Abstract
Cancer progression is a complex multistep process comprising of angiogenesis of the primary tumor, its invasion into the surrounding stroma and its migration to distant organs to produce metastases. Nutritional compounds of the “capsaicinoid” family regulate angiogenesis, invasion and metastasis of tumors. Capsaicinoids display robust anti-angiogenic activity in both cell culture and mice models. However, conflicting reports exist about the effect of capsaicinoids on invasion of metastasis of cancers. While some published reports have described an anti-invasive and anti-metastatic role for capsaicinoids, others have argued that capsaicinoids stimulate invasion and metastasis of cancers. The present review article summarizes these findings involving the bioactivity of capsaicin in angiogenesis, invasion and metastasis of cancer. A survey of literature indicate that they are several articles summarizing the growth-inhibitory activity of capsaicinoids but few describe its effects on angiogenesis, invasion and metastasis in detail. Our review article fills this gap of knowledge. The discovery of a second generation of natural and synthetic capsaicin analogs (with anti-tumor activity) will pave the way to improved strategies for the treatment of several human cancers.
Keywords: Capsaicinoids, angiogenesis, epithelial-to-mesenchymal transition (EMT), migration, invasion, metastasis
Introduction
Capsaicin is the spicy ingredient found in chili peppers (Capsicum frutescens) or hot chili red peppers (Capsucum annum L) [1–3]. The term “capsaicinoid” refers to capsaicin-like compounds found in different strains of chili peppers. The amount of capsaicin present in chili peppers defines the pungency and spiciness of the chili pepper. It is generally believed that such pungent capsaicin-compounds were synthesized by the chili plants as a defense mechanism against fungi, microbes and herbivores. The majority of capsaicin is found in the placental tissue and lesser amounts have been detected in seeds and pericarp portion of Capsicum. The heat index of capsaicin is measured by of the Scoville scale; the higher the Scoville index, the hotter is the pepper [4, 5] .For example, the mild bell pepper has an average Scoville index of 300units, whereas the hot “orange habanero peppers” have a Scoville index of about 400,000 units (Figure 1A). Pure capsaicin gas the highest Scoville scale value of 16 million units.
Figure 1.
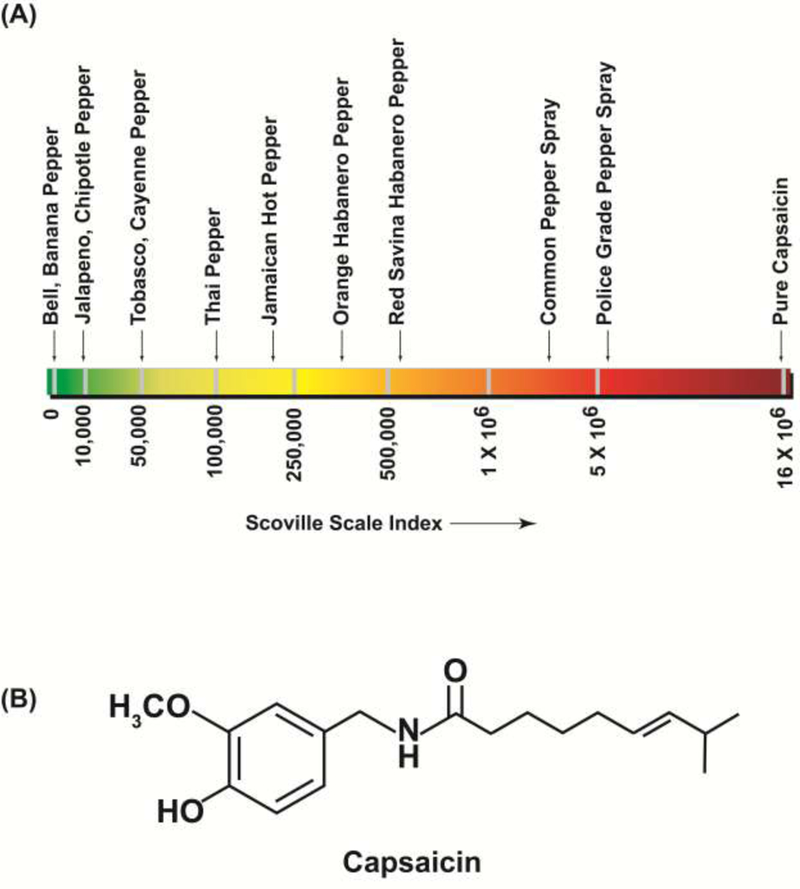
The hotness and pungency of chili peppers is measured by the Scoville scale. The higher the Scoville unit, the more “spicy and pungent” the pepper. Pure Capsaicin has the highest Scoville index and bell peppers have the lowest Scoville value.
Capsaicin (trans-8-methyl-N-vanillyl-6-nonemide) is a crystalline colorless odorless alkaloid (Figure1B) with the molecular formula C18H27NO3 [6]. It is a lipophilic compound (Molecular weight =305.4g/mol) which is insoluble in water but readily soluble in non-polar solvents like ethanol and DMSO. Capsaicin displays cis-trans isomerism because the double bond prevents internal rotation [6–8]. Naturally occurring capsaicin is the cis-isoform. Apart from capsaicin, there are many naturally occurring capsaicinoids in hot peppers (Capsicum annum and Capsaicum frutenscens) namely dihydrocapsaicin, nondihydrocapsaicin, norcapsaicin, homocapsaicin and homodihydrocapsaicin [9]. Capsaicinoids are synthesized in the placenta of chili pepper fruit by enzymatic condensation (mediated by capsaicin synthase) of vanillylamine and different fatty acid side chains [1, 6, 10]. Interestingly, non-pungent capsaicin-like compounds have been isolated from Japanese CH-19 sweet papers [2, 11, 12]. These compounds are collectively called as capsinoids. The compounds in the capsinoid family include capsiate, dihydrocapsiate and nor dihydrocapsiate. Capsaicin displays a spectrum of biological activities. Traditionally, it has been used as a pain-relieving agent in patches, creams and lotions [10, 13, 14]. However, it has also shown to possess anti-inflammatory effects, antioxidant activity and anti-obesity properties [1, 2, 7, 10, 15–19]. Conflicting data exist anti-cancer activity of capsaicinoids. A majority of published reports have revealed that low doses of capsaicin suppress the growth of many types of human cancers. Capsaicin has also been found to sensitize neoplastic cells to the apoptotic activity of chemotherapeutic drugs [20–22]. However, a few research papers have shown that capsaicin has tumor-promoting activities in skin cancer, breast cancer and colon cancer [15, 23, 24]. These tumor-promoting activities were observed when high doses of capsaicin was used to treat the cancer cells. It may also be possible that the anti-neoplastic activity of capsaicin depends on the nature of the cancer; skin and colon cancers may be unique in the fact that capsaicin induces proliferative effects in these cancers. The biological activity of capsiate and its related compounds have not been studied extensively [2, 11, 12]. It seems that the bioactivity of capsiate-like compounds is similar to that of capsaicinoids. However, an advantage of capsiate (and its related compounds) is that they do not produce the “burning sensation” associated with capsaicin.
The pain-reliving activity of capsaicin is mediated by its binding to the transient receptor potential vanilloid (TRPV) superfamily of ion-channel receptors [25, 26]. Although, the TRPV family of receptors have six members (TRPV1-TRPV6) [16, 25, 26]. Several capsaicin-like compounds have been isolated and characterized from different species of chili peppers [2, 12]. Similarly, rational chemical synthesis have led to the discovery of capsaicin analogs that resemble capsaicin in their structure and bioactivity [11, 21]. These natural and synthetic capsaicin-like compounds are collectively known as capsaicinoids. All capsaicinoids are agonists of the TRPV1 receptor [27, 28]. However, recent studies have shown that some of the biological effects of capsaicinoids may be independent of TRPV receptors [29–32].
With the exception of a limited number of studies that reported that the long-term administration of capsaicin induced neoplastic changes in the liver and cecum [33], promoted the survival and growth of bladder, colon and skin cancers [15, 24, 33, 34] the overwhelming majority of published data show that capsaicin displays potent anti-tumor activity in a diverse array of human cancers [35–40]. Early research demonstrated that capsaicinoids display robust chemopreventive activities, inhibiting carcinogenesis in lung, prostate, pancreatic, breast and skin cancer [1, 38, 41–44]. Subsequently, published reports have confirmed the anti-cancer activity of capsaicin in human breast, lung, prostate, gastric, renal, oral and hepatocellular carcinoma [1, 7, 17]. The anti-cancer activity of capsaicin has been found to be mediated via TRPV-dependent, as well as TRPV-independent, mechanisms [17, 45–48]. Apart from their effects on primary tumors, capsaicinoids have been found to regulate tumor angiogenesis and metastasis; two key processes in cancer progression. There are several in-depth review articles describing the growth-inhibitory activity of capsaicinoids. However, there is a paucity of reviews which systematically document the effect of capsaicinoids on tumor angiogenesis, epithelial-to mesenchymal transition (EMT), invasion and metastasis. The present article fills this void of knowledge. The first portion of this manuscript describes the anti-angiogenic activity of capsaicinoids in tumor angiogenesis. Subsequently, we discuss the bioactivity of capsaicinoids on the individual steps of the metastatic cascade, namely EMT, migration and invasion. Finally, we describe the therapeutic applications of capsaicin in controlling cancer pain in patients.
