Abstract
A recent study in Nature (Szczerba et al. 2019;566:553–557) reports that the association of neutrophils with circulating tumor cells (CTCs) in the blood of patients with breast cancer can promote CTC proliferation and metastasis. These findings reveal a new mechanism by which the innate immune system may be co-opted to drive tumor progression.
Circulating tumor cells (CTCs) are found in the bloodstream of patients with cancer. Consistent with the notion that these tumor cells originate from primary tumor sites and seed metastases, CTC abundance correlates with poor prognosis and metastasis for certain types of cancer, including prostate, breast, colorectal, lung, and bladder [1]. CTCs serve as a relatively safe and easy-to-obtain ‘liquid biopsy’; indeed, CTCs have been used as a putative indicator of cancer diagnosis and prognosis, and have been relied upon to provide molecular information that can guide therapeutic decisions [1]. CTCs are occasionally found in the blood as either single cells, bound together in tight ‘microclusters’, and/or clustered together with immune cells [2]. Moreover, previous studies have shown that clusters can be endowed with enhanced metastatic ability, both in mouse models and in patients [3], but the function of CTC–immune cell clusters remains almost completely unexplored.
Neutrophils are a component of the innate immune system and the most abundant white blood cell (WBC) in the human circulation. They are also a type of myeloid cell that is rapidly mobilized from the bone marrow as one of the first lines of defense against infection. Neutrophils have been increasingly studied in the context of cancer because they have been associated with both anti- and protumorigenic activities. Evidence for protumor functions found in patients and mouse models has included suppression of T cell effector functions, promotion of angiogenesis, production of reactive oxygen species (ROS), formation of neutrophil extracellular traps (NETs, via a process known as ‘NETosis’), extracellular matrix remodeling, among others [4]. Aside from functions within the primary tumor site, neutrophils also have a high degree of plasticity and have been implicated in enabling metastasis [5].
In the recent publication by the Aceto group [6], Szczerba et al. examined blood samples from 70 patients with breast cancer and detected CTCs in nearly half of the cases. Of these, most were single CTCs, whereas smaller subsets were either CTC clusters (8.6%) or CTCs coupled to WBCs (3.4%). Most of the CTC-associated WBCs in these patients were myeloid cells, with similar findings in mouse models of breast cancer. Through single-cell RNA-sequencing (scRNA-seq) analysis, the authors revealed that CTC–myeloid clusters largely comprised lymphocyte antigen 6 complex, locus G (LY6G)+ neutrophils with a protumor ‘N2-like’ signature, representing a polarized state associated with immune suppression. A main finding was that the presence of CTC–neutrophil clusters correlated with worse prognosis in patients, compared to single CTCs or CTC clusters.
To determine whether the CTC–neutrophil clusters were of functional significance, the authors compared the ability of either single CTCs, dissociated CTC clusters, or dissociated CTC–neutrophil clusters isolated from tumor-bearing mice to promote metastases in mouse models: either immunocompetent (MMTV-PyMT and BALB/c-4T1-GFP) or immunocompromised [NOD-scid-Il2rgnull (NSG) mice bearing 4T1, LM2, and BR16 xenografts]. A significant finding was that CTCs from neutrophil-associated clusters led to more rapid metastases and shorter survival of mice relative to CTCs alone. This suggested that CTCs associated with neutrophils are more ‘aggressive’ (i.e., enabling metastasis) than their single or clustered counterparts. Differential gene expression analysis of scRNA-seq data suggested that CTCs coupled to neutrophils, compared with other CTCs, exhibited increased cell cycle and DNA replication programs, validated by Ki67 expression. The authors reasoned that this enhanced proliferative capacity supported the ability of CTCs to proliferate and survive in the bloodstream and, ultimately, to seed metastases. Four cytokines were frequently expressed by CTC-associated neutrophils and, of these, interleukin (IL6) and IL1B expression conferred a proliferative advantage to CTC–neutrophil clusters relative to untreated controls, or to 4T1 cells lacking IL6 and IL1B receptors [6].
