Abstract
Background:
Individuals with extreme food avoidance such as Avoidant Restrictive Food Intake Disorder (ARFID) experience impairing physical and mental health consequences from nutrition of insufficient variety or/and quantity. Identifying mechanisms contributing to food avoidance is essential to develop effective interventions. Anxiety figures prominently in theoretical models of food avoidance; however, there is limited evidence that repeated exposures to foods increases approach behavior in ARFID. Studying disgust, and relationships between disgust and anxiety, may offer novel insights, as disgust is functionally associated with avoidance of contamination from pathogens (as may occur via ingestion) and is largely resistant to extinction.
Methods:
This exploratory, cross-sectional study included data from 1644 adults who completed an online questionnaire. Participant responses were used to measure ARFID classification, picky eating, sensory sensitivity, disgust, and anxiety. Structural equation modeling tested a measurement model of latent disgust and anxiety factors as measured by self-reported frequency of disgust and anxiety reactions. Mediational models were used to explore causal ordering.
Results:
A latent disgust factor was more strongly related to severity of picky eating (B ≈ 0.4) and ARFID classification (B ≈ 0.6) than the latent anxiety factor (B ≈ 0.1). Disgust partially mediated the association between anxiety and picky eating and fully mediated the association between anxiety and ARFID. Models testing the reverse causal ordering demonstrated poorer fit. Findings suggest anxiety may be associated with food avoidance in part due to increased disgust.
Conclusions:
Disgust may play a prominent role in food avoidance. Findings may inform novel approaches to treatment.
Keywords: ARFID, Anxiety, Disgust, structural equation modeling, exposure, picky eating
Introduction
Avoidant Restrictive Food Intake Disorder (ARFID) was codified in the Diagnostic and Statistical Manual for Mental Disorders, 5th edition (American Psychiatric Association, 2013) to characterize individuals who engage in clinically impairing food restriction/avoidance without exhibiting the weight and shape concerns associated with anorexia nervosa or bulimia nervosa. ARFID is an elaboration and expansion of the diagnosis Feeding Disorder of Infancy and Early Childhood (American Psychiatric Association, 2000). This re-articulation allows for diverse presentations that may have unique (or overlapping) motivations for food avoidance (Katzman, Norris, & Zucker, 2019). We examine the role of negative affect, particularly anxiety and disgust, in contributing to food avoidance/restriction in adults with ARFID.
Anxiety figures prominently in theoretical models of food avoidance. Evidence indicates elevated anxiety symptoms in children with ARFID or selective eating (Farrow & Coulthard, 2012; Norris et al., 2014). Additionally, one putative motivation for food avoidance in ARFID is fearing negative consequences of eating (e.g., choking or gagging, American Psychiatric Association, 2013; Fisher et al., 2014). Indeed, some ARFID cases characterized by fear of choking appear to respond to exposure based treatments (de Roos & de Jongh, 2008). However, unlike anxiety disorders where exposure-based treatments have been highly efficacious (Kaczkurkin & Foa, 2015), daily exposure to the sights, smells, and innocuous consequences of others’ food consumption alone does not seem to increase approach behavior in many with persisting food avoidance/ARFID (Mascola, Bryson, & Agras, 2010; Wildes, Zucker, & Marcus, 2012).
Potential hypotheses for why exposures do not increase approach behavior in selective eating/ARFID (Mascola et al., 2010; Zucker et al., 2018) include that individuals’ cognitive formulations prevent learning via experience (Clark & Beck, 2010) or that food ingestion may be essential for exposures to increase sustained approach (Wardle et al., 2003). Disgust is implicated in the development of a range of anxiety disorders and OCD symptoms (Muris, van der Heiden, & Rassin, 2008; Olatunji, Cisler, McKay, & Phillips, 2010; Olatunji, Ebesutani, Haidt, & Sawchuk, 2014). However, disgust has not been fully explored in understanding disorders of food avoidance, and may also play a critical role (Anderson et al., 2018; Attwood & Scarpa, 2013; Davey, Buckland, Tantow, & Dallos, 1998; Hildebrandt et al., 2015; Kauer, Pelchat, Rozin, & Zickgraf, 2015; Troop, Murphy, Bramon, & Treasure, 2000).
According to some theorists, the primary function of disgust is to help humans avoid poisons and pathogens (Curtis, 2011; Curtis, de Barra, & Aunger, 2011; Rozin & Fallon, 1987). Affective motivational systems, such as disgust, that dictate an individual’s probability of accepting or rejecting nutrition, thus may be paramount to survival and healthy development of eating behaviors. One prominent theory of disgust (Rozin & Fallon, 1987; Rozin, Haidt, & McCauley, 2008) formulates disgust as having originated as an adaptive food rejection response, noting the relationship between a physiological correlate of disgust (nausea) and the expulsion of inappropriate foods; furthermore, they note that disgusting objects tend to be appraised as distasteful (possessing aversive sensory properties such as a bad taste, smell, or texture). Key to this conceptualization is that the disgust experience and reaction (the curled-up lip, the scrunched nose, the head turn) happens before—and presumably prevents—ingestion or contact with a possibly spoiled or unsafe substance (Curtis et al., 2011; Tybur, Lieberman, Kurzban, & DeScioli, 2013).
The disgust reaction may initially be triggered by sensitivities to the experience of a certain odor, texture, or visual anomaly that leads to subsequent avoidance. As emotional reactions occur, in part, in response to perceptions, the intensity of sensory experiences and corresponding strong emotional reactions may be linked. Correspondingly, individual differences in sensitivity to sensory features of smell, texture, and visual features (e.g., a lower threshold for experiencing a sensory experience as strong or atypical) may index a propensity for an individual to experience heightened disgust (e.g. Kauer et al., 2015; Mataix-Cols et al., 2008; Sherlock, Zietsch, Tybur, & Jern, 2016). Indeed, adult picky eaters who endorse higher levels of disgust sensitivity are more likely to refuse foods that are mixed or “lumpy” and report more intense taste responses than non-picky adults (Kauer et al., 2015). Someone sensitive to visual flaws or details (such as a brown spot on a French fry) may also experience an aberrant visual feature as signaling danger. Thus, it is interesting to consider whether sensitivity in a given sensory modality increases intensity of emotional experiences generally, or to particular emotions specifically.
Greater precision in characterizing the nature of sensory sensitivities and ARFID etiology might mean, for example, discovering that sound sensitivity is linked more closely with anxiety while sensitivity to smell is more related to disgust. To this point, evidence indicates that more individuals are able to identify smells that elicit disgust than can identify odors that elicit anxiety (Croy, Olgun, & Joraschky, 2011). Moreover, a near infra-red spectroscopy study reported increased hemodynamic responses and temporal-parietal activation to sounds associated with fear (i.e., screams of fear/pain) compared to sounds of disgust (i.e., vomiting/diarrhea) (Köchel, Schöngassner, & Schienle, 2013). Taken together with evidence that an individual’s smell-taste capacity and endocrine system may be related to features of anorexia nervosa, another restrictive eating disorder (Fernández-Aranda et al., 2016), these findings suggest that identifying potentially distinct sensory pathways related to disgust and anxiety may help to better understand restrictive eating disorders such as ARFID. As such, greater precision characterizing the nature of sensory sensitivities may help differentiate the phenomenology of disgust relative to anxiety, especially as it concerns the pathophysiology of ARFID.