1. Capsaicinoids and Angiogenesis
Angiogenesis refers to the growth of new blood vessels from pre-existing vasculature. It is a complex multistep process involving endothelial cell activation, cell proliferation, invasion, chemotactic migration and differentiation into new blood vessels[49]. The acquisition of an angiogenic phenotype is considered to be a vital step in tumor progression [50, 51]. Tumors need to recruit blood vessels in order to obtain oxygen and nutrients necessary for their growth, as well as to dispose of metabolic waste products. Furthermore, endothelial cells secrete growth factors which stimulate tumor growth in an autocrine and paracrine manner [52, 53]. The onset of angiogenesis coincides with increased entry of neoplastic cells into circulation and thus facilitates metastasis [50, 54]. Several congruent studies indicate that the transition from an in situ carcinoma to invasive cancer (for solid tumors) must be accompanied by neovascularization. Therefore, the suppression of angiogenesis is a highly effective strategy for the treatment of multiple cancers [52, 55, 56], one for which capsaicinoids may have relevant therapeutic potential.
Min et al., (2004) studied the effects of capsaicin on angiogenesis using multiple model systems [57]. They observed that capsaicin suppressed vascular endothelial growth factor (VEGF)-induced angiogenesis in Matrigel model systems, ex vivo rat aortic rings models, chicken chorioallantoic membrane (CAM) models[35] and in vivo Matrigel plug experiments [57]. Capsaicin robustly inhibited VEGF-induced endothelial cell proliferation and invasion [57]. Similarly, the non-pungent capsinoids, capsiate and dihydrocapsiate inhibited VEGF-induced angiogenesis in both cell culture and mouse models [58]. Capsiate and dihydrocapsiate inhibited VEGF-induced endothelial permeability and formation of cell-cell junctions[59] . The anti-angiogenic activity of capsiate and dihydrocapsiate was found to be independent of the TRPV1 receptor [58]. In fact, the activation of the TRPV1 receptor by another capsaicin-like compound, evodiamine (derived from tetradium or bee tree), stimulates angiogenesis in Matrigel Plug model [60]. The pro-angiogenic activity of evodiamine was studied in human aortic endothelial cells (HAEC) in the context of cardiovascular disease and involved activation of the nitric oxide pathway [60]. Min et al., (2004) observed that capsaicin suppressed VEGF-induced angiogenesis by a mechanism distinct from the activation of KDR/Flk-1 VEGF receptor [61, 62]. The anti-angiogenic activity of capsaicin correlated with decreased expression of cyclin D1, which in turn translated to decreased phosphorylation of Rb, leading to G1 arrest of human umbilical cord endothelial cells (HUVEC)[57]. Furthermore, capsaicin also suppressed VEGF-induced activation of focal adhesion kinase (FAK) and p38 kinase (Figure 2 )[57]. Molecular docking studies showed that capsaicin and capsiate could directly bind to Src kinase in a hydrophobic cleft near the ATP binding site [58]. However, the authors did not confirm the results of molecular modeling by binding experiments showing a direct association between capsaicin and Src.
Figure 2.
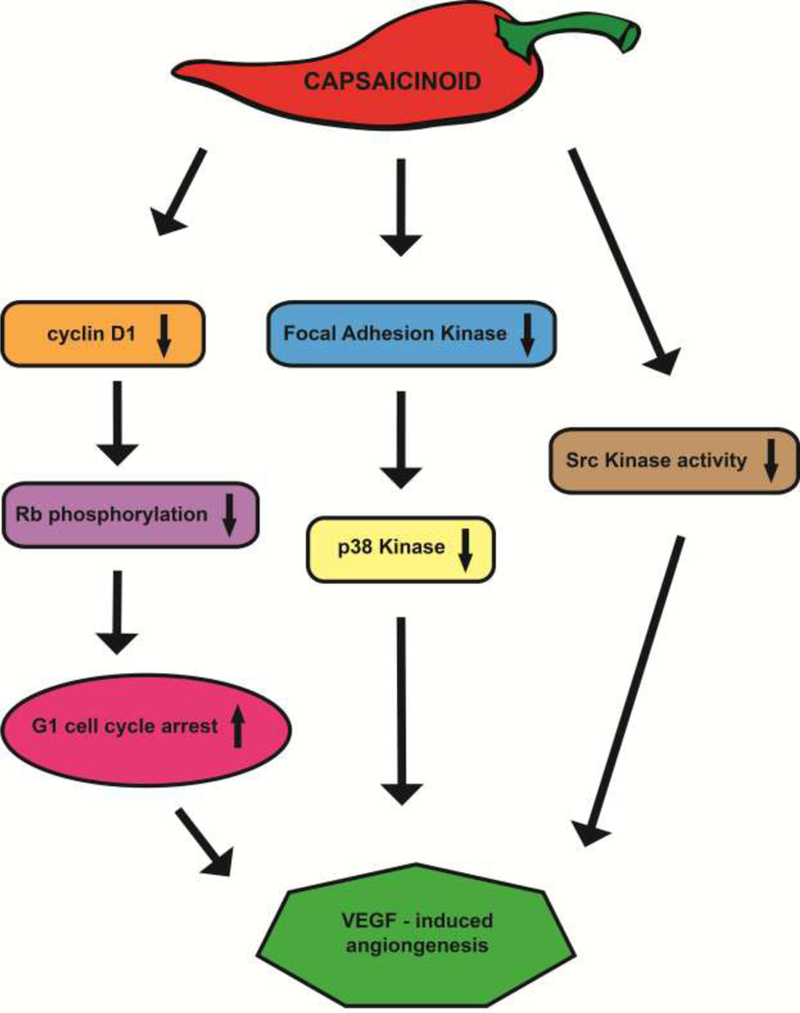
A flow chart representing the signaling pathways by which capsaicinoids suppress VEGF-induced angiogenesis.
Conflicting reports exist on the effect of capsaicin on the expression of the angiogenic growth factor VEGF. Patel et al., (2002) showed that capsaicin enhanced VEGF levels by increasing the DNA binding activity of hypoxia inducible factor alpha (HIF-1α) on the VEGF promoter in human melanoma cells [63]. In contrast, subsequent studies showed that capsaicin suppressed production of VEGF from myeloma and non-small lung cancer (NSCLC) cells [64, 65]. Chakraborty et al., (2014) showed that capsaicin decreased VEGF secretion via the p53-scaffold matrix-associated region-1 (SMAR1) pathway in NSCLC cells [65]. The anti-angiogenic activity of capsaicin was mediated by stabilization of p53 (mediated by upregulation of SMAR1) and subsequent degradation of HIF-1α, resulting in decreased transcription of VEGF. The question arises whether the conflicting observations were due to cell type or capsaicin. Furthermore, the treatment of human NSCLC with capsaicin induced repression of cyclooxygenase-2 (COX-2) activity and production of prostaglandin-2 (PGE2), which in turn blocked nuclear localization and transcriptional activation of HIF-1α, causing decrease in VEGF production [65]. Such conflicting results involving the effect of capsaicin on VEGF production may be explained by differences in the type of human cancer (melanoma versus myeloma and NSCLC) being used in the studies.
2. Capsaicinoids and Epithelial-to-Mesenchymal Transition (EMT)
The ability of neoplastic cells to travel away from the primary tumor to distant sites is essential for metastasis. EMT is believed to be one of the initial steps of metastasis and refers to the trans-differentiation of epithelial cells to motile mesenchymal cells [66, 67]. The process of EMT also confers epithelial neoplastic cells with increased invasiveness and the ability to degrade the extracellular matrix proteins [66]. The EMT program is not a binary on/off switch transitioning cells from the epithelial to the mesenchymal phenotype[67, 68], but rather a spectrum of molecular changes in which intermediate mixed epithelial/mesenchymal phenotypes [69, 70] have been detected (Figure 3). The acquisition of EMT coincides with loss of epithelial biomarkers like E-cadherin, beta-catenin, Zona occudens-1 (ZO-1) and increased expression of mesenchymal proteins like fibronectin, vimentin and N-cadherin[67, 71, 72]. Such molecular changes are regulated by specific EMT-transcription factors like Twist, [73, 74] Snail, Slug [75] and Zeb1[76]. Other transcription factors like Zeb2, Foxc2, Prrxl (and many others) have also been shown to be capable of inducing a subset of the EMT process [67, 71].
Figure 3.
