Abstract
Retinal iron accumulation has been implicated in the pathogenesis of age-related macular degeneration (AMD) and other neurodegenerative diseases. The retina and the brain are protected from the systemic circulation by the blood retinal barrier (BRB) and blood brain barrier (BBB), respectively. Iron levels within the retina and brain need to be tightly regulated to prevent oxidative injury. The method of iron entry through the retina and brain vascular endothelial cells (r&bVECs), an essential component of the BRB and BBB, is not fully understood. However, localization of the cellular iron exporter, ferroportin (Fpn), to the abluminal membrane of these cells, leads to the hypothesis that Fpn may play an important role in the import of iron across the BRB and BBB. To test this hypothesis, a mouse model with deletion of Fpn within the VECs in both the retina and the brain was developed through tail vein injection of AAV9-Ple261(CLDN5)-icre to both experimental Fpnf/f, and control Fpn+/+ mice at P21. Mice were aged to 9 mo and changes in retinal and brain iron distribution were observed. In vivo fundus imaging and quantitative serum iron detection were used for model validation. Eyes and brains were collected for immunofluorescence. Deletion of Fpn from the retinal and brain VECs leads to ferritin-L accumulation, an indicator of elevated iron levels, in the retinal and brain VECs. This occurred despite lower serum iron levels in the experimental mice. This result suggests that Fpn normally transfers iron from retinal and brain VECs into the retina and brain. These results help to better define the method of retina and brain iron import and will increase understanding of neurodegenerative diseases involving iron accumulation.
Keywords: Iron, ferroportin, age-related macular degeneration (AMD), retina, retinal vascular endothelium, ferritin
1. Introduction
Iron is essential for cellular survival, but excess iron loading within tissues can lead to cellular damage because iron catalyzes Fenton chemistry, leading to the production of hydroxyl free radicals. Because iron is absorbed through the diet, but not excreted from the body, it accumulates in tissues with age, potentially leading to age-related oxidative injury (Song and Dunaief, 2013). The photoreceptors are particularly susceptible to damage caused by iron overload due to factors such as high retinal oxygen tension, high metabolic rate, and high concentration of polyunsaturated fatty acids. For largely unknown reasons, individuals with certain neurodegenerative diseases such as Alzheimer Disease (Ayton et al., 2015; Smith et al., 2010), Parkinson Disease (Lee and Andersen, 2010) and age-related macular degeneration (AMD) (Dunaief, 2006; Hahn et al., 2003) have elevated iron levels within the brain or the retina.
Elevated blood iron levels can affect retinal iron levels and retinal health. We recently published a case study that describes a middle-aged patient that developed early onset AMD after receiving IV iron supplementation (Song et al., 2016). Consistent with this observation, mice with elevated serum iron levels develop retinal iron overload and subsequent retinal degeneration and dysfunction (Gnana-Prakasam et al., 2012; Hadziahmetovic et al., 2011a, 2011c; Song et al., 2016; Theurl et al., 2016a). Together, these clinical observations and animal models suggest that iron dysregulation may play an important role in the development and exacerbation of neurodegeneration and that elevated blood iron levels may impact retinal iron levels and retinal health. However, the mechanisms of iron transfer from the blood to the retina and brain are incompletely understood.
The cellular iron regulatory system involves several iron handling proteins that regulate cellular iron import, storage, and export. The majority of non-heme iron in the blood and extracellular fluid is complexed with the iron transport protein, transferrin (Tf). After binding two atoms of ferric iron, the iron-laden Tf (holo-Tf) binds to the iron importer, transferrin receptor (TfR) on the cell surface, and the entire complex is endocytosed, a process known as transferrin-bound iron (TBI) import (Steere et al., 2012). Once in the acidified environment of the endosome, iron dissociates from Tf and is released into the cytosol through the iron/proton symporter divalent metal transporter 1 (Dmt1) (Dautry-Varsat et al., 1983; Yamashiro and Maxfield, 1984). Cytosolic iron levels are tightly regulated; within the cytosol, iron can be trafficked to the mitochondria, to the ER to be incorporated as a cofactor in many proteins, or safely stored within the iron storage protein, ferritin (Arosio et al., 2009). Cellular iron can be exported out of the cell through the only known mammalian iron exporter, ferroportin (Fpn) (Donovan et al., 2005; Nemeth et al., 2004). Regulation of the expression of Fpn by the regulatory iron hormone hepcidin (Hepc) is essential for regulation of the systemic iron pool. Hepc, also known as hepcidin antimicrobial peptide (Hamp), is a 25 aa peptide hormone that is produced primarily by hepatocytes, and binds to the extracellular domain of Fpn on the cell surface, leading to its internalization and degradation, effectively preventing cellular iron export and limiting the amount of iron that gets into the serum or extracellular fluid (Ganz, 2004). Fpn-mediated iron export is further regulated by the homologous multi-copper ferroxidases, ceruloplasmin (Cp) and hephaestin (Heph), which aid in iron export by oxidizing Fe2+ exported through Fpn to Fe3+, which can bind to transferrin in the serum or extracellular fluid (De Domenico et al., 2007; Syed et al., 2002).
The retina and brain are protected from the free-flow of solutes and nutrients due to the presence of the blood-retinal barrier (BRB) or blood-brain barrier (BBB), respectively. Unlike other organs, where the systemic iron pool largely regulates tissue iron levels, iron cannot freely enter and exit the retina, but rather needs to be transported across the BRB or BBB. The BRB is composed of two components, an inner and an outer barrier. The inner BRB is composed of tight junctions between the retinal vascular endothelial cells (rVEC) and contributions by other components including pericytes and Muller cell endfeet (Klaassen et al., 2013). The outer BRB is formed by the tight junctions between the retinal pigment epithelium (RPE), separating the retina from the choriocapillaris. The BBB is similarly composed of tight junctions between brain vascular endothelial cells (bVEC) (Ballabh et al., 2004; Rouault and Cooperman, 2006).
In order for iron to be imported into the retina or brain, it must pass through the rVECs or bVECs. First, iron enters the VECs through TBI import via TfR expressed on the luminal membrane of the vascular endothelium (Moos, 2002) (Jefferies et al., 1984; Kissel et al., 1998; Moos, 1996). Non-transferrin bound iron (NTBI) import is a second possible mechanism for iron entry into the VECs, however, prior studies imply that TBI import is the major mechanism (McCarthy and Kosman, 2013).
Although the mechanism for iron entry into rVECs or bVECs through TBI import is well understood, there are two main theories on the mechanism of iron transfer across the abluminal membranes of rVECs and bVECs into the retina or brain parenchyma. One theory suggests that holo-Tf is exported whole via transcytosis through the VEC, thus the regulation of retina or brain iron entry depends entirely on TBI iron input. Evidence for Tf transcytosis include the observation that mice perfused with radiolabeled transferrin accumulate the radiolabel in both bVECs and within the brain parenchyma, suggesting that transferrin is transported intact across the endothelium(Fishman et al., 1987). Additionally, monoclonal antibodies against TfR (OX-26) are able to cross the BBB (Friden et al., 1991; Pardridge et al., 1991). In addition, the presence of Dmt1, the iron transporter needed to move iron from the endosome to the cytosol, in bVECs is debated (Burdo et al., 2001; Knutson et al., 2004; Moos and Morgan, 2003), calling into question whether iron that has been endocytosed has the ability to be transported out of the endosome into the cytosol.
It is unlikely, however, that Tf transcytosis is the only method for iron transport across r&bVECs, as this mechanism does not consider how the endothelium, which is itself very metabolically active, is able to utilize iron for its own needs, or additionally how the brain is able to regulate the amount of iron entry if iron is solely transported through Tf transcytosis. Astrocytes, which are closely associated with blood vessels within the brain, express proteins that may regulate iron entry across the VECs, including Cp and Hepc (McCarthy and Kosman, 2014). Both proteins regulate the expression or function of Fpn, suggesting that a second mechanism for iron entry into the retina or brain is Fpn-mediated iron export.
