Abstract
l‐homoarginine is an endogenous, non‐proteinogenic amino acid that has emerged as a new player in health and disease. Specifically, low l‐homoarginine levels are associated with cardiovascular diseases, stroke, and reduced kidney function. However, the role of l‐homoarginine in the pathogenesis of diabetic nephropathy (DN) is not known. Experiments were conducted in 6‐week‐old Ins2Akita mice supplemented with l‐homoarginine via drinking water or mini osmotic pump for 12 weeks. Both plasma and kidney l‐homoarginine levels were significantly reduced in diabetic mice compared to nondiabetic controls. Untreated Ins2Akita mice showed significant increases in urinary albumin excretion, histological changes, glomerular macrophage recruitment, the inflammatory cytokine KC‐GRO/CXCL1, and urinary thiobarbituric acid reactive substances (TBARS) excretion as an indicator of oxidative stress, along with a significant reduction in kidney nitrate + nitrite levels compared to control mice at 18 weeks of age. In contrast, l‐homoarginine supplementation for 12 weeks in Ins2Akita mice, via either drinking water or mini osmotic pump, significantly reduced albuminuria, renal histological changes, glomerular macrophage recruitment, KC‐GRO/CXCL1 levels, urinary TBARS excretion, and largely restored kidney nitrate + nitrite levels. These data demonstrate that l‐homoarginine supplementation attenuates specific features of DN in mice and could be a potential new therapeutic tool for treating diabetic patients.
Keywords: Diabetic nephropathy, l‐homoarginine, nitric oxide
Introduction
In the United States, diabetes is the major cause of end‐stage renal disease (ESRD), accounting for more than 40% of ESRD patients, making it a heavy burden for the health care system (Boyle et al. 2010). Early histological changes in diabetic nephropathy (DN) include glomerular hyperfiltration, glomerular hypertrophy, followed by glomerular basement membrane thickening, endothelium dysfunction, and mesangial matrix accumulation. As the disease progresses, the urinary albumin excretion rate (UAER) increases, which leads to glomerular sclerosis and ultimately ESRD.
l‐homoarginine is an endogenous amino acid in many species that is not involved in protein synthesis (Pilz et al. 2015). l‐homoarginine is synthesized by mitochondrial arginine:glycine amidinotransferase (AGAT) in kidneys using l‐arginine and l‐lysine as substrates (Ryan and Wells 1964; Choe et al. 2013) and is distinguished from l‐arginine by an additional methylene group in its structure (Pilz et al. 2015).
Plasma concentrations of homoarginine are inversely correlated with the risk of cardiovascular disease and overall mortality (Marz et al. 2010; Jud et al. 2018), increased risk for fatal strokes (Haghikia et al. 2017), congestive heart failure and left ventricular hypertrophy (Atzler et al. 2013, 2017, Pilz et al. 2014a; Bahls et al. 2018), aging (Marz et al. 2010; Atzler et al. 2014b), smoking (Zwan et al. 2013; Sobczak et al. 2014; Atzler et al. 2014a; Vogl et al. 2015), body mass index (Marz et al. 2010; Atzler et al. 2014a; Pilz et al. 2014b), and pregnancy (Valtonen et al. 2008). In addition, the effects of homoarginine on nitric oxide (NO) synthesis have been linked with increased risk of stroke and atherosclerosis as a reduction in homoarginine levels increased the aortic intima‐media thickness (Haghikia et al. 2017). Low plasma homoarginine concentration is also significantly associated with the progression of chronic kidney disease (Drechsler et al. 2013) and progression to dialysis and mortality (Ravani et al. 2013), indicating that low plasma homoarginine concentrations might be an early indicator of kidney failure and a potential target for the prevention of disease progression. However, the role of homoarginine in the pathogenesis of DN is not known.
These findings suggested that changes in l‐homoarginine levels or metabolism may be involved in chronic renal injury. However, there is little information regarding the possible role of l‐homoarginine in diabetic renal injury.
Therefore, we investigated whether changes in l‐homoarginine levels play a role in DN. Using diabetic Ins2Akita mice as a model of type‐1 diabetes, this current study demonstrates that plasma and kidney homoarginine levels were reduced in diabetic mice compared to nondiabetic control mice. l‐homoarginine supplementation significantly reduced kidney injury, as evidenced by reduced albuminuria, histologic kidney damage, glomerular macrophage recruitment, inflammatory cytokines, urinary thiobarbituric acid reactive substances (TBARS) excretion, along with significant increases in kidney nitrate + nitrite levels. Taken together, our data suggest that l‐homoarginine supplementation might be a promising therapeutic modality for DN.
Materials and Methods
Animal model
All animal experiments were performed in Ins2Akita and their wild‐type littermates (DBA background; Jackson Laboratories, Bar Harbor, ME). The experiments started at 6 weeks of age. As recommended by the Animal Models of Diabetes Complication Consortium as an optimal model of DN, Ins2Akita mice develop hyperglycemia at 3–4 weeks of age (Breyer et al. 2005; Brosius et al. 2009). All the mice were euthanized at 18 weeks of age. Mouse plasma and 24‐hour urine were collected, and kidneys were removed for further studies. All the animal experiments were approved by Pennsylvania State University College of Medicine Institutional Animal Care and Use Committee.
l‐homoarginine administration
Ins2Akita mice at 6 weeks of age were supplemented with l‐homoarginine (Sigma, St. Louis MO, Cat #: H1007) for 12 weeks either in drinking water at a concentration of 50 mg/L or by continuous subcutaneous infusion via a mini osmotic pump (Alzet, Durect, Palo Alto, CA) at a dose of 0.72 mg/kg/day (Pump was changed after 6 weeks). All mice had free access to drinking water.
Immunohistochemistry
Mouse kidney tissues were fixed in 10% formalin and embedded in paraffin, and 3‐μm sections were cut. Immunohistochemistry was performed on paraffin‐embedded sections with anti‐mouse Mac‐2 antibody (clone M3/38; Cedarlane, Burlington, NC) as previously described (You et al. 2013a, 2014). Twenty glomeruli were examined at 40× in a blinded manner. Images were taken with an Olympus BX51 microscope and DP71 digital camera using Microsuite Basic 2.6 image software. Images were obtained with 100× (oil) objective with a total magnification of 1000×.
