations of follicular, marginal zone B cells, and plasma cells. We did not find any differences in these cell populations (data not shown). Interestingly, we found that the protected mice had significantly higher numbers of follicular B cells than the prechallenge immunized, adjuvant-alone immunized, and naive mice (P < 0.0001) (Fig. 4A, bottom). Follicular B cells promote effective primary immune responses and establish isotype switching and B-cell memory with the help of follicular T helper (Tfh) cells. We further investigated the levels of Tfh cells. CD4+ Tfh cells have been shown to be critical for the generation of B-cell responses. We found a significant decrease in Tfh cells in the protected mice compared to the levels in the naive (P < 0.0001), adjuvant-alone (P < 0.0001), and prechallenge immunized mice (P < 0.001) (Fig. 4B). The decrease may be a result of consumption of Tfh cells to activate B cells, to ultimately provide protection against the sporozoite challenge.
FIG 4.
Comparison of B cells and follicular B and follicular T-helper cells in C57BL/6 mouse groups by flow cytometry. Groups (n = 6) comprised rPyCSP + adjuvant (prechallenge), naive (unimmunized), adjuvant-alone, and protected mice. Protected mice were immunized with rPyCSP and challenged with Py17XNL sporozoites and did not develop blood stage infection. Splenocytes were isolated 14 days after sporozoite challenge. (A) Top, B cells were quantitated in live single splenocytes by gating on the CD19+ cell marker. Bottom, CD19+ B cells were gated on IgD and IgM to get IgMlo IgDhi, IgMhi IgDhi, and IgMhi IgDlo subpopulations. The IgMlo IgDhi population was further gated on CD38 and CD23 to quantitate follicular B cells in mice. (B) CD4+ T cells were gated on CXCR5 and PD-1 to quantitate follicular T-helper cells in mice. Representative dot plots are shown. All error bars show standard deviations of the mean values. *, P < 0.01; **, P < 0.001; ***, P < 0.0001 by Student’s t test.
DISCUSSION
The most advanced subunit malaria vaccine, RTS,S, induced modest, short-lived immunity in clinical studies in children and adults living in Africa. Moreover, the mechanism of RTS,S-induced immunity remains incompletely defined, which can be attributed at least in part to limitations in the types of immunological studies that can be performed in humans. The main object of this study was to use the P. yoelii sporozoite challenge model to better understand the correlates of sterilizing immunity induced by a CSP subunit-based vaccine in mice.
We found that immunization with rPyCSP in Montanide ISA 51 induced sterilizing immunity against sporozoite challenge infection in mice with two different genetic backgrounds, C57BL/6 and BALB/c (Table 1). On the other hand, 100% of mice that received Montanide ISA 51 alone or no immunization displayed no immunity and developed blood stage parasite infection. This immunity is primarily antibody mediated, since in contrast to wild-type mice, 100% of B-cell-KO mice immunized with rPyCSP had no protective immunity against P. yoelii sporozoite challenge (Table 2). We further provide evidence that the antibodies generated upon rPyCSP immunization are protective in in vitro and passive-transfer studies. The sera obtained from protected mice were able to neutralize sporozoites in vitro and render them noninfectious, unable to cause blood stage parasite infections, in WT and IFN-γ-KO mice (Table 3). Additionally, the neutralized sporozoites failed to invade the livers of mice; the copy numbers of P. yoelii 18S rRNA were significantly lower in the livers of mice that received the immune antibody-treated sporozoites than in mice that received the sporozoites treated with antibody from nonimmunized mice (Table 4). These observations firmly established that the rPyCSP-based vaccine is capable of inducing protective levels of neutralizing antibodies that prevent sporozoites from entering liver cells or establishing a subsequent blood stage parasite infection.
