Abstract
Aneurysmal subarachnoid hemorrhage has a high mortality rate and, for those who survive this devastating injury, can lead to life-long impairment. Clinical trials have demonstrated that cerebral vasospasm of larger extraparenchymal vessels is not the sole contributor to neurologic outcome. Recently, the focus of intense investigation has turned to mechanisms of early brain injury that may play a larger role in outcome, including neuroinflammation and microvascular dysfunction. Extravasated blood after aneurysm rupture results in a robust inflammatory response characterized by activation of microglia, upregulation of cellular adhesion molecules, recruitment of peripheral immune cells, as well as impaired neurovascular coupling, disruption of the blood-brain barrier, and imbalances in endogenous vasodilators and vasoconstrictors. Each of these phenomena are either directly or indirectly associated with neuronal death and brain injury. Here, we review recent studies investigating these various mechanisms in experimental models of subarachnoid hemorrhage with special emphasis on neuroinflammation and its effect on microvascular dysfunction. We discuss the various therapeutic targets that have risen from these mechanistic studies and suggest the utility of a multi-targeted approach to preventing delayed injury and improving outcome after SAH.
Keywords: Early brain injury, microvascular dysfunction, neuroinflammation, subarachnoid hemorrhage, vasospasm
Introduction
Non-traumatic subarachnoid hemorrhage (SAH) is a devastating neurological emergency resulting most commonly from the rupture of cerebral aneurysms. It occurs in 7.2–10.5 out of 100,000 people and accounts for approximately 5% of strokes annually.1, 2 While the incidence of aneurysmal SAH is lower compared to that of ischemic stroke, it occurs in younger patient populations, has a higher mortality, and confers a significant impairment on quality of life. Long-term disability and mortality from SAH are estimated to account for up to 27% of all stroke-related years of potential life lost before age 65.3 For those who survive, life expectancy is greatly reduced with reported excess mortality rates of approximately 17% at 20 years compared to the general population.4 Mortality after SAH can occur at various time points after aneurysmal rupture. Approximately 10–15% of patients die before receiving medical attention.1, 5 This is likely due to a sharp rise in intracranial pressure that reduces cerebral perfusion, inducing global cerebral ischemia and resulting in metabolic crisis. For those who make it to the hospital, another 25% may succumb within the first 24–72 hours.5 This is a critical window during which multiple pathogenic processes are occurring that are collectively referred to as early brain injury (EBI). Clinically, severity of EBI is determined by various admission factors which include hemorrhage volume, level of consciousness, and presence of neurological deficits. Various mechanisms have been investigated to target EBI after SAH, including a robust inflammatory response, cerebral edema, and microvascular dysfunction throughout the brain.6
Of those who survive the initial 24–72 hours, there is additional risk of delayed cerebral vasospasm and ischemia which occur in 70% and 30% of patients, respectively.7–9 Vasospasm typically affects the medium- and large-sized intracranial arteries and occurs within days 3 and 14 after SAH. This luminal narrowing has been associated with delayed cerebral ischemia (DCI), cerebral infarction, and long-term neurocognitive impairment. Clinical deterioration caused by DCI is a distinct entity that presents with focal neurological deficits or a decrease in Glasgow Coma Scale of at least two points for at least one hour.10 Hypoperfusion from DCI may progress to infarction in some cases, detected via CT, MRI, or on autopsy.1, 10 For decades, cerebral vasospasm was the subject of intense investigation as it was attributed to be the principal contributor to poor outcome. However, recent clinical trials using the endothelin-1 (ET-1) receptor antagonist clazosentan demonstrated a reduction in the incidence of angiographic vasospasm, but no significant change in functional outcome or mortality.11–13 The results of these trials served as a major turning point in the field and highlighted the need for further investigation into other pathogenic mechanisms of brain injury after SAH.
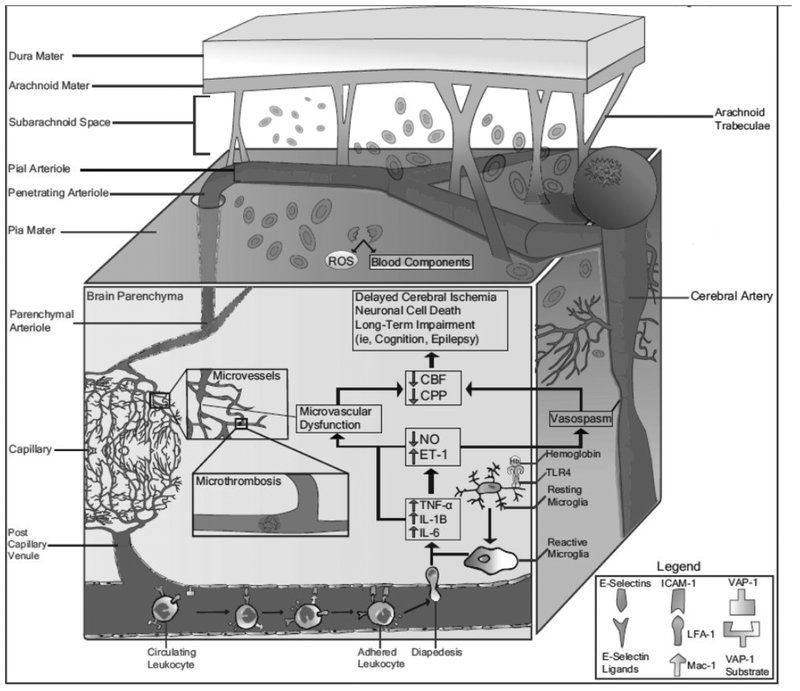
Several mechanisms during the acute phase of SAH contribute to DCI and poor outcome. These include neuroinflammation, microthrombosis, cortical spreading depolarizations, disrupted integrity of the blood-brain barrier (BBB), and microvascular dysfunction in addition to well-studied macrovascular cerebral vasospasm.6 There are additional systemic responses after SAH including stress hyperglycemia, fever, infection, and dysregulation of coagulation pathways which may also affect clinical outcome, although systemic complications are not the focus of our present review. Recently, our lab has focused on two of these phenomena and the complex interplay between them – neuroinflammation and microvascular dysfunction. Results from our work and that of others suggest that these pathophysiological processes play a highly influential role during the EBI phase and set the stage for long-term complications and outcome (Figure 1). Further, these events may be interrelated as inflammatory responses following SAH may result in microvascular dysfunction, which in turn could drive further inflammation.
Figure 1. Neuroinflammation and microvascular dysfunction after aneurysmal subarachnoid hemorrhage.
In addition to the well-studied phenomenon of cerebral vasospasm, release of blood products into the subarachnoid space can trigger a robust inflammatory response consisting of activated microglia, secretion of pro-inflammatory cytokines, increased expression of CAMs, peripheral leukocyte recruitment, and BBB disruption. This can further contribute to microvascular dysfunction including arteriolar vasoconstriction, microthrombosis, and imbalance of endogenous vasoconstrictors and vasodilators, which further compromises CBF and drives delayed ischemic damage.
There are excellent reviews describing the association of inflammation with hemostasis, dysregulation of large conduit arterial tone, and DCI.14–16 This review summarizes the efforts to date investigating the role of neuroinflammation and microvascular dysfunction during EBI as well as mechanistic and therapeutic targets that may ameliorate EBI and improve outcome after SAH (Table 1). By highlighting various mechanisms contributing to EBI and long-term outcome, we suggest a multifactorial approach to the management of brain injury following SAH.
Table 1.
Major strategies in experimental subarachnoid hemorrhage aimed at targeting neuroinflammation and/or microvascular dysfunction.
| Major Experimental Targets | Genetic or Pharmacologic Approach |
Results | Translated to SAH Patients |
Reference(s) |
|---|---|---|---|---|
| Pattern-Recognition Receptors | ||||
| Toll-like Receptor 4 (TLR4) | TLR4−/− mice | Decreased vasospasm and neuronal apoptosis on days 7 and 15 after SAH | Not applicable | (27) |
| TLR4 antagonists (IAXO-102, TAK-242) | Higher neurological scores and reduced brain water content at 24 hours compared to controls, reduced BBB disruption with decreased MMP-9 and preserved tight junctions | No | (45) | |
| Resident Cells of the CNS | ||||
| Microglia | Clodronate liposomes | Depletion of microglia results in significant ablation of vasospasm at day 7 and 15 in mice, reduced neuronal death at day 7 but not at day 15 compared to vehicle-treated controls | No | (27) |
| CS11b-HSVTK+/− mice | Depletion of microglia results in reduced neuronal cell death | Not applicable | (31) | |
| Pro-inflammatory Cytokines | ||||
| Interleukin-1 (IL-1) | IL-1 receptor antagonist (IL-1Ra) | In rodents, treatment resulted in decreased BBB breakdown and subsequent brain injury | Yes. The SCILSAH study has shown safety, tolerability, and effective reduction in peripheral inflammatory markers, supporting a Phase III clinical trial | (42–44) |
| Cellular Adhesion Molecules | ||||
| CD11/CD18 (includes LFA-1 and Mac-1, also known as CD11a/CD18 and CD11b/CD18, respectively) | Anti-LFA-1 antibody | Reduction in femoral artery spasm following blood exposure in rats | No | (63) |
| Anti-CD11/CD18 antibody | Reduction in non-human primate cerebral vasospasm from baseline angiography compared to vehicle-treated animals; Reduction of rabbit basilar artery spasm and increased peripheral white blood cell count | No | (64–66) | |
| Intercellular adhesion molecule-1 (ICAM-1) | Anti-ICAM-1 antibody | Reduction in rabbit basilar artery spasm, synergistic with effects of anti- CD18 Ab; reduction in femoral artery spasm following blood exposure in rats | No | (63,65) |
| Vascular adhesion protein-1 (VAP-1) | LJP-1586 | Inhibition of VAP-1 results in reduced leukocyte adhesion and infiltration, enhanced microvascular reactivity, and improved shortterm neurologic outcome | No | (67,69) |
| Peripheral Immune Cells | ||||
| Neutrophils | Anti-neutrophil serum | Reduction in leukocyte infiltration into CNS, preservation of pial arteriolar dilating function, and protection of neurobehavioral l function; reduction in vascular collagenase activity | No, may prolong bleeding time from ruptured artery based on preclinical data | (69,70) |
| Anti-Ly6G/C antibody | Reduction in middle cerebral artery (MCA) vasospasm and improved behavioral testing via Ymaze and Barnes maze tests; reduced cerebral inflammation and decreased impairment in long-term potentiation (LTP) at day 6 after SAH in mice | No | (68,71) | |
| Lymphocytes | Corticosteroids (Dexamethasone, Methylprednisolone, etc.) | Reduced alterations in contractile and cytoskeletal proteins of rabbit cerebral arteries; decreased CSF citrulline (contributor to NO production) and leukocytosis; mixed results related to effect on vasospasm | Yes, with conflicting results. Overall, no strong evidence of beneficial or adverse effect | (76–78,82) |
| Cyclosporine | Reduction in canine basilar artery vasospasm with prophylactic treatment; reduction in neuronal apoptosis and BBB disruption in mice with improved neurological outcome | Yes, with conflicting results. Some have shown improved neurological outcome while others have shown no effect on vasospasm or DCI | (79–81,83,84) | |
| Fingolimod (FTY-720) | Reduced intravascular leukocyte adhesion to pial venules, preserved pial arteriolar reactivity, improved neurological outcome | No | (85) | |
| Endogenous Vasoconstrictors | ||||
| Endothelin-1 (ET-1) | Clazosentan (ET-1 receptor antagonist) | Prophylactic treatment in rats prevented continued reduction in cerebral blood flow after acute hypoperfusion; reduced largeartery vasospasm but did not prevent formation of microthrombi, neuronal cell death, or loss of LTP | Yes, reduction in angiographic vasospasm but no statistically significant effect on morbidity, mortality, or functional outcome | (11,12,97,98) |
| 20-hydroxyeicosatetranoic acid (20-HETE) | TS-011, 17-octadecynoic acid, HET0016 (selective CYP450 inhibitors) | Pre-treatment resulted in faster recovery of cerebral blood flow in the acute setting following SAH; reversal of delayed vasospasm in vitro and in vivo | No | (163–165) |
| Endogenous Vasodilators | ||||
| Nitric Oxide (NO) | NO donors (Larginine, Snitrosoglutathione, sodium nitroprusside, transdermal nitroglycerin, etc.) | Improved CBF recovery, reduction in cerebral vasospasm, decreased glutamate excitotoxicity, and transient decrease in systemic blood pressure | Yes, with conflicting results. Side effects including systemic hypotension, headache, and rebound hypertension possible, limiting clinical use. | (135–137,140–143) |