According to this theory, iron is dissociated from Tf within the acidified endosome, exported out of the endosome through Dmt1, and is subsequently transported across the abluminal membrane into the retina or brain through Fpn-mediated iron export. Evidence for this theory includes the observation that Fpn is present on the abluminal membrane of both the bVECs (McCarthy and Kosman, 2013; Wu et al., 2004) and the rVECs (Theurl et al., 2016a), indicating a directionality of iron transport across the abluminal membrane. Additionally, mice with a defect in Dmt1 have iron deficiency in their brains (Burdo et al., 1999), suggesting that Dmt1-mediated iron exit from endosomes is necessary for iron transport into the brain through the BBB.
Our lab has previously demonstrated the importance of the Hepc/Fpn axis in regulating retinal iron levels, indicating that Fpn is an essential component of retinal iron transport. Fpn is localized to three locations within the retina, the abluminal membrane of the rVECs, the basal membrane of the RPE, and the Müller cell endfeet (Theurl et al., 2016a). The localization of Fpn in those specific regions of the retina, combined with the localization of TfR to the rVECs (Gnana-Prakasam et al., 2010), suggests a route of iron transport across the retina: It is imported through the rVECs, where it can then diffuse throughout the retina, and exported from the retina through the RPE into the choriocapillaris or into the vitreous through the Müller cell endfeet.
We have generated further evidence that regulation of Fpn on the rVECs is important for retinal iron transport, as mice that are given intravitreal injections of AAV-Hepc have iron accumulation within the rVECs compared to control mice that received intravitreal injection of AAV-LacZ (Theurl et al., 2016a). These data imply that the overexpression of Hepc within the retina may reduce Fpn expression on the rVECs, ultimately leading to iron loading within the rVECs (Theurl et al., 2016a). Consistent with this data, treatment of bovine retinal endothelial cell culture (BREC) with Hepc leads to reduced Fpn protein levels as well as reduced iron export (Hadziahmetovic et al., 2011a). These data together strongly suggest that Fpn plays an important role in retinal iron import, however, no direct in vivo evidence for the role of Fpn in retinal or brain iron transport has been reported. In this study, we developed a transgenic model that uses AAV-mediated delivery of a retina and brain vascular endothelium specific promoter driving Creexpression in a Fpn floxed mouse strain, leading to deletion of Fpn within the r&bVECs, to test its role in iron export from these cells.
2. Materials and Methods
2.1. Generation of r&bVEC-specific Fpn knockout mice
C57BL/6J mice carrying a floxed Fpn allele were generated as previously described (Lakhal-Littleton et al., 2015). The Fpn floxed strain was crossed with a strain containing a cre-activated GFP reporter (CAG-GFP) (Kawamoto et al., 2000). To create the rVEC and bVEC-specific Fpn KO, cre driven by a Ple (Pleiades) Claudin5 (CLDN5) promoter Ple261(CLDN5)-icre (pEMS1982) was packaged into AAV9 to produce AAV9-Ple261(CLDN5)-icre. Note: icre is improved cre recombinase (Shimshek et al., 2002). Ple261(CLDN5)-icre was previously demonstrated to transduce brain and retinal vascular endothelium in adult mice (De Leeuw et al., 2016). All control (Fpn+/+, CAG-GFP+) and experimental mice (Fpnf/f, CAG-GFP+) were injected with the AAV9-Ple261(CLDN5)-icre into the tail vein at P21 using 1.03x1013 vg/ml in a volume of 50 ul with a 31-gauge needle and a 0.3 cc syringe. Micron III in vivo retinal photography (Micron III, Phoenix Research Laboratories, Inc., Pleasanton, CA, USA) was used to verify GFP expression within the rVEC one month post injection. Experimental and control mice were aged to 2, 6 and 9 months and euthanized. Both control and experimental mice were on a C57BL/6J background and both males and females were used in this study. All mice were negative for the rd1 and rd8 alleles. All mice were fed a standard laboratory diet with 300 ppm iron and given free access to water. All mice were maintained in a temperature-controlled room at 21–23°C under dim cyclic light (12 h: 12 h light-dark cycle) during the experiments. Experimental procedures were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmology and vision research. All protocols were approved by the animal care review board of the University i of Pennsylvania.
2.2. Fundus imaging
In vivo fundus imaging was used to visualize GFP expression within the rVECs 1 month post injection. Mice were anesthetized with an intraperitoneal injection of (in mg/kg body ) weight): 80 ketamine (Par Pharmaceutical, Spring Valley, NY, USA), 10 xylazine (Lloyd Inc., Shenandoah, IA, USA) and 2 acepromazine (Boehringer Ingelheim Vetmedica, Inc. St. Joseph, MO, USA). Pupils were then dilated with 1% tropicamide (Akorn, Inc., Lake Forest, IL, USA). Once anesthetized adequately, mice were placed on a padded stage. Color and autofluorescence images were acquired using a fundus camera (Micron III, Phoenix Research Laboratories, Inc., Pleasanton, CA, USA).
2.3. Fixation of eyes and brains and preparation of eyecups
Eyes were collected at three time points: 2 mo, 6 mo and 9 mo, while brains were collected at the 2 mo and 9 mo time points. Eyes were enucleated immediately after euthanasia and fixed for 15 minutes in 4% paraformaldehyde and eyecups were created by removing the cornea and lens. Brains were collected immediately after euthanasia and fixed for 120 minutes in 4% paraformaldehyde. Eyecups and brains were dehydrated overnight in 30% sucrose and embedded in Tissue-Tek OCT (Sakura Finetek, Torrance, CA, USA).
2.4. Immunofluorescence
Immunofluorescence imaging was performed on 10 μm thick cryosections, as described previously (Hadziahmetovic et al., 2011a). Antibodies used: chicken anti-GFP (1:500; Abcam, Cambridge, MA, USA), rabbit anti-CD31 (1:100; Abcam, Cambridge, MA, USA), and rabbit anti-light ferritin (E17) (1:2500; P. Arosio, University of Brescia, Italy). Control sections were treated identically but with omission of primary antibody. Sections were analyzed by fluorescence microscopy with identical exposure parameters across genotype using Nikon Elements software (Melville, NY, USA). The percentage of retinal blood vessels that were co-stained with GFP and CD31 was determined by a masked observer who counted the number of CD31+ cells per section and then determined the percentage of CD31+ cells that were GFP+. The percentage of GFP+ retinal blood vessels or brain blood vessels that were also Ferritin-L+ was determined by a masked observer who counted the number of GFP+ cells per sections and then determined the percentage of GFP+ cells that were also Ferritin-L+.
2.5. Dissection of Mouse RPE, retinas and brains for RT-PCR
Mice were euthanized, and the brain was immediately removed, flash-frozen and stored at −80°C. The eyes were also immediately enucleated. Anterior segments were removed, and retinas were completely dissected away from the underlying RPE. Retinas were then flash-frozen and stored at −80°C. RPE cells were isolated from other ocular structures using enzymatic (dispase and hyaluronidase) digestion and mechanical dissection, as previously described (Wolkow et al., 2012).
2.6. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
RNA isolation was performed according to the manufacturer’s protocol (RNeasy Kit; Qiagen, Valencia, CA, USA). cDNA was synthesized with reverse transcription reagents (TaqMan Reverse Transcription Reagents; Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol. Gene expression of transferrin receptor (Tfrc) was analyzed using quantitative real-time PCR as previously described (Hadziahmetovic et al., 2011a). Gene expression assays (TaqMan; Applied Biosystems, Foster City, CA, USA) were used for qPCR analysis. Real-time RT-PCR was performed on a commercial sequence detection system (ABI Prism 7500; Applied Biosystems, Darmstadt, Germany). All reactions were performed in technical triplicates (N=3-10 mice per genotype). Probes used were as follows: Tfrc (Mm00441941), Gapdh served as an internal control (4352932E).
2.7. Quantitative serum iron detection
Blood was collected from anesthetized animals by retro-orbital bleeding. Blood was collected in BD microtainer blood collection tubes (BD Biosciences, San Jose, CA, USA) and spun down for 30 min at 3000 rpm. Serum was collected and stored at −20C. Serum Fe status was analyzed by quantifying total serum iron using an Iron/TIBC testing kit (Pointe Scientific, Inc., Canton, MI, USA).
