Abstract
Objective:
Behavioral variant frontotemporal dementia (bvFTD), is commonly considered the cognitive presentation of the frontotemporal dementia-motor neuron disease (FTD-MND) spectrum disorder. We evaluated the prevalence of primary progressive aphasia in a series of pathologically confirmed cases of FTD-MND spectrum.
Methods:
Pathologically confirmed cases of frontotemporal lobar degeneration-motor neuron disease (FTLD-MND) were obtained from the UCSF brain bank. Cases were analyzed for presence of language impairment via retrospective chart review of research visits that include neurologic exam, in-depth cognitive testing and magnetic resonance imaging (MRI) imaging. Forty one cases were included. Thirty two were diagnosed with FTD-MND, while nine cases were diagnosed as MND-only from clinical evaluation.
Results:
Ten FTLD-MND cases (31%) presented with prominent or isolated language involvement consistent with a diagnosis of primary progressive aphasia (PPA), which we called progressive aphasia with motor neuron disease (PA-MND). Of these, three cases that mirrored the non-fluent variant of PPA (nfvPPA) were named nfvPA-MND. The imaging pattern of these nfvPA-MND showed atrophy strictly confined to the frontal and anterior temporal language cortical areas. Another group of seven cases that resembled patients with the semantic variant PPA (svPPA) were named svPA-MND. The group of svPPA-MND on imaging analysis showed selective atrophy of the temporal lobe and orbitofrontal cortex.
Conclusions:
Language impairment was a frequent phenotype of FTD-MND associated with focal atrophy patterns within the language networks. This data suggest patients with FTD-MND can present quite often with language phenotype of nfvPPA and svPPA, as opposed to exclusive bvFTD symptoms.
Keywords: Neuropathology, imaging, dementia, primary progressive aphasia, motor neuron disease, frontotemporal dementia, amyotrophic lateral sclerosis
1. Introduction
Frontotemporal dementia (FTD) and motor neuron disease (MND), once thought to be separate entities, are now considered a spectrum of disease with genetic and neuropathological overlap (1–4). In this FTD-MND continuum, MND and FTD forms represent the opposite ends of the spectrum: most of the cases lie between these two “pure” phenotypes, showing a certain degree of cognitive/behavioral impairment or motor involvement. Indeed, in the last decades progressive motor neuron dysfunction has been increasingly recognized in FTD cases ((5), inserire cit 13–15 tesi): nearly 60% of all FTD cases are found with EMG signs of MND, with 10–15% of cases showing clinical sign of motor involvement. Besides, various degrees of executive or behavioral dysfunction has long been recognized in MND (6), leading to the development of criteria for the diagnosis of fronto-temporal dysfunction in amyotrophic lateral sclerosis (ALS), which have been recently revised (4,7). In particular, accordingly to different series, nearly 50% of MND patients develop a certain degree of cognitive decline along the course of the disease, reaching threshold for full FTD diagnosis in 25% of the cases (7). In addition, behavioral/cognitive impairment fastens functional decline and shortens survival in the MND patient population {Elamin, 2011 #1433; Montuschi, 2015 #1302}.
The cognitive profile of the FTD-MND spectrum is most often described as social cognition and behavioral disturbances or executive dysfunction, compatible with a diagnosis of behavioral variant of FTD (bvFTD){Phukan, 2007 #1440; Mioshi, 2014 #242; Woollacott, 2016 #1319; Lansdall, 2017 #1312}. Although speech and language disorders associated with MND have been previously investigated and a few cases of primary progressive aphasia (PPA) with MND have also been described {Caselli, 1993 #1425; Tsuchiya, 2000 #1311; da Rocha, 2007 #1426; Ostberg, 2011 #1431}, an isolated speech and language disorder is not considered the most common presentation of FTD-MND. It is also still a matter of debate if language impairment in MND patients represents true language deficit or downstream manifestation of other compromised cognitive functions (4). Only in very recent years, some studies have drawn attention to the possibility that language dysfunction could recur as frequently as the executive dysfunction in ALS (8–14) and their contribution was revised in a recent review (15). Another study considered the relative frequencies of FTD phenotypes (bvFTD, semantic dementia and progressive non-fluent aphasia) in a clinical consecutive cohort of patients diagnosed with FTD with or without associated ALS; they concluded that pure language phenotypes (semantic dementia and progressive non-fluent aphasia) occur infrequently in FTD cases with coexistent ALS (16). In another paper, the authors focused on the different symptoms’ profile of bvFTD as opposed to bvFTD with ALS (termed ALS-FTD), the same authors observed a comparatively greater degree of language impairment in the ALS-FTD group (17).
Nevertheless, thus far the relation between isolated progressive language impairment, as to say PPA phenotype, and MND, with support of extensive language assessment, imaging data, and their correlation with pathological diagnosis, have never been systematically investigated. In this study, we aimed to investigate the occurrence of PPA diagnosis and the profile of language deficits within a large cohort of patients with FTLD-MND pathological diagnosis and with extensive clinical, neuropsychological neuroimaging pattern characterization.
2. Methods
2.1. Study population
We systematically screened the University of California, San Francisco (UCSF) Neurodegenerative disease brain bank (NDBB) to identify all cases with postmortem pathological diagnosis of FTLD-MND or MND (specifically ALS) available in the NDBB from January 1999 to December 2014. Along with this major neuropathological inclusion criteria, cases were included in the study also if at least one magnetic resonance imaging (MRI) acquisition and cognitive testing, as well as extensive clinical information, were available. Overall, 41 patients were recruited. Thirty two cases had a pathological diagnosis of FTLD-MND, while 9 cases had ALS.
We then retrospectively looked at the clinical diagnosis of patients. Of the 32 cases with FTLD-MND pathological diagnosis, 18 cases had been diagnosed as bvFTD with associated MND, while four cases as bvFTD with no clinical signs of MND: since the cognitive phenotype of these 22 cases corresponded to bvFTD, we defined this group of patients as bvFTD-MND. Ten cases were instead initially diagnosed as PPA (Table 1). We thus defined this group as affected by progressive aphasia with MND (PA-MND), as not all cases could be defined as “primary” since motor symptoms were also present at the time of first PPA diagnosis or soon after. We further defined three of these 10 PA-MND cases as affected by non-fluent aphasia (nfvPA-MND) and seven by semantic variant aphasia (svPA-MND).
Table 1.
Diagnostic criteria for PPA fulfilled by each PA-MND case.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| nfvPPA | Core features | ||||||||||
| agrammatism in language production | ✓ | ✓ | |||||||||
| apraxia of speech | ✓ | ✓ | |||||||||
| Supporting features | |||||||||||
| impaired comprehension of complex sentences | ✓ | ✓ | ✓ | ||||||||
| spared single-word comprehension | ✓ | ✓ | ✓ | ||||||||
| spared object knowledge | ✓ | ||||||||||
| svPPA | Core features | ||||||||||
| impaired confrontation naming | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Impaired single-word comprehension | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Supporting features | |||||||||||
| impaired object knowledge | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| surface dyslexia or dysgraphia | ✓ | ✓ | ✓ | ✓ | |||||||
| spared repetition | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | □ | ||||
| spared speech production | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
nfvPA-MND: non fluent variant of progressive aphasia with motor neuron disease; svPA-MND: semantic variant of progressive aphasia with motor neuron disease.
We finally compared these two PA-MND sub-groups to a group of nfvPPA and svPPA cases selected from the UCSF-MAC PPA pathological cohort and matched for age, gender, functional (Clinical Dementia Rating Scale [CDR]), and cognitive performance (Mini–mental state examination [MMSE]).