| Inhaled NO | Reduction in microvascular constriction with limited effects on large artery spasms, decreased cerebral edema, hippocampal neuronal loss, and mortality; improved neurological outcome | No | (133) | |
| Phosphodiesterase (PDE)-V inhibitors (sildenafil) | Reduction of vasospasm and neuronal cell death with improved neurological outcome in mice | Yes, Phase I study demonstrate d safety and tolerability, with some data suggesting potential improvement of vasospasm | (144,145) | |
| Vascular smooth muscle cell (vSMC) relaxation | PDE-III inhibitors (milrinone) | Prevented angiographic vasospasm in canine model; improved CBF and neurobehavioral l outcome, reduced DCI in mice | Yes, reduction in delayed cerebral vasospasm warranting further study | (146–151) |
| Magnesium sulfate | Reduction of infarct size, reversal of vasospasm in vivo and in vitro, and improved cerebral blood flow recovery | Yes, MASH-II and IMAGES trials failed to show clinical benefit | (152–158) |
Neuroinflammation
Immediately after aneurysm rupture, blood rushes into the subarachnoid space under arterial pressure. This leads to a sharp rise in intracranial pressure and reduction in cerebral blood flow (CBF), which compromises tissue perfusion and causes diffuse brain injury and potential death. In addition, extravasated red blood cells (RBCs) in the subarachnoid space undergo degradation, releasing a host of bioactive and potentially toxic molecules including hemoglobin, methemoglobin, bilirubin, coagulation factors such as fibrinogen, and more (Figure 1).16–22 Several of these molecules, including free hemoglobin and its subsequent byproducts, have long been associated with the development of cerebral vasospasm and outcome.16–23 Experiments have suggested that mechanisms responsible for this may include production of free radicals and other metabolites with vasoconstrictive and pro-inflammatory properties. Bilirubin oxidation products (BOXes) formed from the breakdown of hemoglobin have peak concentrations correlating with the occurrence of vasospasm.21, 22 Several endogenous scavenging mechanisms in place, such as the CD163-haptoglobin-hemoglobin system, may act to may help clear toxic hemoglobin and its metabolites; however, studies suggest that these systems may quickly become saturated following SAH.24 While direct interaction with the cerebral vasculature is one mechanism by which these molecules can produce long-term impairments, they may also interact with neurons, glia, and immune cells as blood products contact adjacent tissue or infiltrate brain parenchyma via paravascular spaces and disrupt normal flow between interstitial fluid and cerebrospinal fluid (CSF).25, 26 The interactions between blood products and cells throughout the central nervous system (CNS) results in a cascade of molecular events triggered initially during EBI that may persist and result in both acute and delayed brain injury.
Microglial Response
One of the first cells to respond to these extravasated RBC products is microglia, the resident immune cell of the CNS. Under normal physiological circumstances, microglia are the main immune cell actively surveying the CNS, which is otherwise somewhat restricted from peripheral immune cell trafficking. Blood products such as hemoglobin have been shown to bind to pattern recognition receptors (PRRs), such as toll-like receptor 4 (TLR4), on the surface of immune cells such as microglia (Figure 1).18, 27 Activation of TLR4 and other PRRs can lead to activation of downstream inflammatory signaling cascades including NF-kB, MyD88/TRIF, and MAPK pathways.16, 27–29 This results in activation of microglia, which take on a more amoeboid shape and release pro-inflammatory cytokines. In animal models of SAH, a robust increase in microglia and pro-inflammatory cytokine expression throughout the brain was associated with long-term sensorimotor deficits.30 Depletion of microglia using CD11b HSVTK+/− mice attenuated neuronal loss after experimental SAH.31 Further, increased microglial expression of heme oxygenase-1, responsible for the metabolism of free heme, has been shown to reduce neuronal cell death, vasospasm, and cognitive impairment in murine models.32 While most studies have investigated the deleterious effects of microglial activation and promotion of a pro-inflammatory environment, it is also well-known that microglia can be polarized to a more anti-inflammatory phenotype.33 Potential therapeutic strategies early after SAH may thus seek to promote the activation of these microglia towards an anti-inflammatory phenotype which may confer neuroprotective benefits,34 including but not limited to effects on neurogenesis and neurorepair which have been suggested in other forms of stroke.
Cytokines and Secreted Proteins
Several pro-inflammatory cytokines including interleukin-1 (IL-1), IL-6, tumor necrosis factor-a (TNF-a), and others have been demonstrated to be upregulated in CSF and serum after SAH in humans and animal models (Figure 1).35, 36 Pro-inflammatory cytokines can potentiate brain damage by triggering apoptotic pathways, interfering with the balance of endogenous vasodilators and vasoconstrictors, activating clotting factors leading to microthrombosis, and recruiting peripheral immune cells via upregulation of cellular adhesion molecules (CAMs). This initial release of cytokines and chemokines occurs from resident cells of the CNS such as microglia, but subsequent infiltration of peripheral immune cells further drives production of cytokines within the subarachnoid space and the brain parenchyma. IL-1, in particular, increases BBB permeability, enhances glial-mediated neurotoxicity, and promotes ischemic changes after SAH in preclinical models.37–41 Based on these results in experimental SAH and other forms of stroke, the SCIL-SAH study targeted the pro-inflammatory cytokines IL-1 and downstream IL-6 using the naturally occurring IL-1 receptor antagonist anakinra in humans.42–44 In addition to being safe and well-tolerated, results of the Phase II trial demonstrated reduction of IL-6, C reactive protein (CRP), and fibrinogen in the active arm, supporting a Phase III trial to investigate the effect on outcome.42, 43 Upstream targeting of TLR4 in experimental SAH through genetic knockouts and pharmacologic interventions has also been effective at reducing vasospasm and improving outcome highlighting the contribution of immune mechanisms to vascular tone regulation.16, 27, 45
Additional secreted immune molecules further promote inflammation and worsen outcome after SAH. Previous studies have demonstrated an association between elevated CSF and plasma levels of complement proteins C3a and C4a, and outcome.46, 47 In addition, alterations in members of the mannose-binding lectin pathway of complement activation, such as ficolin-1, have been described.48, 49 Changes in complement proteins are an attractive target for further investigation given their proposed role in microglial-mediated synaptic alterations during development and aberrant reactivation in neurologic disease.50–53 In contrast, anti-inflammatory mediators, such as IL-10 and various fatty acid-derived lipid mediators, may promote resolution of inflammation.54, 55 The results of these clinical and preclinical studies suggest a highly significant contribution of microglia, pro-inflammatory cytokines, and other secreted factors to poor outcome after SAH (Table 1).
Cellular Adhesion Molecules
The development of a pro-inflammatory state induced by secretion of cytokines and chemokines following SAH is also associated with increased expression of CAMs on the surface of endothelium, platelets, and leukocytes. CAMs such as E-selectin, vascular adhesion protein-1 (VAP-1), vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), macrophage-1 antigen (Mac-1), and lymphocyte function-associated antigen-1 (LFA-1) promote leukocyte adhesion of immune cells to luminal endothelia.56–58 This robust inflammatory response leads to increased BBB permeability which facilitates the infiltration of peripheral leukocytes into the brain parenchyma (Figure 1). CAMs including E-selectin, VCAM-1, and ICAM-1 are elevated in the CSF of SAH patients, and these elevations correlate with the occurrence of vasospasm and DCI.59–62 In animal models, similar elevations in CAM expression have been detected, and treatment with anti-CAM antibodies such as anti-ICAM1, anti-LFA-1, and anti-Mac-1 resulted in reduced leukocyte infiltration and arterial narrowing (Table 1).56, 58, 63–66 In our own lab, we have targeted endothelial VAP-1 using the VAP-1 inhibitor LJP-1586 in rats during the period of EBI, and demonstrated reduced leukocyte adhesion, enhanced microvascular reactivity, and improved short-term neurologic outcome.67 Most strategies targeting CAMs are non-selective inhibitors of leukocyte adhesion, and thus the neurological benefits derived from CAM inhibition may be from neutrophils, monocytes, lymphocytes, or a combination. Future studies may seek to identify and target CAM pathways which selectively block infiltration of specific peripheral immune cell populations.
Peripheral Immune Cells
Recruitment of peripheral immune cells into the brain after SAH is a well-documented phenomenon and occurs early in the course of disease in response to increased expression of chemokines and CAMs. Infiltrating leukocytes attempt to phagocytose RBCs and debris induced by aneurysm rupture. Leukocyte adhesion to pial venules occurs within 48 hours after SAH, corresponding to the period of EBI.67–69 The earliest peripheral immune cell to infiltrate the CNS after SAH is the neutrophil, believed to enter the CNS within 24–48 hours after injury.63, 64, 67, 69 Induction of neutropenia using anti-rat neutrophil serum reduced leukocyte adhesion to pial vessels and improved neurologic outcome suggesting that neutrophils play a predominant role in poor outcome following experimental SAH.69 These observations were also described in other studies which either depleted neutrophils or limited their function.68–70 Further, Provencio et al. (2016) showed that neutrophil depletion using an anti-Ly6G/C antibody after SAH results in improved spatial memory six days after SAH, and that this is mediated largely by attenuating dysfunction in long-term potentiation via NMDA receptors.71 In SAH patients, CSF neutrophil content has been shown to be an independent predictor of other delayed events including vasospasm.72 The extent of neutrophil infiltration into the subarachnoid space and CNS parenchyma helps determine the extent of the acute inflammatory response, which in turn may influence delayed events such as vasospasm and neurologic outcome.
Some studies have shown a significant role of other infiltrating leukocyte subtypes. Infiltrating monocytes enter the subarachnoid space and CNS parenchyma as active macrophages around 2–5 days post-SAH, and like neutrophils engage in phagocytosis of RBCs, clots, and debris.73 Increased migration of monocytes across the cerebral microvasculature has been shown in vivo as well as in vitro using monocyte migration assays.74 Further, due to the number of overlapping markers between monocyte-derived macrophages and resident microglia, few studies have successfully distinguished between the contribution of these two cell populations in SAH. Recent studies using RNA sequencing technology have successfully identified unique markers of resident microglia in the CNS (e.g. Tmem119).75 Future studies in SAH would benefit from using more specific microglial and infiltrating monocyte markers to differentiate the unique role of these cells.