Renal histopathology
Kidneys were fixed in 4% paraformaldehyde, embedded in paraffin, and 5‐μm sections were cut. Sections were stained with periodic acid–Schiff (PAS) stain, all glomeruli were examined at 400× in a blinded manner, and scores were averaged. All images were obtained with a Nikon Eclipse E600 microscope and Nikon DXM1200 camera. Images were taken at 400x magnification.
Analytical methodology
Blood glucose was measured using Accu‐Chek glucometer and urine albumin concentration was measured by ELISA using an Albuwell M kit (Exocell, Philadelphia, PA) as described previously (Awad et al. 2011, 2015b; Morris et al. 2017). Urinary TBARS Assay was performed according to the instruction provided by Models of Diabetic Complication Consortium (AMDCC) as described previously (You et al. 2013b, 2014). Plasma and kidney arginine levels were determined by liquid chromatography–mass spectroscopy and amino acid assays as previously described (You et al. 2014).
Homoarginine assay
Mouse kidneys were homogenized in 300‐μL 3M perchloric acid (Fisher Scientific, Fair Lawn, NJ) per 10 mg of tissue. After centrifugation at 4°C, the supernatants were used for amino acid assay as previously described (You et al. 2014).
Kidney nitrate and nitrite assay
Kidney lysates were homogenized in 1 × PBS, pH 7.4, and centrifuged at 4°C. Supernatants were transferred to a clean tube and protein concentration was determined by BCA protein assay. Nitrate and nitrite were measured using a Nitrate/Nitrite Colorimetric Assay Kit (Cayman Chemical, Ann Arbor, MI, cat #: 780001) according to manufacturer's instructions (You et al. 2014).
MSD multi‐spot assay
Mouse KC‐GRO/CXCL1 was measured in 90 µg of kidney lysate protein using a Multi‐spot assay system (Mouse proinflammatory panel 1, Meso Scale Diagnostics, Rockville, MD) performed as per manufacturer's instructions.
Statistical analysis
Comparisons between groups were conducted using SPSS software (version 19.0, SPSS, Chicago, IL). Results are expressed as mean ± SEM. One‐way ANOVA was used to compare significance between more than two groups. A P value of <0.05 represented significant difference.
Results
Type‐1 diabetes reduces plasma and kidney l‐homoarginine levels
Plasma samples from 18‐week‐old diabetic mice were analyzed for l‐homoarginine levels. Both plasma and kidney l‐homoarginine levels were significantly reduced in type‐1 diabetic mice compared to nondiabetic control mice (Fig. 1), indicating that diabetes alters circulating l‐homoarginine levels.
Figure 1.
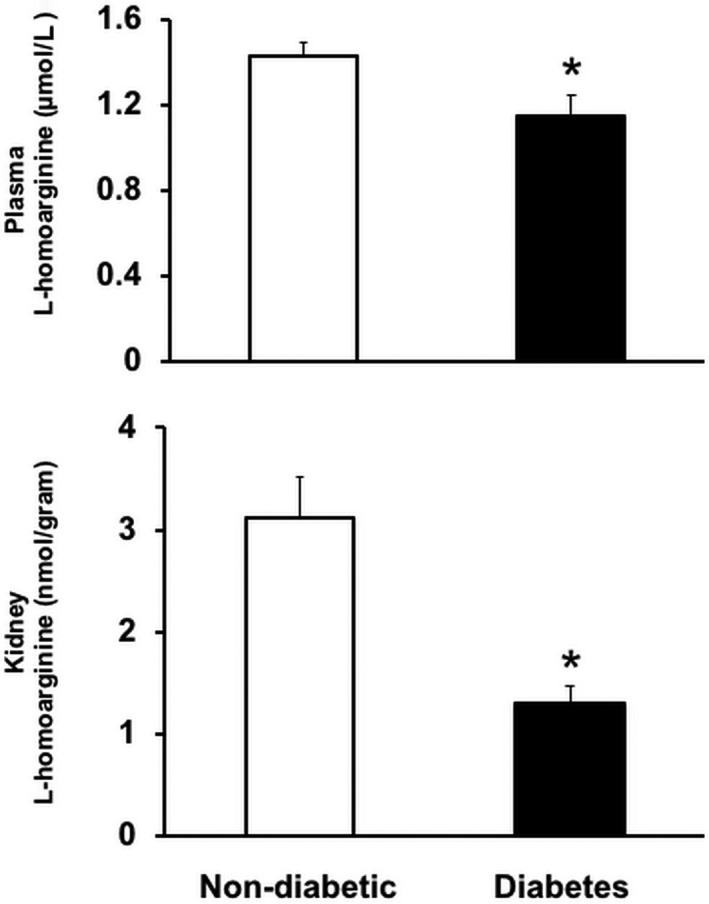
Plasma and kidney l‐homoarginine levels were reduced in type‐1 diabetic mice. Plasma and kidney samples from diabetic and nondiabetic mice at 18 weeks of age were used to measure l‐homoarginine levels using a liquid chromatography–mass spectrometry system. Results are presented as mean ± SEM. *P < 0.05 compared to nondiabetic mice.
l‐homoarginine supplementation did not alter gross characteristics of diabetic mice
l‐homoarginine supplementation via drinking water or osmotic pump had no effect on the increased blood glucose levels, increased water consumption, decreased body weight, or increased kidney weight/body weight ratio in diabetic mice (Table 1).
Table 1.
