Abstract
Background and objectives:
This paper aims to describe and compare the characteristics of two stroke populations in Singapore and in St. Louis, USA, and to document thrombolysis rates and contrast factors associated with its uptake in both populations.
Methods:
The stroke populations described were from the Singapore Stroke Registry (SSR) in Singapore and the Cognitive Rehabilitation Research Group Stroke Registry (CRRGSR) in St Louis, Missouri, USA. The registries were compared in terms of demographics and stroke risk factor history. Logistic regression was used to determine factors associated with thrombolysis uptake.
Results:
A total of 39,323 and 8,106 episodes were recorded in SSR and CRRGSR respectively from 2005 to 2012. Compared to CRRGSR, patients in SSR were older, male and from the ethnic majority. Thrombolysis rates in SSR and CRRGSR were 2.5% and 8.2% respectively for the study period. History of ischemic heart disease or atrial fibrillation was associated with increased uptake in both populations, while history of stroke was associated with lower uptake. For SSR, younger age and males were associated with increased uptake, while having a history of smoking or diabetes was associated with decreased uptake. For CRRGSR, ethnic minority status was associated with decreased uptake.
Conclusions:
The comparison of stroke populations in Singapore and St Louis revealed distinct differences in clinico-demographics of the two groups. Thrombolysis uptake was driven by non-ethnicity demographics in Singapore. Ethnicity was the only demographic driver of uptake in the CRRGSR population, highlighting the need to target ethnic minorities in increasing access to thrombolysis.
Keywords: Stroke, thrombolysis, treatment, international comparison
2. Introduction
Thrombolysis via intravenous recombinant tissue plasminogen activator (rtPA) is a recommended treatment for ischemic strokes with optimal recovery rates documented when administered within 3 hours from stroke onset, and moderate recovery for 3 to 4.5 hours from onset [1]. Administration is traditionally carried out within stroke centres or hospitals with expertise and access to facilities such as cerebral angiography [2]. Thrombolysis rates reported in stroke registries vary widely across regions, from 0.6% in Taiwan [3] to 28% in Germany [4], due to different treatment type and time intervals from onset considered. Delivery of treatment has primarily been associated with health system factors, such as time to stroke discovery and expertise [5], but patient-level factors such as demographic characteristics and risk factors have also been influential on delivery of thrombolysis [6]. Hence, there is a need to understand thrombolysis use and its patient-level drivers, as well as its variation across different countries.
The global burden of stroke on populations worldwide is well established, causing the second highest disability adjusted life years (DALYs) in the world [7]. However, few studies focus on contrasting differences in stroke burden between Asian and Western stroke populations [8,9]. A detailed comparison of individual-level stroke data from USA and Singapore will allow an examination of demographic and clinical characteristics of each population and facilitate better understanding of the clinical drivers and health challenges between the Asian and Western contexts. Therefore, the first aim of this paper was to describe and compare the stroke populations from hospitals in Singapore and USA, in terms of demographics, risk factor history and thrombolysis rates. The second aim was to contrast the factors associated with use of thrombolytic therapy between the two registries. This study is the first to explore stroke in two multi-ethnic countries across Asian and Western contexts.
3. Methods
Two stroke registry datasets from Singapore and USA were combined and analyzed. Ethics approval for the analysis was obtained from the Institutional Review Board of the National University of Singapore (NUS) and Washington University in St Louis (WUSTL), and a waiver of patient informed consent was granted by both boards. The methodology of data collection for both datasets have been described in detail elsewhere [10–12]. In brief, the dataset from Singapore consisted of de-identified stroke episodes from the Singapore Stroke Registry (SSR), accessed via the National Registry of Diseases Office (NRDO) under the Ministry of Health (MOH). The SSR received stroke case notifications from (1) all public healthcare institutions via the Hospital In-patient Discharge Summary, (2) MOH via information on medical claims to the government (MediClaims list), and (3) the national death registry. NRDO’s Registry Coordinators verified the cases and extracted the detailed clinical information required by SSR, covering about 94% of strokes occurring in the country.
The stroke registry dataset from the USA consisted of stroke episodes from a prospective cohort of stroke patients served by the Comprehensive Stroke Center at the Barnes-Jewish Hospital (BJH) in St Louis, Missouri, collected by the Cognitive Rehabilitation Research Group (CRRG) at WUSTL. Consent for follow up was given at point of interview during the hospital stay. Data were extracted from the hospital system for patients admitted between 1999 and 2017. Case notes were verified by registry coordinators and all patient information were exported into a standard report form. The de-identified CRRG data was combined with the SSR data in NRDO. Variables common to both registries were identified and reviewed to ensure the same definitions and categorizations were used. Thrombolysis was defined as use of intravenous tissue plasminogen activator (tPA) in both registries.