rPyCSP was highly immunogenic and induced very high levels of CSP-specific IgG antibodies in WT C57BL/6 mice (mean titer of 3.1 × 106) as determined by ELISA, and IgG1 was the most prominent isotype in vaccinated mice. IFN-γ-KO mice displayed the highest levels of IgG1 isotype response, which suggested a lack of proinflammatory response that promoted the induction of superior antigen-specific Th2-type antibodies. The lack of CSP-specific antibodies in B-cell-KO mice further confirms the antibody-dependent nature of immunity in the P. yoelii murine model. Most surprisingly, a reasonably high level of CSP-specific IgG (5.1 × 104) and higher IgG1 isotype levels were also induced in the CD4+-KO mice, which is consistent with the sterilizing immunity observed in 30% of vaccinated mice. The CSP-specific antibodies, albeit at lower levels, in the absence of CD4+ T cell help are a matter for further investigation. CD8-KO mice also generated lower levels of IgG and isotype antibodies and showed protective immunity in only 44% of immunized mice, suggesting a role for CD8 cells in protection induced by a recombinant-CSP-based malaria vaccine. The association between anti-CSP antibody titers and protection from infection (Fig. 2) is consistent with data from other vaccine studies (31, 32), as well as data from mouse models where RTS,S-induced anti-CSP antibodies have been shown to inhibit sporozoite invasion (12). Furthermore, high antibody titers are needed for protection (Fig. 2A). rPyCSP immunization favors the production of IgG1 antibody responses that are protective in nature and thus polarizes induction of strong humoral responses of the Th2 type (Fig. 2C). We have previously shown in an experimental cerebral malaria (ECM) model that, in infection with virulent malaria parasites, immune modulation resulted in a shift toward a Th2-type response that may help to ameliorate the most severe consequences of malaria immunopathogenesis (33).
Immunity induced in mice by irradiated malaria sporozoite vaccines is shown to be CD8+ T cell and IFN-γ dependent (34). Interestingly, the protection induced by rPyCSP immunization is IFN-γ independent, as C57BL/6 IFN-γ-KO mice also developed a level of sterilizing immunity similar to that in the WT mice (Table 2). Another striking observation is that rPyCSP-immunized C57BL/6 CD4- and CD8-KO mice also developed a partial protection against sporozoite infection (Table 2). How CD4-KO mice were also able a mount a moderate level of antibody response and protective immunity in some mice is not clear. These results point toward the existence of PyCSP-induced protective immune mechanism(s) that are independent of CD4+/CD8+ T cells and are being further investigated in our laboratory. In the RTS,S vaccination studies, the combination of a strong CD4+ T cell response with the high levels of anti-CSP antibody titers is important to protect against malaria illness (17). By flow cytometric analysis, we found a significant increase of total CD4+ and CD8+ T cells in the spleens of protected mice (Fig. 3). In the RTS,S study, the magnitudes of the RTS,S-induced antibody and cell-mediated responses were correlated (5), and the most prominent CSP-specific CD4+ T-cell phenotype induced was IL-2 (6, 30, 35) at 1 month postvaccination with RTS,S/AS01 or RTS,S/AS02. Consistent with these findings, we observed significantly elevated levels of CD4+ T cells producing IL-2 (Fig. 3A) in protected mice. Such IL-2+ CD4+ T cells could provide help to antibody-producing B cells. We also observed significant increases in CD4+ T cells producing IL-4 and TNF-α (Fig. 3A). CSP-specific TNF-α+ CD4+ T cells have been associated with protection in RTS,S clinical trials (6). Combined IL-2/IL-4 responses have been observed in P. falciparum-specific cellular immune responses after immunization with the RTS,S/AS02D candidate malaria vaccine in infants (36). In this regard, our mouse model is reminiscent of the immunological findings of RTS,S clinical trials.
In the RTS,S studies, protective immunity was linked to PfCSP-specific CD4+ and the possible involvement of CD8+ T cells producing IFN-γ (37). Our results differ from the RTS,S findings. WT mice showed no significant change in levels of CD4+ T cells secreting IFN-γ (Fig. 3A) and decreased CD8+ IFN-γ levels (Fig. 3B). In a previous study in a mouse model, T lymphocytes were shown to not require IFN-γ production for antiparasitic activity after CSP epitope immunization (28). This is to note that in different mouse models, delivery platforms evaluated in mice with different backgrounds most often induce protection by different mechanisms (21).
Another significant finding in our study was that the protected mice had significantly higher levels of follicular B cells (Fig. 4A, bottom). Follicular B cells promote effective primary immune responses and establish isotype switching and B-cell memory with the help of follicular Th cells (38, 39). We found a decrease in CD4+ PD1+ CXCR5+ follicular helper T (Tfh) cells in the protected mice compared to the levels in the naive and unchallenged immunized mice (Fig. 4B). This may be a result of the usage of Tfh cells to activate B cells, which may be responsible for providing protection against the sporozoite challenge. Recently, it has been shown that malaria parasite infection leads to a Th1-type cytokine response that causes a decrease in circulating PD1+ CXCR5+ Tfh cells (40). Although the protected mice are not infected, exposure to sporozoites in immunized mice may be responsible for the observed decrease in the Tfh cell population. Whether exposure to sporozoites attenuated by gamma irradiation, genetic modification, or heat would also lead to a decrease in the Tfh cell population remains to be seen.
