Postinfluenza methicillin-resistant Staphylococcus aureus (MRSA) infection can quickly develop into severe, necrotizing pneumonia, causing over 50% mortality despite antibiotic treatments. In this study, we investigated the efficacy of antibiotic therapies and the impact of S. aureus alpha-toxin in a model of lethal influenza virus and MRSA coinfection.
KEYWORDS: MRSA, alpha-toxin, antibiotic therapy, coinfection, influenza, pneumonia
ABSTRACT
Postinfluenza methicillin-resistant Staphylococcus aureus (MRSA) infection can quickly develop into severe, necrotizing pneumonia, causing over 50% mortality despite antibiotic treatments. In this study, we investigated the efficacy of antibiotic therapies and the impact of S. aureus alpha-toxin in a model of lethal influenza virus and MRSA coinfection. We demonstrate that antibiotics primarily attenuate alpha-toxin-induced acute lethality, even though both alpha-toxin-dependent and -independent mechanisms significantly contribute to animal mortality after coinfection. Furthermore, we found that the protein synthesis-suppressing antibiotic linezolid has an advantageous therapeutic effect on alpha-toxin-induced lung damage, as measured by protein leak and lactate dehydrogenase (LDH) activity. Importantly, using a Panton-Valentine leucocidin (PVL)-negative MRSA isolate from patient sputum, we show that linezolid therapy significantly improves animal survival from postinfluenza MRSA pneumonia compared with vancomycin treatment. Rather than improved viral or bacterial control, this advantageous therapeutic effect is associated with a significantly attenuated proinflammatory cytokine response and acute lung damage in linezolid-treated mice. Together, our findings not only establish a critical role of alpha-toxin in the extreme mortality of secondary MRSA pneumonia after influenza but also provide support for the possibility that linezolid could be a more effective treatment than vancomycin to improve disease outcomes.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is a significant cause of severe pneumonia in this antibiotic era. In particular, MRSA has emerged as an important contributor to the morbidity and mortality associated with influenza virus infection. Influenza virus and MRSA coinfection can quickly develop into severe, necrotizing pneumonia, causing over 50% mortality despite antibiotic treatment (1). However, an incomplete understanding of coinfection pathogenesis has hindered the development of effective therapies.
Vancomycin and linezolid are two frontline antibiotics for the treatment of MRSA pneumonia (2). In a retrospective analysis, initial therapy with linezolid was associated with significantly better clinical cure rates than initial therapy with vancomycin in patients with hospital-associated MRSA (HA-MRSA) pneumonia (3, 4). However, the results of a post hoc analysis of these studies are controversial (5, 6). Animal studies suggest that linezolid has a unique immunomodulatory effect in a model of sublethal influenza virus and community-associated MRSA (CA-MRSA) coinfection (7). Nonetheless, its therapeutic effect on disease cure and host survival remains unclear. As a result, vancomycin continues to be commonly used for treating patients with MRSA pneumonia.
The pore-forming cytotoxin alpha-hemolysin (alpha-toxin, Hla) is an essential virulence factor in S. aureus pneumonia, including cases caused by CA-MRSA and HA-MRSA (8). It has been shown that alpha-toxin directly disrupts the epithelial barrier and induces mortality, whereas its neutralization mitigates disease in animals (9, 10). However, the role of alpha-toxin in influenza virus and S. aureus coinfection remains uncertain (11). To our knowledge, no study has hitherto examined the influence of alpha-toxin on antibiotic efficacy during the treatment of secondary MRSA pneumonia that occurs after influenza.
In this study, we investigated the impact of alpha-toxin expression on the pathogenesis and the efficacy of antibiotic therapies in mouse models of lethal influenza virus and MRSA coinfection. Our results suggest that antibiotics primarily ameliorate alpha-toxin-induced acute animal death. Compared with vancomycin therapy, linezolid therapy significantly attenuated alpha-toxin-induced lung damage and improved the survival outcome in a more clinically relevant mouse model of secondary MRSA pneumonia. Together, our findings provide direct evidence for the pathogenic role of alpha-toxin and the advantageous effect of linezolid therapy during influenza virus and S. aureus coinfection.
RESULTS
Antibiotic treatment decreases alpha-toxin-induced animal death during postinfluenza CA-MRSA infection.
Bacterial strain LAC is a well-characterized USA300 CA-MRSA clone that is often used in laboratory research (12). To investigate whether alpha-toxin is critically involved in lethal influenza virus and CA-MRSA coinfection, we used isogenic strain LAC-JE2 (a plasmid-cured derivative of LAC) with a deletion of the hla gene (13). Furthermore, we determined whether alpha-toxin expression influences the therapeutic effect of vancomycin and linezolid, two frontline antibiotics for the treatment of MRSA pneumonia. Specifically, wild-type C57BL/6 mice were inoculated with a sublethal dose of A/Puerto Rico/8/1934 (PR8) virus, followed by MRSA infection 7 days later. Starting 2 h after bacterial infection, all animals, including mice infected with bacteria alone, were treated with antibiotics or phosphate-buffered saline (PBS) as a control.