Myeloid-derived ROS have been previously shown to increase mutational burden in mouse intestinal tumor cells [7], prompting the authors to investigate the incidence of mutations in CTCs. Although the overall mutational burden was unaltered across CTC types, CTCs from neutrophil clusters harbored recurrent mutations in certain genes, such as transducin-like enhancer of split-1 (Tle1), encoding a protein that can suppress inflammation. Moreover, lentiviral introduction of mutant Tle1 into tumor cells was sufficient to increase neutrophil abundance and CTC–neutrophil clusters in NSG mice compared with controls in which tumor cells with wild-type Tle1 were introduced [6].
The authors sought to determine whether altering the number of tumor-associated neutrophils (TANs) could impact the quantity of CTC–neutrophil clusters in the blood of tumor-bearing NSG mice. Indeed, treatment with either LY6G-blocking antibodies to decrease TANs, or granulocyte-colony stimulating factor (G-CSF) to increase TANs, led to decreased and increased CTC-neutrophil clusters, respectively, without affecting primary tumor size. Accordingly, the extent of metastasis positively correlated with the quantity of CTC–neutrophil clusters. Altogether, these data suggest that specific somatic mutations present in CTCs lead to neutrophil recruitment at primary tumors to promote the formation of CTC– neutrophil clusters and metastasis [6]. While not investigated in this study, blockade of the neutrophil receptor C-X-C motif chemokine receptor 2 (CXCR2) is predicted to phenocopy LY6G blockade, which is a clinically relevant approach, since CXCR2 inhibitors have been tested in clinical trials [8] (Figure 1), and LY6G is a mouse-specific protein with no known ortholog in humans.
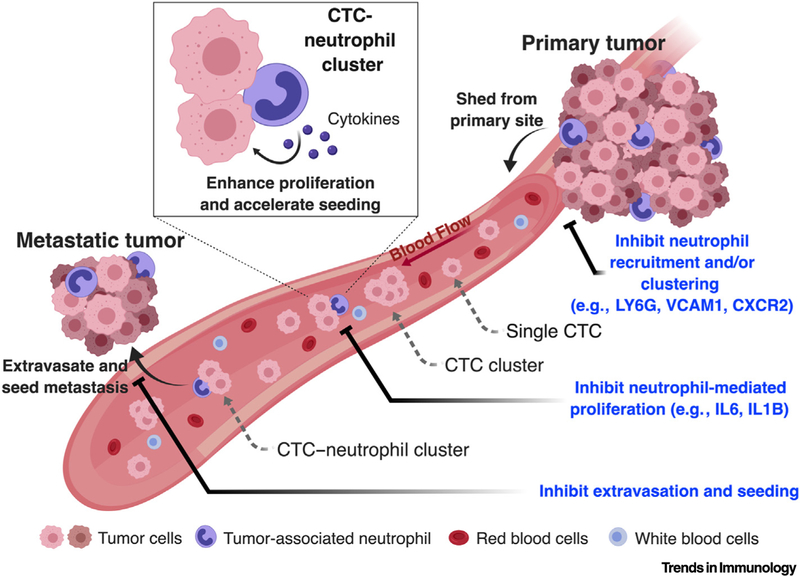
Figure 1. Circulating Tumor Cell (CTC)–Neutrophil Clusters Can Have Increased Metastatic Potential in Breast Cancer.
CTCs are shed from primary breast tumor sites and travel through the bloodstream mostly as single CTCs, but some can be detected as CTC clusters or CTC–neutrophil clusters in mice and humans. Relative to CTC clusters or single CTCs, CTCs from neutrophil clusters exhibit increased proliferation that may be driven, in part, by cytokines such as interleukin (IL)6 and IL1B, expressed by neutrophils. CTCs from neutrophil clusters also exhibit an enhanced ability to promote metastases in multiple mouse tumor models. Genetic and pharmacological inhibition of distinct steps of this cascade, such as vascular cell adhesion molecule 1 (VCAM1), lymphocyte antigen 6 complex, locus G (LY6G) or C-X-C motif chemokine receptor 2 (CXCR2) blockade might bear potential in suppressing tumor metastasis.