Characteristics of mice.
| Nondiabetic | Diabetes | Diabetes + oral HA | Diabetes + pump HA | |
|---|---|---|---|---|
| Number | 10 | 9 | 9 | 7 |
| BW (g) | 33 ± 0.8 | 26 ± 0.5a | 25 ± 0.7a | 27 ± 0.8b |
| BG (mg/dL) | 156 ± 11 | 501 ± 65a | 542 ± 50a | 638 ± 42a |
| WI (mL/24 h) | 1.9 ± 0.4 | 6.8 ± 0.4a | 5.6 ± 0.7c | 7.5 ± 0.7a |
| KW/BW | 0.96 ± 0.04 | 1.38 ± 0.08c | 1.35 ± 0.08c | 1.25 ± 0.06b |
Diabetic mice were supplemented with homoarginine via drinking water or osmotic pumps for 12 weeks. HA, homoarginine; BW, Body weight; BG, Blood glucose; WI, Water intake; KW/BW, kidney weight/body weight.
P < 0.0001.
P < 0.001.
P < 0.0005 versus nondiabetic.
l‐homoarginine supplementation reduced albuminuria in diabetic mice
As shown in Figure 2, Ins2Akita mice displayed significantly higher UAER than nondiabetic controls. l‐homoarginine supplementation by either drinking water or osmotic pumps for 12 weeks in Ins2Akita mice significantly reduced UAER compared to untreated Ins2Akita mice. In addition, l‐homoarginine supplementation by drinking water in Ins2Akita mice significantly reduced UAER at week 9 compared to untreated Ins2Akita mice. Importantly, l‐homoarginine supplementation significantly increased kidney homoarginine levels (Fig. 3).
Figure 2.
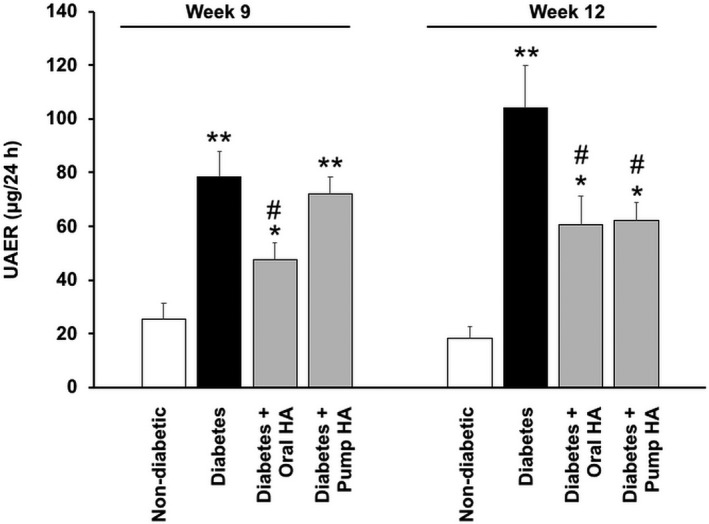
l‐homoarginine supplementation reduces urine albuminuria in diabetic mice. l‐homoarginine was supplemented to Ins2Akita mice for 12 weeks via either drinking water or mini osmotic pumps as indicated. Twenty‐four hour urine was collected for the measurement of UAER after 9 and 12 weeks of supplementation. Data are presented as mean ± SEM. *P < 0.05; **P < 0.01 compared to nondiabetic controls mice; # P < 0.05 compared to diabetic mice.
Figure 3.
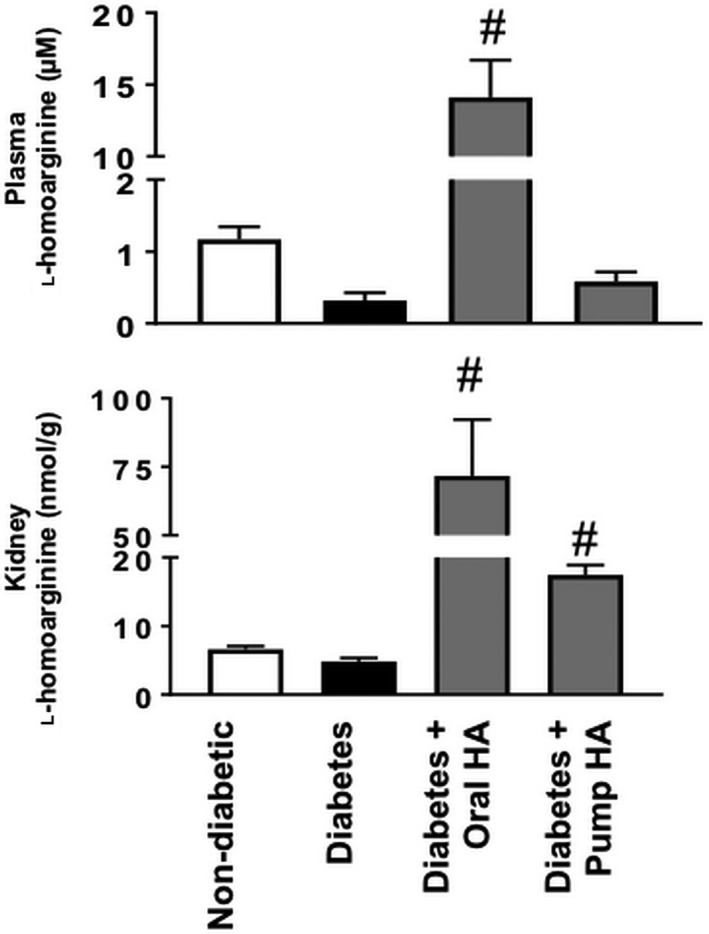
l‐homoarginine supplementation increased kidney homoarginine levels in diabetic mice. Plasma and kidney samples were used after 12 weeks of diabetes using a liquid chromatography–mass spectrometry system or ELISA assay. Results are presented as mean ± SEM. # P < 0.0001 compared to diabetic mice.
l‐homoarginine supplementation decreased renal histological changes in diabetic mice
Periodic acid–Schiff staining showed significantly increased glomerular cellularity and mesangial expansion at 18 weeks of age in Ins2Akita mice versus nondiabetic controls (score of 1.5 vs. 0.25, respectively). l‐homoarginine supplementation by either drinking water or osmotic pumps for 12 weeks in Ins2Akita mice significantly reduced glomerular changes (score of 0.625 and 0.375, respectively) compared to untreated Ins2Akita mice (Fig. 4).