The following groups were, therefore, considered for analysis: 10 cases of PA-MND (3 nfvPA-MND and 7 svPA-MND), 22 cases of bvFTD-MND, and 9 cases of ALS.
The whole cohort was tested for presence of genetic mutations known to be associated with the FTD-MND spectrum, with 16 cases showing the C9orf72 gene mutation (1 in the PA-MND group, 12 in the bvFTD-MND group, and 3 cases in the ALS group).
All participants gave written informed consent and the study was approved by the UCSF Committee on Human Research.
2.2. Clinical and cognitive evaluation
Clinical evaluations of the study patients took place at the UCSF-Memory and Aging Center (MAC) between 1999 and 2014 through a semi-structured history and neurological examination by a behavioral neurologist, a caregiver interview by a nurse, a structural brain MRI and a neuropsychological evaluation.
When collecting the neurological history of each patient, a set of standardized questions directed to probe cognitive and behavioral as well as motor symptoms are asked, with particular attention to documenting first symptoms.
The neuropsychological evaluation consists of a standardized battery of cognitive tests administered by a neuropsychologist (UCSF neuropsychological and UCSF speech and language batteries, as previously described (18–20).
For general functioning and cognition, the following tests/scales were considered: the MMSE and the Geriatric Dementia Scale (GDS) as measures of general cognition function; the CDR Scale Total and Sum of Boxes as a measure of functional performance; the Neuropsychiatric Inventory (NPI) and the Total Behavior Rating as measures of neuropsychiatric symptomatology.
For evaluation of main cognitive domains, the following subtest of the UCSF Neuropsychological Battery were considered: the California Verbal Learning Test (CVLT) (immediate and delayed free recall) and the Benson figure recall for verbal and visuo-spatial memory, respectively; Benson Figure copy for visuospatial skills; Modified Trails, Calculation and Digits Backward for attention, working memory and executive functions.
Specific aspects of language function were investigated considering subtests from the UCSF Neuropsychological Battery (available for all cases) and tests from the UCSF Battery Speech and Language Batteries (available for subset of patients in the PA-MND and the bvFTD-MND groups). These included: the spontaneous speech section of the Western Aphasia Battery (WAB) for evaluation of speech fluency and syntactic production; the repetition section of the WAB for repetition ability; syntactic comprehension abilities with the sentence comprehension test and the syntax comprehension subtest of the Curtiss-Yamada Comprehensive Language evaluation (CYCLE)-Receptive test; the Peabody Picture Vocabulary Test (PPVT)-revised for single word comprehension; the Boston Naming Test (BNT) for confrontation naming; D-words per minute and animals per minute to evaluate phonemic and category fluency, respectively; and the Pyramids and Palm Trees Picture version for semantic associations; the Oral reading Regularity section (regular and irregular words) of the Psycholinguistic Assessments of Language Processing Abilities (PALPA) battery for surface dyslexia.
Results of each patient group were compared to the others and to a reference group of 21 healthy controls.
2.3. Statistical analyses for demographics, cognitive, speech, and language evaluation
Taking into account sample size, data analysis was performed using non-parametric tests (Kruskal–Wallis and Mann–Whitney U tests) and χ2 test for categorical data. A p value <0.05 was considered statistically significant.
2.4. Neuropathology
Pathological diagnosis was assessed for each case with postmortem autopsy and based on consensus criteria for FTLD (21) and Alzheimer disease (22,23). All FTD-MND cases had FTLD-TDP pathology (24). In the PA-MND group, six cases presented TDP type-B (TDP-B), two cases TDP type-A (TDP-A), and two cases TDP type-C (TDP-C) pathology. Specifically, of the three nfvPA-MND cases, one had TDP-A and two TDP-B pathology, while of the seven svPA-MND cases two had TDP-C, one TDP-A, and four TDP-B pathology, respectively. In the bvFTD-MND group, 15 cases were TDP-B, while 7 cases were TDP of unclassifiable type. Eight of the nine ALS cases presented TDP pathology (25), while one case presented NOS (non-identifiable inclusion protein).
Alzheimer’s disease neuropathological change (ADNC) also coexisted in some cases. Specifically, three cases in the PA-MND group (all with low ADNC grade); ten cases in the bvFTD-MND group (eight with low ADNC, two with intermediate ADNC); four cases in the ALS group (two with low and two with intermediate ADNC).
2.5. Neuroimaging
T1 image acquisitions were obtained on either a 1.5T (18 cases), 3T (10 cases), or 4T scanner (13 cases), as previously described (18,26,27). The first available MRI acquisition was used for each patient. All except two subjects underwent structural MRI within one year of their evaluation at UCSF; one subject underwent MRI scan 15 months prior and the other 21 months after the first evaluation.
An imaging control group of 32 healthy subjects recruited from the UCSF-MAC healthy aging cohort was used, matched with the patient groups by gender, age at scan, handedness, education, and scanner type (24 males, mean age 61.8 ± 7.5 years, 28 right-handed, mean education 16.7 ±2.1 years; nine subjects on 1.5T, 18 on 3T, and five on 4T MRI scanner).
Voxel-based morphometry (VBM) in SPM12 (Statistical Parametric Mapping; Wellcome Department of Imaging Neuroscience, London, UK) was performed to investigate gray matter (GM) volume differences using the Diffeomorphic anatomical registration through exponentiated Lie algebra (DARTEL) toolbox running under Matlab R2013a (MathWorks, Natick, MA) according to standard procedures described elsewhere (28). The images were then smoothed with a Gaussian kernel (8 mm FWHM).
Statistical analysis was performed using the general linear model in SPM12. Whole brain analyses of differences in GM were investigated using an analysis of covariance (ANCOVA) test across groups, including age, gender, total intracranial volume (TIV), and scanner type as nuisance variables.
Corrected level of significance for family wise error (p < 0.05 FWE) was considered when comparing patients to controls, except for groups <10 cases, for which uncorrected (p < 0.001) level of significance was accepted. Direct comparison between patients’ groups did not allow the survival of significant result from the analysis.
The SPM Anatomy toolbox version 2.0 was used to report the anatomical localization of the results.
3. Results
3.1. Demographics
Characteristics of study groups and their comparison are reported in Tables 2 and 3. PA-MND, bvFTD-MND, and ALS groups showed similar distribution of gender, handedness, and level of education. They also had comparable age at first symptom onset (cognitive or motor, depending on group), at motor symptoms onset, and at first evaluation (Figure 1).
Table 2.
Demographic data of PA-MND, bvFTD-MND and ALS group.
| PA-MND | bvFTD-MND | ALS | |||
|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | Controls | SR |
| Demographic data | |||||
| Gender (M/F) | 7/3 | 16/6 | 7/2 | 7/14 | |
| Handedness (L/R) | 10/0 | 21/1 | 9/0 | 19/2 | |
| Education, years | 16.5 ±2.5 | 14.8 ± 2.7 | 15.3 ± 2.1 | 17.5 ± 2.2 | |
| Age at onset (general), years | 56.6 ±6.4 | 54.5 ± 8.5 | 58.1 ± 10.4 | n/a | |
| Age at onset (motor), years | 62.8 ±7.9 | 60.2 ± 7.8 | 58.2 ± 10.0 | n/a | |
| Age at initial evaluation, years | 61.7 ±9.0 | 59.8 ± 7.4 | 59.8 ± 10.4 | 67.0 ± 8.0 | |
| Illness duration at initial evaluation, year | 5.0 ±4.6 | 5.2 ± 4.3 | 1.8 ± 1.0 | n/a | 2 vs. 3 |
| Presence of motor symptoms at initial evaluation | 6/10 | 15/22 | 9/9 | n/a | |
| Presence of motor symptoms at any point | 9/10 | 18/22 | 9/9 | n/a | |
| Survival, years | 9.1 ±6.4 | 8.0 ± 6.3 | 3.4 ± 1.9 | n/a | 1 vs. 3, 2 vs. 3 |
PA-MND: progressive aphasia with motor neuron disease; bvFTD-MND: behavioral variant frontotemporal dementia with motor neuron disease; ALS: amyotrophic lateral sclerosis; SR: significant results for comparison between groups (p < 0.05)
Reported values are mean ± SD.