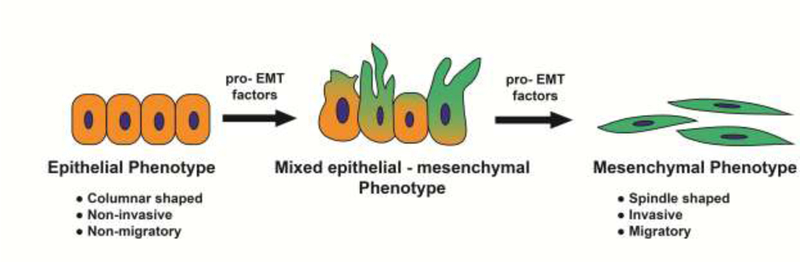
A simplified schematic of epithelial-to-mesenchymal transition in human cancer cells. The induction of EMT is a spectrum of biochemical mechanisms where epithelial, mesenchymal and mixed epithelial-mesenchymal phenotypes are observed.
The effect of capsaicin on EMT is controversial. Yang et al., (2013) treated SW480 human colon cancer cells with 100 μM capsaicin for 48 hours. Subsequently, they analyzed the cell lysates for EMT-biomarkers [77]. They observed that capsaicin decreased the expression of E-cadherin and increased the levels of mesenchymal proteins like vimentin and N-cadherin. Taken together, capsaicin promoted EMT in SW480 cells at a high concentration of 100μM [77]. Capsaicin also increased the expression of matrix metalloproteinase (MMP)-2 and MMP-9 suggesting that it endowed pro-migratory and pro-invasive properties on human colon cancer cells. Furthermore, the treatment with HCT-116 human colon cancer cells with 1–10 μM capsaicin led to induction of the EMT program [77]. Geng et al., (2016) observed that high doses of capsaicin (50 mg capsaicin/kg body weight administered intragastrically) promoted EMT in the urethane-induced lung carcinogenesis [78] model. Immunohistochemical (IHC) analysis showed a decrease in E-cadherin with concomitant increase in N-cadherin in the lungs of mice administered with capsaicin [78]. A drawback of these studies is that the authors only analyzed one epithelial protein and one mesenchymal biomarker. As mentioned above, data from several research labs have highlighted the existence of a “partial EMT” state where the cells retain a mixture of epithelial and mesenchymal traits [67, 68, 71, 79]. Therefore, analysis of a panel of epithelial and mesenchymal proteins would have enabled the authors to better characterize capsaicin-induced EMT in the lung carcinogenesis model.
The EMT-inhibitory activity of capsaicin was studied in human cholangiocarcinoma cells. Wutka et al., (2014) observed that capsaicin suppressed EMT in two cholangiocarcinoma cell lines (SZ-1 and TFK-1) in a time- and concentration-dependent manner [80]. Two concentrations of capsaicin (150 μM and 200 μM) increased the expression of E-cadherin with concomitant decrease in mesenchymal protein vimentin levels in both cell lines. The maximal suppression of EMT by capsaicin was achieved 48–96 hours post treatment [80]. Notably, capsaicin decreased the levels of N-cadherin in SZ-1 cells, but not in TFK-1 cells. Such observations show that the effects of capsaicin on EMT are critically dependent on the nature of the cell line used. Capsaicin blocked EMT in TSGH, 5637 and T24 human bladder cancer cells. The EMT-inhibitory activity of capsaicin correlated to the inhibition of tumor associated NADH oxidase (tNOX) levels in TSGH and T24 cells [81]. Innovative studies by Amatini et al., (2016) showed that high doses of capsaicin may induce capsaicin-resistance (CPS-R) in human bladder cancer cells [82]. A subset of cells survive the high concentrations of capsaicin by undergoing autophagy. These CPS-R bladder cancer cells display typical mesenchymal morphology along with overexpression of mesenchymal biomarkers like vimentin, EMT transcription factors Zeb 2, α5-, β1-integrin subunits and integrin-like kinase [82]. Further support for the EMT-inhibitory activity of capsaicin was provided by Xu et al.,(2018) who observed that capsaicin downregulated the expression of EMT transcription factors Snail1, Twist1, as well as MMP-2 and 9 in human thyroid carcinoma cells[83]. Capsaicin-induced suppression of Twist1 and MMP-9 was abrogated by the TRPV1 antagonist capsazepine, suggesting that the TRPV1 receptors played an integral role in the anti-EMT activity of capsaicin in thyroid carcinoma cells[83]. Dai et al., (2018) measured the growth-inhibitory activity of a combination of capsaicin and sorafenib in hepatocellular cancers[84]. They observed that the combination of 80 μM capsaicin and 4 μM sorafenib suppressed the induction of EMT better than either drug given alone[84]. There was a robust upregulation of E-cadherin and concomitant decrease of N-cadherin and vimentin in the cancer cells treated with a combination of capsaicin and sorafenib [84]. These observations suggest that capsaicin sensitizes human cancer cells to the anti-EMT activity of standard chemotherapeutic drugs used in the clinic.
3. Capsaicinoids and tumor migration, invasion
The migration and invasion of cancer cells into the surrounding stroma, blood vessels and lymph nodes is a vital step in the metastatic pathway [85, 86]. Cell migration measures the ability of cells to travel under the influence of chemotactic gradient. Cell invasion measures the ability of cells to degrade basement membrane proteins and subsequently migrate in response to chemotactic stimuli. Conflicting reports exist on the impact of capsaicin on tumor cell migration and invasion. Data by Yang et al., (2013) also show that capsaicin stimulates the invasion of human colon carcinoma cells via the Akt/mTOR and STAT-3 dependent pathways [77]. Aside from the research paper of Yang et al. (2013), the bulk of published reports show that capsaicin displays anti-migratory and anti-invasive activity in breast cancer, melanoma, thyroid cancer, bladder cancer, small cell lung cancer (SCLC) and cholangiocarcinoma[80–83, 87, 88]. The anti-migratory and anti-invasive activity of capsaicin in thyroid cancer was found to be mediated via a TRPV1-dependent mechanism [83]. Caprodossi et al., (2011) studied the impact of TRPV1 status on the invasion-regulatory properties of capsaicin[89]. They observed that capsaicin increases the invasion of TRPV1-null 5637 human bladder cancer cells. The pro-invasive activity of capsaicin coincides with increased production of insulin growth factor (IGF-1), granzyme A (GZMA) and activation of MMP-9 in the TRPV-null cells. Furthermore capsaicin induced cytoskeletal remodeling in 5637 cells[89]. Gene expression analysis showed that capsaicin elevated the levels of pro-angiogenic genes like angiopoietins, VEGF, MMP1, MMP-1, tissue inhibitor of matrix metalloproteinase 1 (TIMP1), NM23A and S100A in TRPV1-null cells[89]. Finally, the authors re-expressed TRPV1 in 5637 cells by transient transfection and subsequently treated the cells with 100 μM capsaicin[89]. They observed that capsaicin displayed anti-invasive and growth-inhibitory activity in TRPV1+/+ 5637 cells[89]. Therefore, the loss of TRPV1 causes capsaicin to promote invasion, whereas its re-expression has the opposite effect. These findings align well with clinical observations that high grade invasive bladder cancers display decreased TRPV1 expression relative to low grade tumors [90, 91]. The differential TRPV1 expression may, at least, in part explain the conflicting data involving the effects of capsaicin on tumor invasion. It is tempting to speculate that the status of TRPV receptors on neoplastic cells may play a role in the pro-invasive versus anti-invasive activity of capsaicin in human cancers.
Capsaicin recruits multiple signaling mechanisms to regulate migration and invasion. These include activation of EMT, AMP activated protein kinase (AMPK), MMP signaling pathway, elevation of intracellular calcium, VEGF, regulation of Wnt-Hedgehog, tNOX, Akt, MMPs and inhibition of epidermal growth factor receptor (EGFR), ERK, p38 MAP kinase, Rac1, NF-kB, AP-1[80–83, 87, 88]. Apart from its bioactivity as a single agent, capsaicin synergizes with established chemotherapeutic drugs to potently suppress the migration and invasion of tumor cells. Dai et al., (2018) investigated the combinatorial anti-migratory and anti-invasive activity of capsaicin and sorafenib was in LM3 hepatocellular carcinoma cells. Wound healing migration assays showed that the combination of 80 μM capsaicin and 4 μM sorafenib suppressed migration to a greater magnitude than either sorafenib or capsaicin alone[84]. The abovementioned drug combination also suppressed invasion of LM3 cells. The combination of 80 μM capsaicin and 4 μM sorafenib inhibited the induction of EMT and decreased levels of MMP-2 and MMP-9 (in LM3 cells) better than either drug used alone[84]. Studies by Wang et al., (2018) explored the anti-invasive activity of the combination of cisplatin and capsaicin in human osteosarcoma cells. The authors chose to study the anti-invasive activity of cisplatin and capsaicin in three osteosarcoma cell lines namely MG63, 143B and HOS cells [92]. The combination of 16.7 μM cisplatin and 100 μM capsaicin suppressed invasion (in all the three cell lines more robustly than either agent administered alone [92]. The anti-invasive activity of capsaicin and cisplatin was associated with decreased expression and activity of MMP-2 and MMP-9. The combination of capsaicin and cisplatin resulted in the greatest magnitude of reduction of MMP2 and MMP9 expression (and activity) relative to the drugs as single agents [92].