2.8. Statistical Analysis
Mean ± SEM was calculated for each group. Student’s two-group, two-tailed t-test was used for statistical analysis. All statistical analyses were performed using GraphPad Prism 5.0 (San Diego, CA, USA).
3. Results
3.1. Validation of r&bVEC-specific Fpn KO model
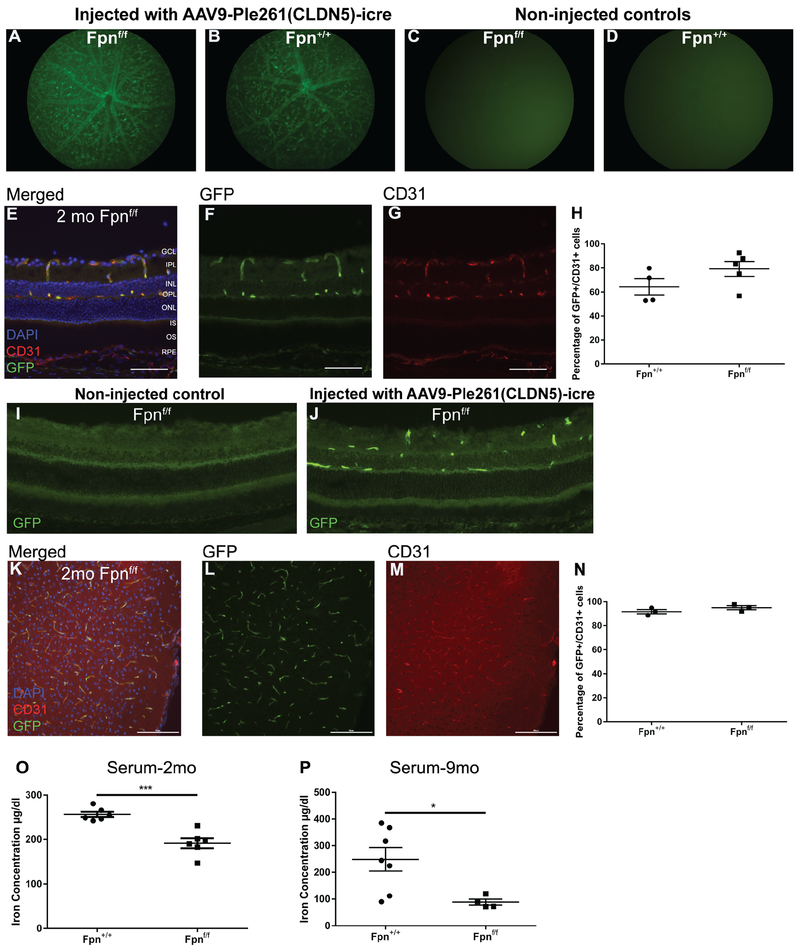
Experimental (Fpnf/f, CAG-GFP+) and control (Fpn+/+, CAG-GFP+) mice were injected with 50 μl of AAV9-Ple261(CLDN5)-icre into the tail vein at p21. To verify successful delivery of the AAV9-Ple261(CLDN5)-icre to rVECs, fundus photography was performed 1 month post-injection to visualize GFP expression within the retinal vasculature. GFP is expressed by the CAG-GFP floxed reporter that is activated if cre is expressed in the cell. GFP fluorescence was observed within blood vessels in the retina of both the experimental Fpnf/f (Figure 1A) and control Fpn+/+ mice (Figure 1B) one month post injection. There was no GFP fluorescence observed in the fundus of either Fpnf/f (Figure 1C) or Fpn+/+ (Figure 1D) mice that were not injected with the AAV9-Ple261(CLDN5)-icre vector. To determine the efficiency of AAV9-Ple261(CLDN5)-icre delivery to rVECs, cryosections from injected Fpnf/f and Fpn+/+ mice at 2 mo were co-labeled with GFP and CD31, a vascular endothelial cell marker, to determine what percentage of vascular endothelial cells were also GFP+ (Figure 1E–G). We observed that the majority of CD31+ rVECs in both the Fpn+/+ and Fpnf/f retinas were also GFP+, indicating that the majority of the rVECs had been transduced with the AAV9-Ple261(CLDN5)-icre (Figure 1H). A non-injected Fpnf/f control and Fpnf/f mouse injected with AAV9-Ple261(CLDN5)-icre vector were labeled with anti-GFP to compare background immunofluorescence labeling to specific labeling of rVECs (Figure 1I,J). We also observed a similarly high percentage of bVEC transduction, with the majority of CD31+ endothelial cells also co-labeling with GFP in the cortices of 2 mo experimental and control mice (Figure 1K–N).
Figure 1. Validation of rVEC and bVEC Fpn-deletion model.
(A): Representative in vivo green fluorescence fundus images of 2 mo Fpnf/f experimental mouse 1 month post injection of AAV9-Ple261(CLDN5)-icre demonstrates fluorescence in retinal vasculature. (B): Representative in vivo green fluorescence fundus image of 2 mo Fpn+/+ control mice 1 month post injection of AAV9-Ple261(CLDN5)-icre. (C): Representative in vivo green fluorescence fundus image of 2 mo Fpnflox/flox mouse that was not injected with AAV9-Ple261(CLDN5)-icre vector. (D): Representative in vivo green fluorescence fundus image of 2 mo Fpn+/+ mouse that was not injected with AAV9-Ple261(CLDN5)-icre vector. (E-G): Representative retinal GFP and CD31 co-immunolabeling to determine the efficacy of tail vein injection in 2 mo Fpnf/f experimental mouse. Scale bars, 50 μm. (H): Quantification of retinal CD31/GFP co-labeling in 2 mo Fpn+/+ control and Fpnf/f experimental mice. GFP immunolabeling of non-injected Fpnf/f control (I) and Fpnf/f injected with AAV9-Ple261(CLDN5)-icre vector (J). (K-M): Representative GFP and CD31 immunolabeling in the cortex of a 2 mo Fpnf/f experimental mouse to determine the efficacy of tail vein injection. Scale bars, 100 μm (N): Quantification of CD31/GFP co-labeling in the cortex of 2 mo control and experimental mice. (O): Serum iron concentration is decreased in Fpnf/f experimental mice versus Fpn+/+ controls at 2 mo time point. (P): Serum iron concentration is decreased in Fpnf/f experimental mice versus Fpn+/+ controls at 9 mo time point. Statistical analysis was performed using Student’s two-group, two-sided t-test. * p<0.05, *** p<.001. Abbreviations: GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium.
Although the CLDN5-icre was expected to transduce only neuronal vascular endothelial cells, there was also unexpected GFP expression outside the CNS in the injected mice. In addition to the GFP expression observed in the rVECs and bVECs, there was also GFP expression in hepatocytes of experimental and control mice (data not shown). Hepatocyte transduction may account for the observed 25.4% reduction of serum iron levels in experimental mice at 2 mo (Figure 1O) and the 64.4% reduction of serum iron levels in experimental mice at 9 mo (Figure 1P).
3.2. rVEC-specific Fpn KO leads to iron loading in rVECs
To determine how r&bVEC-specific Fpn KO altered VEC iron levels, ferritin-L (Ft-L) immunostaining of retinal and brain cryosections was performed. Ft-L is a subunit of ferritin, the intracellular iron storage protein. Ft-L protein levels are regulated by the IRE/IRP pathway and are a validated measure of tissue iron levels (Hadziahmetovic et al., 2011c, 2011a; Hahn et al., 2004; Theurl et al., 2016a). When tissue iron levels are high, Ft-L protein levels are upregulated by the decrease in binding of the iron regulatory proteins 1 and 2 (IRP1/2) to the 5’ iron responsive element (IRE) on Ft-L mRNA (Harford et al., 2006; Muckenthaler et al., 1998).