To understand the potential contribution of disgust to eating disorders, it is crucial to acknowledge that disgust and anxiety may not be operating completely independently of one another. Indeed, disgust has been linked to the development and maintenance of other anxiety disorders (e.g., Olatunji et al., 2010) while, in some cases, disgust reactions may be manifestations of heath anxiety or a fear of sickness (e.g., Goetz, Lee, Cougle, & Turkel, 2013; Hedman et al., 2016). Moreover, in a study by Marzillier and Davey (2005), the investigators found that induced anxiety produced increases in reported disgust, while induced disgust showed no effect on reported anxiety. Disgust and anxiety are supported by distinct neural systems and may respond differently to exposure and extinction paradigms (for example, see the classic study on the one-trial avoidance learning associated with conditioned taste aversion [Garcia, Kimeldorf, & Koelling, 1955]). Thus the boundaries and overlap of these affective motivational systems are complex.
The current exploratory study looked at the contributions of sensory sensitivity, anxiety, and disgust experience to elucidate the potential role of disgust in food avoidance in ARFID. Given the known relationships between anxiety and disgust, we chose to do a mediation analysis of the relative contributions of disgust and anxiety to ARFID diagnosis. We hypothesized that anxiety, as a future-oriented emotion, operates to influence food avoidance, in part, through the anticipation of prior or novel disgusting experiences. As such, disgust would partially mediate the relationship between anxiety and ARFID diagnosis. Our cross-sectional design precludes causal inferences. However, results may provide more information about the complex relationship between these emotions, may help identify a more proximal target for treatment, and may serve as a springboard with which to consider alternative approaches. For example, better understanding of the potential role played by disgust in the maintenance of food avoidance may guide development of interventions that can complement/are less reliant on exposure-based approaches.
Using an online sample of 1644 adults self-identifying as “picky eaters,” we hypothesized that: (1) food related disgust and anxiety would contribute unique variance to an ARFID classification and picky eating severity; (2) disgust would be a stronger correlate of ARFID diagnosis/picky eating severity than anxiety; (3) disgust would be more strongly associated with sensitivity to taste and smell relative to anxiety; and (4) relationships of anxiety to ARFID and picky eating would be partially mediated by disgust. To clarify the possible symptom profile related to ARFID symptomatology, we tested models that examine the relative contributions of anxiety and disgust in an adult sample. This was done to add to emerging research investigating disgust in ARFID (Ellis et al., 2018; Kauer et al., 2015) and to help develop a more comprehensive understanding of ARFID and food avoidance.
Methods
Participants
Participants were recruited via links in articles on adult picky eating and a southeastern academic medical center website (http://dukedpn.qualtrics.com/jfe/form/SV_3mZELWkUIl4Y4Sx). The survey went online October 25, 2012 and included validated questionnaires and questions on demographics, eating habits, sensory sensitivity, disgust, and anxiety. Individuals self-selected to participate by clicking the link, completing a mandatory electronic consent process, and filling-out questions. Stipulations for inclusion were that participants were ≥18 years of age, self-identified as “picky-eaters,” and that picky eating was not due to: medical conditions, structural/physical limitations affecting eating, food allergies, or pregnancy. At data extraction (December 15, 2016), 2,002 participants had filled out the survey. Nine participants were excluded after indicating they were under 18 years of age, 349 individuals were excluded due to a comorbid threshold or subthreshold eating disorder diagnosis (further detail in results) and data analysis was conducted on the remaining 1644. All research activities were approved by the Duke Medical Center Institutional Review Board prior to data collection (Protocol #00019967).
Measures
Demographics were assessed through questions adapted from the United States Census. Disgust was assessed with a 25-item version of the Disgust Scale-Revised (DS-R, Haidt, McCauley, & Rozin, 1994; Olatunji, Williams, et al., 2007) measuring three domains: (1) core disgust (food, animals, bodily functions), (2) animal-reminder disgust (death), and (3) contamination disgust (disease transmission). The DS-R has two question sets with different response options, a 5-point Likert scale (strongly disagree to strongly agree) and a six-point “not disgusting at all” to “extremely disgusting” scale. Internal consistency estimates are above .70 (Olatunji, Williams, et al., 2007; van Overveld, de Jong, Peters, & Schouten, 2011). The measure had good internal consistency in the current sample (Cronbach’s alpha = .88).
Disgust and anxious reactions associated with selective eating /ARFID as well as sensory sensitivities to food were also assessed via investigator crafted questions presented as part of a 20-item questionnaire. Items assessing anxious and disgusted reactions were developed using parallel structure such that the only thing that differed between items was the emotional state (anxious, disgusted). Further, affective state was assessed in response to the presentation of a new food and a familiar food that had previously been disliked (e.g., Do you feel disgusted/anxious when presented with a new/disliked food?). Additional questions assessed sensory experience, the perceived relationship between sensory experience and food avoidance, experiences of gagging, and social discomfort with eating (see Appendix 1). Questions were presented on a five-point Likert scale (“all the time” to “rarely or never”).
Item responses on the 20-item questionnaire as a whole reflected good internal consistency (Cronbach’s alpha =.88). Questions particular to disgust reactions (“Do you feel disgusted when presented with a new food?”; “Do you feel disgusted when presented with a disliked food”); gagging (“Do you gag when tasting a new food?”, “Do you gag when tasting a disliked food?) and food-related anxiety (“Do you feel afraid or nervous when presented with a new food?”; and “Do you feel afraid or nervous when presented with a disliked food”) were included in statistical models. Item responses to the four items assessing disgust and gagging also reflected good internal consistency (Cronbach’s alpha = .83) as did those assessing anxiety related to being presented with a new or disliked food (Cronbach’s alpha =.086). Finally, sums of the texture and smell items from the same 20-item scale were included and covariation of items examined to understand potential group differences associated with ARFID classification.
Symptoms of OCD were measured with the Maudsley Obsessive Compulsive Inventory (MOCI, Hodgson & Rachman, 1977) a 30 item, true-false response measure. The MOCI has good convergent validity with other measures of OCD and adequate internal consistency (Cronbach’s alpha =.65-.89) (Emmelkamp, Kraaijkamp, & Van den Hout, 1999; Olatunji, Williams, et al., 2007). The MOCI contains items that may be relevant for picky/selective eating such as contamination concerns (Bryant‐Waugh, Markham, Kreipe, & Walsh, 2010) and demonstrated adequate reliability in the current sample (Cronbach’s alpha = .65). Eating disorder symptoms were assessed with the Eating Disorder Diagnostic Scale (EDDS, Stice, Telch, & Rizvi, 2000) a 22-item self-report measure based on DSM-IV (American Psychiatric Association, 2000) criteria for anorexia nervosa, bulimia nervosa, and binge eating disorder. The EDDS has strong test-retest validity (Kappa=.71-.95) and criterion validity (Kappa=.74-.93) (Stice, Fisher, & Martinez, 2004; Stice et al., 2000). It showed adequate internal consistency in the current sample (Cronbach’s alpha =.78).
