Abstract
Animal models suggest a protective role of antioxidants against the adverse effect of di-2-ethylhexyl phthalate (DEHP) on insulin resistance. However, no epidemiologic study has examined the effects observed in the animal model. We conduct a study to examine associations of urinary concentrations of phthalate metabolites (individually and as a mixture) with insulin resistance, along with potential effect modification by serum antioxidant concentrations. This cross-sectional study included 1,605 participants (51% males) aged 12–85 from the National Health and Nutrition Examination Surveys (2003–2006). Urinary concentrations of 9 phthalate metabolites were measured from spot urine samples. Antioxidant (vitamin A, C, E, and carotenoids) concentrations were measured from a fasting serum sample. We used Bayesian Kernel Machine Regression (BKMR) to evaluate associations between phthalate metabolite mixtures and insulin resistance, and examined whether serum antioxidant levels modified these associations, while accounting for the correlations of multiple concurrent exposures. A change in urinary ΣDEHP concentrations from the 25th to the 75th percentile was associated with a higher log HOMA-IR of 0.07 (95% CI = 0.01, 0.14) (4.85% increase in HOMA-IR). In contrast, the same change in urinary monoethyl phthalate (MEP) was associated with a lower HOMA-IR of −0.07 (95% CI = −0.14, −0.02) (6.68% decrease in HOMA-IR). The positive association between ΣDEHP and HOMA-IR became weaker at higher concentrations of serum β-carotene. The relationship between MEP and HOMA-IR, however, was not modified by the serum antioxidants examined. The remaining phthalate metabolites were unrelated to HOMA-IR. In this cross-sectional study, the positive association between DEHP exposure and insulin resistance weakened among participants with higher concentrations of serum β-carotene. As this is the first human report on the protective role of serum β-carotene on DEHP induced insulin resistance, future studies are needed.
Keywords: Phthalates, insulin resistance, NHANES, antioxidants, diabetes
Introduction
Phthalates are ubiquitous environmental contaminants and there is widespread human exposure (Romero-Franco et al., 2011; Schettler, 2006). Phthalates consist of dialkyl esters or alkyl and aryl esters of orthophthalic acid, which can be classified into two groups in terms of their molecular weight and properties. High molecular weight phthalates, such as di-2-ethylhexyl phthalate (DEHP), are commonly used in industry as plasticizers to soften polyvinyl chloride plastics. Low molecular weight phthalates, such as di-ethyl phthalate (DEP) and di-n-butyl phthalate (DnBP) are widely used in personal care products (Hauser and Calafat, 2005; Marie et al., 2015).
In the literature, phthalates have been reported to be associated with increased risk of insulin resistance (IR) or type 2 diabetes (James-Todd et al., 2012; Kim et al., 2013; Stahlhut et al., 2007; Svensson et al., 2011; Trasande et al., 2013; Viswanathan et al., 2017), and increased oxidative stress has been suggested as a potential mechanism (Kim et al., 2013). An in vitro study has shown that DEHP increased reactive oxygen species and decreased expression of antioxidant enzymes in a dose-response manner (Cho et al., 2015). Epidemiological studies have also found a positive relationship between phthalate exposure and malondialdehyde (MDA) levels, an oxidative stress biomarker (Hong et al., 2009; Kim et al., 2013).
The role of oxidative stress in IR has also been supported in several studies (Goldstein et al., 2005; Schulz et al., 2007). The proposed mechanism of oxidative stress leading to down-regulation of the cellular responses to insulin is complicated, involving increased serine/threonine phosphorylation of insulin receptor substance-1 (IRS1) and disturbance of cellular redistribution of insulin signaling components, which in turn, reducing transcription of glucose transporter (Bloch-Damti and Bashan, 2005; Lamb and Goldstein, 2008; Morino et al., 2006). Antioxidants, known to ameliorate the adverse effects of oxidative stress, therefore, may be a promising target for preventing oxidative stress-induced IR (Pisoschi and Pop, 2015). While animal studies have suggested a protective role of antioxidants on phthalate induced IR (Rajesh et al., 2013; Srinivasan et al., 2011), to our knowledge, no study has examined this relationship in humans.
As humans are concurrently exposed to different phthalates, it is important to consider phthalates as a mixture when studying the associations between phthalates and adverse health outcomes (Bobb et al., 2015). In the present study, we examined the dose-response relationships between individual urinary concentrations of phthalate metabolites and IR accounting for the concomitant concentrations within the chemical mixture. We also examined whether concentrations of antioxidants modified the associations of phthalate exposures with a biomarker of IR.