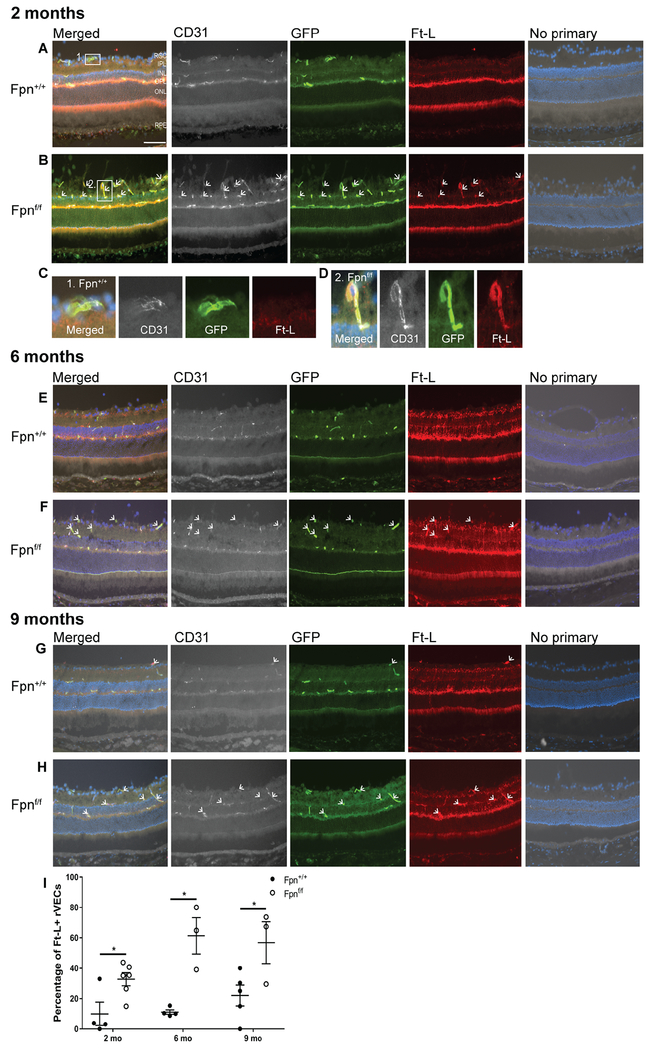
In order to determine whether deletion of Fpn affected VEC iron levels, the retinas of AAV9-Ple261(CLDN5)-icre injected mice of genotype Fpnf/f were compared to Fpn+/+ mice injected with the same virus. VECs that had been transduced with cre were identified as CD31+/GFP+ cells. The percentage of CD31/+GFP+ VECs that were Ft-L positive was then compared across the two genotypes. In the retinas of Fpnf/f experimental mice at ages 2 mo (Figure 2B, arrows), 6 mo (Figure 2F, arrows), and 9 mo (Figure 2H, arrows), there was an increased percentage of GFP+ rVECs that were co-labeled with Ft-L compared to the age-matched Fpn+/+ controls (Figure 2A,E,G). An example of a CD31+, GFP+, Ft-L− rVEC from the 2 mo control retina is presented in Figure 2C, while an example of a CD31+, GFP+, Ft-L+ rVEC from a 2 mo experimental retina is presented in Figure 2D. The percentage of Ft-L/GFP co-labeling in the rVECs at the 2, 6, and 9 mo time points is quantified in Figure 2I.
Figure 2. Fpn-deletion in the r&bVECs leads to Ft-L accumulation in GFP+ blood vessels in the retina.
At the 2 mo time point, there was increased Ft-L labeling within the GFP+ rVECs (rVECs were identified by co-labeling with CD31) of Fpnf/f experimental mice (B) compared to age-matched controls (A). (C): A magnified image of a Ft-L−, GFP+ rVEC from the 2 mo Fpn+/+ control retina. (D): A magnified image of a Ft-L+, GFP+ rVEC from the 2 mo Fpnf/f experimental retina. At the 6 mo time point, there was increased Ft-L labeling within the GFP+ rVECs of experimental mice (F) compared to age-matched controls (E). At the 9 mo time point, there was increased Ft-L labeling within the GFP+ rVECs of experimental mice (H) versus controls (G). Scale bar, 50 μm. Quantification of percentage of Ft-L positive, GFP+ blood vessels in retinas at three time points (I). White arrows point to Ft-L+, CD31+, GFP+ rVECs. * p<0.05, *** p<.001. Abbreviations: GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium.
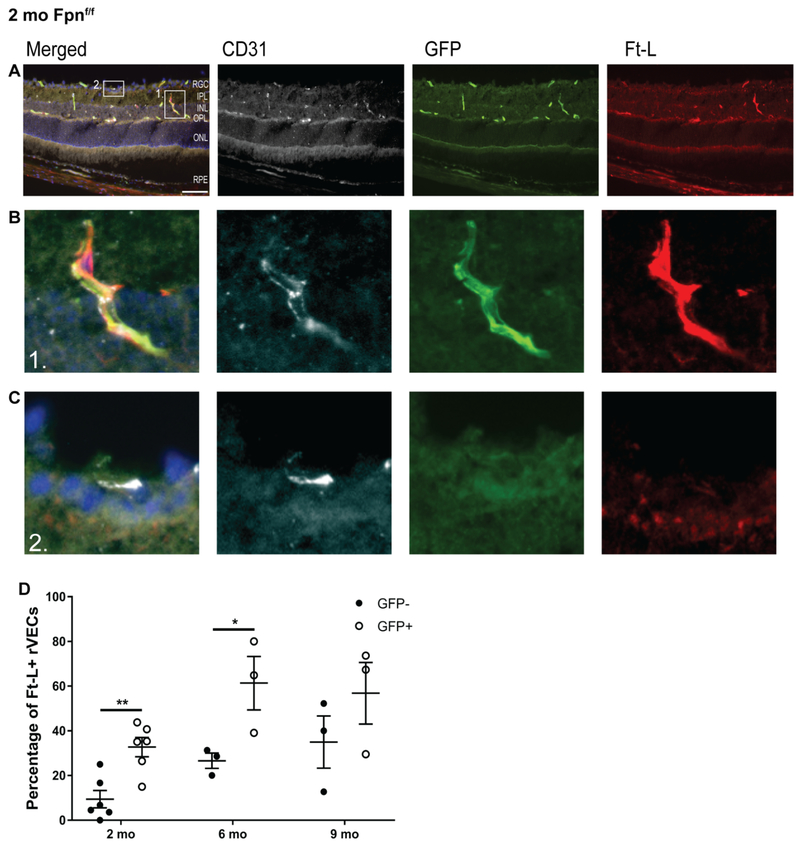
To test whether the Ft-L accumulation within the rVECs of the Fpnf/f experimental mice may have been caused by changes in blood iron levels, the percentage of Ft-L+ rVECs that were both GFP+ and CD31+ was compared to the percentage of FT-L+ rVECs that were CD31+, but GFP−. This measurement was collected in order to determine whether the presence of GFP, and thus the KO of Fpn was more likely to result in Ft-L accumulation within the rVECs compared to non-transduced (GFP−) rVECs. A representative image of the retina of a 2 mo Fpnf/f mouse that is immunolabeled with CD31, GFP, and Ft-L is presented in Figure 3A. We found that the majority of strongly Ft-L+ rVECs were GFP+ and CD31+ (Figure 3B), while rVECs that were either not Ft-L+ or were weakly Ft-L were GFP− and CD31+ (Figure 3C). At ages 2 mo, 6 mo, and 9mo, the percentage of Ft-L+ rVECs that were both GFP+ and CD31+ were greater than the percentage of Ft-L+ rVECs that were GFP− and CD31+ (Figure 3D), and this difference was significant at ages 2 and 6mo. These data indicate that the loss of Fpn, as indicated by GFP expression within the rVECs, was the cause of elevated Ft-L levels in the retina, at least at the 2 mo and 6 mo time points.
Figure 3. Ft-L accumulation occurs in rVECs that lack Fpn.
Representative images from a 2 mo Fpnf/f experimental retina that is immunolabeled with CD31, GFP, and Ft-L (A). Magnified images (from box#1 in panel A) of a CD31+, GFP+, Ft-L+ rVEC (B). Magnified images (from box#2 in panel A) of a CD31+, GFP− rVEC with very faint Ft-L immuno labeling (C). Quantification of the percentage of Ft-L+ rVECs that are both CD31 and GFP+ compared to the percentage of Ft-L+ rVECs that are CD31+, but GFP−. Scale bar, 50 μm. Statistical analysis was performed using Student’s two-group, two-sided t-test.* p<0.05, ** p<0.01.Abbreviations: GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium.