Lymphocytes as a prominent cell type of adaptive immunity may also play a role in post-SAH pathophysiology; however, studies are limited and inconsistent. Therapeutic strategies targeting T lymphocytes such as corticosteroids or cyclosporine have shown efficacy in some studies;76–81 however, evidence supporting clinical use is lacking due to conflicting results or increased risk of adverse events.56, 82–84 Studies in our lab have employed the immunomodulatory agent fingolimod (FTY720) in a preclinical rat model of SAH.85, 86 Fingolimod is a well-tolerated, orally bioavailable, FDA-approved drug currently used for multiple sclerosis that acts as a sphingosine-1-phosphate (S1P) analog.87 Its mechanism of action involves reversible phosphorylation and activation by sphingosine-kinase 2, which allows it to recognize and downregulate G-coupled S1P receptor (S1PR) type 1 expressed on peripheral lymphocytes. The immunomodulatory effect of fingolimod results from the sequestration of circulating mature lymphocytes in peripheral lymphoid tissues resulting in lymphopenia.88 In addition, fingolimod crosses the BBB and binds to S1PRs expressed on CNS cells including neurons, oligodendrocytes, astrocytes, microglia, and brain endothelia, resulting in direct effects on these cells.86, 89, 90 Studies in ischemic stroke models, for example, have shown that fingolimod is neuroprotective, can enhance remyelination, restore BBB integrity, and reduce microglial activation and astrogliosis.86 Treatment with fingolimod reduces intravascular leukocyte adhesion to pial venules, preserves pial arteriolar reactivity, and improves neurologic function at 48 hours after SAH in a rat model.85 While the benefit of immunomodulators such as fingolimod has been demonstrated in experimental SAH EBI, other readily available biomarkers can be used clinically to evaluate immune responses following cerebral hemorrhage. One such marker is the serum neutrophil-to-lymphocyte ratio (NLR), which has not been thoroughly studied in EBI but has been associated with DCI.91–93 Taken together, alterations in leukocyte trafficking after SAH seem to play an important role in driving outcome; however, the role of resident versus infiltrating immune cells in SAH-associated EBI remains a key area of investigation.
Microvascular Dysfunction
SAH-associated vascular dysfunction of large intracranial arteries has been the principal focus of preclinical and clinical studies over the last several decades. However, while large-vessel angiographic vasospasm is observed in up to 70% of patients, DCI is only observed in up to half of them.94, 95 Also, neurologic deterioration and radiologic evidence of cerebral ischemia can occur in the absence of vasospasm, and the reversal of vasospasm using ET-1 inhibitors does not demonstrably influence outcome despite some evidence from preclinical studies.95–98 Moreover, the only medication that has been shown to be beneficial in SAH, the calcium-channel blocker nimodipine, does not seem to have a significant effect on vasospasm.99 However, nimodipine does in fact appear to inhibit vasoconstriction at the level of small-diameter arterioles,100 suggesting that targeting microvascular dysfunction could improve outcomes after SAH. The phase 3, multicenter, randomized NEWTON 2 trial was designed to compare the effect of an extended-release microparticle nimodipine preparation delivered directly to CSF to oral nimodipine.101 However, skepticism exists in relation to the efficacy of this approach as the Data Monitoring Committee has recommended discontinuation of the study due to low probability of meeting its primary endpoint for favorable outcome.102 This highlights the need for further mechanistic understanding of microvascular changes after SAH and identification of therapeutic targets.
Blood Vessel Reactivity
It has been estimated that at least 50% of the cerebrovascular resistance lies in arterioles and precapillary segments. Despite their central role in hemodynamic control, the contribution of microvessels to SAH outcome has received little attention. Increasing evidence suggests that microvascular dysfunction is associated with both EBI and DCI.103, 104 Various cell types within the cerebral microvasculature including endothelia, pericytes, and vascular smooth muscle cells (vSMCs) engage in constant communication with surrounding neurons and glia, collectively forming a functional neurovascular unit. The intense cross-talk between these various cells under normal conditions results in changes in microvascular tone and tissue perfusion in response to neuronal energetic needs.103, 105, 106 This process, called neurovascular coupling, is coordinated by neurons and astrocytes, which typically respond to increased extracellular glutamate and transmit signals to vSMCs in arterioles to promote vasodilation and enhanced blood flow in response to neuronal activity and increasing metabolic demands.107 Following SAH, there appears to be inversion of neurovascular coupling starting 24 to 96 hours after injury, whereby neuronal activity instead promotes a vasoconstrictive response in arterioles.108, 109 This aberrant response to neuronal activity creates a mismatch between neuronal energetic needs and blood flow that can further potentiate brain injury.
Further evidence from our lab and others has suggested that significant microvascular dysfunction after SAH occurs at the level of arterioles. Several studies have attempted to investigate microvascular reactivity after experimental SAH via direct visualization of vessels in vivo.67, 69, 85 Under normal conditions, cortical activation (achieved via sciatic nerve stimulation) or topical treatment of pial vessels with vasoactive agents, including adenosine, acetylcholine, nitric oxide (NO) donors, or carbon dioxide, result in pial arteriolar dilation. However, in the rodent model of SAH, impaired microvascular reactivity in response to these interventions was observed.17, 67, 103 These changes peak at 48 hours and slowly resolve within the subsequent 5–7 days post injury. In addition, Friedrich et al. (2012) showed that greater than 70% of arterioles were constricted in diameter up to 72 hours after SAH, with smaller arterioles having more constriction.103 Similar findings have been reported in other studies, and have described arteriolar constrictions in a “pearl string” pattern.57, 103, 110 Interestingly, microthombi which have commonly been observed throughout the brain following SAH, are commonly found in areas of arteriolar constriction.6, 103, 105 Microthrombosis further compromises cerebral perfusion and can lead to ischemia and neuronal cell death (Figure 1). Activation of the coagulation cascade, formation of microthrombi, and neuroinflammation are closely linked to one another through a process known as thromboinflammation.6, 14, 111, 112 Microthrombosis has been reviewed elsewhere.103, 113
Several other structural and cellular changes within the cerebral microvasculature can be observed after SAH. Microvilli have been shown to develop and extrude from the vessel wall, forming blebs that can detach from the basal lamina and obstruct the lumen.104, 106 In addition to affecting blood flow directly, these changes can lead to exposure of the basal lamina, triggering both platelet and leukocyte adhesion, promoting microthrombosis and neuroinflammation, respectively. The role of pericytes in various cerebrovascular conditions including SAH has received increasing attention for their contribution to vessel tone and alterations in CBF.114–116 Li et. al. (2016) showed that penetration of hemoglobin into the brain parenchyma following SAH in a rat model resulted in phenotypic transformation of pericytes to a hypercontractile form that resulted in reduction in microvessel diameter.116 This transformation was further shown to be dependent on reduction in NO/cyclic guanosine monophosphate (cGMP) signaling, a well-documented phenomenon after SAH described below. In addition to pericyte-mediated vasoconstriction, swelling of astrocytic end feet can further compromise blood flow.57, 104, 106 Astrocytes also appear to serve as a source of the endogenous vasoconstrictor ET-1 and undergo proliferation after SAH in the cortex and hippocampus.117, 118 Further, astrocytes following exposure to CSF containing blood appear to enter a metabolic crisis related to release of intracellular pools of calcium that also underlie alterations in neurovascular coupling.108, 119 Taken together, it appears that several cell types within the neurovascular unit collectively drive microvascular dysfunction after SAH.
Blood-Brain Barrier
SAH also disrupts the integrity of the BBB, which can further compromise cerebral perfusion and facilitate neuroinflammation. Leakage of endogenous proteins and injected dyes normally restricted from the CNS have been observed after experimental SAH.57, 110 This increased permeability of the BBB drives cerebral edema and intracranial hypertension which further compromise cerebral perfusion. Mechanistically, loss of BBB integrity has been associated with the upregulation of matrix metalloproteinases (MMPs) and other proteases which degrade tight junctions and the basal lamina.16, 120 MMP-9 in particular has emerged as a key player in post-SAH pathophysiology based on several studies in patients and animal models, contributing to global cerebral edema following degradation of extracellular matrix proteins and disruption of tight junctions.121 Increased expression and subsequent activation of MMP-9 can occur in response to reactive oxygen species and pro-inflammatory cytokines such as TNF-a and IL-17, all of which are increased after SAH.122 The source of MMP-9 in SAH is not well described, but evidence obtained in ischemia-reperfusion models indicates that neutrophils may constitute the major source of MMP-9 acting on the BBB.121 MMP-9 can also drive neuroinflammation via activation of pro-inflammatory signals and clotting factors, triggering a positive feedback loop promoting thromboinflammation and neurotoxicity.122 Indeed, increased MMP-9 in both plasma and CSF of SAH patients have been observed and some studies have shown a correlation with the extent of EBI, vasospasm, and DCI.123–125 The correlation of MMP-9 with vasospasm in human cohorts; however, remains controversial.125 Beyond MMP-9, recent studies have also shown upregulation of sulfonylurea receptor 1-transient receptor potential melastatin 4 (Sur1-Trpm4) after SAH in rats, and that this upregulation is associated with BBB dysfunction, neuroinflammation, and deficits in spatial learning and memory.126 Importantly, blockade of this channel using antisense oligonucleotides or the Sur1 inhibitor glibenclamide reduced these deficits.126 While a detailed review of BBB changes after SAH is outside the scope of this review, it is clear that disruption of the BBB after SAH is closely linked to neuroinflammation and contributes to poor outcome.
Vasoconstrictors and Vasodilators
As mentioned above, SAH also results in an imbalance between endogenous vasoconstrictors (i.e., ET-1) and vasodilators (i.e., NO). Changes in these vasoactive substances may also be triggered by the initial inflammatory response following SAH (Figure 1).127, 128 Studies of SAH patients have shown that activated mononuclear leukocytes in CSF synthesize and release ET-1, and that this occurs in parallel with the release of pro-inflammatory cytokines such as IL-1b.128 ET-1 was the target of previous clinical trials targeting large conduit arteries using the ET-1 receptor antagonist clazosentan; however, despite a reduction in large-vessel vasospasm, there was no improvement in long-term functional outcome.11–13 Clazosentan is currently being re-examined in a more focused manner in the REACT trial (clinical trial registration number , clinicaltrials.gov). Although results have not yet been published, the aim of this trial is to identify subgroups of patients which may derive benefit from targeting ET-1.
NO is an alternative target gaining attraction due to its ability to induce vascular dilation via cGMP-dependent relaxation of vSMCs and its involvement in the inflammatory response.129–132 A constant supply of NO is important under normal homeostatic conditions in the maintenance of arteriolar diameter, in addition to preventing the activation of platelets and leukocytes.106 This constitutive production of NO is mostly provided by neuronal nitric oxide synthase (nNOS) and endothelial NOS (eNOS), whereas NO involved in inflammatory processes is mainly produced by inducible NOS (iNOS).127 Immediately following SAH, different mechanisms result in reduced bioavailability of NO, including decreased synthesis, uncoupling of eNOS, endothelial damage, upregulation of endogenous NOS inhibitors (such as asymmetric dimethyl arginine), and sequestration of NO by various byproducts including hemoglobin and reactive oxygen species through a sink effect.106, 116, 131 Free hemoglobin released from dying RBCs can undergo oxidation and serve as a strong NO scavenger in addition to suppression of NO signaling.116 NOS uncoupling refers to a pathological condition by which the NO synthesized by this enzyme reacts with superoxide anion (•O2−) and forms the reactive nitrogen species peroxynitrite (ONOO−) which has toxic effects on lipids, genetic material, and proteins, and contributes to endothelial dysfunction, vasoconstriction, and thrombosis.104, 106 Different NOS isoforms also undergo different changes following SAH – nNOS is primarily downregulated, iNOS is primarily upregulated, and eNOS undergoes complex changes characterized by decreased endothelial expression and increased parenchymal expression.127 Upregulation of iNOS by cells such as microglia or astrocytes can generate large amounts of NO that leads to downstream inflammation and cytotoxicity through uncoupling.127 This suggests that rather than a simplistic model of decreased NO following SAH, the specific enzymes producing NO at a particular time and place may in fact regulate both neuroinflammation and microvascular function.