3.1. Statistical analysis
Only ischemic strokes that occurred between 1st January 2005 and 31st December 2012 (the overlapping period) in both registries were included in this study, using the definition of stroke by the World Health Organization with supporting radiological data and a set of International Classification of Diseases codes for ischemic stroke [10]. The two stroke populations were compared in terms of demographic factors and history of risk factors. The thrombolysis rates for the study period and by year were also assessed. Ethnicities in both populations were regrouped into majority and minority groups. For the SSR, patients of Chinese ethnicity were identified as the majority while Malay, Indian and other ethnicities were grouped together as the ethnic minority. For the CRRGSR, Caucasians were identified as the majority while African-American, Hispanic, Asian and other ethnicities were grouped as the ethnic minority.
To examine the factors associated with thrombolysis, logistic regression was used with uptake as the outcome and models were built using pooled data from both countries, as well as for each country separately. Unadjusted logistic regression was used to identify factors independently associated with uptake, using a p-value criterion of 0.05 and below for statistical significance. For multivariable analysis, all factors were included in the same model and a backward stepwise approach was implemented for variable selection, using the abovementioned p-value criterion to obtain a parsimonious model. Odds ratios and their corresponding 95% confidence intervals (CIs) were reported for each factor. Sensitivity analyses were carried out by building the same regression models using the respective ethnic groups of the two populations. All analyses were performed in RStudio [13].
4. Results
4.1. Clinico-demographic profile of both stroke populations
A total of 47,429 ischemic strokes were recorded from 2005 to 2012, with 39,323 and 8,106 episodes recorded in the SSR and CRRGSR respectively (Table 1). The two stroke populations differed in most demographic characteristics, with a larger proportion of patients in the SSR being above 65 years old and belonging to the male sex or ethnic majority. The distributions of risk factors for stroke differed significantly between the two populations, with patients in the CRRGSR exhibiting a higher prevalence across all investigated factors, except for diabetes mellitus (SSR: 40.4%; CRRGSR: 23.5%). For thrombolysis rates, 2.5% of episodes in the SSR were treated with thrombolysis, a lower rate in comparison to 8.2% in the CRRGSR (Table 1). Examining the rates by year, uptake increased over the study period in both countries (Figure 1).
Table 1.
Characteristics of study populations from SSR (Singapore) and CRRGSR (St Louis, Missouri, USA)
| Combined (N=47,429) | SSR (N=39,323) | CRRGSR (N=8,106) | |
|---|---|---|---|
| N (%) | N (%) | ||
| Year of admission | |||
| 2005 | 5,332 (11.2%) | 4,662 (11.9%) | 670 (8.3%) |
| 2006 | 5,317 (11.2%) | 4,600 (11.7%) | 717 (8.8%) |
| 2007 | 5,641 (11.9%) | 4,801 (12.2%) | 840 (10.4%) |
| 2008 | 5,678 (12.0%) | 4,689 (11.9%) | 989 (12.2%) |
| 2009 | 5,979 (12.6%) | 4,887 (12.4%) | 1,092 (13.5%) |
| 2010 | 6,248 (13.2%) | 5,025 (12.8%) | 1,223 (15.1%) |
| 2011 | 6,591 (13.9%) | 5,219 (13.3%) | 1,372 (16.9%) |
| 2012 | 6,643 (14.0%) | 5,440 (13.8%) | 1,203 (14.8%) |
| Age at stroke onset (years) | |||