Material and methods
Subjects
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative survey conducted by the Centers for Disease Control and Prevention’s (CDC) National Center for Health Statistics. The cross-sectional survey includes measurement of the health and nutritional status, and chemicals and metabolites in blood and urine of the U.S. population (NCHS, 2017). In this study, we used the NHANES survey from 2003–2006 (NCHS, 2009a) because only these year cycles had complete data for urinary phthalate metabolites, serum antioxidants, and insulin resistance measurements. Specifically, among the 20,470 individuals who participated in this time period, urinary phthalate metabolites were measured in 5,335 urinary samples, vitamin A, C, E, and carotenoids were analyzed in 16,068 serum samples, and plasma levels of glucose and insulin were measured in 6,708 samples; all three sets of measures were available in 1,746 participants. We excluded participants with fasting time less than 8 hours or taking insulin, leaving a total of 1,605 participants in the final analysis. Information on demographics and anthropometric measurements were obtained by household interview using questionnaires or provided by Mobile Exam Center (a mobile health examination unit constructed from four to five connecting tractor trailers) (NCHS, 2009a).
Ethical approval was not necessary for this study. The data used in this study is publicly accessible (https://www.cdc.gov/nchs/nhanes/index.htm).
Exposure assessment—urinary phthalate metabolite concentrations
A total of 13 phthalate metabolites were measured in both NHANES 2003–2004 and 2005–2006. For this analysis, we included nine phthalate metabolites for which at least 60% of study subjects had concentrations above the limit of detection (LOD): mono (2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), monobenzyl phthalate (MBzP), monobutyl phthalate (MBP), mono-isobutyl phthalate (MiBP), monoethyl phthalate (MEP), and mono (3-carboxypropyl) phthalate (MCPP). We did not include monocyclohexyl phthalate (MCHP), monooctyl phthalate (MnOP), monoisononyl phthalate (MiNP), and monomethyl phthalate (MMP) because of the low percentage of detectable concentrations. The measurements utilized high-performance liquid chromatography-electrospray ionization tandem mass spectrometry for the quantitative detection in the urine of the phthalate metabolites. Details on protocols are available on the NHANES website (NCHS, 2008a; NCHS, 2010). Concentrations of the phthalates metabolites below the limit of detection (LOD) were replaced by LOD/√2. (NCHS, 2008b; NCHS, 2010).
We calculated the molar sum of DEHP metabolites (ΣDEHP) by dividing each metabolite concentration by its molecular weight and then summing: ΣDEHP =[MEHP (µg/L) × (1/278.34 (g/mol))] + [MEHHP (µg/L) × (1/294.34 (g/mol))] + [MEOHP (µg/L) × (1/292.33 (g/mol))] + [MECPP (µg/L) × (1/308.33 (g/mol))] (Hauser et al., 2016).
Outcome assessment—insulin resistance
Fasting glucose was measured by a hexokinase method. Insulin was measured using an ELISA immunoassay. Details on protocols for biochemical analyses are available on the NHANES website (NCHS, 2016a; NCHS, 2016b). We calculated homeostatic model assessment of insulin resistance (HOMA-IR) by multiplying fasting plasma glucose in mmol/L by fasting serum insulin in mU/mL and dividing by 22.5 (Matthews et al., 1985).
Measurement of serum antioxidants
The serum antioxidants in this analysis included vitamin A (retinol), vitamin C, vitamin E (α-tocopherol), and carotenoids (including α-carotene, β-carotene, β-cryptoxanthin, lycopene, and lutein/zeaxanthin). Vitamin A, E, and carotenoids were measured using high-performance liquid chromatography (HPLC) with multiwavelength photodiode-array absorbance detection (NCHS, 2011a; NCHS, 2011b). Vitamin C was measured by isocratic HPLC with electrochemical detection at 650 mV (NCHS, 2009b).
Statistical analysis
Urinary concentrations of phthalate metabolites, serum concentrations of antioxidants, and HOMA-IR were all log-transformed to normalize distributions. Potential confounders were selected based on prior knowledge, including age, gender, body mass index (BMI), race/ethnicity, education levels, poverty-income ratio, self-reported physical activity, serum continue, and urinary creatinine (James-Todd et al., 2012; Stahlhut et al., 2007; Trasande et al., 2013). Spearman correlations were used to assess the correlations of phthalate metabolites with serum antioxidants.
We first used multivariable linear regression models to evaluate the associations of individual log-transformed urinary phthalates metabolites with HOMA-IR. To enhance clinical interpretation, the mean differences in log HOMA-IR were translated to “a percent change in mean outcome per unit change in phthalate exposure” using the equation: [exp (β) − 1] × 100%. To account for simultaneous exposures to multiple concurrent exposures, we further applied Bayesian kernel machine regression (BKMR) (Bobb et al., 2015) using the bkmr package in R (Bobb et al., 2018). BKMR is a non-parametric approach to evaluate dose-response relationships and possible interactions between exposures and outcome associations. Details of this methodology have been described elsewhere (Bobb et al., 2015; Chiu et al., 2018; Valeri et al., 2017).