Therapeutic targeting of NO may thus serve two purposes – reduction of pro-inflammatory mediators and attenuation of microvascular dysfunction. After SAH, infiltrating immune cells such as neutrophils or macrophages may increase production of inflammatory reactive nitrogen species through upregulation of iNOS while impairments in constitutive NO signaling can interfere with microvascular function.127 Attempts to restore the balance of constitutive NO production have been effective in experimental models, including the use of genetic elimination of eNOS and more clinically-relevant NO supplementation using pharmacological NO donors and inhaled NO.56, 133–135 Drugs such as L-arginine and S-nitrosoglutathione showed efficacy in improving outcome after SAH in animal models,136, 137 but were associated with drops in systemic blood pressure.133, 138 However, inhaled NO has limited effects on systemic blood pressure and was shown in rodents to reduce the number and severity of microvascular constrictions with subsequent reduction in mortality and improvement in outcome.133 In SAH patients, NO donors including sodium nitroprusside and transdermal nitroglycerin have been used.139 Some of these studies showed promise; however, they were underpowered and side effects of systemic hypotension, headache, and rebound hypertension limit routine use.139–143
Beyond targeting NO directly, many therapeutic strategies have sought to modulate NO production by interfering with vSMC relaxation in other ways. Phosphodiesterase V (PDE-V) is a key regulator of the eNOS-NO-cGMP pathway that hydrolyzes cGMP and prevents vSMC relaxation and subsequent vasodilation. Inhibition of PDE-V using sildenafil showed promising results in experimental SAH and was recently tested in a phase I safety and proof-of-concept trial.144, 145 Other PDE inhibitors were tested which have more direct effects on vSMCs themselves, including the PDE-III inhibitor milrinone which showed some efficacy at reducing vasospasm and improving outcome (Table 1).146–151 Besides PDE inhibitors, magnesium sulfate showed promise in experimental SAH with reduction in cerebral infarct size, reversal of vasospasm, and improved cerebral perfusion based on its ability to promote vSMC relaxation.152–155 However, two large phase III clinical trials failed to demonstrate clinical benefit.156–158 Some recent studies have suggested that the use of higher dose magnesium sulfate may have some benefit, although this deserves further study.159 Thus, although targeting of vasoconstrictive mediators such as ET-1 did not appear to improve long-term outcome after SAH, perhaps targeting dysfunction of vasodilatory molecules such as NO may prove efficacious.
In addition to ET-1 and NO, additional potent vasomodulators have been described which may serve as therapeutic targets. Such targets include arachidonic acid and its metabolites.160, 161 One of the most well-studied of this family is 20-hydroxyeicosatetraenoic acid (20-HETE), shown to be elevated following SAH in patients and animal models.160–163 Produced by cytochrome P450 enzymes in vSMCs, neurons, and glia, 20-HETE can induce vasoconstriction.160 Mechanistically, 20-HETE levels are increased following loss or scavenging of NO.160, 161 Selective inhibition of 20-HETE synthesis using pharmacological inhibitors reverses delayed vasospasm and improves acute CBF recovery (Table 1).163–165 20-HETE levels are elevated in the CSF of SAH patients, and this elevation is associated with acute and long-term outcomes.166, 167 Another arachidonic acid metabolite, 14,15-epoxyeicosatrienoic acid (14,15-EET), may be protective against the actions of 20-HETE.168 The cumulative data suggests that arachidonic acid metabolites play an active role in SAH pathophysiology, and may offer novel therapeutic targets.
Discussion
Through extensive has become investigation of neuroinflammation and microvascular dysfunction after SAH, it clear that they play an important role in EBI and contribute to poor outcome. These two mechanisms are also tightly linked, as pro-inflammatory signaling can promote disruption of the microvasculature and vice versa (Figure 1). The release of RBC components such as hemoglobin into the subarachnoid space following aneurysmal rupture likely triggers an initial inflammatory reaction by microglia, which secrete numerous pro-inflammatory chemokines and cytokines. These signals increase expression of CAMs on endothelia, drive peripheral leukocyte transmigration, and may also promote microvascular dysfunction. Meanwhile, changes in NO bioavailability coupled with damage to BBB and neurovascular unit dysfunction likely compromise vascular tone regulation and lead to the formation of microthrombi. One exciting area of investigation is the role of cortical spreading depolarizations after SAH, which may be related to both neuroinflammation and microvascular dysfunction.169–171 Taking into account the various mechanistic changes occurring in the brain after SAH, management must be comprehensive and play close attention to acute and delayed brain injury as well as systemic complications. Systemic complications of SAH include hyperglycemia, fever, infection, and dysregulation of coagulation cascades, all of which can influence clinical outcome. Glycosylated hemoglobin, monomeric CRP, and other biochemical mediators have been associated with outcome in other stroke subtypes.172, 173 However, their value in SAH has not been conclusively demonstrated. While large vessel cerebral vasospasm likely contributes to poor outcome, it is no longer believed to be the determining factor and additional studies of neuroinflammation and microvascular dysfunction will likely provide both mechanistic information and therapeutic targets (Table 1).
Despite the evidence of a clear role of neuroinflammation and microvascular dysfunction in poor outcomes after SAH, there are some limitations to current studies. One such limitation is the lack of a standardized animal model of SAH. Studies use a variety of different animal models ranging from autologous blood injection to endovascular perforation models, contributing to variability both within species and between species.30, 174, 175 Within these models, those such as the endovascular perforation model have a relatively high mortality rate, and thus studies may only be conducted on those animals which survive and thus may have limited EBI. The endovascular perforation model provides translational relevance to human SAH by recapitulating a hemorrhagic lesion under arterial pressure but may also have high variability in the location and severity of hemorrhage. The more commonly used blood injection models are easier to control and have lower mortality rates; however, a limitation to these models is that they do not reproduce the complex hemodynamic changes seen in SAH and therefore may have limited EBI.176 One additional shortcoming of using rodent models to investigate the role of inflammation in human SAH is related to differences in immune responses across species, including the major immune cells involved in the response as well as the timeline and major signaling pathways.177, 178 At the level of clinical studies, neuroinflammation and microvascular dysfunction are much more difficult to assess compared to large vessel vasospasm, although studies for various biomarkers in both the peripheral blood and CSF are underway.
Future experimental studies in preclinical models should focus on a multi-pronged effect of targeting both neuroinflammation and microvascular dysfunction to improve outcomes after SAH. While single molecular targets have shown promise in experimental SAH, they have not easily translated to success in clinical trials. Targeting neuroinflammation may alleviate the microvascular dysfunction observed after SAH, as the initial inflammatory response may serve as the primordial factor that contributes to abnormal vascular reactivity and imbalance of endogenous vasodilators and vasoconstrictors. Further, several drugs currently under investigation show promise in targeting both neuroinflammation and microvascular dysfunction, and may be effective in improving outcomes after SAH. Statins were considered an attractive target due to their pleiotropic effects including anti-inflammation, neuroprotection, and increase in eNOS.179–181 However, clinical trials have had less success demonstrating a significant effect on DCI, infarction, or mortality.182–184 More promising results have been obtained with low-dose, unfractionated heparin, which has several biologic effects independent of its anticoagulant properties. By complexing with oxyhemoglobin, heparin can block the formation of free radicals and act as an antagonist to ET-1-mediated vasoconstriction and cytokine-mediated neuroinflammation.185–188 Recently, clinical trials of low-dose unfractionated heparin in SAH patients have shown a favorable safety profile, reduction in DCI without a change in angiographic vasospasm, and improved cognitive outcomes.187, 189 These data have supported the initiation of an ongoing, large-scale, randomized control trial, the Aneurysmal Subarachnoid Hemorrhage Trial Randomizing Heparin (ASTROH, clinical trial registration no. , clinicaltrials.gov).
In summary, a body of evidence supports the notion that the pathophysiology of brain injury in SAH is multifactorial and targeting only one process will likely be insufficient to derive clinical benefit. The complex interplay of microvascular dysfunction and neuroinflammation point to new and exciting areas of current investigation that may result in the development of new therapeutics that reduce long-term impairments after SAH.