These results offer several important lessons for the design of next-generation recombinant-CSP-based malaria vaccines. An important factor behind the sterilizing immunity induced by the rPyCSP vaccine could be that it contains the entire CSP sequence (without the signal and cytoplasmic domains). On the other hand, the RTS,S vaccine lacks the biologically relevant amino acid region upstream from the NANP repeats (41) and a part of the COOH terminus sequence which contains a well-characterized helper T cell epitope (42). Furthermore, an adjuvant formulation that leads to a shift in the Th2-type antibodies may induce a more effective sterilizing immunity. Finally, this experimental PyCSP-sporozoite challenge model allows evaluation of immunogen-adjuvant dosing and novel adjuvants, longevity of immunity, allelic polymorphism, and the effect of prior malaria parasite exposure in the search for more effective pre-erythrocytic-stage malaria vaccines.
MATERIALS AND METHODS
Construction of rPyCSP expression plasmid.
The PyCSP gene encoding amino acids P20 to S369 was PCR amplified using genomic DNA prepared from blood stage P. yoelii strain 17XNL parasites. The following primer pair was used for PCR amplification: sense, 5′-GAAGCTAGCCCAGGATATGGACAAAATAAAAGT-3′, and antisense, 5′-ATGAGGGCCCTGAACATTTATCCATTTTACAAAT-3′. The resultant 1,050-nucleotide PCR product was digested with NheI and ApaI restriction enzymes and cloned at compatible sites in a yeast expression plasmid, pREU-3. The nucleotide sequence of the CSP gene insert in the recombinant plasmid was confirmed by automated DNA sequencing.
Recombinant expression and purification of PyCSP.
PyCSP was recombinantly expressed in Saccharomyces cerevisiae and purified using a procedure described previously (43, 44). Briefly, the recombinant PyCSP-pREU-3 plasmid was electroporated into S. cerevisiae strain 2905/6 and the Trp+ recombinant yeast was grown at 30°C with Trp-selective protein expression medium. The recombinant cells were induced for 18 to 24 h with ethanol, and secreted rPyCSP was microfiltered, ultrafiltered, and then diafiltered with a 10-kDa spiral fiber filter. The His6 PyCSP was then purified by batch binding to Ni-nitrilotriacetic acid (NTA) agarose (Qiagen, Chatsworth, CA) at 4°C for 12 to 18 h, washing the Ni-NTA resin with 2× phosphate-buffered saline (PBS), and recovering the rPyCSP from the resin with 0.25 M sodium acetate, pH 4.5 (43). After elution from the Ni-NTA column, the rPyCSP was applied to a size exclusion column (S-75) and buffer exchanged into 1× PBS, pH 7.4. The protein concentration of rPyCSP was determined using the bicinchoninic acid (BCA) protein assay reagent (Pierce, Rockford, IL). rPyCSP was analyzed by running 0.1 μg of protein on 4 to 12% morpholineethanesulfonic acid (MES) NuPAGE Bis-Tris gel (Life Technologies), followed by staining with Coomassie blue. Enhanced chemiluminescence (ECL)-Western blot analysis was performed to characterize the immunoreactivity of rPyCSP. Briefly, the rPyCSP profile from the gel was electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane and probed with the anti-PyCSP monoclonal antibody (MAb) NYS1, followed by goat anti-mouse alkaline phosphatase (AP)-conjugated antibody. Finally, the membrane was reacted with the ECL substrate solution (Western Star immunodetection system; Life Technologies), and data were digitally captured on the C-Digit blot scanner (Li-Cor).
Mice.