In our case, let X the vector of the mixture of components, i.e., xi=( ΣDEHPi, MEPi, MBPi, MiBPi, MBzPi, MCPPi, vitamin Ai, vitamin Ci, vitamin Ei, α–carotenei, β-carotenei, β-cryptoxanthini, lycopenei, lutein/zeaxanthini)T and Z the vector of potential confounders. For each subject i=1, …,n, the BKMR model is given by Yi=h(xi) +βTZi+ei, where the function h(xi) is an exposure-response function that accommodates nonlinearity and/or interaction among the mixture components. Z=Z1,…, Zp are p potential confounders. We used the Gaussian kernel function, which accommodates a broad range of underlying functional forms for h() and has been previously applied in other studies (Chiu et al., 2018; Valeri et al., 2017). Because some phthalate metabolites are highly correlated, we used a hierarchical variable selection approach (based on the principal component analysis results) to estimate the exposure-response surface of the relationship between night phthalate metabolites and insulin resistance. DEHP metabolites, MBzP, MiBP, MBP, and MCPP were designated as group 1, MEP alone was group 2, α-carotene and β-carotene were group 3, and the other vitamins and carotenoids were group 4. Fitting a BKMR model will estimate the exposure-response function h() while incorporating the uncertainty due to the estimation of high dimensional exposures and multiple-testing penalty. After fitting the model, we summarize results by plotting dose-response relationships between each individual phthalate metabolite and log HOMA-IR, with 95% credible intervals (95% CI). We also present the mean difference in log HOMA-IR per an interquartile (25th to 75th) change in a log phthalate metabolite concentration. To enhance clinical interpretation, these mean differences in log HOMA-IR were also translated to a percent change in mean outcome by exponentiating the corresponding estimates. To assess possible effect modification by various antioxidant levels, we plotted a dose-response relationship of a phthalate metabolite with HOMA-IR at various quantile concentrations (i.e., 25th, 50th, 75th quantiles) of an antioxidant. When presenting the results of BKMR analyses, we set the remaining metabolites in the models at their median concentrations. Lastly, to formally test for the presence of effect modification by serum levels of a specific nutrient (<median, >=median), we used multivariable linear models adding a cross-product term of phthalate and an antioxidant. When there was suggestive evidence of effect modification (p-value <0.10), we stratified the analyses by high (>=median) and low (<median) antioxidant groups. Analyses were performed in R software version 3.5.2.
Results
Baseline characteristics of the study population.
Our analysis included 1,605 participants from NHANES (2003–2006). Mean [standard deviation (SD)] age was 37.4 (21.5) years and BMI was 27.1 (7.1) kg/m2 (Table 1). Most participants were non-Hispanic whites (43%) and had not completed a high school education (46%). Their mean (SD) serum cotinine and urinary creatinine were 48.1 (113.6) ng/mL and 145.2 (83.5) mg/dL, respectively. The mean (SD) glucose, insulin, and HOMA-IR were 98.29 (24.89) mg/dL, 11.18 (9.67) μ U/mL, and 2.79 (2.71), respectively.
Table 1.
Baseline characteristics among 1,605 participants in the NHANES 2003–2006
| Characteristic | Mean ± SD or N (%) |
|---|---|
| Age (years) | 37.44 ± 21.51 |
| Gender | |
| Female | 788 (49.10) |
| Male | 817 (50.90) |
| Body mass index (kg/m2) | 27.12 ± 7.05 |
| Race | |
| Mexican American | 393 (24.48) |
| Other Hispanic | 60 (3.74) |
| Non-Hispanic White | 698 (43.49) |
| Non-Hispanic Black | 380 (23.68) |
| Other Race | 74 (4.61) |
| Education level | |
| Less than high school | 736 (45.86) |
| High school grad/GED or equivalent | 309 (19.25) |
| Some college or above | 560 (34.89) |
| Physical activitya | |
| No | 1079 (67.23) |
| Moderate activity over past 30 days | 294 (18.32) |
| Vigorous activity over past 30 days | 232 (14.45) |
| Serum cotinine (ng/mL) | 48.09 ± 113.56 |
| Urinary creatinine (mg/dL) | 145.16 ± 83.46 |
| Poverty-income ratio | 2.52 ± 1.63 |
| Glucose (mg/dL) | 98.29 ± 24.89 |
| Insulin (µU/mL) | 11.18 ± 9.67 |
| HOMA-IR | 2.79 ± 2.71 |
GED: General Educational Development; Poverty-income ratio: the ratio of a family’s income to the US Census Bureau’s poverty threshold;
: any moderate (light sweating or a slight to moderate increase in breathing or heart rate) or vigorous (heavy sweating or large increases in breathing or heart rate) activities for at least 10 minutes over past 30 days.
The distribution of the urinary phthalate metabolites and serum antioxidants is shown in Table S1. As shown in Supplemental Figure S1, the urinary phthalate metabolites were positively correlated with each other, with correlation coefficients ranging from 0.21 to 0.72 (p-values <.0001). Most of the serum antioxidants were also positively correlated with each other except for vitamin A and β-cryptoxanthin (r = −0.003, p-value= 0.92) (Supplemental Figure S2).