Sources of Funding:
Mr. Geraghty receives grant support from the National Institute of Neurological Disorders and Stroke (Grant No. 1F31NS105525–01A1).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Disclosures: None
References
- 1.Suarez JI, Tarr RW and Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med 2006. DOI: 354/4/387 [pii]. [DOI] [PubMed] [Google Scholar]
- 2.Rincon F, Rossenwasser RH and Dumont A. The epidemiology of admissions of nontraumatic subarachnoid hemorrhage in the United States. Neurosurgery 2013. DOI: 10.1227/01.neu.0000430290.93304.33 [doi]. [DOI] [PubMed] [Google Scholar]
- 3.Johnston SC, Selvin S and Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology 1998. [DOI] [PubMed] [Google Scholar]
- 4.Huhtakangas J, Lehto H, Seppa K, et al. Long-Term Excess Mortality After Aneurysmal Subarachnoid Hemorrhage: Patients With Multiple Aneurysms at Risk. Stroke 2015. DOI: 10.1161/STROKEAHA.115.009288 [doi]. [DOI] [PubMed] [Google Scholar]
- 5.Broderick JP, Brott TG, Duldner JE, et al. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 1994. [DOI] [PubMed] [Google Scholar]
- 6.Geraghty JR and Testai FD. Delayed Cerebral Ischemia after Subarachnoid Hemorrhage: Beyond Vasospasm and Towards a Multifactorial Pathophysiology. Curr Atheroscler Rep 2017. DOI: 10.1007/s11883-017-0690-x [doi]. [DOI] [PubMed] [Google Scholar]
- 7.Dorsch NW and King MT. A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage Part I: Incidence and effects. J Clin Neurosci 1994. DOI: 0967-5868(94)90005-1 [pii]. [DOI] [PubMed] [Google Scholar]
- 8.Pegoli M, Mandrekar J, Rabinstein AA, et al. Predictors of excellent functional outcome in aneurysmal subarachnoid hemorrhage. J Neurosurg 2015. DOI: 10.3171/2014.10.JNS14290 [doi]. [DOI] [PubMed] [Google Scholar]
- 9.Crowley RW, Medel R, Dumont AS, et al. Angiographic vasospasm is strongly correlated with cerebral infarction after subarachnoid hemorrhage. Stroke 2011. DOI: 10.1161/STROKEAHA.110.597005 [doi]. [DOI] [PubMed] [Google Scholar]
- 10.Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010. DOI: 10.1161/STROKEAHA.110.589275 [doi]. [DOI] [PubMed] [Google Scholar]
- 11.Macdonald RL, Kassell NF, Mayer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke 2008. DOI: 10.1161/STROKEAHA.108.519942 [doi]. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald RL, Higashida RT, Keller E, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol 2011. DOI: 10.1016/S1474-4422(11)70108-9 [doi]. [DOI] [PubMed] [Google Scholar]
- 13.Meyers PM and Connolly ES Jr. Stroke: disappointing results for clazosentan in CONSCIOUS-2. Nat Rev Neurol 2011. DOI: 10.1038/nrneurol.2011.168 [doi]. [DOI] [PubMed] [Google Scholar]
- 14.McBride DW, Blackburn SL, Peeyush KT, et al. The Role of Thromboinflammation in Delayed Cerebral Ischemia after Subarachnoid Hemorrhage. Front Neurol 2017. DOI: 10.3389/fneur.2017.00555 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Oliveira Manoel AL and Macdonald RL. Neuroinflammation as a Target for Intervention in Subarachnoid Hemorrhage. Front Neurol 2018. DOI: 10.3389/fneur.2018.00292 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucke-Wold BP, Logsdon AF, Manoranjan B, et al. Aneurysmal Subarachnoid Hemorrhage and Neuroinflammation: A Comprehensive Review. Int J Mol Sci 2016. DOI: 10.3390/ijms17040497 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Britz GW, Meno JR, Park IS, et al. Time-dependent alterations in functional and pharmacological arteriolar reactivity after subarachnoid hemorrhage. Stroke 2007. DOI: >01.STR.0000259853.43084.03 [pii]. [DOI] [PubMed] [Google Scholar]
- 18.Kwon MS, Woo SK, Kurland DB, et al. Methemoglobin is an endogenous toll-like receptor 4 ligand-relevance to subarachnoid hemorrhage. Int J Mol Sci 2015. DOI: 10.3390/ijms16035028 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayberg MR, Okada T and Bark DH. The role of hemoglobin in arterial narrowing after subarachnoid hemorrhage. J Neurosurg 1990. DOI: 10.3171/jns.1990.72.4.0634 [doi]. [DOI] [PubMed] [Google Scholar]
- 20.Macdonald RL and Weir BK. A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke 1991. [DOI] [PubMed] [Google Scholar]
- 21.Pyne-Geithman GJ, Morgan CJ, Wagner K, et al. Bilirubin production and oxidation in CSF of patients with cerebral vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab 2005. DOI: 9600101 [pii]. [DOI] [PubMed] [Google Scholar]
- 22.Rapoport RM. Bilirubin Oxidation Products and Cerebral Vasoconstriction. Front Pharmacol 2018. DOI: 10.3389/fphar.2018.00303 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hugelshofer M, Sikorski CM, Seule M, et al. Cell-Free Oxyhemoglobin in Cerebrospinal Fluid after Aneurysmal Subarachnoid Hemorrhage: A Biomarker and Potential Therapeutic Target. World Neurosurg 2018. DOI: S1878-8750(18)31924-7 [pii]. [DOI] [PubMed] [Google Scholar]
- 24.Galea J, Cruickshank G, Teeling JL, et al. The intrathecal CD163-haptoglobin-hemoglobin scavenging system in subarachnoid hemorrhage. J Neurochem 2012. DOI: 10.1111/j.1471-4159.2012.07716.x [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golanov EV, Bovshik EI, Wong KK, et al. Subarachnoid hemorrhage - Induced block of cerebrospinal fluid flow: Role of brain coagulation factor III (tissue factor). J Cereb Blood Flow Metab 2017. DOI: 10.1177/0271678X17701157 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo C, Yao X, Li J, et al. Paravascular pathways contribute to vasculitis and neuroinflammation after subarachnoid hemorrhage independently of glymphatic control. Cell Death Dis 2016. DOI: 10.1038/cddis.2016.63 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanafy KA. The role of microglia and the TLR4 pathway in neuronal apoptosis and vasospasm after subarachnoid hemorrhage. J Neuroinflammation 2013. DOI: 10.1186/1742-2094-10-83 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin S, Yin Q, Zhong Q, et al. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J Neuroinflammation 2012. DOI: 10.1186/1742-2094-9-46 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki H, Hasegawa Y, Kanamaru K, et al. Mitogen-activated protein kinases in cerebral vasospasm after subarachnoid hemorrhage: a review. Acta Neurochir Suppl 2011. DOI: 10.1007/978-3-7091-0353-1_23 [doi]. [DOI] [PubMed] [Google Scholar]
- 30.Kooijman E, Nijboer CH, van Velthoven CT, et al. Long-term functional consequences and ongoing cerebral inflammation after subarachnoid hemorrhage in the rat. PLoS One 2014. DOI: 10.1371/journal.pone.0090584 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider UC, Davids AM, Brandenburg S, et al. Microglia inflict delayed brain injury after subarachnoid hemorrhage. Acta Neuropathol 2015. DOI: 10.1007/s00401-015-1440-1 [doi]. [DOI] [PubMed] [Google Scholar]
- 32.Schallner N, Pandit R, LeBlanc R 3rd,, et al. Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. J Clin Invest 2015. DOI: 10.1172/JCI78443 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu X, Leak RK, Shi Y, et al. Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol 2015. DOI: 10.1038/nrneurol.2014.207 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tobin MK, Bonds JA, Minshall RD, et al. Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab 2014. DOI: 10.1038/jcbfm.2014.130 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muroi C, Hugelshofer M, Seule M, et al. Correlation among systemic inflammatory parameter, occurrence of delayed neurological deficits, and outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery 2013. DOI: 10.1227/NEU.0b013e31828048ce [doi]. [DOI] [PubMed] [Google Scholar]
- 36.McMahon CJ, Hopkins S, Vail A, et al. Inflammation as a predictor for delayed cerebral ischemia after aneurysmal subarachnoid haemorrhage. J Neurointerv Surg 2013. DOI: 10.1136/neurintsurg-2012-010386 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thornton P, Pinteaux E, Gibson RM, et al. Interleukin-1-induced neurotoxicity is mediated by glia and requires caspase activation and free radical release. J Neurochem 2006. DOI: JNC3872 [pii]. [DOI] [PubMed] [Google Scholar]
- 38.Blamire AM, Anthony DC, Rajagopalan B, et al. Interleukin-1beta -induced changes in blood-brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: a magnetic resonance study. J Neurosci 2000. DOI: 20/21/8153 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenhalgh AD, Brough D, Robinson EM, et al. Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology. Dis Model Mech 2012. DOI: 10.1242/dmm.008557 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larysz-Brysz M, Lewin-Kowalik J, Czuba Z, et al. Interleukin-1beta increases release of endothelin-1 and tumor necrosis factor as well as reactive oxygen species by peripheral leukocytes during experimental subarachnoid hemorrhage. Curr Neurovasc Res 2012. DOI: CNR-EPUB-20120523-8 [pii]. [DOI] [PubMed] [Google Scholar]
- 41.Sozen T, Tsuchiyama R, Hasegawa Y, et al. Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke 2009. DOI: 10.1161/STROKEAHA.109.549592 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh N, Hopkins SJ, Hulme S, et al. The effect of intravenous interleukin-1 receptor antagonist on inflammatory mediators in cerebrospinal fluid after subarachnoid haemorrhage: a phase II randomised controlled trial. J Neuroinflammation 2014. DOI: 10.1186/1742-2094-11-1 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galea J, Ogungbenro K, Hulme S, et al. Reduction of inflammation after administration of interleukin-1 receptor antagonist following aneurysmal subarachnoid hemorrhage: results of the Subcutaneous Interleukin-1Ra in SAH (SCIL-SAH) study. J Neurosurg 2017. DOI: 10.3171/2016.9.JNS16615 [doi]. [DOI] [PubMed] [Google Scholar]
- 44.Greenhalgh AD, Brough D, Robinson EM, et al. Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology. Dis Model Mech 2012. DOI: 10.1242/dmm.008557 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okada T, Kawakita F, Nishikawa H, et al. Selective Toll-Like Receptor 4 Antagonists Prevent Acute Blood-Brain Barrier Disruption After Subarachnoid Hemorrhage in Mice. Mol Neurobiol 2018. DOI: 10.1007/s12035-018-1145-2 [doi]. [DOI] [PubMed] [Google Scholar]
- 46.Kasuya H and Shimizu T. Activated complement components C3a and C4a in cerebrospinal fluid and plasma following subarachnoid hemorrhage. J Neurosurg 1989. DOI: 10.3171/jns.1989.71.5.0741 [doi]. [DOI] [PubMed] [Google Scholar]
- 47.Mack WJ, Ducruet AF, Hickman ZL, et al. Early plasma complement C3a levels correlate with functional outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery 2007. DOI: 10.1227/01.NEU.0000255518.96837.8E [doi]. [DOI] [PubMed] [Google Scholar]
- 48.Llull L, Thiel S, Amaro S, et al. Ficolin-1 Levels in Patients Developing Vasospasm and Cerebral Ischemia After Spontaneous Subarachnoid Hemorrhage. Mol Neurobiol 2017. DOI: 10.1007/s12035-016-0180-0 [doi]. [DOI] [PubMed] [Google Scholar]
- 49.Sandgaard E, Troldborg A, Lauridsen SV, et al. Changes in the Lectin Pathway Following Intracerebral or Spontaneous Subarachnoid Hemorrhage. Mol Neurobiol 2018. DOI: 10.1007/s12035-018-1066-0 [doi]. [DOI] [PubMed] [Google Scholar]
- 50.Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012. DOI: 10.1016/j.neuron.2012.03.026 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong S, Beja-Glasser VF, Nfonoyim BM, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016. DOI: 10.1126/science.aad8373 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevens B, Allen NJ, Vazquez LE, et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007. DOI: S0092-8674(07)01355-4 [pii]. [DOI] [PubMed] [Google Scholar]