Table 3.
Demographic and cognitive data of nfvPA-MND, svPA-MND, and bvFTD-MND.
| nfvPA-MND | svPA-MND | bvFTD-MND | ||
|---|---|---|---|---|
| Group | 1 | 2 | 3 | Controls |
| Demographic data | ||||
| Gender (M/F) | 2/1 | 5/2 | 16/6 | 7/14 |
| Handedness (R/L) | 3/0 | 7/0 | 21/1 | 19/2 |
| Education, years | 16.0 ± 2.0 | 16.7 ± 2.9 | 14.8 ±2.7 | 17.5 ±2.2 |
| Age at onset (general), years | 55.0 ± 6.2 | 57.3 ± 6.8 | 54.5 ±8.5 | n/a |
| Age at onset (motor), years | 56.0 ± 6.2 | 65.7 ± 6.8 | 60.2 ±7.8 | n/a |
| Delay between cognitive and motor onset (median), years | 0.92 | 9.75 | 3.83 | n/a |
| Age at initial evaluation, years | 56.7 ± 6.4 | 63.9 ± 9.5 | 59.8 ±7.4 | 67.0 ±8.0 |
| Illness duration at initial evaluation, years | 1.4 ± 0.2 | 6.6 ± 4.7 | 5.2 ±4.3 | n/a |
| Presence of motor symptoms at initial evaluation | 3/3 | 4/7 | 15/22 | n/a |
| Presence of motor symptoms at any point | 3/3 | 6/7 | 18/22 | n/a |
| Survival, years | 3.8 ± 0.6 | 11.6 ± 6.0 | 8.0 ±6.3 | n/a |
nfv PA-MND: non-fluent variant of progressive aphasia with motor neuron disease; svPA-MND: semantic variant of progressive aphasia with motor neuron disease; bvFTD-MND: behavioral variant frontotemporal dementia with motor neuron disease; ALS: amyotrophic lateral sclerosis
Reported values are mean ± SD.
Figure 1.
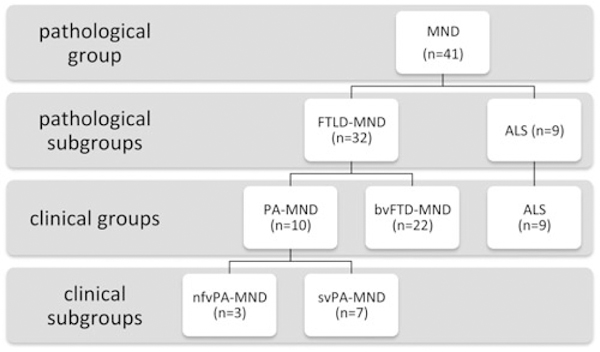
Flowchart of case selection on pathological criteria and classification by clinical diagnosis.
Regarding the type of the first symptom reported, all 10 PA-MND cases referred language difficulties as the earliest and major complain, even if one nfvPA-MND and four svPA-MND patients reported concurrent and synchronous behavioral change.
For the bvFTD-MND group, all the 22 patients reported first changes in personality/behavior, but two also reported simultaneous memory impairment and two reported speech difficulties.
Median time of motor symptoms occurrence from disease onset significantly differed between groups: 0.92 years for nfvPA-MND, 9.75 years for svPA-MND, with intermediate value of 3.83 years for bvFTD-MND (Figure 2).
Figure 2.
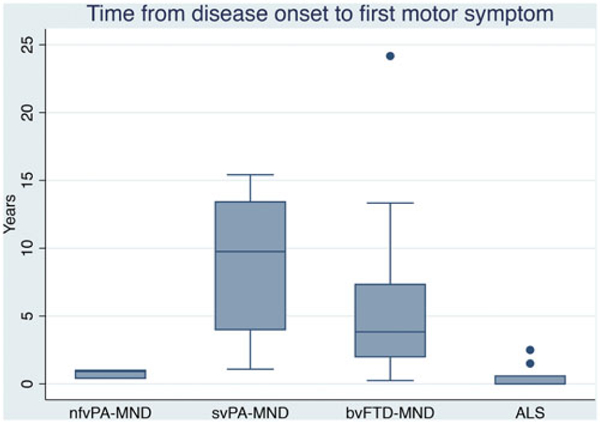
Time from disease onset to first motor symptom in nfvPA-MND, svPA-MND, bvFTD-MND, and ALS group.
Motor symptoms were the initial complain for six of the nine ALS cases, while two patient complained of subtle memory problems in the previous period (30 and 7 months before motor onset) and one presented slight behavioral change 18 months before motor disease onset. The degree of these cognitive symptoms was never sufficient to warrant a clinical diagnosis of dementia during life and, for the two cases with memory loss, this could be accountable by the presence of AD copathology at autopsy.
Regarding illness duration at first evaluation, ALS group appeared to show up early compared to PA-MND and bvFTD-MND.
When looking at PA-MND subgroups, nfvPA-MND and svPA-MND, they were similar for age at onset for cognitive symptoms (average 55 and 57.3, respectively), while there was a trend of difference for age of onset for motor symptom, even not reaching statistical significance (56 and 65.7, respectively, p = 0.06).
Survival was significantly longer in the PA-MND and bvFTD-MND group compared to the ALS group (p < 0.001, Table 2), and this finding appears to be drag by the svPA-MND cases, which show mean disease duration of 11.6 years compared to the 3.8 mean disease duration of the nfvPA-MND group (Table 3).
3.2. Patients’ complains and symptoms at first evaluation
All the four major groups presented with some degree of cognitive symptoms including the group of ALS cases (Table 4).
Table 4.
Symptoms and complaints of patients at first evaluation.