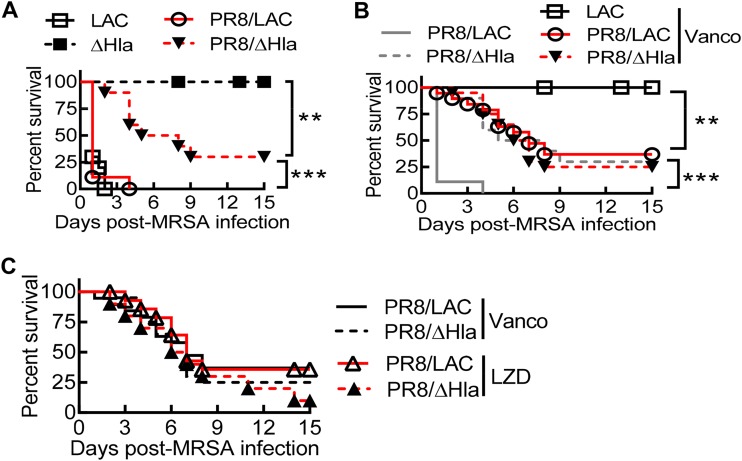
In the absence of antibiotic treatment, mice infected with an hla deletion mutant (the Δhla mutant) exhibited 100% survival, while intranasal (i.n.) infection with alpha-toxin-expressing LAC-JE2 (LAC) resulted in 100% mortality (Fig. 1A). This result is in agreement with earlier reports demonstrating that alpha-toxin plays an essential role in the pathogenesis of S. aureus pneumonia. As expected, nearly all mice died within 24 h after PR8/LAC coinfection. Interestingly, prior PR8 infection led to the death of a majority of the animals during secondary Δhla mutant infection (Fig. 1A). This suggests that alpha-toxin-independent mechanisms contribute significantly to coinfection lethality. Of note, the initiation of vancomycin therapy at 2 h after infection provided complete protection in mice infected with LAC alone, while the majority (∼75%) of the animals still succumbed to PR8/LAC coinfection (Fig. 1B). These observations indicate that prior influenza virus infection impairs the therapeutic effect of antibiotics against S. aureus pneumonia.
FIG 1.
Hla expression and antibiotic inefficacy against postinfluenza CA-MRSA pneumonia. (A and B) Survival after infection with LAC or the Δhla mutant of naive C57BL/6 mice and C57BL/6 mice on day 7 after PR8 infection (n ≥ 8 mice/group). (C) Survival of C57BL/6 mice (n ≥ 10 mice/group) superchallenged with LAC or the Δhla mutant on day 7 after PR8 virus infection. Starting at 2 h after MRSA infection, mice were treated daily with the antibiotic vancomycin (Vanco) or linezolid (LZD) or with PBS as a control. P values were determined by the log-rank test. **, P < 0.01; ***, P < 0.001. The data shown are representative of those from at least two independent experiments.
Nonetheless, compared with the 100% mortality in the PBS-treated controls, the initiation of vancomycin therapy at 2 h after infection significantly improved animal survival from PR8/LAC coinfection (Fig. 1B). Surprisingly, following PR8/Δhla mutant coinfection, mice that received vancomycin treatment did not exhibit any improved survival compared with their PBS-treated counterparts (Fig. 1B), indicating that acute antibiotic therapy does not improve protection against alpha-toxin-independent mortality. Indeed, the survival outcomes were comparable between mice with PR8/LAC and mice with PR8/Δhla mutant coinfection, regardless of vancomycin or linezolid treatment at 2 h after infection (Fig. 1C). Together, these results suggest that despite the significant contribution of alpha-toxin-independent mechanisms to pathogenesis, acute antibiotic treatment primarily attenuates alpha-toxin-induced lethality during influenza virus/S. aureus coinfection.
Linezolid treatment ameliorates alpha-toxin-induced acute lung damage during postinfluenza CA-MRSA infection.
Studies in animal models demonstrated that alpha-toxin-induced lung injury occurs very early following S. aureus infection (14). To determine whether prompt antibiotic therapy is essential for protection against alpha-toxin-induced mortality, we initiated antibiotic treatment 4 h after bacterial infection. Treatment with vancomycin or linezolid rescued the majority of animals from otherwise lethal LAC infection alone (Fig. 2A), while nearly all mice succumbed to influenza virus/LAC coinfection despite antibiotic therapies (Fig. 2B). As a result, under this antibiotic treatment regimen, PR8/Δhla mutant-coinfected mice exhibited significantly increased survival compared with those with PR8/LAC coinfection (Fig. 2B). This suggests that the initiation of antibiotic treatment at 4 h is too late to rescue animals from alpha-toxin toxicity during coinfection. Together, these findings provide support for the suggestion that prompt antibiotic treatment is essential to prevent alpha-toxin-induced lethality during postinfluenza MRSA pneumonia.
FIG 2.
Hla expression during coinfection promotes animal death, despite acute antibiotic therapy. (A) Survival of C57BL/6 mice (n =10) infected with LAC or the Δhla mutant alone. (B) Survival of C57BL/6 mice (n ≥ 10 mice/group) superchallenged with LAC or the Δhla mutant on day 7 after PR8 virus infection. C57BL/6 mice were infected with PR8 and 7 days later were superchallenged with LAC or the Δhla mutant. (C to E) At 24 h after infection, the lungs were analyzed for bacterial (C) and viral (D) burdens and neutrophil numbers (E) in BALF (mean ± SD, n = 5). Starting at 4 h after MRSA infection, all mice were treated daily with the antibiotic vancomycin (Vanco) or linezolid (LZD). PMN, polymorphonuclear leukocyte. *, P < 0.05, log-rank test. The data shown are representative of those from two independent experiments.
