Development of long-term memory is crucial for vaccine-induced adaptive immunity against infectious diseases such as Staphylococcus aureus infection. Toxic shock syndrome toxin 1 (TSST-1), one of the superantigens produced by S. aureus, is a possible vaccine candidate against infectious diseases caused by this pathogen.
KEYWORDS: IL-10, IL-17, Staphylococcus aureus, Th17, toxic shock syndrome toxin 1
ABSTRACT
Development of long-term memory is crucial for vaccine-induced adaptive immunity against infectious diseases such as Staphylococcus aureus infection. Toxic shock syndrome toxin 1 (TSST-1), one of the superantigens produced by S. aureus, is a possible vaccine candidate against infectious diseases caused by this pathogen. We previously reported that vaccination with less toxic mutant TSST-1 (mTSST-1) induced T helper 17 (Th17) cells and elicited interleukin-17A (IL-17A)-mediated protection against S. aureus infection 1 week after vaccination. In the present study, we investigated the host immune response induced by mTSST-1 vaccination in the memory phase, 12 weeks after the final vaccination. The protective effect and IL-17A production after vaccination with mTSST-1 were eliminated because of IL-10 production. In the presence of IL-10-neutralizing monoclonal antibody (mAb), IL-17A production was restored in culture supernatants of CD4+ T cells and macrophages sorted from the spleens of vaccinated mice. Vaccinated mice treated with anti-IL-10 mAb were protected against systemic S. aureus infection in the memory phase. From these results, it was suggested that IL-10 produced in the memory phase suppresses the IL-17A-dependent vaccine effect through downregulation of IL-17A production.
INTRODUCTION
Staphylococcus aureus, a Gram-positive extracellular bacterium, remains one of the most frequent causes of infectious diseases. The diseases caused by this bacterium range from superficial skin infections to life-threatening conditions such as bacteremia, endocarditis, pneumonia, and toxic shock syndrome (TSS) (1). Treatment of S. aureus infections has been increasingly difficult because methicillin-resistant S. aureus (MRSA) strains with resistance to clinically relevant antibiotics have emerged worldwide (2). Therefore, development of effective vaccines to prevent S. aureus infection is urgently needed.
There have been many studies on development of vaccines against S. aureus infection which target bacterial surface components and virulence factors, including capsular polysaccharides, surface antigens, alpha-hemolysin, and Panton-Valentine leucocidin (3). Although the induction of antibody production has been shown to be a critical marker for vaccine efficacy against S. aureus infection, recent studies indicate that T helper 17 (Th17) cell-mediated immunity is important for host defense against this bacterium (3, 4). It has been evidenced that Th17 cells play a critical role in protection against infection with extracellular pathogens, including S. aureus (5, 6), Klebsiella pneumoniae (7), Streptococcus pyogenes (8), and Candida albicans (9), through the production of interleukin-17 (IL-17), which induces chemokine-mediated effective recruitment of neutrophils (10, 11).
Among staphylococcal virulence factors, toxic shock syndrome toxin 1 (TSST-1) is a possible vaccine candidate against S. aureus infection. TSST-1 is one of the superantigens produced by pathogenic strains of S. aureus and is recognized as an important factor causing staphylococcal TSS, which manifests as fever, rash, desquamation, and hypotension (12). Although the prevalence of TSST-1-producing MRSA isolates varies among countries and areas, a majority of MRSA strains isolated from Japanese hospitals have been reported to carry the TSST-1-encoding gene (tst) (13–16).
TSST-1 with a histidine-to-alanine mutation at residue 135 (mutant TSST-1 [mTSST-1]) has been shown to be less toxic, as revealed by in vitro and in vivo experiments in which mTSST-1 showed less superantigenic activity and toxicity in an animal model (17–19). Vaccination with mTSST-1 induced a protective effect against a lethal challenge with recombinant TSST-1 potentiated with lipopolysaccharide or lethal S. aureus infection (20, 21). We previously reported that vaccination with mTSST-1 induced differentiation of Th17 cells and that mTSST-1-vaccinated mice produced a significant amount of IL-17A by Th17 cells but not IL-17-producing CD4+ T cell receptor γδ-positive (TCRγδ+) cells after challenge with S. aureus 1 week after the final vaccination (22). Moreover, vaccinated wild-type but not IL-17-deficient mice elicited protection against systemic S. aureus infection by inducing the recruitment of neutrophils and macrophages to the infectious foci (22).
Development of long-term memory is crucial for vaccine-induced adaptive immunity. Th1 and Th2 lineages are mutually exclusive and stable in their phenotypes and functions. However, plasticity of the Th17 lineage has been argued. For instance, tuberculosis subunit vaccine-induced Th17 memory cells exhibit lineage stability by retaining both phenotypic and functional properties for nearly 2 years in mice (23). It was also reported that Th17 cells are comparatively stable and retain the potential to produce IL-17 in the environment of allergic inflammation (24). In contrast to these reports, it was documented that Th17 cells exhibit a great degree of plasticity, which allows functional adaptation to various physiological situations, acquiring the capacity to produce gamma interferon (IFN-γ) or IL-10 as well as IL-17 or completely altering profiles of cytokine production (25–28). Therefore, it is not clear whether Th17 cells are stable and terminally differentiated or exhibit considerable plasticity.
In this study, we investigated the functional properties of Th17 cells and the protective effect against S. aureus infection induced by mTSST-1 vaccination in the memory phase, 12 weeks after the final vaccination. The protective effect of vaccination with mTSST-1 was eliminated because of IL-10 production. The protective effect was recovered by blockade of IL-10. We demonstrate that Th17-mediated adaptive protection against systemic S. aureus infection is masked by a change to an IL-10-dominant response in the memory phase during vaccination with mTSST-1.
RESULTS
Altered cytokine profile in spleen cells of mTSST-1-vaccinated mice in the memory phase.
We previously reported that vaccination with mTSST-1 induced antigen-specific Th17 cells 1 week after the final vaccination in mice (22). However, Th17 cell subpopulations were reported to display a high grade of plasticity and enter the memory pool less efficiently (25–28). Hence, we evaluated cytokine profiles of spleen cell cultures stimulated with mTSST-1 2 weeks and 12 weeks after the final vaccination. The level of IL-17A but not IL-10 production was higher in the culture supernatants of spleen cells of mTSST-1-vaccinated mice than in those of nonvaccinated mice 2 weeks after the final vaccination (Fig. 1A). Twelve weeks after the final vaccination, IL-10 production was significantly augmented by stimulation with mTSST-1 in the spleen cell cultures of vaccinated mice (Fig. 1B). In contrast, the production of neither IL-17A nor IFN-γ was upregulated by mTSST-1 stimulation (Fig. 1B). These results suggested that the production of IL-10 is induced instead of IL-17A by the specific antigen in the memory phase of vaccination with mTSST-1.
