Abstract
Cell-autonomous endothelial cell (EC) fibroblast growth factor receptor (FGFR) signaling through FGFR1/2 is essential for injury-induced wound vascularization and pathologic neovascularization as in blinding eye diseases such as age-related macular degeneration. Which FGF ligand(s) is critical in regulating angiogenesis is unknown. Utilizing ex vivo models of choroidal endothelial sprouting and in vivo models of choroidal neovascularization (CNV), we demonstrate here that only FGF2 is the essential ligand. Though FGF-FGFR signaling can activate multiple intracellular signaling pathways, we show that FGF2 regulates pathogenic angiogenesis via STAT3 activation. The identification of FGF2 as a critical mediator in aberrant neovascularization provides a new opportunity for developing multi-target therapies in blinding eye diseases especially given the limitations of anti-VEGF monotherapy.
Keywords: choroidal neovascularization, endothelial cell, fibroblast growth factor, STAT3, choroid sprouting, macular degeneration, retina
1. Introduction
The FGF family is composed of at least 18 signaling ligands, which interact with four cell surface tyrosine kinase receptors, FGFRs 1–4, in complex with heparan sulfate proteoglycan (HPSG) (Ornitz and Itoh, 2015). The endothelial cell response to FGF signals has been characterized using in vitro and in vivo models of angiogenesis (Chen et al., 2016; Gospodarowicz et al., 1978; Murakami et al., 2008; Oladipupo et al., 2014; Yamada et al., 2000). Using mice deficient in Fgfr1 and Fgfr2 in cells of both endothelial and hematopoietic lineages, we discovered that endothelial FGFR1/2 signaling is not required for embryonic development or for maintaining vascular integrity and function under homeostatic physiological conditions, despite the well-established role for FGF signaling in endothelial cells for vascular development in vitro. In contrast, when subjected to either skin or eye injury, or hypoxia-induced pathologic angiogenesis (House et al., 2016), mice lacking endothelial FGFR1/2 display a significant reduction in neovascular growth and tissue repair (Oladipupo et al., 2014).
Pathologic neovascularization is central to diverse disease processes including cancer, atherosclerosis and blinding eye diseases. In the eye, pathologic neovascularization can cause blindness in advanced age-related macular degeneration (AMD), the leading cause of blindness in people over 50 years of age in the industrialized world (Sene and Apte, 2014). The exudative or neovascular form of AMD is devastating as it causes the majority of acute vision loss and is characterized by the development of abnormal and leaky blood vessels underneath the retina called CNV. Although VEGF plays a significant role in the pathogenesis of CNV in AMD, it is becoming increasingly clear that VEGF-independent pathways also play a key role in the development and growth of CNV (Oladipupo et al., 2014; Sene and Apte, 2014; Sene et al., 2015; Stahl et al., 2009). In addition, resistance to anti-VEGF therapy and persistence of disease despite VEGF neutralization remains a significant clinical hurdle (Bakall et al., 2013; Gasperini et al., 2012; Spooner et al., 2018; Wagle et al., 2011; Yonekawa et al., 2013). Therefore, elucidating VEGF-independent pathways that promote CNV in diseases such as AMD is a high priority. Given the importance of the FGF-FGFR signaling pathway in promoting CNV, we conducted a series of experiments to identify the FGF ligand(s) critical to pathologic angiogenesis. Using established murine models of CNV and ex vivo models of vascular proliferation using explanted choroidal tissue, we identified FGF2 as the pathogenic ligand that regulates vascular endothelial proliferation and CNV via intracellular STAT3 activation. These findings have potential clinical implications given the high interest in developing multi-target therapeutic approaches that complement VEGF-based therapies in AMD.
2. Materials and Methods
2.1. Animals
All animal experiments and experimental procedures were approved by the Institutional Animal Care and Use Committee and performed in accordance with the Washington University School of Medicine Animal Care and Use guidelines as well as ARVO Guidelines for Use of Animals in Research.
C57BL/6J mice (age <3 months) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice genetically deficient in Fgf2 (Fgf2−/−), Fgf8ER-TAM Cre and Fgf9ER-TAM Cre, and Fgfr1/2/3Tie2-Cre mice in which Fgfr1, Fgfr2 and Fgfr3 genes were inactivated in Tie2-Cre–expressing endothelial cells as well as their littermates with the un-recombined Fgfr1/2/3f/f alleles, lacking the Tie2-Cre allele, were generated from published mouse lines (Colvin et al., 1996; Colvin et al., 2001; Deng et al., 1996; Meyers et al., 1998; Trokovic et al., 2003; Xu et al., 1998; Zhou et al., 1998). For the induction of ER cre in Fgf8ER-TAM Cre and Fgf9ER-TAM Cre mice, animals were fed 500mg/kg Tamoxifen-containing chow (TD150858; Envigo, Summerset, NJ) ad libitum for 2 weeks.