While the majority of studies have focused on the use of capsaicin as an anti-cancer agent, the clinical development of capsaicin as anti-cancer drug is problematic due to its unfavorable side effect profile. Several convergent studies have shown that systemic administration of capsaicin in humans leads to intense gut pain, hyperalgesia, stomach cramps and nausea. Clinical studies show that these adverse side effects have led patients to abandon taking the oral capsaicin [10, 93–95]. This drawback can be circumvented by the discovery of capsaicin analogs, which retain the anti-tumor activity of capsaicin but do not produce the “heat-sensation” of capsaicin. Structure Activity Relationship (SAR) studies show that the structure of capsaicin can be divided into three pharmacophore-regions namely Region A, B, and C (Figure 4) [96]. The addition of long chain unsaturated fatty acyl groups to the Region C of capsaicin generates non-pungent capsaicin analogs[96]. Studies in our laboratory compared the anti-invasive activity of capsaicin and two capsaicin analogs arvanil and olvanil in human SCLC [87]. Arvanil and olvanil showed better anti-invasive activity than capsaicin in a panel of human SCLC cell lines. The anti-invasive activity of arvanil and olvanil was independent of TRPV1 and mediated by activation of the AMPK pathway [87]. Lee et al., (2017) investigated the anti-invasive activity of the capsaicin analog capsazepine in human prostate cancer cells [97]. They observed that capsazepine robustly suppressed the invasion of DU145 human prostate cancer cells via inhibition of the JAK/STAT3 pathway.
Figure 4.
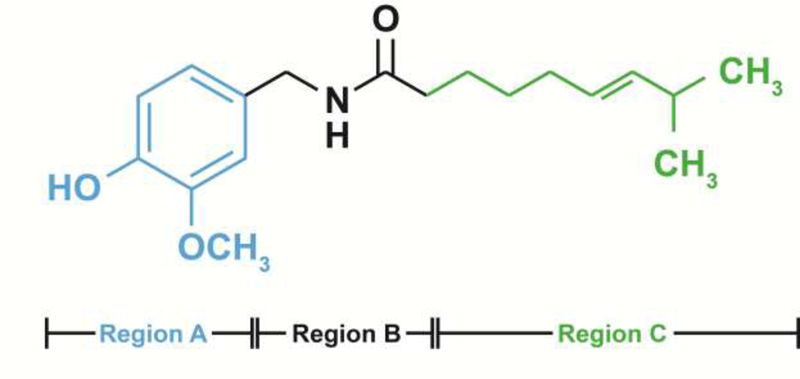
Structure of capsaicinoids. The blue structural moiety represents Region A; the red portion of the structure represents Region B; the green alkyl side chain represents Region C.
4. Capsaicinoids and Metastasis
Initial research studied the impact of the sensory effects of capsaicin on tumor metastasis [14, 98]. The administration of high doses of capsaicin causes deactivation of sensory neurons[10]. Erin et al. (2004), used high doses of capsaicin (125 mg capsaicin/kg body weight) to ablate the activity of systemic sensory neurons and studied the effects of such denervation on the metastasis of mammary cancers [23]. The authors used the orthotopic 4T1 syngeneic mouse model of metastasis in their studies. The mice were pretreated with 125mg capsaicin/kg bodyweight (injected subcutaneously into the fat tissues if the neck0 for two consecutive days, designated as day 1 and 2 of the study [23]. 4T1 murine mammary carcinoma cells were orthotopically injected into the right axillary mammary fat pad 7–21 days after the administration of capsaicin. The inactivation of sensory neurons (by capsaicin) promoted the metastasis of mammary tumors to the lungs and heart. Notably, the high doses of capsaicin did not affect the growth rate of the primary tumor [23]. A subsequent published report by the same research group compared gene expression patterns (in the primary tumors) between the vehicle-treated and capsaicin-treated mice [99]. They identified a group of seventeen genes that were decreased in primary tumors of capsaicin treated mice versus vehicle treated mice. Interestingly, all seventeen identified genes regulate growth, differentiation and progression of human cancers [99].
Yang et al., (2013) investigated the effect of capsaicin on the metastasis of CT26 murine colorectal carcinoma cells [77]. They treated CT26 cells with 100μM capsaicin for 48 hours and then intravenously injected these cells in mice. After 15 days, and their lungs of mice were examined for metastatic nodules [77]. The authors observed that CT26 treated with 100μM capsaicin produced a greater number of lung metastatic nodules than vehicle-treated mice. A noteworthy aspect of this study was that CT26 cells were pretreated with a rather high concentration of capsaicin (100μM) prior to being injected in mice [77]. Another important point was that the authors did not use orthotopic models to measure metastasis. They injected the CT26 cells directly into the tail vein of mice, which is not a true representation of metastasis, since it circumvents important events of the metastatic pathway like migration, EMT and invasion of cancer cells [77].
Venier et al. (2015) studied the anti-metastatic activity of capsaicin in the transgenic TRAMP model of prostate cancer [100]. The authors administered 5 mg capsaicin/kg body weight by oral gavage three times a week. The administration of capsaicin significantly lowered metastatic burden in TRAMP mice model. The capsaicin-treated mice had also higher non-cancerous intraepithelial neoplasia (PIN) relative to the control group. IHC experiments showed an elevation of the tumor suppressor p27Kip1 in the tumors of capsaicin-treated TRAMP mice. This study seems to suggest that capsaicin may possess robust anti-metastatic activity [100]. A recent publication by Kandagalla et al., (2019) used computational approaches to determine the impact of capsaicin on TGF-β-induced metastasis [101]. They observed that capsaicin targets several key genes in the TGF-β signaling pathway [101].
5. Capsicinoids and Cancer Pain
The occurrence of pain is a common symptom of cancer patients, especially those who present with advanced or terminal disease. It is estimated that over 70% of cancer patients experience pain during and after their treatment [102, 103]. Chemotherapy-induced peripheral neuropathy along with chronic pain is often observed in cancer patients. A diverse array of chemotherapeutic drugs like taxol, vincristine, oxaliplatin and bortezomib induce peripheral neuropathy in patients [104]. The pain-relieving activity of capsaicin is mediated by the TRPV1 receptor [25–27]. Although, acute exposure to capsaicin activates theTRPV1 receptor causing a burning sensation, prolonged exposure to capsaicin desensitizes TRPV1 and produces relief from pain. Another approach has been to explore the pain-relieving activity of TRPV1 antagonists [4, 7, 8, 10, 13, 14, 16, 105].
Quetenza is a high dose 8% capsaicin patch (also called NGX-4010) has been found to decrease neuropathic pain after a single application within an hour for about 12 weeks. Each NGX-2010 patch contains 179 mg of capsaicin [4, 10]. Anand et al. (2019) examined the effect of the Quentenza patch on sixteen patients suffering from chemotherapy-induced peripheral neuropathy (CIPN). The patients were administered the capsaicin patch on their feet for 30 minutes for a total period of there months [104]. The study found that patients reported a decrease in spontaneous pain, touch-induced pain and cold-evoked pain. After the treatment period, a skin biopsy was done in both the placebo and capsaicin-treated group of patients. The skin biopsies from placebo-treated patients showed loss of intra-epidermal and sub-epidermal nerve fibers. In contrast, the skin biopsies from capsaicin-treated patients showed a significant increase (towards normalization) of intra-epidermal and sub-epidermal nerve fibers [104]. Additionally, levels of key signaling proteins like nerve growth factor, Neurotrophin-3 were normalized in the capsaicin–treated patients. Taken together, the NGX-2010 capsaicin patch provides relief from chronic cancer pain and accelerates regeneration of sensory nerve fibers.
A prevalent side effect of chemotherapy and radiation therapy is the high incidence of oral mucositis (painful ulcers in the throat and oral cavity) in patients [106, 107]. Currently, there are no therapies to relieve the pain of these lesions. Berger et al., (1995) investigated the potential of capsaicin to diminish the pain caused by oral mucositis. They administered 5–9ppm capsaicin (as cayenne peppers) in candy. They made two strengths of capsaicin-candy, the first one contained 5–9ppm capsaicin and second contained half the amount of capsaicin [108]. The study was performed in 11 patients and all of them reported pain relief after using the capsaicin-containing candy. The capsaicin candies did not totally abolish the pain but minimized the discomfort due to oral mucositis [108]. Out of the eleven patients two opted for candies containing half the dose of capsaicin. The authors observed that even candies containing lower doses of capsaicin provided substantial pain relief to the patients [108].