FIG 1.
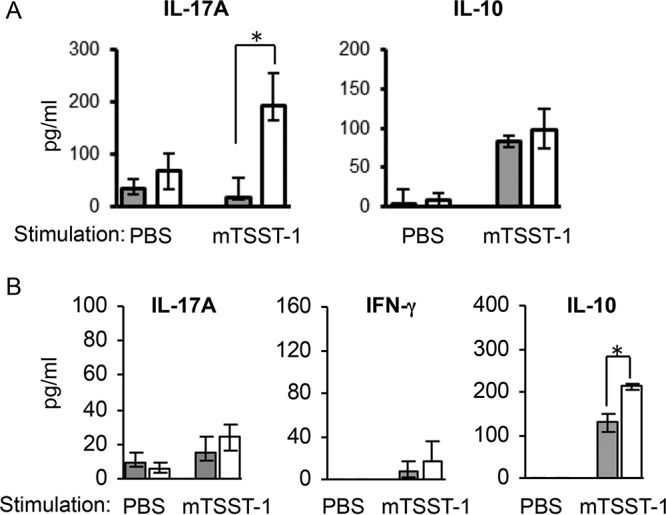
Cytokine responses in spleen cells of mTSST-1-vaccinated mice in the effector phase and the memory phase. Mice were vaccinated with mTSST-1 plus alum (white bars) or alum only (gray bars). Spleen cells of both groups of mice were prepared 2 weeks (A) and 12 weeks (B) after the final vaccination. These cells were stimulated with mTSST-1. Concentrations of IL-17A, IFN-γ, and IL-10 in the culture supernatants were quantified by an ELISA. Data in panel A are expressed as medians ± interquartile ranges for a group of 4 mice from 2 independent experiments. Data in panel B are expressed as medians ± interquartile ranges for a group of 6 mice from 2 independent experiments (B). An asterisk represents a statistically significant difference from the nonvaccinated group at a P value of <0.05.
Augmented IL-10 production but not IL-17 production in memory T cells of mTSST-1-vaccinated mice.
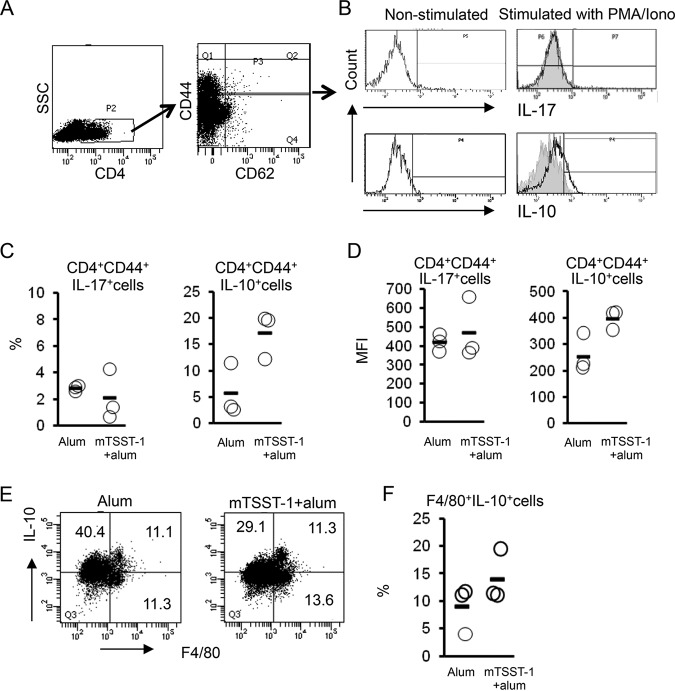
To investigate the cellular source of IL-10, flow cytometric analysis was performed using spleen cells isolated from mTSST-1-vaccinated mice in the memory phase. The spleen cells were obtained 12 weeks after the final vaccination and stimulated with p-phorbol-12-myristate-13-acetate (PMA) and ionomycin. The stimulated cells were gated on CD4 and then gated on CD44 and CD62L. A large amount of CD4+ CD44+ CD62low effector memory T (TEM) cells was observed in the spleens of the vaccinated mice, whereas only a small population of CD4+ CD44+ CD62high central memory T (TCM) cells was detected (Fig. 2A). Flow cytometric analysis showed that the population of IL-17A-producing memory Th cells in the spleens of mTSST-1-vaccinated mice was similar to that in nonvaccinated mice, whereas IL-10-producing memory Th cells were increased in the vaccinated mice (Fig. 2B to D). To determine whether the source of IL-10 is associated with macrophages, flow cytometric analysis using a macrophage marker was performed. Spleen cells obtained from vaccinated or nonvaccinated mice 12 weeks after the final vaccination were stimulated with mTSST-1. Flow cytometric analysis revealed that the ratio of IL-10-producing F4/80+ macrophages in the spleens of vaccinated mice was similar to that in nonvaccinated mice (Fig. 2E and F). Although the spleen contains several other immune cells that are capable of IL-10 production, such as B, NK, and NKT cells (29, 30), our results suggested that TEM cells are one of the major IL-10-producing populations in the memory phase of mTSST-1 vaccination.
FIG 2.
IL-17A and IL-10 production by CD4+ T cells and macrophages of mTSST-1-vaccinated mice in the memory phase. Mice were vaccinated with mTSST-1 plus alum or alum only. The spleen cells of both groups of mice were prepared 12 weeks after the final vaccination. (A) Spleen cells were gated on CD4 and then gated on CD44 and CD62L. SSC, side scatter. (B) Intracellular IL-17A or IL-10 expression in CD4+ CD44+ T cells of vaccinated mice or nonvaccinated mice was assessed by flow cytometry. Data are displayed in an overlay histogram. Black tracings represent nonstimulated or PMA- and ionomycin (Iono)-stimulated CD4+ CD44+ T cells in the spleens of vaccinated mice. Gray-shaded histograms represent PMA- and ionomycin-stimulated CD4+ CD44+ T cells of nonvaccinated mice. (C) Frequencies (indicated by circles) of IL-17A+ cells and IL-10+ cells in CD4+ CD44+ T cells. Horizontal lines indicate group means. Each result represents data for a group of 3 mice. (D) Mean fluorescence intensities (MFI) (indicated by circles) of IL-17A+ cells and IL-10+ cells in CD4+ CD44+ T cells. Horizontal lines indicate group means. (E) F4/80+ IL-10+ cells in spleen cells of vaccinated mice and nonvaccinated mice were assessed by flow cytometry. The results shown are representative of data from experiments. (F) Frequencies (indicated by circles) of F4/80+ IL-10+ cells. Each result represents data for a group of 3 mice.
Cytokine mRNA expression in the spleen and liver of mTSST-1-vaccinated and S. aureus-infected mice in the memory phase.