2.2. Antibodies
Neutralizing antibody against mouse FGF2 was purchased from Abcam (ab33103, Cambridge, MA) and its IgG isotype control from R&D Systems Inc. (MAB002, R&D Systems Inc., Minneapolis, MN). Antibodies used in western blotting were purchased from Cell Signaling Technology (Danvers MA): rabbit polyclonal antibodies against phospho-STAT1 (Tyr705, #9167), phospho-STAT3 (Tyr705, #9145), phospho-STAT5 (Tyr694, #4322), phospho-PLCγ1 (#2821), phospho-MEK1/2 (#9121), phospho-AKT (#9271), STAT1 (#9172), STAT3 (#12640), STAT5 (#94205), PLCγ1 (#2822), MEK1/2 (#9122) and AKT (#9272); and Sigma-Aldrich (St. Louis, MO): mouse monoclonal antibody against β-ACTIN (A2228).
2.3. Choroid sprouting assay
Choroid sprouting assay was performed as previously described with slight modification (Shao et al., 2013). Briefly, the RPE-choroid-sclera complex (“choroid explant”) was dissected from mouse eyes, segmented to create multiple samples of similar size and shape to either receive later treatment or serve as vehicle control for that choroid sample set, and immediately placed on the surface of growth factor-reduced Matrigel™ (Corning, NY) in 24-well tissue culture plates. Choroid explants were incubated in 500μL CSC complete medium (Cell Systems, Cat. 420–500) activated with growth factor boost and supplemented with 1% Penicillin/Streptomycin (GIBCO, Grand Island, NY) and 5 μg/mL of Plasmocin™ (Invitrogen, Cat. Ant-mpt) at 37°C with 5% CO2 for 48 hours before any treatment. For FGF stimulation experiment, choroid explants were treated with FGFs (FGF2: SRP4038, FGF9: SRP4054, Sigma-Aldrich; FGF8: ab205522, Abcam) in the presence of 1μg/ml heparan sulfate (Sigma-Aldrich). For sprouting inhibition experiment, either vehicle (DMSO, Sigma-Aldrich) or STAT3 inhibitor (Stattic, R&D Systems Inc.) was added to the medium. The final concentration of DMSO is 0.2% (v/v). Medium was changed every 48 hours. Phase contrast photos of individual explants were taken every other day for 8 days post-dissection using an Olympus Microfire camera on an Olympus CKX41 microscope. The area of neovascular growth extending beyond the explant tissue area was measured for each sample. Each measurement of vascular area was normalized to the area of growth for vehicle-treated samples originating from the same choroid for each timepoint and the resulting data is expressed in relative units.
2.4. Laser-induced choroidal neovascularization
Mice were anesthetized by intraperitoneal injection of ketamine hydrochloride (100mgkg−1) and xylazine (13.4mg kg−1). Pupils were dilated with 1% tropicamide. Induction of CNV was performed with an Argon laser as previously described with a slight modification ((Nakamura et al., 2015a); (Apte et al., 2006); (Kelly et al., 2007); (Sene et al., 2013). Briefly, after anesthesia induction and pupil dilation, four laser spots were placed around the optic disc (200mW, 0.1s, 100μm spot size) using a slit-lamp delivery system with a cover glass as a contact lens. Laser spots with vitreous, retinal, or subretinal hemorrhage were excluded from the analysis.
For measurement of CNV, RPE-choroid complex flat mounts were made as previously described (Ban et al., 2017; Nakamura et al., 2015a; Sene et al., 2013). Briefly, seven days after injury, mice were anesthetized and perfused with 2000μl of 5mg/ml FITC-labeled dextran (M.W. 2,000,000. Sigma-Aldrich) via the left ventricle. Eyes were enucleated and fixed immediately in 2% PFA for 30 minutes. Eyes were then washed with phosphate buffered saline (PBS) and the RPE-choroid complex was flat mounted onto a glass slide. Z-stack images of CNV spots were acquired using an Olympus Fluoview1000 confocal microscope and processed using ImageJ software (NIH, Bethesda, MD) to create pseudo volumetric 2D images. Pixel intensity was quantified using Metamorph software analysis (Sunnyvale, CA).
For FGF2 treatment, intravitreal injection of 1μl FGF2 or vehicle (PBS) was performed using a 30-gauge needle immediately after the laser injury.
For FGF2 neutralizing antibody treatment, intravitreal injection of either 1μl neutralizing antibody against FGF2 (ab33103, Abcam) or 1μl its IgG isotype control (MAB002, R&D Systems Inc.) was performed as described above.