Six- to 8-week-old female C57BL/6, BALB/c, CD4-knockout (KO) (B6.129S2-Cd4tm1Mak), CD8-KO (B6.129P2-β2mtm1Unc), IFN-γ-KO (B6.129S7-Ifnγtm1Ts), and B-cell-KO (B6.129S2-Igh-6tm1Cgn) female mice (C57BL/6 background) were purchased from the Jackson Laboratories (Bar Harbor, ME). All experimental animals were housed, fed, and used in accordance with guidelines set forth in the National Institutes of Health manual Guide for the Care and Use of Laboratory Animals (45). The Center for Biologics Evaluation and Research Animal Care and Use Committee approved the animal study protocol. The study was performed under CBER animal study protocol number 2002-21.
Immunizations.
Mice were immunized with an emulsion of 20 μg of rPyCSP and 25 μg of CpG oligodeoxynucleotide (ODN) 1826 (Coley Pharmaceuticals) in 100 μl of PBS and 100 μl of Montanide ISA51 (Seppic, Inc., Fairfield, NJ) as an adjuvant. Control mice received 25 μg of CpG ODN 1826 in 100 μl of PBS and 100 μl of Montanide ISA51 without rPyCSP. Each mouse received three immunizations with 200 μl of vaccine, with each dose delivered by the subcutaneous route at 3-week intervals. Serum samples were taken from each mouse at the time of each immunization and 2 days prior to sporozoite challenge.
For flow cytometry experiments, 6- to 8-week old female C57BL/6 mice were purchased from Jackson Laboratories. Mice were immunized with an emulsion of 20 μg of rPyCSP in 100 μl of PBS and 100 μl of Freund’s adjuvant. Each mouse received three immunizations with 200 μl of vaccine, with each dose delivered by the subcutaneous route at 3-week intervals. The immunized mice were divided into two groups, and half were challenged with 100 P. yoelii 17XNL sporozoites through the intravenous route. Naive mice that did not receive any immunization served as unimmunized control mice.
Sporozoite challenge and determination of protection against malaria.
Mice were injected intravenously with 100 P. yoelii 17XNL (clone 1.1) sporozoites on day 14 after the third immunization. Beginning on day 4 post-sporozoite challenge, thin blood films were made every day for the next 10 days by pricking at tail bases. Blood smears were Giemsa stained, and the presence or absence of blood form parasites was determined with a light microscope. Mice were considered to have developed sterilizing immunity if asexual blood stage parasites were not detected by day 14 postchallenge.
IgG isotype ELISA.
Titers of rPyCSP-specific total IgG or IgG isotypes IgG1, IgG2a, IgG2b, and IgG3 in the sera of vaccinated mice were assessed by enzyme-linked immunosorbent assay (ELISA). Briefly, 50 μl of a 1-μg/ml solution of rPyCSP in PBS, pH 7.4, was used to coat the wells of flat-bottom Immulon II ELISA plates (Dynatech Laboratories, Chantilly, VA) overnight at 4°C. The wells were blocked by incubation with 5% bovine serum albumin (BSA) in PBST (1× PBS containing 0.05% Tween 20) at 37°C. Amounts of 50 μl of 2-fold serial dilutions of the test sera or a control serum in 1% BSA–PBST were added to the wells and incubated for 60 min at 37°C. Following three washes, amounts of 100 μl of an appropriate dilution of anti-mouse IgG-, IgG1-, IgG2a-, IgG2b-, or IG3-specific antibody conjugated to alkaline phosphatase (Promega, Inc., Madison, WI) were added. After incubation for 1 h at 37°C, the plates were washed three times and then phosphatase substrate tablets (Sigma-Aldrich, Saint Louis, MO) were diluted in diethanolamine (Pierce Scientific, Rockford, IL) and 100 μl was added to each well; the reaction was allowed to proceed at room temperature. The optical density values for plates were read at 405 nm with an ELISA reader. The ELISA values shown are calculated values determined as interpolated titers at an optical density of 0.5.
Assays of protective immunity.
(i) In vitro sporozoite neutralization assay. Five hundred P. yoelii 17XNL (clone 1.1) sporozoites were incubated with pooled sera from protected and nonprotected groups of mice for 45 min at room temperature. Naive mice were challenged intravenously with sporozoites that were preincubated with antibody. Blood samples from tail-pricked mice were smeared from day 4 to day 14 postchallenge. Mice were considered protected from malaria if the Giemsa-stained blood films were negative for blood stage parasites until day 14 postchallenge.