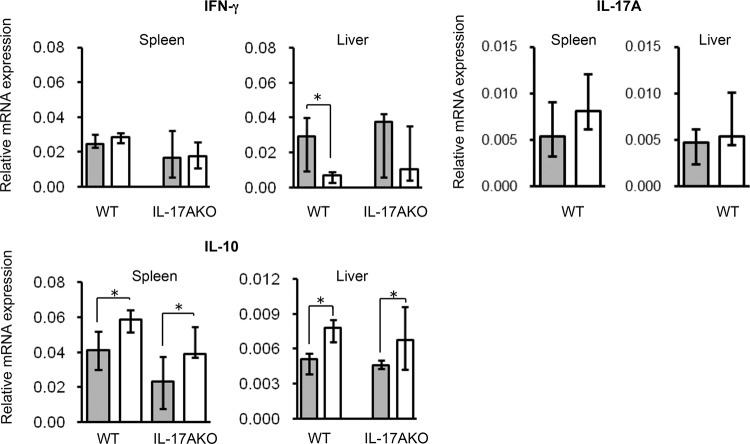
mTSST-1-vaccinated and nonvaccinated mice were infected with S. aureus in the memory phase, and cytokine mRNA expression was measured 24 h after infection. As shown in Fig. 3, IFN-γ mRNA expression in the spleens of mTSST-1-vaccinated mice was comparable to that in nonvaccinated mice. The IFN-γ mRNA expression level in the livers of vaccinated mice was lower than that in nonvaccinated mice. IL-17A mRNA expression in the spleens and livers of vaccinated mice was similar to that in nonvaccinated mice. On the other hand, the IL-10 mRNA expression level in the spleens and livers of mTSST-1-vaccinated mice was higher than that in nonvaccinated mice.
FIG 3.
Cytokine mRNA expression in the spleens and livers of mTSST-1-vaccinated mice in the memory phase. Wild-type (WT) and IL-17A knockout (IL-17AKO) mice were vaccinated with mTSST-1 plus alum (white bars) or alum only (gray bars). The spleens and livers of both groups of mice were excised 24 h after infection. Total mRNA was extracted, and IL-17A, IFN-γ, and IL-10 mRNA expression in the organs was measured as described in Materials and Methods. Data are expressed as medians ± interquartile ranges for a group of 8 mice from 2 independent experiments. An asterisk represents a statistically significant difference from the nonvaccinated group at a P value of <0.05.
Impaired host resistance of mTSST-1-vaccinated mice against systemic S. aureus infection in the memory phase.
We reported previously that vaccination with mTSST-1 elicited Th17-dependent and IL-17-mediated host defense against systemic S. aureus infection 1 week after the final vaccination (22). However, IL-17 production was downregulated and IL-10 production was upregulated in mTSST-1-vaccinated mice 12 weeks after the final vaccination (Fig. 1 to 3). Hence, we evaluated whether the protective effect of mTSST-1 vaccination is maintained in the memory phase. IL-17A-deficient and wild-type mice were vaccinated with mTSST-1 and infected with 1 × 108 CFU of S. aureus intravenously 12 weeks after the final vaccination, and their survival rates were observed for 14 days. Most of the mTSST-1-vaccinated wild-type mice and IL-17A-deficient mice died, as did the nonvaccinated wild-type mice and IL-17A-deficient mice, until day 14 after infection (Fig. 4A). Next, mice were infected with 5 × 107 CFU of S. aureus intravenously 12 weeks after the final vaccination to assess bacterial elimination from the spleens and livers. The bacterial numbers in the organs were comparable among these 4 groups on day 3 after infection (Fig. 4B). These results demonstrated that IL-17-mediated protective immunity by vaccination with mTSST-1 is not elicited in the memory phase.
FIG 4.
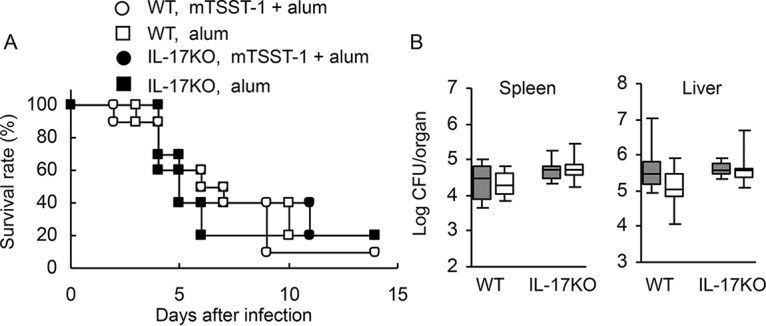
Survival rates and bacterial numbers in the organs of vaccinated or nonvaccinated wild-type and IL-17A-deficient mice in the memory phase. Wild-type and IL-17A-deficient mice were vaccinated with mTSST-1 plus alum or alum only. These mice were infected with S. aureus 12 weeks after the final vaccination. (A) Deaths of mice were observed for 14 days after infection. Each result represents data for a group of 10 mice from 2 independent experiments. (B) Bacterial numbers in the spleens and livers of vaccinated (open boxes) and nonvaccinated (shaded boxes) mice were determined on day 3 after infection. Each result represents data for a group of 8 mice from 2 independent experiments. Data are presented as box plots, with the boxes representing the interquartile ranges. The median value is represented by the line across each box.
Neutralization of IL-10 enhances IL-17 production in CD4+ cells of mTSST-1-vaccinated mice.
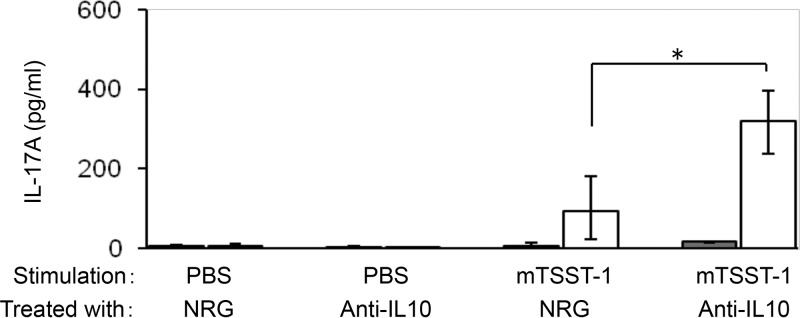
IL-10 has been known to suppress Th cell responses (31–34). To evaluate whether IL-10 is involved in the decreased IL-17 production by CD4+ cells of mTSST-1-vaccinated mice, CD4+ cells and macrophages of mTSST-1-vaccinated or nonvaccinated mice were obtained 12 weeks after the final vaccination and stimulated with mTSST-1 in the presence of anti-IL-10 monoclonal antibody (mAb) or normal rat globulin (NRG). Although anti-IL-10 mAb failed to affect IL-17 production under conditions without mTSST-1 stimulation, IL-17 production in the culture supernatant of CD4+ cells and macrophages of the vaccinated but not the nonvaccinated mice was significantly augmented by stimulation with mTSST-1 in the presence of anti-IL-10 mAb (Fig. 5). These results indicated that the diminution of the IL-17A response in the memory phase is not due to a disappearance or unresponsiveness of IL-17A-producing cells, but the IL-17A response is suppressed by the superior production of IL-10 which is produced after stimulating the cells with mTSST-1.