2.5. Western blot analysis
Laser rupture of the Bruch’s membrane of C57BL/6J wild type mice was conducted as described above. Three days post CNV induction, RPE-choroid complex were collected and sonicated in lysis buffer supplemented with proteinase and phosphatase inhibitors. After being centrifuged at 14,000 × g for 20 min, the supernatants were collected for western blot analysis. The electrophoresis and transfer were performed as previously described (Sene et al., 2013; Nakamura et al., 2015b; Ban et al., 2017). The membranes were then blocked for 2 hours at room temperature with 5% (w/v) bovine serum albumin (BSA; Sigma-Aldrich) in TBS with 0.1% Tween-20 (0.1% TBST), and incubated with the antibodies against phospho-Stat1 (1:1000), phospho-Stat3 (1: 2000), phospho-Stat5 (1:1000), phospho-PLCγ1 (1: 1000), phospho-MEK1/2 (1:1000), phospho-AKT (1:1000) (Cell Signalling Technologies, Danvers, MA; #9145, 4322, 2821, 9121, and 4058, respectively), and monoclonal β-actin (1: 5000; A5316, Sigma) overnight at 4°C. Next, the membranes were washed with 0.1% TBST and incubated with secondary antibodies conjugated to either IRDye 800CW or IRDye 680LT (1:5,000; LI-COR, Lincoln, NE) for 60 min at room temperature, respectively. Immunoreactive bands were visualized using the Odyssey Infrared Imaging System (LI-COR). Then the membranes were stripped using Re-Blot Plus Western Blot Recycling Kit (EMD Milipore, Corp. Cat No.2500), and reprobed with antibodies against Stat1 (1:1000), Stat3 (1:1000), Stat5 (1:1000), PLCγ1 (1:1000), MEK1/2 (1:1000) and Akt (1:1000), respectively (Cell Signalling Technologies; 9172, 12640, 9272, 2282, and 9122). The membranes were then incubated with secondary antibodies and immunoreactive bands were visualized as described above.
2.6. Statistical analysis
Data are presented as mean±SEM. Statistical evaluations were performed using GraphPad Prism Software version 6.0 (GraphPad, San Diego, CA). One-way or 2-way mixed analysis of variance (ANOVA) with Bonferroni post test, or two-tailed Student’s unpaired t-test was used for comparison among groups. The accepted level of significance for all tests was p<0.05.
3. Results
3.1. FGF2 stimulates vascular sprouting from choroid explants
In prior studies, we demonstrated that after injury to induce CNV, Fgf2, Fgf8 and Fgf9 were the only FGF ligands whose expression was significantly upregulated in addition to anticipated upregulated of Vegfa (Oladipupo et al., 2014). In those studies, we also demonstrated a VEGF-independent and critical role for FGFR1/2 signaling in the vascular endothelial cell response to injury. These data suggested the possibility that FGF ligands induced during tissue injury were important in the VEGF-independent vascular response.
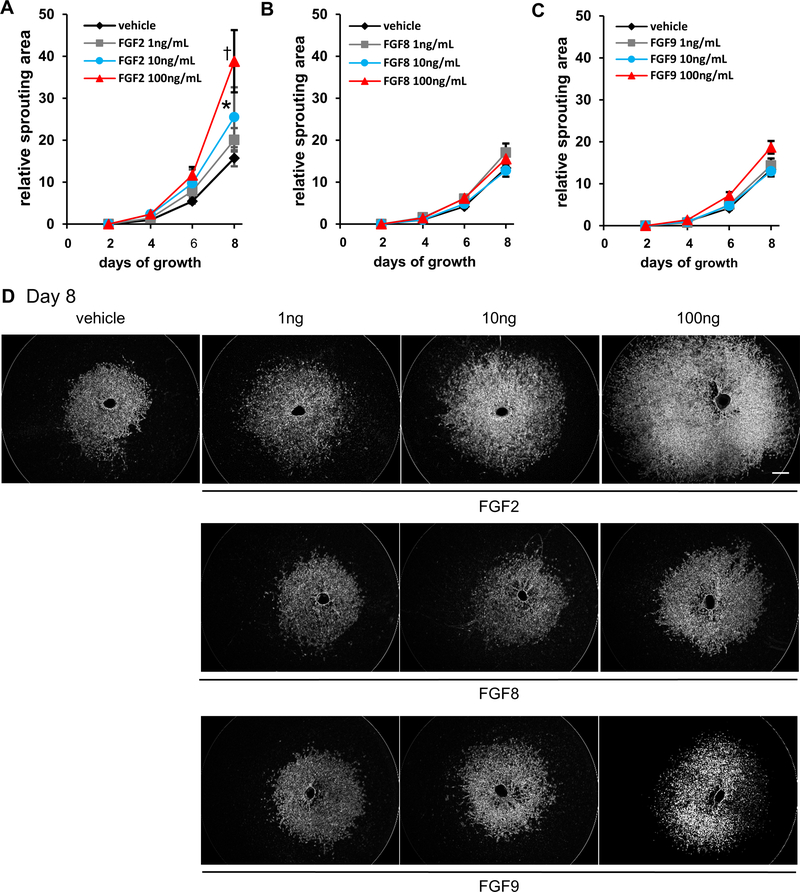
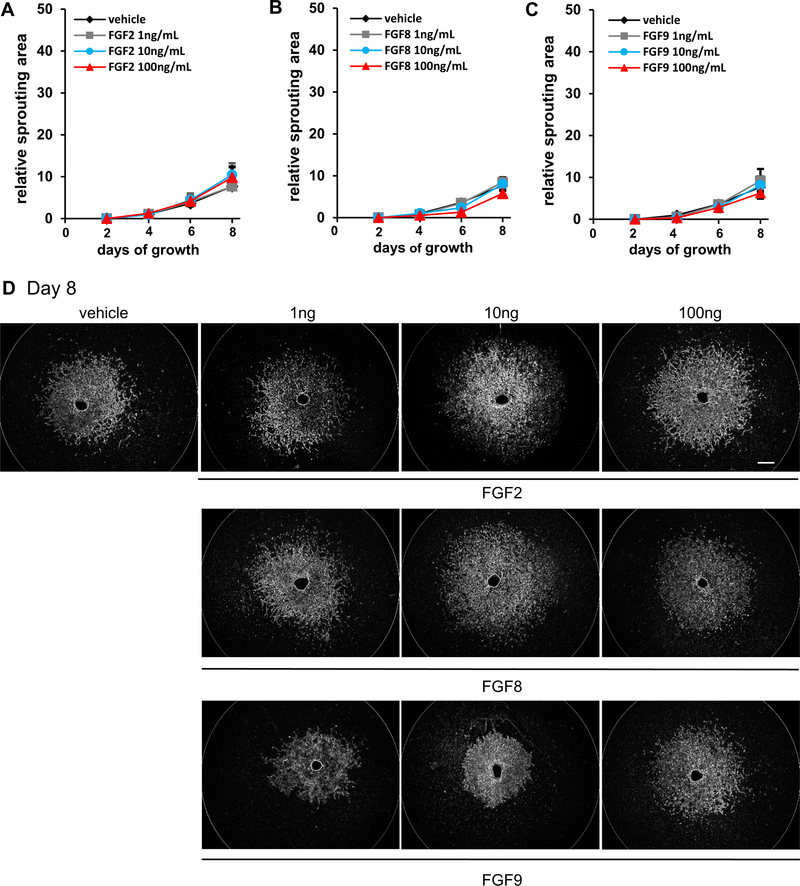