A relatively rare side effect of cancer treatment is long term postsurgical neuropathic pain. Surgical resection of the tumor may cause transection, contusion, stretching, or inflammation of the nerves, producing chronic pain symptoms in the patient [109]. Early studies by Watson et al, (1989) investigated the effect of 0.025% capsaicin cream on post-mastectomy pain in eighteen patients. Their data reveal that 12 out of the 18 patients experienced a 50% (or greater) relief in their pain symptoms [110]. Such observations were in alignment with the findings of Dini et al., (1993) who tested the pain-relieving activity of 0.025% capsaicin cream in 21 patients suffering from post-mastectomy pain [111]. They observed that 58% of the patients receiving capsaicin cream experienced a substantial reduction in pain. Eleven of the thirteen responding patients continue to experience improvement in pain symptoms for three months after of stopping topical-capsaicin therapy
Ellison et al. (1997) explored whether the topical administration of capsaicin cream could alleviate postsurgical neuropathic pain. The study was performed in 99 patients who had undergone surgical procedures namely mastectomy, thoracotomy, amputation or any surgical procedure as part of their cancer treatment regimen [112]. After randomization, the treatment group received 0.075% capsaicin cream to be applied 4 times every day for a period of 8 weeks. The authors observed that 0.075% capsaicin caused initial adverse reactions like burning sensation and redness of skin as well as more coughing in patients. The number of patients who decided to discontinue the cream was similar to that of the placebo group indicating that capsaicin was well tolerated in patients. At the end of study 60% of patients (in the capsaicin-treated) group reported pain-relief relative to 18% in the placebo-cream-treated patients [112]. Capsaicin-induced pain relief was maximal at the end of 4 weeks and remained constant thereafter.
The capsaicinoid resiniferatoxin (RTX) has been observed to display potent analgesic activity in several animal models [113]. Mendez et al. (2006) examined the analgesic effect of RTX in mice models of osteosarcoma. NCTC-2472 murine osteosarcoma cells were injected into the tibial medullar cavity to induce osteosarcoma in mice [114]. The subcutaneous injection of a single dose of RTX (0.01–0.1mg/kg bodyweight) inhibited osteosarcoma-induced hyperalgesia in a dose-dependent manner. The pain-relieving activity of RTX (administered via intrathecal injection) was also observed in canine models of spontaneous osteosarcoma [115, 116]. The pathophysiology of canine osteosarcoma resembles human bone cancers. The results obtained in these mice and canine models has formed the basis of a Phase 1 clinical trial involving intrathecal RTX injections to relieve cancer pain in patients. The trial is still ongoing but preliminary results show that patients experience improvement in pain symptoms after RTX treatment [113].
The TRPV1 receptor is known to play a vital role in stimulating pain symptoms in arising from heat, acidosis and extracellular protons [26]. Tumors progression occurs in an acidotic environment [117]. This raises to the possibility that disruption of TRPV1 function by TRPV1 antagonists may alleviate pain symptoms. Several research papers have shown that the capsazepine or ABT102 potently decreases pain symptoms in mouse models of bone cancer [114, 118]. TRPV1 antagonists like capsazepine and SB366791 potentiate the pain relieving activity of cannabinoid drugs and morphine [119–122]. The administration of TRPV1 antagonists did not cause discomfort or gross toxicity to mice. Such studies will facilitate the development of TRPV1-based pain relieving drugs in human cancers.
6. Conclusions and Future Directions
A survey of literature show that conflicting reports exist on the effect of capsaicin on invasion, EMT and metastasis. A noticeable caveat of the majority these studies is that they have been mostly done with high concentrations of capsaicin in cell culture systems and not in animal models. Such facts underscore the need for further in-depth studies in animal models to precisely delineate the effects of capsaicin on the EMT, migration, invasion and metastasis of human cancers. Several model systems have shown that capsaicin and capsiate inhibit VEGF-induced angiogenesis activity in endothelial cells. The treatment of capsaicin causes robust downregulation of the angiogenic growth factor VEGF in myelomas and NSCLC. All these findings suggest that capsaicin is an anti-angiogenic nutritional compound. An important biological activity of capsainoids is their ability to relive cancer pain symptoms. The combination of capsaicinoids with standard chemotheraputic drugs has a two-fold advantage. Firstly, capsaicinoids may potentiate the activity of chemotherapy and second it may provide pain relief for cancer patients
An exciting development in the field of capsaicin-based drug design has been the discovery of non-pungent capsaicin-analogs which retain the anti-tumor activity of capsaicin. It is hoped that future studies will identify a new generation of capsaicin mimetics with potent anti-metastatic activity in human cancers.
7. Acknowledgements
We acknowledge Dr. S. Chellappan and his laboratory for their continuous support. SDR and JCM are recipients of NSF-SURE and WV-NASA Space Consortium undergraduate fellowships respectively. PD and MAV is supported by a National Institutes of Health R15 Academic Research Enhancement Award (Grants 1R15CA161491–01A1 and 2R15CA161491–02).
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Srinivasan K, Biological Activities of Red Pepper (Capsicum annuum) and Its Pungent Principle Capsaicin: A Review, Crit Rev Food Sci Nutr 56(9) (2016) 1488–1500. [DOI] [PubMed] [Google Scholar]
- [2].Naves ER, de Avila Silva L, Sulpice R, Araujo WL, Nunes-Nesi A, Peres LEP, Zsogon A, Capsaicinoids: Pungency beyond Capsicum, Trends Plant Sci 24(2) (2019) 109–120. [DOI] [PubMed] [Google Scholar]
- [3].Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin, Int J Toxicol 26 Suppl 1 (2007) 3–106. [DOI] [PubMed] [Google Scholar]
- [4].Kulkarni YA, Suryavanshi SV, Auti ST, Gaikwad AB, Capsicum: A Natural Pain Modulator, Nutritional Modulators of Pain in the Aging Population (2017) 107–119. [Google Scholar]
- [5].Lau JK, Brown KC, Dom AM, Dasgupta P, Capsaicin: Potential Applications in Cancer Therapy, Bentham Press Inc, London, United Kingdom: 2012. [Google Scholar]
- [6].Reyes-Escogido Mde L, Gonzalez-Mondragon EG, Vazquez-Tzompantzi E, Chemical and pharmacological aspects of capsaicin, Molecules 16(2) (2011) 1253–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Basith S, Cui M, Hong S, Choi S, Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases, Molecules 21(8) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fattori V, Hohmann MS, Rossaneis AC, Pinho-Ribeiro FA, Verri WA, Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses, Molecules 21(7) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Reilly CA, Crouch DJ, Yost GS, Quantitative analysis of capsaicinoids in fresh peppers, oleoresin capsicum and pepper spray products, J Forensic Sci 46(3) (2001) 502–9. [PubMed] [Google Scholar]
- [10].O’Neill J, Brock C, Olesen AE, Andresen T, Nilsson M, Dickenson AH, Unravelling the mystery of capsaicin: a tool to understand and treat pain, Pharmacol Rev 64(4) (2012) 939–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kobata K, Sugawara M, Mimura M, Yazawa S, Watanabe T, Potent production of capsaicinoids and capsinoids by Capsicum peppers, J Agric Food Chem 61(46) (2013) 11127–32. [DOI] [PubMed] [Google Scholar]
- [12].Lu M, Ho CT, Huang Q, Extraction, bioavailability, and bioefficacy of capsaicinoids, J Food Drug Anal 25(1) (2017) 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chung MK, Campbell JN, Use of Capsaicin to Treat Pain: Mechanistic and Therapeutic Considerations, Pharmaceuticals (Basel) 9(4) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Frias B, Merighi A, Capsaicin, Nociception and Pain, Molecules 21(6) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bode AM, Dong Z, The two faces of capsaicin, Cancer Res 71(8) (2011) 2809–14. [DOI] [PubMed] [Google Scholar]