FIG 5.
IL-17A production by CD4+ T cells of mTSST-1-vaccinated mice in the presence of anti-IL-10 mAb. CD4+ T cells and F4/80+ macrophages of vaccinated (open bars) and nonvaccinated (shaded bars) mice 12 weeks after the final vaccination were sorted and incubated with mTSST-1 in the presence of anti-IL-10 mAb or NRG for 48 h. IL-17A concentrations in the cell culture supernatants were quantified by ELISAs. Data are expressed as medians ± interquartile ranges for a group of 4 to 6 mice from 2 independent experiments. An asterisk represents a statistically significant difference from the nonvaccinated group at a P value of <0.05.
Neutralization of IL-10 restores IL-17A mRNA expression in the spleens of mTSST-1-vaccinated mice after S. aureus infection.
Twelve weeks after the final vaccination, mTSST-1-vaccinated and nonvaccinated mice were administered either anti-IL-10 mAb or NRG and infected with S. aureus 24 h after administration. Spleens of the infected mice were taken 24 h after infection. Anti-IL-10 mAb administration failed to enhance IFN-γ mRNA expression in the spleens of both vaccinated and nonvaccinated wild-type mice (Fig. 6A). On the other hand, IL-17A mRNA expression was restored by anti-IL-10 mAb administration in the spleens of mTSST-1-vaccinated but not in nonvaccinated mice (Fig. 6B).
FIG 6.
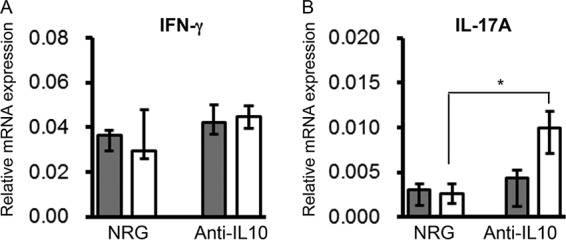
Cytokine mRNA expression in spleens of mTSST-1-vaccinated and anti-IL-10 mAb-treated mice after S. aureus infection in the memory phase. Wild-type mice were vaccinated with mTSST-1 plus alum (white bars) or alum only (gray bars). Twelve weeks after the final vaccination, mice were treated with anti-IL-10 mAb or NRG 1 day before infection. Spleens were excised 24 h after infection. Total mRNA was extracted, and IFN-γ (A) and IL-17A (B) mRNA expression levels in the spleens were measured as described in Materials and Methods. Data are expressed as medians ± interquartile ranges for a group of 8 mice from 2 independent experiments. An asterisk represents a statistically significant difference from the nonvaccinated group at a P value of <0.05.
Neutralization of IL-10 restores the protective effect of mTSST-1 vaccination against systemic S. aureus infection in the memory phase.
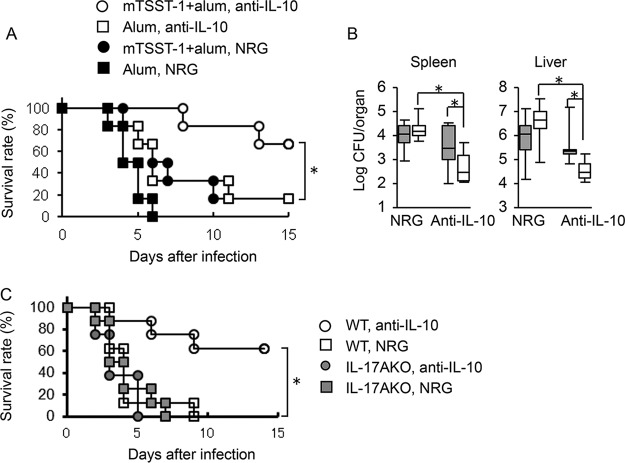
As neutralization of IL-10 by the corresponding mAb restored IL-17 production and mRNA expression in mTSST-1-vaccinated mice in vitro and in vivo (Fig. 5 and 6), we investigated whether the administration of neutralizing anti-IL-10 mAb is able to restore IL-17-mediated host defense against systemic S. aureus infection. Twelve weeks after the final vaccination with mTSST-1, the mice were administered anti-IL-10 mAb or NRG and then challenged with S. aureus 24 h later. The survival rate of the vaccinated and anti-IL-10 mAb-administered mice was 66.7% 14 days after challenge, whereas that of the vaccinated and NRG-administered mice and the nonvaccinated and anti-IL-10 mAb-injected mice was 16.7% equally (Fig. 7A). The bacterial numbers in the spleens and livers were also determined on day 3 after infection. Numbers of S. aureus cells in the spleens and livers of the vaccinated and anti-IL-10 mAb-administered mice were reduced significantly, compared with those in the mTSST-1-vaccinated and NRG-administered mice and the nonvaccinated and anti-IL-10 mAb-administered mice (Fig. 7B). The survival rate of the vaccinated wild-type mice was improved by the administration of anti-IL-10 mAb, whereas that of the IL-17A-deficient vaccinated mice was not (Fig. 7C). These results suggested that the IL-17A-mediated protective effect in mTSST-1-vaccinated mice is suppressed by the superiorly produced IL-10 in the memory phase.
FIG 7.
Effect of anti-IL-10 mAb administration on survival rates and bacterial numbers in the organs of mTSST-1-vaccinated mice in the memory phase. Mice were vaccinated with mTSST-1 plus alum or alum only. Both groups of mice were administered anti-IL-10 mAb or NRG 12 weeks after the final vaccination and then infected with S. aureus 24 h later. (A) The deaths of mice were observed for 14 days after infection. Each result represents data for a group of 9 mice from 2 independent experiments. (B) Bacterial numbers in the spleens and livers of vaccinated (open boxes) and nonvaccinated (shaded boxes) mice were determined on day 3 after infection. Each result represents data for a group of 8 to 9 mice from 3 independent experiments. Data are presented as box plots, with the boxes representing the interquartile ranges. The median value is represented by the line across each box. (C) mTSST-1-vaccinated wild-type and IL-17A knockout mice were administered anti-IL-10 mAb or NRG 12 weeks after the final vaccination and then infected with S. aureus 24 h later. Each result represents data for a group of 8 to 9 mice from 2 or 3 independent experiments. An asterisk represents a statistically significant difference at a P value of <0.05.