To further identify which FGF ligand(s) is critical to ocular neovascularization, we utilized the choroidal endothelial sprouting assay, a well-established ex vivo model of microvascular angiogenesis (Shao et al., 2013). We compared the effect of various doses of either FGF2, FGF8 or FGF9 on the proliferation of choroidal endothelial cells. Of note, only FGF2 stimulated vascular sprouting from choroid explants of C57BL/6J mice in a dose-dependent manner (normalized sprouting area relative to vehicle control for each choroid at day 8 post-explant was 25.5 ± 7.1, p<0.05 for 10ng FGF2 and 38.8 ± 7.4, p<0.01 for 100ng FGF2 as compared to 15.7 ± 1.9. n=9–11 for vehicle controls, 2-way mixed ANOVA with Bonferroni’s multiple comparison test. Fig. 1, A and D top panel). In contrast, neither FGF8 (n=14–16) nor FGF9 (n=10–14), affected vascular sprouting from choroid explants as compared to controls (Fig. 1, B–D bottom panels). Furthermore, the effect of FGF2 on vascular sprouting was completely eliminated if the choroidal explants were isolated from Fgfr1/2/3Tie2-Cre−/− mice where FGFR1/2/3 are selectively deficient in endothelial cells (Fig. 2, A and D top panel). As expected, neither FGF8 nor FGF9 effected vascular sprouting in Fgfr1/2/3Tie2-Cre−/− explants (Fig. 2, B–D bottom panel). These results suggest that among FGF ligands upregulated after injury, FGF2 but not FGF8 or 9, regulated choroidal endothelial cell sprouting. In addition, the effect of FGF2 required endothelial FGFR signaling as demonstrated by the failure to induce sprouting in choroidal explants isolated from mice lacking vascular endothelial FGFR signaling.
Fig. 1. FGF2, but not FGF8 or FGF9, stimulates neovascular sprouting.
FGF2 stimulated neovascular sprouting in a dose-dependent manner in C57Bl/6J choroid explants by 8 days post-treatment at doses of 10 or 100 ng/ml (n=9–11; 15.7 ± 1.9 vs. 25.5 ± 7.1 relative sprouting area (RSA) (10ng/mL); *p<0.05, and 15.7 ± 1.9 vs. 38.8 ± 7.4 RSA (100ng/mL); † p<0.01; 2-way mixed ANOVA with Bonferroni’s multiple comparison test). (A). Neither FGF8 (n=14–16; 13.4 ± 0.73 vs. 15.5 ± 1.5 RSA (100ng/mL) (B) nor FGF9 (n=10–14; 13.4 ± 0.73 vs. 18.7 ± 1.5 RSA) (C) stimulated choroidal sprouting regardless of dose. Representative pictures of choroidal explants treated with FGF2, FGF8 or FGF9 (D). Scale bar: 500 μm.
Fig. 2. Neovascularization stimulated by FGF2 requires endothelial FGFR.
Neither FGF2 (9.8 ± 2.4 RSA at 100ng/mL) (A), FGF8 (5.7 ± 0.88 RSA at 100ng/mL) (B) nor FGF9 (6.2 ± 1.3 RSA at 100ng/mL) (C) ligands stimulated choroidal sprouting in FGFR1/2/3−/− endothelial cell triple knockout mouse choroid explants (vs. vehicle control 7.7 ± 0.93 RSA at 100ng/mL; n=3–8; no significance in 2-way mixed ANOVA with Bonferroni’s multiple comparison test). Representative pictures of choroidal spouting treatments (D) Scale bar: 500 μm.