- 53.Schartz ND, Wyatt-Johnson SK, Price LR, et al. Status epilepticus triggers long-lasting activation of complement C1q-C3 signaling in the hippocampus that correlates with seizure frequency in experimental epilepsy. Neurobiol Dis 2018. DOI: S0969-9961(17)30239-5 [pii]. [DOI] [PubMed] [Google Scholar]
- 54.Farooqui AA. N-3 Fatty Acid-Derived Lipid Mediators in the Brain: New Weapons Against Oxidative Stress and Inflammation. Curr Med Chem 2012. DOI: BSP/CMC/E-Pub/2012/044 [pii]. [DOI] [PubMed] [Google Scholar]
- 55.Garcia JM, Stillings SA, Leclerc JL, et al. Role of Interleukin-10 in Acute Brain Injuries. Front Neurol 2017. DOI: 10.3389/fneur.2017.00244 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pradilla G, Chaichana KL, Hoang S, et al. Inflammation and cerebral vasospasm after subarachnoid hemorrhage. Neurosurg Clin N Am 2010. DOI: 10.1016/j.nec.2009.10.008 [doi]. [DOI] [PubMed] [Google Scholar]
- 57.Tso MK and Macdonald RL. Acute microvascular changes after subarachnoid hemorrhage and transient global cerebral ischemia. Stroke Res Treat 2013. DOI: 10.1155/2013/425281 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frijns CJ and Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke 2002. [DOI] [PubMed] [Google Scholar]
- 59.Polin RS, Bavbek M, Shaffrey ME, et al. Detection of soluble E-selectin, ICAM-1, VCAM-1, and L-selectin in the cerebrospinal fluid of patients after subarachnoid hemorrhage. J Neurosurg 1998. DOI: 10.3171/jns.1998.89.4.0559 [doi]. [DOI] [PubMed] [Google Scholar]
- 60.Mocco J, Mack WJ, Kim GH, et al. Rise in serum soluble intercellular adhesion molecule-1 levels with vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg 2002. DOI: 10.3171/jns.2002.97.3.0537 [doi]. [DOI] [PubMed] [Google Scholar]
- 61.Kaynar MY, Tanriverdi T, Kafadar AM, et al. Detection of soluble intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in both cerebrospinal fluid and serum of patients after aneurysmal subarachnoid hemorrhage. J Neurosurg 2004. DOI: 10.3171/jns.2004.101.6.1030 [doi]. [DOI] [PubMed] [Google Scholar]
- 62.Rothoerl RD, Schebesch KM, Kubitza M, et al. ICAM-1 and VCAM-1 expression following aneurysmal subarachnoid hemorrhage and their possible role in the pathophysiology of subsequent ischemic deficits. Cerebrovasc Dis 2006. DOI: 93243 [pii]. [DOI] [PubMed] [Google Scholar]
- 63.Clatterbuck RE, Oshiro EM, Hoffman PA, et al. Inhibition of vasospasm with lymphocyte function-associated antigen-1 monoclonal antibody in a femoral artery model in rats. J Neurosurg 2002. DOI: 10.3171/jns.2002.97.3.0676 [doi]. [DOI] [PubMed] [Google Scholar]
- 64.Pradilla G, Wang PP, Legnani FG, et al. Prevention of vasospasm by anti-CD11/CD18 monoclonal antibody therapy following subarachnoid hemorrhage in rabbits. J Neurosurg 2004. DOI: 10.3171/jns.2004.101.1.0088 [doi]. [DOI] [PubMed] [Google Scholar]
- 65.Bavbek M, Polin R, Kwan AL, et al. Monoclonal antibodies against ICAM-1 and CD18 attenuate cerebral vasospasm after experimental subarachnoid hemorrhage in rabbits. Stroke 1998. [DOI] [PubMed] [Google Scholar]
- 66.Clatterbuck RE, Gailloud P, Ogata L, et al. Prevention of cerebral vasospasm by a humanized anti-CD11/CD18 monoclonal antibody administered after experimental subarachnoid hemorrhage in nonhuman primates. J Neurosurg 2003. DOI: 10.3171/jns.2003.99.2.0376 [doi]. [DOI] [PubMed] [Google Scholar]
- 67.Xu HL, Garcia M, Testai F, et al. Pharmacologic blockade of vascular adhesion protein-1 lessens neurologic dysfunction in rats subjected to subarachnoid hemorrhage. Brain Res 2014. DOI: 10.1016/j.brainres.2014.08.036 [doi]. [DOI] [PubMed] [Google Scholar]
- 68.Provencio JJ, Altay T, Smithason S, et al. Depletion of Ly6G/C(+) cells ameliorates delayed cerebral vasospasm in subarachnoid hemorrhage. J Neuroimmunol 2011. DOI: 10.1016/j.jneuroim.2010.10.016 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu H, Testai FD, Valyi-Nagy T, et al. VAP-1 blockade prevents subarachnoid hemorrhage-associated cerebrovascular dilating dysfunction via repression of a neutrophil recruitment-related mechanism. Brain Res 2015. DOI: 10.1016/j.brainres.2015.01.047 [doi]. [DOI] [PubMed] [Google Scholar]
- 70.Friedrich V, Flores R, Muller A, et al. Reduction of neutrophil activity decreases early microvascular injury after subarachnoid haemorrhage. J Neuroinflammation 2011. DOI: 10.1186/1742-2094-8-103 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Provencio JJ, Swank V, Lu H, et al. Neutrophil depletion after subarachnoid hemorrhage improves memory via NMDA receptors. Brain Behav Immun 2016. DOI: 10.1016/j.bbi.2016.02.007 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Provencio JJ, Fu X, Siu A, et al. CSF neutrophils are implicated in the development of vasospasm in subarachnoid hemorrhage. Neurocrit Care 2010. DOI: 10.1007/s12028-009-9308-7 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jackowski A, Crockard A, Burnstock G, et al. The time course of intracranial pathophysiological changes following experimental subarachnoid haemorrhage in the rat. J Cereb Blood Flow Metab 1990. DOI: 10.1038/jcbfm.1990.140 [doi]. [DOI] [PubMed] [Google Scholar]
- 74.Schneider UC, Schiffler J, Hakiy N, et al. Functional analysis of Pro-inflammatory properties within the cerebrospinal fluid after subarachnoid hemorrhage in vivo and in vitro. J Neuroinflammation 2012. DOI: 10.1186/1742-2094-9-28 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bennett ML, Bennett FC, Liddelow SA, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A 2016. DOI: 10.1073/pnas.1525528113 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spratt DE, Reddy VK, Choxi AA, et al. Dexamethasone significantly attenuates sub-arachnoid hemorrhage-induced elevation in cerebrospinal fluid citrulline and leukocytes. J Neurosurg Sci 2012. DOI: R38122281 [pii]. [PubMed] [Google Scholar]
- 77.Gomis P, Tran-Dinh YR, Sercombe C, et al. Dexamethasone preventing contractile and cytoskeletal protein changes in the rabbit basilar artery after subarachnoid hemorrhage. J Neurosurg 2005. DOI: 10.3171/jns.2005.102.4.0715 [doi]. [DOI] [PubMed] [Google Scholar]
- 78.Chyatte D Prevention of chronic cerebral vasospasm in dogs with ibuprofen and high-dose methylprednisolone. Stroke 1989. [DOI] [PubMed] [Google Scholar]
- 79.Peterson JW, Nishizawa S, Hackett JD, et al. Cyclosporine A reduces cerebral vasospasm after subarachnoid hemorrhage in dogs. Stroke 1990. [DOI] [PubMed] [Google Scholar]
- 80.Dai Y, Sun Q, Zhang X, et al. Cyclosporin A ameliorates early brain injury after subarachnoid hemorrhage through inhibition of a Nur77 dependent apoptosis pathway. Brain Res 2014. DOI: 10.1016/j.brainres.2014.01.052 [doi]. [DOI] [PubMed] [Google Scholar]
- 81.Pan P, Zhang X, Li Q, et al. Cyclosporine A alleviated matrix metalloproteinase 9 associated blood-brain barrier disruption after subarachnoid hemorrhage in mice. Neurosci Lett 2017. DOI: S0304-3940(17)30280-X [pii]. [DOI] [PubMed] [Google Scholar]
- 82.Feigin VL, Anderson N, Rinkel GJ, et al. Corticosteroids for aneurysmal subarachnoid haemorrhage and primary intracerebral haemorrhage. Cochrane Database Syst Rev 2005. DOI: 10.1002/14651858.CD004583.pub2 [doi]. [DOI] [PubMed] [Google Scholar]
- 83.Ryba M, Pastuszko M, Iwanska K, et al. Cyclosporine A prevents neurological deterioration of patients with SAH--a preliminary report. Acta Neurochir (Wien) 1991. [DOI] [PubMed] [Google Scholar]
- 84.Manno EM, Gress DR, Ogilvy CS, et al. The safety and efficacy of cyclosporine A in the prevention of vasospasm in patients with Fisher grade 3 subarachnoid hemorrhages: a pilot study. Neurosurgery 1997. [DOI] [PubMed] [Google Scholar]
- 85.Xu HL, Pelligrino DA, Paisansathan C, et al. Protective role of fingolimod (FTY720) in rats subjected to subarachnoid hemorrhage. J Neuroinflammation 2015. DOI: 10.1186/s12974-015-0234-7 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li W, Xu H and Testai FD. Mechanism of Action and Clinical Potential of Fingolimod for the Treatment of Stroke. Front Neurol 2016. DOI: 10.3389/fneur.2016.00139 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chun J and Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 2010. DOI: 10.1097/WNF.0b013e3181cbf825 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baer A, Colon-Moran W and Bhattarai N. Characterization of the effects of immunomodulatory drug fingolimod (FTY720) on human T cell receptor signaling pathways. Sci Rep 2018. DOI: 10.1038/s41598-018-29355-0 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Groves A, Kihara Y and Chun J. Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci 2013. DOI: 10.1016/j.jns.2013.02.011 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brunkhorst R, Vutukuri R and Pfeilschifter W. Fingolimod for the treatment of neurological diseases-state of play and future perspectives. Front Cell Neurosci 2014. DOI: 10.3389/fncel.2014.00283 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tao C, Wang J, Hu X, et al. Clinical Value of Neutrophil to Lymphocyte and Platelet to Lymphocyte Ratio After Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 2017. DOI: 10.1007/s12028-016-0332-0 [doi]. [DOI] [PubMed] [Google Scholar]
- 92.Lattanzi S, Cagnetti C, Provinciali L, et al. Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. [doi]. of acute intracerebral hemorrhage. J Neurol Sci 2018. DOI: S0022–510X(18)30054–6 [pii]. Oncotarget 2017. DOI: 10.18632/oncotarget.15423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lattanzi S, Cagnetti C, Rinaldi C, et al. Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neurol Sci 2018. DOI: S0022-510X(18)30054-6 [pii]. [DOI] [PubMed] [Google Scholar]
- 94.Crowley RW, Medel R, Dumont AS, et al. Angiographic vasospasm is strongly correlated with cerebral infarction after subarachnoid hemorrhage. Stroke 2011. DOI: 10.1161/STROKEAHA.110.597005 [doi]. [DOI] [PubMed] [Google Scholar]
- 95.Hijdra A, Van Gijn J, Stefanko S, et al. Delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: clinicoanatomic correlations. Neurology 1986. [DOI] [PubMed] [Google Scholar]
- 96.Vergouwen MD, Ilodigwe D and Macdonald RL. Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke 2011. DOI: 10.1161/STROKEAHA.110.597914 [doi]. [DOI] [PubMed] [Google Scholar]
- 97.Schubert GA, Schilling L and Thome C. Clazosentan, an endothelin receptor antagonist, prevents early hypoperfusion during the acute phase of massive experimental subarachnoid hemorrhage: a laser Doppler flowmetry study in rats. J Neurosurg 2008. DOI: 10.3171/JNS.2008.109.12.1134 [doi]. [DOI] [PubMed] [Google Scholar]
- 98.Chen G, Tariq A, Ai J, et al. Different effects of clazosentan on consequences of subarachnoid hemorrhage in rats. Brain Res 2011. DOI: 10.1016/j.brainres.2011.03.068 [doi]. [DOI] [PubMed] [Google Scholar]
- 99.Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012. DOI: 10.1161/STR.0b013e3182587839 [doi]. [DOI] [PubMed] [Google Scholar]
- 100.Wellman GC and Koide M. Impact of subarachnoid hemorrhage on parenchymal arteriolar function. Acta Neurochir Suppl 2013. DOI: 10.1007/978-3-7091-1192-5_33 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hanggi D, Etminan N, Mayer SA, et al. Clinical Trial Protocol: Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy, and Safety Study Comparing EG-1962 to Standard of Care Oral Nimodipine in Adults with Aneurysmal Subarachnoid Hemorrhage [NEWTON-2 (Nimodipine Microparticles to Enhance Recovery While Reducing TOxicity After SubarachNoid Hemorrhage). Neurocrit Care 2018. DOI: 10.1007/s12028-018-0575-z [doi]. [DOI] [PubMed] [Google Scholar]
- 102.Edge Therapeutics I Edge Therapeutics Provides Update following Interim Analysis of Phase 3 NEWTON 2 Study of EG-1962 in Aneurysmal Subarachnoid Hemorrhage, http://investors.edgetherapeutics.com/phoenix.zhtml?c=253911&p=irol-newsArticle&ID=2340075 (2018, accessed January 28, 2019).