Finally, the authors performed an elegant CRISPR-Cas9-based mini-screen focused on genes that might be required for the formation of CTC–neutrophil interactions, specifically, cell–cell junction pairs. They looked for short guide RNAs (sgRNAs) that were lost in CTC–neutrophil clusters compared with CTCs alone, and consistently identified a deficiency in the expression of vascular cell adhesion molecule 1 (Vcam1), which prevented CTC–neutrophil cluster formation. Thus, these findings bear translational relevance because approaches to block VCAM1 or other candidate targets involved in mediating CTC–neutrophil interactions could ultimately be exploited to inhibit the formation of metastases (Figure 1).
Collectively, this report reveals interesting and unexpected roles for neutrophils in breast cancer metastasis, and raises new questions that warrant further study. For example, what is the function of other CTC–immune cell clusters identified in patients with cancer, such as the CTC–T cell clusters found in a small percentage of patients with breast cancer in this study? How common are these clusters across cancer types, and do they have unique functions beyond what has been identified in breast cancer? Do other cancer types exhibiting increased numbers of TANs (e.g., lung squamous cell carcinoma) harbor enhanced numbers of CTC–neutrophil clusters and metastases? Given that G-CSF is used to treat neutropenia in patients with cancer, modulating G-CSF concentrations may be therapeutically relevant, because G-CSF overexpression could increase TAN numbers and promote metastases in mouse models of breast cancer relative to controls [6]. Therefore, is such a mechanism also present in patients? If so, could there be a safer way to treat neutropenia without increasing TANs or CTC– neutrophil clusters?
TANs, as well as the neutrophil-to-lymphocyte ratio (NLR), correlate with poor outcome in certain malignancies, as well as with a poor response to immunotherapy [5]. Furthermore, TANs release arginase-1, cytokines, proteases, and other factors that contribute to shaping the local immune microenvironment [9]. Thus, can neutrophils also protect tumor cells from immune attack in the bloodstream and at metastatic sites? It remains unknown what functions of neutrophils may be required for CTC proliferation and metastases. For example, could NETosis (implicated in metastases [10]) promote cluster shedding and intravasation, and would blocking NETosis prevent cluster formation? Are neutrophil-derived ROS or protease cathepsin G required for cluster formation, and do they also impact metastases? How do CTC–neutrophil clusters enable metastasis? This study has provided interesting advances in the field, while also opening a plethora of questions regarding the role of neutrophils in cancer. Further mechanistic insights into this process are required; nevertheless, these questions are relevant in that they may ultimately lead to potential therapeutic strategies to prevent or inhibit metastasis.
Acknowledgments
T.G.O. is supported by National Institutes of Health R01 (5R01CA187457-05).
References
- 1.Poudineh M et al. (2018) Profiling circulating tumour cells and other biomarkers of invasive cancers. Nat. Biomed. Eng 2, 72–84 [DOI] [PubMed] [Google Scholar]
- 2.Sarioglu AF et al. (2015) A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat. Methods 12, 685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aceto N et al. (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffelt SB et al. (2016) Neutrophils in cancer: neutral no more. Nat. Rev. Cancer 16, 431–446 [DOI] [PubMed] [Google Scholar]
- 5.Mollinedo F (2019) Neutrophil degranulation, plasticity, and cancer metastasis. Trends Immunol 40, 228–242 [DOI] [PubMed] [Google Scholar]
- 6.Szczerba BM et al. (2019) Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566, 553–557 [DOI] [PubMed] [Google Scholar]
- 7.Canli Ö et al. (2017) Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell 32, 869–883.e5 [DOI] [PubMed] [Google Scholar]
- 8.O’Byrne PM et al. (2016) Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: a randomised, double-blind, placebo-controlled trial. Lancet Respir. Med 4, 797–806 [DOI] [PubMed] [Google Scholar]
- 9.Leliefeld PHC et al. (2015) How neutrophils shape adaptive immune responses. Front. Immunol 6, 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albrengues J et al. (2018) Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, eaao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]