Linezolid has been suggested to have better lung tissue penetration than vancomycin (15). We observed that mice that received linezolid therapy tended to have an extended survival time (P = 0.1) during PR8/LAC coinfection compared with that for their vancomycin-treated counterparts (Fig. 2B). We thus examined the effect of alpha-toxin expression and antibiotic therapies on lung bacterial and viral control. Nonetheless, following either vancomycin or linezolid treatment at 4 h after infection, lung bacterial and viral burdens were comparable between mice with PR8/LAC and mice with PR8/Δhla mutant coinfections. These results suggest that the survival outcome is not directly associated with reductions in the bacterial or viral burden (Fig. 2C and D). Furthermore, flow cytometry analysis of bronchoalveolar lavage (BAL) fluid (BALF) cells showed that the number of neutrophils (CD11b+ Ly6G+) was comparable among all experimental groups (Fig. 2E), suggesting that the pathogenic role of alpha-toxin is not directly associated with increased neutrophil accumulation.
At the same time, tumor necrosis factor alpha (TNF-α) production tended to be lower in linezolid-treated mice than vancomycin-treated mice, even though this difference was not statistically significant in this PR8/CA-MRSA coinfection model (Fig. 3A). The levels of gamma interferon (IFN-γ) were comparable among all coinfected groups. Conversely, we observed significantly increased levels of interleukin-6 (IL-6) production in PR8/LAC-coinfected mice compared with PR8/Δhla mutant-coinfected animals during vancomycin treatment. Of particular interest, this increased IL-6 production was blunted after linezolid treatment (Fig. 3A). A similar inhibitory effect of linezolid on alpha-toxin-induced TNF-α and IL-6 production was observed when antibiotic treatment was initiated 2 h after MRSA superinfection (see Fig. S1A in the supplemental material), even though there were no differences in animal survival under this antibiotic treatment regimen (Fig. 1C).
FIG 3.
Linezolid attenuates alpha-toxin-induced acute lung damage during coinfection. C57BL/6 mice were infected with PR8 and 7 days later were superchallenged with LAC or the Δhla mutant. Starting at 4 h after bacterial infection, all mice were treated with the antibiotic vancomycin (Vanco) or linezolid (LZD). BALF samples were analyzed for the levels of TNF-α, IL-6, and IFN-γ (A) (mean ± SEM), total protein (mean ± SD) (B), and LDH (mean ± SEM) (C) at 24 h after infection (n = 5). AU, arbitrary units. The data in panel B represent protein levels relative to those in naive mice. P values were determined by Tukey’s multiple-comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The data shown are representative of those from two independent experiments.
We next assessed protein leak and lactate dehydrogenase (LDH) activity in the airway fluid as indications of lung tissue damage. After vancomycin treatment, the levels of protein and LDH activity in BALF were significantly higher in mice with PR8/LAC coinfection than in mice with PR8/Δhla mutant coinfection (Fig. 3B and C). In contrast, the level of these tissue damage indicators became comparable between mice with PR8/LAC and mice with PR8/Δhla mutant coinfection after linezolid therapy, regardless of the initiation of treatment at 2 h (Fig. S1B) or 4 h (Fig. 3B and C) after bacterial infection. Together, these data suggest not only that alpha-toxin promotes IL-6 production and exacerbates acute lung damage during influenza virus/CA-MRSA coinfection but also that linezolid therapy is more effective than vancomycin therapy in preventing these alpha-toxin-induced pathological changes.
Treatment with linezolid improves animal survival from postinfluenza MRSA pneumonia.