DISCUSSION
There has been a consensus that antibodies complete host defense against S. aureus infection in adaptive immunity. This concept was overlooked by a recent paper concerning immunity to S. aureus (35). Our previous study showed that vaccination with mTSST-1 or the fibrinogen-binding domain of clumping factor A provided protection against lethal S. aureus infection but that only antibody failed to give complete protection (21, 36). Similar results were shown for other potential vaccines for S. aureus infection, including iron-regulated surface determinant B (V710 vaccine) and C. albicans adhesion agglutin-like sequence 3 protein (37–40). Alternatively, these studies suggested that Th17 response-mediated protective immunity plays a critical role in vaccine effectiveness against S. aureus infection.
We previously reported that vaccination with mTSST-1 induced Th17 cells and elicited IL-17-mediated immune responses to S. aureus systemic infection 1 week after the final vaccination (22). However, Th17 cells have been shown to exhibit a high degree of plasticity and may not stably maintain their function to produce lineage-specific cytokines (25–27). These studies raise the question of whether a long-term Th17-based vaccine is able to be established or not. Hence, we evaluated IL-17A production in the spleen cells of mTSST-1-vaccinated mice in the effector phase 2 weeks after the final vaccination and in the memory phase 12 weeks after the final vaccination. Although these cells produce a large amount of IL-17A in the effector phase (Fig. 1A), the spleen cells of vaccinated mice failed to produce IL-17A in the memory phase (Fig. 1B). These results suggested that the Th response of mTSST-1-vaccinated mice fails to stably maintain its function to produce IL-17 in the memory phase.
Memory CD4+ T cells are subdivided into TEM cells and TCM cells. TEM cells, which show low surface expression levels of CD62L, are subsequently recruited to the infection sites to combat microbial infections. In contrast, TCM cells express CD62L highly on the cell surface and expand in lymphoid tissues after restimulation with the antigen (41). Vaccine-specific CD4+ T cells have been reported to consist mainly of the TEM type, whereas bystander memory T cells mainly exhibit a TCM phenotype (42). Consistent with data from this report, the CD4+ CD44+ memory T cell subset in the spleen of mTSST-1-vaccinated mice mainly expressed a low level of CD62L (Fig. 2A), which correlates with the TEM phenotype.
It was reported previously that IL-17 was released by CD8+ cells and that γδ T cells were a significant source of IL-17 (43). However, our previous study revealed that mTSST-1 vaccination did not induce IL-17A-producing γδ T cells in mice (22). Our present results suggested that TEM cells induced by mTSST-1 vaccination might be the major IL-17A-producing cells, although there is a possibility that other cells, such as CD8+ T cells, also produce IL-17A. Moreover, we also show that these TEM cells exhibit plasticity that fails to continue the stable maintenance of their function to produce IL-17A.
It was suggested previously that in vivo- but not in vitro-generated Th17 cells retain their phenotype (23). Th17 cells generated in vitro were converted into Th1 or Th2 cells in response to IL-12 and IL-4, respectively, whereas in vivo-generated Th17 cells were stable, and their phenotype was regulated by neither IL-12 nor IL-4 (44). In contrast, Th17 cells under chronic inflammatory conditions of experimental autoimmune encephalomyelitis reportedly produce IFN-γ but not IL-17A (28). In addition, Th17 cells in the memory phase of acute cutaneous infection with C. albicans produce neither IFN-γ nor IL-17A (28). Both in vivo-primed C. albicans- and S. aureus-specific memory Th17 cells downregulate IL-17A production, whereas S. aureus-specific clones upregulate IL-10 production by restimulation, and the increased amount of IL-10 produced in memory T cells is significantly inhibited by IL-1β (27). In the present study, it is possible that TEM cells induced by mTSST-1 vaccination altered their phenotype in the memory phase to produce a significant amount of IL-10 but not IL-17A (Fig. 2B to D). In the in vitro human system, Zielinski et al. demonstrated that S. aureus-specific memory Th17 cells show a marked induction of IL-10 and reduced expression of IL-17 5 days after restimulation, but the levels of these cytokines reverse after extended incubation beyond 5 days (27). In our hands, this possibility is not conceivable because the survival rates of mTSST-1-vaccinated and nonvaccinated mice were similar for 14 days after infection (Fig. 4A).
Spleen cells of nonvaccinated mice produced levels of IL-10 similar to those in mTSST-1-vaccinated mice by stimulation with mTSST-1 2 weeks after the final vaccination (Fig. 1A), while the level of IL-10 production in the spleen cells of the vaccinated mice was higher than that in the nonvaccinated mice in the memory phase (Fig. 1B). Spleen cells include several types of IL-10-producing immune and nonimmune cells, such as CD8+ cells, B cells, and endothelial cells (29, 30). Although the involvement of other cell types excluding TEM cells and macrophages was not investigated in our study, specific TEM cells are suggested to be one of the major populations of IL-10-producing cells in the memory phase of mTSST-1 vaccination.
IL-10 has been reported to modulate the host immune response during S. aureus infection, such as downregulation of the adaptive Th17 responses and suppression of macrophage and neutrophil migration and functions (31). The elevated IL-10 level allows S. aureus to spread and cause severe invasive infections (32). IL-10 produced by neutrophils specifically targets IL-10 receptor α (IL-10Rα)-expressing Th17 cells, and IL-17A production is shut down in mycobacterial infection (33). IL-17A-producing CD4+ T cells express IL-10Rα, and both Th17 and Th1 cells are controlled in an IL-10-dependent manner during intestinal inflammation (34). In the present study, IL-17A production was upregulated in the cultures of CD4+ T cells and macrophages of vaccinated mice by stimulation with mTSST-1 in the presence of anti-IL-10 mAb (Fig. 5). IL-10-producing cells in the memory phase of mTSST-1-vaccinated mice were not macrophages, but TEM cells were one of the major populations of IL-10-producing cells (Fig. 2). Thus, it is possible that IL-10 produced by mTSST-1-specific TEM cells downregulates IL-17A production through autocrine signaling via their IL-10R.
Consistent with the restored ability of TEM cells to produce IL-17A by neutralizing IL-10 in vitro (Fig. 5), IL-17A mRNA expression in the spleens of mTSST-1-vaccinated mice was increased by injection of anti-IL-10 mAb but not NRG after S. aureus infection (Fig. 6), and vaccination with mTSST-1 elicited a protective effect in anti-IL-10 mAb-injected wild-type but not IL-17A-deficient mice during the memory phase (Fig. 7). It was reported previously that Th17 cells can transdifferentiate into regulatory T (Treg) cells (45). However, upregulation of Foxp3, the master gene of Treg cells, was not observed in the spleen cells of mTSST-1-vaccinated mice 12 weeks after the final vaccination (data not shown), indicating that IL-10 production might not be due to Treg cells, which are transdifferentiated from Th17 cells.
