Abstract
Background
Approximately 50% of patients with newly diagnosed non‐small cell lung cancer (NSCLC) are over 70 years of age at diagnosis. Despite this fact, these patients are underrepresented in randomized controlled trials (RCTs). As a consequence, the most appropriate regimens for these patients are controversial, and the role of single‐agent or combination therapy is unclear. In this setting, a critical systematic review of RCTs in this group of patients is warranted.
Objectives
To assess the effectiveness and safety of different cytotoxic chemotherapy regimens for previously untreated elderly patients with advanced (stage IIIB and IV) NSCLC. To also assess the impact of cytotoxic chemotherapy on quality of life.
Search methods
We searched the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 10), MEDLINE (1966 to 31 October 2014), EMBASE (1974 to 31 October 2014), and Latin American Caribbean Health Sciences Literature (LILACS) (1982 to 31 October 2014). In addition, we handsearched the proceedings of major conferences, reference lists from relevant resources, and the ClinicalTrial.gov database.
Selection criteria
We included only RCTs that compared non‐platinum single‐agent therapy versus non‐platinum combination therapy, or non‐platinum therapy versus platinum combination therapy in patients over 70 years of age with advanced NSCLC. We allowed inclusion of RCTs specifically designed for the elderly population and those designed for elderly subgroup analyses.
Data collection and analysis
Two review authors independently assessed search results, and a third review author resolved disagreements. We analyzed the following endpoints: overall survival (OS), one‐year survival rate (1yOS), progression‐free survival (PFS), objective response rate (ORR), major adverse events, and quality of life (QoL).
Main results
We included 51 trials in the review: non‐platinum single‐agent therapy versus non‐platinum combination therapy (seven trials) and non‐platinum combination therapy versus platinum combination therapy (44 trials).
Non‐platinum single‐agent versus non‐platinum combination therapy
Low‐quality evidence suggests that these treatments have similar effects on overall survival (hazard ratio (HR) 0.92, 95% confidence interval (CI) 0.72 to 1.17; participants = 1062; five RCTs), 1yOS (risk ratio (RR) 0.88, 95% CI 0.73 to 1.07; participants = 992; four RCTs), and PFS (HR 0.94, 95% CI 0.83 to 1.07; participants = 942; four RCTs). Non‐platinum combination therapy may better improve ORR compared with non‐platinum single‐agent therapy (RR 1.79, 95% CI 1.41 to 2.26; participants = 1014; five RCTs; low‐quality evidence).
Differences in effects on major adverse events between treatment groups were as follows: anemia: RR 1.10, 95% 0.53 to 2.31; participants = 983; four RCTs; very low‐quality evidence; neutropenia: RR 1.26, 95% CI 0.96 to 1.65; participants = 983; four RCTs; low‐quality evidence; and thrombocytopenia: RR 1.45, 95% CI 0.73 to 2.89; participants = 914; three RCTs; very low‐quality evidence. Only two RCTs assessed quality of life; however, we were unable to perform a meta‐analysis because of the paucity of available data.
Non‐platinum therapy versus platinum combination therapy
Platinum combination therapy probably improves OS (HR 0.76, 95% CI 0.69 to 0.85; participants = 1705; 13 RCTs; moderate‐quality evidence), 1yOS (RR 0.89, 95% CI 0.82 to 0.96; participants = 813; 13 RCTs; moderate‐quality evidence), and ORR (RR 1.57, 95% CI 1.32 to 1.85; participants = 1432; 11 RCTs; moderate‐quality evidence) compared with non‐platinum therapies. Platinum combination therapy may also improve PFS, although our confidence in this finding is limited because the quality of evidence was low (HR 0.76, 95% CI 0.61 to 0.93; participants = 1273; nine RCTs).
Effects on major adverse events between treatment groups were as follows: anemia: RR 2.53, 95% CI 1.70 to 3.76; participants = 1437; 11 RCTs; low‐quality evidence; thrombocytopenia: RR 3.59, 95% CI 2.22 to 5.82; participants = 1260; nine RCTs; low‐quality evidence; fatigue: RR 1.56, 95% CI 1.02 to 2.38; participants = 1150; seven RCTs; emesis: RR 3.64, 95% CI 1.82 to 7.29; participants = 1193; eight RCTs; and peripheral neuropathy: RR 7.02, 95% CI 2.42 to 20.41; participants = 776; five RCTs; low‐quality evidence. Only five RCTs assessed QoL; however, we were unable to perform a meta‐analysis because of the paucity of available data.
Authors' conclusions
In people over the age of 70 with advanced NSCLC who do not have significant co‐morbidities, increased survival with platinum combination therapy needs to be balanced against higher risk of major adverse events when compared with non‐platinum therapy. For people who are not suitable candidates for platinum treatment, we have found low‐quality evidence suggesting that non‐platinum combination and single‐agent therapy regimens have similar effects on survival. We are uncertain as to the comparability of their adverse event profiles. Additional evidence on quality of life gathered from additional studies is needed to help inform decision making.
Plain language summary
Comparing different types of chemotherapy for treatment of older people with advanced lung cancer
Background
Worldwide, lung cancer is responsible for most cases of cancer‐related death among individuals of both sexes. For adult patients with advanced disease, therapy regimens based on the combination of cisplatin or carboplatin with a different agent are considered the standard of care. However, few elderly patients have been included in relevant trials for chemotherapy, raising concerns about the safety and efficacy of such regimens, which are considered the standard of care for adult patients. As a consequence, older patients are often treated with less intense chemotherapy regimens.
Review objectives
Our objectives were to investigate the effects of different chemotherapy regimens (non‐platinum single‐agent, non‐platinum combination, and platinum combination) on survival, quality of life, tumor shrinkage, and toxicity in older people with advanced lung cancer.
Study characteristics
We performed a systematic search (up to 31 October 2014) for trials that compared non‐platinum single‐agent therapy versus non‐platinum combination therapy or non‐platinum combination therapy versus platinum combination therapy in patients over 70 years of age who have advanced non‐small cell lung cancer. We included in the review a total of 51 studies (seven studies in the non‐platinum single‐agent therapy vs non‐platinum combination therapy group and 44 studies in the non‐platinum combination therapy vs platinum combination therapy group); however, we were able to include only 19 studies in the meta‐analysis.
Key results
Non‐platinum single‐agent versus non‐platinum combination therapy
We analyzed five trials involving 1294 participants. We found that these regimens are equally effective for survival. However, combinations of non‐platinum agents are associated with a greater chance of decreasing tumor size. We also found that these regimens are similar regarding chance of major toxicity such as low hemoglobin levels, platelets, and white cell counts (neutrophils). Only two trials assessed the impact of treatment on quality of life, and we were not able to combine these results because of lack of information.
Non‐platinum therapy versus platinum combination therapy
We analyzed 14 trials involving 1705 elderly participants. We found that platinum therapy is associated with longer survival and greater chance of decreasing tumor size among elderly patients. However, we found that these regimens are more toxic than those based on non‐platinum agents and provide greater risk of low hemoglobin and platelet levels, fatigue, nausea or vomiting, and numbness or tingling in the hands and feet. Only five trials assessed the impact of treatment on quality of life, and we were not able to combine these results because of lack of information.
Quality of evidence
Non‐platinum single‐agent versus non‐platinum combination therapy
We downgraded to low the quality of evidence on survival because different results were reported across studies, and because three included trials were stopped early, which also influenced the quality of evidence for chance of decreasing tumor size and low hemoglobin, platelet, and white cell counts. For theses outcomes, issues with study design were also a matter of concern, leading to low quality of evidence.
Non‐platinum combination versus platinum combination therapy
We downgraded to moderate the quality of evidence on the benefit of platinum combination therapy for survival based on inclusion of nine trials that were not specifically designed for older patients. Other issues with study design influenced the quality of evidence on interval to tumor growth after start of treatment, rate of tumor shrinkage, and toxicity. Regarding low hemoglobin and platelet levels, we further reduced the quality of evidence to low because of imprecision of reported results. We recognize that other limitations such as age alone might not be adequate criteria for selection of the best treatment. Older people can be very different from one another in terms of other health conditions associated with aging. Older patients included in randomized trials were selected through strict eligibility criteria that excluded most patients with other health problems. Therefore, we believe that these results must be interpreted with clinical judgement applied regarding selection of an appropriate treatment regimen.
Summary of findings
Summary of findings for the main comparison. Non‐platinum single‐agent versus non‐platinum combination treatment for non‐small cell lung cancer in the elderly population.
| Non‐platinun single‐agent versus non‐platinum combination treatment for non‐small cell lung cancer in the elderly population | ||||||
| Patient or population: non‐small cell lung cancer in the elderly population Setting: first‐line chemotherapy for advanced non‐small cell lung cancer in elderly participants Intervention: non‐platinum combination Comparison: non‐platinum single agent | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Numberof participants (studies) | Quality of evidence (GRADE) | Comments | |
| Risk with non‐platinum single agent | Risk with non‐platinum combination | |||||
| Overall survival (OS) | Study population | HR 0.92 (0.72‐1.17) | 1294 (5 RCTs) | ⨁⨁◯◯ Lowa,b | ||
| Not applicable | Not applicable | |||||
| 1‐Year survival rate (OS1y) | Study population | RR 0.88 (0.73‐1.07) | 993 (4 RCTs) | ⨁⨁◯◯ Lowa,b | ||
| 677 per 1000 | 596 per 1000 (494‐724) | |||||
| Moderate | ||||||
| 680 per 1000 | 598 per 1000 (496‐728) | |||||
| Progression‐free survival | Study population | HR 0.94 (0.83‐1.07) | 1105 (4 RCTs) | ⨁⨁◯◯ Lowa,c | ||
| Not applicable | Not applicable | |||||
| Objective response rate (ORR) | Study population | RR 1.79 (1.41‐2.26) | 1014 (5 RCTs) | ⨁⨁◯◯ Lowa,c | ||
| 168 per 1000 | 301 per 1000 (237‐380) | |||||
| Moderate | ||||||
| 143 per 1000 | 256 per 1000 (201‐323) | |||||
| Grade 3 and 4 hematological adverse events (AE) ‐ anemia | Study population | RR 1.18 (0.57‐2.40) | 983 (4 RCTs) | ⨁◯◯◯ Very lowa,c,d | ||
| 30 per 1000 | 35 per 1000 (17‐71) | |||||
| Moderate | ||||||
| 28 per 1000 | 33 per 1000 (16‐67) | |||||
| Grade 3 and 4 hematological adverse events (AE) ‐ neutropenia | Study population | RR 1.19 (0.93‐1.54) | 1064 (5 RCTs) | ⨁⨁◯◯ Lowa,c | ||
| 172 per 1000 | 204 per 1000 (160‐264) | |||||
| Moderate | ||||||
| 164 per 1000 | 195 per 1000 (153‐253) | |||||
| Grade 3 and 4 hematological adverse events (AE) ‐ thrombocytopenia | Study population | RR 1.58 (0.82‐3.04) | 995 (4 RCTs) | ⨁◯◯◯ Very lowa,c,d | ||
| 25 per 1000 | 39 per 1000 (20‐75) | |||||
| Moderate | ||||||
| 31 per 1000 | 49 per 1000 (26‐95) | |||||
| Quality of life (QoL) | Only 2 RCTs assessed quality of life; however, we were not able to perform a meta‐analysis because of the paucity of available data | ‐ | (2 RCTs) | |||
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect, but it may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded the quality of evidence by one level because of serious risk of bias based on the large number (three RCTs) of prematurely interrupted trials.
bWe downgraded the quality of evidence by one level because of serious inconsistency (I2 > 50%).
cWe downgraded the quality of evidence by one level because of absence of blinding in the design of most RCTs.
dWe downgraded the quality of evidence by one level because of imprecision based on the wide confidence interval.