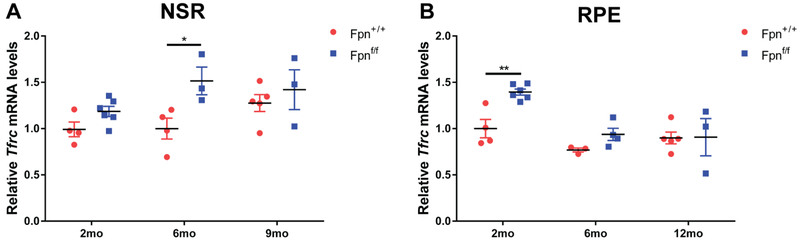
Iron levels within the neurosensory retina (NSR) and RPE were also determined using mRNA levels of the iron importer, transferrin receptor (Tfrc). There was an increase in Tfrc mRNA levels in the NSR of Fpnf/f experimental mice at the 6 mo time point compared to age-matched controls (Figure 4A) and increased Tfrc in the RPE at the 2 mo time point (Figure 4B). An increase in Tfrc mRNA levels in the NSR and RPE in the experimental mice indicates that these tissues were relatively iron deficient compared to the control NSR and RPE at those time points.
Figure 4. Transferrin receptor mRNA levels in the neurosensory retina and RPE of rVEC-specific Fpn KO mice.
Graphs of relative transferrin receptor (Tfrc) mRNA levels determined by qPCR in neurosensory retina (NSR) (A) and RPE (B) of r&bVEC-specific Fpn KO mice compared to controls at 2 mo, 6 mo and 9 mo. Statistical analysis was performed using Student’s two-group, two-sided t-test.* p<0.05, ** p<0.01.
3.3. bVEC-specific Fpn KO leads to iron loading in bVECs
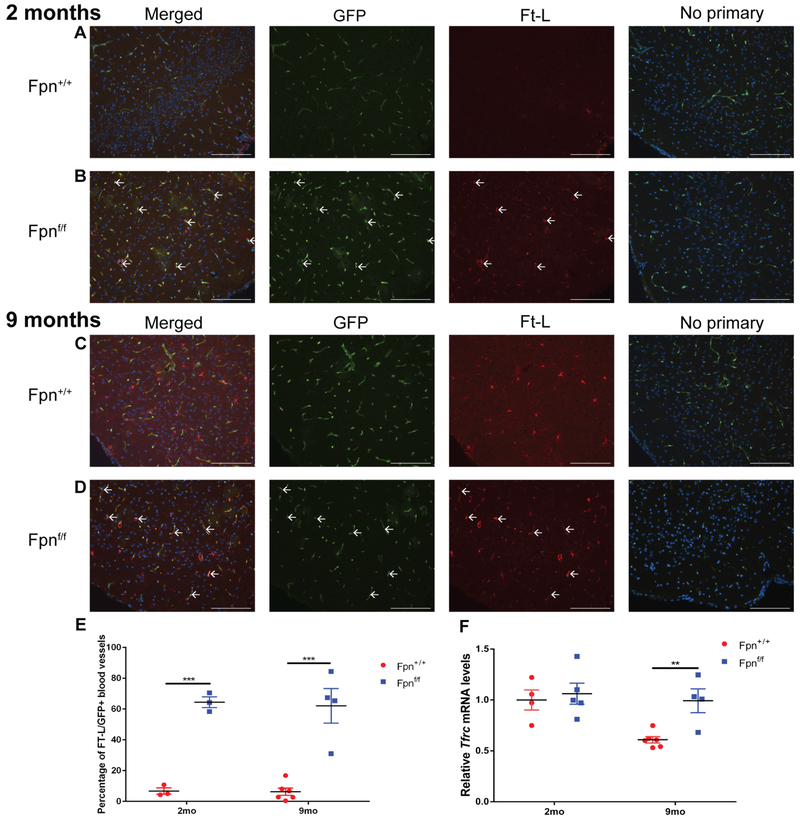
Ft-L/GFP co-labeling in the brain cortex was also increased with bVEC-specific Fpn KO. At the 2 mo (Figure 5B) and 9 mo (Figure 5D) time points, there was an increase in the proportion of Ft-L+,GFP+ bVECs compared to age-matched controls (Figure 5A,C). At the 2 mo time point, there was very little Ft-L labeling in any cell type within the cortex of the control mice (Figure 5A), however, by the 9 mo time point, there was increased labeling within arborized brain parenchymal cells in the control mice (Figure 5C). In contrast, there was no increase in parenchymal staining in the 9 mo Fpnf/f experimental mice (Figure 5D). The percentage of Ft-L/GFP colabeling in the cortex at the 2 mo and 9 mo time points is quantified in Figure 5E. There were also increased Tfrc mRNA levels within the cortex of the bVEC-specific Fpn KO mice compared to controls at the 9 mo time point (Figure 5F).
Figure 5. Fpn-deletion in the bVECs leads to Ft-L accumulation in GFP+ blood vessels in the brain.
There was increased Ft-L labeling within the GFP+ bVECs at both the 2 mo (B) and 9 mo (D) time points compared to controls (A,C). Scale bars, 100 μm. White arrows point to Ft-L+ bVECs. Quantification of percentage of Ft-L positive, GFP+ blood vessels in the brain at the two time points (E). Graph of relative transferrin receptor (Tfrc) mRNA levels determined by qPCR in the cortex of r&bVEC-specific Fpn KO mice compared to controls at 2 mo and 9 mo (F). Statistical analysis was performed using Student’s two-group, two-sided t-test. ** p<0.01, *** p<.001.
4. Discussion
In this study, a novel in-vivo model of r&bVEC-specific Fpn KO was developed to test the role of Fpn in r&bVEC-mediated iron transport into the retina and the brain. Using this model, we determined that Fpn exports iron from r&bVECs, most likely promoting iron import from the blood into the retina and brain. To delete Fpn in the rVEC and bVEC, we used IV injection of AAV9-Ple261(CLDN5)-icre in a Fpn floxed strain, expressing a cre-mediated GFP reporter. The specificity of the AAV9-Ple261(CLDN5)-icre vector was verified using fundus photography and immunolabeling with GFP and CD31 antibodies. Within the retina and the brain, the only cells that were GFP+ after injection were CD31+ vascular endothelial cells, indicating that Fpn had been specifically deleted from the endothelial cells and not in any other retinal or neuronal cell within the CNS. In addition to GFP expression in r&bVECs, there was GFP expression in hepatocytes, which may have resulted in the decreased serum iron levels observed in the experimental mice compared to control mice.
Despite the decreased serum iron levels observed in the experimental mice, deletion of r&bVEC Fpn led to Ft-L accumulation within the retinal GFP+ rVECs at the 2, 6, and 9mo time points. The bVECs had a similar response to Fpn deletion, with Ft-L accumulation within the GFP+ bVECs in the experimental mice at both the 2 mo and the 9 mo time points relative to control mice. The accumulation of Ft-L within the r&bVECs is indirect, but highly validated, evidence that the loss of Fpn in these cells is preventing iron from being exported, ultimately leading to elevated VEC iron levels.
The fact that not all the GFP+ rVECs in the Fpnf/f experimental mice were iron loaded suggests that the rVECs may have a non-Fpn-mediated mechanism to slowly export iron. In the retina, the percentage of Ft-L+, GFP+ rVECs was higher than the percentage of Ft-L+, GFP− rVECs at the 2 mo and 6 mo time point, but there was no significant difference between these two populations at the 9 mo time point. These results imply that the Ft-L accumulation due to Fpn KO may be transient in rVECs. There are several possible explanations for transient Ft-L accumulation in the rVECs following rVEC-specific Fpn KO. First, the ferritin within the rVECs may be secreted out of the rVECs over time into the retinal parenchyma, leading to reduced iron levels in the rVECs despite a lack of Fpn on the abluminal membrane. Although ferritin is typically considered an intracellular storage protein, it is found in small quantities within the serum, and therefore can be secreted. Ferritin lacks the signal peptide that is necessary for classical ER-Golgi mediated secretion; it is instead secreted through a non-classical lysosomal secretion pathway (Cohen et al., 2010; Truman-Rosentsvit et al., 2018). In this non-classical pathway, ferritin is secreted from the cell by secretory lysosomes or exosomes. Many cells within the retina express the ferritin-L receptor, Scara5, indicating that ferritin secretion and ferritin uptake may occur in the retina (Mendes-Jorge et al., 2014). Ferritin secretion across the rVECs has not been studied extensively, so it is unclear whether this process occurs under physiologic conditions or alternatively occurs only when the Fpn-mediated iron export pathway has been disrupted.