- 103.Friedrich B, Muller F, Feiler S, et al. Experimental subarachnoid hemorrhage causes early and long-lasting microarterial constriction and microthrombosis: an in-vivo microscopy study. J Cereb Blood Flow Metab 2012. DOI: 10.1038/jcbfm.2011.154 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ostergaard L, Aamand R, Karabegovic S, et al. The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J Cereb Blood Flow Metab 2013. DOI: 10.1038/jcbfm.2013.173 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sabri M, Ai J, Lakovic K, et al. Mechanisms of microthrombosis and microcirculatory constriction after experimental subarachnoid hemorrhage. Acta Neurochir Suppl 2013. DOI: 10.1007/978-3-7091-1192-5_35 [doi]. [DOI] [PubMed] [Google Scholar]
- 106.Sehba FA and Friedrich V. Cerebral microvasculature is an early target of subarachnoid hemorrhage. Acta Neurochir Suppl 2013. DOI: 10.1007/978-3-7091-1192-5_37 [doi]. [DOI] [PubMed] [Google Scholar]
- 107.Phillips AA, Chan FH, Zheng MM, et al. Neurovascular coupling in humans: Physiology, methodological advances and clinical implications. J Cereb Blood Flow Metab 2016. DOI: 10.1177/0271678X15617954 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pappas AC, Koide M and Wellman GC. Astrocyte Ca2+ Signaling Drives Inversion of Neurovascular Coupling after Subarachnoid Hemorrhage. J Neurosci 2015. DOI: 10.1523/JNEUROSCI.1551-15.2015 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Balbi M, Koide M, Wellman GC, et al. Inversion of neurovascular coupling after subarachnoid hemorrhage in vivo. J Cereb Blood Flow Metab 2017. DOI: 10.1177/0271678X16686595 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Germano A, d’Avella D, Cicciarello R, et al. Blood-brain barrier permeability changes after experimental subarachnoid hemorrhage. Neurosurgery 1992. [DOI] [PubMed] [Google Scholar]
- 111.Frontera JA, Provencio JJ, Sehba FA, et al. The Role of Platelet Activation and Inflammation in Early Brain Injury Following Subarachnoid Hemorrhage. Neurocrit Care 2017. DOI: 10.1007/s12028-016-0292-4 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.El Amki M, Dubois M, Lefevre-Scelles A, et al. Long-Lasting Cerebral Vasospasm, Microthrombosis, Apoptosis and Paravascular Alterations Associated with Neurological Deficits in a Mouse Model of Subarachnoid Hemorrhage. Mol Neurobiol 2017. DOI: 10.1007/s12035-017-0514-6 [doi]. [DOI] [PubMed] [Google Scholar]
- 113.Sabri M, Ai J, Lakovic K, et al. Mechanisms of microthrombi formation after experimental subarachnoid hemorrhage. Neuroscience 2012. DOI: 10.1016/j.neuroscience.2012.08.002 [doi]. [DOI] [PubMed] [Google Scholar]
- 114.Sweeney MD, Ayyadurai S and Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 2016. DOI: 10.1038/nn.4288 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Winkler EA, Birk H, Burkhardt JK, et al. Reductions in brain pericytes are associated with arteriovenous malformation vascular instability. J Neurosurg 2018. DOI: 10.3171/2017.6.JNS17860 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li Q, Chen Y, Li B, et al. Hemoglobin induced NO/cGMP suppression Deteriorate Microcirculation via Pericyte Phenotype Transformation after Subarachnoid Hemorrhage in Rats. Sci Rep 2016. DOI: 10.1038/srep22070 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pluta RM, Boock RJ, Afshar JK, et al. Source and cause of endothelin-1 release into cerebrospinal fluid after subarachnoid hemorrhage. J Neurosurg 1997. DOI: 10.3171/jns.1997.87.2.0287 [doi]. [DOI] [PubMed] [Google Scholar]
- 118.Sabri M, Kawashima A, Ai J, et al. Neuronal and astrocytic apoptosis after subarachnoid hemorrhage: a possible cause for poor prognosis. Brain Res 2008. DOI: 10.1016/j.brainres.2008.08.031 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kasseckert SA, Shahzad T, Miqdad M, et al. The mechanisms of energy crisis in human astrocytes after subarachnoid hemorrhage. Neurosurgery 2013. DOI: 10.1227/NEU.0b013e31827d0de7 [doi]. [DOI] [PubMed] [Google Scholar]
- 120.Sehba FA, Mostafa G, Knopman J, et al. Acute alterations in microvascular basal lamina after subarachnoid hemorrhage. J Neurosurg 2004. DOI: 10.3171/jns.2004.101.4.0633 [doi]. [DOI] [PubMed] [Google Scholar]
- 121.Hayman EG, Wessell A, Gerzanich V, et al. Mechanisms of Global Cerebral Edema Formation in Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 2017. DOI: 10.1007/s12028-016-0354-7 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rempe RG, Hartz AM and Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J Cereb Blood Flow Metab 2016. DOI: 10.1177/0271678X16655551 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fischer M, Dietmann A, Beer R, et al. Differential regulation of matrix-metalloproteinases and their tissue inhibitors in patients with aneurysmal subarachnoid hemorrhage. PLoS One 2013. DOI: 10.1371/journal.pone.0059952 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Triglia T, Mezzapesa A, Martin JC, et al. Early matrix metalloproteinase-9 concentration in the first 48 h after aneurysmal subarachnoid haemorrhage predicts delayed cerebral ischaemia: An observational study. Eur J Anaesthesiol 2016. DOI: 10.1097/EJA.0000000000000494 [doi]. [DOI] [PubMed] [Google Scholar]
- 125.Chou SH, Feske SK, Simmons SL, et al. Elevated peripheral neutrophils and matrix metalloproteinase 9 as biomarkers of functional outcome following subarachnoid hemorrhage. Transl Stroke Res 2011. DOI: 10.1007/s12975-011-0117-x [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tosun C, Kurland DB, Mehta R, et al. Inhibition of the Sur1-Trpm4 channel reduces neuroinflammation and cognitive impairment in subarachnoid hemorrhage. Stroke 2013. DOI: 10.1161/STROKEAHA.113.002904 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Iqbal S, Hayman EG, Hong C, et al. Inducible nitric oxide synthase (NOS-2) in subarachnoid hemorrhage: Regulatory mechanisms and therapeutic implications. Brain Circ 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fassbender K, Hodapp B, Rossol S, et al. Endothelin-1 in subarachnoid hemorrhage: An acute-phase reactant produced by cerebrospinal fluid leukocytes. Stroke 2000. [DOI] [PubMed] [Google Scholar]
- 129.Schwartz AY, Sehba FA and Bederson JB. Decreased nitric oxide availability contributes to acute cerebral ischemia after subarachnoid hemorrhage. Neurosurgery 2000. [DOI] [PubMed] [Google Scholar]
- 130.Sehba FA and Bederson JB. Nitric oxide in early brain injury after subarachnoid hemorrhage. Acta Neurochir Suppl 2011. DOI: 10.1007/978-3-7091-0353-1_18 [doi]. [DOI] [PubMed] [Google Scholar]
- 131.Vellimana AK, Milner E, Azad TD, et al. Endothelial nitric oxide synthase mediates endogenous protection against subarachnoid hemorrhage-induced cerebral vasospasm. Stroke 2011. DOI: 10.1161/STROKEAHA.110.607200 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garry PS, Ezra M, Rowland MJ, et al. The role of the nitric oxide pathway in brain injury and its treatment--from bench to bedside. Exp Neurol 2015. DOI: 10.1016/j.expneurol.2014.10.017 [doi]. [DOI] [PubMed] [Google Scholar]
- 133.Terpolilli NA, Feiler S, Dienel A, et al. Nitric oxide inhalation reduces brain damage, prevents mortality, and improves neurological outcome after subarachnoid hemorrhage by resolving early pial microvasospasms. J Cereb Blood Flow Metab 2016. DOI: 0271678X15605848 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sabri M, Ai J, Lass E, et al. Genetic elimination of eNOS reduces secondary complications of experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab 2013. DOI: 10.1038/jcbfm.2013.49 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sehba FA, Friedrich V Jr, Makonnen G, et al. Acute cerebral vascular injury after subarachnoid hemorrhage and its prevention by administration of a nitric oxide donor. J Neurosurg 2007. DOI: 10.3171/jns.2007.106.2.321 [doi]. [DOI] [PubMed] [Google Scholar]
- 136.Pluta RM, Oldfield EH and Boock RJ. Reversal and prevention of cerebral vasospasm by intracarotid infusions of nitric oxide donors in a primate model of subarachnoid hemorrhage. J Neurosurg 1997. DOI: 10.3171/jns.1997.87.5.0746 [doi]. [DOI] [PubMed] [Google Scholar]
- 137.Sehba FA, Ding WH, Chereshnev I, et al. Effects of S-nitrosoglutathione on acute vasoconstriction and glutamate release after subarachnoid hemorrhage. Stroke 1999. [DOI] [PubMed] [Google Scholar]
- 138.Bath PM, Krishnan K and Appleton JP. Nitric oxide donors (nitrates), L-arginine, or nitric oxide synthase inhibitors for acute stroke. Cochrane Database Syst Rev 2017. DOI: 10.1002/14651858.CD000398.pub2 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Siuta M, Zuckerman SL and Mocco J. Nitric oxide in cerebral vasospasm: theories, measurement, and treatment. Neurol Res Int 2013. DOI: 10.1155/2013/972417 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reinert M, Wiest R, Barth L, et al. Transdermal nitroglycerin in patients with subarachnoid hemorrhage. Neurol Res 2004. DOI: 10.1179/016164104225015976 [doi]. [DOI] [PubMed] [Google Scholar]
- 141.Pachl J, Haninec P, Tencer T, et al. The effect of subarachnoid sodium nitroprusside on the prevention of vasospasm in subarachnoid haemorrhage. Acta Neurochir Suppl 2005. [DOI] [PubMed] [Google Scholar]
- 142.Raabe A, Zimmermann M, Setzer M, et al. Effect of intraventricular sodium nitroprusside on cerebral hemodynamics and oxygenation in poor-grade aneurysm patients with severe, medically refractory vasospasm. Neurosurgery 2002. [DOI] [PubMed] [Google Scholar]
- 143.Lesley WS, Lazo A, Chaloupka JC, et al. Successful treatment of cerebral vasospasm by use of transdermal nitroglycerin ointment (Nitropaste). AJNR Am J Neuroradiol 2003. [PMC free article] [PubMed] [Google Scholar]
- 144.Washington CW, Derdeyn CP, Dhar R, et al. A Phase I proof-of-concept and safety trial of sildenafil to treat cerebral vasospasm following subarachnoid hemorrhage. J Neurosurg 2016. DOI: 10.3171/2015.2.JNS142752 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Han BH, Vellimana AK, Zhou ML, et al. Phosphodiesterase 5 inhibition attenuates cerebral vasospasm and improves functional recovery after experimental subarachnoid hemorrhage. Neurosurgery 2012. DOI: 10.1227/NEU.0b013e31822ec2b0 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Khajavi K, Ayzman I, Shearer D, et al. Prevention of chronic cerebral vasospasm in dogs with milrinone. Neurosurgery 1997. [DOI] [PubMed] [Google Scholar]