| Median (IQR) | 68.0 (58.0–78.0) | 69.0 (59.0–78.0) | 64.0 (53.0–76.0) |
| Range | 15.0–115.0 | 15.0–115.0 | 18.0–90.0 |
| Age group | |||
| <50 years | 4,829 (10.2%) | 3,382 (8.6%) | 1,447 (17.9%) |
| 50–64 years | 14,606 (30.8%) | 11,920 (30.3%) | 2,686 (33.1%) |
| ≥65 years | 27,994 (59.0%) | 24,021 (61.1%) | 3,973 (49.0%) |
| Sex | |||
| Female | 21,436(45.2%) | 17,228 (43.8%) | 4,208 (51.9%) |
| Male | 25,993 (54.8%) | 22,095 (56.2%) | 3,898 (48.1%) |
| Ethnic group | |||
| Majority | 34,043 (71.8%) | 29,519 (75.1%) | 4,524 (55.8%) |
| Minority | 13,386 (28.2%) | 9,804 (24.9%) | 3,582 (44.2%) |
| Risk factors for stroke | |||
| Transient ischemic attack (TIA) | 2,797 (5.9%) | 1,766 (4.5%) | 1,031 (12.7%) |
| Stroke | 12,665 (26.7%) | 10,237 (26.0%) | 2,428 (30.0%) |
| Hypertension | 36,206 (76.3%) | 29,868 (76.0%) | 6,338 (78.2%) |
| Diabetes mellitus | 17,781 (37.5%) | 15,876 (40.4%) | 1,905 (23.5%) |
| Ischemic heart disease | 12,092 (25.5%) | 9,612 (24.4%) | 2,480 (30.6%) |
| Atrial fibrillation | 5,808 (12.2%) | 4,636 (11.8%) | 1,172 (14.5%) |
| Valvular heart disease | 1,302 (2.7%) | 898 (2.3%) | 404 (5.0%) |
| Peripheral heart disease | 1,996 (4.2%) | 1,408 (3.6%) | 588 (7.3%) |
| Smoking | 18,421 (38.8%) | 15,169 (38.6%) | 3,252 (40.1%) |
| Inpatient events | |||
| CT/Head scan | 46,292 (97.6%) | 39,237 (99.8%) | 7,055 (87.0%) |
| Thrombolysis | 1,647 (3.5%) | 985 (2.5%) | 662 (8.2%) |
| Length of stay (days) | |||
| Median (IQR) | 5.0(3.0–10.0) | 6.0 (3.0–11.0) | 3.0 (2.0–7.0) |
| Maximum | 400.0 | 388.0 | 400.0 |
| Discharge destination | |||
| Deceased | 3,317(7.0%) | 2,957 (7.5%) | 360 (4.4%) |
| Home (no rehabilitation) | 17,812(37.6%) | 15,915 (40.5%) | 1,897 (23.4%) |
| Home (with rehabilitation) | 8,039 (16.9%) | 5,915 (15.0%) | 2,124 (26.2%) |
| Nursing home | 2,534 (5.3%) | 1,655 (4.2%) | 879 (10.8%) |
| Other hospitals | 3,071 (6.5%) | 2,891 (7.4%) | 180 (2.2%) |
| Inpatient rehabilitation hospital | 12,656 (26.7%) | 9,990 (25.4%) | 2,666 (32.9%) |
SSR: Singapore Stroke Registry; CRRGSR: Cognitive Rehabilitation Research Group Stroke Registry; IQR: Interquartile range
Figure 1. Thrombolysis rates over time in CRRGSR and SSR.
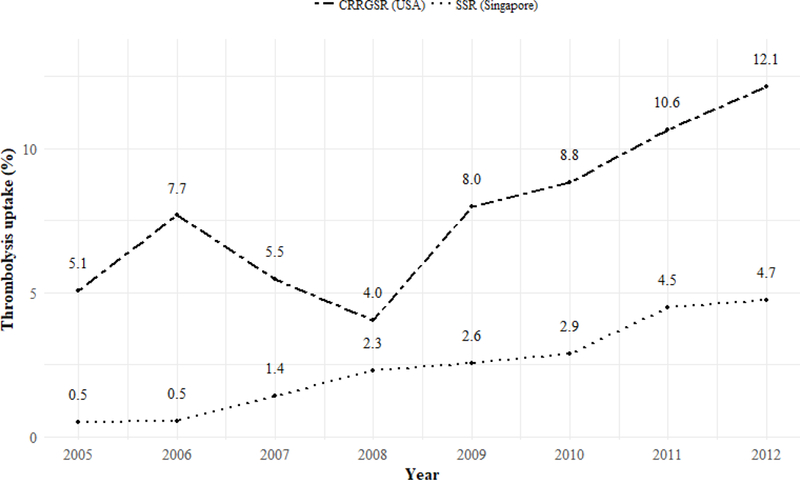
SSR: Singapore Stroke Registry; CRRGSR: Cognitive Rehabilitation Research Group Stroke Registry
4.2. Factors associated with thrombolysis uptake
From bivariable analysis of pooled information from both stroke populations (Table 2), being of older age was associated with lower thrombolysis uptake (Odds Ratio=0.99, 95% confidence interval: 0.99–0.99), while male sex (1.17, 1.06–1.29) and ethnic minority (1.16, 1.04–1.29) were associated with higher thrombolysis uptake. Clinical risk factors such as having a history of transient ischemic attack (TIA) (1.28, 1.06–1.54), ischemic heart disease (1.30, 1.17–1.45) or atrial fibrillation (1.71, 1.51–1.94) were also associated with higher uptake. In contrast, history of stroke (0.58, 0.51–0.66) or diabetes (0.67, 0.60–0.74) were associated with lower rates of thrombolysis. In addition, uptake was significantly higher in all years after 2005, with the highest in 2012 (5.99, 4.53–7.93). In the multivariable analysis, younger age (0.99, 0.99–1.00), males (1.20, 1.08–1.33), history of ischemic heart disease (1.29, 1.15–1.46) and atrial fibrillation (1.74, 1.51–1.99) remained associated with thrombolysis use upon admission, while history of stroke (0.53, 0.47–0.61) or diabetes (0.81, 0.72–0.90) were associated with lower uptake.