(ii) Parasite load in liver. To determine whether sporozoites were able to invade liver cells following antibody treatment in vitro, naive BALB/c mice were challenged with neutralized sporozoites. Livers were harvested 40 h postchallenge for detection and quantification of the P. yoelii 18S rRNA to assess the sporozoite loads in the livers. Briefly, harvested livers were wrapped in aluminum foil and dipped in liquid nitrogen for 45 s. The frozen livers were crushed between two sheets of aluminum foil, and the pulverized tissue was suspended in 5 ml of TRIzol reagent (Invitrogen, Carlsbad, CA). RNA was extracted using the high pure RNA isolation kit (Roche Diagnostic Corporation, Indianapolis, IN). Amounts of 2 μg of RNA were used for 25 μl of cDNA preparation using the SuperScript II cDNA synthesis kit (Invitrogen, Carlsbad, CA). Amounts of 2 μl of the cDNA were used for real-time PCR amplification of P. yoelii 18S rRNA using the primers described previously (46). Real-time incorporation of SYBR green 1 (iQ Supermix; Bio-Rad, Hercules, CA) was performed using the MyiQ single-color real-time PCR detection system (Bio-Rad, Hercules, CA). The threshold cycle (CT) values were extrapolated from a standard curve generated with known amounts of P. yoelii 18S rRNA plasmid to determine the corresponding P. yoelii 18S rRNA copy numbers. The 18S rRNA copy numbers obtained from different samples were normalized using the mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene (47).
(iii) Flow cytometry. In-depth analysis of immune cell subsets was performed by flow cytometry. Briefly, single-cell suspensions of splenocytes were first subjected to ammonium-chloride-potassium (ACK) lysis of erythrocytes, washed, and seeded at 1 × 106 cells per sample. Samples were then labeled with eFluor 506 viability dye (eBioscience, San Diego, CA, USA), incubated with anti-mouse CD16/32 antibody (Biolegend, San Diego, CA, USA), and stained with antibodies specific for fluorescein isothiocyanate (FITC)–anti-T-cell receptor alpha/beta (TCRαβ), peridinin chlorophyll protein (PerCP)–anti-CD4, allophycocyanin (APC)-Cy7–anti-CD8, phycoerythrin (PE)–anti-IL-2, FITC–TNF-α, APC–anti-IFN-γ, FITC–anti-CD19, Pacific Blue (PB)–anti-IgD, APC–anti-IgM, PE–anti-CD 23, PerCP-Cy5.5–anti-CD38, APC-Cy7, and anti-CD21 antibodies in Hanks balanced salt solution (HBSS) containing 1% BSA for 30 min at 4°C, washed three times in HBSS containing 0.1% BSA, fixed, and then acquired on an LSR II flow cytometer using FACSDiva (BD Biosciences, San Jose, CA, USA) and analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
ACKNOWLEDGMENTS
We thank Anthony W. Stowers for assistance with the expression of the recombinant rPyCSP. We thank Mark Kukuruga and Adovi Akue at the Center for Biologics Evaluation and Research (CBER) for maintenance of flow cytometers in the CBER core facility. We also thank the veterinary staff at CBER for the care and maintenance of mice.
Richard L. Shimp, Jr., is supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.WHO. 2018. World malaria report 2018. World Health Organization, Geneva, Switzerland: https://www.who.int/malaria/publications/world-malaria-report-2018/report/en/. [Google Scholar]
- 2.Kappe SH, Buscaglia CA, Nussenzweig V. 2004. Plasmodium sporozoite molecular cell biology. Annu Rev Cell Dev Biol 20:29–59. doi: 10.1146/annurev.cellbio.20.011603.150935. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. 2010. From the circumsporozoite protein to the RTS,S/AS candidate vaccine. Hum Vaccin 6:90–96. doi: 10.4161/hv.6.1.9677. [DOI] [PubMed] [Google Scholar]
- 4.Ballou WR. 2009. The development of the RTS,S malaria vaccine candidate: challenges and lessons. Parasite Immunol 31:492–500. doi: 10.1111/j.1365-3024.2009.01143.x. [DOI] [PubMed] [Google Scholar]