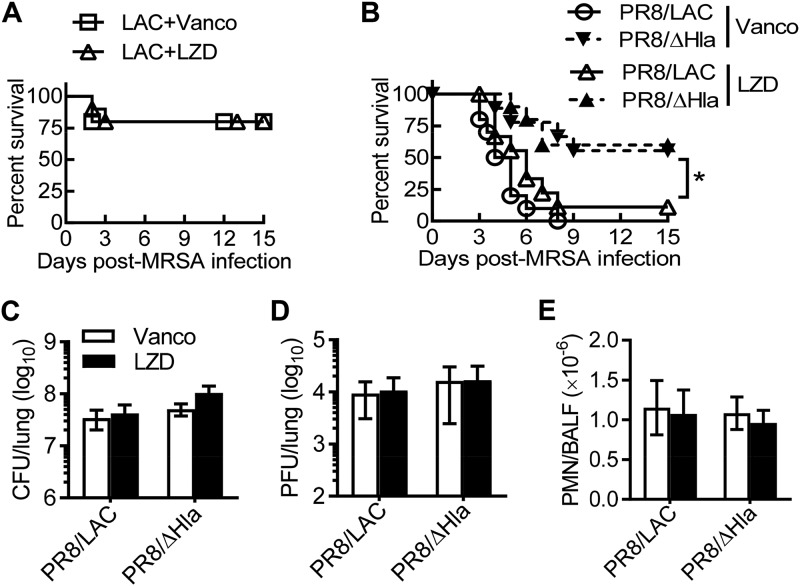
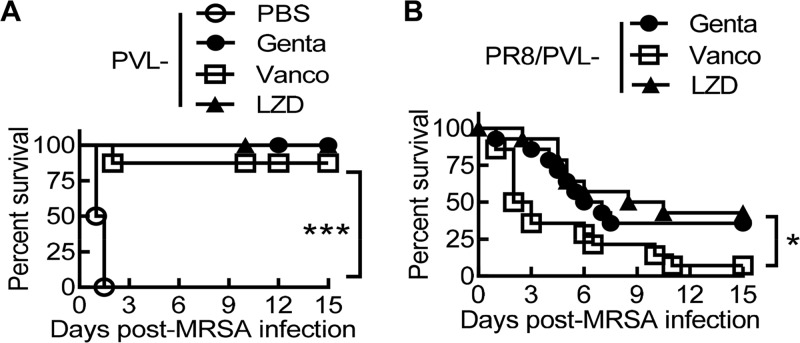
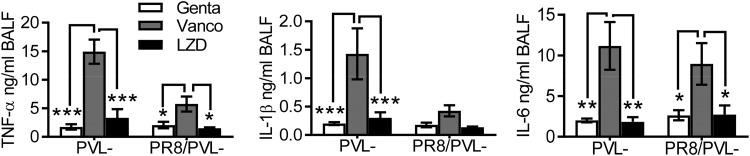
Considering that CA-MRSA USA300 has generally been implicated in skin and soft tissue infections, we next used ATCC MRSA strain BAA-1695, a Panton-Valentine leucocidin (PVL)-negative (PVL−) isolate from patient sputum (13), to determine whether linezolid is a better therapy for secondary pneumonia after influenza. We have previously shown that prior influenza impairs the bactericidal effect of the aminoglycoside antibiotic gentamicin (16). Thus, we evaluated the therapeutic effect of vancomycin and linezolid side-by-side in a comparison with gentamicin during PVL− MRSA single infection (Fig. 4A) and PR8/PVL− MRSA coinfection (Fig. 4B). We found that the therapeutic efficacy of these antibiotics differed significantly in this PR8/PVL− MRSA coinfection model. Specifically, mice that received linezolid or gentamicin therapy exhibited a significantly improved survival outcome compared with that for their vancomycin-treated counterparts (Fig. 4B).
FIG 4.
Linezolid improves animal survival during secondary infection with a pvl-negative MRSA isolate after influenza. (A) Survival of C57BL/6 mice infected with MRSA BAA-1695 (PVL−) alone (n ≥ 8 mice/group). (B) Survival of C57BL/6 mice (n ≥ 14 mice/group) superchallenged with BAA-1695 on day 7 after PR8 virus infection (PR8/PVL− MRSA). Starting 2 h after PVL− MRSA infection, all mice were treated daily with the antibiotic gentamicin (Genta), vancomycin (Vanco), or linezolid (LZD) or with PBS. P values were determined by the log-rank test. *, P < 0.05; ***, P < 0.001. The data shown are representative of those from two independent experiments.
We next examined the lung bacterial burden as an indication of the bactericidal effect of these antibiotics in vivo. In agreement with their improved survival rate, mice that received gentamicin therapy had significant decreased bacterial burdens compared with their vancomycin-treated counterparts during PVL− MRSA single infection and PR8/PVL− MRSA coinfection (Fig. 5A). Conversely, mice that received linezolid therapy did not exhibit any reduction in bacterial burden but, rather, exhibited a significant increase in viral titer compared with the vancomycin-treated animals (Fig. 5A and B). Thus, the acute inflammation during vancomycin treatment may actually facilitate viral clearance. We have recently reported that influenza virus-induced monocytes serve as reservoirs to protect MRSA from killing by antibiotics in this coinfection model (16). Nonetheless, we did not detect a differential effect of antibiotics on monocyte (CD11b+ Ly6C+) or neutrophil (CD11b+ Ly6G+) accumulation in the lung (Fig. 5C). These results indicate that the advantageous therapeutic efficacy of linezolid is not due to improved lung viral and bacterial control or decreased inflammatory cell infiltration compared with those achieved with vancomycin treatment.
FIG 5.
Linezolid treatment does not enhance lung bacterial clearance compared with vancomycin. C57BL/6 mice were infected with PR8 and 7 days later were superchallenged with MRSA BAA-1695 (PVL−). Starting at 2 h after bacterial infection, mice were treated with the antibiotic gentamicin (Genta), vancomycin (Vanco), or linezolid (LZD) or with PBS. (A and B) Lungs were analyzed for the bacterial (A) and viral (B) burdens, (C) Airway neutrophil and monocyte numbers at 24 h (mean ± SD, n = 5). P values were determined by Tukey’s multiple-comparison test. *, P < 0.05; **, P < 0.01. The data shown are representative of those from two independent experiments. Mϕ, macrophage.
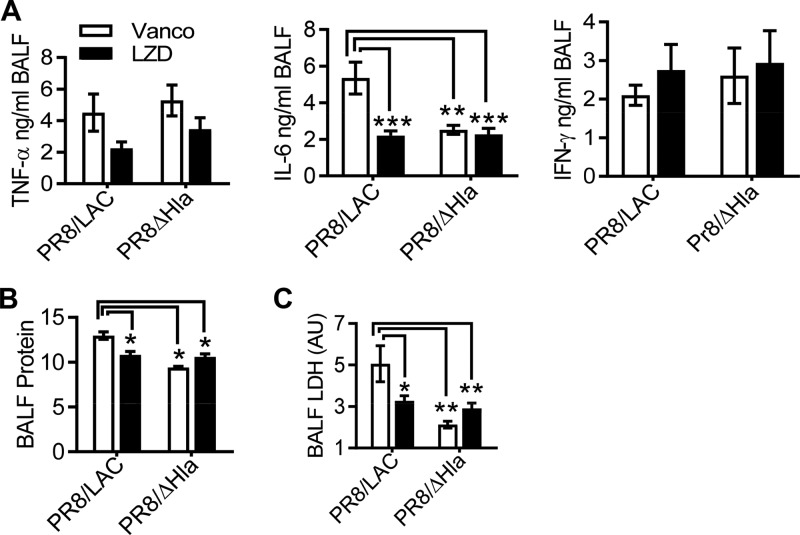
Treatment with linezolid attenuates proinflammatory cytokine production and acute lung injury during MRSA pneumonia.