The relationship of phthalate metabolites with HOMA-IR
In multivariable linear regression models for examining associations of individual phthalate metabolites and individual antioxidants with HOMA-IR (Table 2), we found that MBP, MiBP, MCPP, and ΣDEHP were positively associated with HOMA-IR (beta ranges from 0.043 (p-value = 0.01) for MCPP (4.39% increase per unit change) to 0.058 (p-value <.01) for both MBP and MiBP (5.97% increase per unit change), while MEP was borderline negatively associated with log HOMA-IR (beta=−0.024, p-value = 0.06) (2.4% decrease per unit change). On the other hand, most antioxidants, including vitamin C, α–carotene, β-carotene, β-cryptoxanthin, lycopene, and lutein/zeaxanthin, were negatively associated with HOMA-IR (beta ranges from −0.179 (p-value <.0001) for β-carotene (19.6% decrease per unit change) to −0.053 (p-value = 0.04) (5.4% decrease per unit change) for vitamin C except for vitamin A, which had a positive association with HOMA-IR (beta=0.215, p-value <.001, 24.0% increase per unit change).
Table 2.
The associations of each phthalate metabolite or antioxidant with HOMA-IR among 1,605 participants based on the multiple linear regression model.
| Urinary phthalate metabolites | β (95%CI) | P-value |
|---|---|---|
| MBzP1 | 0.018 (−0.013, 0.049) | 0.26 |
| MBP1 | 0.058 (0.021, 0.094) | <.01 |
| MiBP1 | 0.058 (0.023, 0.092) | <.01 |
| MEP1 | −0.024 (−0.048, 0.001) | 0.06 |
| MCPP1 | 0.043 (0.009, 0.077) | 0.01 |
| ΣDEHP1 | 0.055 (0.027, 0.083) | <.001 |
| Vitamin A2 | 0.215 (0.090, 0.339) | <.001 |
| Vitamin C2 | −0.053 (−0.103, −0.003) | 0.04 |
| Vitamin E2 | −0.002 (−0.100, 0.096) | 0.97 |
| α–carotene2 | −0.110 (−0.149, −0.070) | <.0001 |
| β-carotene2 | −0.179 (−0.226, −0.132) | <.0001 |
| β-cryptoxanthin2 | −0.117 (−0.170, −0.064) | <.0001 |
| Lycopene2 | −0.053 (−0.119, 0.012) | 0.11 |
| Lutein/zeaxanthin2 | −0.139 (−0.215, −0.063) | <.001 |
All models were adjusted for age, gender, BMI, race, education level, physical activity, serum cotinine, urinary creatinine, and poverty-income ratio.
Urinary phthalate, serum antioxidants, and HOMA-IR were log transformed.
β represents the one unit change in log HOMA-IR per one unit increase in log urinary concentrations of the phthalate.
β represents the one unit change in HOMA-IR per one unit increase in log urinary concentrations of the antioxidant.
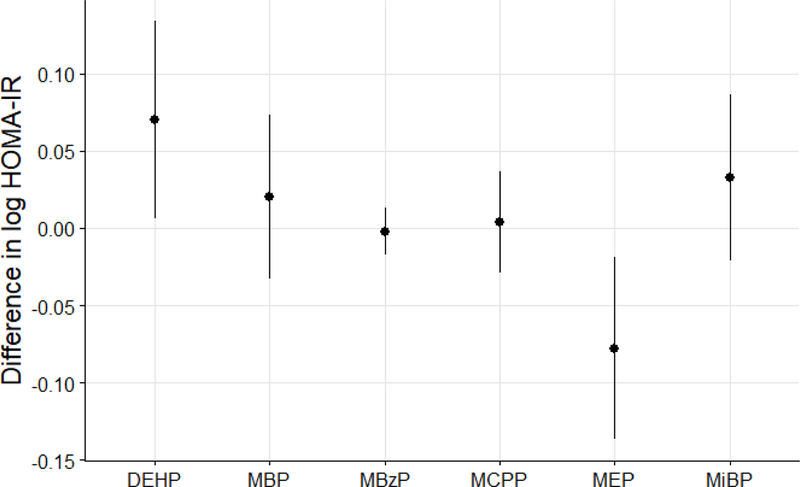
Because the associations observed in Table 2 may be confounded by other phthalate metabolites, we used the BKMR approach to assess the relationships between individual phthalate metabolites and HOMA-IR while accounting for correlations between these exposures. We observed a positive dose-response relationship between ΣDEHP and HOMA-IR and a negative, U-shaped relationship between MEP and HOMA-IR (Figures 1A and 1B). For the remaining phthalate metabolites, there was no evidence of relationships with HOMA-IR (Supplemental Figure S3). For better interpretation, we also quantified the magnitude of these associations (Figure 2). Specifically, a change in ΣDEHP concentrations from its 25th to 75th percentile was associated with a higher log HOMA-IR of 0.07 (95% CI = 0.01, 0.14) (4.85% increase in HOMA-IR). In contrast, a change in MEP concentrations from its 25th to 75th percentile was associated with a lower HOMA-IR of −0.07 (95% CI = −0.14, −0.02) (6.68% decrease in HOMA-IR). Other phthalate metabolites were not associated with HOMA-IR. In addition, the relationships of ΣDEHP or MEP with HOMA-IR were not modified by other phthalates (Supplemental Figure S4 and S5).