- 5.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, Schwenk R, Nielsen RA, Debebe Z, Pinelis E, Juompan L, Williams J, Dowler M, Stewart VA, Wirtz RA, Dubois MC, Lievens M, Cohen J, Ballou WR, Heppner DG Jr, RTS,S Vaccine Evaluation Group. 2009. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis 200:337–346. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 6.Olotu A, Moris P, Mwacharo J, Vekemans J, Kimani D, Janssens M, Kai O, Jongert E, Lievens M, Leach A, Villafana T, Savarese B, Marsh K, Cohen J, Bejon P. 2011. Circumsporozoite-specific T cell responses in children vaccinated with RTS,S/AS01E and protection against P. falciparum clinical malaria. PLoS One 6:e25786. doi: 10.1371/journal.pone.0025786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmuller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Nhamuave A, Quelhas D, Bassat Q, Mandjate S, Macete E, Alonso P, Abdulla S, Salim N, Juma O, Shomari M, Shubis K, Machera F, Hamad AS, Minja R, Mtoro A, Sykes A, Ahmed S, Urassa AM, Ali AM, Mwangoka G, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Tahita MC, Kabore W, Ouedraogo S, Sandrine Y, Guiguemde RT, Ouedraogo JB, Hamel MJ, Kariuki S, Odero C, et al. 2011. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 365:1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 8.RTS,S Clinical Trials Partnership, Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmuller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Machevo S, Acacio S, Bulo H, Sigauque B, Macete E, Alonso P, Abdulla S, Salim N, Minja R, Mpina M, Ahmed S, Ali AM, Mtoro AT, Hamad AS, Mutani P, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Bihoun B, Guiraud I, Kabore B, Sombie O, Guiguemde RT, Ouedraogo JB, Hamel MJ, Kariuki S, Oneko M, Odero C, Otieno K, Awino N, McMorrow M, Muturi-Kioi V, Laserson KF, Slutsker L, et al. 2012. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.RTS,S Clinical Trials Partnership. 2015. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olotu A, Fegan G, Wambua J, Nyangweso G, Leach A, Lievens M, Kaslow DC, Njuguna P, Marsh K, Bejon P. 2016. Seven-year efficacy of RTS,S/AS01 malaria vaccine among young African children. N Engl J Med 374:2519–2529. doi: 10.1056/NEJMoa1515257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra S, Nussenzweig RS, Nussenzweig V. 2012. Antibodies to Plasmodium circumsporozoite protein (CSP) inhibit sporozoite's cell traversal activity. J Immunol Methods 377:47–52. doi: 10.1016/j.jim.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foquet L, Hermsen CC, van Gemert GJ, Van Braeckel E, Weening KE, Sauerwein R, Meuleman P, Leroux-Roels G. 2014. Vaccine-induced monoclonal antibodies targeting circumsporozoite protein prevent Plasmodium falciparum infection. J Clin Invest 124:140–144. doi: 10.1172/JCI70349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pied S, Roland J, Louise A, Voegtle D, Soulard V, Mazier D, Cazenave PA. 2000. Liver CD4− CD8− NK1.1+ TCR alpha beta intermediate cells increase during experimental malaria infection and are able to exhibit inhibitory activity against the parasite liver stage in vitro. J Immunol 164:1463–1469. doi: 10.4049/jimmunol.164.3.1463. [DOI] [PubMed] [Google Scholar]
- 14.Roland J, Soulard V, Sellier C, Drapier AM, Di Santo JP, Cazenave PA, Pied S. 2006. NK cell responses to Plasmodium infection and control of intrahepatic parasite development. J Immunol 177:1229–1239. doi: 10.4049/jimmunol.177.2.1229. [DOI] [PubMed] [Google Scholar]
- 15.Zavala F, Tam JP, Hollingdale MR, Cochrane AH, Quakyi I, Nussenzweig RS, Nussenzweig V. 1985. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science 228:1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]
- 16.John CC, Tande AJ, Moormann AM, Sumba PO, Lanar DE, Min XM, Kazura JW. 2008. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J Infect Dis 197:519–526. doi: 10.1086/526787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White MT, Bejon P, Olotu A, Griffin JT, Riley EM, Kester KE, Ockenhouse CF, Ghani AC. 2013. The relationship between RTS,S vaccine-induced antibodies, CD4(+) T cell responses and protection against Plasmodium falciparum infection. PLoS One 8:e61395. doi: 10.1371/journal.pone.0061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cochrane AH, Ockenhouse CF, Nussenzweig RS. 1984. Identification of circumsporozoite proteins in individual malaria-infected mosquitoes by Western blot analysis. J Immunol Methods 71:241–245. doi: 10.1016/0022-1759(84)90070-X. [DOI] [PubMed] [Google Scholar]