We have previously shown that after appropriate antibiotic treatment, inflammatory lung damage, rather than bacterial outgrowth, is the fundamental determinant of the coinfection outcome (17). In agreement with this notion, mice that received linezolid therapy had significantly reduced TNF-α and IL-6 levels compared to their vancomycin-treated counterparts during PVL− MRSA single infection and PR8/PVL− MRSA coinfection (Fig. 6). We next examined whether linezolid treatment attenuated acute lung injury downstream of the proinflammatory cytokine response. As expected, PR8/PVL− MRSA-coinfected mice exhibited exacerbated lung injury compared with the corresponding controls infected with PVL− MRSA alone, as evidenced by prominent LDH levels in BALF (Fig. 7). Importantly, compared with vancomycin treatment, linezolid treatment significantly reduced the levels of albumin and LDH activity in BALF during both infection with PVL− MRSA alone and coinfection (Fig. 7B and C). Together, these data suggest that linezolid treatment attenuates proinflammatory cytokine production and acute lung damage during PVL− MRSA pneumonia, in agreement with its advantageous effect on animal survival from influenza virus and PVL− MRSA coinfection.
FIG 6.
Linezolid reduces proinflammatory cytokine production compared with vancomycin. C57BL/6 mice were infected with PR8 and 7 days later were superchallenged with MRSA BAA-1695 (PVL−). Starting 2 h after bacterial infection, mice were treated with the antibiotic gentamicin (Genta), vancomycin (Vanco), or linezolid (LZD). BALF samples were analyzed for TNF-α, IL-1β, and IL-6 levels at 24 h (mean ± SEM, n = 5). P values were determined by Tukey’s multiple-comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The data shown are representative of those from two independent experiments.
FIG 7.
Linezolid therapy attenuates acute lung damage compared with vancomycin. C57BL/6 mice were infected with PR8 and 7 days later were superchallenged with MRSA BAA-1695 (PVL−). Starting at 2 h after bacterial infection, the mice were treated with the antibiotic gentamicin (Genta), vancomycin (Vanco), or linezolid (LZD). BALF samples were analyzed for total protein (A), albumin (B), and LDH (C) levels at 24 h (mean ± SD, n = 5). AU, arbitrary units. The data in panel A represent protein levels relative to those in naive mice. P values were determined by Tukey’s multiple-comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.01. The data shown are representative of those from two independent experiments.
DISCUSSION
It is well established that alpha-toxin mediates key virulence in animal models of S. aureus pneumonia. In this study, we show that alpha-toxin also plays a critical role in acute animal death during influenza virus/MRSA coinfection. However, in contrast to MRSA single infection, alpha-toxin-independent mechanisms are sufficient to induce animal mortality during influenza virus/MRSA coinfection. Furthermore, acute antibiotic treatment attenuates alpha-toxin-dependent but not -independent mortality during coinfection. Importantly, we demonstrate that linezolid therapy is more effective than vancomycin therapy in preventing alpha-toxin-induced acute lung damage during postinfluenza MRSA pneumonia.
Alpha-toxin primarily induces alveolar injury through cytolysis of host epithelial cells and leukocytes in animal models of S. aureus pneumonia (8). At subcytolytic concentrations, alpha-toxin stimulates the proinflammatory cytokine response, thereby promoting acute lung damage (18). On the other hand, the role of this toxin in influenza virus/S. aureus coinfection remains uncertain (11). This is likely due to the fact that the reported animal models are not sensitive enough to discern the role of specific virulence factors in coinfection (19–21). To our knowledge, we provide the first in vivo evidence here that alpha-toxin-induced damage is critically responsible for acute animal death during coinfection, despite antibiotic treatment. These data are in agreement with those from a study by Yu et al, in which they demonstrated a correlation between alpha-toxin exposure and a worsened disease outcome in children with influenza-complicated MRSA pneumonia (11).
We have recently shown that gentamicin therapy has an improved effect on lung bacterial control compared with vancomycin (16). This beneficial effect may explain the advantageous impact of gentamicin therapy on the coinfection outcome. Of note, the MIC values of antibiotics for MRSA BAA-1695 are ≤12.5 μg/ml for gentamicin, ≤2.5 μg/ml for linezolid, and ≤1.17 μg/ml for vancomycin in vitro (17). Despite that, we found that linezolid-treated mice harbored bacteria comparable to those harbored by their vancomycin-treated counterparts during MRSA single infection and coinfection. Considering the critical role of alpha-toxin in acute lung damage, together with the finding that neither vancomycin nor linezolid therapy attenuates alpha-toxin-independent lethality, inhibition of alpha-toxin expression is likely the mechanism contributing to the advantageous outcome of the protein synthesis-suppressing antibiotic linezolid in our coinfection model. In agreement with this suggestion, during MRSA infection alone, it has been shown that treatment with linezolid is associated with reduced levels of staphylococcal toxins in animal models of sepsis (22) and necrotizing pneumonia (23) compared with those found after treatment with vancomycin.