Figure 1.
Dose-response function (95% credible intervals) between selected metabolite concentrations (i.e., A) ΣDEHP and B) MEP) and HOMA-IR while fixing other phthalate metabolite concentrations at median values. The results were estimated by Bayesian kernel machine regression, adjusting for age, gender, BMI, race, education level, physical activity, serum cotinine, urinary creatinine, and poverty-income ratio.
Urinary phthalate, serum antioxidants, and HOMA-IR were log transformed.
Figure 2.
Mean differences in HOMA-IR (log-transformed, estimates and 95% confidence intervals) as a function of the concentrations of urinary phthalate metabolites. Point estimates showed the difference in mean HOMA-IR (log-transformed) when each urinary phthalate metabolite (log-transformed) was increased from the 25th to the 75th percentile of its distribution, while fixing other urinary phthalate metabolite concentrations at their median concentrations. The results were estimated by Bayesian Kernel Machine Regression, adjusting for age, gender, BMI, race, education level, physical activity, serum cotinine, urinary creatinine, and poverty-income ratio.
Effect modification by serum antioxidants
When simultaneously accounting for all other antioxidants, we found the dose-response relationship between ΣDEHP and HOMA-IR varied according to serum concentrations of β-carotene. Specifically, the positive association of ΣDEHP with HOMA-IR weakened with higher concentrations of β-carotene (Figure 3). However, the dose-response relationship for ΣDEHP and HOMA-IR was similar to varying levels of other antioxidants (Supplemental Figure S6). On the other hand, the dose-response relationship for MEP and HOMA-IR was not modified by any antioxidant examined (Supplemental Figure S7).
Figure 3.
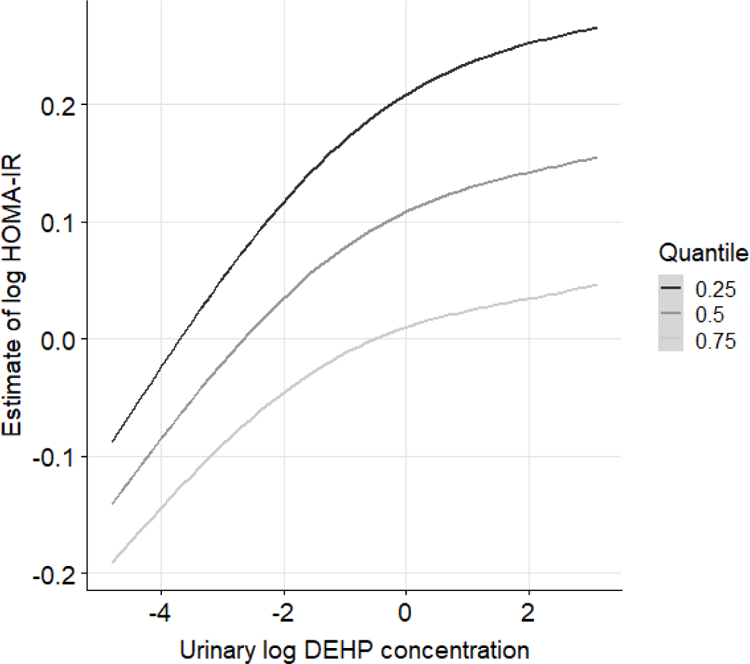
Bivariate exposure–response functions between log ΣDEHP and log HOMA-IR when log β-carotene concentrations at the 25th, 50th, or 75th percentile, while fixing other urinary phthalate metabolite concentrations at their median concentrations.
The results were estimated by Bayesian Kernel Machine Regression, adjusting for age, gender, BMI, race, education level, physical activity, serum cotinine, urinary creatinine, and poverty-income ratio.
Lastly, when formally testing for the presence of interactions between ΣDEHP and β-carotene as suggested by the BKMR, using cross-product terms in our multivariable linear regression model, there was a suggested interaction between ΣDEHP and β-carotene (p for interaction = 0.06). The positive association between ΣDEHP and HOMA were also weaker among participants with high concentrations of serum β-carotene than those with low concentrations (Table S2).
Discussion
In the population-based sample of US adults during 2003–2006, we found several urinary metabolites of several individual phthalates were associated with HOMA-IR in multivariable linear regression analyses. However, when we used BKMR to account for correlations among the metabolites, only DEHP had a positive association with HOMA-IR whereas MEP had an inverse association with it. Furthermore, we found that the positive association between DEHP and HOMA-IR weakened across increasing serum concentrations of β-carotene, suggesting that β-carotene might mitigate DEHP induced insulin resistance.