Summary of findings 2. Non‐platinum therapies compared with platinum combination for non‐small cell lung cancer in the elderly population.
| Non‐platinum therapies compared with platinum combination for non‐small cell lung cancer in the elderly population | ||||||
| Patient or population: non‐small cell lung cancer in the elderly population Setting: first‐line chemotherapy for advanced non‐small cell lung cancer in elderly patients Intervention: platinum combination Comparison: non‐platinum therapies | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Numberof participants (studies) | Quality of evidence (GRADE) | Comments | |
| Risk with non‐platinum therapies | Risk with platinum combination | |||||
| Overall survival | Study population | HR 0.76 (0.69‐0.85) | (13 RCTs) | ⨁⨁⨁◯ Moderatea | ||
| Not applicable | Not applicable | |||||
| 1‐Year survival rate | Study population | RR 0.89 (0.82‐0.96) | 813 (13 RCTs) | ⨁⨁⨁◯ Moderatea | ||
| 714 per 1000 | 635 per 1000 (585‐685) | |||||
| Moderate | ||||||
| 667 per 1000 | 593 per 1000 (547‐640) | |||||
| Progression‐free survival | Study population | HR 0.76 (0.61‐0.93) | (9 RCTs) | ⨁⨁◯◯ Lowa,b,c | ||
| Not applicable | Not applicable | |||||
| Objective response rate (ORR) | Study population | RR 1.57 (1.32‐1.85) | 1432 (11 RCTs) | ⨁⨁⨁◯ Moderatea,b | ||
| 218 per 1000 | 342 per 1000 (288‐403) | |||||
| Moderate | ||||||
| 246 per 1000 | 386 per 1000 (325‐455) | |||||
| Grade 3 or higher hematological toxicity for platinum therapies ‐ anemia | Study population | RR 2.53 (1.70‐3.76) | 1437 (11 RCTs) | ⨁⨁◯◯ Lowa,b,d | ||
| 41 per 1000 | 105 per 1000 (70‐156) | |||||
| Moderate | ||||||
| 26 per 1000 | 65 per 1000 (44‐96) | |||||
| Grade 3 or higher hematological toxicity for platinum therapies ‐ thrombocytopenia | Study population | RR 3.59 (2.22‐5.82) | 1260 (9 RCTs) | ⨁⨁◯◯ Lowa,b,d | ||
| 28 per 1000 | 101 per 1000 (63‐164) | |||||
| Moderate | ||||||
| 26 per 1000 | 92 per 1000 (57‐149) | |||||
| Grade 3 or higher Non‐Hematological Toxicity for Platinum‐based therapies ‐ Peripheral neuropathy | Study population | RR 7.02 (2.42‐20.41) | 776 (5 RCTs) | ⨁⨁◯◯ Lowa,b,d | ||
| 5 per 1000 | 36 per 1000 (12‐104) | |||||
| Quality of life (QoL) | Only 5 RCTs assessed quality of life; however, we were not able to perform a meta‐analysis because of the paucity of available data | ‐ | (5 studies) | |||
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded the quality of evidence by one level because of serious risk of bias due to inclusion of unplanned elderly subgroup analysis.
bWe downgraded the quality of evidence by one level because of serious risk of bias. A large number of trials were open‐label. We considered absence of blinding as introducing potential risk for PFS, ORR, and adverse events.
cWe downgraded the quality of evidence by one level because of serious inconsistency (I2 = 63%; P value = 0.005). We explored reasons for heterogeneity by performing a subgroup analysis by type of non‐platinum therapy, type of platinum therapy, and trial design. We found heterogeneity only in the subgroup on the non‐platinum control arm (I2 = 79%); on the carboplatin‐based combination (I2 = 85%), and on elderly‐specific trials (I2 = 85%).
dWe downgraded the quality of evidence by one level because of serious imprecision (few events and wide confidence interval observed).
Background
Description of the condition
Worldwide, lung cancer is the most common malignancy among men and the second most common among women, with an estimated 1.6 million new cases in 2008; it is responsible for most of the cancer‐related deaths reported in both sexes (American Cancer Society 2011). It is estimated that in 2012, 56% of new cases were diagnosed at advanced stages of disease. Therefore, a large number of patients will be candidates for palliative chemotherapy.
Approximately 50% of patients newly diagnosed with non‐small cell lung cancer (NSCLC) are older than 70 years of age at diagnosis (Davidoff 2010). Despite this fact, these patients are underrepresented in randomized controlled trials (RCTs), resulting in lack of reliable information about treatment effectiveness and safety for patients in this age group (Hutchins 1999; Talarico 2004). In clinical practice, this lack of information has led many clinicians to deliver suboptimal treatment based on the presumption of poor tolerance of treatment (Quoix 2011a). Recognition of this limitation has prompted investigators to design randomized studies specifically focused on this population; nonetheless, the best way of treating this important group of patients remains to be determined.
Description of the intervention
For patients with advanced NSCLC with a good performance status (PS), platinum regimens are considered standard first‐line treatment. However, debate about the most appropriate regimen for older patients is ongoing. Only recently, few RCTs allowed inclusion of elderly patients. A subgroup analysis of a meta‐analysis of individual participant data from 16 RCTs addressed the role of chemotherapy for this subgroup. Elderly individuals accounted for 26.9% of all participants, and analyses suggested similar benefit across younger and older participants, confirming the benefit of chemotherapy over best supportive care (BSC) (NSCLC Collaborative Group 2010; NSCLC Meta‐Analyses Collaborative Group 2008). Nevertheless, concern about specific issues related to the older patient has led to trials specifically addressing the issue of chemotherapy in this population. One of the first RCTs to evaluate the role of chemotherapy in older patients was stopped early because of poor accrual; investigators randomly assigned 191 participants older than 70 years of age to vinorelbine monotherapy or BSC (Gridelli 2001). This study showed better overall survival (OS) in the treatment arm than in the BSC arm. Since that time, other RCTs have sought the most appropriate regimen for this population by examining the role of different cytotoxic single agents and combined chemotherapy agents containing or not containing platinum.
How the intervention might work
Cytotoxic chemotherapy comprises a variety of drugs with different mechanisms of action, which are aimed at stopping cell division and consequently tumor growth. Cytotoxic chemotherapy has been selected as the main treatment for a variety of solid tumors, reducing risk of death and disease progression. However, it is also associated with numerous adverse events, which may be more common among older patients with significant co‐morbidities that affect their ability to tolerate and continue with treatment.
Why it is important to do this review
Today, no chemotherapy regimen is accepted as the standard of care for elderly patients. The best treatment approach for elderly patients with advanced NSCLC must be carefully balanced between efficacy and safety. The impact of more active regimens containing platinum compounds or newer drugs with better toxicity profiles remains to be defined with regard to benefits for survival and quality of life (QoL). A systematic review of RCTs for this group of patients is crucial and warranted.
Objectives
To assess the effectiveness and safety of different cytotoxic chemotherapy regimens for previously untreated elderly patients with advanced (stage IIIB and IV) NSCLC. To also assess the impact of cytotoxic chemotherapy on QoL.
Methods
Criteria for considering studies for this review
Types of studies
We considered only RCTs that compared different chemotherapy regimens containing cytotoxic drugs alone or in combination for previously untreated patients with advanced NSCLC. We screened all RCTs regardless of age eligibility criteria, and we allowed inclusion of RCTs specifically designed for the elderly population and those that included this population as a subgroup. We classified included chemotherapy regimens as non‐platinum monotherapy, non‐platinum combination therapy, and platinum combination therapy. We did not include RCTs that included a BSC alone comparison group or that investigated the role of antiangiogenic drugs or tyrosine kinase inhibitors, given alone or in combination with cytotoxic chemotherapy. We also excluded non‐randomized studies and quasi‐RCTs .
Types of participants
We included patients 70 years of age and older with previously untreated and histologically confirmed NSCLC, with metastatic disease and/or pleural effusion (stage IIIB or IV). We allowed inclusion of patients enrolled in trials specifically designed for the elderly population or included as a subgroup in general adult population RCTs.
Types of interventions
We classified chemotherapy regimens into three categories.
Non‐platinum monotherapy.
Non‐platinum combination therapy.
Platinum combination therapy.
We considered trials comparing these compounds, whatever the numbers.
Categories were compared according to the following.
Non‐platinum monotherapy versus non‐platinum combination therapy.
Non‐platinum therapy (given as a single agent or in combination) versus platinum combination therapy.
Types of outcome measures
Primary outcomes
Overall survival (OS).
Quality of life (QoL).
Secondary outcomes
One‐year survival rate (1yOS).
Progression‐free survival (PFS).
Objective response rate (ORR), classified according to Response Evaluation Criteria in Solid Tumors (RECIST) (Therasse 2000), World Healh Organization (WHO) criteria, or individual study criteria.
Serious adverse events (grade 3 or above, according to WHO or National Cancer Institute Common Toxicity Criteria (NCI‐CTC) (NCI Common Toxicity Criteria)).
Search methods for identification of studies
We implemented an electronic search strategy according to recommendations of the Cochrane Lung Cancer Review Group. We applied no data or language restrictions.
Electronic searches
We searched the following databases.
The Cochrane Central Register of Controlled Trias (CENTRAL; latest issue) (Appendix 1).
MEDLINE (via OVID) (from 1966 to 31 October 2014) (Appendix 2).
EMBASE (via Elsevier) (from 1974 to 31 October 2014) (Appendix 3).
Latin American Caribbean Health Sciences Literature (LILACS) (from 1982 to 31 October 2014) (Appendix 4).
We used validated filters to retrieve clinical trials from MEDLINE and EMBASE (Higgins 2011).
Searching other resources
We performed a handsearch of the following sources with the goal of identifying RCTs that might have been reported in abstract form, or that might have been missed by the search strategy described above.
Proceedings of meetings of the American Society of Clinical Oncology (ASCO) (from 1990 to 31 October 2014).
Proceedings of the International Association for the Study of Lung Cancer (IASLC) World Lung Cancer Conference (from 1990 to 31 October 2014).
Proceedings of the European Society of Medical Oncology (ESMO) (from 1990 to 31 October 2014).
Proceedings of the European Cancer Conference Organization (ECCO) (from 1990 to 31 October 2014).
We also searched reference lists included in relevant studies, and we contacted professionals with expertise in these areas to ask about ongoing or unpublished trials. We searched ClinicalTrial.gov database for registered RCTs.
Data collection and analysis
Selection of studies
Two review authors (FNS and MRSC) independently evaluated titles and abstracts obtained through the search. We obtained full‐text articles on potentially relevant studies for further analysis. We included studies that fulfilled the inclusion criteria and did not meet the exclusion criteria. A third review author (RR) independently evaluated studies when the two previous review authors did not agree.