Prophylactic treatment with linezolid has been suggested to improve disease outcomes by inhibiting IFN-γ expression during postinfluenza pneumococcal pneumonia (24). However, we did not detect a differential regulatory effect of antibiotics on influenza virus-induced IFN-γ production during therapeutic treatment (Fig. 3A). Using a sublethal coinfection model, it has been shown that linezolid reduces S. aureus PVL production and thereby attenuates neutrophil-mediated acute lung injury during CA-MRSA pneumonia after influenza (7). In contrast, during lethal influenza virus/CA-MRSA coinfection, we found that linezolid treatment decreases alpha-toxin-induced IL-6 production and acute lung injury but has no significant effect on neutrophil accumulation compared with the effect of vancomycin therapy. Importantly, we demonstrate that linezolid therapy significantly improved animal survival from secondary MRSA pneumonia caused by a pvl-negative isolate. These findings indicate that the beneficial effect of linezolid is independent of PVL regulation during lethal influenza virus and MRSA coinfection.
In the absence of alpha-toxin expression, intranasal infection with the hla mutant alone did not cause any animal deaths. However, prior influenza virus infection led to significant deaths among mice challenged with the LAC Δhla mutant (Fig. 1A). These results suggest that influenza virus infection primarily enhances S. aureus virulence through alpha-toxin-independent mechanisms. The alpha-toxin-independent lung damage and mortality may result from the host response to other bacterial virulence factors, such as cell wall components and protein A (25–29). Nonetheless, future studies are necessarily to clarify these alpha-toxin-independent pathogenic mechanisms during influenza virus and S. aureus coinfection.
Linezolid therapy has been reported to produce a survival advantage in patients with nosocomial MRSA pneumonia (3, 4). A recent study also suggested that vancomycin is insufficient to treat MRSA pneumonia in children with influenza-related critical illness (30). In animal studies, it has been shown that treatment with linezolid but not vancomycin significantly delayed weight loss compared with placebo during postinfluenza MRSA pneumonia (31). In agreement with these reported findings, here we provide direct evidence for the advantageous effect of linezolid therapy compared with vancomycin therapy in protection against lethal influenza virus/MRSA coinfection. In conclusion, our data not only demonstrate that alpha-toxin exacerbates acute animal death but also provide support for the suggestion that linezolid may be more efficacious as a first-line treatment to improve disease outcomes during secondary MRSA pneumonia after influenza.
MATERIALS AND METHODS
Murine model of viral and bacterial infections.
Specific-pathogen-free, C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA) and The Jackson Laboratory (Bar Harbor, ME). All animal experiments were approved by University of Nebraska Medical Center, and all experiments were carried out in accordance with University of Nebraska Medical Center Assurance of Compliance with PHS Policy on Humane Care and Use of Laboratory Animals, which is on file with the Office of Protection from Research Risks, NIH. For bacterial challenge, 8- to 12-week-old sex- and weight-matched mice were anesthetized and infected intranasally (i.n.) with 50 μl of PBS containing 5 × 108 CFU of S. aureus from overnight cultures. Bacterial burdens in the lungs and bronchoalveolar lavage fluid (BALF) were measured by sacrificing infected mice at 24 h and plating serial 10-fold dilutions of each sample onto blood agar plates. The plates were incubated at 37°C overnight, and the CFU were enumerated 24 h later. To induce postinfluenza S. aureus pneumonia, mice were challenged with 50 PFU/mouse A/Puerto Rico/8/1934 (PR8) virus 7 days prior to bacterial infection (13, 17).
BALF cell analysis.
Bronchoalveolar lavage (BAL) fluid (BALF) samples were collected by making an incision in the trachea and lavaging the lung twice with 0.8 ml PBS, pH 7.4. Total cell counts were determined using a hemacytometer.
For flow cytometry analysis, BALF or lung cells were incubated with monoclonal antibody (MAb) 2.4G2 against Fcγ receptor II/III (FcγRII/III) and stained with allophycocyanin-conjugated anti-CD11c (BioLegend), Brilliant Ultraviolet 395-conjugated anti-CD11b (BD Biosciences), phycoerythrin (PE)-Cy7-conjugated anti-Ly6G (clone 1A8; BioLegend), peridinin chlorophyll protein-Cy5.5-conjugated (eBioscience), or fluorescein isothiocyanate-conjugated anti-Ly6C (BD Biosciences) and PE-conjugated anti-Siglec-F (BD Biosciences) MAbs. The stained cells were analyzed on a BD LSR II green flow cytometer using BD FACSDiva and FlowJo software analysis (16).
Determination of cytokine production by ELISA.
BALF samples were harvested and assayed for TNF-α, IL-1β, IL-6, and IFN-γ by enzyme-linked immunosorbent assay (ELISA) using commercially available kits from BD Biosciences.
Evaluation of airway damage.
BALF samples were harvested and assayed for albumin by ELISA using a commercially available kit from Bethyl Laboratories (Montgomery, TX). Total protein levels and lactic acid dehydrogenase (LDH) activities in BALF were analyzed by use of a micro-bicinchoninic acid protein assay kit (Thermo Scientific) and an LDH cytotoxicity assay kit (Thermo Scientific), respectively.
Treatment with antibiotics.
Mice were subcutaneously (s.c.) injected with a therapeutic dose of vancomycin (300 mg/kg of body weight) or gentamicin (100 mg/kg) beginning 2 or 4 h after MRSA infection, which was then followed by treatment with 150 mg/kg/day for vancomycin or 50 mg/kg/day for gentamicin. For linezolid (provided by Pfizer) treatment, mice were treated s.c. at 50 mg/kg/day beginning 2 or 4 h after MRSA infection. Control mice received PBS. For survival studies, all antibiotic treatment and sham injections continued through day 10 after MRSA infection.