- [16].Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D, The capsaicin receptor: a heat-activated ion channel in the pain pathway, Nature 389(6653) (1997) 816–24. [DOI] [PubMed] [Google Scholar]
- [17].Chapa-Oliver AM, Mejia-Teniente L, Capsaicin: From Plants to a Cancer-Suppressing Agent, Molecules 21(8) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fecher-Trost C, Weissgerber P, Wissenbach U, TRPV6 channels, Handbook of experimental pharmacology 222 (2014) 359–84. [DOI] [PubMed] [Google Scholar]
- [19].Reilly CA, Taylor JL, Lanza DL, Carr BA, Crouch DJ, Yost GS, Capsaicinoids cause inflammation and epithelial cell death through activation of vanilloid receptors, Toxicol Sci 73(1) (2003) 170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huh HC, Lee SY, Lee SK, Park NH, Han IS, Capsaicin induces apoptosis of Cisplatin-resistant stomach cancer cells by causing degradation of Cisplatin-inducible aurora-a protein, Nutr Cancer 63(7) (2011) 1095–103. [DOI] [PubMed] [Google Scholar]
- [21].Friedman JR, Nolan NA, Brown KC, Miles SL, Akers AT, Colclough KW, Seidler JM, Rimoldi JM, Valentovic MA, Dasgupta P, Anticancer Activity of Natural and Synthetic Capsaicin Analogs, J Pharmacol Exp Ther 364(3) (2018) 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Friedman JR, Perry HE, Brown KC, Gao Y, Lin J, Stevenson CD, Hurley JD, Nolan NA, Akers AT, Chen YC, Denning KL, Brown LG, Dasgupta P, Capsaicin synergizes with camptothecin to induce increased apoptosis in human small cell lung cancers via the calpain pathway, Biochem Pharmacol 129 (2017) 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Erin N, Boyer PJ, Bonneau RH, Clawson GA, Welch DR, Capsaicin-mediated denervation of sensory neurons promotes mammary tumor metastasis to lung and heart, Anticancer Res 24(2B) (2004) 1003–9. [PubMed] [Google Scholar]
- [24].Toth B, Gannett P, Carcinogenicity of lifelong administration of capsaicin of hot pepper in mice, In Vivo 6(1) (1992) 59–63. [PubMed] [Google Scholar]
- [25].Yang F, Zheng J, Understand spiciness: mechanism of TRPV1 channel activation by capsaicin, Protein Cell 8(3) (2017) 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Satheesh NJ, Uehara Y, Fedotova J, Pohanka M, Busselberg D, Kruzliak P, TRPV currents and their role in the nociception and neuroplasticity, Neuropeptides 57 (2016) 1–8. [DOI] [PubMed] [Google Scholar]
- [27].Darre L, Domene C, Binding of Capsaicin to the TRPV1 Ion Channel, Mol Pharm 12(12) (2015) 4454–65. [DOI] [PubMed] [Google Scholar]
- [28].Darre L, Furini S, Domene C, Permeation and dynamics of an open-activated TRPV1 channel, J Mol Biol 427(2) (2015) 537–49. [DOI] [PubMed] [Google Scholar]
- [29].Mori A, Lehmann S, O’Kelly J, Kumagai T, Desmond JC, Pervan M, McBride WH, Kizaki M, Koeffler HP, Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells, Cancer Res 66(6) (2006) 3222–9. [DOI] [PubMed] [Google Scholar]
- [30].Athanasiou A, Smith PA, Vakilpour S, Kumaran NM, Turner AE, Bagiokou D, Layfield R, Ray DE, Westwell AD, Alexander SP, Kendall DA, Lobo DN, Watson SA, Lophatanon A, Muir KA, Guo DA, Bates TE, Vanilloid receptor agonists and antagonists are mitochondrial inhibitors: how vanilloids cause non-vanilloid receptor mediated cell death, Biochem Biophys Res Commun 354(1) (2007) 50–5. [DOI] [PubMed] [Google Scholar]
- [31].Hail N Jr., Lotan R, Examining the role of mitochondrial respiration in vanilloid-induced apoptosis, J Natl Cancer Inst 94(17) (2002) 1281–92. [DOI] [PubMed] [Google Scholar]
- [32].Szallasi A, Blumberg PM, Vanilloid (Capsaicin) receptors and mechanisms, Pharmacol Rev 51(2) (1999) 159–212. [PubMed] [Google Scholar]
- [33].Hoch-Ligeti C, Production of liver tumours by dietary means; effect of feeding chilies [Capsicum frutescens and annuum (Linn.)] to rats, Acta Unio Int Contra Cancrum 7(3) (1951) 606–11. [PubMed] [Google Scholar]
- [34].Georgescu SR, Sarbu MI, Matei C, Ilie MA, Caruntu C, Constantin C, Neagu M, Tampa M, Capsaicin: Friend or Foe in Skin Cancer and Other Related Malignancies?, Nutrients 9(12) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Brown KC, Witte TR, Hardman WE, Luo H, Chen YC, Carpenter AB, Lau JK, Dasgupta P, Capsaicin displays anti-proliferative activity against human small cell lung cancer in cell culture and nude mice models via the E2F pathway, PLoS One 5(4) (2010) e10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chou CC, Wu YC, Wang YF, Chou MJ, Kuo SJ, Chen DR, Capsaicin-induced apoptosis in human breast cancer MCF-7 cells through caspase-independent pathway, Oncol Rep 21(3) (2009) 665–71. [PubMed] [Google Scholar]
- [37].Chow J, Norng M, Zhang J, Chai J, TRPV6 mediates capsaicin-induced apoptosis in gastric cancer cells--Mechanisms behind a possible new “hot” cancer treatment, Biochim Biophys Acta 1773(4) (2007) 565–76. [DOI] [PubMed] [Google Scholar]
- [38].Hartel M, di Mola FF, Selvaggi F, Mascetta G, Wente MN, Felix K, Giese NA, Hinz U, Di Sebastiano P, Buchler MW, Friess H, Vanilloids in pancreatic cancer: potential for chemotherapy and pain management, Gut 55(4) (2006) 519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Minna JD, Kurie JM, Jacks T, A big step in the study of small cell lung cancer, Cancer Cell 4(3) (2003) 163–6. [DOI] [PubMed] [Google Scholar]
- [40].Venier NA, Colquhoun AJ, Sasaki H, Kiss A, Sugar L, Adomat H, Fleshner NE, Klotz LH, Venkateswaran V, Capsaicin: a novel radio-sensitizing agent for prostate cancer, Prostate 75(2) (2015) 113–25. [DOI] [PubMed] [Google Scholar]
- [41].Aggarwal BB, Kunnumakkara AB, Harikumar KB, Tharakan ST, Sung B, Anand P, Potential of spice-derived phytochemicals for cancer prevention, Planta Med 74(13) (2008) 1560–9. [DOI] [PubMed] [Google Scholar]
- [42].Anandakumar P, Kamaraj S, Jagan S, Ramakrishnan G, Vinodhkumar R, Devaki T, Stabilization of pulmonary mitochondrial enzyme system by capsaicin during benzo(a)pyrene induced experimental lung cancer, Biomed Pharmacother (2007). [DOI] [PubMed] [Google Scholar]
- [43].Malagarie-Cazenave S, Olea-Herrero N, Vara D, Diaz-Laviada I, Capsaicin, a component of red peppers, induces expression of androgen receptor via PI3K and MAPK pathways in prostate LNCaP cells, FEBS Lett 583(1) (2009) 141–7. [DOI] [PubMed] [Google Scholar]
- [44].Sanchez AM, Sanchez MG, Malagarie-Cazenave S, Olea N, Diaz-Laviada I, Induction of apoptosis in prostate tumor PC-3 cells and inhibition of xenograft prostate tumor growth by the vanilloid capsaicin, Apoptosis 11(1) (2006) 89–99. [DOI] [PubMed] [Google Scholar]
- [45].Cho SC, Lee H, Choi BY, An updated review on molecular mechanisms underlying the anticancer effects of capsaicin, Food Sci Biotechnol 26(1) (2017) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Clark R, Lee SH, Anticancer Properties of Capsaicin Against Human Cancer, Anticancer Res 36(3) (2016) 837–43. [PubMed] [Google Scholar]
- [47].Ziglioli F, Frattini A, Maestroni U, Dinale F, Ciufifeda M, Cortellini P, Vanilloid-mediated apoptosis in prostate cancer cells through a TRPV-1 dependent and a TRPV-1-independent mechanism, Acta Biomed 80(1) (2009) 13–20. [PubMed] [Google Scholar]
- [48].Le Pechoux C, Laplanche A, Faivre-Finn C, Ciuleanu T, Wanders R, Lerouge D, Keus R, Hatton M, Videtic GM, Senan S, Wolfson A, Jones R, Arriagada R, Quoix E, Dunant A, Prophylactic G Cranial Irradiation Collaborative, Clinical neurological outcome and quality of life among patients with limited small-cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99–01, EORTC 22003–08004, RTOG 0212 and IFCT 99–01), Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 22(5) (2011) 1154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Potente M, Gerhardt H, Carmeliet P, Basic and therapeutic aspects of angiogenesis, Cell 146(6) (2011) 873–87. [DOI] [PubMed] [Google Scholar]
- [50].Folkman J, Role of angiogenesis in tumor growth and metastasis, Semin Oncol 29(6 Suppl 16) (2002) 15–8. [DOI] [PubMed] [Google Scholar]
- [51].Lin Z, Zhang Q, Luo W, Angiogenesis inhibitors as therapeutic agents in cancer: Challenges and future directions, Eur J Pharmacol 793 (2016) 76–81. [DOI] [PubMed] [Google Scholar]