Phthalates may affect glucose metabolism or insulin resistance through several different pathways. Studies have suggested that phthalates can bind to human peroxisome proliferator-activated receptors (PPAR) alpha and gamma and upregulate the genes associated with adipogenesis and insulin resistance (Desvergne et al., 2009). Another important mechanism by which phthalates could be involved in diabetogenesis is through increased oxidative stress (Robertson et al., 2004). DEHP might induce oxidative stress and decrease expression of antioxidant enzymes (Cho et al., 2015), which in turn, impair insulin signaling and result in insulin resistance (Bloch-Damti and Bashan, 2005; Lamb and Goldstein, 2008; Morino et al., 2006). Several epidemiological studies have evaluated the association between urinary phthalate metabolites and diabetes mellitus or insulin resistance. A majority of the studies have found that DEHP was positively associated with insulin resistance or diabetes mellitus (Chen et al., 2017; Dirinck et al., 2015; Huang et al., 2014; James-Todd et al., 2012; Kim et al., 2013; Smerieri et al., 2015; Trasande et al., 2013), although a few studies did not find an association. (Kataria et al., 2017; Sun et al., 2014). Furthermore, in a prospective study of 560 elderly participants, Kim et al. found that exposure to urinary concentrations of DEHP metabolites was associated with higher HOMA-IR as well as higher malondialdehyde, an oxidative stress biomarker. In addition, increased malondialdehyde was also associated with glucose and HOMA-IR, suggesting that DEHP may induce insulin resistance through increased oxidative stress level (Kim et al., 2013). Regarding the association of MEP with insulin resistance, only a few studies have found a positive association (Dirinck et al., 2015; Stahlhut et al., 2007) whereas most found no association (Chen et al., 2017; Goodman et al., 2014; Huang et al., 2014; James-Todd et al., 2012; Trasande et al., 2013) between MEP and insulin resistance. Of note, however, two of the null studies found a negative trend between MEP and HOMR-IR although it did not reach a statistical significance (James-Todd et al., 2012; Trasande et al., 2013). These inconsistent results with our study may be due to the non-linear relationship between MEP and HOMA-IR identified in our study. Nonetheless, it is important to point out that these previous studies analyzed one phthalate metabolite at a time without accounting for other concurrent phthalates that are possibly associated with each other and with the outcomes.
Antioxidants are known to prevent oxidative damage by trapping free radicals and inhibits reactive oxygen species (Betteridge, 2000). Therefore, antioxidants may have a protective role against DEHP induced insulin resistance. In addition, certain antioxidants such as beta-carotene have been shown to inhibit proliferator-activated receptors (PPARs), which may protect against adiposity and insulin resistance (Ziouzenkova and Plutzky, 2008). In experimental studies, rats treated with DEHP showed impaired insulin signal transduction and elevated blood glucose levels compared with controls. In contrast, rats treated with DEHP plus vitamin C and E showed no difference in insulin signal transduction and glucose levels compared with controls, suggesting that antioxidant vitamin C and E may have a protective role against the adverse effect of DEHP on insulin resistance and insulin signaling (Rajesh et al., 2013; Srinivasan et al., 2011). To our knowledge, our study is the first human study examining the interactive effects of antioxidants on the associations between phthalates and HOMR-IR. It is important to note that the effectiveness of carotenoids as antioxidants might vary according to their interactions with other antioxidants (Young and Lowe, 2001). Thus, in the present study, when examining the interactive effect of β-carotene on DEHP and HOMA-IR, we simultaneously accounted for other antioxidants and phthalates in the same model. We found that the positive association between ΣDEHP and HOMA-IR attenuated with higher serum concentrations of β-carotene, but not with higher concentrations of vitamin C or vitamin E. The reasons for the inconsistency between our current study and past experimental studies are not clear. One possible explanation is that β-carotene is a more lipophilic antioxidant than vitamin C and E, making it more efficient at scavenging free radicals within the lipophilic compartment (Niki et al., 1995; Shite et al., 2001). The other possible explanation is that vitamin C and E have a shorter half-life (8–40 days for Vitamin C; 44 hours for vitamin E (α-tocopherol); 37 days for beta-carotene) (Burri et al., 2001; Duconge et al., 2008; Tan et al., 2012). Thus, there may be misclassification of vitamin C and E levels. Future examination of the effect of different antioxidants on DEHP-induced insulin resistance is warranted.
Strengths of our study include a large sample size with sufficient statistical power to assess potential interactions. Also, we used BKMR accounting for potential interactions between phthalate mixtures and antioxidants. An additional strength is the use of objectively measured biomarkers in the analysis. The most important limitation of the study is its cross-sectional design, limiting our ability to assess causality. In addition, we only have a one-time assessment of the serum antioxidants, urinary concentrations of phthalate metabolites, and HOMA-IR, which may introduce misclassification of exposures and outcomes.