Table 2.
Logistic regression analyses of thrombolysis rates, using pooled data
| Bivariable OR (95% CI) | Multivariable OR (95% CI) | |
|---|---|---|
| Country | ||
| SG | 1.00 | 1.00 |
| USA | 3.46 (3.13–3.83) *** | 3.10 (2.79–3.45) *** |
| Demographics | ||
| Age at admission | 0.99 (0.99–0.99) *** | 0.99 (0.99–1.00) *** |
| Sex | ||
| Male | 1.17 (1.06–1.29) ** | 1.20 (1.08–1.33) *** |
| Ethnicity | ||
| Minority | 1.16 (1.04–1.29) ** | |
| Admission year | ||
| 2005 | 1.00 *** | 1.00 *** |
| 2006 | 1.40 (0.99–1.97) | 1.36 (0.97–1.92) |
| 2007 | 1.91 (1.39–2.63) | 1.80 (1.30–2.48) |
| 2008 | 2.48 (1.82–3.37) | 2.26 (1.66–3.08) |
| 2009 | 3.40 (2.53–4.57) | 3.06 (2.28–4.12) |
| 2010 | 3.91 (2.92–5.22) | 3.42 (2.56–4.58) |
| 2011 | 5.66 (4.28–7.50) | 4.87 (3.67–6.46) |
| 2012 | 5.99 (4.53–7.93) | 5.40 (4.07–7.15) |
| Risk factors | ||
| TIA | 1.28 (1.06–1.54) * | |
| Previous stroke | 0.58 (0.51–0.66) *** | 0.53 (0.47–0.61) *** |
| Hypertension | 0.94 (0.84–1.05) | |
| Diabetes mellitus | 0.67 (0.60–0.74) *** | 0.81 (0.72–0.90) *** |
| Ischemic heart disease | 1.30 (1.17–1.45) *** | 1.29 (1.15–1.46) *** |
| Atrial fibrillation | 1.71 (1.51–1.94) *** | 1.74 (1.51–1.99) *** |
| Valvular heart disease | 1.26 (0.96–1.65) | |
| Peripheral heart disease | 1.17 (0.93–1.47) | |
| Smoking | 0.99 (0.90–1.10) | |
p<0.05;
p<0.01;
p<0.001;
SSR: Singapore Stroke Registry; CRRGSR: Cognitive Rehabilitation Research Group Stroke Registry; OR: Odds ratio; CI: Confidence interval
Examining only the SSR population (Table 3), similar bivariable associations with thrombolysis uptake were observed, except for ethnicity (1.12, 0.97–1.29) and history of TIA (1.24, 0.94–1.64) which were no longer statistically significant. In the multivariable analysis, smoking was additionally found to be associated with lower uptake (0.78, 0.67–0.90). Sensitivity analyses did not show any statistically significant association of the different ethnic minorities with thrombolysis uptake (Table S1). The uptake increased significantly from 2007 onwards (2007: 2.78, 1.73–4.47; 2012: 9.35, 6.09–14.36).
Table 3.