Statistics.
Significant differences between experimental groups were determined using an analysis of variance followed by Tukey’s multiple-comparison test in GraphPad Prism (version 7) software (La Jolla, CA). Survival analyses were performed using the log-rank test. For all analyses, a P value of <0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Center for Staphylococcal Research (CSR) for providing LAC-JE2 and the hla mutant. We also thank the University of Nebraska Medical Center (UNMC) Flow Cytometry Research Facility for assistance with fluorescence-activated cell sorting analysis.
The UNMC Flow Cytometry Research Facility is supported by state funds from the Nebraska Research Initiative (NRI) and a support grant from the National Cancer Institute’s Fred and Pamela Buffett Cancer Center. This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute, grant R01 HL118408 to K.S.
We have no conflicting financial interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00538-19.
REFERENCES
- 1.Vardakas KZ, Matthaiou DK, Falagas ME. 2009. Incidence, characteristics and outcomes of patients with severe community acquired-MRSA pneumonia. Eur Respir J 34:1148–1158. doi: 10.1183/09031936.00041009. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society, Infectious Diseases Society of America. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 3.Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. 2003. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 124:1789–1797. doi: 10.1016/S0012-3692(15)33412-7. [DOI] [PubMed] [Google Scholar]
- 4.Kollef MH, Rello J, Cammarata SK, Croos-Dabrera RV, Wunderink RG. 2004. Clinical cure and survival in Gram-positive ventilator-associated pneumonia: retrospective analysis of two double-blind studies comparing linezolid with vancomycin. Intensive Care Med 30:388–394. doi: 10.1007/s00134-003-2088-1. [DOI] [PubMed] [Google Scholar]
- 5.Wilke MH, Becker K, Kloss S, Heimann SM, Goldmann A, Weber B, Pletz MW, Simon P, Petrik C. 2017. Treatment of MRSA pneumonia: clinical and economic comparison of linezolid vs. vancomycin—a retrospective analysis of medical charts and re-imbursement data of real-life patient populations. GMS Infect Dis 5:Doc02. doi: 10.3205/id000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pletz MW, Burkhardt O, Welte T. 2010. Nosocomial methicillin-resistant Staphylococcus aureus (MRSA) pneumonia: linezolid or vancomycin?—comparison of pharmacology and clinical efficacy. Eur J Med Res 15:507–513. doi: 10.1186/2047-783x-15-12-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhan U, Podsiad AB, Kovach MA, Ballinger MN, Keshamouni V, Standiford TJ. 2015. Linezolid has unique immunomodulatory effects in postinfluenza community acquired MRSA pneumonia. PLoS One 10:e0114574. doi: 10.1371/journal.pone.0114574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, Wang Y, Bubeck Wardenburg J. 2011. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med 17:1310–1314. doi: 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, Datta V, Ren S, Feng H, Zinsou R, Keller A, O'Day T, Du Q, Cheng L, Damschroder M, Robbie G, Suzich J, Stover CK, Sellman BR. 2014. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother 58:1108–1117. doi: 10.1128/AAC.02190-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diep BA, Hilliard JJ, Le VT, Tkaczyk C, Le HN, Tran VG, Rao RL, Dip EC, Pereira-Franchi EP, Cha P, Jacobson S, Broome R, Cheng LI, Weiss W, Prokai L, Nguyen V, Stover CK, Sellman BR. 2017. Targeting alpha-toxin to mitigate its lethal toxicity in ferret and rabbit models of Staphylococcus aureus necrotizing pneumonia. Antimicrob Agents Chemother 61:e02456-16. doi: 10.1128/AAC.02456-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu KO, Randolph AG, Agan AA, Yip WK, Truemper EJ, Weiss SL, Ackerman KG, Schwarz AJ, Giuliano JS Jr, Hall MW, Bubeck Wardenburg J, Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) PICFlu Study Group PALISI PICFlu Study Group. 2016. Staphylococcus aureus alpha-toxin response distinguishes respiratory virus-methicillin-resistant S. aureus coinfection in children. J Infect Dis 214:1638–1646. doi: 10.1093/infdis/jiw441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung GY, Wang R, Khan BA, Sturdevant DE, Otto M. 2011. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect Immun 79:1927–1935. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yajjala VK, Thomas VC, Bauer C, Scherr TD, Fischer KJ, Fey PD, Bayles KW, Kielian T, Sun K. 2016. Resistance to acute macrophage killing promotes airway fitness of prevalent community-acquired Staphylococcus aureus strains. J Immunol 196:4196–4203. doi: 10.4049/jimmunol.1600081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stulik L, Rouha H, Labrousse D, Visram ZC, Badarau A, Maierhofer B, Groß K, Weber S, Kramarić MD, Glojnarić I, Nagy G, Croisier D, Nagy E. 2019. Preventing lung pathology and mortality in rabbit Staphylococcus aureus pneumonia models with cytotoxin-neutralizing monoclonal IgGs penetrating the epithelial lining fluid. Sci Rep 9:5339. doi: 10.1038/s41598-019-41826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiem S, Schentag JJ. 2014. Interpretation of epithelial lining fluid concentrations of antibiotics against methicillin resistant Staphylococcus aureus. Infect Chemother 46:219–225. doi: 10.3947/ic.2014.46.