Data extraction and management
Two review authors (FNS and MRSC) independently retrieved and recorded data from selected trials onto a data collection form. A third review author (RR) resolved disagreements between the two previous review authors. We stored references using RevMan 5.3 (Review Manager).
We included on the data collection form the following information derived from individual studies.:
Source (e.g. study identification, citation).
Eligibility criteria.
Methods (e.g. study design, method of allocation, allocation concealment, blinding, risk of bias, type of analysis).
Participants (e.g. number, age, sex, stage, performance status, histological type).
Interventions (e.g. chemotherapy regimen, treatment schedule, length of treatment).
Outcome measures (e.g. OS, PFS, adverse events, QoL assessment).
Results for each outcome of interest.
Assessment of risk of bias in included studies
Assessment of risk of bias was composed of a domain‐based evaluation based on the 'Risk of bias' (RoB) tool described in Chapter 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We evaluated six domains: random sequence generation; allocation concealment; blinding of outcome assessment; incomplete data outcomes; selective outcome reporting; and other bias. The domain from the RoB tool called 'blinding of participants and personnel' was not evaluated because of intrinsic difficulties associated with blinding of participants and healthcare providers included in a chemotherapy trial that provided different regimens. We judged each domain as having high risk of bias when results were seriously weakened by the plausible bias; low risk of bias when results were unlikely to be seriously altered by the plausible bias; and unclear risk of bias when insufficient details were included in the report, or when, despite sufficient details, risk of bias was unknown (for details, see Table 8.5c of the Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2011). We analyzed each individual trial for every domain that had only one entry per trial for the following domains: random sequence generation, allocation concealment, and selective outcome reporting. For the other domains, we allowed two entries per trial: one for objective outcomes (OS) and another for subjective outcomes (all other outcomes).
Two review authors (FNS and TBC) assessed independently the risk of bias for each study. A third review author (RR) resolved disagreements between the two previous review authors.
Measures of treatment effect
We presented results for time‐to‐event outcomes (such as OS and PFS) as hazard ratios (HRs) and 95% confidence intervals (CIs). We extracted the HR for each individual trial directly from published data, when available, or indirectly using reported summary statistics or Kaplan‐Meier curves according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; Parmar 1998; Tierney 2007). We presented the treatment effects of dichotomous outcomes (such as ORR and serious adverse events) as risk ratios (RRs) and 95% CIs.
Unit of analysis issues
We analyzed each eligible trial for potential unit of analysis errors such as using non‐standard trial design (cluster randomization, cross‐over trial, studies with more than one intervention) or reporting multiple observations for the same outcome. We evaluated all trials with potential unit of analysis errors according to criteria provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
When possible, we contacted study authors to request missing data for relevant trials. We explicitly described attempts to provide values for missing data by any method. In studies for which data could not be obtained despite contact with the study author, we assessed the impact of missing data on risk of bias and described this assessment in the Discussion section.
Assessment of heterogeneity
We evaluated heterogeneity between studies by using the I2 statistic. We considered an I2 value greater than 50% as showing substantial heterogeneity (Higgins 2011). In cases of absence of heterogeneity, we used a fixed‐effect model for analysis. When we observed heterogeneity among studies, we explored clinical and methodological differences as potential causes, and we used a random‐effects model for analysis. We also explored heterogeneity in the subgroup analysis by type of trial (elderly specific or elderly subgroup analysis), type of platinum (cisplatin‐based or carboplatin‐based), and type of non‐platinum therapy (combination or single agent).
Assessment of reporting biases
For studies at high risk of reporting bias, we attempted to retrieve full data sets or reasons for non‐reporting of some data outcomes by contacting study authors. We also searched for protocol versions of included trials.
Data synthesis
We used RevMan 5.3 (Review Manager) to summarize the data of interest and to produce forest plot graphics, using a fixed‐effect model. For time‐to‐event outcomes, we combined data using the generic inverse variance method, and we presented measurements of treatment effects as HRs and 95% CIs. For dichotomous outcomes, we used the Mantel‐Haenszel method, and we presented measurements of treatment effects as risk ratios (RRs) with 95% CIs. When data aggregation was not feasible, we discussed and presented the results in table format.
We presented two ‘Summary of findings’ (SoF) tables, one for each major comparison, according to recommendations provided in Chapter 11.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included data on the following outcomes: OS, QoL, 1yOS, PFS, ORR, serious hematological adverse events, and serious non‐hematological adverse events. As we were unable to perform a meta‐analysis of QoL data, we described assessment of these data in each trial under Effects of interventions, and we presented these results narratively in SoF tables. We presented measurements of treatment effects as HRs and 95% CIs for time‐to‐event outcomes. For dichotomous data outcomes, we presented results as absolute risk values in the 'Assumed control risk' and 'Corresponding intervention risk' columns, and as measurements of relative risk such as RRs with 95% CIs. We presented data regarding numbers of participants and studies for these outcomes, assessment of overall quality of evidence (using the grading system developed by the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) Working Group (GRADE Working Group 2004)), and all appropriate comments. We calculated assumed risks on the basis of risks observed in the control arm for each comparison: non‐platinum single‐agent arm (for the non‐platinum single‐agent therapy vs non‐platinum combination therapy comparison) and non‐platinum therapy arm (for the non‐platinum combination therapy vs platinum combination therapy comparison).
Subgroup analysis and investigation of heterogeneity
We performed an exploratory between‐trial subgroup analysis for platinum combination therapy versus non‐platinum combination therapy according to the following.
Type of non‐platinum therapy (single agent or combination).
Type of platinum agent (cisplatin‐based or carboplatin‐based).
Type of trial (trial specifically designed for the elderly or elderly subgroup analysis).
Sensitivity analysis
We performed a sensitivity analysis to evaluate the robustness of study results by:
excluding one by one trials with high risk of bias and trials with unclear risk of bias;
excluding all trials with high risk of bias or unclear risk of bias;
excluding one by one trials without age restriction to the elderly population;
excluding all trials without age restriction to the elderly population; and
excluding unpublished and prematurely interrupted trials.
Results
Description of studies
Results of the search
By implementing our search strategy, we identified 4648 manuscripts: 3047 from MEDLINE, 865 from EMBASE, 815 from CENTRAL, and 19 from LILACS. Among these, we considered 61 manuscripts from 56 trials to be relevant and requiring full‐text analysis: eight trials for non‐platinum single‐agent therapy versus non‐platinum combination therapy and 48 trials for non‐platinum combination therapy versus platinum combination therapy. After careful evaluation, we excluded 14 trials for methodological reasons or for absence of elderly patients among the study population: three for non‐platinum single‐agent therapy versus non‐platinum combination therapy and 11 for non‐platinum combination therapy versus platinum combination therapy. We identified nine additional trials through handsearching and analyzed them as full‐text articles (Abe 2011; Chen 2008; Comella 2004; Depierre 1994; Lilenbaum 2005b; Quoix 2011b; Rijavec 2010;Tsukada 2007; Zukin 2013). Therefore, we included 51 trials in the systematic review, distributed as follows among comparison groups: seven for non‐platinum single‐agent therapy versus non‐platinum combination therapy and 44 for non‐platinum combination therapy versus platinum combination therapy (Figure 1).
1.

Search strategy flowchart.
Included studies
See Characteristics of included studies.
Non‐platinum single‐agent versus non‐platinum combination therapy
We included seven RCTs involving 1514 elderly patients for the non‐platinum single‐agent and non‐platinum combination comparison. Four RCTs were designed specifically for patients over 70 years of age (Frasci 2001; Gridelli 2003; Karampeazis 2010; Rijavec 2010). Two RCTs were designed for patients considered elderly or unfit for platinum regimens (Comella 2004; Hainsworth 2007). Georgoulias 2008 included adult patients but allowed inclusion of patients over 70 years of age; we obtained these data from study authors upon direct request.
Frasci 2001 conducted a randomized phase III trial enrolling only elderly patients, defined as older than 70 years. Participants were randomly assigned to vinorelbine single‐agent (V arm) or vinorelbine‐gemcitabine combination (VG arm) treatment. The study was planned for accrual of 120 participants in each arm; however, it was prematurely stopped after the first planned interim analysis with 60 participants in each arm showed increased risk of death in the vinorelbine arm.
Gridelli 2003 randomly assigned 698 elderly participants in a three‐arm study: vinorelbine single‐agent (V arm); gemcitabine single‐agent (G arm); and vinorelbine‐gemcitabine combination (VG arm) treatment. This study was designed for comparison of overall survival between single‐agent arms separately from the combination arm.
Karampeazis 2010 conducted a phase III trial that enrolled only patients over 70 years of age. Participants were randomly assigned to gemcitabine single‐agent (the G arm) or gemcitabine‐docetaxel combination (the GD arm) treatment. After a third of planned participants had been enrolled, the study was prematurely terminated because of slow accrual. Results are available only in abstract form with follow‐up of 10.27 months. Contact with study authors yielded additional data on 106 participants with longer median follow‐up of 16.27 months. Rijavec 2010 conducted a phase II trial enrolling 75 participants randomly assigned (69 assessable) to docetaxel single‐agent or docetaxel‐gemcitabine combination. Eligibility criteria allowed inclusion of patients older than 70 years with stage III or IV NSCLC and Eastern Cooperative Oncology Group (ECOG) PS of 0 to 2. The study was designed to select a treatment regimen for further study, and more than eight objective responses would be required to select the winner. The primary outcome was response rate according to RECIST; secondary outcomes consisted of toxicity, time‐to‐progression, and survival. The trial was prematurely stopped because of slow accrual. Results are available only in abstract form.
Comella 2004 performed a phase III randomized trial in which participants were assigned to gemcitabine single‐agent, paclitaxel single‐agent, gemcitabine‐paclitaxel combination, or gemcitabine‐vinorelbine combination. Eligibility criteria allowed inclusion of patients over 70 years of age with ECOG PS of 0 to 2 and younger patients with ECOG PS of 2. The study was designed to detect an OS advantage of combination treatment over either single‐agent treatment. Investigators planned to accrue 520 participants to detect improvement in median OS from 5 to 7.5 months. However, after publication of Gridelli 2003, the trial was prematurely terminated for ethical reasons and slow accrual, enrolling only 264 participants. Among the study population (intention‐to‐treat (ITT)), 83.4% (220) were over 70 years of age. Study authors did not present a separate analysis on elderly participants.
Hainsworth 2007 allowed inclusion of patients older than 65 years or younger considered by study investigators as poor candidates for platinum therapy on the basis of co‐morbidities or poor performance status. Participants were randomly assigned to weekly docetaxel (D arm) or docetaxel‐gemcitabine combination (DG arm) treatment. This trial accrued a total of 345 participants, including 232 over the age of 70. No data specifically on this subgroup were available. A total of 223 participants were classified as elderly with good ECOG PS. Despite multiple attempts, no additional data could be retrieved.