Picky eating classification was based on responses to “Do you consider yourself to be a picky eater?” presented on a five-point Likert scale (“all of the time” to “rarely or never”). An exploratory ARFID classification was based on questions reflecting diagnostic criteria (American Psychiatric Association, 2013): individuals had to (1) consider themselves a picky eater “all of the time;” (2) indicate their eating problems led to significant: weight loss, nutritional deficiency, and/or interference with job functioning, relationships and/or avoidance of social situations involving food, and (3) not have anorexia or bulimia nervosa (as determined by responses on the EDDS). Individuals meeting threshold or subthreshold symptoms of anorexia nervosa, bulimia nervosa, or binge eating disorder were excluded from the ARFID group.
Data Analysis
To identify which measures might best assess latent constructs of anxiety and disgust and show the strongest associations with ARFID diagnosis and picky eating, two exploratory principal components analyses (PCA) with promax rotation (eigenvalues > 1) were conducted using IBM SPSS® version 25. The first PCA included the twelve items from the DS-R Core Disgust subscale (Haidt et al., 1994; Olatunji, Williams, et al., 2007) as well as the four items related specifically to food gagging and disgust as described above. The second PCA included the thirty MOCI items (Hodgson & Rachman, 1977) as well as the two items specifically assessing anxiety related to food. Listwise deletion was used to exclude the 4.5% of participants with missing data. There were no significant differences between participants with and without missing data on the variables included in the PCAs. Factor scores from these two PCAs were calculated using the standard regression method in SPSS® in order to examine bivariate correlations with picky eating and ARFID classification.
Results from the bivariate correlations were used to test a measurement model of disgust and anxiety latent factors. Sums of texture and smell items also were included in this model representing a sensory sensitivities factor. After identifying a measurement model representing anxiety, disgust, and sensory sensitivities, SEMs were constructed to predict two separate outcomes: severity of picky eating and ARFID diagnosis. Mediation models of indirect and direct effects were also tested to identify potential causal ordering of the latent factors as predictors of these two outcomes.
All SEMS and measurement models were conducted using Mplus (Muthén & Muthén, 2010). For SEMs with ARFID classification as the outcome, we used the weighted least squares means and variance adjusted (WLSMV) algorithm, which is appropriate for categorical and nonmultivariate normal data in large samples (Flora & Curran, 2004). Maximum likelihood estimation was used for all other models. For the mediation models, indirect and direct effects were computed using bias-corrected bootstrapping procedures with 5000 samples (MacKinnon, Lockwood, & Williams, 2004). Standard methods for assessing goodness of fit were used, including the maximum likelihood goodness-of-fit chi-square test (p > 0.05), the comparative fit index (CFI > 0.95), and the root mean square error of approximation (RMSEA < 0.08)(Kline, 2011). Missingness was unrelated to the variables included in the analyses, and we thus employed full information maximum likelihood imputation as implemented by Mplus (Enders, 2010). To examine whether sex moderated our results, we conducted goodness of fit comparisons between the models for males and females. This analysis indicated that a model with different path weights by sex did not provide a better fit than a model with equal weights, suggesting that the paths did not significantly differ by sex. Hence, results focus on models with equal weights for sex. The results of these analyses can be found in Supplemental Materials 1.
Results
Initially, 2002 individuals completed the survey at the time of data extraction. Nine individuals were excluded due to age (< 18 years), 223 participants were excluded who met criteria for at least one other eating disorder, and 126 were excluded who reported subthreshold eating disorder symptoms, leaving a final sample of 1644. Table 1a presents the demographic features and scale scores of the sample, broken down by ARFID classification. Of the resulting sample (N=1,644), 1,144 (69.1%) met criteria for ARFID. Overall, the sample was 26.8% male, predominately white (90.2%), highly educated (48.6% had a 4-year-college degree or greater), and between 18 and 34 years old (69.8%). The average age was 30.9 ± 15.7 (standard deviation). Groups (ARFID vs. No ARFID) significantly differed on levels of Core Disgust, Food Disgust, and Food Anxiety (p < .001), Table 1b. See Supplemental Materials 2 for additional descriptive data such as group differences in experiences of gagging across levels of picky eating (e.g., gagging in response to new foods, F(2) = 105, p < .001: ARFID>High Picky>Low Picky).
Table 1.
| Table 1a. Demographic and Clinical Profile of SampleA | ||
|---|---|---|
| Features | N
(%)†
ARFID (1144) |
N
(%)†
No ARFID (500) |
| Sex of Selective Eater | ||
| Male | 510 (25.6) | 125 (25.0) |
| Female | 1440 (72.2) | 360 (72.0) |
| Not Reported | 43 (2.2) | 15 (3.0) |
| Age of Selective Eater | ||
| 18 to 19 | 126 (11.0) | 46 (9.2) |
| 20 to 24 | 331 (28.9) | 102 (20.4) |
| 25 to 34 | 371 (32.4) | 171 (34.2) |
| 35 to 44 | 162 (14.2) | 94 (18.8) |
| 45 to 54 | 108 (9.4) | 61 (12.2) |
| 55 to 64 | 38 (3.3) | 20 (4.0) |
| 65 years and over | 8 (0.7) | 6 (1.2) |
| Race/Ethnicity of Selective Eater | ||
| White | 1036 (90.6) | 446 (89.2) |
| African American/Black | 28 (2.4) | 15 (3.0) |
| Hispanic | 43 (3.8) | 12 (2.4) |
| Asian | 4 (0.3) | 11(2.2) |
| Native American | 9 (0.8) | 4 (0.8) |
| Other | 24 (2.1) | 10 (2.0) |
| Not Reported | 2 (0.4) | |
| Highest Level of Education | ||
| Less than High School | 14 (1.2) | 2 (0.4) |
| High School / GED | 134 (11.7) | 49 (9.8) |
| Some College | 387 (33.8) | 119 (23.8) |
| 2-year College Degree | 107 (9.4) | 32 (6.4) |
| 4-year College Degree | 351 (30.7) | 173 (34.6) |
| Master’s Degree | 123 (10.8) | 90 (18.0) |
| Doctoral Degree | 8 (0.7) | 15 (3.0) |
| Professional Degree (JD, MD) | 19 (1.7) | 18 (3.6) |
| Not Reported | 1 (0.1) | 2 (0.4) |
| Table 1b. Demographic and Clinical Profile of Sample ContinuedB | ||||
|---|---|---|---|---|
| Features | ARFID* | No ARFID* | ||
| Mean (Standard Deviation) |
95th% Confidence Interval for Mean |
Mean (Standard Deviation) |
95th% Confidence Interval for Mean |
|
| Average of Food Disgust ItemsD | 4.02 (.83)** | 3.97-4.07 | 3.08 (1.04)** | 2.98-3.17 |
| Disgust Scale Revised– Average Animal ReminderC | 2.21 (.94) | 2.16-2.27 | 2.14 (.90) | 2.06-2.22 |
| Disgust Scale Revised– Average ContaminationC | 1.43 (.86) | 1.38-1.48 | 1.39 (.82) | 1.32-1.47 |
| Disgust Scale Revised – Average Core DisgustC | 2.65 (.68)** | 2.61-2.69 | 2.49 (.70)** | 2.43-2.56 |
| Average of Food Anxiety ItemsD | 4.34 (.93)** | 4.29-4.40 | 3.29 (1.34)** | 3.16-3.40 |
| Maudsley Obsessive Compulsive Inventory | 7.21 (4.92) | 6.92-7.51 | 6.82 (4.77) | 6.39-7.25 |
Notes:
The initial sample at data extraction included 2002 participants. Nine were excluded due to age and 349 due to eating disorder diagnosis, resulting in a somple of 1644.