3.2. FGF2 promotes choroidal neovascularization in mice
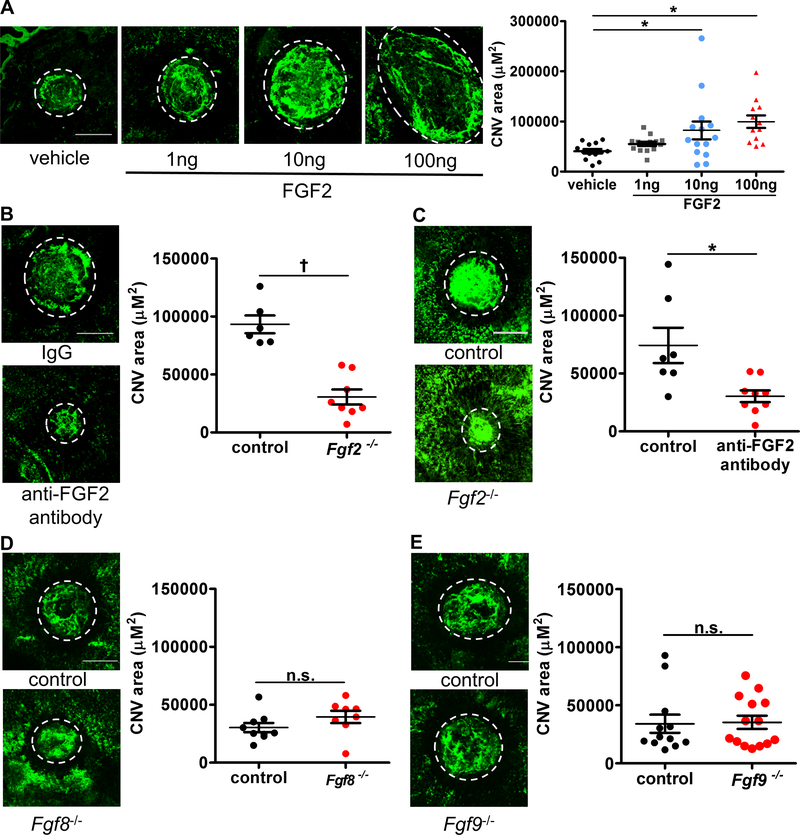
Based on the above results, we next investigated whether FGF2 stimulated angiogenesis in the previously described laser injury-induced CNV mouse model. FGF2 (1ng, 10ng or 100ng) or PBS was injected into the vitreous immediately after the laser injury and evaluation of CNV was performed 7 days later by confocal microscopy. As compared to control mice that received PBS injection (40599.3 ± 4508.1 μm2), FGF2 promoted CNV formation in a dose-dependent manner, particularly at a dose of 10ng or 100ng (82273.4 ± 17833.4 μm2, p<0.01, and 93878.9 ± 12475.6 μm2, p<0.01, respectively. n=13–16, one-way ANOVA with Bonferroni’s multiple comparison test. Fig. 3A). On the other hand, intravitreal injection of anti-FGF2 neutralizing antibody significantly suppressed CNV formation as compared to the injection of control antibody (30369.2 ± 5010.7 μm2 vs. 74338.9 ± 15250.2 μm2, p<0.05, respectively. n=7–9, Mann-Whitney u-test. Fig. 3B). To confirm the role of FGF in promoting CNV, we used Fgf2−/− mice that lacked expression of the FGF2 protein. In Fgf2−/− mice, CNV was significantly reduced as compared to littermate controls (93264 ± 7690 μm2 vs. 30613 ± 6476 μm2, p<0.0001, respectively. n=6–8, two-tailed Student’s unpaired t-test. Fig. 3C). No difference in CNV formation was noted in mice lacking FGF8 (30356 ± 3989 μm2 vs. 39511 ± 5310, n=8–9) (Fig. 3D or FGF9 (34114 ± 7775 μm2 vs. 35336 ± 9459 μm2) (Fig. 3F) expression compared to their respective littermate controls. Collectively, those results demonstrate that FGF2, but not FGF8 or FGF9, plays an important, pro-angiogenic role in choroidal neovascularization.
Fig. 3. FGF2, but not FGF8 or FGF9 stimulates pathogenic CNV after laser induction.
FGF2 stimulated CNV formation following laser injury in C57Bl/6J mice in a dose-dependent manner, reaching significance at doses of 10 ng and 100 ng compared to mice receiving vehicle control (n=13–16; 40599.3 ± 4508.1 μm2 vs 82273.4 ± 17833.4 μm2, *p<0.01 (10ng) or 93878.9 ± 12475.6 μm2, *p<0.01 (100ng); one-way ANOVA with Bonferroni’s multiple comparison test (A). Neutralizing antibody against FGF2 significantly suppressed CNV formation (n=7–9; 30369.2 ± 5010.7 μm2 vs. 74338.9 ± 15250.2 μm2, *p<0.05; two-tailed Student’s unpaired t-test (B). Furthermore, CNV formation was impaired in Fgf2−/− mice as compare to its littermates (n=6–7; 93264 ± 7690 μm2 vs. 26699 ± 5958 μm2, †p<0.0001; two-tailed Student’s unpaired t-test) (C) while deletion of Fgf8 (D) (n=8; n.s.= no significance by two-tailed Student’s unpaired t-test) or Fgf9 (E) (n=13–15; n.s.= no significance by two-tailed Student’s unpaired t-test) did not significantly modify CNV volumes compared to their respective littermate controls. Scale bar: 200 μm.