- 147.Arakawa Y, Kikuta K, Hojo M, et al. Milrinone for the treatment of cerebral vasospasm after subarachnoid hemorrhage: report of seven cases. Neurosurgery 2001. [DOI] [PubMed] [Google Scholar]
- 148.Fraticelli AT, Cholley BP, Losser MR, et al. Milrinone for the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 2008. DOI: 10.1161/STROKEAHA.107.492447 [doi]. [DOI] [PubMed] [Google Scholar]
- 149.Romero CM, Morales D, Reccius A, et al. Milrinone as a rescue therapy for symptomatic refractory cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Neurocrit Care 2009. DOI: 10.1007/s12028-008-9048-0 [doi]. [DOI] [PubMed] [Google Scholar]
- 150.Lannes M, Teitelbaum J, del Pilar Cortes M, et al. Milrinone and homeostasis to treat cerebral vasospasm associated with subarachnoid hemorrhage: the Montreal Neurological Hospital protocol. Neurocrit Care 2012. DOI: 10.1007/s12028-012-9701-5 [doi]. [DOI] [PubMed] [Google Scholar]
- 151.Mutoh T, Mutoh T, Sasaki K, et al. Neurocardiac protection with milrinone for restoring acute cerebral hypoperfusion and delayed ischemic injury after experimental subarachnoid hemorrhage. Neurosci Lett 2017. DOI: S0304-3940(17)30018-6 [pii]. [DOI] [PubMed] [Google Scholar]
- 152.Pyne GJ, Cadoux-Hudson TA and Clark JF. Magnesium protection against in vitro cerebral vasospasm after subarachnoid haemorrhage. Br J Neurosurg 2001. [DOI] [PubMed] [Google Scholar]
- 153.Ram Z, Sadeh M, Shacked I, et al. Magnesium sulfate reverses experimental delayed cerebral vasospasm after subarachnoid hemorrhage in rats. Stroke 1991. [DOI] [PubMed] [Google Scholar]
- 154.Mori K, Miyazaki M, Iwata J, et al. Intracisternal infusion of magnesium sulfate solution improved reduced cerebral blood flow induced by experimental subarachnoid hemorrhage in the rat. Neurosurg Rev 2008. DOI: 10.1007/s10143-008-0122-z [doi]. [DOI] [PubMed] [Google Scholar]
- 155.Mori K, Miyazaki M, Hara Y, et al. Novel vasodilatory effect of intracisternal injection of magnesium sulfate solution on spastic cerebral arteries in the canine two-hemorrhage model of subarachnoid hemorrhage. J Neurosurg 2009. DOI: 10.3171/2008.4.17494 [doi]. [DOI] [PubMed] [Google Scholar]
- 156.Dorhout Mees SM, Algra A, Wong GK, et al. Early Magnesium Treatment After Aneurysmal Subarachnoid Hemorrhage: Individual Patient Data Meta-Analysis. Stroke 2015. DOI: 10.1161/STROKEAHA.115.010575 [doi]. [DOI] [PubMed] [Google Scholar]
- 157.Dorhout Mees SM, Algra A, Vandertop WP, et al. Magnesium for aneurysmal subarachnoid haemorrhage (MASH-2): a randomised placebo-controlled trial. Lancet 2012. DOI: 10.1016/S0140-6736(12)60724-7 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wong GK, Poon WS, Chan MT, et al. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage (IMASH): a randomized, double-blinded, placebo-controlled, multicenter phase III trial. Stroke 2010. DOI: 10.1161/STROKEAHA.109.571125 [doi]. [DOI] [PubMed] [Google Scholar]
- 159.Kunze E, Lilla N, Stetter C, et al. Magnesium Protects in Episodes of Critical Perfusion after Aneurysmal SAH. Transl Neurosci 2018. DOI: 10.1515/tnsci-2018-0016 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fan F, Ge Y, Lv W, et al. Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front Biosci (Landmark Ed) 2016. DOI: 4465 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Takeuchi K, Miyata N, Renic M, et al. Hemoglobin, NO, and 20-HETE interactions in mediating cerebral vasoconstriction following SAH. Am J Physiol Regul Integr Comp Physiol 2006. DOI: 00445.2005 [pii]. [DOI] [PubMed] [Google Scholar]
- 162.Kehl F, Cambj-Sapunar L, Maier KG, et al. 20-HETE contributes to the acute fall in cerebral blood flow after subarachnoid hemorrhage in the rat. Am J Physiol Heart Circ Physiol 2002. DOI: 10.1152/ajpheart.00924.2001 [doi]. [DOI] [PubMed] [Google Scholar]
- 163.Hacein-Bey L, Harder DR, Meier HT, et al. Reversal of delayed vasospasm by TS-011 in the dual hemorrhage dog model of subarachnoid hemorrhage. AJNR Am J Neuroradiol 2006. DOI: 27/6/1350 [pii]. [PMC free article] [PubMed] [Google Scholar]
- 164.Miyata N, Seki T, Tanaka Y, et al. Beneficial effects of a new 20-hydroxyeicosatetraenoic acid synthesis inhibitor, TS-011 [N-(3-chloro-4-morpholin-4-yl) phenyl-N’-hydroxyimido formamide], on hemorrhagic and ischemic stroke. J Pharmacol Exp Ther 2005. DOI: jpet.105.083964 [pii]. [DOI] [PubMed] [Google Scholar]
- 165.Takeuchi K, Renic M, Bohman QC, et al. Reversal of delayed vasospasm by an inhibitor of the synthesis of 20-HETE. Am J Physiol Heart Circ Physiol 2005. DOI: 00556.2005 [pii]. [DOI] [PubMed] [Google Scholar]
- 166.Donnelly MK, Crago EA, Conley YP, et al. 20-HETE is associated with unfavorable outcomes in subarachnoid hemorrhage patients. J Cereb Blood Flow Metab 2015. DOI: 10.1038/jcbfm.2015.75 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Crago EA, Thampatty BP, Sherwood PR, et al. Cerebrospinal fluid 20-HETE is associated with delayed cerebral ischemia and poor outcomes after aneurysmal subarachnoid hemorrhage. Stroke 2011. DOI: 10.1161/STROKEAHA.110.605816 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Siler DA, Martini RP, Ward JP, et al. Protective role of p450 epoxyeicosanoids in subarachnoid hemorrhage. Neurocrit Care 2015. DOI: 10.1007/s12028-014-0011-y [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dreier JP, Woitzik J, Fabricius M, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain 2006. DOI: awl297 [pii]. [DOI] [PubMed] [Google Scholar]
- 170.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 2011. DOI: 10.1038/nm.2333 [doi]. [DOI] [PubMed] [Google Scholar]
- 171.Shibata M and Suzuki N. Exploring the role of microglia in cortical spreading depression in neurological disease. J Cereb Blood Flow Metab 2017. DOI: 10.1177/0271678X17690537 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Di Napoli M, Slevin M, Popa-Wagner A, et al. Monomeric C-Reactive Protein and Cerebral Hemorrhage: From Bench to Bedside. Front Immunol 2018. DOI: 10.3389/fimmu.2018.01921 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lattanzi S, Bartolini M, Provinciali L, et al. Glycosylated Hemoglobin and Functional Outcome after Acute Ischemic Stroke. J Stroke Cerebrovasc Dis 2016. DOI: S1052-3057(16)00160-9 [pii]. [DOI] [PubMed] [Google Scholar]
- 174.Lee JY, Sagher O, Keep R, et al. Comparison of experimental rat models of early brain injury after subarachnoid hemorrhage. Neurosurgery 2009. DOI: 10.1227/01.NEU.0000345649.78556.26 [doi]. [DOI] [PubMed] [Google Scholar]
- 175.Prunell GF, Mathiesen T, Diemer NH, et al. Experimental subarachnoid hemorrhage: subarachnoid blood volume, mortality rate, neuronal death, cerebral blood flow, and perfusion pressure in three different rat models. Neurosurgery 2003. [DOI] [PubMed] [Google Scholar]
- 176.Leclerc JL, Garcia JM, Diller MA, et al. A Comparison of Pathophysiology in Humans and Rodent Models of Subarachnoid Hemorrhage. Front Mol Neurosci 2018. DOI: 10.3389/fnmol.2018.00071 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mestas J and Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 2004. [DOI] [PubMed] [Google Scholar]
- 178.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 2013. DOI: 10.1073/pnas.1222878110 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ayer RE, Ostrowski RP, Sugawara T, et al. Statin-induced T-lymphocyte modulation and neuroprotection following experimental subarachnoid hemorrhage. Acta Neurochir Suppl 2013. DOI: 10.1007/978-3-7091-1192-5_46 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sugawara T, Ayer R, Jadhav V, et al. Simvastatin attenuation of cerebral vasospasm after subarachnoid hemorrhage in rats via increased phosphorylation of Akt and endothelial nitric oxide synthase. J Neurosci Res 2008. DOI: 10.1002/jnr.21807 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sabri M, Ai J, Marsden PA, et al. Simvastatin re-couples dysfunctional endothelial nitric oxide synthase in experimental subarachnoid hemorrhage. PLoS One 2011. DOI: 10.1371/journal.pone.0017062 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kirkpatrick PJ, Turner CL, Smith C, et al. Simvastatin in aneurysmal subarachnoid haemorrhage (STASH): a multicentre randomised phase 3 trial. Lancet Neurol 2014. DOI: 10.1016/S1474-4422(14)70084-5 [doi]. [DOI] [PubMed] [Google Scholar]
- 183.Naraoka M, Matsuda N, Shimamura N, et al. Long-acting statin for aneurysmal subarachnoid hemorrhage: A randomized, double-blind, placebo-controlled trial. J Cereb Blood Flow Metab 2018. DOI: 10.1177/0271678X17724682 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shen J, Huang KY, Zhu Y, et al. Effect of statin treatment on vasospasm-related morbidity and functional outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurosurg 2017. DOI: 10.3171/2016.5.JNS152900 [doi]. [DOI] [PubMed] [Google Scholar]
- 185.Khattar NK and James RF. Heparin: The Silver Bullet of Aneurysmal Subarachnoid Hemorrhage?. Front Neurol 2018. DOI: 10.3389/fneur.2018.00097 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Simard JM, Tosun C, Ivanova S, et al. Heparin reduces neuroinflammation and transsynaptic neuronal apoptosis in a model of subarachnoid hemorrhage. Transl Stroke Res 2012. DOI: 10.1007/s12975-012-0166-9 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Simard JM, Aldrich EF, Schreibman D, et al. Low-dose intravenous heparin infusion in patients with aneurysmal subarachnoid hemorrhage: a preliminary assessment. J Neurosurg 2013. DOI: 10.3171/2013.8.JNS1337 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Simard JM, Schreibman D, Aldrich EF, et al. Unfractionated heparin: multitargeted therapy for delayed neurological deficits induced by subarachnoid hemorrhage. Neurocrit Care 2010. DOI: 10.1007/s12028-010-9435-1 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.James RF, Khattar NK, Aljuboori ZS, et al. Continuous infusion of low-dose unfractionated heparin after aneurysmal subarachnoid hemorrhage: a preliminary study of cognitive outcomes. J Neurosurg 2018. DOI: 10.3171/2017.11.JNS17894 [doi]. [DOI] [PubMed] [Google Scholar]