The model was computed on 1644 subjects with missing data imputed. For scale scores, we present raw scores without imputed values. Sample size ranges from 1549-1639.
It is recommended that average scores for the Disgust-Scale-Revised be computed.
To be consistent with Disgust-Scale-Revised Scoring, we present the average of food disgust and food anxiety items.
p < .001
Preliminary Analyses for Model Construction
The first PCA identified four factors: gagging and disgust to food items loaded together on Factor 1; the Core Disgust subscale items from the DS-R loaded on Factors 2-4 (see Supplemental Materials 3). The second PCA identified eight factors: anxiety to food items loaded on Factor 4; the MOCI items loaded on Factors 1-3 and 5-8 (see Supplemental Materials 3). Factor scores from these PCAs were calculated and bivariate correlations were conducted. These results showed that Factor 1 from the first PCA including the gagging and disgust to food items was positively related to picky eating (r = 0.426) and ARFID classification (r = 0.438) (p’s < 0.001). Factors 2 through 4 from the first PCA were either negatively (p’s < 0.001) or weakly positively related to (p’s < 0.05) picky eating and ARFID diagnosis (see Table 2). Similarly, Factor 4 from the second PCA including the anxiety to food items was positively related to picky eating (r = 0.398) and ARFID classification (r = 0.407) (p’s < 0.001). Factors 1-3 and 5-8 from the second PCA were either unrelated (all p’s > 0.075) or weakly negatively (p’s < 0.05) related to picky eating and ARFID diagnosis. These results suggest that the gagging, disgust, and anxiety to food items most strongly relate to picky eating and ARFID classification.
Table 2.
Bivariate Correlations of Factors from the Principal Components Analyses (PCAs) and ARFID Diagnosis and Picky Eating.
| Factor Scores | ARFID Diagnosis | Picky Eating |
|---|---|---|
| PCA 1 | ||
| Factor 1 | 0.438*** | 0.426*** |
| Factor 2 | −0.091*** | −0.093*** |
| Factor 3 | −0.124*** | −0.102*** |
| Factor 4 | 0.051* | 0.057* |
| PCA 2 | ||
| Factor 1 | −0.015 | 0.002 |
| Factor 2 | 0.014 | 0.008 |
| Factor 3 | −0.045 | −0.027 |
| Factor 4 | 0.407*** | 0.398*** |
| Factor 5 | −0.014 | −0.014 |
| Factor 6 | −0.036 | −0.017 |
| Factor 7 | −0.025 | −0.011 |
| Factor 8 | −0.058* | −0.008 |
Note. PCA 1 included the Core Disgust subscale items from the DS-R and the gagging and disgust to food items. PCA 2 included the MOCI items and the anxiety to food items. Factor 1 from PCA 1 includes the gagging and disgust to food items. Factors 2-4 from PCA 1 include the Core Disgust subscale items. Factors 1-3 and 5-8 from PCA 2 include MOCI items. Factor 4 from PCA 2 includes anxiety to food items.
p < 0.05,
p < 0.001.
Based on the PCA and bivariate correlation results above, the measurement model was constructed such that the two disgust items and the two gagging to food items loaded on the latent disgust factor and the two anxiety to food items loaded on the latent anxiety factor. Sums of texture and smell items loaded on a sensory sensitivities latent factor as well. Disgust, anxiety, and sensory sensitivities factors could correlate. An examination of residuals indicated the presence of correlated errors between the two gagging items (“gagging to new foods”, “gagging to disliked foods”), the two disgust items (“disgust to new foods”, “disgust to disliked foods”), the three dislike items (“disgust to disliked foods,” “gagging to disliked foods”, “anxiety to disliked foods”), which were added to the model. Although the final model fit the data adequately (X2(12) =163.131, p<0.001; CFI=0.978; TLI=0.948; RMSEA=0.088), the sensory sensitivities and disgust factors were so highly correlated (r=0.917) it was not possible to distinguish them, which provided support for our third hypothesis that disgust would be a stronger predictor of sensory sensitivities than anxiety. Thus, we removed the sensory sensitivities factor from the model. We also removed the gagging items from the disgust factor and allowed them to load on the anxiety factor to determine whether these items better represented latent anxiety. This model did not fit the data well (X2(2)=173.501, p<0.001; CFI=0.969; TLI=0.769; RMSEA=0.228), suggesting that gagging may better represent latent disgust. The final model with gagging items loading on the disgust factor and the removal of the sensory sensitivities factor fit the data well: X2(3)=1.736, p=0.629; CFI=1.00; TLI=1.00; RMSEA<0.001. The disgust and anxiety latent factors were strongly correlated (r=0.772). All subsequent SEM and mediation analyses are presented in Table 3 and included this basic structure.
Table 3.
Goodness of Fit Indices and Standardized Weights of Paths in Structural Equation and Mediation Models for Picky Eating and ARFID Diagnosis Outcomes†
| Path/Goodness of Fit | Picky Eating | ARFID | ||||
|---|---|---|---|---|---|---|
| Estimate | S.E. | P-Value | Estimate | S.E | P-Value | |
| SEM | ||||||
| Disgust→DV | 0.407 | 0.049 | <0.001 | 0.605 | 0.061 | <0.001 |
| Anxiety→DV | 0.141 | 0.047 | 0.003 | 0.056 | 0.058 | 0.337 |
| Disgust⇔Anxiety | 0.769 | 0.018 | <0.001 | 0.769 | 0.017 | <0.001 |
| Mediation Models | ||||||
| Path A: Disgust→Anxiety | 0.769 | 0.023 | <0.001 | 0.769 | 0.024 | <0.001 |
| Path B: Anxiety→DV | 0.141 | 0.054 | 0.009 | 0.055 | 0.066 | 0.404 |
| Path C: Disgust→DV | 0.523 | 0.023 | <0.001 | 0.651 | 0.028 | <0.001 |
| IE: Disgust→Anxiety→DV | 0.109 | 0.041 | 0.008 | 0.042 | 0.050 | 0.402 |
| DE: Disgust→DV | 0.407 | 0.052 | <0.001 | 0.605 | 0.068 | <0.001 |
| Path A: Anxiety→Disgust | 0.769 | 0.023 | <0.001 | 0.769 | 0.024 | <0.001 |
| Path B: Disgust→DV | 0.407 | 0.052 | <0.001 | 0.606 | 0.068 | <0.001 |
| Path C: Anxiety→DV | 0.435 | 0.022 | <0.001 | 0.517 | 0.026 | <0.001 |
| IE: Anxiety→Disgust→DV | 0.313 | 0.044 | <0.001 | 0.465 | 0.060 | <0.001 |
| DE: Anxiety→DV | 0.141 | 0.054 | 0.009 | 0.055 | 0.066 | 0.406 |
| Model Fit | ||||||
| X2/df | 26.419/7 | 17.059/7 | ||||
| CFI | 0.997 | 0.994 | ||||
| TLI | 0.990 | 0.982 | ||||
| RMSEA (90% CI) | 0.041 (0.025, 0.058) | 0.030 (0.012, 0.048) | ||||
Results are shown for each structural equation and mediation model with picky eating (left panel) and ARFID diagnosis (right panel) as two separate outcomes. N=1644. Abbreviations: ARFID: Avoidant/Restrictive Food Intake Disorder; S.E. = standard error; IE = indirect effect; DE = direct effect (including mediator in the model); DV = dependent variable; → = “predicts;” ⇔ =“correlates with;” df = degrees of freedom; CFI = Comparative Fit Index; TLI = Tucker-Lewis Index; RMSEA = root mean square error of approximation.