3.3. Activation of the STAT3 pathway occurs after induction of CNV
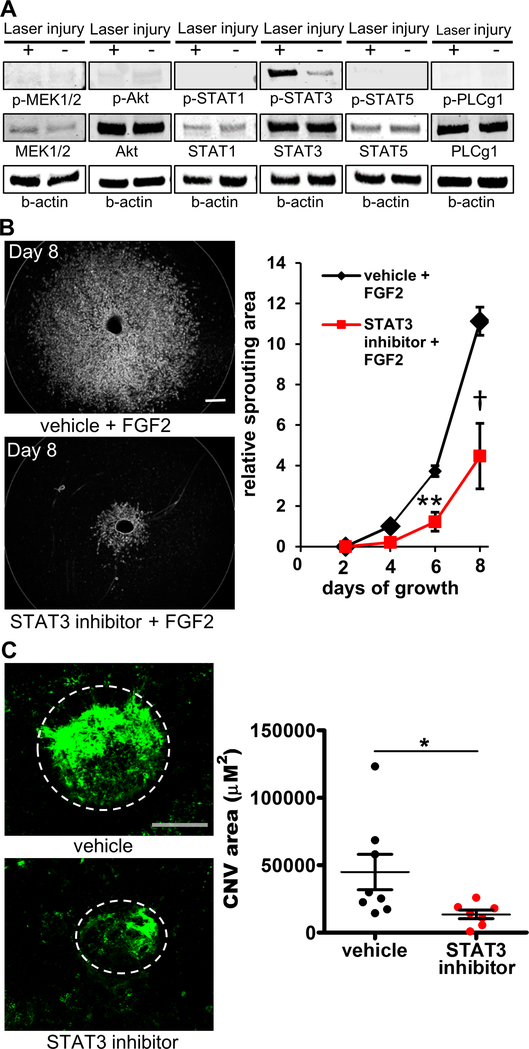
Next, we investigated which FGF2-mediated intracellular signaling pathway(s) were potentially critical to ocular neovascularization given the robust phenotype above. We induced laser injury in the choroid of right eye of C57BL/6J wild type mice and used the left eye as an uninjured control. Three days after the injury, eyes were enucleated and the choroid-RPE complex was harvested and processed for immunoblotting. As shown in Fig. 4A, among the four intracellular signaling pathways that are known to be activated by FGF2 (RAS-RAF-MAPK, PI3K-AKT, STAT1,3,5 and PLCγ pathways), only STAT3 was activated following laser injury while the other signaling mediators including MEK1/2 in RAS-RAF-MAPK pathway, AKT in PI3K-AKT pathway, PLCγ1 in PLCγ-regulated pathways, and STAT1 and STAT5 were not activated.
Fig. 4. The STAT3 signaling pathway is essential for CNV growth.
Western blotting demonstrates that the STAT3 pathway in the RPE-choroid complex is activated following the induction of choroidal neovascularization, while other FGF2-induced intracellular signaling pathways remain inactivate (A). Inhibition of STAT3 by the small molecule, Stattic, significantly suppressed choroidal sprouting 8 days post-explant dissection (n=6; 4.5 ± 0.9 vs 9.3 ± 0.8; *p<0.05; 2-way mixed ANOVA with Bonferroni’s multiple comparison test) (B). STAT3 inhibition also significantly suppressed CNV formation in vivo (n=7–8; 13496.7 ± 3202 μm2 vs. 44932.3 ± 13152.9 μm2, *p<0.05; two-tailed Student’s unpaired t-test) (C) Scale bar: 200 μm.
3.4. FGF2 stimulates choroidal neovascularization via STAT3 activation
Based on these results, we next examined whether STAT3 inhibition might suppress ocular angiogenesis in both the ex vivo and in vivo models presented above. Stattic, a small-molecule inhibitor of STAT3 activation and dimerization, significantly suppressed FGF2-induced choroidal sprouting by day 8 as compared to choroid explants treated with vehicle (relative sprouting area at day 8 is 4.5 ± 0.9 vs 9.3 ± 0.8, respectively. n=6, 2-way mixed ANOVA with Bonferroni’s multiple comparison test. Fig. 4B). In the injury-induced CNV model, Stattic significantly suppressed CNV development (13496.7 ± 3202 μm2 vs. 44932.3 ± 13152.9 μm2, p<0.05, respectively. n=7–8, two-tailed Student’s unpaired t-test. Fig. 4C). These results suggest that FGF2 may signal through the STAT3 pathway in endothelial cells to regulate ocular neovascularization.
4. Discussion
Pathologic neovascularization is critical in cancers, atherosclerosis and blinding eye diseases including AMD and diabetic retinopathy. VEGF is an important driver of neovascularization in these diseases and therapies that target VEGF can reduce pathogenic vascularization in many of these diseases (Apte et al., 2019). In many advanced/metastatic cancers, anti-VEGF therapies are approved by regulatory authorities for therapy in combination with other chemotherapeutic agents (Duh et al., 2017; Miller et al., 2013; Sene and Apte, 2014; Sene et al., 2015).