An alternative Fpn-independent mechanism for the export of iron out of rVECs is the redistribution of iron across rVECs via transport through gap junctions, which allow the flux of small molecules less than 1kDa across the cell membranes of adjoining cells (Meşe et al., 2007; Nagasawa et al., 2006). Gap junctions occur between endothelial cells throughout the body, including within endothelial cells within the brain and the retina (Nagasawa et al., 2006; Sato et al., 2002). The passage of ions and other small molecules through gap junctions allows for communication and coordinated actions between neighboring cells in multi-cellular tissues and is essential for vascular endothelial cell function.In the rVECs of rVEC-specific Fpn KO mice, the cells that lack Fpn accumulate iron and increase expression of Ft-L over time, while the adjoining rVECs that were not transduced and therefore still express Fpn do not become iron loaded. The iron loaded rVEC may transport iron across gap junctions to a Fpn-expressing rVEC, which can then transport iron into the retina. This hypothesis may help to explain why we observed a greater percentage of Ft-L+ VECs in the brain of Fpnf/f experimental mice compared to the percentage observed in the VECs in the retina. The transduction efficiency in the bVECs was higher than in the rVECs, and therefore, although iron may be transported across gap junctions in the bVECs, very few of the bVECs could then export iron into the brain parenchyma due to the loss of Fpn on the abluminal membrane.
The observation that Tfrc mRNA levels were increased at the 2 mo time point in the RPE of the experimental mice suggests that the RPE is partially dependent on the neural retina for a portion of its iron content. One of the essential functions that the RPE performs is the daily phagocytosis of the iron-laden photoreceptor outer segments (Song and Dunaief, 2013). If the photoreceptors in the rVEC Fpn KO mice are iron deficient due to lack of iron export from the rVECs, then this deficiency may result in a subsequent decrease in RPE iron content. The RPE can increase expression of Tfrc, leading to increased iron import from the choriocapillaris. The lack of change in Tfrc mRNA levels at the later 6 mo and 9 mo time points in the RPE indicates that the increase in Tfrc mRNA at the 2 mo time point may be adequately compensating for the loss of iron from phagocytosis of photoreceptor outer segments.
Experimental mice had diminished retinal and brain iron levels, assessed by transferrin receptor qPCR. It is likely that this decrease in CNS iron resulted from both impaired Fpn-mediated iron import into the CNS and the 25.4% percent lower serum iron levels in the experimental mice, although it is not possible to weigh the relative influences of these two mechanisms on CNS iron levels. Additional studies in which Fpn deletion occurs exclusively in the VECs are needed.
Off-target transduction of hepatocytes resulting in Fpn deletion in these liver cells diminished serum iron levels, making interpretation of the VEC phenotype more complex. However, lowered serum iron levels would be expected to cause diminished, not increased, VEC iron and ferritin levels. Also, the fact that in transduced Fpnf/f mice, GFP+ VECs were more likely to be ferritin positive than were GFP− VECs supports cell-autonomous effects of VEC Fpn deletion.
Prior studies showing Fpn localization on the abluminal surface of VECs and hepcidin-sensitive iron transport across the abluminal surface of cultured VECs (Theurl et al., 2016b), as well as the observation that systemic Hepc−/− mice have iron overload in the NSR and RPE, but have no rVEC Ft-L accumulation (Hadziahmetovic et al., 2011b), suggest that the Fpn/Hepc axis is necessary for regulation of retinal iron transport. The data presented herein indicate that Fpn is a VEC iron exporter in retina and brain, and suggest that Fpn transports iron into the retinal and brain parenchyma. As such, Fpn could become a pharmacologic target to modulate CNS iron levels.
Highlights.
Iron is transiently trapped in retinal vascular endothelial cells after ferroportin deletion
Iron is trapped in brain vascular endothelial cells after ferroportin deletion
Ferroportin on vascular endothelial cells may be essential for iron transport into the retina and brain
Acknowledgements:
Funding from NIH/NEI EY015240, the UPenn Vision Science Training Grant (5T32EY007035-37), Research to Prevent Blindness, the F.M. Kirby Foundation, a gift in memory of Lee F. Mauger, MD, the Paul and Evanina Bell Mackall Foundation Trust, and grant MEYS LO1419. The development of Ple261(CLDN5)-icre was supported by Genome British Columbia grant AGCPCanEuCre-01 to EMS.
Nonstandard Abbreviations:
- AMD
age-related macular degeneration
- Tf
transferrin
- TfR
transferrin receptor
- Dmt1
divalent metal transporter 1
- Fpn
ferroportin
- Hepc
Hepcidin
- Cp
ceruloplasmin
- Heph
hephaestin
- Ft-L
ferritin-L
- TBI
transferrin bound iron
- NTBI
non-transferrin bound iron
- RPE
retinal pigment epithelium
- NSR
neurosensory retina
- BRB
blood-retinal barrier
- BBB
blood-brain barrier
- rVECs
retinal vascular endothelial cells
- bVECs
brain vascular endothelial cells
- Ple
Pleiades Promoter Project
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arosio P, Ingrassia R, Cavadini P, 2009. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta - Gen. Subj 10.1016/j.bbagen.2008.09.004 [DOI] [PubMed] [Google Scholar]
- Ayton S, Faux NG, Bush AI, Weiner MW, Aisen P, Petersen R, Jack CR Jr., Jagust W, Trojanowki JQ, Toga AW, Beckett L, Green RC, Saykin AJ, Morris J, Shaw LM, Khachaturian Z, Sorensen G, Kuller L, Raichle M, Paul S, Davies P, Fillit H, Hefti F, Holtzman D, Marcel Mesulam M, Potter W, Snyder P, Schwartz A, Montine T, Thomas RG, Donohue M, Walter S, Gessert D, Sather T, Jiminez G, Harvey D, Bernstein M, Fox N, Thompson P, Schuff N, Borowski B, Gunter J, Senjem M, Vemuri P, Jones D, Kantarci K, Ward C, Koeppe R. a., Foster N, Reiman EM, Chen K, Mathis C, Landau S, Cairns NJ, Householder E, Taylor-Reinwald L, Lee V, Korecka M, Figurski M, Crawford K, Neu S, Foroud TM, Potkin S, Shen L, Faber K, Kim S, Nho K, Thal L, Buckholtz N, Albert M, Frank R, Hsiao J, Kaye J, Quinn J, Lind B, Carter R, Dolen S, Schneider LS, Pawluczyk S, Beccera M, Teodoro L, Spann BM, Brewer J, Vanderswag H, Fleisher A, Heidebrink JL, Lord JL, Mason SS, Albers CS, Knopman D, Johnson K, Doody RS, Villanueva-Meyer J, Chowdhury M, Rountree S, Dang M, Stern Y, Honig LS, Bell KL, Ances B, Carroll M, Leon S, Mintun M. a., Schneider S, Oliver A, Marson D, Griffith R, Clark D, Geldmacher D, Brockington J, Roberson E, Grossman H, Mitsis E, DeToledo-Morrell L, Shah RC, Duara R, Varon D, Greig MT, Roberts P, Albert M, Onyike C, D’Agostino D II, Kielb S, Galvin JE, Cerbone B, Michel C. a., Rusinek H, de Leon MJ, Glodzik L, De Santi S, Murali Doraiswamy P, Petrella JR, Wong TZ, Arnold SE, Karlawish JH, Wolk D, Smith CD, Jicha G, Hardy P, Sinha P, Oates E, Conrad G, Lopez OL, Oakley M, Simpson DM, Porsteinsson AP, Goldstein BS, Martin K, Makino KM, Saleem Ismail M, Brand C, Mulnard R. a., Thai G, Mc-Adams-Ortiz C, Womack