Structural Equation and Mediation Models
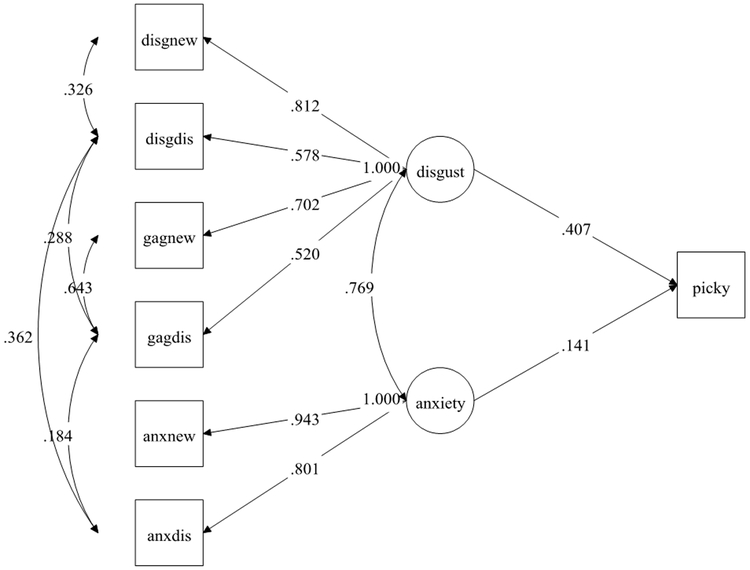
The latent disgust factor predicted picky eating frequency (standardized B=0.407) approximately four times more strongly than the latent anxiety factor (standardized B=0.141; Table 3 and Figure 1), suggesting that disgust may be a better predictor of picky eating behavior than anxiety in support of our first and second hypotheses. Mediation models testing whether disgust mediated anxiety or anxiety mediated disgust in predicting the outcomes were conducted to explore the complex relationship between anxiety and disgust to generate ideas for future research. These analyses showed that both disgust (standardized B=0.313) and anxiety (standardized B=0.109) partially mediated prediction of picky eating, as the direct effects remained significant (p’s < 0.01) with the addition of the mediator in the models (Table 3). However, disgust served as a stronger mediator in predicting picky eating than anxiety.
Figure 1.
Structural Equation Model of disgust and anxiety latent factors predicting picky eating†
† Standardized weights are shown. N=1644.
Abbreviations: Disgnew = disgust to a new food; disgdis = disgust to a disliked food; gagnew = gagging to a new food; gagdis = gagging to a disliked food; anxnew = anxiety to a new food; anxdis = anxiety to a disliked food; disgust = disgust latent factor; anxiety = anxiety latent factor; picky = are you a picky eater (yes=picky all the time/no).
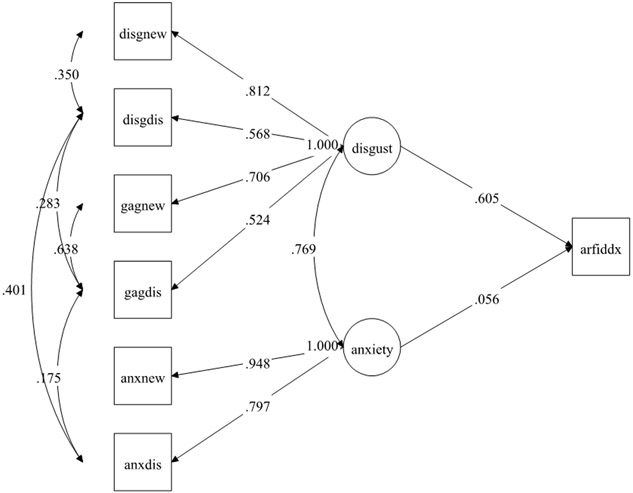
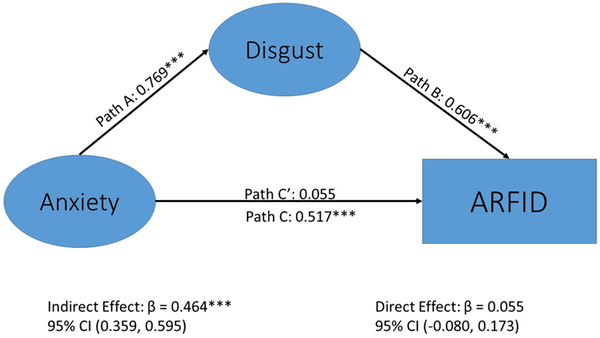
Like picky eating results, disgust was a much stronger predictor of the study-derived ARFID classification (standardized B=0.605) than anxiety (standardized B=0.056; Table 3, Figure 2), which was unrelated to ARFID classification. However, in this case, disgust fully mediated (standardized B=0.465) the association between anxiety and ARFID classification given that the direct effect of anxiety to ARFID became nonsignificant (p = 0.404) when the mediator was added to the model. Alternatively, anxiety was not a significant mediator of the association between disgust and ARFID classification (standardized B=0.042, p = 0.402) with the addition of the direct effect of disgust to ARFID in the model (Table 3, Figure 3). This suggests that the indirect effect of anxiety to disgust predicting ARFID diagnosis better explained the data than the indirect effect of disgust to anxiety predicting ARFID diagnosis , which supports our fourth hypothesis.
Figure 2.
Structural Equation Model of disgust and anxiety latent factors predicting Avoidant/Restrictive Food Intake Disorder diagnosis†
†Standardized weights are shown. N=1644.
Abbreviations: disgnew = disgust to a new food; disgdis = disgust to a disliked food; gagnew = gagging to a new food; gagdis = gagging to a disliked food; anxnew = anxiety to a new food; anxdis = anxiety to a disliked food; disgust = disgust latent factor; anxiety = anxiety latent factor; arfiddx = do you have a diagnosis of avoidant restrictive food intake disorder (ARFID; yes/no response).
Figure 3.