As in cancer, several anti-VEGF agents have been demonstrated to prevent vision loss in eye diseases including AMD and diabetic retinopathy (Aiello, 2005; Heier et al., 2012). A key therapeutic challenge in eye diseases is the clear evidence of under-responsiveness to VEGF-targeted therapy over the long term, the increased therapeutic burden associated with the need for chronic intraocular therapy, and the resistance to therapy that develops in some patients (Apte, 2016). This may be similar to what has been known for a long time with cancer therapy where multiple therapeutic agents that target diverse molecular pathways usually lead to optimal clinical outcomes (Gerber and Ferrara, 2005). As such, there is a strong need to identify novel, VEGF-independent pathways that can be developed for eye diseases but this effort has been hampered by recent therapeutic failures.
Here, using both in vivo and in vitro models, we identify FGF2 as the critical ligand that activates Stat3 via FGFR signaling and promotes pathologic neovascularization. Given that our previous studies demonstrate that the FGFR signaling pathway may be VEGF independent, this finding is potentially therapeutically relevant in opening a novel vista for development where either FGF2 or STAT3 may be targeted in order to reduce pathogenic neovascularization. This is especially exciting given the compounds that target both these molecules are currently being tested in clinical trials. Due to the potential binding of FGF2 to VEGFR2, it remains possible that FGF2 could contribute to VEGF signaling to some degree. Although a significant amount of work needs to be done in the translational aspects in determining whether targeting these pathways is safe and efficacious, it remains possible that should these pathways have therapeutic value. Such therapeutic agents could be combined with current anti-VEGF regimens in order to enhance efficacy and reduce the risk of vision loss in these devastating diseases.
Highlights.
Fibroblast growth factor-2 (FGF-2) regulates choroidal neovascularization
FGF-2 avtivates intracellular stat3
The identification of the fgf-2/stat3 axis presents novel therapeutic opportunities
Acknowledgments
Grant information
This work was supported by NIH Grants R21 EY026707-01 (R.S.A. and D.M.O.), R01 EY019287-06 (R.S.A.), P30 EY02687 (Vision Core Grant); the Starr Foundation (R.S.A.); the Carl Marshall Reeves and Mildred Almen Reeves Foundation (R.S.A.); the Bill and Emily Kuzma Family Gift for Retinal Research (R.S.A.); a Physician Scientist Award and a Nelson Trust Award from Research to Prevent Blindness (R.S.A.); the Jeffrey Fort Innovation Fund (R.S.A.); the Glenn Foundation for Medical Research and the Thome Foundation (R.S.A.). Additional funding comes from an unrestricted grant to the Department of Ophthalmology and Visual Sciences of Washington University School of Medicine from Research to Prevent Blindness. Z.D. was supported by the VitreoRetinal Surgery Foundation.
Footnotes
Conflicts of interest
The authors have declared that no conflict of interest exists.
Financial disclosure
The authors have no financial or property interest in the materials or methods described herein.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello LP, 2005. Angiogenic pathways in diabetic retinopathy. N Engl J Med 353, 839–841. [DOI] [PubMed] [Google Scholar]
- Apte RS, 2016. What Is Chronic or Persistent Diabetic Macular Edema and How Should It Be Treated? JAMA Ophthalmol 134, 285–286. [DOI] [PubMed] [Google Scholar]
- Apte RS, Chen DS, Ferrara N, 2019. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 176, 1248–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte RS, Richter J, Herndon J, Ferguson TA, 2006. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med. 3, e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakall B, Folk JC, Boldt HC, Sohn EH, Stone EM, Russell SR, Mahajan VB, 2013. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol 156, 15–22 e11. [DOI] [PubMed] [Google Scholar]
- Ban N, Siegfried CJ, Lin JB, Shui YB, Sein J, Pita-Thomas W, Sene A, Santeford A, Gordon M, Lamb R, Dong Z, Kelly SC, Cavalli V, Yoshino J, Apte RS, 2017. GDF15 is elevated in mice following retinal ganglion cell death and in glaucoma patients. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PY, Qin L, Li G, Tellides G, Simons M, 2016. Fibroblast growth factor (FGF) signaling regulates transforming growth factor beta (TGFbeta)-dependent smooth muscle cell phenotype modulation. Sci Rep 6, 33407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM, 1996. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet 12, 390–397. [DOI] [PubMed] [Google Scholar]
- Colvin JS, White AC, Pratt SJ, Ornitz DM, 2001. Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development 128, 2095–2106. [DOI] [PubMed] [Google Scholar]
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P, 1996. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84, 911–921. [DOI] [PubMed] [Google Scholar]
- Duh EJ, Sun JK, Stitt AW, 2017. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperini JL, Fawzi AA, Khondkaryan A, Lam L, Chong LP, Eliott D, Walsh AC, Hwang J, Sadda SR, 2012. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol 96, 14–20. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Ferrara N, 2005. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res 65, 671–680. [PubMed] [Google Scholar]