Figure 4.
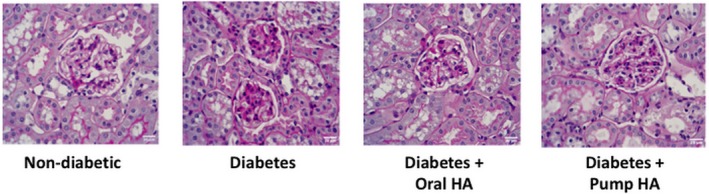
Effects of l‐homoarginine on kidney pathology. Paraffin‐embedded mouse kidney sections were subjected to periodic acid–Schiff (PAS) staining. Scale bar represents 20 µm.
l‐homoarginine supplementation reduces glomerular macrophage infiltration in diabetic mice
Consistent with previous studies (You et al. 2013a; Awad et al. 2015a), Mac‐2 staining in untreated Ins2Akita mice showed significant increases in glomerular macrophages compared with untreated controls. l‐homoarginine supplementation by either drinking water or osmotic pumps for 12 weeks in Ins2Akita mice had significantly reduced glomerular macrophage recruitment compared to untreated Ins2Akita mice (Fig. 5).
Figure 5.
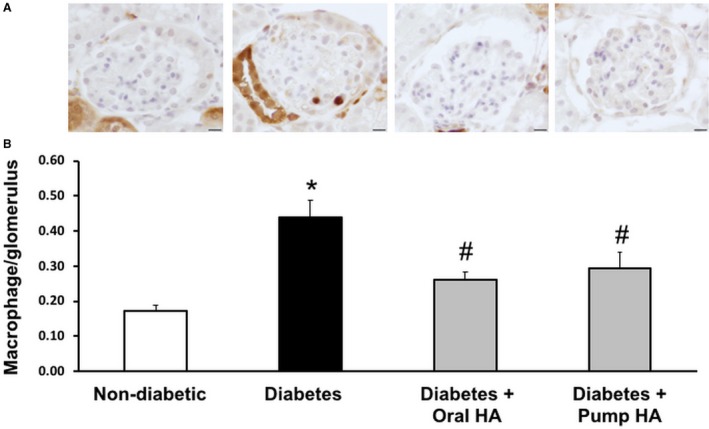
l‐homoarginine supplementation reduces glomerular macrophage infiltration in diabetic mice. (A) Glomerular macrophage recruitment was visualized by immunohistochemical staining using Mac‐2 antibody 12 weeks after l‐homoarginine supplementation. Macrophage numbers per glomerulus were counted in a blinded manner. Scale bar in images represents 10 µm. (B) The number of glomerular macrophages was counted in 20 glomeruli per section (number of macrophages in glomeruli divided by the number of glomeruli) in blinded fashion under 40× magnification and averaged. Results are presented as mean ± SEM. *P < 0.01 compared to nondiabetic control mice; # P < 0.05 compared to diabetic mice. N = 10 for nondiabetic, 9 for diabetes and diabetes + oral HA, 7 for diabetes + pump HA.
l‐homoarginine supplementation reduces kidney KC‐GRO (CXCL1) level in diabetic mice
We demonstrated previously that inflammation is a critical determinant in the pathophysiology of DN (Awad et al. 2011; You et al. 2017). KC‐GRO/CXCL1 is a cytokine that plays an important role in leukocyte recruitment, and mediates inflammation in diabetes (Citro et al. 2015). Kidney KC‐GRO/CXCL1 significantly increased in untreated Ins2Akita mice compared to nondiabetic controls, an effect that was attenuated by oral l‐homoarginine supplementation (Fig. 6).
Figure 6.
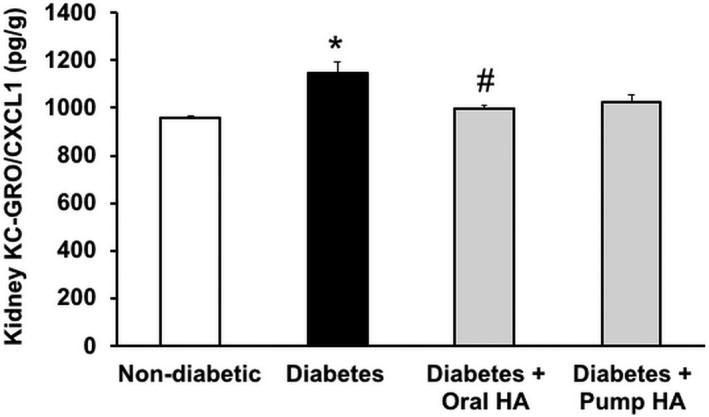
l‐homoarginine supplementation reduces kidney KC‐GRO/CXCL1 levels in diabetic mice. A MSD multi‐spot assay was used to measure kidney KC‐GRO levels. Data are presented as mean ± SEM. *P < 0.0005 compared to nondiabetic control mice; # P < 0.005 compared to diabetic mice.
l‐homoarginine supplementation reduces TBARS, an indicator of oxidative stress in diabetic mice
Urinary TBARS levels were used as an indicator of oxidative stress as previously reported (You et al. 2013b, 2014). Whereas levels of urinary TBARS greatly increased in untreated Ins2Akita mice compared to nondiabetic controls; urinary TBARS excretion was significantly attenuated in diabetic mice that received l‐homoarginine supplementation compared to untreated Ins2Akita mice (Fig. 7).
Figure 7.