Mediation model of disgust as a significant mediator of the association between anxiety and Avoidant/Restrictive Food Intake Disorder diagnosis†
Standardized weights are shown. N=1644. The direct path from anxiety to ARFID diagnosis (β=.517, p < .001) becomes non-significant with the addition of disgust as a mediator (β=.055, p = .406). The indirect path of anxiety mediated by disgust was significant, β=.465, p < .001. Abbreviations: disgnew = disgust to a new food; disgdis = disgust to a disliked food; gagnew = gagging to a new food; gagdis = gagging to a disliked food; anxnew = anxiety to a new food; anxdis = anxiety to a disliked food; disgust = disgust latent factor; anxiety = anxiety latent factor; arfiddx = do you have a diagnosis of avoidant restrictive food intake disorder (ARFID; yes/no response). *** = p < .001. †See Table 3 for results with Picky Eating as the outcome.
Discussion
Results suggest that disgust is strongly associated to picky eating severity and ARFID classification. Both SEM and mediation models were consistent with this conclusion: mediation models aimed at better understanding differential contributions of disgust and anxiety demonstrated that a model positioning disgust as a mediator of the association between anxiety and picky eating/ARFID provided a more robust model fit than when anxiety was positioned as a mediator of the association between disgust and picky eating/ARFID. Data also revealed that the association of disgust with sensory features of smell, taste, and texture was so robust as to preclude model fitting due to collinearity. Given proven associations between anxiety and disgust (e.g. Goetz, Lee & Cougle, 2013; Hedman et al., 2016; Olatunji et al., 2010) and because cross-sectional data cannot adjudicate the causal order of these factors, we offer tentative hypotheses to motivate further exploration.
Exaggerated disgust experience may establish initial learning of a stimulus as potentially noxious, and maintain subsequent avoidance via the potency of visceral memories (Mayer, 2011). Indeed, as shown here, disgust is associated with feelings of nausea/gastrointestinal malaise. In a seminal study of conditioned taste aversion (Garcia et al., 1955), the (often single) pairing of a taste with gastrointestinal discomfort resulted in a potent form of avoidance learning resistant to extinction and maintaining of avoidance. Although conditioned taste aversion is not a proxy for disgust and may reflect other processes, the resistance to extinction draws into question the role of anxiety and provides a new avenue for thinking about complementary interventions. If primarily to avoid pathogens, disgust would be highly sensitized to stimuli that could violate or penetrate a protective body boundary (e.g., food). The strong association (r = .92) of disgust with sensory features associated with eating (e.g., smell) is not surprising given these features may signal contamination. Seemingly, for our participants, disgust motivates avoidance of potentially contaminating stimuli. However, experimental evidence of disgust generalization is limited. Perhaps the fear-learning architecture is co-opted to support elaborate avoidance behaviors and situations motivated by disgust, a potential mechanism supported by distinct neural circuitry constituting fear relative to disgust learning (Hildebrandt et al., 2015). Thus, consistent with our findings, disgust would have a strong direct association with food avoidance and more strongly mediate the association between anxiety and food avoidance than vice-versa. Longitudinal research is needed to test these hypotheses.
Our findings suggest that interventions developed primarily for anxiety may have limited efficacy in managing food avoidance (but see Anderson et al. 2018 for evidence of sex differences). Disgust has been found to be more resistant to extinction than anxiety with some reports indicating a failure for extinction processes to occur (Engelhard, Leer, Lange, & Olatunji, 2014; Mason & Richardson, 2010; Olatunji, Forsyth, & Cherian, 2007). Repeated presentations of a food may not reduce the disgust reaction precisely because disgust is an adaptive strategy for disease avoidance and, as such, less susceptible to extinction via repeated exposure. Yet, research on the developmental course of picky eating, suggests that a certain subset of picky children “grow out of it” with age. Further, individuals sometimes do repeatedly approach disgusting things (e.g., medical provider treating gangrene). Thus, approaching disgusting stimuli may require a highly valued motivation (Charland, 2011). As such, the approach may become associated with the valued motivation rather than changing the nature of the disgusting stimuli itself. It could be that, in conditions of pathology, the intensity of aversion cannot be superseded by valued motivations or that a powerful motivator has not been identified or is challenging to identify due to deficits in approach motivation more broadly. As such, treatments for ARFID may require flexible, nuanced approaches taking into account all of the potential motivations for food avoidance (e.g. Brigham, Manzo, Eddy & Thomas, 2018; Thomas et al. 2017). Indeed, it may be that the goal of treatment in ARFID for those with severe sensory aversion is the capacity to approach and consume foods without the expectation that food preferences will change. This would be a significant change in terms of aligning expectations for treatment.
Limitations
Limitations include the non-representative, self-selected sample and the cross-sectional design, which precludes causal inferences based on the results of the mediation analyses. ARFID classification was derived based on a study-specific algorithm meant to reflect diagnostic criteria. The high percentage of those meeting for the study-derived ARFID classification in this sample may reflect that only highly motivated individuals would participate in such a project. As such, these estimates may be conservative as we lack a non-picky control group but rather use a control group with less severe pickiness. Further, our sample was racially homogenous (90% white) and findings may not generalize. Although consistent with other studies (Mascola et al., 2010), we only used one item to assess degree of picky eating and diagnosis of ARFID was via self-report (note, validated measures of ARFID as assessed via self-report had not yet been published at the time this study was conducted). Importantly, despite including questions related to food neophobic and expected taste aversion within our anxiety construct (see Appendix 1) we cannot be certain that our measure of disgust does not encompass some expected taste dislike or food neophobia. It is also possible that investigator-constructed items were correlated in factor analyses due to method variance. However, the finding that these items loaded on separate factors in an exploratory analysis of all items lessens this concern. Finally, we did not include a validated measure of sensory sensitivity but rather employed face valid items that directly linked sensory experience to food avoidance.
Future Directions
This study highlights the need for considering disgust, and its possible relationship with anxiety, in selective eating and ARFID—especially with high levels of sensory sensitivity. Given exciting findings regarding the up-regulation of the disgust system during times in which immunity may be compromised or the need for protection from pathogens greater (e.g., pregnancy, sickness [Curtis et al., 2011; Fessler, Eng, & Navarrete, 2005; Stevenson et al., 2012])–the role of disgust in food avoidance may lead to interventions targeted at key developmental phases or vulnerable periods. Incorporating measurement tools that capture food-related disgust (Ammann, Hartmann, & Siegrist, 2018; Hartmann & Siegrist, 2018), changing the context of the experience of disgust (e.g., making it playful), creating developmentally sensitive tools to help children and adolescents define and pursue valued goals, and exploring whether and/or when food preferences change may be important domains for future research.