Georgoulias 2008 randomly assigned 312 adult participants with no upper age limit to docetaxel single‐agent (D arm) or docetaxel‐gemcitabine combination (DG arm) treatment. After the first interim analysis showing improvement in OS in favor of docetaxel and gemcitabine combination over docetaxel single‐agent treatment (9.4 months vs 8.3 months; P value = 0.037), this trial was interrupted prematurely. Researchers included 81 participants over 70 years of age. Upon contact with study authors, we retrieved data regarding demographics and elderly subgroup analysis.
Non‐platinum therapy versus platinum combination therapy
For this comparison, we included 44 RCTs enrolling patients over 70 years of age. Six RCTs were specifically designed for the elderly (Abe 2011; Chen 2008; Lou 2010; Quoix 2011b; Tsukada 2007; Zhang 2006), and all used a non‐platinum single‐agent control arm. A total of 38 RCTs were designed for adult patients and applied no restriction for inclusion of patients older than 70 years. Among these, 26 RCTs did not provide enough information on the number of elderly enrolled and did not perform a separate analysis on this subgroup; therefore, we considered these data as missing (Table 3). The remaining 12 RCTs included 1294 elderly participants, representing 25.2% of the ITT population (n = 5130) (Boni 2012; Flotten 2012; Georgoulias 2001; Georgoulias 2004; Georgoulias 2005; Kubota 2008; Laack 2004; Lilenbaum 2005; Sederholm 2005; Treat 2010; Zukin 2013; Zwitter 2010). Among the non‐platinum therapies used in control arms for those 12 trials, five used non‐platinum single‐agent treatment (Georgoulias 2004; Lilenbaum 2005; Sederholm 2005; Zukin 2013; Zwitter 2010) and seven non‐platinum combination (Boni 2012; Flotten 2012; Georgoulias 2001; Georgoulias 2005; Kubota 2008; Laack 2004; Treat 2010).
1. Trials included for N‐based versus P‐based comparison with no information on the elderly.
| Non‐platinum single‐agent versus platinum‐based combination treatment | ||||
| Author, Year | Intervention | ITT population | Median age (range) | Elderly |
| Vansteenkiste 2001 | G arm: gemcitabine 1000 mg/m2 D1, D8, D15 q28 days | 84 | 63.7 (55.5–71.9) | NA |
| CV arm: cisplatin 100 mg/m2 + vindesine 3 mg/m2 D1, D15 q 28 days | 85 | 63.1 (54.5–71.7) | NA | |
| Le Chevalier 1994 | N arm: vinorelbine 30 mg/m2 weekly | 206 | 60 (NA) | NA |
| PN arm: cisplatin 120 mg/m2 D1, D29, then every 6 weeks + vinorelbine 30 mg/m2 weekly | 206 | 59 (NA) | NA | |
| PV arm: cisplatin 120 mg/m2 D1, D29, then every 6 weeks + vindesine 3 mg/m2 weekly for 6 weeks, then every 2 weeks | 200 | 59 (NA) | NA | |
| Depierre 1994 | PV arm: cisplatin 80 mg/m2 on D1 every 3 weeks and vinorelbine 30 mg/m2 weekly | 121 | 59.2 (NA) | NA |
| V arm: vinorelbine 30 mg/m2 weekly | 119 | 58.8 (NA) | NA | |
| Manegold 1998 (Europe) | G arm: gemcitabine 1000 mg/m2 D1, D8, D15 q28 days | 71 | 59 (32‐80) | NA |
| EP arm: cisplatin 100 mg/m2 D1 + etoposide 100 mg/m2 D1, D2, D3 q28 days | 75 | 59 (33–78) | NA | |
| Manegold 1998 (Taiwan) | G arm: gemcitabine 1250 mg/m2 D1, D8, D15 q28 days | 27 | 63 (36–75) | NA |
| EP arm: cisplatin 100 mg/m2 D1 + etoposide 100 mg/m2 D1, D2, D3 q28 days | 26 | 60 (35–75) | NA | |
| Perng 1997 | G arm: gemcitabine 1250 mg/m2 D1, D8, D15 q28 days | 27 | 63 (36–75) | NA |
| EP arm: cisplatin 80 mg/m2 D1 + etoposide 80 mg/m2 D1, D2, D3 q28 days | 26 | 63 (35‐75) | NA | |
| Jeremic 1997 | E arm: etoposide 50 mg/m2/d D1 to D21 every 28 days | 59 | NA | NA |
| EP arm: carboplatin 400 mg/m2 D1 + etoposide 50 mg/m2 D1 to D21 every 28 days | 58 | NA | NA | |
| Rosso 1988 | E arm: etoposide 120 mg/m2 D1, D2, D3 q21 days | 113 | NA | NA |
| EP arm: cisplatin 60 mg/m2 D1, D2 + etoposide 120 mg/m2 D1, D2, D3 | 103 | NA | NA | |
| Total | 1366 | NA | ||
| Non‐platinum combination versus platinum combination | ||||
| Author, Year | Intervention | ITT population | Median age (range) | Elderly |
| Berghmans 2013 | GIP arm: gemcitabine 1000 mg/m2 on days 1 and 8 + ifosfamide 3000 mg/m2 on day 1 + cisplatin 50 mg/m2 on day 1 | 231 | 58 (29‐78) | NA |
| DP arm: docetaxel 75 mg/m2 + cisplatin 50 mg/m2 on day 1 | 233 | 58 (28‐81) | NA | |
| IG arm: ifosfamide 3000 mg/m2 + gemcitabine 1000 mg/m2 on days 1 and 8 | 229 | 59 (30‐84) | NA | |
| Saito 2012 | CP arm: carboplatin AUC6 plus paclitaxel 200 mg/m2 on day 1, every 3 weeks | 41 | 65 (20‐77) | NA |
| GV arm: gemcitabine 1000 mg/m2 plus vinorelbine 25 mg/m2 on days 1 and 8, every 3 weeks | 43 | 67 (34‐76) | NA | |
| Hsu 2008 | GE arm: gemcitabine 1000 mg/m2 on days 1, 8, 15 plus epirubicin 70 mg/m2 | 43 | 62.3 (33.9–78.6) | NA |
| GP arm: gemcitabine 1000 mg/m2 on days 1, 8, 15 plus cisplatin on day 15 | 42 | 60.9 (37.6–76) | NA | |
| Gricorescu 2007 | GV/GI arm: gemcitabine 1000 mg/m2 plus vinorelbine 25 mg/m2 on days 1 and 8 for 2 cycles, followed by gemcitabine 1000 mg/m2 on days 1 and 8 plus ifosfamide 2000 mg/m2 on day 1 for 2 cycles | 50 | 59 (NA) | NA |
| GP arm: gemcitabine 1250 mg/m2 on days 1 and 8 plus cisplatin 70 mg/m2 on day 1 for 4 cycles | 52 | 56 (NA) | NA | |
| Yamamoto 2006 | GV arm: gemcitabine 1000 mg/m2 plus vinorelbine 25 mg/m2 on days 1 and 8 | 64 | 62 (36‐74) | NA |
| GC arm: gemcitabine 1000 mg/m2 on days 1 and 8 plus carboplatin AUC5 on day 1 | 64 | 60 (30‐74) | NA | |
| Katakami 2006 | DG arm: docetaxel 60 mg/m2 on day 1 plus gemcitabine 800 mg/m2 on days 1 and 8, every 3 weeks | 65 | NA | NA |
| CD arm: cisplatin 80 mg/m2 plus docetaxel 60 mg/m2 on day 1, every 3 weeks. | 68 | NA | NA | |
| Mok 2005 | GE arm: gemcitabine 1000 mg/m2 on days 1, 8, and 15 plus etoposide 50 mg/m2 p.o. from day 1 through day 14, every 4 weeks | 45 | 61 (38‐70) | NA |
| GP arm: cisplatin 75 mg/m2 on day 1 plus gemcitabine 1000 mg/m2 on days 1, 8, and 15, every 4 weeks | 44 | 56 (23‐72) | NA | |
| Tan 2005 | GV arm: gemcitabine 1000 mg/m2 plus vinorelbine 25 mg/m2 on days 1 and 8, every 3 weeks | 157 | 57 (29–74) | NA |
| CV arm: carboplatin AUC5 on day 1 plus vinorelbine 30 mg/m2 on days 1 and 8, every 3 weeks | 159 | 60 (30–75) | NA | |
| Pujol 2005 | GD arm: gemcitabine 1000 mg/m2 days 1 and 8 plus docetaxel 85 mg/m2 day 8, every 3 weeks | 155 | 60 (37–75) | NA |
| CV arm: cisplatin 100 mg/m2 on day 1 plus vinorelbine 30 mg/m2 on days 1, 8, 15, and 28, every 4 weeks for 6 cycles | 156 | 57 (39–75) | NA | |
| Lilenbaum 2005b | GV arm: vinorelbine 25 mg/m2 plus gemcitabine 1000 mg/m2 on days 1 and 8, every 3 weeks | 82 | 66 (42–86) | NA |
| CP arm: paclitaxel 200 mg/m2 plus carboplatin AUC6 on day 1, every 3 weeks | 83 | 63 (38–86) | NA | |
| Stathopoulos 2004 | PV arm: paclitaxel 135 mg/m2 plus vinorelbine 25 mg/m2 on day 1, every 2 weeks | 175 | 65 (36‐84) | NA |
| CP arm: carboplatin AUC6 and paclitaxel 175 mg/m2 on day 1, every 3 weeks | 185 | 65 (30‐83) | NA | |
| Yamamoto 2004 | DI arm: docetaxel 60 mg/m2 on day 8 and irinotecan 60 mg/m2 on days 1 and 8, every 3 weeks | 57 | 60 (42‐77) | NA |
| CD arm: cisplatin 80 mg/m2 and docetaxel 60 mg/m2 on day 1, every 3 weeks | 51 | 62 (39‐74) | NA | |
| Alberola 2003 | GV‐VI arm: gemcitabine 1000 mg/m2 and vinorelbine 30 mg/m2 on days 1 and 8, every 3 weeks for 3 cycles, followed by vinorelbine 30 mg/m2 on days 1 and 8 plus ifosfamide 3 g/m2 on day 1 for 3 cycles | 187 | 60 (33‐76) | NA |
| GC arm: cisplatin 100 mg/m2 on day 1 plus gemcitabine 1250 mg/m2 on days 1 and 8, every 3 weeks | 182 | 59 (39‐74) | NA | |
| CGV arm: cisplatin 100 mg/m2 on day 1 plus gemcitabine 1000 mg/m2 plus vinorelbine 25 mg/m2 on days 1 and 8, every 3 weeks | 188 | 59 (33‐75) | NA | |
| Smit 2003 | GPac arm: paclitaxel 175 mg/m2 on day 1 plus gemcitabine 1250 mg/m2 on days 1 and 8, every 3 weeks | 161 | 56 (31‐75) | NA |
| CG arm: cisplatin 80 mg/m2 on day 1 plus gemcitabine 1250 mg/m2 on days 1 and 8, every 3 weeks | 160 | 57 (28‐75) | NA | |
| CP arm: cisplatin 80 mg/m2 plus paclitaxel 175 mg/m2 on day 1, every 3 weeks | 159 | 57 (27‐75) | NA | |
| Wachters 2003 | GE arm: epirubicin 70 mg/m2 on day 1 plus gemcitabine 1125 mg/m2 on days 1 and 8, every 3 weeks | 121 | 60 (32‐76) | NA |
| CG arm: cisplatin 80 mg/m2 on day 2 plus gemcitabine 1125 mg/m2 on days 1 and 8, every 3 weeks | 119 | 60 (29‐80) | NA | |
| Sculier 2002 | IG arm: ifosfamide 4500 mg/m2 on day 1 plus gemcitabine 1000 mg/m2 on days 1, 8, and 15, every 4 weeks | 94 | 52 > 60 years old | NA |
| CCI arm: cisplatin 60 mg/m2 and carboplatin AUC3 and ifosfamide 4500 mg/m2 over 18‐hour i.v. infusion on day 1, every 4 weeks | 94 | 45 > 60 years old | NA | |
| CCG arm: cisplatin 60 mg/m2 on day 1 plus carboplatin AUC3 on day 1 and gemcitabine 1000 mg/m2 on days 1, 8, and 15, every 4 weeks | 92 | 52 > 60 years old | NA | |
| Chen 2002 | PG arm: paclitaxel 175 mg/m2 on day 1 and gemcitabine 1000 mg/m2 on days 1 and 8, every 3 weeks | 45 | 67 (35‐80) | NA |
| CP arm: carboplatin AUC7 on day 1 plus paclitaxel 175 mg/m2 on day 1, every 3 weeks | 45 | 64 (37‐77) | NA | |
| Buccheri 1997 | MACC arm: methotrexate 40 mg/m2, doxorubicin 40 mg/m2 i.v., cyclophosphamide 400 mg/m2 i.v. infusion, and lomustine 30 mg/m2 per os on day 1, every 3 weeks | 78 | 64 (NA) | NA |
| MVP arm: mitomycin C 10 mg/m2, vinblastine 6 mg/m2, and cisplatin 40 mg/m2 i.v. infusions on day 1, every 3 weeks | 78 | 65 (NA) | NA | |
| Hara 1990 | MCT arm: mitomycin C 4 mg/body i.v. infusion and cytosine arabinoside 30 mg/body on days 1, 4, 14, 21, and 28 and tegafur 600 mg orally every day | 67 | 63 (37‐75) | NA |
| CAPM arm: cyclophosphamide 400 mg/m2 i.v. infusion on day 1, Adriamycin 30 mg/m2 i.v. infusion on day 1, cisplatin 60 mg/m2 i.v. infusion on day 1, and mitomycin C 3 mg/m2 i.v. infusion on day 1 | 69 | 60 (27‐75) | NA | |
| Total | 5040 | |||
Non‐platinum single‐agent versus platinum combination therapy
We included 12 RCTs for comparison between non‐platinum single‐agent and platinum combination therapy. Six RCTs were specifically designed for the elderly population with an accrual of 1013 participants (Abe 2011; Chen 2008; Lou 2010; Quoix 2011b;Tsukada 2007; Zhang 2006), and five were elderly subgroup analyses of RCTs designed for the adult population involving a total of 1783 adult participants; 468 (26.25%) were 70 years of age or older (Georgoulias 2004; Lilenbaum 2005; Sederholm 2005; Zukin 2013; Zwitter 2010).