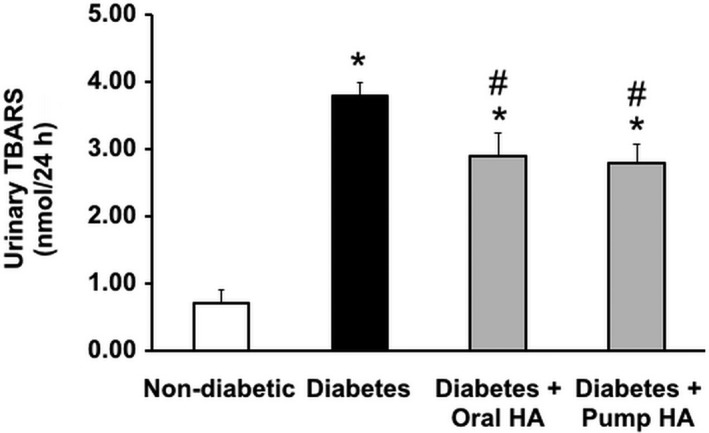
l‐homoarginine supplementation reduces urinary TBARS excretion in diabetic mice. Twenty‐four hour urine was collected at 18 weeks of age for the measurement of TBARS. Data are presented as mean ± SEM. *P < 0.0001 compared to nondiabetic controls mice; # P < 0.05 compared to diabetic mice.
l‐homoarginine supplementation increases kidney nitrate and nitrite production in diabetic mice
Nitric oxide plays a critical role in DN. Previous reports demonstrated that NO inhibition or deficiency exacerbates kidney dysfunction in diabetic mice (Zhao et al. 2006; Wang et al. 2011). Consistent with our previous report (You et al. 2015), kidney nitrate + nitrite levels were significantly reduced in Ins2Akita mice compared to nondiabetic controls. Oral l‐homoarginine supplementation significantly increased kidney nitrate + nitrite levels compared to untreated Ins2Akita mice (Fig. 8).
Figure 8.
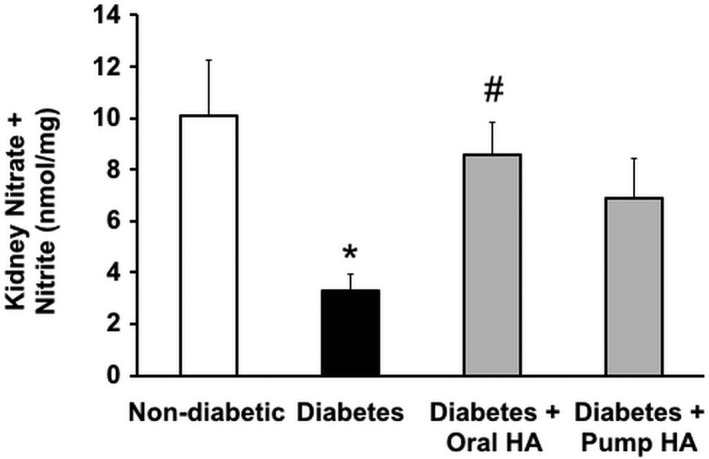
l‐homoarginine supplementation increases renal nitrate and nitrite concentration in diabetic mice. Mouse kidney lysates were used to measure nitrate and nitrite concentration after 12 weeks of l‐homoarginine supplementation. Data are presented as mean ± SEM. *P < 0.01 compared to nondiabetic control mice; # P < 0.05 compared to diabetic mice.
l‐homoarginine supplementation did not affect arginine levels in diabetic mice
As arginase‐2 inhibition or deletion ameliorates diabetic kidney injury (Morris et al. 2011; You et al. 2013b, 2014) and arginase inhibition prevents the reduction in kidney nitrate + nitrite levels in diabetic mice (You et al. 2015), we investigated whether homoarginine has any effect on l‐arginine levels in circulation and in the kidneys. Although plasma l‐arginine levels were shown to be reduced in streptozotocin‐induced diabetic DBA/2J mice, (You et al. 2014) plasma l‐arginine levels have not been reported previously for diabetic Ins2Akita mice. We therefore evaluated l‐arginine availability in Ins2Akita mice as indicated by levels of l‐arginine in plasma and kidney. As shown in Figure 9, diabetes significantly reduced the levels of l‐arginine in both plasma and kidney, but l‐homoarginine supplementation did not restore l‐arginine levels to nondiabetic levels in either plasma or kidney.
Figure 9.
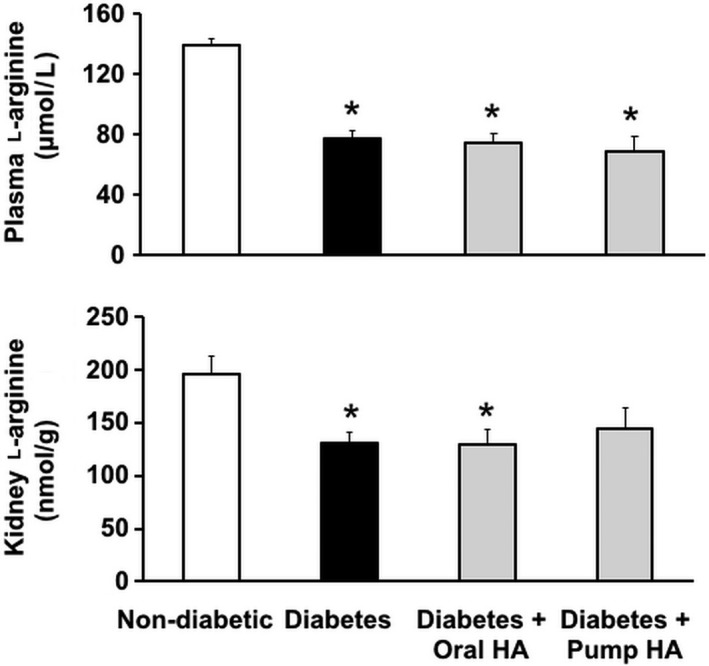
The effect of l‐homoarginine supplementation on l‐arginine levels. Mouse plasma and kidney tissues were prepared to measure l‐arginine levels using a liquid chromatography–mass spectrometry system. Data are mean ± SEM. *P < 0.01 compared to nondiabetic control mice.