- 19.Santoro F, Cochrane AH, Nussenzweig V, Nardin EH, Nussenzweig RS, Gwadz RW, Ferreira A. 1983. Structural similarities among the protective antigens of sporozoites from different species of malaria parasites. J Biol Chem 258:3341–3345. [PubMed] [Google Scholar]
- 20.Doolan DL, Hoffman SL. 1999. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J Immunol 163:884–892. [PubMed] [Google Scholar]
- 21.Doolan DL, Hoffman SL. 2000. The complexity of protective immunity against liver-stage malaria. J Immunol 165:1453–1462. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 22.Schofield L, Ferreira A, Altszuler R, Nussenzweig V, Nussenzweig RS. 1987. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J Immunol 139:2020–2025. [PubMed] [Google Scholar]
- 23.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. 1987. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330:664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 24.Tsuji M, Miyahira Y, Nussenzweig RS, Aguet M, Reichel M, Zavala F. 1995. Development of antimalaria immunity in mice lacking IFN-gamma receptor. J Immunol 154:5338–5344. [PubMed] [Google Scholar]
- 25.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 26.Kitamura D, Roes J, Kuhn R, Rajewsky K. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues M, Nussenzweig RS, Zavala F. 1993. The relative contribution of antibodies, CD4+ and CD8+ T cells to sporozoite-induced protection against malaria. Immunology 80:1–5. [PMC free article] [PubMed] [Google Scholar]
- 28.Chakravarty S, Baldeviano GC, Overstreet MG, Zavala F. 2008. Effector CD8+ T lymphocytes against liver stages of Plasmodium yoelii do not require gamma interferon for antiparasite activity. Infect Immun 76:3628–3631. doi: 10.1128/IAI.00471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheiblhofer S, Chen D, Weiss R, Khan F, Mostbock S, Fegeding K, Leitner WW, Thalhamer J, Lyon JA. 2001. Removal of the circumsporozoite protein (CSP) glycosylphosphatidylinositol signal sequence from a CSP DNA vaccine enhances induction of CSP-specific Th2 type immune responses and improves protection against malaria infection. Eur J Immunol 31:692–698. doi:. [DOI] [PubMed] [Google Scholar]
- 30.Ansong D, Asante KP, Vekemans J, Owusu SK, Owusu R, Brobby NA, Dosoo D, Osei-Akoto A, Osei-Kwakye K, Asafo-Adjei E, Boahen KO, Sylverken J, Adjei G, Sambian D, Apanga S, Kayan K, Janssens MH, Lievens MJ, Olivier AC, Jongert E, Dubois P, Savarese BM, Cohen J, Antwi S, Greenwood BM, Evans JA, Agbenyega T, Moris PJ, Owusu-Agyei S. 2011. T cell responses to the RTS,S/AS01(E) and RTS,S/AS02(D) malaria candidate vaccines administered according to different schedules to Ghanaian children. PLoS One 6:e18891. doi: 10.1371/journal.pone.0018891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bijker EM, Bastiaens GJ, Teirlinck AC, van Gemert GJ, Graumans W, van de Vegte-Bolmer M, Siebelink-Stoter R, Arens T, Teelen K, Nahrendorf W, Remarque EJ, Roeffen W, Jansens A, Zimmerman D, Vos M, van Schaijk BC, Wiersma J, van der Ven AJ, de Mast Q, van Lieshout L, Verweij JJ, Hermsen CC, Scholzen A, Sauerwein RW. 2013. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci U S A 110:7862–7867. doi: 10.1073/pnas.1220360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White MT, Bejon P, Olotu A, Griffin JT, Bojang K, Lusingu J, Salim N, Abdulla S, Otsyula N, Agnandji ST, Lell B, Asante KP, Owusu-Agyei S, Mahama E, Agbenyega T, Ansong D, Sacarlal J, Aponte JJ, Ghani AC. 2014. A combined analysis of immunogenicity, antibody kinetics and vaccine efficacy from phase 2 trials of the RTS,S malaria vaccine. BMC Med 12:117. doi: 10.1186/PREACCEPT-5212460251240508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oakley MS, Sahu BR, Lotspeich-Cole L, Solanki NR, Majam V, Pham PT, Banerjee R, Kozakai Y, Derrick SC, Kumar S, Morris SL. 2013. The transcription factor T-bet regulates parasitemia and promotes pathogenesis during Plasmodium berghei ANKA murine malaria. J Immunol 191:4699–4708. doi: 10.4049/jimmunol.1300396. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt NW, Butler NS, Badovinac VP, Harty JT. 2010. Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog 6:e1000998. doi: 10.1371/journal.ppat.1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ndungu FM, Mwacharo J, Kimani D, Kai O, Moris P, Jongert E, Vekemans J, Olotu A, Bejon P. 2012. A statistical interaction between circumsporozoite protein-specific T cell and antibody responses and risk of clinical malaria episodes following vaccination with RTS,S/AS01E. PLoS One 7:e52870. doi: 10.1371/journal.pone.0052870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbosa A, Naniche D, Aponte JJ, Manaca MN, Mandomando I, Aide P, Sacarlal J, Renom M, Lafuente S, Ballou WR, Alonso PL. 2009. Plasmodium falciparum-specific cellular immune responses after immunization with the RTS,S/AS02D candidate malaria vaccine in infants living in an area of high endemicity in Mozambique. Infect Immun 77:4502–4509. doi: 10.1128/IAI.00442-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun P, Schwenk R, White K, Stoute JA, Cohen J, Ballou WR, Voss G, Kester KE, Heppner DG, Krzych U. 2003. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. J Immunol 171:6961–6967. doi: 10.4049/jimmunol.171.12.6961. [DOI] [PubMed] [Google Scholar]
- 38.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H. 2011. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Figueiredo MM, Costa PAC, Diniz SQ, Henriques PM, Kano FS, Tada MS, Pereira DB, Soares IS, Martins-Filho OA, Jankovic D, Gazzinelli RT, Antonelli L. 2017. T follicular helper cells regulate the activation of B lymphocytes and antibody production during Plasmodium vivax infection. PLoS Pathog 13:e1006484. doi: 10.1371/journal.ppat.1006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obeng-Adjei N, Portugal S, Tran TM, Yazew TB, Skinner J, Li S, Jain A, Felgner PL, Doumbo OK, Kayentao K, Ongoiba A, Traore B, Crompton PD. 2015. Circulating Th1-cell-type Tfh cells that exhibit impaired B cell help are preferentially activated during acute malaria in children. Cell Rep 13:425–439. doi: 10.1016/j.celrep.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kisalu NK, Idris AH, Weidle C, Flores-Garcia Y, Flynn BJ, Sack BK, Murphy S, Schon A, Freire E, Francica JR, Miller AB, Gregory J, March S, Liao HX, Haynes BF, Wiehe K, Trama AM, Saunders KO, Gladden MA, Monroe A, Bonsignori M, Kanekiyo M, Wheatley AK, McDermott AB, Farney SK, Chuang GY, Zhang B, Kc N, Chakravarty S, Kwong PD, Sinnis P, Bhatia SN, Kappe SHI, Sim BKL, Hoffman SL, Zavala F, Pancera M, Seder RA. 2018. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat Med 24:408–416. doi: 10.1038/nm.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rathore D, Sacci JB, de la Vega P, McCutchan TF. 2002. Binding and invasion of liver cells by Plasmodium falciparum sporozoites. J Biol Chem 277:7092–7098. doi: 10.1074/jbc.M106862200. [DOI] [PubMed] [Google Scholar]
- 43.Kaslow DC, Shiloach J. 1994. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate: TBV25H expressed in yeast and purified using nickel-NTA agarose. Nat Biotechnol 12:494–499. doi: 10.1038/nbt0594-494. [DOI] [PubMed] [Google Scholar]
- 44.Tian JH, Miller LH, Kaslow DC, Ahlers J, Good MF, Alling DW, Berzofsky JA, Kumar S. 1996. Genetic regulation of protective immune response in congenic strains of mice vaccinated with a subunit malaria vaccine. J Immunol 157:1176–1183. [PubMed] [Google Scholar]
- 45.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 46.Bruna-Romero O, Hafalla JC, Gonzalez-Aseguinolaza G, Sano G, Tsuji M, Zavala F. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int J Parasitol 31:1499–1502. doi: 10.1016/S0020-7519(01)00265-X. [DOI] [PubMed] [Google Scholar]
- 47.Overbergh L, Valckx D, Waer M, Mathieu C. 1999. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]