Abe 2011 performed a phase III randomized trial in which participants older than 70 years were randomly assigned to docetaxel or combination cisplatin and docetaxel. The study was designed for accrual of 380 participants, with OS as the primary outcome. Results were presented at the American Society of Clinical Oncology (ASCO) meeting in 2011; however, no full‐text article was available for analysis. The trial was prematurely interrupted after the interim analysis showed a low probability of achieving the primary endpoint. A total of 221 participants were assessable for this analysis. Chen 2008 conducted a randomized phase II trial in which 65 participants over 70 years of age were assigned to vinorelbine single‐agent or cisplatin‐vinorelbine combination. This study was designed to accrue at least 28 qualified participants in each treatment arm, with the objective of detecting a 10% difference in response rate in favor of the best treatment arm. Lou 2010 conducted a small trial in China in which 68 participants over 70 years of age were randomly assigned to gemcitabine single‐agent (G arm) or carboplatin‐gemcitabine combination (CG arm) treatment. The full‐text article was available only in the Chinese language. Study authors did not provide details on the randomization process nor on the primary endpoint of the study. Quoix 2011b conducted the largest randomized phase III trial specifically designed for the elderly. In all, 451 participants between 70 and 89 years of age were randomly assigned to non‐platinum monotherapy (vinorelbine or gemcitabine as single‐agent) or platinum combination (carboplatin and weekly paclitaxel).
Tsukada 2007 performed a randomized phase III trial in which elderly participants, defined as older than 70 years of age, were assigned to weekly docetaxel (D arm) or a combination of cisplatin 25 mg/m2 and docetaxel 20 mg/m2 on days 1, 8, and 15 (DP arm). The study was planned to include 115 in each treatment arm, to detect an overall survival advantage in favor of the DP arm. After the second interim analysis, involving 112 assessable participants, the Data and Safety Monitoring Board (DSMB) recommended early termination of the study based on the strong indication that the cisplatin‐containing regimen was superior for the subgroup of participants between 70 and 74 years of age.
Zhang 2006 conducted a randomized trial enrolling 96 patients between 65 and 80 years old. Participants were randomly assigned to one of three treatment arms: paclitaxel as single‐agent (P arm); cisplatin‐paclitaxel combination (CisP arm), or carboplatin‐paclitaxel combination (CarP arm) treatment. The full‐text article was available only in the Chinese language. Study authors did not provide details on the randomization process nor on objectives of the study.
Georgoulias 2004 accrued 339 adult patients for a phase III trial. Participants younger than 75 years were randomly assigned to receive docetaxel as single‐agent (D arm) or cisplatin‐docetaxel combination (CD arm) treatment. The primary objective of the study was to detect an overall survival difference between treatment arms. An exploratory elderly subgroup analysis involving 71 participants was available from unpublished data through direct contact with study authors. Lilenbaum 2005 randomly assigned 561 eligible adult participants to paclitaxel single‐agent (P arm) or carboplatin‐paclitaxel combination (CP arm) treatment. The study was designed to detect 30% improvement in OS in the CP over the P arm. Median age for the ITT population was 64 years (range 31 to 86), with no imbalance between treatment arms. In all, 178 (18%) participants had ECOG PS of 2 at baseline, and 155 participants were 70 years of age or older. Planned subgroup analysis by age was performed on OS, 1yOS, and RR. No data on geriatric scales were collected, and we obtained no additional data on the elderly participants.
Sederholm 2005 conducted a randomized phase III trial in which 334 participants older than 18 years were assigned to gemcitabine monotherapy (G arm) or carboplatin‐gemcitabine combination (CG arm) treatment. The study was planned to detect OS differences between treatment arms. A total of 37% (126) of study participants were over 70 years of age. No elderly subgroup analysis was planned, and we obtained no data for an exploratory analysis through direct contact with study authors.
Zukin 2013 conducted a multi‐center randomized phase III trial enrolling adult patients with ECOG PS of 2. A total of 217 participants were randomly assigned to pemetrexed as single‐agent (P arm) or carboplatin‐pemetrexed combination (CP arm) treatment. The primary objective of the study was to compare overall survival between treatment arms among participants with ECOG PS of 2. Secondary outcomes were PFS, RR, and toxicity. Response was assessed according to RECIST criteria, and toxicity according to NCI‐CTC. In all, 74 individuals 70 years of age or older were included in trial. No data on geriatric scales were collected. Exploratory subgroup analyses on these participants were available for OS and RR. Despite multiple attempts, we retrieved no additional data on demographics, PFS, nor toxicity.
Zwitter 2010 conducted a phase II trial in which 112 participants were randomly assigned to gemcitabine single‐agent (G arm) or low‐dose cisplatin‐gemcitabine combination (CG arm) treatment. Eligible patients were considered poor candidates for platinum combination. A total of 42 patients older than 70 years were included in the trial. Despite contact with study authors, we retrieved no data on elderly subgroup analysis.
We also identified three ongoing clinical trials (NCT01405586; NCT01593293; NCT01656551), specifically designed for the elderly population and fulfilling our eligibility criteria. Results from these studies are not yet available.
Non‐platinum combination versus platinum combination therapy
We included 26 RCTs that allowed inclusion of elderly patients for comparison between non‐platinum combination and platinum combination treatment. None were specifically designed for the elderly population. A total of 19 RCTs involving 4800 adult participants did not report information regarding numbers nor outcome data for the elderly subgroup (Table 3). Only seven trials reported the number of elderly patients enrolled; among a total of 3567 adult participants, 826 (23.2%) were 70 years of age or older (Boni 2012; Flotten 2012; Georgoulias 2001; Georgoulias 2005; Kubota 2008; Laack 2004; Treat 2010).
Only Flotten 2012 presented an elderly subgroup analysis (n = 74) on OS. After contacting study authors, we retrieved unpublished additional data from five RCTs regarding the post hoc elderly subgroup analysis, which involved 414 participants included in the meta‐analysis (Boni 2012; Georgoulias 2001; Georgoulias 2005; Kubota 2008; Laack 2004).
Boni 2012 conducted a multi‐center phase III trial in which 433 adult participants, with no upper age limit, were randomly assigned to four treatment arms: cisplatin‐gemcitabine combination (CG arm); cisplatin‐gemcitabine‐ifosfamide combination (CGI arm); gemcitabine‐vinorelbine combination (GV arm); and gemcitabine‐ifosfamide‐vinorelbine combination (GIN arm). The study was designed for a 2 × 2 factorial analysis of OS on (1) platinum (CG and CGI arms) versus non‐platinum (GV and GIV arms) comparisons; and (2) two‐drug regimen (CG and GV arms) versus three‐drug regimen (GIN and CGI arms) comparisons. Elderly subgroup analysis was not planned. Through direct contact with study authors, we obtained unpublished data from an exploratory analysis involving 101 elderly participants. Flotten 2012 reported results of a randomized phase III trial in which 444 participants with no upper age limit were randomly assigned to vinorelbine‐gemcitabine combination (VG arm) or carboplatin‐vinorelbine combination (VC arm) treatment. Participants 75 years of age or older had a 25% dose reduction in their chemotherapy regimen. The study was planned to detect increased 1yOS. In all, 74 participants were older than 75 years. A post hoc analysis of OS in the elderly subgroup was presented. Despite making direct contact with study authors, we retrieved no additional data. Georgoulias 2001 randomly assigned 441 adult participants younger than 75 years to docetaxel‐gemcitabine (DG arm) or cisplatin‐docetaxel combinations (CD arm) treatment. Primary outcomes were RR and time‐to‐progression (TTP). The study was planned to enroll 412 participants to detect 12% improvement in RR with platinum combination over non‐platinum combination. Through contact with study authors, we obtained exploratory subgroup analyses on 71 elderly participants (17.5% of the ITT population). Georgoulias 2005 conducted a phase III trial with 413 participants between 18 and 75 years of age randomly assigned to docetaxel‐gemcitabine combination (DG arm) or cisplatin‐vinorelbine combination (CV arm) treatment. The study was planned to detect a four‐month difference in OS between treatment arms. Unplanned elderly subgroup analysis involving 81 participants (19.6% of ITT population) was provided upon contact with study authors. For this population, median ages were 72 (range 70 to 75) and 73 (range 70 to 78) years; 53.8% and 44.2% had PS of 0; 46.2% and 55.8% had squamous cell histology; and 53.8% and 60.5% had stage IV for DG and CV arms, respectively. Kubota 2008 enrolled 401 patients in a phase III trial (Japan Multinational Trial Organization (JMTO) LC00–03) in which adult participants with no upper age limit were randomly assigned to vinorelbine‐gemcitabine combination for three cycles followed by docetaxel single‐agent (VGD arm) or carboplatin‐paclitaxel combination (CP arm) treatment. This study was designed to detect improvement in OS with the non‐platinum combination over the platinum combination. Investigators performed a QoL assessment on an additional study (BRI LC03‐01), which screened 109 of 401 participants (Kawahara 2011). Through direct contact with study authors, we retrieved an unpublished exploratory subgroup analysis on patients over 70 years of age. A total of 118 participants were included in this analysis, representing 30% of the ITT population. Laack 2004 performed a multi‐center, randomized phase III trial in which 300 adult participants between 18 and 75 years of age were randomly assigned to gemcitabine‐vinorelbine combination (GV arm) or gemcitabine‐vinorelbine‐cisplatin combination (GVP arm) treatment. This study was planned to detect overall survival improvement in favor of platinum combination. The study protocol allowed inclusion of patients over 70 years but not over 75 years of age. The trial was not planned for an elderly subgroup analysis; however, we obtained a post hoc analysis upon direct request to the study author. In all, 43 participants older than 70 years were included in the study, with 16 assigned to the GV arm and 27 to the GVP arm.