| nfvPA-MND | svPA-MND | bvFTD-MND | |||
|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | ALS 4 |
SR |
| Behavior | |||||
| Apathy | 33 | 43 | 59 | 56 | |
| Impulsiveness | 33 | 29 | 32 | 11 | |
| Obsessive-compulsive behavior | 0 | 57 | 59 | 0 | 2 vs. 4, 3 vs. 4 |
| Disinhibition | 0 | 71 | 50 | 11 | 2 vs. 4 |
| Emotional blunting | 67 | 29 | 64 | 11 | 3 vs. 4 |
| Personality changes | 33 | 57 | 45 | 22 | |
| Mood changes/depression | 33 | 43 | 18 | 56 | |
| Mental rigidity | 67 | 29 | 32 | 11 | |
| Loss of empathy | 0 | 29 | 27 | 0 | |
| Delusions/hallucinations | 0 | 0 | 27 | 0 | |
| Aggressiveness/irritability | 33 | 14 | 23 | 11 | |
| Anxiety | 33 | 29 | 9 | 11 | |
| Eating changes | 33 | 14 | 23 | 0 | |
| Emotional lability | 100 | 0 | 5 | 22 | 1 vs. 2, 1 vs. 3, 1 vs. 4 |
| Memory loss | 33 | 100 | 64 | 22 | 1 vs. 2, 2 vs. 4 |
| Frontal/executive | 0 | 57 | 82 | 33 | 1 vs. 3, 3 vs. 4 |
| Language | |||||
| Reduced speech output | 100 | 57 | 45 | 22 | 1 vs. 4 |
| Impaired comprehension | 67 | 57 | 27 | 0 | 1 vs. 4, 2 vs. 4 |
| Word finding problem | 100 | 100 | 55 | 33 | 2 vs. 3, 2 vs. 4 |
| Semantic impairment | 0 | 57 | 9 | 0 | 2 vs. 3, 2 vs. 4 |
| Simplified speech | 67 | 43 | 9 | 0 | 1 vs. 3, 1 vs. 4 |
| Impaired writing | 33 | 29 | 32 | 33 | |
| Impaired reading | 33 | 29 | 14 | 22 | |
| Echolalia | 0 | 0 | 14 | 0 | |
| Impaired spelling | 0 | 0 | 9 | 0 | |
| Motor impairment | |||||
| Dysarthria, slurring of speech | 67 | 14 | 23 | 89 | 2 vs. 4, 3 vs. 4 |
| Swallowing difficulties | 100 | 29 | 50 | 67 | |
| UL motor impairment | 33 | 29 | 32 | 78 | |
| LL motor impairment | 0 | 0 | 14 | 78 | 1 vs. 4, 2 vs. 4, 3 vs. 4 |
| Fasciculation | 67 | 29 | 36 | 56 | |
| Muscle cramps | 33 | 43 | 18 | 33 | |
| Gait/balance | 0 | 0 | 36 | 67 | 2 vs. 4 |
| Falls | 0 | 14 | 41 | 67 | |
| Tremor | 0 | 29 | 14 | 0 | |
UL: upper limbs; LL: lower limbs. nfvPA-MND: non-fluent variant of progressive aphasia with motor neuron disease; svPA-MND: semantic variant of progressive aphasia with motor neuron disease; bvFTD-MND: behavioral variant frontotemporal dementia with motor neuron disease; ALS: amyotrophic lateral sclerosis; SR: significant results for comparison between groups (p < 0.05)
Reported values are percentage of symptoms presence upon total.
Among the behavioral complaints, obsessive-compulsive and repetitive behaviors were significantly more prevalent as early manifestations in the svPA-MND and bvFTD-MND compared to the other two groups, in which were completely absent. Also, disinhibition was significantly more often reported in the svPA-MND compared to ALS, as well as emotional blunting in the bvFTD-MND group compared to ALS.
Emotional lability (or pseudobulbar affect) characterized all nfvPA-MND patients, significantly more than the ALS, in which the symptom was still present, and the bvFTD-MND and svPA-MND in which it was very rarely reported.
Memory loss was reported by all svPA-MND, in which was significantly more prevalent than the nfvPA-MND and the ALS group.
Frontal/dysexecutive symptoms were much more frequent in bvFTD-MND compared to nfvPA-MND and ALS, which intermediate prevalence in the svPA-MND group.
In the language domain, the most frequently reported symptoms in the nfvPA-MND included reduced verbal output, impaired comprehension and simplified speech, which were significantly more prevalent than in the ALS. Simplified speech was also significantly more present in this group compared to bvFTD-MND. svPA-MND patients mainly complained of word finding problems and semantic loss compared to bvFTD-MND and ALS; they also significantly differed for impaired comprehension from ALS.
Concerning motor symptoms, all groups showed some degree of motor complaints at first visit. svPA-MND distinguished for low prevalence of bulbar symptoms (dysarthria and slurring of speech) compared to ALS, similarly to bvFTD-MND. Lower limb symptoms were unfrequently reported compared in nfvPA-MND and svPA-MND as well as bvFTD-MND compared to ALS. Difficulties in gait or balance were reported more often in the ALS group, with significant values with regard to the svPA-MND.
3.3. Neurologic findings
In the motor domain, a striking asymmetric motor weakness was noted in the nfvPA-MND group, with significantly higher prevalence compared to both svPA-MND and bvFTD-MND (Table 5). On the other hand, weakness in lower limbs was significantly more frequent in ALS compared to nfvPA-MND and bvFTD-MND.
Table 5.
Neurological signs at first evaluation.
| nfvPA-MND | svPA-MND | bvFTD-MND | ALS | ||
|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | 4 | SR |
| Motor | |||||
| VII cn palsy | 33 | 0 | 18 | 44 | |
| IX cn palsy | 33 | 0 | 5 | 33 | |
| XII cn palsy | 0 | 0 | 9 | 0 | |
| UL weakness | 100 | 43 | 41 | 89 | 3 vs. 4 |
| UL weakness asymmetry | 100 | 14 | 23 | 56 | 1 vs. 2, 1 vs. 3 |
| LL weakness | 0 | 29 | 27 | 78 | 1 vs. 4, 3 vs. 4 |
| LL weakness asymmetry | 0 | 14 | 14 | 44 | |
| Tongue weakness | 67 | 0 | 23 | 67 | 1 vs. 2, 2 vs. 4, 3 vs. 4 |
| Tongue atrophy | 0 | 0 | 18 | 33 | |
| UL atrophy | 33 | 43 | 41 | 56 | |
| LL atrophy | 0 | 0 | 27 | 22 | |
| Tongue fasciculations | 33 | 0 | 41 | 11 | |
| UL fasciculations | 100 | 43 | 45 | 33 | |
| LL fasciculations | 67 | 29 | 36 | 33 | |
| Limbs’ spasticity | 33 | 0 | 23 | 44 | |
| Reflexes | |||||
| Pseudobulbar affect | 100 | 0 | 9 | 78 | 1 vs. 2, 1 vs. 3, 2 vs. 4, 3 vs. 4 |
| Frontal release signs | 33 | 14 | 41 | 33 | |
| Jaw jerk | 67 | 0 | 32 | 22 | |
| UL hyperreflexia | 100 | 0 | 45 | 89 | 1 vs. 2, 2 vs. 4, 3 vs. 4 |
| LL hyperreflexia | 100 | 0 | 45 | 89 | 1 vs. 2, 2 vs. 4, 3 vs. 4 |
| Hyperreflexia asymmetry | 100 | 14 | 18 | 11 | 1 vs. 2, 1 vs. 3, 1 vs. 4 |
| Hoffmann’s sign | 0 | 0 | 32 | 33 | |
| Babinski’s sign | 0 | 14 | 9 f | 56 | 3 vs. 4 |
| Language | |||||
| Apraxia of speech | 33 | 0 | 0 | 0 | |
| Effortful speech | 67 | 0 | 9 | 11 | 1 vs. 2, 1 vs. 3 |
| Agrammatism | 33 | 0 | 9 | 0 | |
| Reduced verbal output | 67 | 29 | 23 | 0 | 1 vs. 4 |
| Naming deficit | 67 | 71 | 9 | 0 | 2 vs. 3, 2 vs. 4 |
| Semantic impairment | 0 | 71 | 5 | 0 | 2 vs. 3, 2 vs. 4 |
| Dysarthria | 100 | 0 | 32 | 100 | 1 vs. 2, 2 vs. 4, 3 vs. 4 |
| Comprehension deficit | 67 | 14 | 18 | 0 | 1 vs. 4 |
| Word-finding pauses | 0 | 43 | 14 | 0 | |
| Repetition | 33 | 14 | 9 | 0 | |
| Simplified speech | 33 | 0 | 0 | 0 | |
cn: cranial nerve; UL: upper limbs; LL: lower limbs. nfvPA-MND: non-fluent variant of progressive aphasia with motor neuron disease; svPA-MND: semantic variant of progressive aphasia with motor neuron disease; bvFTD-MND: behavioral variant frontotemporal dementia with motor neuron disease; ALS: amyotrophic lateral sclerosis. SR: significant results for comparison between groups (p < 0.05)
Reported values are percentage of symptoms presence upon total.