In conclusion, in this cross-sectional study, we found urinary DEHP metabolite concentrations were positively associated with HOMA-IR, whereas urinary MEP was inversely associated with HOMA-IR after accounting for complex mixtures of phthalates. In addition, the positive association between ΣDEHP and HOMA weakened among participants with higher concentrations of serum β-carotene. To our knowledge, this is the first human study to address a potential protective effect of serum β-carotene on the urinary ΣDEHP induced insulin resistance. Future studies, especially using multiple urine samples and prospective design, are warranted to verify this association.
Supplementary Material
Highlights.
Bayesian kernel machine regression was used to model the exposure-response function
Multiple concurrent exposures were taken into account in our analysis
DEHP was associated with higher insulin resistance
MEP was associated with lower insulin resistance
β-carotene might mitigate the adverse effect of DEHP on insulin resistance
Acknowledgments
Dr. Chiu acknowledged Amazon Web Service (AWS) Research Grant and the NVIDIA Graphics Processing Unit (GPU) grant. Dr. Chavarro was supported by grant P30DK046200 from the National Institutes of Health. Dr. Li was supported by grant CMU107-Z-0 from China Medical University
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest and Funding Disclosure:
The authors declare no conflict of interest.
References
- Betteridge DJ, 2000. What is oxidative stress? Metabolism 49, 3–8. [DOI] [PubMed] [Google Scholar]
- Bloch-Damti A, Bashan N, 2005. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid Redox Signal 7, 1553–67. [DOI] [PubMed] [Google Scholar]
- Bobb JF, et al. , 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 17, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, et al. , 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16, 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri BJ, et al. , 2001. Serum carotenoid depletion follows first-order kinetics in healthy adult women fed naturally low carotenoid diets. J Nutr 131, 2096–100. [DOI] [PubMed] [Google Scholar]
- Chen SY, et al. , 2017. Mono-2-ethylhexyl phthalate associated with insulin resistance and lower testosterone levels in a young population. Environ Pollut 225, 112–117. [DOI] [PubMed] [Google Scholar]
- Chiu YH, et al. , 2018. Evaluating effects of prenatal exposure to phthalate mixtures on birth weight: A comparison of three statistical approaches. Environ Int 113, 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YJ, et al. , 2015. Di-(2-ethylhexyl)-phthalate induces oxidative stress in human endometrial stromal cells in vitro. Mol Cell Endocrinol 407, 9–17. [DOI] [PubMed] [Google Scholar]
- Desvergne B, et al. , 2009. PPAR-mediated activity of phthalates: A link to the obesity epidemic? Mol Cell Endocrinol 304, 43–8. [DOI] [PubMed] [Google Scholar]
- Dirinck E, et al. , 2015. Urinary phthalate metabolites are associated with insulin resistance in obese subjects. Environ Res 137, 419–23. [DOI] [PubMed] [Google Scholar]
- Duconge J, et al. , 2008. Pharmacokinetics of vitamin C: insights into the oral and intravenous administration of ascorbate. P R Health Sci J 27, 7–19. [PubMed] [Google Scholar]
- Goldstein BJ, et al. , 2005. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes 54, 311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M, et al. , 2014. Do phthalates act as obesogens in humans? A systematic review of the epidemiological literature. Crit Rev Toxicol 44, 151–75. [DOI] [PubMed] [Google Scholar]
- Hauser R, Calafat A, 2005. Phthalates and human health. Occupational and environmental medicine 62, 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, et al. , 2016. Urinary Phthalate Metabolite Concentrations and Reproductive Outcomes among Women Undergoing in Vitro Fertilization: Results from the EARTH Study. Environ Health Perspect 124, 831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YC, et al. , 2009. Community level exposure to chemicals and oxidative stress in adult population. Toxicol Lett 184, 139–44. [DOI] [PubMed] [Google Scholar]
- Huang T, et al. , 2014. Gender and racial/ethnic differences in the associations of urinary phthalate metabolites with markers of diabetes risk: National Health and Nutrition Examination Survey 2001–2008. Environ Health 13, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd T, et al. , 2012. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ Health Perspect 120, 1307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataria A, et al. , 2017. Exposure to bisphenols and phthalates and association with oxidant stress, insulin resistance, and endothelial dysfunction in children. Pediatr Res 81, 857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, et al. , 2013. Diethylhexyl phthalates is associated with insulin resistance via oxidative stress in the elderly: a panel study. PloS one 8, e71392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RE, Goldstein BJ, 2008. Modulating an oxidative-inflammatory cascade: potential new treatment strategy for improving glucose metabolism, insulin resistance, and vascular function. Int J Clin Pract 62, 1087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie C, et al. , 2015. Obstetrical outcomes and biomarkers to assess exposure to phthalates: A review. Environ Int 83, 116–36. [DOI] [PubMed] [Google Scholar]
- Matthews DR, et al. , 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–9. [DOI] [PubMed] [Google Scholar]
- Morino K, et al. , 2006. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55 Suppl 2, S9–s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCHS, 2008a. National Health and Nutrition Examination Survey 2003–2004: Data documentation, codebook, and frequencies: Phthalates - urine (L24PH_C)
- NCHS, National Health and Nutrition Examination Survey: 2003–2004 data documentation, codebook, and frequencies 2008b.