K, Mathews D, Quiceno M, Diaz-Arrastia R, King R, Weiner M, Martin-Cook K, DeVous M, Levey AI, Lah JJ, Cellar JS, Burns JM, Anderson HS, Swerdlow RH, Apostolova L, Tingus K, Woo E, Silverman DHS, Lu PH, Bartzokis G, Graff-Radford NR, Parfitt F, Kendall T, Johnson H, Farlow MR, Hake AM, Matthews BR, Herring S, Hunt C, van Dyck CH, Carson RE, MacAvoy MG, Chertkow H, Bergman H, Hosein C, Black S, Stefanovic B, Caldwell C, Robin Hsiung G-Y, Feldman H, Mudge B, Assaly M, Kertesz A, Rogers J, Bernick C, Munic D, Kerwin D, Mesulam M-M, Lipowski K, Wu C-K, Johnson N, Sadowsky C, Martinez W, Villena T, Scott Turner R, Johnson K, Reynolds B, Sperling R. a., Johnson K. a., Marshall G, Frey M, Lane B, Rosen A, Tinklenberg J, Sabbagh MN, Belden CM, Jacobson S. a., Sirrel S. a., Kowall N, Killiany R, Budson AE, Norbash A, Johnson PL, Allard J, Lerner A, Ogrocki P, Hudson L, Fletcher E, Carmichael O, Olichney J, DeCarli C, Kittur S, Borrie M, Lee T-Y, Bartha R, Johnson S, Asthana S, Carlsson CM, Potkin SG, Preda A, Nguyen D, Tariot P, Reeder S, Bates V, Capote H, Rainka M, Scharre DW, Kataki M, Adeli A, Zimmerman E. a., Celmins D, Brown AD, Pearlson GD, Blank K, Anderson K, Santulli RB, Kitzmiller TJ, Schwartz ES, Sink KM, Williamson JD, Garg P, Watkins F, Ott BR, Querfurth H, Tremont G, Salloway S, Malloy P, Correia S, Rosen HJ, Miller BL, Mintzer J, Spicer K, Bachman D, Finger E, Pasternak S, Rachinsky I, Drost D, Pomara N, Hernando R, Sarrael A, Schultz SK, Boles Ponto LL, Shim H, Elizabeth Smith K, Relkin N, Chaing G, Raudin L, Smith A, Fargher K, Ashok Raj B, Neylan T, Grafman J, Davis M, Morrison R, Hayes J, Finley S, Friedl K, Fleischman D, Arfanakis K, James O, Massoglia D, Jay Fruehling J, Harding S, Peskind ER, Petrie EC, Li G, Yesavage J. a., Taylor JL, Furst AJ, 2015. Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat. Commun 6, 6760 10.1038/ncomms7760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M, 2004. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis 10.1016/j.nbd.2003.12.016 [DOI] [PubMed] [Google Scholar]
- Burdo JR, Martin J, Menzies SL, Dolan KG, Romano MA, Fletcher RJ, Garrick MD, Garrick LM, Connor JR, 1999. Cellular distribution of iron in the brain of the Belgrade rat. Neuroscience 93, 1189–1196. 10.1016/S0306-4522(99)00207-9 [DOI] [PubMed] [Google Scholar]
- Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, Dolan KG, Haile DJ, Beard JL, Connor JR, 2001. Distribution of Divalent Metal Transporter 1 and Metal Transport Protein 1 in the normal and Belgrade rat. J. Neurosci. Res 66, 1198–1207. 10.1002/jnr.1256 [DOI] [PubMed] [Google Scholar]
- Cohen LA, Gutierrez L, Weiss A, Leichtmann-Bardoogo Y, Zhang DL, Crooks DR, Sougrat R, Morgenstern A, Galy B, Hentze MW, Lazaro FJ, Rouault TA, Meyron-Holtz EG, 2010. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood 116, 1574–1584. 10.1182/blood-2009-11-253815 [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A, Ciechanover A, Lodish HF, 1983. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 10.1073/pnas.80.8.2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Domenico I, Ward DM, di Patti MCB, Jeong SY, David S, Musci G, Kaplan J, 2007. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J. 26, 2823–31. 10.1038/sj.emboj.7601735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leeuw CN, Korecki AJ, Berry GE, Hickmott JW, Lam SL, Lengyell TC, Bonaguro RJ, Borretta LJ, Chopra V, Chou AY, D’Souza CA, Kaspieva O, Laprise S, Mclnerny SC, Portales-Casamar E, Swanson-Newman MI, Wong K, Yang GS, Zhou M, Jones SJM, Holt RA, Asokan A, Goldowitz D, Wasserman WW, Simpson EM, 2016. RAAV-compatible MiniPromoters for restricted expression in the brain and eye. Mol. Brain. 10.1186/s13041-016-0232-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC, 2005. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 1, 191–200. 10.1016/j.cmet.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Dunaief JL, 2006. Iron induced oxidative damage as a potential factor in age-related macular degeneration: The Cogan lecture. Investig. Ophthalmol. Vis. Sci 47, 4660–4664. 10.1167/iovs.06-0568 [DOI] [PubMed] [Google Scholar]
- Fishman JB, Rubin JB, Handrahan JV, Connor JR, Fine RE, 1987. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J. Neurosci. Res 10.1002/jnr.490180206 [DOI] [PubMed] [Google Scholar]
- Friden PM, Walus LR, Musso GF, Taylor M.a., Malfroy B, Starzyk RM, 1991. Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc. Natl. Acad. Sci 10.1073/pnas.88.11.4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T, 2004. Hepcidin in iron metabolism. Curr. Opin. Hematol 10.1097/00062752-200407000-00004 [DOI] [PubMed] [Google Scholar]
- Gnana-Prakasam JP, Martin PM, Smith SB, Ganapathy V, 2010. Expression and function of iron-regulatory proteins in retina. IUBMB Life. 10.1002/iub.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnana-Prakasam JP, Tawfik A, Romej M, Ananth S, Martin PM, Smith SB, Ganapathy V, 2012. Iron-mediated retinal degeneration in haemojuvelin-knockout mice. Biochem. J 10.1042/BJ20111148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadziahmetovic M, Song Y, Ponnuru P, Iacovelli J, Hunter A, Haddad N, Beard J, Connor JR, Vaulont S, Dunaief JL, 2011a. Age-dependent retinal iron accumulation and degeneration in hepcidin knockout mice. Investig. Ophthalmol. Vis. Sci 52, 109–118. 10.1167/iovs.10-6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadziahmetovic M, Song Y, Ponnuru P, Iacovelli J, Hunter A, Haddad N, Beard J, Connor JR, Vaulont S, Dunaief JL, 2011b. Age-dependent retinal iron accumulation and degeneration in hepcidin knockout mice. Investig. Ophthalmol. Vis. Sci 52, 109–118. 10.1167/iovs.10-6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadziahmetovic M, Song Y, Wolkow N, Iacovelli J, Kautz L, Roth MP, Dunaief JL, 2011c. Bmp6 regulates retinal iron homeostasis and has altered expression in age-related macular degeneration. Am. J. Pathol 179, 335–348. 10.1016/j.ajpath.2011.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn P, Milam AH, Dunaief JL, 2003. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch’s membrane. Arch. Ophthalmol 121, 1099–1105. 10.1001/archopht.121.8.1099 [DOI] [PubMed] [Google Scholar]
- Hahn P, Qian Y, Dentchev T, Chen L, Beard J, Harris ZL, Dunaief JL, 2004. Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc Natl Acad Sci U S A 101, 13850–13855. 10.1073/pnas.0405146101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford JB, Klausner RD, McBride OW, Samaniego F, Burgess WH, Tang CK, Kaptain S, Rouault TA, Haile DJ, 2006. Cloning of the cDNA encoding an RNA regulatory protein--the human iron-responsive element-binding protein. Proc. Natl. Acad. Sci 87, 7958–7962. 10.1073/pnas.87.20.7958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY, 1984. Transferrin receptor on endothelium of brain capillaries. Nature. 5 10.1038/312162a0 [DOI] [PubMed] [Google Scholar]
- Kawamoto S, Niwa H, Tashiro F, Sano S, Kondoh G, Takeda J, Tabayashi K, Miyazaki JI, 2000. A novel reporter mouse strain that expresses enhanced green fluorescent protein upon Cre-mediated recombination. FEBS Lett. 10.1016/S0014-5793(00)01338-7 [DOI] [PubMed] [Google Scholar]