Tongue weakness was noted in higher proportion in the nfvPA-MND group compared to svPA-MND, as well as in the ALS compared to both svPA-MND and bvFTD-MND.
Overall, hyperreflexia and pseudobulbar affect were specific objective findings of the nfvPA-MND group, with significantly higher prevalence compared to svPA-MND. The same pattern was found in the ALS group compared both svPA-MND and bvFTD-MND. Hyperreflexia asymmetry resulted significantly more present in the nfvPA-MND compared to all the other groups.
In language evaluation, the nfvPA-MND group was characterized by effortful speech compared to svPA-MND and bvFTD-MND, and reduced verbal output and comprehension deficits compared to ALS.
The svPA-MND group distinguished for significantly more naming deficit and semantic impairment cioared ti bvFTD-MND and ALS.
Dysarthria was significantly more prevalent in the nfvPA-MND group compared to svPA-MND, and in the ALS group compared to svPA-MND and bvFTD-MND.
3.4. Cognitive findings
3.4.1. PA-MND vs. bvFTD-MND and ALS.
Overall, PA-MND cases were less impaired at first evaluation compared to bvFTD-MND cases in general functioning scores (MMSE and CDR scores, Table 6). However, both groups were more impaired than ALS.
Table 6.
Cognitive and language findings of PA-MND, bvFTD-MND, and ALS group at presentation.
| PA-MND | bvFTD-MND | ALS | |||
|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | Controls | SR |
| General functioning | |||||
| MMSE | 26.3 ± 1.9 | 24.7 ±4.7 | 28.8 ±1.3 | 29.7 ± 0.5 | 1 vs. 3, 2 vs. 3 |
| CDR total | 0.7 ± 0.5 | 1.2 ±0.7 | 0.4 ±0.6 | 0.0 ± 0.0 | 1 vs. 2, 2 vs. 3 |
| CDR sum of boxes | 4.0 ± 2.8 | 6.4 ±3.9 | 2.0 ±2.5 | 0.0 ± 0.0 | 2 vs. 3 |
| NPI total | 33.9 ± 18.6 | 34.9 ±20.0 | 17.4 ±20 | 0.5 ± 0.7 | |
| GDS total | 9.2 ± 5.5 | 5.7 ±4.1 | 9.1 ±4.0 | 3.9 ± 3.4 | 2 vs. 3 |
| Total behavior ratings | 2.0 ± 1.4 | 5.3 ±5.4 | 1.5 ±1.3 | 0.0 ± 0.0 | |
| Cognition | |||||
| CVLT-MS total free recall | 18.1 ± 5.5 | 21.5 ±6.1 | 25.6 ±4.3 | 29.9 ± 3.3 | 1 vs. 3 |
| CVLT-MS free recall at 10min | 3.4 ± 2.6 | 3.9 ±2.5 | 5.5 ±2.4 | 7.9 ± 1.0 | |
| Benson figure recall | 9.7 ± 2.9 | 8.2 ±4.7 | 11.1 ±2.1 | 12.8 ± 3.2 | |
| Benson figure copy | 15.5 ± 1.0 | 14.5 ±2.7 | 15.0 ±1.6 | 16.3 ± 1.0 | |
| Modified trails | 0.02 ± 0.01 | 0.03 ±0.02 | 0.03 ±0.01 | 0.04 ± 0.02 | |
| Calculation | 4.4 ± 1.0 | 3.6 ±1.6 | 4.2 ±1.1 | 4.9 ± 0.3 | |
| Digits backward | 4.1 ± 1.2 | 3.9 ±1.7 | 4.5 ±1.6 | 5.3 ± 1.3 | |
| Speech and language | |||||
| Sentence repetition (5) | 3.8 ± 1.3 | 3.9 ±1.3 | 4.3 ±1.1 | 5.0 ± 0.0 | |
| Verbal agility (6) | 4.2 ± 2.5 | 3.5 ±2.1 | 4.2 ±1.5 | 5.7 ± 0.5 | |
| Sentence comprehension (5) | 4.4 ± 0.9 | 3.8 ±0.7 | 4.2 ±0.8 | 5.0 ± 0.0 | |
| Single-word comprehension (16) | 12.0 ± 2.4 | 13.2 ±3.2 | 14.7 ±1.2 | 15.7 ± 0.6 | 1 vs. 3 |
| Phonemic fluency (D-words/min) | 6.8 ± 4.0 | 6.2 ±4.1 | 11.0 ±3.7 | 15.2 ± 4.7 | 1 vs. 3, 2 vs. 3 |
| Semantic fluency (animals/min) | 7.5 ± 2.6 | 10.7 ±6.1 | 16.2 ±6.8 | 23.9 ± 5.4 | 1 vs. 3 |
| Boston Naming Test (15) | 7.9 ± 4.3 | 12.3 ±2.1 | 13.4 ±1.3 | 14.8 ± 0.7 | 1 vs. 2, 1 vs. 3 |
| PPTP total (52) | 43.3 ± 10.0 | 45.4 ±5.4 | 49.7 ±3.0 | 51.7 ± 0.6 | 2 vs. 3 |
MMSE: Mini–mental state examination; CDR: Clinical Dementia Rating; NPI: Neuropsychiatric Inventory; GDS: Geriatric Dementia Scale; CVLT-MS: California verbal learning test-mental status; PPTP: pyramids and palm trees-pictures; AOS: apraxia of speech; MSE: motor speech evaluation; WAB: western aphasia battery; CYCLE: Curtiss-Yamada comprehensive language evaluation; n/a = not applicable or not available; PALPA: psycholinguistic assessments of language processing in aphasia; PA-MND: progressive aphasia with motor neuron disease; bvFTD-MND: behavioral variant frontotemporal dementia with motor neuron disease; ALS: amyotrophic lateral sclerosis; SR: significant results for comparison between groups (p < 0.05)
Reported values are mean ± SD
GDS scores were significantly lower in the bvFTD-MND group compared to PA-MND and ALS.
Neuropsychiatric symptoms were much more prevalent in both PA-MND and bvFTD-MND groups compared to ALS as evidenced by NPI scores.
In the speech and language subset of tests, PA-MND performed significantly worse than the ALS group in the single-word comprehension test, phonemic fluency, semantic fluency, and BNT. The BNT was also the only test showing a significantly lower performance in PA-MND compared with bvFTD-MND group. PA-MND did not show significantly poorer performance in the verbal agility, sentence comprehension, and sentence repetition tests compared to bvFTD-MND. The bvFTD-MND group showed significant lower scores in the phonemic fluency and the PPTP tests compared to the ALS group.
Overall, there was no correlation of disease severity with cognitive parameters observed, except for bvFTD-MND patients who showed an inverse correlation between CDR scores and BNT, PPVT, and semantic fluency performance.
3.4.2. nfvPA-MND and svPA-MND vs. bvFTD-MND.
When the PA-MND group is split into the two phenotypic variants (Table 7), nfvPA-MND and svPA-MND, a number of differences in test performance were found.
Table 7.