Logistic regression analyses of thrombolysis rates, using country-specific data
| SSRSSR (N=39,323) | CRRGSR (N=8,106) | |||
|---|---|---|---|---|
| Bivariable OR (95% CI) |
Multivariable OR (95% CI) |
Bivariable OR (95% CI) |
Multivariable OR (95% CI) |
|
| Demographics | ||||
| Age at admission | 0.98 (0.98–0.99) *** | 0.98 (0.98–0.99) *** | 1.01 (1.00–1.01) ** | |
| Sex | ||||
| Male | 1.37 (1.20–1.56) *** | 1.38 (1.18–1.60) *** | 1.13 (0.96–1.33) | |
| Ethnicity | ||||
| Minority | 1.12 (0.97–1.29) | 0.73 (0.62–0.86) *** | 0.83 (0.70–0.98) * | |
| Admission year | ||||
| 2005 | 1.00 *** | 1.00 *** | 1.00 *** | 1.00 *** |
| 2006 | 1.06 (0.60–1.88) | 1.04 (0.58–1.84) | 1.55 (1.00–2.42) | 1.55 (1.00–2.42) |
| 2007 | 2.90 (1.80–4.66) | 2.78 (1.73–4.47) | 1.08 (0.69–1.71) | 1.07 (0.68–1.69) |
| 2008 | 4.76 (3.03–7.47) | 4.56 (2.90–7.17) | 0.79 (0.49–1.26) | 0.80 (0.50–1.28) |
| 2009 | 5.29 (3.39–8.27) | 4.98 (3.19–7.79) | 1.62 (1.08–2.44) | 1.65 (1.09–2.48) |
| 2010 | 5.99 (3.85–9.32) | 5.62 (3.61–8.75) | 1.81 (1.22–2.70) | 1.82 (1.22–2.71) |
| 2011 | 9.47 (6.16–14.56) | 8.85 (5.75–13.61) | 2.23 (1.52–3.27) | 2.23 (1.51–3.28) |
| 2012 | 10.04 (6.54–15.41) | 9.35 (6.09–14.36) | 2.58 (1.76–3.80) | 2.59 (1.76–3.81) |
| Risk factors | ||||
| TIA | 1.24 (0.94–1.64) | 0.76 (0.58–0.98) * | ||
| Previous stroke | 0.46 (0.39–0.55) *** | 0.48 (0.40–0.58) *** | 0.66 (0.55–0.80) *** | 0.66 (0.54–0.80) *** |
| Hypertension | 0.89 (0.77–1.03) | 0.94 (0.78–1.14) | ||
| Diabetes mellitus | 0.69 (0.60–0.79) *** | 0.72 (0.63–0.83) *** | 1.03 (0.86–1.24) | |
| Ischemic heart disease | 1.20 (1.04–1.38) * | 1.41 (1.21–1.65) *** | 1.23 (1.04–1.45) * | 1.22 (1.03–1.45) * |
| Atrial fibrillation | 1.70 (1.44–2.00) *** | 1.89 (1.58–2.26) *** | 1.53 (1.25–1.88) *** | 1.45 (1.18–1.79) *** |
| Valvular heart disease | 1.16 (0.78–1.73) | 0.90 (0.61–1.31) | ||
| Peripheral heart disease | 0.93 (0.66–1.32) | 0.98 (0.72–1.33) | ||
| Smoking | 0.99 (0.87–1.12) | 0.78 (0.67–0.90) *** | 0.96 (0.81–1.12) | |
p<0.05;
p<0.01;
p<0.001;
SSR: Singapore Stroke Registry; CRRGSR: Cognitive Rehabilitation Research Group Stroke Registry; OR: Odds ratio; CI: Confidence interval
For the CRRGSR population, bivariable associations were found similar to that in the pooled analysis, except for sex (1.13, 0.96–1.33) and history of diabetes (1.03, 0.86–1.24) which were no longer statistically significant (Table 3). In multivariable analysis, history of ischemic heart disease (1.22, 1.03–1.45) and atrial fibrillation (1.45, 1.18–1.79), were found to be associated with greater uptake of thrombolysis, while ethnic minority status (0.83, 0.70–0.98) and history of stroke (0.66, 0.54–0.80) were associated with lower uptake. Uptake increased significantly only from 2009 onwards (2009: 1.65, 1.09–2.48; 2012: 2.59, 1.76–3.81). Sensitivity analyses revealed the African-American minority, constituting 42.7% of the population, to be driving the association of the ethnic minority with lower thrombolysis uptake (Table S1).