Treat 2010 randomly assigned 1135 adult participants with no upper age limit to one of three arms: carboplatin‐gemcitabine combination (CG arm); gemcitabine‐paclitaxel combination (GP arm); or carboplatin‐paclitaxel combination (CP arm) treatment. The study was designed for three pairwise comparisons on overall survival: CG versus GP arm, CP versus GP arm, and CG versus CP arm. The primary endpoint was OS. Ansari 2011 published a post hoc analysis on different subgroups based on age (< 70; 70 to 74; 75 to 79; and ≥ 80 years old). A total of 338 participants were older than 70 years, representing 29.8% of the ITT population. Differences in OS, TTP, and RR among participants younger or older than 70 years were not statistically significant. Unplanned analysis on the elderly subgroup by treatment arm was not performed; therefore we were not able to include these data in the meta‐analysis.
Excluded studies
See Characteristics of excluded studies.
Non‐platinum single‐agent versus non‐platinum combination therapy
After full‐text analysis, we excluded three RCTs from this review because of lack of eligibility criteria, specifically for the treatment regimens used (De Marinis 1999; Gridelli 2007; Rocha Lima 2004).
De Marinis 1999 conducted a randomized trial including only individuals older than 70 years. A total of 153 participants were randomly assigned to four treatment arms: lonidamine single‐agent (L arm); vindesine single‐agent (V arm); lonidamine‐vindesine combination (LV arm); and best supportive care (BSC arm). This study was designed to assess ORR and OS in a 2 × 2 factorial analysis of BSC and V arms versus L and LV arms (effect of lonidamine) and of BSC and L arms versus V and LV arms (effect of vindesine). Results were based on 126 participants after 27 had been excluded from four poorly performing centers. Analysis of data on non‐platinum single‐agent versus non‐platinum combination treatment was not planned and was not available. We considered uncertain the activity of lonidamine for treatment of advanced NSCLC. Gridelli 2007 randomly assigned 87 participants to two arms: (1) pemetrexed; or (2) pemetrexed and gemcitabine as sequential therapy. Eligibility criteria required participants older than 70 years or younger but considered poor candidates for platinum therapy. We did not consider sequential therapy as a non‐platinum combination. Rocha Lima 2004 published a phase II trial in which 78 participants were randomly assigned to two non‐platinum combinations: (1) gemcitabine and irinotecan; and (2) gemcitabine‐docetaxel treatment. Inclusion criteria allowed participants 18 to 75 years of age; however, neither the number of elderly participants nor a subgroup analysis was presented.
Non‐platinum therapy versus platinum combination therapy
After full‐text analysis, we excluded 11 RCTs from this review.
The main reason for exclusion was absence of elderly participants from the trial. Randomized controlled trials published by Colucci 1997; Comella 2007; Gridelli 1996; Gridelli 2003; Morabito 2013; and Novello 2009 excluded patients older than 70 years of age. In RCTs published by Binder 2007, Greco 2002, and Rubio 2009, no participants 70 years of age or older were randomly assigned, even though elderly patients were included in the study.
Gebbia 2003 conducted a phase III trial in which 400 participants were randomly assigned to four different strategies with platinum combinations as follows: (1) gemcitabine‐Ifosfamide for two cycles followed by cisplatin‐vinorelbine; (2) cisplatin‐vinorelbine for two cycles followed by gemcitabine‐ifosfamide; (3) vinorelbine‐cisplatin; and (4) cisplatin‐gemcitabine. Even though eligibility criteria allowed inclusion of patients older than 70 years, the trial design did not allow comparison between non‐platinum combinations and platinum combinations.
Zatloukal 2008 randomly assigned 62 participants to (1) cisplatin 75 mg/m2 and larotaxel 50 mg/m2 on day 1, or (2) gemcitabine 800 mg/m2 on days 1 and 8 plus larotaxel 50 mg/m2 on day 8. We considered larotaxel an investigational drug, whose activity is not well established. Therefore, we excluded this RCT from our review.
Risk of bias in included studies
2.
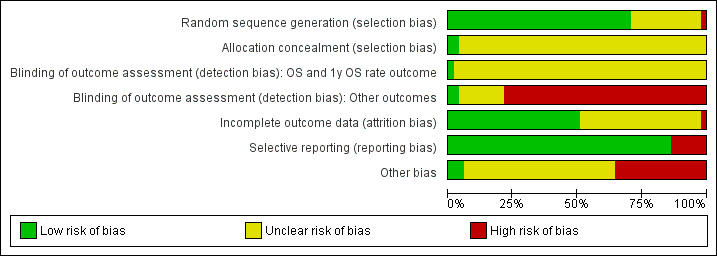
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
3.
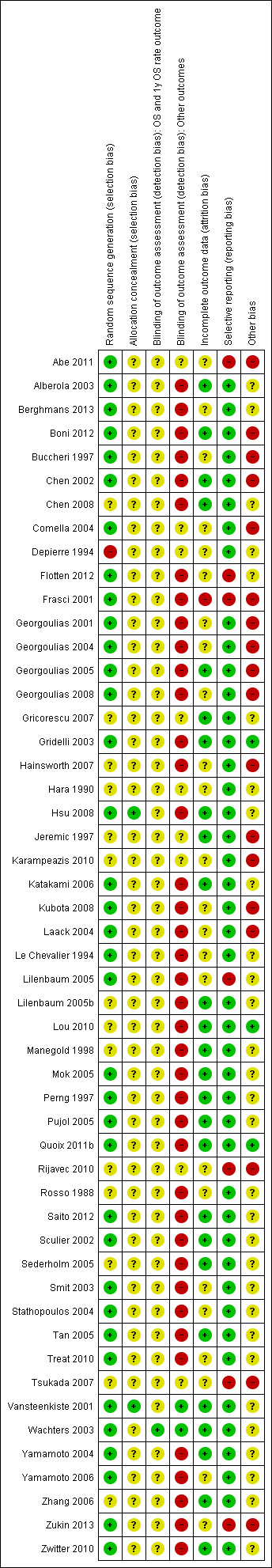
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Non‐platinum single‐agent versus non‐platinum combination therapy
Hainsworth 2007, Karampeazis 2010, and Rijavec 2010 had unclear risk of bias for random sequence generation. Karampeazis 2010 and Rijavec 2010 were reported in abstract form only, and no information on the randomization process was provided. Hainsworth 2007, although reported in a full‐text article, provided insufficient information on the allocation process.
Comella 2004, Frasci 2001, Georgoulias 2008, and Gridelli 2003 had low risk of bias for random sequence generation.
We classified all RCTs as having unclear risk of bias for allocation concealment.
Non‐platinum therapy versus platinum combination therapy
No RCTs were at high risk of selection bias.
Chen 2008, Lou 2010, Tsukada 2007, and Zhang 2006 reported insufficient information about random sequence generation. Therefore, we considered these trials to have unclear risk of bias. We considered Gricorescu 2007, Hara 1990, Jeremic 1997, Katakami 2006, Lilenbaum 2005b, Manegold 1998, Rosso 1988, and Sederholm 2005 to have unclear risk of bias. However, their results were not included in the meta‐analysis because a separate elderly subgroup analysis was not performed.
Abe 2011, Boni 2012, Chen 2002, Flotten 2012, Georgoulias 2001, Georgoulias 2004, Georgoulias 2005, Kubota 2008, Laack 2004, Lilenbaum 2005, Quoix 2011b, Sederholm 2005, Treat 2010, and Zukin 2013 showed no evidence of bias for random sequence generation. Alberola 2003, Berghmans 2013, Buccheri 1997, Depierre 1994, Hsu 2008, Le Chevalier 1994, Mok 2005, Perng 1997, Pujol 2005, Saito 2012, Sculier 2002, Smit 2003, Stathopoulos 2004, Tan 2005, Vansteenkiste 2001, Wachters 2003, Yamamoto 2004, Yamamoto 2006, and Zwitter 2010 also showed no evidence of bias for random sequence generation. However, their results were not included in the meta‐analysis because an elderly subgroup analysis was not performed.
All RCTs, except Hsu 2008 and Vansteenkiste 2001, provided inadequate information for an appropriate judgement on allocation concealment. Therefore, we considered these trials to have unclear risk of bias for allocation concealment.
Blinding
Non‐platinum single‐agent versus non‐platinum combination therapy
Comella 2004, Karampeazis 2010, and Rijavec 2010 showed unclear risk of performance and detection bias. Karampeazis 2010 and Rijavec 2010 were presented in abstract form only and provided no information on blinding of assessment.
Frasci 2001, Georgoulias 2008, Gridelli 2003, and Hainsworth 2007 were designed as open‐label trials. We considered them as having unclear risk of bias for OS and 1yOS outcomes and high risk of bias for PFS, ORR, and toxicity.
Non‐platinum therapy versus platinum combination therapy
Boni 2012, Chen 2008, Flotten 2012, Georgoulias 2001, Georgoulias 2004, Georgoulias 2005, Kubota 2008, Laack 2004, Lilenbaum 2005, Lou 2010, Quoix 2011b, Treat 2010, Zhang 2006, Zukin 2013, and Zwitter 2010 were designed as open‐label trials. We considered them to have unclear risk of detection bias for OS and 1yOS and high risk of bias for PFS, ORR, and toxicity. Abe 2011 and Tsukada 2007 provided insufficient information to allow adequate judgement of detection bias. Therefore, we classified these trials as having unclear risk of bias.