Cognitive and language findings of nfvPA-MND, svPA-MND, and bvFTD-MND at presentation.
| PA-MND | bvFTD-MND | ALS | |||
|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | Controls | SR |
| General functioning | |||||
| MMSE | 25.3 ±3.1 | 26.7 ±1.2 | 24.6 ± 4.7 | 29.7 ± 0.5 | |
| CDR Total | 0.5 ±0.0 | 0.8 ±0.6 | 1.2 ± 0.7 | 0.0 ± 0.0 | |
| CDR Sum of boxes | 2.5 ±1.3 | 4.7 ±3.2 | 6.4 ± 3.9 | 0.0 ± 0.0 | |
| NPI Total | 22.0 ± 3.6 | 41.0 ±20.8 | 34.9 ±20.0 | 0.5 ± 0.7 | |
| GDS Total | 10.0 ±7.2 | 8.9 ±5.2 | 5.7 ± 4.1 | 3.9 ± 3.4 | |
| Total behavior ratings | 1, 3 | n/a | 5.3 ± 5.4 | 0.0 ± 0.0 | |
| Cognition | |||||
| CVLT-MS Total free recall | 23.3 ±6.7 | 15.9 ±3.3 | 21.5 ± 6.1 | 29.9 ± 3.3 | 2 vs. 3 |
| CVLT-MS free recall at 10min | 6.3 ±2.5 | 2.1 ±1.3 | 3.9 ± 2.5 | 7.9 ± 1.0 | 1 vs. 2 |
| Benson figure recall | 11.7 ±1.5 | 8.9 ±3.0 | 8.2 ± 4.7 | 12.8 ± 3.2 | |
| Benson figure copy | 15.3 ±0.6 | 15.6 ±1.1 | 14.5 ± 2.7 | 16.3 ± 1.0 | |
| Modified Trails | 0.02, 0.02 | 0.02 ±0.02 | 0.03 ± 0.02 | 0.04 ± 0.02 | |
| Calculation | 3.7 ±1.5 | 4.8 ±0.5 | 3.6 ± 1.6 | 4.9± 0.3 | |
| Digits Backward | 3.3 ±0.6 | 4.4 ±1.3 | 3.9 ± 1.7 | 5.3 ± 1.3 | |
| Speech and language | |||||
| AOS Severity (MSE, 7) | 2 | 0.0 ±0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1 vs. 2, 1 vs. 3 |
| Dysarthria Severity (MSE, 7) | 5 | 0.0 ±0.0 | 1.3 ± 1.2 | 0.0 ± 0.0 | 1 vs. 2 |
| Verbal agility (6) | 0, 4 | 5.7 ±0.6 | 3.5 ± 2.1 | 5.5± 0.5 | |
| Speech fluency (WAB, 10) | 6 | 9.7 ±0.6 | 8.3 ± 3.1 | 10.0 ± 0.0 | |
| Information Content (WAB, 10) | 10 | 9.0 ±1.0 | 8.3 ± 3.2 | 4.3 ± 5.1 | |
| Sentence repetition (5) | 2, 5 | 4.0 ±1.0 | 3.9 ± 1.3 | 5.0 ± 0.0 | |
| Syntax comprehension (short CYCLE, 55) | 39 | 50.5 ±5.1 | 49 ± 9.5 | 54.3 ± 1.1 | |
| Sentence comprehension (5) | 3, 4 | 5.0 ±0.0 | 3.8 ± 0.7 | 5.0 ± 0.0 | 1 vs. 2, 2 vs. 3 |
| Single-word comprehension (PPVT, 16) | 14.3 ±1.5 | 10.2 ±0.5 | 13.2 ± 3.2 | 15.7 ± 0.6 | 1 vs. 2, 2 vs. 3 |
| Boston Naming Test (15) | 11.7 ±2.1 | 6.3 ±4.0 | 12.3 ± 2.1 | 14.8 ± 0.7 | 2 vs. 3 |
| Phonemic fluency (D-words/min) | 3.0 ±1.7 | 8.4 ±3.5 | 6.2 ± 4.1 | 15.2 ± 4.7 | 1 vs. 2 |
| Semantic fluency (animals/min) | 9.0 ±3.6 | 6.9 ±1.9 | 10.7 ± 6.1 | 23.9 ± 5.4 | |
| PPTP Total (52) | 50.0 ±1.0 | 36.7 ±10.7 | 45.4 ± 5.4 | 51.7 ± 0.6 | |
| Reading, regular words (PALPA, 30) | n/a | 29.7 ±0.6 | 30.0 ± 0.0 | 30.0 ± 0.0 | |
| Reading, irregular words (PALPA, 30) | n/a | 25 ±6.2 | 29.5 ± 0.7 | 29.8 ± 0.4 | |
MMSE: Mini–mental state examination; CDR: Clinical Dementia Rating; NPI: Neuropsychiatric Inventory; GDS: Geriatric Dementia Scale; CVLT-MS: California verbal learning test-mental tatus; PPTP: pyramids and palm trees-pictures; AOS: apraxia of speech; MSE: motor speech evaluation; WAB: western aphasia battery; CYCLE: Curtiss-Yamada comprehensive language evaluation; n/a = not applicable or not available; PALPA: psycholinguistic assessments of language processing in aphasia; nfvPA-MND: non fluent variant of progressive aphasia with motor neuron disease; svPA-MND: semantic variant of progressive aphasia with motor neuron disease; bvFTD-MND: behavioral variant frontotemporal dementia with motor neuron disease. SR: significant results for comparison between groups (p < 0.05)
Reported values are mean ± SD.
NfvPA-MND reached higher apraxia of speech (AOS) scores than svPA-MND, as well as dysarthria score, even though only one case in nfvPA-MND group had the formal scores available. NfvPA-MND achieved lower sentence comprehension scores than svPA-MND of borderline significance (p = 0.05). This group was better in single words comprehension, while showed much lower scores for phonemic fluency per minute.
On the other side, svPA-MND performed significantly worse than bvFTD-MND in verbal memory (CVLT-MS total free recall), single word comprehension (PPVT) and BNT (p = 0.001), while they appeared better in sentence comprehension.
3.4.3. nfvPA-MND vs. nfvPPA-TDP and svPA-MND vs. svPPA.
Cognitive profile comparison between each PA-MND subgroup and the corresponding PPA groups are shown in Supplementary Table 1.
3.5. Imaging findings
3.5.1. PA-MND vs. bvFTD-MND and ALS.
The PA-MND (n = 10) group compared to controls showed peak of GM atrophy in the left temporal pole, hippocampus, fusiform gyrus, amygdala, and inferior temporal gyrus, with a specular area localized in the right parahippocampal gyrus (Figure 3(a)). Also, the left inferior frontal gyrus, pars opercularis and the precentral gyrus appeared atrophic. In the bvFTD-MND (n = 22) group, atrophy emerged mainly in the left insular lobe, left inferior frontal gyrus pars triangularis and orbitalis, and, symmetrically, in the right insular lobe and putamen; also the left temporal pole, amygdala, hippocampus, rectal and superior orbital gyrus were involved, as long with left thalamus (Figure 3(b)). Also right anterior and midcingulate cortex were atrophic. A smaller cluster in the left inferior frontal gyrus at the edge with the precentral gyrus (Area 44) was also found. ALS (n = 9) group showed atrophy mainly in the left precentral gyrus (Area 4p, Figure 3(c)).
Figure 3.