5. Discussion/Conclusion
The comparison of stroke populations from hospitals in Singapore and St Louis revealed distinct differences in clinico-demographics of the two groups. Findings revealed differing age distributions between the two registries, with younger patients in the CRRGSR than in the SSR. Hypertension, smoking, ischemic heart disease, diabetes and prior stroke remain the top risk factors for both populations. This similarity in risk factor profile across these Western and Asian populations is consistent with the findings from comparative studies [8,9], including the Global Burden of Disease Study 2013 [14]. With diabetes within the top 10 attributable risk factors for stroke in the high-income Asia-Pacific region [14], the implications of an increasing diabetes prevalence would include an increase in stroke burden in later years, motivating the need to ensure that the burden of diabetes in Singapore is addressed to tackle the future stroke burden in the country.
The increase in thrombolysis rates over time suggest increased awareness and advocacy of thrombolysis as an established stroke treatment [15]. The higher thrombolysis rates in the CRRGSR demonstrate the importance of system-level programs to increase quality of care and can be attributed to a decade long nationwide effort to improve hospital processes [16]. The Get With The Guidelines (GWTG) campaign by the American Stroke Association (ASA) in the USA requires enrolled hospitals to achieve certain standards of care, such as meeting targeted rates of brain scans and thrombolysis of patients. The association also recognizes hospitals which achieve excellence in these areas, encouraging greater adherence to clinical guidelines and subsequently increasing thrombolysis rates. In Singapore, the publication of the first set of Clinical Practice Guidelines (CPG) for stroke and transient ischemic attacks in 2008 by the Ministry of Health could have contributed to the steady increase in thrombolysis rates in ensuing years. The CPG recommended the use of tPA for thrombolysis within 3 hours of stroke onset [17]. This recommendation was revised in 2013 (after the period of study), in accordance with newer clinical evidence which demonstrated benefit to patients treated with tPA during the 3 to 4.5 hour window period [18]. In addition to the use of the CPG, implementation of a guideline similar to GWTG in Singapore could potentially encourage a further system-level increase in thrombolysis rates.
The results of the multivariable analysis suggest differing barriers to stroke thrombolysis in both countries. Uptake of treatment appears to be dependent on age, sex and risk factors of stroke patients in Singapore. We found females and smokers were associated with lower thrombolysis uptake in the SSR, which are in agreement with previous studies [19,20]. The lower thrombolysis rates observed in females could potentially be attributed to delay in arrival time or higher stroke severity, which would preclude them from treatment [20,21]. The lower treatment rates in smokers may be attributed to higher stroke severity and subsequently treatment ineligibility for smokers with small-vessel occlusions [22]. However, we were unable to perform a subgroup analysis to confirm this as ischemic stroke subtype information was not collected in this study. In the USA, ethnicity in contrast appears to be a dominant factor in the delivery of stroke treatment to a patient. The evident ethnic disparity in thrombolysis rates in the CRRGSR is supported by the wealth of literature indicating racial-ethnic disparities in stroke care between white and minority communities [23–26]. Our results support the discussion advocating greater measures to narrow the gap in care for the minorities in the country and to address the underlying drivers of this disparity [23].
This study has several strengths. This study examined two multi-ethnic stroke populations using individual-level data, revealing associations of various factors with thrombolysis uptake. The completeness of coverage by the respective registries, as well as the use of retrospective data from hospital records of all stroke admissions minimized selection and recall bias. Any bias was also likely to be non-differential across the patients who received and did not receive thrombolysis. This was because the registries were set up with collecting routine stroke data as a primary purpose, and information collected would not be associated with the outcome of thrombolysis or any specific characteristics of the patients. Attrition bias was minimal as all data was collected for a single admission, without the need for follow up. As a nationwide stroke registry, the SSR would have reached all hospitalizations related to stroke in public hospitals, covering around 94% of all stroke cases in Singapore. Information collected is routine and standardized from the different hospitals, ensuring quality of the data. The CRRGSR was also comprehensive in coverage as it covered all patients in the region of St Louis, Missouri who sought care for stroke at BJH, which is the largest hospital in the state.