Discussion
l‐homoarginine has a well‐established role to alter endothelial function in cardiovascular diseases, yet its role in diabetic kidney injury has not previously been determined. This study shows that homoarginine supplementation mediates renal tissue protection as demonstrated by a reduction in albuminuria, histopathological changes, and kidney macrophage recruitment during diabetes. We speculate that the effect of homoarginine in the kidney is mainly mediated via increased NO production as indicated by increased kidney nitrate + nitrite levels in diabetic mice. These findings reveal an important role for homoarginine in the pathogenesis of DN and provide evidence for homoarginine supplementation as potential therapeutic modality for treating diabetic patients.
Although l‐homoarginine has been known for a century, its role in physiology and pathophysiology remains unclear. l‐homoarginine is synthesized by mitochondrial AGAT in kidneys using l‐arginine and l‐lysine as substrates (Ryan and Wells 1964; Choe et al. 2013). Clinical studies of l‐arginine metabolites indicated that reduced l‐homoarginine levels were correlated with coronary heart diseases, peripheral arterial occlusive disease, stroke, and overall mortality (Marz et al. 2010; Ravani et al. 2013; Drechsler et al. 2015; Pilz et al. 2015). Choe et al. (2013) reported that elevated l‐homoarginine levels were associated with reduced all‐cause mortality in patients with ischemic stroke and that low l‐homoarginine appears to be related to poor outcome after ischemic stroke. Moreover, l‐homoarginine supplementation significantly improved cerebral damage and neurological deficits in mouse models with stroke (Haghikia et al. 2017).
Clinical investigations demonstrated that l‐homoarginine concentrations are reduced in patients with impaired kidney function. The European mild to moderate kidney disease study showed that plasma l‐homoarginine levels were significantly associated with reduced glomerular filtration rate (GFR) and proteinuria (Ravani et al. 2013), and low plasma l‐homoarginine concentrations were identified as a predictor of kidney progression (Drechsler et al. 2013; Ravani et al. 2013). Importantly, l‐homoarginine supplementation has been shown to be safe and well tolerated in young volunteers (Atzler et al. 2016). In addition, supplementation of homoarginine to control mice did not alter any hemodynamic parameters or body weight despite increasing plasma homoarginine levels (Atzler et al. 2017; Karetnikova et al. 2019). Although reduced l‐homoarginine levels are a marker for cardiovascular and kidney disease (Morris et al. 2011; Jud et al. 2018; Martens‐Lobenhoffer et al. 2018), the role of l‐homoarginine in diabetes remains controversial (Carmann et al. 2015; Krebs et al. 2015). In a diet‐induced obese mouse model, Stockebrand et al. (2015) reported that l‐homoarginine supplementation reduced blood glucose and stimulated insulin production, despite having no effect on body weight or glucose tolerance. Although we found reduced plasma l‐homoarginine levels in diabetic Ins2Akita mice, our data showed no effect of l‐homoarginine supplementation on elevated blood glucose levels in diabetic Ins2Akita mice. However, l‐homoarginine supplementation did attenuate diabetes‐induced reductions in albuminuria, kidney histological changes, glomerular macrophage recruitment, KC‐GRO/CXCL1 levels, urinary TBARS excretion, and restored kidney nitrate + nitrite levels.
Although diabetes significantly reduced l‐arginine levels in plasma and kidney tissues as reported previously (Morris et al. 2011; You et al. 2013b, 2014), l‐homoarginine supplementation did not affect these parameters in our current study, indicating that the renal protective effect of l‐homoarginine is likely unrelated to reduced l‐arginine availability. However, our results indicate possible direct or indirect effects of l‐homoarginine on NO production. This notion is derived from our data showing restoration of kidney nitrate + nitrite levels and reduced urinary TBARS in diabetic mice. The effects of homoarginine on NO synthesis have been linked with increased risk of stroke and atherosclerosis (Haghikia et al. 2017). DN is associated with endothelial nitric oxide synthase (eNOS) uncoupling, increased reactive oxygen species (ROS), and oxidative stress in diabetic kidneys (Faria et al. 2012). The fact that l‐homoarginine is a weak substrate for NOS (Hecker et al. 1991; Moali et al. 1998) raises the possibility that it may directly affect NO production. Alternatively, the effects of l‐homoarginine on kidney nitrate + nitrite may be mediated indirectly, for example, via changes in levels of asymmetric dimethylarginine (ADMA), an endogenous inhibitor of NOS. The basis for the possible effects of l‐homoarginine on NO production in DN is not known and will be investigated in future studies.
In summary, this study demonstrates that l‐homoarginine is reduced in diabetic mice and that l‐homoarginine supplementation protects against specific features of renal damage in diabetes, thus suggesting that l‐homoarginine supplementation may be useful in treatment of DN. Future research will determine the specific mechanisms by which l‐homoarginine affects DN and whether l‐homoarginine administration alters specific aspects of arginine metabolism that are altered in diabetes.
Conflict of Interest
All authors declare no conflict of interest.
Wetzel M. D., Gao T., Venkatachalam M., Morris S. M. Jr., Awad A. S.. l‐Homoarginine supplementation prevents diabetic kidney damage. Physiol Rep, 7 (18), 2019, e14235, 10.14814/phy2.14235
Funding information
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK‐094930 and DK‐094930S1.