- [52].Folkman J, Tumor angiogenesis: therapeutic implications, N Engl J Med 285(21) (1971) 1182–6. [DOI] [PubMed] [Google Scholar]
- [53].Prager GW, Poettler M, Angiogenesis in cancer. Basic mechanisms and therapeutic advances, Hamostaseologie 32(2) (2012) 105–14. [DOI] [PubMed] [Google Scholar]
- [54].Fidler IJ, Angiogenesis and cancer metastasis, Cancer J 6 Suppl 2 (2000) S134–41. [PubMed] [Google Scholar]
- [55].Rajabi M, Mousa SA, The Role of Angiogenesis in Cancer Treatment, Biomedicines 5(2) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ye W, The Complexity of Translating Anti-angiogenesis Therapy from Basic Science to the Clinic, Dev Cell 37(2) (2016) 114–25. [DOI] [PubMed] [Google Scholar]
- [57].Min JK, Han KY, Kim EC, Kim YM, Lee SW, Kim OH, Kim KW, Gho YS, Kwon YG, Capsaicin inhibits in vitro and in vivo angiogenesis, Cancer Res 64(2) (2004) 644–51. [DOI] [PubMed] [Google Scholar]
- [58].Pyun BJ, Choi S, Lee Y, Kim TW, Min JK, Kim Y, Kim BD, Kim JH, Kim TY, Kim YM, Kwon YG, Capsiate, a nonpungent capsaicin-like compound, inhibits angiogenesis and vascular permeability via a direct inhibition of Src kinase activity, Cancer Res 68(1) (2008) 227–35. [DOI] [PubMed] [Google Scholar]
- [59].Bates DO, Vascular endothelial growth factors and vascular permeability, Cardiovasc Res 87(2) (2010) 262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ching LC, Kou YR, Shyue SK, Su KH, Wei J, Cheng LC, Yu YB, Pan CC, Lee TS, Molecular mechanisms of activation of endothelial nitric oxide synthase mediated by transient receptor potential vanilloid type 1, Cardiovasc Res 91(3) (2011) 492–501. [DOI] [PubMed] [Google Scholar]
- [61].Li B, Ogasawara AK, Yang R, Wei W, He GW, Zioncheck TF, Bunting S, de Vos AM, Jin H, KDR (VEGF receptor 2) is the major mediator for the hypotensive effect of VEGF, Hypertension 39(6) (2002) 1095–100. [DOI] [PubMed] [Google Scholar]
- [62].Yang S, Toy K, Ingle G, Zlot C, Williams PM, Fuh G, Li B, de Vos A, Gerritsen ME, Vascular endothelial growth factor-induced genes in human umbilical vein endothelial cells: relative roles of KDR and Flt-1 receptors, Arterioscler Thromb Vasc Biol 22(11) (2002) 1797–803. [DOI] [PubMed] [Google Scholar]
- [63].Patel PS, Yang S, Li A, Varney ML, Singh RK, Capsaicin regulates vascular endothelial cell growth factor expression by modulation of hypoxia inducing factor-1alpha in human malignant melanoma cells, J Cancer Res Clin Oncol 128(9) (2002) 461–8. [DOI] [PubMed] [Google Scholar]
- [64].Bhutani M, Pathak AK, Nair AS, Kunnumakkara AB, Guha S, Sethi G, Aggarwal BB, Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation, Clin Cancer Res 13(10) (2007) 3024–32. [DOI] [PubMed] [Google Scholar]
- [65].Chakraborty S, Adhikary A, Mazumdar M, Mukherjee S, Bhattacharjee P, Guha D, Choudhuri T, Chattopadhyay S, Sa G, Sen A, Das T, Capsaicin-induced activation of p53-SMAR1 auto-regulatory loop down-regulates VEGF in non-small cell lung cancer to restrain angiogenesis, PLoS One 9(6) (2014) e99743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Brabletz T, Kalluri R, Nieto MA, Weinberg RA, EMT in cancer, Nat Rev Cancer 18(2) (2018) 128–134. [DOI] [PubMed] [Google Scholar]
- [67].Zhang Y, Weinberg RA, Epithelial-to-mesenchymal transition in cancer: complexity and opportunities, Front Med 12(4) (2018) 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Liao TT, Yang MH, Revisiting epithelial-mesenchymal transition in cancer metastasis: the connection between epithelial plasticity and stemness, Mol Oncol 11(7) (2017) 792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Roche J, The Epithelial-to-Mesenchymal Transition in Cancer, Cancers (Basel) 10(2) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].He P, Qiu K, Jia Y, Modeling of mesenchymal hybrid epithelial state and phenotypic transitions in EMT and MET processes of cancer cells, Sci Rep 8(1) (2018) 14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lamouille S, Xu J, Derynck R, Molecular mechanisms of epithelial-mesenchymal transition, Nat Rev Mol Cell Biol 15(3) (2014) 178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zeisberg M, Neilson EG, Biomarkers for epithelial-mesenchymal transitions, J Clin Invest 119(6) (2009) 1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chen S, Chen JZ, Zhang JQ, Chen HX, Yan ML, Huang L, Tian YF, Chen YL, Wang YD, Hypoxia induces TWIST-activated epithelial-mesenchymal transition and proliferation of pancreatic cancer cells in vitro and in nude mice, Cancer Lett 383(1) (2016) 73–84. [DOI] [PubMed] [Google Scholar]
- [74].Wang Y, Liu J, Ying X, Lin PC, Zhou BP, Twist-mediated Epithelial-mesenchymal Transition Promotes Breast Tumor Cell Invasion via Inhibition of Hippo Pathway, Sci Rep 6 (2016) 24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Haslehurst AM, Koti M, Dharsee M, Nuin P, Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, Davey S, Squire J, Park PC, Feilotter H, EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer, BMC Cancer 12 (2012) 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG, Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer, Clin Cancer Res 13(16) (2007) 4769–76. [DOI] [PubMed] [Google Scholar]
- [77].Yang J, Li TZ, Xu GH, Luo BB, Chen YX, Zhang T, Low-concentration capsaicin promotes colorectal cancer metastasis by triggering ROS production and modulating Akt/mTOR and STAT-3 pathways, Neoplasma 60(4) (2013) 364–72. [DOI] [PubMed] [Google Scholar]
- [78].Geng S, Zheng Y, Meng M, Guo Z, Cao N, Ma X, Du Z, Li J, Duan Y, Du G, Gingerol Reverses the Cancer-Promoting Effect of Capsaicin by Increased TRPV1 Level in a Urethane-Induced Lung Carcinogenic Model, J Agric Food Chem 64(31) (2016) 6203–11. [DOI] [PubMed] [Google Scholar]
- [79].Diepenbruck M, Christofori G, Epithelial-mesenchymal transition (EMT) and metastasis: yes, no, maybe?, Curr Opin Cell Biol 43 (2016) 7–13. [DOI] [PubMed] [Google Scholar]
- [80].Wutka A, Palagani V, Barat S, Chen X, El Khatib M, Gotze J, Belahmer H, Zender S, Bozko P, Malek NP, Plentz RR, Capsaicin treatment attenuates cholangiocarcinoma carcinogenesis, PLoS One 9(4) (2014) e95605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lin MH, Lee YH, Cheng HL, Chen HY, Jhuang FH, Chueh PJ, Capsaicin Inhibits Multiple Bladder Cancer Cell Phenotypes by Inhibiting Tumor-Associated NADH Oxidase (tNOX) and Sirtuin1 (SIRT1), Molecules 21(7) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Amantini C, Morelli MB, Nabissi M, Cardinali C, Santoni M, Gismondi A, Santoni G, Capsaicin triggers autophagic cell survival which drives epithelial mesenchymal transition and chemoresistance in bladder cancer cells in an Hedgehog-dependent manner, Oncotarget 7(31) (2016) 50180–50194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Xu S, Zhang L, Cheng X, Yu H, Bao J, Lu R, Capsaicin inhibits the metastasis of human papillary thyroid carcinoma BCPAP cells through the modulation of the TRPV1 channel, Food Funct 9(1) (2018) 344–354. [DOI] [PubMed] [Google Scholar]
- [84].Dai N, Ye R, He Q, Guo P, Chen H, Zhang Q, Capsaicin and sorafenib combination treatment exerts synergistic antihepatocellular carcinoma activity by suppressing EGFR and PI3K/Akt/mTOR signaling, Oncol Rep 40(6) (2018) 3235–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Levine MD, Liotta LA, Stracke ML, Stimulation and regulation of tumor cell motility in invasion and metastasis, EXS 74 (1995) 157–79. [DOI] [PubMed] [Google Scholar]
- [86].Petruzzelli GJ, The biology of tumor invasion, angiogenesis and lymph node metastasis, ORL J Otorhinolaryngol Relat Spec 62(4) (2000) 178–85. [DOI] [PubMed] [Google Scholar]
- [87].Hurley JD, Akers AT, Friedman JR, Nolan NA, Brown KC, Dasgupta P, Non-pungent long chain capsaicin-analogs arvanil and olvanil display better anti-invasive activity than capsaicin in human small cell lung cancers, Cell Adh Migr 11(1) (2017) 80–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lee GR, Jang SH, Kim CJ, Kim AR, Yoon DJ, Park NH, Han IS, Capsaicin suppresses the migration of cholangiocarcinoma cells by down-regulating matrix metalloproteinase-9 expression via the AMPK-NF-kappaB signaling pathway, Clin Exp Metastasis 31(8) (2014) 897–907. [DOI] [PubMed] [Google Scholar]