Limitations of this study include the inability to capture the data of stroke patients who sought help at the private hospitals in Singapore or other hospitals in St Louis. The results obtained may not also be generalizable to other stroke populations in the rest of the United States due to variations in demographic makeup. Consequently, the findings of this study cannot be generalized to reflect any nation-level differences in characteristics or trends. Furthermore, BJH as a regional stroke center receives stroke patients from neighboring sites who may have already received thrombolysis before transferring to BJH. The treatment rates reported in this paper would then reflect the thrombolysis usage in the hospital itself, and would be a under-estimate of the rate in the state. In addition, the uptake of thrombolysis was modeled in this paper to be dependent on patient-centric factors, and did not account for the impact of health system factors affecting the administration of thrombolysis [5,6]. In particular, history of medication use and door-or onset-to-needle time were not included as they were not available for the study period in both datasets. Also, stroke severity, measured by indices such as the National Institutes of Health Stroke Scale (NIHSS) [27], was similarly not available in the SSR for the study period as it was not routinely collected in hospitals in Singapore, limiting any conclusions to be made for the association between the stroke severity and thrombolysis uptake. The findings could also be affected by differences in treatment indications between the two populations, such as indications for patients aged 80 and above within 4.5 hours of stroke onset by the ASA but not by the CPG. The lack of such information related to the eligibility of stroke patients arriving at the hospital could also potentially pose as confounders in identifying factors associated with thrombolysis uptake and should be obtained to refine the analysis in future work.
The detailed comparison of stroke registry data from Singapore and St Louis suggest that the characteristics of stroke patients in the two populations generally differ. Rates of thrombolysis was lower in Singapore than in the CRRGSR. Factors influencing the administration of thrombolysis upon admission were similar in clinical risk factors across the two registries, but differed in demographic aspects, with age and sex driving uptake in Singapore, and ethnicity in the CRRG stroke population. However, both trends in uptake appear to be on the rise, suggesting that the increasing awareness and advocacy of thrombolysis may be translating to greater use over the period of 2005 to 2012.
Supplementary Material
7.1. Acknowledgements
We thank the National Registry of Disease Office in the Ministry of Health, Singapore; as well as the following who helped with the development of the Singapore Stroke Registry: Aftab Ahmad; Yan Hoon Ang; Hui Meng Chang; Keng He Kong; Sherry Young; Kok Foo Tang; Kee Seng Chia; Seang Mei Saw; C Rajasoorya; and Noor Hafizah. We also thank the following who supported the maintenance of the Cognitive Rehabilitation Research Group Stroke Registry: Ojoyi Agbo; Alexis Young; and Jack Baty.
7.4. Sources of Funding
This study was supported by the Washington University’s Institute of Public Health, Harvey A. Friedman Center for Aging, Global Aging Initiative; and the James S. McDonnell Foundation 21st Century Science Initiative in Cognitive Rehabilitation – Collaborative Award (grant no. 220020413). A.W.K.W.’s effort was supported in part by the National Institutes of Health (NIH) –Eunice K. Shriver National Institute of Child Health and Human Development (National Center for Medical Rehabilitation Research) (NCMRR), USA (K12HD055931). G.C.H.K’s effort was supported by the Singapore Ministry of Health’s National Medical Research Council under the Centre Grant Programme - Singapore Population Health Improvement Centre (NMRC/CG/C026/2017_NUHS). The contents do not necessarily represent the policies of the Ministry of Health, Singapore and the NIH-NICHD (NCMRR).
Footnotes
Statement of Ethics
The NUS Institutional Review Board approved a waiver of written consent for this study.
Disclosure Statement
The authors declare no conflicts of interest.
8. References
- 1.Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJB, Demaerschalk BM, et al. : Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 2.Venketasubramanian N, Pwee KH, Chen CPL: Singapore Ministry of Health Clinical Practice Guidelines on Stroke and Transient Ischemic Attacks. Int J Stroke 2011;6:251–258. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh CY, Chen CH, Chen YC, Kao Yang YH: National survey of thrombolytic therapy for acute ischemic stroke in Taiwan 2003–2010. J Stroke Cerebrovasc Dis 2013;22:e620–7. [DOI] [PubMed] [Google Scholar]
- 4.Gumbinger C, Reuter B, Hacke W, Sauer T, Bruder I, Diehm C, et al. : Restriction of therapy mainly explains lower thrombolysis rates in reduced stroke service levels. Neurology 2016;86:1975–1983. [DOI] [PubMed] [Google Scholar]