Supplementary Material
Acknowledgments
Funding source: All phases of the study were supported by the National Institute of Mental Health (R21-MH-097959) and the Duke Institute for Brain Sciences
Abbreviations:
- ARFID:
Avoidant Restrictive Food Intake Disorder
Appendix 1: The following questions were presented as part of an online questionnaire
Instructions: Please select the response that best describes your CURRENT experiences
| All of the time (1) |
More than half the time (2) |
About half the time (3) |
Less than half the time (4) |
Rarely or never (5) |
||
|---|---|---|---|---|---|---|
| 1. | Are you willing to try a food you have never eaten before? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 2. | Do you get anxious about social situations because you will be expected to eat? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 3. | Do you avoid social situations that involve food? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 4. | Do you lie to avoid eating in social situations? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 5. | Do you gag when tasting a new food? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 6. | Do you gag when tasting a disliked food? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 7. | Do you feel disgusted when presented with a new food? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 8. | Do you feel disgusted when presented with a disliked food? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 9. | Do you feel afraid or nervous when presented with a new food? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 10. | Do you feel afraid or nervous when presented with a disliked food? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 11. | Are you sensitive to the smells of food? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 12. | Does sensitivity to smells keep you from trying new foods? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 13. | Does sensitivity to smells keep you from eating a variety of foods? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 14. | Does sensitivity to smells keep you from participating in social gatherings with food? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 15. | Does sensitivity to food smells make you gag? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 16. | Are you sensitive to the textures of food? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 17. | Does sensitivity to the textures of food keep you from trying new foods? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 18. | Does sensitivity to food textures keep you from eating a variety of foods? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 19. | Does sensitivity to the textures of foods keep you from participating in social gatherings with food? | ❍ | ❍ | ❍ | ❍ | ❍ |
| 20. | Does sensitivity to food texture make you gag? | ❍ | ❍ | ❍ | ❍ | ❍ |
Footnotes
Financial disclosure: The authors have indicated they have no financial relationships relevant to this article to disclose.
Conflict of interest: Dr. Marsha D. Marcus, PhD is on the Scientific Advisory Board of WW International, Inc.
Clinical trial registration: N/A
References
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders-text revision (4th ed.). Washington, D.C.: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Washington, DC: American Psychiatric Association [Google Scholar]
- Ammann J, Hartmann C, & Siegrist M (2018). Development and validation of the Food Disgust Picture Scale. Appetite, 125, 367–379. [DOI] [PubMed] [Google Scholar]
- Anderson LM, Reilly EE, Thomas JJ, Eddy KT, Franko DL, Hormes JM, & Anderson DA (2018). Associations among fear, disgust, and eating pathology in undergraduate men and women. Appetite, 125, 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood T, & Scarpa A (2013). Modifications of cognitive-behavioral therapy for children and adolescents with high-functioning ASD and their common difficulties In Scarpa A, Williams White S, Attwood T, Scarpa A, Williams White S, & Attwood T (Eds.), CBT for children and adolescents with high-functioning autism spectrum disorders. (pp. 27–44). New York, NY, US: Guilford Press. [Google Scholar]
- Brigham KS, Manzo LD, Eddy KT, & Thomas JJ (2018). Evaluation and treatment of avoidant/restrictive food intake disorder (ARFID) in adolescents. Current pediatrics reports, 6(2), 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant‐Waugh R, Markham L, Kreipe RE, & Walsh BT (2010). Feeding and eating disorders in childhood. International Journal of Eating Disorders, 43(2), 98–111. [DOI] [PubMed] [Google Scholar]
- Charland LC (2011). Moral undertow and the passions: Two challenges for contemporary emotion regulation. Emotion Review, 3(1), 83–91. doi: 10.1177/1754073910380967 [DOI] [Google Scholar]
- Clark DA, & Beck AT (2010). Cognitive theory and therapy of anxiety and depression: Convergence with neurobiological findings. Trends Cogn Sci, 14(9), 418–424. [DOI] [PubMed] [Google Scholar]
- Croy I, Olgun S, & Joraschky P (2011). Basic emotions elicited by odors and pictures. Emotion, 11(6), 1331. [DOI] [PubMed] [Google Scholar]
- Curtis V (2011). Why disgust matters. Phil. Trans. R. Soc. B, 366(1583), 3478–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis V, de Barra M, & Aunger R (2011). Disgust as an adaptive system for disease avoidance behaviour. Philos Trans R Soc Lond B Biol Sci, 366(1563), 389–401. doi: 10.1098/rstb.2010.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey GC, Buckland G, Tantow B, & Dallos R (1998). Disgust and eating disorders. European Eating Disorders Review: The Professional Journal of the Eating Disorders Association, 6(3), 201–211. [Google Scholar]
- de Roos C, & de Jongh A (2008). EMDR treatment of children and adolescents with a choking phobia. Journal of EMDR Practice and Research, 2(3), 201–211. [Google Scholar]
- Ellis JM, Schenk RR, Galloway AT, Zickgraf HF, Webb RM, & Martz DM (2018). A multidimensional approach to understanding the potential risk factors and covariates of adult picky eating. Appetite, 125, 1–9. [DOI] [PubMed] [Google Scholar]
- Emmelkamp P, Kraaijkamp H, & Van den Hout M (1999). Assessment of obsessive-compulsive disorder. Behavior Modification, 23(2), 269–279. [DOI] [PubMed] [Google Scholar]
- Enders CK (2010). Applied missing data analysis: Guilford Press. [Google Scholar]
- Engelhard IM, Leer A, Lange E, & Olatunji BO (2014). Shaking that icky feeling: effects of extinction and counterconditioning on disgust-related evaluative learning. Behavior therapy, 45(5), 708–719. [DOI] [PubMed] [Google Scholar]
- Farrow CV, & Coulthard H (2012). Relationships between sensory sensitivity, anxiety and selective eating in children. Appetite, 58(3), 842–846. [DOI] [PubMed] [Google Scholar]
- Fernández-Aranda F, Agüera Z, Fernández-García JC, Garrido-Sanchez L, Alcaide-Torres J, Tinahones FJ, . . . Cebolla A (2016). Smell–taste dysfunctions in extreme weight/eating conditions: analysis of hormonal and psychological interactions. Endocrine, 51(2), 256–267. [DOI] [PubMed] [Google Scholar]
- Fessler DMT, Eng SJ, & Navarrete CD (2005). Elevated disgust sensitivity in the first trimester of pregnancy: Evidence supporting the compensatory prophylaxis hypothesis. Evolution and Human Behavior, 26(4), 344–351. doi: 10.1016/j.evolhumbehav.2004.12.001 [DOI] [Google Scholar]
- Fisher MM, Rosen DS, Ornstein RM, Mammel KA, Katzman DK, Rome ES, . . . Walsh BT (2014). Characteristics of avoidant/restrictive food intake disorder in children and adolescents: a “new disorder” in DSM-5. Journal of Adolescent Health, 55(1), 49–52. [DOI] [PubMed] [Google Scholar]
- Flora DB, & Curran PJ (2004). An empirical evaluation of alternative methods of estimation for confirmatory factor analysis with ordinal data. Psychological methods, 9(4), 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ, & Koelling RA (1955). Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science, 122(3160), 157–158. [PubMed] [Google Scholar]