- Kissel K, Hamm S, Schulz M, Vecchi A, Garlanda C, Engelhardt B, 1998. Immunohistochemical localization of the murine transferrin receptor (TfR) on blood-tissue 5 barriers using a novel anti-TfR monoclonal antibody. Histochem. Cell Biol. 10.1007/s004180050266 [DOI] [PubMed] [Google Scholar]
- Klaassen I, Van Noorden, C.J. F, Schlingemann RO, 2013. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological 7 conditions. Prog. Retin. Eye Res. 34, 19–48. 10.1016/j.preteyeres.2013.02.001 [DOI] [PubMed] [Google Scholar]
- Knutson M, Menzies S, Connor J, Wessling-Resnick M, 2004. Developmental, regional, and cellular expression of SFT/UbcH5A and DMT1 mRNA in brain. J. Neurosci. Res. 10.1002/jnr.20113 [DOI] [PubMed] [Google Scholar]
- Lakhal-Littleton S, Wolna M, Carr CA, Miller JJJ, Christian HC, Ball V, Santos A, Diaz R, Biggs D, Stillion R, Holdship P, Larner F, Tyler DJ, Clarke K, Davies B, Robbins PA, 2015. Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function. Proc. Natl. Acad. Sci 10.1073/pnas.1422373112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Andersen JK, 2010. Iron elevations in the aging Parkinsonian brain: A consequence of impaired iron homeostasis? J. Neurochem 10.1111/j.1471-4159.2009.06470.x [DOI] [PubMed] [Google Scholar]
- McCarthy RC, Kosman DJ, 2014. Glial cell ceruloplasmin and hepcidin differentially regulate iron efflux from brain microvascular endothelial cells. PLoS One. 10.1371/journal.pone.0089003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy RC, Kosman DJ, 2013. Ferroportin and exocytoplasmic ferroxidase activity are required for brain microvascular endothelial cell iron efflux. J. Biol. Chem 288, 17932–17940. 10.1074/jbc.M113.455428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-Jorge L, Ramos D, Valença A, López-Luppo M, Pires VMR, Catita J, Nacher V, Navarro M, Carretero A, Rodriguez-Baeza A, Ruberte J, 2014. L-ferritin binding to Scara5: A new iron traffic pathway potentially implicated in retinopathy. PLoS One 9 10.1371/journal.pone.0106974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meşe G, Richard G, White TW, 2007. Gap junctions: Basic structure and function. J. Invest. Dermatol 10.1038/sj.jid.5700770 [DOI] [PubMed] [Google Scholar]
- Moos T, 2002. Brain iron homeostasis. Dan. Med. Bull 10.1016/0162-0134(92)84071-T [DOI] [PubMed] [Google Scholar]
- Moos T, 1996. Immunohistochemical localization of intraneuronal transferrin receptor immunoreactivity in the adult mouse central nervous system. J. Comp. Neurol [DOI] [PubMed] [Google Scholar]
- Moos T, Morgan EH, 2003. The significance of the mutated divalent metal transporter (DMT1) on iron transport into the Belgrade rat brain. J. Neurochem 10.1046/j.1471-4159.2003.02142.x [DOI] [PubMed] [Google Scholar]
- Muckenthaler M, Gray NK, Hentze MW, 1998. IRP-1 binding to ferritin mRNA prevents the recruitment of the small ribosomal subunit by the cap-binding complex elF4F. Mol. Cell 10.1016/S1097-2765(00)80282-8 [DOI] [PubMed] [Google Scholar]
- Nagasawa K, Chiba H, Fujita H, Kojima T, Saito T, Endo T, Sawada N, 2006. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J. Cell. Physiol 208, 123–132. 10.1002/jcp.20647 [DOI] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J, 2004. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–3. 10.1126/science.1104742 [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Buciak JL, Friden PM, 1991. Selective transport of an anti-transferrin receptor antibody through the blood-brain barrier in vivo. J. Pharmacol. Exp. Ther 10.1007/s00330-011-2364-3 [DOI] [PubMed] [Google Scholar]
- Rouault TA, Cooperman S, 2006. Brain Iron Metabolism. Semin. Pediatr. Neurol 10.1016/j.spen.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Sato T, Haimovici R, Kao R, Li AF, Roy S, 2002. Downregulation of connexin 43 expression by high glucose reduces gap junction activity in microvascular endothelial cells. Diabetes 51, 1565–1571. 10.2337/diabetes.51.5.1565 [DOI] [PubMed] [Google Scholar]
- Shimshek DR, Kim J, Hubner MR, Spergel DJ, Buchholz F, Casanova E, Stewart AF, Seeburg PH, Sprengel R, 2002. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis. 10.1002/gene.10023 [DOI] [PubMed] [Google Scholar]
- Smith M. a, Zhu X, Tabaton M, Liu G, McKeel DW, Cohen ML, Wang X, Siedlak SL, Dwyer BE, Hayashi T, Nakamura M, Nunomura A, Perry G, 2010. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J. Alzheimers. Dis 19, 363–72. 10.3233/JAD-2010-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Dunaief JL, 2013. Retinal iron homeostasis in health and disease. Front. Aging Neurosci. 5, 24 10.3389/fnagi.2013.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Kanu LN, Li Y, Kelly KL, Bhuyan RK, Aleman T, Morgan JIW, Dunaief JL, 2016. AMD-like retinopathy associated with intravenous iron. Exp. Eye Res. 151, 122–133. 10.1016/j.exer.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AN, Byrne SL, Chasteen ND, Mason AB, 2012. Kinetics of iron release from transferrin bound to the transferrin receptor at endosomal pH. Biochim. Biophys. Acta - Gen. Subj. 10.1016/j.bbagen.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed B. a, Beaumont NJ, Patel A, Naylor CE, Bayele HK, Joannou CL, Rowe PSN, Evans RW, Srai SKS, 2002. Analysis of the human hephaestin gene and protein: comparative modelling of the N-terminus ecto-domain based upon ceruloplasmin. Protein Eng. 15, 205–14. [DOI] [PubMed] [Google Scholar]
- Theurl M, Song D, Clark E, Sterling J, Grieco S, Altamura S, Galy B, Hentze M, Muckenthaler MU, Dunaief JL, 2016a. Mice with hepcidin-resistant ferroportin accumulate iron in the retina. FASEB J. 30, 813–823. 10.1096/fj.15-276758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurl M, Song D, Clark E, Sterling J, Grieco S, Altamura S, Galy B, Hentze M, Muckenthaler MU, Dunaief JL, 2016b. Mice with hepcidin-resistant ferroportin accumulate iron in the retina. FASEB J. 30, 813–23. 10.1096/fj.15-276758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman-Rosentsvit M, Berenbaum D, Spektor L, Cohen LA, Belizowsky-Moshe S, Lifshitz L, Ma J, Li W, Kesselman E, Abutbul-Ionita I, Danino D, Gutierrez L, Li H, Li K, Lou H, Regoni M, Poli M, Glaser F, Rouault TA, Meyron-Holtz EG, 2018. Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood 131, 342–352. 10.1182/blood-2017-02-768580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkow N, Song D, Song Y, Chu S, Hadziahmetovic M, Lee JC, Iacovelli J, Grieco S, Dunaief JL, 2012. Ferroxidase hephaestin’s cell-autonomous role in the retinal pigment epithelium. Am. J. Pathol 180, 1614–1624. 10.1016/j.ajpath.2011.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJC, Leenders AGM, Cooperman S, Meyron-Holtz E, Smith S, Land W, Tsai RYL, Berger UV, Sheng ZH, Rouault TA, 2004. Expression of the iron transporter ferroportin in synaptic vesicles and the blood-brain barrier. Brain Res. 10.1016/j.brainres.2003.10.066 [DOI] [PubMed] [Google Scholar]
- Yamashiro DJ, Maxfield FR, 1984. Acidification of endocytic compartments and the intracellular pathways of ligands and receptors. J. Cell. Biochem 10.1002/jcb.240260404 [DOI] [PubMed] [Google Scholar]