- 5.Paul CL, Ryan A, Rose S, Attia JR, Kerr E, Koller C, et al. : How can we improve stroke thrombolysis rates? A review of health system factors and approaches associated with thrombolysis administration rates in acute stroke care. Implement Sci 2016;11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwan J, Hand P, Sandercock P: A systematic review of barriers to delivery of thrombolysis for acute stroke. Age Ageing 2004;33:116–121. [DOI] [PubMed] [Google Scholar]
- 7.Kassebaum NJ, Arora M, Barber RM, Bhutta ZA, Brown J, Carter A, et al. : Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1603–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Zhou L, Zhang Y, Yi DD, Liu L, Rao W, et al. : Risk Factors of Stroke in Western and Asian Countries: A Systematic Review and Meta-analysis of Prospective Cohort Studies. BMC Public Health 2014;14:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai CF, Anderson N, Thomas B, Sudlow CLM: Risk factors for ischemic stroke and its subtypes in Chinese vs. Caucasians: Systematic review and meta-analysis. Int J Stroke 2015;10:485–493. [DOI] [PubMed] [Google Scholar]
- 10.Venketasubramanian N, Chang HM, Chan BPL, Young SH, Kong KH, Tang KF, et al. : Countrywide stroke incidence, subtypes, management and outcome in a multiethnic Asian population: The Singapore Stroke Registry - methodology. Int J Stroke 2015;10:767–769. [DOI] [PubMed] [Google Scholar]
- 11.Wolf TJ, Baum C, Connor LT: Changing face of stroke: Implications for occupational therapy practice. Am J Occup Ther 2009;63:621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf TJ, Brey JK, Baum C, Connor LT: Activity Participation Differences Between Younger and Older Individuals with Stroke. Brain Impair 2012;13:16–23. [Google Scholar]
- 13.RStudio Team: RStudio: Integrated Development for R 2015;Available from: http://www.rstudio.com
- 14.Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. : Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol 2016;15:913–924. [DOI] [PubMed] [Google Scholar]
- 15.Singer OC, Hamann GF, Misselwitz B, Steinmetz H, Foerch C: Time trends in systemic thrombolysis in a large hospital-based stroke registry. Cerebrovasc Dis 2012;33:316–321. [DOI] [PubMed] [Google Scholar]
- 16.Schwamm LH, Ali SF, Reeves MJ, Smith EE, Saver JL, Messe S, et al. : Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get with the Guidelines-Stroke hospitals. Circ Cardiovasc Qual Outcomes 2013;6:543–549. [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Health Singapore: Stroke and Transient Ischaemic Attacks Assessment, Investigation, Immediate Management and Secondary Prevention. 2009.
- 18.Ministry of Health Singapore: Use of intravenous recombinant tissue plasminogen activator ( rtPA ) in ischaemic stroke patients MOH Clinical Guidance. 2013.
- 19.Eriksson M, Jonsson F, Appelros P, Asberg KH, Norrving B, Stegmayr B, et al. : Dissemination of thrombolysis for acute ischemic stroke across a nation: Experiences from the swedish stroke register, 2003 to 2008. Stroke 2010;41:1115–1122. [DOI] [PubMed] [Google Scholar]
- 20.Reeves M, Bhatt A, Jajou P, Brown M, Lisabeth L: Sex differences in the use of intravenous rt-pa thrombolysis treatment for acute ischemic stroke: A meta-analysis. Stroke 2009;40:1743–1749. [DOI] [PubMed] [Google Scholar]
- 21.De Silva D, Ebinger M, Davis SM: Gender issues in acute stroke thrombolysis. J Clin Neurosci 2009;16:501–504. [DOI] [PubMed] [Google Scholar]
- 22.Weng W, Huang W, Chien Y, Wu C, Su F, Jung H: The impact of smoking on the severity of acute ischemic stroke. J Neurol Sci 2011;308:94–97. [DOI] [PubMed] [Google Scholar]
- 23.Cruz-Flores S, Rabinstein A, Biller J, Elkind MS V, Griffith P, Gorelick PB, et al. : Racial-ethnic disparities in stroke care: The American experience: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2091–2116. [DOI] [PubMed] [Google Scholar]
- 24.Hsia AW, Edwards DF, Morgenstern LB, Jeffrey J, Brown NC, Coles R, et al. : Racial disparities in tpa treatment rate for stroke: a population-based study. Stroke 2011;42:2217–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasr DM, Brinjikji W, Cloft HJ, Rabinstein AA: Racial and ethnic disparities in the use of intravenous recombinant tissue plasminogen activator and outcomes for acute ischemic stroke. J Stroke Cerebrovasc Dis 2013;22:154–160. [DOI] [PubMed] [Google Scholar]
- 26.Boehme AK, Siegler JE, Mullen MT, Albright KC, Lyerly MJ, Monlezun DJ, et al. : Racial and gender differences in stroke severity, outcomes and treatment in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 2014;23:e255–e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brott T, Jr HPA, Olinger CP, Marler JR, Barsan WG, Biller J, et al. : Measurements of acute cerebral infarction: a clinical measurement scale 1989;1:864–871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