References
- Atzler, D. , Rosenberg M., Anderssohn M., Choe C. U., Lutz M., Zugck C., et al. 2013. Homoarginine–an independent marker of mortality in heart failure. Int. J. Cardiol. 168:4907–4909. [DOI] [PubMed] [Google Scholar]
- Atzler, D. , Gore M. O., Ayers C. R., Choe C. U., Boger R. H., de Lemos J. A., et al. 2014a. Homoarginine and cardiovascular outcome in the population‐based Dallas Heart Study. Arterioscler. Thromb. Vasc. Biol. 34:2501–2507. [DOI] [PubMed] [Google Scholar]
- Atzler, D. , Schwedhelm E., Nauck M., Ittermann T., Boger R. H., and Friedrich N.. 2014b. Serum reference intervals of homoarginine, ADMA, and SDMA in the study of health in Pomerania. Clin. Chem. Lab. Med. 52:1835–1842. [DOI] [PubMed] [Google Scholar]
- Atzler, D. , Schonhoff M., Cordts K., Ortland I., Hoppe J., Hummel F. C., et al. 2016. Oral supplementation with l‐homoarginine in young volunteers. Br. J. Clin. Pharmacol. 82:1477–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzler, D. , McAndrew D. J., Cordts K., Schneider J. E., Zervou S., Schwedhelm E., et al. 2017. Dietary supplementation with homoarginine preserves cardiac function in a murine model of post‐myocardial infarction heart failure. Circulation 135:400–402. [DOI] [PubMed] [Google Scholar]
- Awad, A. S. , Kinsey G. R., Khutsishvili K., Gao T., Bolton W. K., and Okusa M. D.. 2011. Monocyte/macrophage chemokine receptor CCR6 mediates diabetic renal injury. Am. J. Physiol. Renal Physiol. 301:F1358–F1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad, A. S. , You H., Gao T., Cooper T. K., Nedospasov S. A., Vacher J., et al. 2015a. Macrophage‐derived tumor necrosis factor‐α mediates diabetic renal injury. Kidney Int. 88:722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad, A. S. , You H., Gao T., Gvritishvili A., Cooper T. K., and Tombran‐Tink J.. 2015b. Delayed treatment with a small pigment epithelium derived factor (PEDF) peptide prevents the progression of diabetic renal injury. PLoS ONE 10:e0133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahls, M. , Atzler D., Markus M. R. P., Friedrich N., Boger R. H., Volzke H., et al. 2018. Low‐Circulating Homoarginine is associated with dilatation and decreased function of the left ventricle in the general population. Biomolecules 8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle, J. P. , Thompson T. J., Gregg E. W., Barker L. E., and Williamson D. F.. 2010. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyer, M. D. , Bottinger E., Brosius F. C. 3rd, Coffman T. M., Harris R. C., Heilig C. W., et al. 2005. Mouse models of diabetic nephropathy. J. Am. Soc. Nephrol. 16:27–45. [DOI] [PubMed] [Google Scholar]
- Brosius, F. C. 3rd , Alpers C. E., Bottinger E. P., Breyer M. D., Coffman T. M., Gurley S. B., et al. 2009. Mouse models of diabetic nephropathy. J. Am. Soc. Nephrol. 20:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmann, C. , Lilienthal E., Weigt‐Usinger K., Schmidt‐Choudhury A., Horster I., Kayacelebi A. A., et al. 2015. The l‐arginine/NO pathway, homoarginine, and nitrite‐dependent renal carbonic anhydrase activity in young people with type 1 diabetes mellitus. Amino Acids 47:1865–1874. [DOI] [PubMed] [Google Scholar]
- Choe, C. U. , Atzler D., Wild P. S., Carter A. M., Boger R. H., Ojeda F., et al. 2013. Homoarginine levels are regulated by l‐arginine:glycine amidinotransferase and affect stroke outcome: results from human and murine studies. Circulation 128:1451–1461. [DOI] [PubMed] [Google Scholar]
- Citro, A. , Cantarelli E., and Piemonti L.. 2015. The CXCR15/2 pathway: involvement in diabetes pathophysiology and potential target for T1D interventions. Curr. Diab. Rep. 15:68. [DOI] [PubMed] [Google Scholar]
- Drechsler, C. , Kollerits B., Meinitzer A., Marz W., Ritz E., Konig P., et al. 2013. Homoarginine and progression of chronic kidney disease: results from the Mild to Moderate Kidney Disease Study. PLoS ONE 8:e63560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler, C. , Pihlstrom H., Meinitzer A., Pilz S., Tomaschitz A., Abedini S., et al. 2015. Homoarginine and clinical outcomes in renal transplant recipients: results from the assessment of lescol in renal transplantation study. Transplantation 99:1470–1476. [DOI] [PubMed] [Google Scholar]
- Faria, A. M. , Papadimitriou A., Silva K. C., Lopes de Faria J. M., and Lopes de Faria J. B.. 2012. Uncoupling endothelial nitric oxide synthase is ameliorated by green tea in experimental diabetes by re‐establishing tetrahydrobiopterin levels. Diabetes 61:1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghikia, A. , Yanchev G. R., Kayacelebi A. A., Hanff E., Bledau N., Widera C., et al. 2017. The role of l‐arginine/l‐homoarginine/nitric oxide pathway for aortic distensibility and intima‐media thickness in stroke patients. Amino Acids 49:1111–1121. [DOI] [PubMed] [Google Scholar]
- Hecker, M. , Walsh D. T., and Vane J. R.. 1991. On the substrate specificity of nitric oxide synthase. FEBS Lett. 294:221–224. [DOI] [PubMed] [Google Scholar]
- Jud, P. , Hafner F., Verheyen N., Meinitzer A., Gary T., Brodmann M., et al. 2018. Homoarginine/ADMA ratio and homoarginine/SDMA ratio as independent predictors of cardiovascular mortality and cardiovascular events in lower extremity arterial disease. Sci. Rep. 8:14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karetnikova, E. S. , Jarzebska N., Markov A. G., Weiss N., Lentz S. R., and Rodionov R. N.. 2019. Is homoarginine a protective cardiovascular risk factor? Arterioscler. Thromb. Vasc. Biol. 39:869–875. ATVBAHA118312218. [DOI] [PubMed] [Google Scholar]