In the present study, we demonstrate that the mTSST-1-induced protective effect was abrogated due to a reduced function of TEM cells to produce IL-17 in the memory phase. It was suggested that IL-10 produced by memory Th17 cells suppressed their ability to produce IL-17 through an autocrine mechanism and that blockade of IL-10 restored IL-17 production in memory Th17 cells and IL-17-mediated protection against systemic S. aureus infection. These findings would be useful for the development of successful Th17-based vaccines against infectious diseases from the viewpoint of IL-10 blockade.
MATERIALS AND METHODS
Animals.
C57BL/6 mice purchased from Clea Japan, Tokyo, Japan, and IL-17A gene-deficient mice on a C57BL/6 background (46) were used in this study. Mice were maintained under specific-pathogen-free conditions at the Institute for Animal Experimentation, Hirosaki University Graduate School of Medicine. Food and water were given ad libitum. All animal experiments were carried out in accordance with the Institutional Animal Care and Use Committees (IACUC)/ethics committees of Hirosaki University.
Expression and purification of mTSST-1.
Expression and purification of mTSST-1 were performed as described previously (21). Briefly, Escherichia coli DH5α cells harboring pGXmTST were cultured in 2× YT medium, which contains 16 g/liter tryptone (BD Diagnostic Systems), 10 g/liter yeast extract (BD Diagnostic Systems), 5 g/liter NaCl (Wako Pure Chemical Industries), and 100 μg/ml of ampicillin, at 37°C with shaking. Next, isopropyl-1-thio-β-d-galactopyranoside was added to a final concentration of 0.5 mM for inducing the expression of the glutathione S-transferase (GST)–mTSST-1 fusion protein. The bacteria were cultured for an additional 3 h and harvested by centrifugation. The fusion protein was extracted from bacterial pellets by using B-PER bacterial protein extraction reagent (Pierce, Rockford, IL). Purification of the fusion protein and removal of the GST tag were performed by using bulk GST purification modules (Amersham Pharmacia Biotech, Piscataway, NJ). The concentrations of mTSST-1 were determined by a Bradford assay (Bio-Rad Laboratories, Hercules, CA). The endotoxin content in the purified materials was determined by a Limulus amebocyte lysate assay (Cape Cod Inc., Falmouth, MA). Th endotoxin concentrations in mTSST-1 were less than 8.6 pg/μg.
Bacterial strain and culture conditions.
S. aureus strain 834, a clinical sepsis isolate (47), was grown in tryptic soy broth (BD Diagnostics Systems, Sparks, MD) for 15 h. The bacterial cells were harvested by centrifugation, washed with sterile phosphate-buffered saline (PBS), and diluted with PBS to appropriate cell concentrations as determined by spectrophotometry at 550 nm.
Vaccination and infection of mice.
For vaccination, purified mTSST-1 was diluted in PBS and emulsified at a 1:1 ratio in alum adjuvant (Pierce, Rockford, IL). Five- to eight-week-old wild-type and IL-17A-deficient mice were subcutaneously injected with 200 μl of the emulsion containing 10 μg of mTSST-1 or PBS as a control on days 0, 14, and 28. In some experiments, the vaccinated mice were injected with 1 mg of rat anti-IL-10 mAb intravenously 24 h before S. aureus infection. Anti-IL-10 mAb was prepared from JES5-2A5 hybridoma cells as described previously (48). To determine the survival rate after bacterial infection, the vaccinated mice were infected with 1 × 108 CFU of S. aureus per mouse intravenously 2 weeks or 12 weeks after the final vaccination. Survival was monitored for 14 days. To estimate bacterial numbers in the organs, the vaccinated mice were challenged with 5 × 107 CFU of S. aureus per mouse 12 weeks after the final vaccination. The number of viable S. aureus bacteria in the organs was determined by preparing organ homogenates in PBS and plating 10-fold serial dilutions on tryptic soy agar (BD Diagnostics Systems). Colonies were counted after 24 h of incubation at 37°C.
Cell culture.
Spleens were collected from mice 2 weeks and 12 weeks after the final vaccination. Spleen cells were obtained by squeezing the spleens in RPMI 1640 medium (Nissui Pharmaceutical Co., Tokyo, Japan) and filtering the cells through stainless steel mesh (size, 100). Erythrocytes were lysed with 0.85% NH4Cl. After washing 3 times with RPMI 1640 medium, the spleen cells were suspended at a concentration of 2 × 106 cells/ml in RPMI 1640 medium supplemented with 10% fetal calf serum (JRH Biosciences, Lenexa, KS), 1% l-glutamine (Wako Pure Chemical Industries, Osaka, Japan), 100 U per ml of penicillin G, and 100 μg per ml of streptomycin and incubated in a 24-well culture plate. For cytokine assays, the spleen cells were incubated at 37°C in the presence of 0.1 μg/ml of mTSST-1 for 48 h. In some experiments, CD4+ cells and macrophages were stimulated with 0.1 μg/ml of mTSST-1 in the presence of 10 μg/ml rat anti-IL-10 mAb or NRG at 37°C for 48 h (49). The culture supernatants were harvested and stored at −80°C until the cytokine assays were performed.
Cytokine assays.
IFN-γ, IL-10, and IL-17A were quantified by double-sandwich enzyme-linked immunosorbent assays (ELISAs). IFN-γ was quantified as described previously (25). IL-10 and IL-17A were quantified using an IL-10 mouse antibody pair (Thermo Fisher Scientific, Waltham, MA) and an IL-17 ELISA (Bender MedSystems GmbH, Vienna, Austria), respectively.
Intracellular cytokine staining, flow cytometric analysis, and cell sorting.