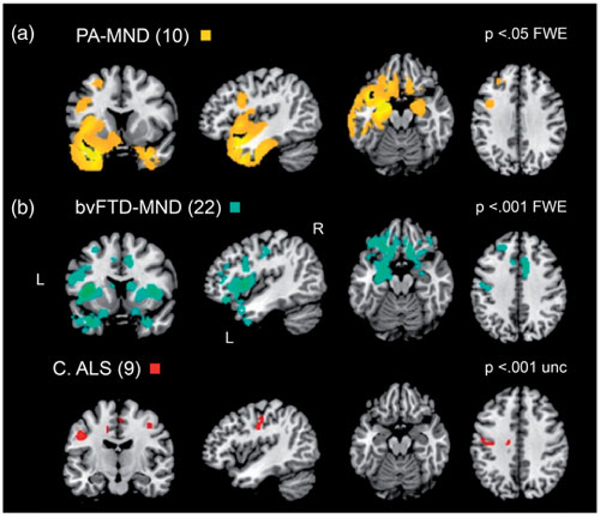
Voxel-based morphometry analysis of gray matter. The main groups were compared to healthy controls group. Results are shown on a template in MNI space (a) PA-MND, 10 subjects, at p < 0.05 FWE (b) bvFTD-MND, 22 subjects, at p < 0.001 FWE C ALS, 9 subjects, at p < 0.001 uncorrected. PA-MND: progressive aphasia with motor neuron disease; bvFTD-MND: behavioral variant frontotemporal dementia with motor neuron disease; ALS: amyotrophic lateral sclerosis.
3.5.2. nfvPA-MND vs. svPA-MND.
Splitting the PA-MND group into nfvPA-MND (n = 3) and svPA-MND (n = 7) cases and comparing the respective areas of atrophy, the nfvPA-MND group showed selective atrophy in the left insula and putamen, the left temporal pole, the left inferior frontal gyrus, pars opercularis and orbitalis, and the left precentral gyrus (Figure 4(a)). Conversely, the svPA-MND group appeared atrophic in the left temporal pole, hippocampus and parahippocampal gyrus, left inferior temporal gyrus, medial temporal pole, and the thalamus. In the right hemisphere, atrophy was distributed with the same pattern, even if with a much smaller proportion (Figure 4(b)).
Figure 4.

Voxel-based morphometry analysis of gray matter in each clinical PA-MND phenotype relative to controls. (a) nfvPA-MND and (b) svPA-MND). Results are shown in MNI space at p < 0.05 FWE. NfvPA-MND: non-fluent progressive aphasia with motor neuron disease; svPA-MND: semantic progressive aphasia with motor neuron disease.
3.5.3. nfvPA-MND vs. nfvPPA and svPA-MND vs. svPPA.
Imaging comparison between PA-MND subgroups and the corresponding PPA groups are shown in the Supplementary Figure 1.
4. Discussion
In this study, we aimed to investigate language function in the FTD-MND spectrum disorder by focusing on prominent and isolated language impairment phenotype. Specifically, this was pursued by looking at language (PPA) vs. behavioral/dysexecutive (bvFTD) cases and by analyzing the type and degree of language deficits in an FTLD-MND pathological cohort.
Quite surprisingly, of 32 FTLD-MND cases, we found that ten cases (31%) were initially diagnosed as PPA (of either non-fluent/agrammatic or semantic variant). This finding is quite in contrast with the usual view that behavioral dysfunction or dysexecutive syndrome, as to say bvFTD, represents the typical cognitive “counterpart” of the FTD-MND spectrum (29). Our results are also inconsistent with those recently yielded by Saxon et al. (JNNP 2017), who found a considerably lower prevalence of semantic dementia and progressive non-fluent aphasia cases in their clinical FTD-ALS population. That study, however, included patients selected on a clinical basis, while our study population was selected on strict pathological grounds (FTLD-MND).
From a clinical prospective, it is also relevant that these PA-MND cases constitute a considerable percentage of our PPA cohort with FTLD pathology, being ten out of 56 cases (18%). Specifically, the nfvPA-MND constitutes 11% (3 out of 28) of nfvPPA pathological cases and 19% (7 out of 36) of svPPA cases.
Focusing on the PA-MND cases, the pattern of language impairment allowed us to separate cases into a non-fluent subgroup (nfvPA-MND) and a semantic subgroup (svPA-MND), closely resembling the nfvPPA and svPPA language phenotypes, respectively. While similar in general functioning, the two groups considerably diverged in general cognitive function and specific language tasks. The nfvPA-MND group performed better in verbal memory tasks than svPA-MND group, which also performed significantly worse than bvFTD-MND. Regarding language tasks, AOS and dysarthria were specific features of the nfvPA-MND group, as well as impaired sentence comprehension and phonemic fluency. Conversely, the svPA-MND distinguished mainly for impairment in single word comprehension (PPVT) and performed worse in naming task compared to bvFTD-MND and nvfPA-MND, though difference could not reach statistical significance likely due to sample size.
The clinical-neuropsychological parallelism found between nfvPA-MND and nfvPPA and between svPA-MND and svPPA was confirmed when looking at the neuroimaging pattern of atrophy: the left BA 44 appeared as the shared key area for non-fluent presentation, with and without MND, while the left temporal lobe emerged as the crucial region responsible for semantic loss in both svPA-MND and svPPA. Conversely, each PA-MND group showed some specific areas of atrophy. As expected, the nfvPA-MND group showed atrophy in the left precentral gyrus, but showed also a partial involvement of the left temporal pole and the right cerebellar hemisphere, which is in line with other studies reporting right cerebellar involvement in MND (PLS and ALS) cases with pseudobulbar affect (30).
In addition to the left temporal pole, in both svPA-MND and svPPA atrophy significantly involved the medial aspect of the temporal lobe, though in svPA-MND atrophy was apparently more confined to this temporal region, with early spreading to the fronto-orbital regions. This finding could explain why all svPA-MND cases presented amnestic and behavioral symptoms as early findings, which specifically relates with fronto-mesial and orbito-frontal regions involvement (31).
Of interest is also the close relation between non-fluent presentation of PA-MND and bulbar signs of motor involvement and the proximity existing between oro-bulbar aspect of the motor cortex, the inferior part of the premotor cortex and the inferior frontal gyrus, pars opercularis could account for this finding, suggesting as previously hypothesized (32) a pattern of TDP disease progression through spreading to contiguous regions. Also, of interest is the observation that two of the three nfvPA-MND cases presented, besides swallowing difficulties, with right upper weakness, a finding in line with a recent study correlating aphasic symptoms in CBS cases with right-sided motor dysfunction (33).
When looking at the timeline of motor symptoms onset, nfPA-MND cases closely resemble ALS, as they develop motor symptoms almost simultaneously with cognitive symptoms. Conversely, motor neuron symptoms in svPA-MND and bvFTD seem to appear significantly later in the disease course. This finding might carry important implication, since the concomitant presence of MND in nfvPA-MND would make nfvPPA very similar to ALS cases regarding disease course and prognosis, while svPA-MND appears characterized by a milder form of MND involvement, with much delayed onset and more indolent course.
Lack of statistical significance in some estimates computed in this study is likely due to the limited sample size, a consequence of the disease rarity and availability of pathological data. Another potential study limitation is the retrospective nature of clinical data. However, the highly standardized procedure to query for clinical details during each clinical assessment at our center makes information bias unlikely. Selection bias due to referrals to UCSF MAC, a specialized tertiary research clinic specialized in cognitive disorders, should be entertained, since in-depth clinical language assessment is not generally performed in the common evaluation setting. Also, the relative frequency of MND cases in our study population is considerably lower than its real occurrence in the FTLD-MND spectrum, thus limiting the generalizability of our finding to the whole FTD- MND disease continuum.
In summary, we found that language presentations of FTD-MND patients are unexpectedly common, calling for rigorous language evaluation as a part of clinical assessment of these patients. Conversely, it appears that signs of MND should be carefully checked in the clinical assessment PPAs, as MND can be frequently associated with this disease.
Supplementary Material
Acknowledgments
Funding
The study was supported by grants from the National Institutes of Health (NINDS R01 NS050915, NIDCD K24DC015544, NIA U01AG052943, NIA P50 AG023501, NIA P01AG019724, K08 AG052648) and AAN/ALSA grant P0519279 and Foundation for the National Institutes of Health.