We also considered Alberola 2003, Berghmans 2013, Buccheri 1997, Chen 2002, Depierre 1994, Gricorescu 2007, Hara 1990, Hsu 2008, Jeremic 1997, Katakami 2006, Le Chevalier 1994, Lilenbaum 2005b, Manegold 1998, Mok 2005, Perng 1997, Pujol 2005, Rosso 1988, Saito 2012, Sculier 2002, Sederholm 2005, Smit 2003, Stathopoulos 2004, Tan 2005, Yamamoto 2004, Yamamoto 2006, and Zwitter 2010; to have unclear risk of detection bias for OS and 1yOS and high risk for the other outcomes because of absence of blinding. However, their results were not included in the meta‐analysis because data on the elderly population were lacking.
Vansteenkiste 2001 and Wachters 2003 were the only RCTs considered to have low risk of detection bias for all outcomes.
Incomplete outcome data
Non‐platinum single‐agent versus non‐platinum combination therapy
Frasci 2001 was the only RCT classified as having high risk of bias for incomplete outcome data. After premature interruption, 21 participants were recruited, but they were not included in the full report.
Comella 2004, Georgoulias 2008, and Hainsworth 2007 reported insufficient data to permit evaluating of risk of attrition bias for the elderly subgroup. Thus, we classified them as having unclear risk of bias.
Karampeazis 2010 and Rijavec 2010 were reported in abstract form only, and we classified them as having unclear risk of bias.
Gridelli 2003 was the only RCT considered to have low risk of attrition bias.
Non‐platinum therapy versus platinum combination therapy
No RCTs included in the systematic review were at high risk of attrition bias.
Abe 2011 and Tsukada 2007 were presented in abstract form only and provided limited data for attrition bias analysis. Flotten 2012, Georgoulias 2001, Georgoulias 2004, Kubota 2008, Laack 2004, Lilenbaum 2005, Treat 2010, and Zukin 2013provided a separate subgroup analysis that was based on assessable elderly participants. However, they reported no information regarding the number of elderly participants not assessable after randomization. We considered these trials to have unclear risk of bias. Berghmans 2013, Buccheri 1997, Depierre 1994, Hara 1990, Le Chevalier 1994, Smit 2003, Stathopoulos 2004, and Yamamoto 2006 also were at unclear risk. However, their results were not included in the meta‐analysis because investigators did not perform a separate elderly subgroup analysis.
Boni 2012, Chen 2008, Georgoulias 2005, Lou 2010, Quoix 2011b, and Zhang 2006 showed no evidence of attrition bias. Chen 2002, Gricorescu 2007, Hsu 2008, Jeremic 1997, Katakami 2006, Lilenbaum 2005b, Manegold 1998, Mok 2005, Perng 1997, Pujol 2005, Rosso 1988, Saito 2012, Sculier 2002, Sederholm 2005, Tan 2005, Vansteenkiste 2001, Wachters 2003, Yamamoto 2004, and Zwitter 2010 also showed no evidence of attrition bias. However, their results were not included in the meta‐analysis because researchers did not perform a separate elderly subgroup analysis.
Selective reporting
Non‐platinum single‐agent versus non‐platinum combination therapy
Frasci 2001 did not include PFS as an outcome in the protocol. Even though it was not planned, we considered the absence of this relevant outcome as introducing high risk of reporting bias.
Rijavec 2010 did not report all outcomes in the abstract. Investigators partially reported participant characteristics and toxicity data. We were not able to obtain further data upon direct contact with study authors, and we have considered this trial to have high risk of reporting bias.
Hainsworth 2007 reported all outcomes for the ITT population. However, study authors did not plan and did not perform a separate analysis on the elderly for all outcomes. They reported a subgroup analysis only for OS among elderly participants with good performance status. Therefore, we have considered this trial to have low risk of reporting bias.
Comella 2004 reported all outcomes for the ITT population, but study authors did not perform an elderly subgroup analysis. We considered this trial as having low risk of selective reporting bias.
Georgoulias 2008 provided data for the unplanned elderly subgroup analysis after we made direct contact with study authors. We considered this study as having low risk of bias.
After we directly contacted study authors, Karampeazis 2010 provided all information regarding participant characteristics and outcome data. Therefore, we considered this study to have low risk of reporting bias.
Gridelli 2003 presented no evidence to suggest selective reporting bias.
Non‐platinum therapy versus platinum combination therapy
Abe 2011 and Tsukada 2007 reported limited numbers of outcomes in the abstract. Flotten 2012, Lilenbaum 2005, and Zukin 2013 reported a planned elderly subgroup analysis on a limited number of outcomes. Flotten 2012 and Zukin 2013 reported an OS subgroup analysis. Lilenbaum 2005 reported OS, 1yOS, and ORR. Therefore, we considered these RCTs to have high risk of reporting bias.
Boni 2012, Chen 2008, Georgoulias 2001, Georgoulias 2004, Georgoulias 2005, Kubota 2008, Laack 2004, Lou 2010, Quoix 2011b, Treat 2010, and Zhang 2006 showed no evidence of selective reporting bias.
Alberola 2003, Berghmans 2013, Buccheri 1997, Chen 2002, Depierre 1994, Gricorescu 2007, Hara 1990, Hsu 2008, Jeremic 1997, Katakami 2006, Le Chevalier 1994, Lilenbaum 2005b, Manegold 1998, Mok 2005, Perng 1997, Pujol 2005, Rosso 1988, Saito 2012, Sculier 2002, Sederholm 2005, Smit 2003, Stathopoulos 2004, Tan 2005, Vansteenkiste 2001, Wachters 2003, Yamamoto 2004, Yamamoto 2006, and Zwitter 2010 showed no evidence of reporting bias. However, their results were not included in the meta‐analysis because investigators did not perform a separate elderly subgroup analysis.
Other potential sources of bias
Non‐platinum single‐agent versus non‐platinum combination therapy
The Georgoulias 2008 trial was designed for the general adult population. Even though it allowed inclusion of patients over 70 years of age, this trial was not planned for an elderly subgroup analysis. We considered this unplanned subgroup analysis to produce high risk of bias.
Comella 2004 and Hainsworth 2007 were designed for elderly and poor performance patients; however, researchers did not perform a separate analysis. We considered these two RCTs to have high risk of bias.
Frasci 2001, Karampeazis 2010, and Rijavec 2010 were prematurely interrupted after interim analyses showed an advantage in favor of the non‐platinum combination (Frasci 2001) and as the result of slow accrual (Karampeazis 2010; Rijavec 2010). We have considered these three RCTs to have high risk of bias.
Gridelli 2003 provided no evidence of other sources of bias.
Non‐platinum therapy versus platinum combination therapy
We included Boni 2012, Georgoulias 2001, Georgoulias 2004, Georgoulias 2005, Kubota 2008, Laack 2004, Treat 2010, and Zukin 2013 despite unplanned subgroup analysis, and we considered these trials to have high risk of bias. Abe 2011 and Tsukada 2007 were prematurely interrupted and were reported as abstracts only. Therefore, we also considered them to have high risk of bias. Jeremic 1997 was prematurely interrupted as the result of personnel issues, and we considered this trial to have high risk of bias. However, we were not able to retrieve data on the elderly subgroup, and its results were not included in the meta‐analysis.
Flotten 2012 and Lilenbaum 2005 were designed for the adult population; however an elderly subgroup analysis was planned in the protocol. Therefore, we considered this trial to have unclear risk of bias.
We found no evidence of other bias in Quoix 2011b.
Alberola 2003, Berghmans 2013, Buccheri 1997, Chen 2002, Depierre 1994, Gricorescu 2007, Hara 1990, Hsu 2008, Katakami 2006, Le Chevalier 1994, Lilenbaum 2005b, Manegold 1998, Mok 2005, Perng 1997, Pujol 2005, Saito 2012, Sculier 2002, Sederholm 2005, Smit 2003, Stathopoulos 2004, Tan 2005, Vansteenkiste 2001, Wachters 2003, Yamamoto 2004, Yamamoto 2006, and Zwitter 2010 were considered to have unclear risk of bias because data regarding inclusion or outcomes of the elderly subgroup were missing.
Effects of interventions
We have summarized the effects of interventions for each comparison in Table 1 and Table 2.
Non‐platinum single‐agent versus non‐platinum combination therapy
Overall survival (OS)
All seven RCTs included for this comparison in the systematic review evaluated OS as an endpoint (Table 4). We excluded Comella 2004 and Hainsworth 2007 from the meta‐analysis because investigators did not perform a separate analysis on participants over 70 years of age. However, we have discussed their results separately.
2. Overall survival (OS) and 1‐year survival (1‐year OS rate) for non‐platinum combination versus non‐platinum single‐agent therapy.
| Author, year | Age of inclusion | n (elderly) | Non‐platinum single‐agent | Non‐platinum combination | HR (95% CI) | P value | ||
| Median OS (95% CI) | 1yOS | Median OS (95% CI) | 1yOS | |||||
| Frasci 2001 | ≥ 70 yo | 120 | 18 weeks (NR) | 13% | 29 weeks (NR) | 30% | 0.48 (0.29‐0.79) | < 0.01 |
| Gridelli 2003 | ≥ 70 yo | 698 | 36 weeks (30‐45)a | 38% | 30 weeks (27‐36) | 30% | 1.17 (0.95‐1.44) | 0.93 |
| 28 weeks (25‐34)b | 28% | 1.06 (0.86‐1.29) | 0.69 | |||||
| Comella 2004c | ≥ 70 yo or < 70 yo and PS 2 | 225 | 5.7 months (3.9‐7.5) | 9.2 months (7.6‐9.2) | 0.76 (0.59‐0.99) | 0.0486 | ||
| Hainsworth 2007 | ≥ 65 yo or PS 2 | 223d | 8.0 months (NR) | 7.2 months (NR) | NR | NR | 0.5 | |
| Georgoulias 2008 | ≥ 18 yo | 81 | 7.7 months (5.86‐9.61) | 31.18% | 7.3 months (4.48‐10.12) | 44.32% | 1.221 (0.724‐2.062) | 0.452 |
| Karampeazis 2010 | ≥ 70 yo | 94 | 12.23 months (NR) | 52.2% | 14.63 months (NR) | 65.7% | 1.478 (0.899‐2.430) | 0.121 |
| Rijavec 2010 | ≥ 70 yo | 69 | 7.2 months (NR) | NR | 7.9 months (NR) | NR | 0.87 (0.52‐1.45) | 0.5916 |
CI: confidence interval; HR: hazard ratio; NR: not reported; PS: performance status; yo: years old.
aCorresponds to results of V arm. bCorresponds to results of G arm. cResults were not presented for elderly subgroup only. dPatients 65 years of age or older with good performance status. Results correspond to OS for the ITT population.
The meta‐analysis of five RCTs involving 1294 participants showed no differences in OS between treatment strategies (hazard ratio (HR) 1.01, 95% confidence interval (CI) 0.89 to 1.15) and significant heterogeneity among trials (I2 = 64%). As a result of the presence of heterogeneity, we performed an analysis using a random‐effects model with no impact on effects of the intervention (HR 0.92, 95% CI 0.72 to 1.17) (Analysis 1.1; Figure 4).