Spleen cells of vaccinated and nonvaccinated mice were prepared 12 weeks after the final vaccination. For flow cytometric analysis of cytokine production in the memory T cell population, the spleen cells were stimulated with 50 ng/ml of PMA (Sigma-Aldrich, St. Louis, MO) and 1 μg/ml of ionomycin (Sigma-Aldrich) at 37°C. For analysis of IL-10 production in macrophages, spleen cells were stimulated with 0.1 μg/ml of mTSST-1. GolgiStop containing monensin (BD Biosciences, San Jose, CA) was added 2 h later. At 6 h of cell stimulation, the cell surface was stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD4 mAb (RM4-5), phycoerythrin (PE)-Cy7-conjugated anti-CD44 mAb (IM7), and allophycocyanin (APC)-conjugated anti-CD62L mAb (MEL-14) (eBioscience, San Diego, CA) or FITC-conjugated anti-F4/80 mAb (CI:A3-1) (BD Biosciences) for 30 min at 4°C. Next, intracellular staining was performed according to the manufacturer’s instructions. Briefly, 1 ml of Cytofix/Cytoperm solution (BD Biosciences) was added to a cell suspension with mild mixing and placed for 15 min at 4°C. The fixed cells were washed with 1 ml of BD Perm/Wash solution twice and stained intracellularly with peridinin chlorophyll protein (PerCP)-Cy5.5-conjugated anti-mouse IL-10 mAb (JES5-16E3; eBioscience) or PE-conjugated anti-mouse IL-17A mAb (TC11-18H10; BD Biosciences) for 30 min at 4°C. The stained cells were analyzed on a FACSAria instrument (BD Biosciences). Isotype controls were used to set gates. The data were analyzed with BD FACSDiva software (BD Biosciences). CD4+ cells and F4/80+ cells were sorted from the spleen cells of mTSST-1-vaccinated or nonvaccinated mice using the FACSAria instrument. The sorted cells were used for cell culture. For flow cytometric cell sorting of CD4+ cells and F4/80+ cells, spleen cells were prepared 12 weeks after the final vaccination and stained with FITC-conjugated anti-CD4 mAb (RM4-5) or FITC-conjugated anti-F4/80 mAb as described above. The stained cells were suspended in sorting buffer (1 mM EDTA and 2% bovine serum albumin in PBS). CD4+ cells and F4/80+ cells were sorted using the FACSAria instrument. The sorted cell subsets were used for cell culture.
Real-time reverse transcription-quantitative PCR.
Spleens and livers of wild-type and IL-17A-deficient mice were taken 24 h after S. aureus infection. Real-time reverse transcription-quantitative PCR (RT-PCR) was performed as described previously, using the following PCR primers (35): forward primer 5′-AGCGGCTGACTGAACTCAGATTGTAG-3′ and reverse primer 5′-GTCACAGTTTTCAGCTGTATAGGG-3′ for IFN-γ, forward primer 5′-GCTCCAGAAGGCCCTCAGA-3′ and reverse primer 5′-AGCTTTCCCTCCGCATTGA-3′ for IL-17A, forward primer 5′-GCAGGGGCCAGTACAGCCGGGA-3′ and reverse primer 5′-CCTCAGCCGCATCCTGAGGGTC-3′ for IL-10, and forward primer 5′-TGAAGGTCGGTGTGAACGGATTTGG-3′ and reverse primer 5′-ACGACATACTCAGCACCAGCATCAC-3′ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Statistical analysis.
Data for bacterial counts are presented as box plots. Boxes represent the interquartile ranges, and the whiskers indicate the ranges of all the data. The median value is represented by a line across each box. Data for cytokine production and percentages of cell populations quantified by flow cytometry are expressed as medians ± interquartile ranges. Statistical analyses were performed via a Mann-Whitney U test. For survival experiments, the Kaplan-Meier method was used to obtain survival fractions, and significance was determined by a log rank test. A P value of <0.05 was considered significant.
ACKNOWLEDGMENTS
This study was supported JSPS KAKENHI grant numbers 23390100, 26460517, 16H5185, and 70598622.
We have no conflict of interest to disclose.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Klein E, Smith DL, Laxminarayan R. 2007. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis 13:1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spellberg B, Daum R. 2012. Development of a vaccine against Staphylococcus aureus. Semin Immunopathol 34:261–280. doi: 10.1007/s00281-011-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler VG Jr, Proctor RA. 2014. Where does a Staphylococcus aureus vaccine stand? Clin Microbiol Infect 20(Suppl 5):66–75. doi: 10.1111/1469-0691.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. 2009. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic response. Immunity 30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. 2010. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest 120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shelito JE, Bagby GJ, Nelson S, Charrler K, Pescon JJ, Kolls JK. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dileepan T, Linehan JL, Moon JJ, Pepper M, Jenkins MK, Cleary PP. 2011. Robust antigen specific Th17 T cell response to group A Streptococcus is dependent on IL-6 and intranasal route of infection. PLoS Pathog 7:e1002252. doi: 10.1371/journal.ppat.1002252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang W, Na L, Fidel PL, Schwarzenberger P. 2004. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis 190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 10.Ouyang W, Kolls JK, Zheng Y. 2008. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis MM, Way SS. 2009. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology 126:177–185. doi: 10.1111/j.1365-2567.2008.03017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasheed JK, Arko RJ, Feeley JC, Chandler FW, Thornsberg C, Gibson RJ, Cohen ML, Jeffries CD, Broome CV. 1985. Acquired ability of Staphylococcus aureus to produce toxic shock-associated protein and resulting illness in a rabbit model. Infect Immun 47:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piao C, Karasawa T, Totsuka K, Uchiyama T, Kikuchi K. 2005. Prospective surveillance of community-onset and healthcare-associated methicillin-resistant Staphylococcus aureus isolated from a university-affiliated hospital in Japan. Microbiol Immunol 49:959–970. doi: 10.1111/j.1348-0421.2005.tb03691. [DOI] [PubMed] [Google Scholar]
- 14.Zaraket H, Otsuka T, Saito K, Dohmae S, Takano T, Higuchi W, Ohkubo T, Ozaki K, Takano M, Reva I, Baranovich T, Yamamoto T. 2007. Molecular characterization of methicillin-resistant Staphylococcus aureus in hospitals in Niigata, Japan: divergence and transmission. Microbiol Immunol 51:171–176. doi: 10.1111/j.1348-0421.2007.tb03898. [DOI] [PubMed] [Google Scholar]
- 15.Hu D-L, Omoe K, Inoue F, Kasai T, Yasujima M, Shinagawa K, Nakane A. 2008. Comparative prevalence of superantigenic toxin genes in meticillin-resistant and meticillin-susceptible Staphylococcus aureus isolates. J Med Microbiol 57:1106–1112. doi: 10.1099/jmm.0.2008/002790-0. [DOI] [PubMed] [Google Scholar]
- 16.Shahini Shams-Abadi M, Halaji M, Hoseini-Alfatemi SM, Gholipour A, Mojtahedi A, Sedigh Ebrahim-Saraie H. 2018. Epidemiology of toxic shock syndrome toxin-1 harboring Staphylococcus aureus obtained from clinical samples in Iran: a systematic review and meta-analysis. Ann Ig 30:391–400. doi: 10.7416/ai.2018.2239. [DOI] [PubMed] [Google Scholar]