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer KJ, Yajjala VK, Bansal S, Bauer C, Chen R, Sun K. 2019. Monocytes represent one source of bacterial shielding from antibiotics following influenza virus infection. J Immunol 202:2027–2034. doi: 10.4049/jimmunol.1801471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun K, Yajjala VK, Bauer C, Talmon GA, Fischer KJ, Kielian T, Metzger DW. 2016. Nox2-derived oxidative stress results in inefficacy of antibiotics against postinfluenza S. aureus pneumonia. J Exp Med 213:1851–1864. doi: 10.1084/jem.20150514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, Jania C, Doerschuk CM, Tilley SL, Duncan JA. 2012. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis 205:807–817. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MH, Arrecubieta C, Martin FJ, Prince A, Borczuk AC, Lowy FD. 2010. A postinfluenza model of Staphylococcus aureus pneumonia. J Infect Dis 201:508–515. doi: 10.1086/650204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iverson AR, Boyd KL, McAuley JL, Plano LR, Hart ME, McCullers JA. 2011. Influenza virus primes mice for pneumonia from Staphylococcus aureus. J Infect Dis 203:880–888. doi: 10.1093/infdis/jiq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson KM, Ramanan K, Clay ME, McHugh KJ, Pilewski MJ, Nickolich KL, Corey C, Shiva S, Wang J, Muzumdar R, Alcorn JF. 2018. The inflammasome potentiates influenza/Staphylococcus aureus superinfection in mice. JCI Insight 3:97470. doi: 10.1172/jci.insight.97470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma-Kuinkel BK, Zhang Y, Yan Q, Ahn SH, Fowler VG Jr. 2013. Host gene expression profiling and in vivo cytokine studies to characterize the role of linezolid and vancomycin in methicillin-resistant Staphylococcus aureus (MRSA) murine sepsis model. PLoS One 8:e60463. doi: 10.1371/journal.pone.0060463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diep BA, Afasizheva A, Le HN, Kajikawa O, Matute-Bello G, Tkaczyk C, Sellman B, Badiou C, Lina G, Chambers HF. 2013. Effects of linezolid on suppressing in vivo production of staphylococcal toxins and improving survival outcomes in a rabbit model of methicillin-resistant Staphylococcus aureus necrotizing pneumonia. J Infect Dis 208:75–82. doi: 10.1093/infdis/jit129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breslow-Deckman JM, Mattingly CM, Birket SE, Hoskins SN, Ho TN, Garvy BA, Feola DJ. 2013. Linezolid decreases susceptibility to secondary bacterial pneumonia postinfluenza infection in mice through its effects on IFN-gamma. J Immunol 191:1792–1799. doi: 10.4049/jimmunol.1300180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaffler H, Demircioglu DD, Kuhner D, Menz S, Bender A, Autenrieth IB, Bodammer P, Lamprecht G, Gotz F, Frick JS. 2014. NOD2 stimulation by Staphylococcus aureus-derived peptidoglycan is boosted by Toll-like receptor 2 costimulation with lipoproteins in dendritic cells. Infect Immun 82:4681–4688. doi: 10.1128/IAI.02043-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin FJ, Gomez MI, Wetzel DM, Memmi G, O’Seaghdha M, Soong G, Schindler C, Prince A. 2009. Staphylococcus aureus activates type I IFN signaling in mice and humans through the Xr repeated sequences of protein A. J Clin Invest 119:1931–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez MI, Lee A, Reddy B, Muir A, Soong G, Pitt A, Cheung A, Prince A. 2004. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med 10:842–848. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- 28.Karlstrom A, Boyd KL, English BK, McCullers JA. 2009. Treatment with protein synthesis inhibitors improves outcomes of secondary bacterial pneumonia after influenza. J Infect Dis 199:311–319. doi: 10.1086/596051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurczynski SJ, Nathani N, Warheit-Niemi HI, Hult EM, Podsiad A, Deng J, Zemans RL, Bhan U, Moore BB. 2018. CCR2 mediates increased susceptibility to post-H1N1 bacterial pneumonia by limiting dendritic cell induction of IL-17. Mucosal Immunol 12:518–530. doi: 10.1038/s41385-018-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Randolph AG, Xu R, Novak T, Newhams MM, Bubeck Wardenburg J, Weiss SL, Sanders RC, Thomas NJ, Hall MW, Tarquinio KM, Cvijanovich N, Gedeit RG, Truemper EJ, Markovitz B, Hartman ME, Ackerman KG, Giuliano JS, Shein SL, Moffitt KL, Kong M, Sanders RC, Hefley G, Tellez D, Typpo K, Markovitz B, Morzov RSP, Graciano AL, Cvijanovich N, Flori H, Brumfield B, Anas N, Schwarz A, Vargas-Shiraishi O, McQuillen P, Sapru A, Mourani P, Czaja A, Carroll C, Giuliano JS, Tala J, Palmieri L, McLaughlin G, Paden M, Tarquinio K, Stone CL, Coates BM, Pinto N, Sullivan J, Montgomery V, Randolph AG, et al. . 2019. Vancomycin monotherapy may be insufficient to treat methicillin-resistant Staphylococcus aureus coinfection in children with influenza-related critical illness. Clin Infect Dis 68:365–372. doi: 10.1093/cid/ciy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, He Y, Xiao K, White JR, Fusco DN, Papanicolaou GA. 2013. Effect of linezolid on clinical severity and pulmonary cytokines in a murine model of influenza A and Staphylococcus aureus coinfection. PLoS One 8:e57483. doi: 10.1371/journal.pone.0057483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.