- Goetz AR, Lee H-J, Cougle JR, & Turkel JE (2013). Disgust propensity and sensitivity: Differential relationships with obsessive-compulsive symptoms and behavioral approach task performance. Journal of Obsessive-Compulsive and Related Disorders, 2(4), 412–419. [Google Scholar]
- Haidt J, McCauley C, & Rozin P (1994). Individual differences in sensitivity to disgust: A scale sampling seven domains of disgust elicitors. Personality and Individual differences, 16(5), 701–713. [Google Scholar]
- Hartmann C, & Siegrist M (2018). Development and validation of the Food Disgust Scale. Food Quality and Preference, 63, 38–50. [DOI] [PubMed] [Google Scholar]
- Hedman E, Lekander M, Karshikoff B, Ljótsson B, Axelsson E, & Axelsson J (2016). Health anxiety in a disease-avoidance framework: Investigation of anxiety, disgust and disease perception in response to sickness cues. Journal of Abnormal Psychology, 125(7), 868. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Grotzinger A, Reddan M, Greif R, Levy I, Goodman W, & Schiller D (2015). Testing the disgust conditioning theory of food-avoidance in adolescents with recent onset anorexia nervosa. Behav Res Ther, 71, 131–138. [DOI] [PubMed] [Google Scholar]
- Hodgson RJ, & Rachman S (1977). Obsessional-compulsive complaints. Behav Res Ther, 15(5), 389–395. [DOI] [PubMed] [Google Scholar]
- Kaczkurkin AN, & Foa EB (2015). Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin Neurosci, 17(3), 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman DK, Norris ML, & Zucker N (2019). Avoidant restrictive food intake disorder. Psychiatric Clinics, 42(1), 45–57. [DOI] [PubMed] [Google Scholar]
- Kauer J, Pelchat ML, Rozin P, & Zickgraf HF (2015). Adult picky eating. Phenomenology, taste sensitivity, and psychological correlates. Appetite, 90, 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline P (2011). Oaxaca-Blinder as a reweighting estimator. American Economic Review, 101(3), 532–537. [Google Scholar]
- Köchel A, Schöngassner F, & Schienle A (2013). Cortical activation during auditory elicitation of fear and disgust: a near-infrared spectroscopy (NIRS) study. Neuroscience Letters, 549, 197–200. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, & Williams J (2004). Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate behavioral research, 39(1), 99–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzillier S, & Davey G (2005). Anxiety and disgust: Evidence for a unidirectional relationship. Cogn Emot, 19(5), 729–750. [Google Scholar]
- Mascola AJ, Bryson SW, & Agras WS (2010). Picky eating during childhood: A longitudinal study to age 11 years. Eat Behav, 11(4), 253–257. doi: 10.1016/j.eatbeh.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason EC, & Richardson R (2010). Looking beyond fear: The extinction of other emotions implicated in anxiety disorders. Journal of Anxiety Disorders, 24(1), 63–70. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, An SK, Lawrence NS, Caseras X, Speckens A, Giampietro V, . . . Phillips ML (2008). Individual differences in disgust sensitivity modulate neural responses to aversive/disgusting stimuli. Eur J Neurosci, 27(11), 3050–3058. doi: 10.1111/j.1460-9568.2008.06311.x [DOI] [PubMed] [Google Scholar]
- Mayer EA (2011). Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci, 12(8), 453–466. doi: 10.1038/nrn3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, van der Heiden S, & Rassin E (2008). Disgust sensitivity and psychopathological symptoms in non-clinical children. Journal of Behavior Therapy and Experimental Psychiatry, 39(2), 133–146. [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2010). Mplus: Statistical analysis with latent variables: User’s guide: Muthén & Muthén Los Angeles. [Google Scholar]
- Norris ML, Robinson A, Obeid N, Harrison M, Spettigue W, & Henderson K (2014). Exploring avoidant/restrictive food intake disorder in eating disordered patients: A descriptive study. International Journal of Eating Disorders, 47(5), 495–499. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Cisler J, McKay D, & Phillips ML (2010). Is disgust associated with psychopathology? Emerging research in the anxiety disorders. Psychiatry research, 175(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Ebesutani C, Haidt J, & Sawchuk CN (2014). Specificity of disgust domains in the prediction of contamination anxiety and avoidance: A multimodal examination. Behavior therapy, 45(4), 469–481. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Forsyth JP, & Cherian A (2007). Evaluative differential conditioning of disgust: A sticky form of relational learning that is resistant to extinction. Journal of Anxiety Disorders, 21(6), 820–834. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Williams NL, Tolin DF, Abramowitz JS, Sawchuk CN, Lohr JM, & Elwood LS (2007). The Disgust Scale: item analysis, factor structure, and suggestions for refinement. Psychological assessment, 19(3), 281. [DOI] [PubMed] [Google Scholar]
- Rozin P, & Fallon AE (1987). A perspective on disgust. Psychological review, 94(1), 23. [PubMed] [Google Scholar]
- Rozin P, Haidt J, & McCauley CR (2008). Disgust In Lewis M, Haviland-Jones J, & Barrett LF (Eds.), Handbook of emotions (3rd ed.). New York: Guilford Press. [Google Scholar]
- Sherlock JM, Zietsch BP, Tybur JM, & Jern P (2016). The quantitative genetics of disgust sensitivity. Emotion, 16(1), 43–51. doi: 10.1037/emo0000101 [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Hodgson D, Oaten MJ, Moussavi M, Langberg R, Case TI, & Barouei J (2012). Disgust elevates core body temperature and up-regulates certain oral immune markers. Brain Behav Immun, 26(7), 1160–1168. doi: 10.1016/j.bbi.2012.07.010 [DOI] [PubMed] [Google Scholar]
- Stice E, Fisher M, & Martinez E (2004). Eating disorder diagnostic scale: additional evidence of reliability and validity. Psychological assessment, 16(1), 60. [DOI] [PubMed] [Google Scholar]
- Stice E, Telch CF, & Rizvi SL (2000). Development and validation of the Eating Disorder Diagnostic Scale: a brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychological assessment, 12(2), 123. [DOI] [PubMed] [Google Scholar]
- Thomas JJ, Lawson EA, Micali N, Misra M, Deckersbach T, & Eddy KT (2017). Avoidant/restrictive food intake disorder: a three-dimensional model of neurobiology with implications for etiology and treatment. Current psychiatry reports, 19(8), 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troop NA, Murphy F, Bramon E, & Treasure JL (2000). Disgust sensitivity in eating disorders: a preliminary investigation. International Journal of Eating Disorders, 27(4), 446–451. [DOI] [PubMed] [Google Scholar]
- Tybur JM, Lieberman D, Kurzban R, & DeScioli P (2013). Disgust: evolved function and structure. Psychol Rev, 120(1), 65–84. doi: 10.1037/a0030778 [DOI] [PubMed] [Google Scholar]
- van Overveld M, de Jong PJ, Peters ML, & Schouten E (2011). The Disgust Scale-R: A valid and reliable index to investigate separate disgust domains? Personality and Individual differences, 51(3), 325–330. [Google Scholar]
- Wardle J, Cooke LJ, Gibson EL, Sapochnik M, Sheiham A, & Lawson M (2003). Increasing children’s acceptance of vegetables; a randomized trial of parent-led exposure. Appetite, 40(2), 155–162. [DOI] [PubMed] [Google Scholar]
- Wildes JE, Zucker NL, & Marcus MD (2012). Picky eating in adults: Results of a web-based survey. International Journal of Eating Disorders, 45(4), 575–582. doi: 10.1002/eat.20975 [DOI] [PubMed] [Google Scholar]
- Zucker N, Arena C, Dable C, Hill J, Hubble C, Sohl E, & Yoon J (2018). Selective Eating: Normative Developmental Phase or Clinical Condition? In Agras WS & Robinson A (Eds.), Oxford Handbook of Eating Disorders (Second ed., pp. 419–437). Oxford, London: Oxford University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.