Declaration of interest
Dr. WWS is funded by the John Douglas French Alzheimer Disease Foundation, Consortium for Frontotemporal Dementia Research, James S. McDonnell Foundation, Larry Hillblom Foundation; has received support for travel by the Alzheimer’s Association; and has received payment for lectures by the Alzheimer’s Association, American Academy of Neurology, and Novartis Korea. BLM serves as board member on the John Douglas French Alzheimer Foundation and Larry L. Hillblom Foundation; serves as a consultant for TauRx Ltd, Allon Therapeutics, Siemens, Bristol-Myers Squibb, the Tau Consortium, and the Consortium for Frontotemporal Research; has received institutional support from Novartis; and is funded by a grant from the state of California. Dr. NO is funded by the American Academy of Neurology and the ALS Association. Dr. NO has received payment for lectures by Avanir’s visiting expert program.
Footnotes
Supplemental data for this article can be accessed here. https://doi.org/10.1080/21678421.2018.1556695
References
- 1.Neary D, Snowden J. Frontal lobe dementia, motor neuron disease, and clinical and neuropathological criteria. J Neurol Neurosurg Psychiatry. 2013;84:713–4. [DOI] [PubMed] [Google Scholar]
- 2.Devenney E, Vucic S, Hodges JR, Kiernan MC. Motor neuron disease-frontotemporal dementia: a clinical continuum. Expert Rev Neurother. 2015;15:509–22. [DOI] [PubMed] [Google Scholar]
- 3.Burrell JR, Halliday GM, Kril JJ, Ittner LM, Gotz J, Kiernan MC, et al. The frontotemporal dementia-motor neuron disease continuum. Lancet. 2016;388:919–31. [DOI] [PubMed] [Google Scholar]
- 4.Strong MJ, Abrahams S, Goldstein LH, Woolley S, McLaughlin P, Snowden J, et al. Amyotrophic lateral sclerosis – frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:153–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burrell JR, Kiernan MC, Vucic S, Hodges JR. Motor neuron dysfunction in frontotemporal dementia. Brain J Neurol. 2011;134:2582–94. [DOI] [PubMed] [Google Scholar]
- 6.Woolley SC, Strong MJ. Frontotemporal dysfunction and dementia in amyotrophic lateral sclerosis. Neurol Clin. 2015;33:787–805. [DOI] [PubMed] [Google Scholar]
- 7.Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10:131–46. [DOI] [PubMed] [Google Scholar]
- 8.Bak TH, Hodges JR. Motor neurone disease, dementia and aphasia: coincidence, co-occurrence or continuum? J Neurol. 2001;248:260–70. [DOI] [PubMed] [Google Scholar]
- 9.Kamminga J, Leslie FV, Hsieh S, Caga J, Mioshi E, Hornberger M, et al. Syntactic comprehension deficits across the FTD-ALS continuum. Neurobiol Aging. 2016; 41:11–18. [DOI] [PubMed] [Google Scholar]
- 10.Hillis AE, Oh S, Ken L. Deterioration of naming nouns versus verbs in primary progressive aphasia. Ann Neurol. 2004;55:268–75. [DOI] [PubMed] [Google Scholar]
- 11.Ash S, Olm C, McMillan CT, Boller A, Irwin DJ, McCluskey L, et al. Deficits in sentence expression in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boeve BF, Graff-Radford NR. Cognitive and behavioral features of c9FTD/ALS. Alzheimers Res Ther. 2012;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor LJ, Brown RG, Tsermentseli S, Al-Chalabi A, Shaw CE, Ellis CM, et al. Is language impairment more common than executive dysfunction in amyotrophic lateral sclerosis? J Neurol Neurosurg Psychiatry. 2013;84:494–8. [DOI] [PubMed] [Google Scholar]
- 14.Tsermentseli S, Leigh PN, Taylor LJ, Radunovic A, Catani M, Goldstein LH. Syntactic processing as a marker for cognitive impairment in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2016; 17:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto-Grau M, Hardiman O, Pender N. The study of language in the amyotrophic lateral sclerosis -frontotemporal spectrum disorder: a systematic review of findings and new perspectives. Neuropsychol Rev. 2018; 28:251–68. [DOI] [PubMed] [Google Scholar]
- 16.Saxon JA, Harris JM, Thompson JC, Jones M, Richardson AMT, Langheinrich T, et al. Semantic dementia, progressive non-fluent aphasia and their association with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2017;88:711–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxon JA, Thompson JC, Jones M, Harris JM, Richardson AM, Langheinrich T, et al. Examining the language and behavioural profile in FTD and ALS-FTD. J Neurol Neurosurg Psychiatry. 2017;88:675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorno-Tempini ML, Rankin KP, Woolley JD, Rosen HJ, Phengrasamy L, Miller BL. Cognitive and behavioral profile in a case of right anterior temporal lobe neurodegeneration. Cortex. 2004;40:631–44. [DOI] [PubMed] [Google Scholar]
- 20.Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord. 2007;21:S23–S30. [DOI] [PubMed] [Google Scholar]
- 21.Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol. 2006; 112:539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The national institute on aging, and Reagan institute working group on diagnostic criteria for the neuropathological assessment of Alzheimer’s disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 23.Lee SE, Rabinovici GD, Mayo MC, Wilson SM, Seeley WW, DeArmond SJ, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol. 2011;70:327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan RH, Shepherd CE, Kril JJ, McCann H, McGeachie A, McGinley C, et al. Classification of FTLD-TDP cases into pathological subtypes using antibodies against phosphorylated and non-phosphorylated TDP43. Acta Neuropathol Commun. 2013;1:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braak H, Ludolph A, Thal DR, Del Tredici K. Amyotrophic lateral sclerosis: dash-like accumulation of phosphorylated TDP-43 in somatodendritic and axonal compartments of somatomotor neurons of the lower brainstem and spinal cord. Acta Neuropathol. 2010;120: 67–74. [DOI] [PubMed] [Google Scholar]
- 26.Bettcher BM, Wilheim R, Rigby T, Green R, Miller JW, Racine CA, et al. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun. 2012;26:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Schuff N, Ching C, Tosun D, Zhan W, Nezamzadeh M, et al. Joint assessment of structural, perfusion, and diffusion MRI in Alzheimer’s disease and frontotemporal dementia. Int J Alzheimer’s Dis. 2011; 2011:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashburner J A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 29.Lillo P, Savage S, Mioshi E, Kiernan MC, Hodges JR. Amyotrophic lateral sclerosis and frontotemporal dementia: a behavioural and cognitive continuum. Amyotroph Lateral Scler. 2012;13:102–9. [DOI] [PubMed] [Google Scholar]
- 30.Floeter MK, Katipally R, Kim MP, Schanz O, Stephen M, Danielian L, et al. Impaired corticopontocerebellar tracts underlie pseudobulbar affect in motor neuron disorders. Neurology. 2014;83:620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melloni M, Urbistondo C, Sedeno L, Gelormini C, Kichic R, Ibanez A. The extended fronto-striatal model of obsessive compulsive disorder: convergence from event-related potentials, neuropsychology and neuroimaging. Front Hum Neurosci. 2012;6:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caso F, Mandelli ML, Henry M, Gesierich B, Bettcher BM, Ogar J, et al. In vivo signatures of nonfluent/agrammatic primary progressive aphasia caused by FTLD pathology. Neurology. 2014;82:239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin J, Bak TH, Rominger A, Mille E, Arzberger T, Giese A, et al. The association of aphasia and right-sided motor impairment in corticobasal syndrome. J Neurol. 2015;262:2241–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