- NCHS, National Health and Nutrition Examination Survey: 2003–2004 data documentation, codebook, and frequencies: Demographic Variables & Sample Weights 2009a.
- NCHS, National Health and Nutrition Examination Survey: 2003–2004 data documentation, codebook, and frequencies: Vitamin C 2009b.
- NCHS, National Health and Nutrition Examination Survey 2005–2006 data documentation, codebook, and frequencies: Phthalates - urine (PHTHTE_D) 2010.
- NCHS, National Health and Nutrition Examination Survey 2005–2006 data documentation, codebook, and frequencies: Vitamin A, vitamin E & carotenoids 2011a.
- NCHS, National Health and Nutrition Examination Survey: 2003–2004 data documentation, codebook, and frequencies: Vitamin A, Vitamin E & Carotenoids 2011b.
- NCHS, National Health and Nutrition Examination Survey 2003–2004 data documentation, codebook, and frequencies: Plasma fasting glucose, serum c-peptide & insulin 2016a.
- NCHS, National Health and Nutrition Examination Survey: 2005–2006 data documentation, codebook, and frequencies. Plasma fasting glucose & insulin (GLU_D) 2016b.
- NCHS, 2017. About the National Health and Nutrition Examination Survey
- Niki E, et al. , 1995. Interaction among vitamin C, vitamin E, and beta-carotene. Am J Clin Nutr 62, 1322s–1326s. [DOI] [PubMed] [Google Scholar]
- Pisoschi AM, Pop A, 2015. The role of antioxidants in the chemistry of oxidative stress: a review. European journal of medicinal chemistry 97, 55–74. [DOI] [PubMed] [Google Scholar]
- Rajesh P, et al. , 2013. Phthalate is associated with insulin resistance in adipose tissue of male rat: role of antioxidant vitamins. J Cell Biochem 114, 558–69. [DOI] [PubMed] [Google Scholar]
- Robertson RP, et al. , 2004. β-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 53, S119–S124. [DOI] [PubMed] [Google Scholar]
- Romero-Franco M, et al. , 2011. Personal care product use and urinary levels of phthalate metabolites in Mexican women. Environ Int 37, 867–71. [DOI] [PubMed] [Google Scholar]
- Schettler T, 2006. Human exposure to phthalates via consumer products. Int J Androl 29, 134–9; discussion 181–5. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, et al. , 2007. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 6, 280–93. [DOI] [PubMed] [Google Scholar]
- Shite J, et al. , 2001. Antioxidant vitamins attenuate oxidative stress and cardiac dysfunction in tachycardia-induced cardiomyopathy. J Am Coll Cardiol 38, 1734–40. [DOI] [PubMed] [Google Scholar]
- Smerieri A, et al. , 2015. Di-(2-ethylhexyl) phthalate metabolites in urine show age-related changes and associations with adiposity and parameters of insulin sensitivity in childhood. PLoS One 10, e0117831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan C, et al. , 2011. Diethyl hexyl phthalate-induced changes in insulin signaling molecules and the protective role of antioxidant vitamins in gastrocnemius muscle of adult male rat. Toxicology and applied pharmacology 257, 155–164. [DOI] [PubMed] [Google Scholar]
- Stahlhut RW, et al. , 2007. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect 115, 876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, et al. , 2014. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ Health Perspect 122, 616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson K, et al. , 2011. Phthalate exposure associated with self-reported diabetes among Mexican women. Environmental Research 111, 792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B, et al. , 2012. Tocotrienols: vitamin E beyond tocopherols CrC press. [Google Scholar]
- Trasande L, et al. , 2013. Urinary phthalates and increased insulin resistance in adolescents. Pediatrics 132, e646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri L, et al. , 2017. The Joint Effect of Prenatal Exposure to Metal Mixtures on Neurodevelopmental Outcomes at 20–40 Months of Age: Evidence from Rural Bangladesh. Environ Health Perspect 125, 067015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan MP, et al. , 2017. Effects of DEHP and its metabolite MEHP on insulin signalling and proteins involved in GLUT4 translocation in cultured L6 myotubes. Toxicology 386, 60–71. [DOI] [PubMed] [Google Scholar]
- Young AJ, Lowe GM, 2001. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys 385, 20–7. [DOI] [PubMed] [Google Scholar]
- Ziouzenkova O, Plutzky J, 2008. Retinoid metabolism and nuclear receptor responses: New insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett 582, 32–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.