- Krebs, A. , Doerfer J., Grunert S. C., Wohrl J., Stier B., Schmidt‐Trucksass A., et al. 2015. Decreased levels of homoarginine and asymmetric dimethylarginine in children with type 1 diabetes: associations with cardiovascular risk factors but no effect by atorvastin. J. Pediatr. Endocrinol. Metab. 28:147–152. [DOI] [PubMed] [Google Scholar]
- Martens‐Lobenhoffer, J. , Emrich I. E., Zawada A. M., Fliser D., Wagenpfeil S., Heine G. H., et al. 2018. l‐Homoarginine and its AGXT2‐metabolite GOCA in chronic kidney disease as markers for clinical status and prognosis. Amino Acids 50:1347–1356. [DOI] [PubMed] [Google Scholar]
- Marz, W. , Meinitzer A., Drechsler C., Pilz S., Krane V., Kleber M. E., et al. 2010. Homoarginine, cardiovascular risk, and mortality. Circulation 122:967–975. [DOI] [PubMed] [Google Scholar]
- Moali, C. , Boucher J. L., Sari M. A., Stuehr D. J., and Mansuy D.. 1998. Substrate specificity of NO synthases: detailed comparison of l‐arginine, homo‐l‐arginine, their N omega‐hydroxy derivatives, and N omega‐hydroxynor‐l‐arginine. Biochemistry 37:10453–10460. [DOI] [PubMed] [Google Scholar]
- Morris, S. M. Jr , Gao T., Cooper T. K., Kepka‐Lenhart D., and Awad A. S.. 2011. Arginase‐2 mediates diabetic renal injury. Diabetes 60:3015–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, S. M. Jr , You H., Gao T., Vacher J., Cooper T. K., and Awad A. S.. 2017. Distinct roles of arginases 1 and 2 in diabetic nephropathy. Am. J. Physiol. Renal Physiol. 313:F899–F905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz, S. , Edelmann F., Meinitzer A., Gelbrich G., Doner U., Dungen H. D., et al. 2014a. Associations of methylarginines and homoarginine with diastolic dysfunction and cardiovascular risk factors in patients with preserved left ventricular ejection fraction. J. Card. Fail. 20:923–930. [DOI] [PubMed] [Google Scholar]
- Pilz, S. , Teerlink T., Scheffer P. G., Meinitzer A., Rutters F., Tomaschitz A., et al. 2014b. Homoarginine and mortality in an older population: the Hoorn study. Eur. J. Clin. Invest. 44:200–208. [DOI] [PubMed] [Google Scholar]
- Pilz, S. , Meinitzer A., Gaksch M., Grubler M., Verheyen N., Drechsler C., et al. 2015. Homoarginine in the renal and cardiovascular systems. Amino Acids 47:1703–1713. [DOI] [PubMed] [Google Scholar]
- Ravani, P. , Maas R., Malberti F., Pecchini P., Mieth M., Quinn R., et al. 2013. Homoarginine and mortality in pre‐dialysis chronic kidney disease (CKD) patients. PLoS ONE 8:e72694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, W. L. , and Wells I. C.. 1964. Homocitrulline and homoarginine synthesis from lysine. Science 144:1122–1127. [DOI] [PubMed] [Google Scholar]
- Sobczak, A. , Prokopowicz A., Radek M., Szula M., Zaciera M., Kurek J., et al. 2014. Tobacco smoking decreases plasma concentration of the emerging cardiovascular risk marker, l‐homoarginine. Circ. J. 78:1254–1258. [DOI] [PubMed] [Google Scholar]
- Stockebrand, M. , Hornig S., Neu A., Atzler D., Cordts K., Boger R. H., et al. 2015. Homoarginine supplementation improves blood glucose in diet‐induced obese mice. Amino Acids 47:1921–1929. [DOI] [PubMed] [Google Scholar]
- Valtonen, P. , Laitinen T., Lyyra‐Laitinen T., Raitakari O. T., Juonala M., Viikari J. S., et al. 2008. Serum l‐homoarginine concentration is elevated during normal pregnancy and is related to flow‐mediated vasodilatation. Circ. J. 72:1879–1884. [DOI] [PubMed] [Google Scholar]
- Vogl, L. , Pohlhammer J., Meinitzer A., Rantner B., Stadler M., Peric S., et al. 2015. Serum concentrations of l‐arginine and l‐homoarginine in male patients with intermittent claudication: a cross‐sectional and prospective investigation in the CAVASIC Study. Atherosclerosis 239:607–614. [DOI] [PubMed] [Google Scholar]
- Wang, C. H. , Li F., Hiller S., Kim H. S., Maeda N., Smithies O., et al. 2011. A modest decrease in endothelial NOS in mice comparable to that associated with human NOS3 variants exacerbates diabetic nephropathy. Proc. Natl. Acad. Sci. USA 108:2070–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, H. , Gao T., Cooper T. K., Brian Reeves W., and Awad A. S.. 2013a. Macrophages directly mediate diabetic renal injury. Am. J. Physiol. Renal Physiol. 305:F1719–F1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, H. , Gao T., Cooper T. K., Morris S. M. Jr, and Awad A. S.. 2013b. Arginase inhibition mediates renal tissue protection in diabetic nephropathy by a nitric oxide synthase 3‐dependent mechanism. Kidney Int. 84:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, H. , Gao T., Cooper T. K., Morris S. M. Jr, and Awad A. S.. 2014. Diabetic nephropathy is resistant to oral l‐arginine or l‐citrulline supplementation. Am. J. Physiol. Renal Physiol. 307:F1292–F1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, H. , Gao T., Cooper T. K., Morris S. M., and Awad A. S.. 2015. Arginase inhibition: a new treatment for preventing progression of established diabetic nephropathy. Am. J. Physiol. Renal Physiol. 309:F447–F455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, H. , Gao T., Raup‐Konsavage W. M., Cooper T. K., Bronson S. K., Reeves W. B., et al. 2017. Podocyte‐specific chemokine (C‐C motif) receptor 2 overexpression mediates diabetic renal injury in mice. Kidney Int. 91:671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. J. , Wang S., Cheng H., Zhang M. Z., Takahashi T., Fogo A. B., et al. 2006. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J. Am. Soc. Nephrol. 17:2664–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zwan, L. P. , Davids M., Scheffer P. G., Dekker J. M., Stehouwer C. D., and Teerlink T.. 2013. l‐Homoarginine and l‐arginine are antagonistically related to blood pressure in an elderly population: the Hoorn study. J. Hypertens. 31:1114–1123. [DOI] [PubMed] [Google Scholar]