- [89].Caprodossi S, Amantini C, Nabissi M, Morelli MB, Farfariello V, Santoni M, Gismondi A, Santoni G, Capsaicin (CPS) promotes a more aggressive gene expression phenotype and invasiveness in null-TRPV1 urothelial cancer cells, Carcinogenesis (2011). [DOI] [PubMed] [Google Scholar]
- [90].Kalogris C, Caprodossi S, Amantini C, Lambertucci F, Nabissi M, Morelli MB, Farfariello V, Filosa A, Emiliozzi MC, Mammana G, Santoni G, Expression of transient receptor potential vanilloid-1 (TRPV1) in urothelial cancers of human bladder: relation to clinicopathological and molecular parameters, Histopathology 57(5) (2010) 744–52. [DOI] [PubMed] [Google Scholar]
- [91].Lazzeri M, Vannucchi MG, Spinelli M, Bizzoco E, Beneforti P, Turini D, Faussone-Pellegrini MS, Transient receptor potential vanilloid type 1 (TRPV1) expression changes from normal urothelium to transitional cell carcinoma of human bladder, Eur Urol 48(4) (2005) 691–8. [DOI] [PubMed] [Google Scholar]
- [92].Wang Y, Deng X, Yu C, Zhao G, Zhou J, Zhang G, Li M, Jiang D, Quan Z, Zhang Y, Synergistic inhibitory effects of capsaicin combined with cisplatin on human osteosarcoma in culture and in xenografts, J Exp Clin Cancer Res 37(1) (2018) 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Drewes AM, Schipper KP, Dimcevski G, Petersen P, Gregersen H, Funch-Jensen P, Arendt-Nielsen L, Gut pain and hyperalgesia induced by capsaicin: a human experimental model, Pain 104(1–2) (2003) 333–41. [DOI] [PubMed] [Google Scholar]
- [94].Hammer J, Effect of repeated capsaicin ingestion on intestinal chemosensation and mechanosensation, Alimentary pharmacology & therapeutics 24(4) (2006) 679–86. [DOI] [PubMed] [Google Scholar]
- [95].LaMotte RH, Lundberg LE, Torebjork HE, Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin, J Physiol 448 (1992) 749–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Huang XF, Xue JY, Jiang AQ, Zhu HL, Capsaicin and its analogues: structure-activity relationship study, Curr Med Chem 20(21) (2013) 2661–72. [DOI] [PubMed] [Google Scholar]
- [97].Lee JH, Kim C, Baek SH, Ko JH, Lee SG, Yang WM, Um JY, Sethi G, Ahn KS, Capsazepine inhibits JAK/STAT3 signaling, tumor growth, and cell survival in prostate cancer, Oncotarget 8(11) (2017) 17700–17711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Baron R, Capsaicin and nociception: from basic mechanisms to novel drugs, Lancet 356(9232) (2000) 785–7. [DOI] [PubMed] [Google Scholar]
- [99].Erin N, Zhao W, Bylander J, Chase G, Clawson G, Capsaicin-induced inactivation of sensory neurons promotes a more aggressive gene expression phenotype in breast cancer cells, Breast Cancer Res Treat 99(3) (2006) 351–64. [DOI] [PubMed] [Google Scholar]
- [100].Venier NA, Yamamoto T, Sugar LM, Adomat H, Fleshner NE, Klotz LH, Venkateswaran V, Capsaicin reduces the metastatic burden in the transgenic adenocarcinoma of the mouse prostate model, Prostate 75(12) (2015) 1300–11. [DOI] [PubMed] [Google Scholar]
- [101].Kandagalla S, B SS, Pavan G, Hani U, H M, Exploring the potential of capsaicin against cancer metastasis based on TGF-beta signaling modulation through module based network pharmacology approach, Curr Drug Discov Technol (2019). [DOI] [PubMed] [Google Scholar]
- [102].Fallon M, Giusti R, Aielli F, Hoskin P, Rolke R, Sharma M, Ripamonti CI, Committee EG, Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines, Ann Oncol 29(Supplement_4) (2018) iv166–iv191. [DOI] [PubMed] [Google Scholar]
- [103].Jara C, Del Barco S, Gravalos C, Hoyos S, Hernandez B, Munoz M, Quintanar T, Meana JA, Rodriguez C, de Las Penas R, SEOM clinical guideline for treatment of cancer pain (2017), Clin Transl Oncol 20(1) (2018) 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Anand P, Elsafa E, Privitera R, Naidoo K, Yiangou Y, Donatien P, Gabra H, Wasan H, Kenny L, Rahemtulla A, Misra P, Rational treatment of chemotherapy-induced peripheral neuropathy with capsaicin 8% patch: from pain relief towards disease modification, J Pain Res 12 (2019) 2039–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Basso L, Altier C, Transient Receptor Potential Channels in neuropathic pain, Curr Opin Pharmacol 32 (2017) 9–15. [DOI] [PubMed] [Google Scholar]
- [106].Chaveli-Lopez B, Oral toxicity produced by chemotherapy: A systematic review, J Clin Exp Dent 6(1) (2014) e81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Chaveli-Lopez B, Bagan-Sebastian JV, Treatment of oral mucositis due to chemotherapy, J Clin Exp Dent 8(2) (2016) e201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Berger A, Henderson M, Nadoolman W, Duffy V, Cooper D, Saberski L, Bartoshuk L, Oral capsaicin provides temporary relief for oral mucositis pain secondary to chemotherapy/radiation therapy, J Pain Symptom Manage 10(3) (1995) 243–8. [DOI] [PubMed] [Google Scholar]
- [109].Zis P, Varrassi G, Painful Peripheral Neuropathy and Cancer, Pain Ther 6(2) (2017) 115–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Watson CP, Evans RJ, Watt VR, The post-mastectomy pain syndrome and the effect of topical capsaicin, Pain 38(2) (1989) 177–86. [DOI] [PubMed] [Google Scholar]
- [111].Dini D, Bertelli G, Gozza A, Forno GG, Treatment of the post-mastectomy pain syndrome with topical capsaicin, Pain 54(2) (1993) 223–6. [DOI] [PubMed] [Google Scholar]
- [112].Ellison N, Loprinzi CL, Kugler J, Hatfield AK, Miser A, Sloan JA, Wender DB, Rowland KM, Molina R, Cascino TL, Vukov AM, Dhaliwal HS, Ghosh C, Phase III placebo-controlled trial of capsaicin cream in the management of surgical neuropathic pain in cancer patients, J Clin Oncol 15(8) (1997) 2974–80. [DOI] [PubMed] [Google Scholar]
- [113].ladarola MJ, Gonnella GL, Resiniferatoxin for Pain Treatment: An Interventional Approach to Personalized Pain Medicine, Open Pain J 6 (2013) 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Menendez L, Juarez L, Garcia E, Garcia-Suarez O, Hidalgo A, Baamonde A, Analgesic effects of capsazepine and resiniferatoxin on bone cancer pain in mice, Neurosci Lett 393(1) (2006) 70–3. [DOI] [PubMed] [Google Scholar]
- [115].Brown DC, Agnello K, Iadarola MJ, Intrathecal resiniferatoxin in a dog model: efficacy in bone cancer pain, Pain 156(6) (2015) 1018–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Brown DC, ladarola MJ, Perkowski SZ, Erin H, Shofer F, Laszlo KJ, Olah Z, Mannes AJ, Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model, Anesthesiology 103(5) (2005) 1052–9. [DOI] [PubMed] [Google Scholar]
- [117].Fidler IJ, Singh RK, Yoneda J, Kumar R, Xu L, Dong Z, Bielenberg DR, McCarty M, Ellis LM, Critical determinants of neoplastic angiogenesis, Cancer J 6 Suppl 3 (2000) S225–36. [PubMed] [Google Scholar]
- [118].Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, Halvorson KG, Poblete J, Chaplan SR, Dubin AE, Carruthers NI, Swanson D, Kuskowski M, Flores CM, Julius D, Mantyh PW, Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain, J Neurosci 25(12) (2005) 3126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Honore P, Chandran P, Hernandez G, Gauvin DM, Mikusa JP, Zhong C, Joshi SK, Ghilardi JR, Sevcik MA, Fryer RM, Segreti JA, Banfor PN, Marsh K, Neelands T, Bayburt E, Daanen JF, Gomtsyan A, Lee CH, Kort ME, Reilly RM, Surowy CS, Kym PR, Mantyh PW, Sullivan JP, Jarvis MF, Faltynek CR, Repeated dosing of ABT-102, a potent and selective TRPV1 antagonist, enhances TRPV1-mediated analgesic activity in rodents, but attenuates antagonist-induced hyperthermia, Pain 142(1–2) (2009) 27–35. [DOI] [PubMed] [Google Scholar]
- [120].Kawamata T, Niiyama Y, Yamamoto J, Furuse S, Reduction of bone cancer pain by CB1 activation and TRPV1 inhibition, J Anesth 24(2) (2010) 328–32. [DOI] [PubMed] [Google Scholar]
- [121].Nguyen TL, Nam YS, Lee SY, Kim HC, Jang CG, Effects of capsazepine, a transient receptor potential vanilloid type 1 antagonist, on morphine-induced antinociception, tolerance, and dependence in mice, Br J Anaesth 105(5) (2010) 668–74. [DOI] [PubMed] [Google Scholar]
- [122].Niiyama Y, Kawamata T, Yamamoto J, Furuse S, Namiki A, SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain, Br J Anaesth 102(2) (2009) 251–8. [DOI] [PubMed] [Google Scholar]