- 17.Bonventre PF, Heeg H, Cullen C, Lian CJ. 1993. Toxicity of recombinant toxic shock syndrome toxin 1 and mutant toxins produced by Staphylococcus aureus in a rabbit infection model of toxic shock syndrome. Infect Immun 61:793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonventre PF, Heeg H, Edwards CK III, Cullen CM. 1995. A mutation at histidine residue 135 of toxic shock syndrome toxin yields an immunogenic protein with minimal toxicity. Infect Immun 63:509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cullen CM, Blanco LR, Bonventre PF, Choi E. 1995. A toxic shock syndrome toxin 1 mutant that defines a functional site critical for T-cell activation. Infect Immun 63:2141–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stiles BG, Krakauer T, Bonventre PF. 1995. Biological activity of toxic shock syndrome toxin 1 and a site-directed mutant, H135A, in a lipopolysaccharide-potentiated mouse lethality model. Infect Immun 63:1229–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu DL, Omoe K, Sasaki S, Sashinami H, Sakuraba H, Yokomizo Y, Shinagawa K, Nakane A. 2003. Vaccination with nontoxic mutant toxic shock syndrome toxin 1 protects against Staphylococcus aureus infection. J Infect Dis 188:743–752. doi: 10.1086/377308. [DOI] [PubMed] [Google Scholar]
- 22.Narita K, Hu DL, Asano K, Nakane A. 2015. Vaccination with non-toxic mutant toxic shock syndrome toxin-1 induces IL-17-dependent protection against Staphylococcus aureus infection. Pathog Dis 73:ftv023. doi: 10.1093/femspd/ftv023. [DOI] [PubMed] [Google Scholar]
- 23.Lindenstrøm T, Woodworth J, Dietrich J, Aagaard C, Anderson P, Agger EM. 2012. Vaccine-induced Th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect Immun 80:3533–3544. doi: 10.1128/IAI.00550-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glosson-Byers NL, Sehra S, Stritesky GL, Yu Q, Awe O, Pham D, Bruns HA, Kaplan MH. 2014. Th17 cells demonstrate stable cytokine production in a proallergic environment. J Immunol 193:2631–2640. doi: 10.4049/jimmunol.1401202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Feri G, Frosali F, Giwici F, Romagnani P, Parronchi P, Torelli F, Maggi E, Romagnani S. 2007. Phenotypic and functional features of human Th17 cells. J Exp Med 204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. 2009. Late developmental plasticity in the T helper 17 lineage. Immunity 30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sailusto F. 2012. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 28.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfor H, Wilhelm C, Tolaini M, Menzel U, Garefalald A, Potocnik AJ, Stockinger B. 2011. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bronte V, Pittet MJ. 2013. The spleen in local and systemic regulation of immunity. Immunity 39:806–818. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saxena A, Khosraviani S, Noel S, Mohan D, Donner T, Hamad AR. 2015. Interleukin-10 paradox: a potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine 74:27–34. doi: 10.1016/j.cyto.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Peres AG, Damian AC, Madrenas J. 2015. Immunomodulation and disease tolerance to Staphylococcus aureus. Pathogens 4:793–815. doi: 10.3390/pathogens4040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose WE, Eickhoff JC, Shukla SK, Pantrangi M, Rooijakkers S, Cosgrove SE, Nizet V, Sakoulas G. 2012. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis 206:1604–1611. doi: 10.1093/infdis/jis552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doz E, Lombard R, Carreras F, Buzoni-Gatel D, Winter N. 2013. Mycobacteria-infected dendritic cells attract neutrophils that produce IL-10 and specifically shut down Th17 CD4 T cells through their IL-10 receptor. J Immunol 191:3818–3826. doi: 10.4049/jimmunol.1300527. [DOI] [PubMed] [Google Scholar]
- 34.Huber S, Gagliani N, Esplugues E, O’Connor W Jr, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, Roncarolo MG, Battaglia M, Flavell RA. 2011. Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagnoli F, Bertholet S, Grandi G. 2012. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol 2:16. doi: 10.3389/fcimb.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narita K, Hu D-L, Mori F, Wakabayashi K, Iwakura Y, Nakane A. 2010. Role of interleukin-17A in cell-mediated protection against Staphylococcus aureus infection in mice immunized with the fibrinogen-binding domain of clumping factor A. Infect Immun 78:4234–4242. doi: 10.1128/IAI.00447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harro CD, Betts RF, Hartzel JS, Onorato MT, Lipka J, Smugen SS, Kartsonis NA. 2012. The immunogenicity and safety of different formulations of a novel Staphylococcus aureus vaccine (V710): results of two phase I studies. Vaccine 30:1729–1736. doi: 10.1016/j.vaccine.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 38.Zorman JK, Esser M, Raedler M, Kreiswirth BN, Ala’Aldeen D, Kartsonis N. 2013. Naturally occurring IgG antibody levels to the Staphylococcus aureus protein IsdB in humans. Hum Vaccin Immunother 9:1857–1864. doi: 10.4161/hv.25253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spellberg B, Ibrahim AS, Yeaman MR, Lin L, Fu Y, Avanesian V, Bayer AS, Filler SG, Lipke P, Otoo H, Edwards JE Jr. 2008. The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus. Infect Immun 76:4574–4580. doi: 10.1128/IAI.00700-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE Jr, Spellberg B. 2009. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allusto F, Geginat J, Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 42.Li CE, Parikh SC, Chudley L, Layfield DM, Ottensmeier CH, Stevenson FK, Di Genova GV. 2015. Vaccination expands antigen-specific CD4+ memory T cells and mobilizes bystander central memory T cells. PLoS One 10:e0136717. doi: 10.1371/journal.pone.0136717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutton CE, Mielke LA, Mills KH. 2012. IL-17-producing γδ T cells and innate lymphoid cells. Eur J Immunol 42:2221–2231. doi: 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- 44.Lexberg MH, Taubner A, Förster A, Albrecht I, Richter A, Kamradt T, Radbruch A, Chang HD. 2008. Th memory for interleukin-17 expression is stable in vivo. Eur J Immunol 38:2654–2664. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- 45.Gagliani N, Vesely MCA, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limón P, Paiva RS, Ching T, Weaver C, Zi X, Pan X, Fan R, Garmire LX, Cotton MJ, Drier Y, Berstein B, Geginat J, Stockinger B, Esplugues S, Huber S, Flavell RA. 2015. TH17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses Immunity 17:375–387. doi: 10.1016/S1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 47.Nakane A, Okamoto M, Asano M, Kohanawa M, Minagawa T. 1995. Endogenous gamma interferon, tumor necrosis factor, and interleukin-6 in Staphylococcus aureus infection in mice. Infect Immun 63:1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasaki S, Nishikawa S, Miura T, Mizuki M, Yamada Y, Madarame H, Tagawa Y-I, Iwakura Y, Nakane A. 2000. Interleukin-4 and interleukin-10 are involved in host resistance to Staphylococcus aureus infection through regulation of gamma interferon. Infect Immun 68:2424–2430. doi: 10.1128/IAI.68.5.2424-2430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakane A, Nishikawa S, Sasaki S, Miura T, Asano M, Kohanawa M, Ishiwata K, Minagawa T. 1996. Endogenous interleukin-4, but not interleukin-10, is involved in suppression of host resistance against Listeria monocytogenes infection in interferon-depleted mice. Infect Immun 64:1252–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]