- Gospodarowicz D, Brown KD, Birdwell CR, Zetter BR, 1978. Control of proliferation of human vascular endothelial cells. Characterization of the response of human umbilical vein endothelial cells to fibroblast growth f actor, epidermal growth factor, and thrombin. J Cell Biol 77, 774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U, Grp VVS, 2012. Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-related Macular Degeneration. Ophthalmology 119, 2537–2548. [DOI] [PubMed] [Google Scholar]
- House SL, Castro AM, Lupu TS, Weinheimer C, Smith C, Kovacs A, Ornitz DM, 2016. Endothelial fibroblast growth factor receptor signaling is required for vascular remodeling following cardiac ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 310, H559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J, Ali Khan A, Yin J, Ferguson TA, Apte RS, 2007. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J. Clin. Invest 117, 3421–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers EN, Lewandoski M, Martin GR, 1998. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet 18, 136–141. [DOI] [PubMed] [Google Scholar]
- Miller JW, Le Couter J, Strauss EC, Ferrara N, 2013. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology 120, 106–114. [DOI] [PubMed] [Google Scholar]
- Murakami M, Nguyen LT, Zhuang ZW, Moodie KL, Carmeliet P, Stan RV, Simons M, 2008. The FGF system has a key role in regulating vascular integrity. J Clin Invest 118, 3355–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R, Sene A, Santeford A, Gdoura A, Kubota S, Zapata N, Apte RS, 2015b. IL10-driven STAT3 signalling in senescent macrophages promotes pathological eye angiogenesis. Nat Commun 6, 7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladipupo SS, Smith C, Santeford A, Park C, Sene A, Wiley LA, Osei-Owusu P, Hsu J, Zapata N, Liu F, Nakamura R, Lavine KJ, Blumer KJ, Choi K, Apte RS, Ornitz DM, 2014. Endothelial cell FGF signaling is required for injury response but not for vascular homeostasis. Proc Natl Acad Sci U S A 111, 13379–13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N, 2015. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol 4, 215–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sene A, Apte RS, 2014. Eyeballing cholesterol efflux and macrophage function in disease pathogenesis. Trends Endocrinol. Metab. 25, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sene A, Chin-Yee D, Apte RS, 2015. Seeing through VEGF: innate and adaptive immunity in pathological angiogenesis in the eye. Trends Mol Med 21, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sene A, Khan AA, Cox D, Nakamura RE, Santeford A, Kim BM, Sidhu R, Onken MD, Harbour JW, Hagbi-Levi S, Chowers I, Edwards PA, Baldan A, Parks JS, Ory DS, Apte RS, 2013. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab. 17, 549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Friedlander M, Hurst CG, Cui Z, Pei DT, Evans LP, Juan AM, Tahiri H, Duhamel F, Chen J, Sapieha P, Chemtob S, Joyal JS, Smith LE, 2013. Choroid sprouting assay: an ex vivo model of microvascular angiogenesis. PLoS One 8, e69552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner K, Hong T, Bahrami B, Chang A, 2018. A meta-analysis of patients with treatment-resistant macular oedema secondary to retinal vein occlusions following switching to aflibercept. Acta Ophthalmol. [DOI] [PubMed] [Google Scholar]
- Stahl A, Paschek L, Martin G, Feltgen N, Hansen LL, Agostini HT, 2009. Combinatory inhibition of VEGF and FGF2 is superior to solitary VEGF inhibition in an in vitro model of RPE-induced angiogenesis. Graefes Arch. Clin. Exp. Ophthalmol 247, 767–773. [DOI] [PubMed] [Google Scholar]
- Trokovic R, Trokovic N, Hernesniemi S, Pirvola U, Vogt Weisenhorn DM, Rossant J, McMahon AP, Wurst W, Partanen J, 2003. FGFR1 is independently required in both developing mid- and hindbrain for sustained response to isthmic signals. EMBO J 22, 1811–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, Kehoe SM, Johannessen CM, Macconaill LE, Hahn WC, Meyerson M, Garraway LA, 2011. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol 29, 3085–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Weinstein M, Li C, Naski M, Cohen RI, Ornitz DM, Leder P, Deng C, 1998. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development 125, 753–765. [DOI] [PubMed] [Google Scholar]
- Yamada H, Yamada E, Kwak N, Ando A, Suzuki A, Esumi N, Zack DJ, Campochiaro PA, 2000. Cell injury unmasks a latent proangiogenic phenotype in mice with increased expression of FGF2 in the retina. J. Cell. Physiol 185, 135–142. [DOI] [PubMed] [Google Scholar]
- Yonekawa Y, Andreoli C, Miller JB, Loewenstein JI, Sobrin L, Eliott D, Vavvas DG, Miller JW, Kim IK, 2013. Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol 156, 29–35 e22. [DOI] [PubMed] [Google Scholar]
- Zhou M, Sutliff RL, Paul RJ, Lorenz JN, Hoying JB, Haudenschild CC, Yin M, Coffin JD, Kong L, Kranias EG, Luo W, Boivin GP, Duffy JJ, Pawlowski SA, Doetschman T, 1998. Fibroblast growth factor 2 control of vascular tone. Nat Med 4, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]