1.1. Analysis.
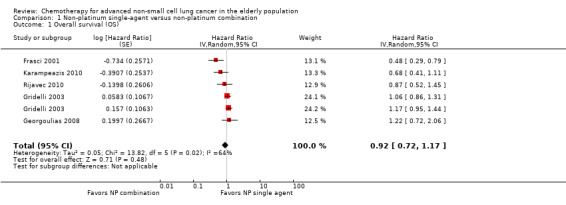
Comparison 1 Non‐platinum single‐agent versus non‐platinum combination, Outcome 1 Overall survival (OS).
4.
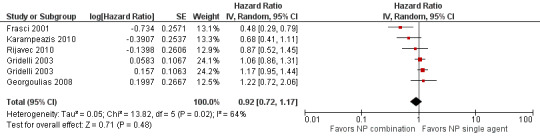
Forest plot of comparison: 1 Non‐platinum single agent vs non‐platinum combination, outcome: 1.1 Overall survival (OS).
Gridelli 2003 was designed for a separate comparison of each single‐agent arm (V arm and G arm) vs the combination arm (VG arm). Therefore, each entry for this trial represents one comparison (V vs VG and G vs VG arm).
Reasons for heterogeneity are not clear, but methodological differences related to inclusion of prematurely interrupted RCTs (Frasci 2001; Karampeazis 2010; Rijavec 2010) or unplanned elderly subgroup analysis (Georgoulias 2008) might be responsible for this finding. Frasci 2001 was the only RCT included in the meta‐analysis that reported advantages of OS with non‐platinum combination over non‐platinum single‐agent treatment (median OS for vinorelbine arm: 18 weeks vs 29 weeks in the vinorelbine plus gemcitabine arm; HR 0.48, 95% CI 0.29 to 0.90; P value < 0.01). When median OS in the single‐agent arm was compared with other trials, this study showed the lowest median OS. Exclusion of Frasci 2001 resulted in lower heterogeneity and no significant change in the effects of interventions (HT 1.05, 95% CI 0.90 to 1.23; I2 = 18%).
Comella 2004 showed an OS advantage in favor of non‐platinum combination over non‐platinum single‐agent therapy (HR 0.76, 95% CI 0.59 to 0.99; P value = 0.0486) in the ITT population. Study authors did not perform a separate analysis on the elderly, who represented 83.3% of the ITT population. We performed an exploratory analysis by including Comella 2004 and noted no significant changes in the effects of interventions (Analysis 2.1; HR 0.88, 95% CI 0.70 to 1.11; six studies).
2.1. Analysis.
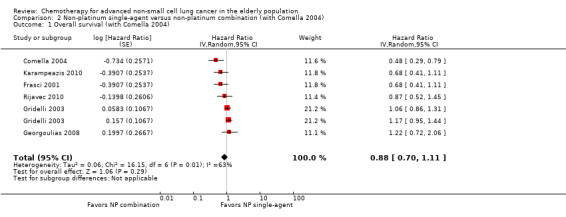
Comparison 2 Non‐platinum single‐agent versus non‐platinum combination (with Comella 2004), Outcome 1 Overall survival (with Comella 2004).
Hainsworth 2007. Even though researchers included elderly patients (defined as patients 65 years of age or older), Hainsworth 2007 allowed inclusion of younger patients with poor performance and those considered poor candidates for platinum therapy. For the ITT population (n = 350), researchers reported no significant differences in OS between the DG arm (5.5 months) and the D arm (5.1 months; P value = 0.65). Study authors reported a subgroup analysis of participants with good performance status, also suggesting no impact on OS for non‐platinum combination over non‐platinum single‐agent treatment (DG arm 7.2 months vs D arm 8.0 months; P value = 0.5). We were not able to estimate the hazard ratio for inclusion of these data in the meta‐analysis.
OS by type of trial
Georgoulias 2008 was the only trial that performed an elderly subgroup analysis for the adult general population. Exclusion of Georgoulias 2008 did not influence the results (HR 0.87, 95% CI 0.66 to 1.15).
Sensitivity analysis
We performed a sensitivity analysis by excluding prematurely interrupted trials (Karampeazis 2010; Rijavec 2010) from which only unpublished data were available; this also did not change the effects of the intervention (HR 0.97, 95% CI 0.72 to 1.31).
We did not perform a sensitivity analysis by excluding trials with unclear or high risk of bias, as none were classified as having low risk of bias for all domains.
Quality of life (QoL)
Only two RCTs included quality of life (QoL) assessment in the trial design (Frasci 2001; Gridelli 2003).
Frasci 2001 assessed QoL by applying a modified Lung Cancer Symptom questionnaire at baseline, at third and sixth cycles, then every 2 months. Study authors considered a minimum change of 10% in the score as classifying improvement or deterioration. In all, 92% (111 of 120) and 81% (35 of 43 alive) completed the QoL assessment at baseline and at 6 months, respectively. Study authors found a higher rate of temporary improvement in the VG arm than in the V arm (26% vs 15%). Time‐to‐deterioration (TTD) analysis showed benefit in favor of combination over non‐platinum single‐agent therapy with median of 29 weeks versus 18 weeks for VG and V arms, respectively.
Gridelli 2003 performed QoL analysis using the European Organization for Reseach and Treatment of Cancer Core Quality of Life Questionnaire (EORTC QLQ‐C30) and the questionnaire module for lung cancer QLQ‐LC13 applied at baseline and after third and sixth cycles. A total of 566 participants (80%) completed the questionnaire at baseline. Rates of missing data were similar between treatment arms. No significant difference was observed in change in QoL and symptom scale findings between treatment arms. Maione 2005 presented a separate analysis in which QoL and activities of daily living (ADL) score at baseline was a prognostic factor in multi‐variate analysis when a Cox model was used. Study authors did not present further summary data on QoL assessment.
We were not able to perform a meta‐analysis because of the paucity of available data.
One‐year survival rate (1yOS)
The meta‐analysis of four RCTs with 993 elderly participants showed no differences in 1yOS between treatment regimens, and heterogeneity was noted among trials (risk ratio (RR) 0.96, 95% CI 0.88 to 1.05; I2 = 61%). A random‐effects model showed no significant changes in results (RR 0.88, 95% CI 0.73 to 1.07) (Analysis 1.2). The reason for heterogeneity is not clear, but it could be explained by the inclusion of prematurely interrupted RCTs (Frasci 2001; Karampeazis 2010) and unplanned elderly subgroup analysis (Georgoulias 2008). Analysis after exclusion of Gridelli 2003 resulted in lower heterogeneity among trials with a 1yOS improvement in favor of the combination arm (RR 0.80, 95% CI 0.68 to 0.94). Gridelli 2003 represents the largest and the only RCT designed specifically for the elderly.
1.2. Analysis.
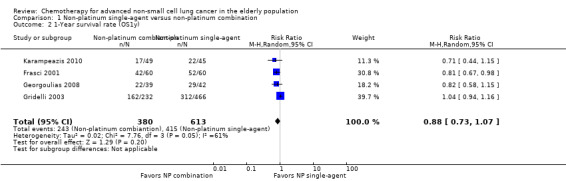
Comparison 1 Non‐platinum single‐agent versus non‐platinum combination, Outcome 2 1‐Year survival rate (OS1y).
Comella 2004 performed a combined analysis of single‐agent (P and G) versus combination (GT and GV) arms. Study authors found a statistically significant difference in 1yOS in favor of the combination regimens (28% vs 39% for single‐agent and combination arms, respectively; P value = 0.028). However, investigators performed no separate analysis for the elderly subgroup. Inclusion of Comella 2004, however, did not change the results (RR 0.88, 95% CI 0.76 to 1.02; participants = 1257; five studies; I2 = 57%; Analysis 2.2).
2.2. Analysis.

Comparison 2 Non‐platinum single‐agent versus non‐platinum combination (with Comella 2004), Outcome 2 1‐Year survival rate.
OS by trial type
Georgoulias 2008 was the only trial designed for the adult population that provided data on elderly subgroup analyses. Exclusion of Georgoulias 2008 did not change the effects of interventions (RR 0.89, 95% CI 0.71 to 1.12).
Sensitivity analysis
We performed a sensitivity analysis by excluding prematurely interrupted trials (Karampeazis 2010), with no changes in results (RR 0.91, 95% CI 0.74 to 1.11).
Progression‐free survival (PFS)
The meta‐analysis of four RCTs involving 942 participants showed no impact on the PFS of non‐platinum combination over non‐platinum single‐agent therapy (HR 0.94, 95% CI 0.83 to 1.07) with low heterogeneity among trials (I2 = 0%) (Analysis 1.3; Figure 5).
1.3. Analysis.
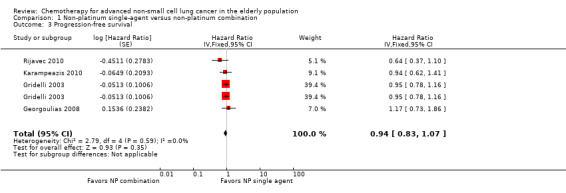
Comparison 1 Non‐platinum single‐agent versus non‐platinum combination, Outcome 3 Progression‐free survival.
5.
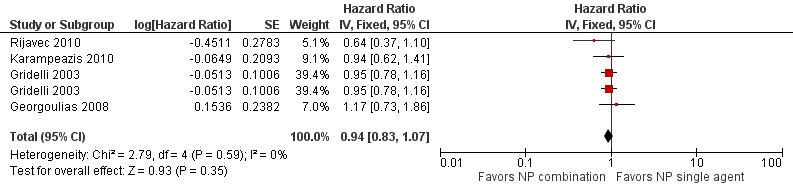
Forest plot of comparison: 1 Non‐platinum single‐agent vs non‐platinum combination, outcome: 1.3 Progression‐free survival.
Comella 2004 reported median failure‐free survival of 4.5 months and 4.1 months for GT and GV arms, respectively, and 3.7 months and 3.1 months for P and G arms, respectively. Differences in failure‐free survival did not reach statistical significance. Study authors defined time‐to‐treatment failure as the time interval from randomization to death, disease progression, or early treatment discontinuation. However, we were not able to retrieve sufficient data for extraction and inclusion in the meta‐analysis. Frasci 2001 was the only RCT that included neither PFS nor TTP analysis.
Sensitivity analysis
We also performed a sensitivity analysis by excluding a prematurely interrupted trial (Rijavec 2010), an elderly subgroup analysis (Georgoulias 2008), and unpublished data (Karampeazis 2010; Rijavec 2010). This did not change the effects of interventions.
Two RCTs evaluated PFS as a secondary outcome (Gridelli 2003; Karampeazis 2010). Comella 2004, Georgoulias 2008, and Rijavec 2010 performed a time‐to‐tumor progression (TTP) analysis, defined as time from randomization to first evidence of disease progression. We performed an exploratory sensitivity analysis by excluding trials that used a TTP endpoint while maintaining no impact of either intervention on the PFS (HR 0.95, 95% CI 0.83 to 1.08).
Objective response rate (ORR)
Five RCTs evaluated ORR as a secondary endpoint. Comella 2004 and Hainsworth 2007 did not report a separate analysis on elderly participants.
The meta‐analysis including 1014 participants assessed from five RCTs showed statistically significant improvement in response rate (RR 1.79, 95% CI 1.41 to 2.26; I2 = 0%) with no heterogeneity among trials (I2 = 0%) (Analysis 1.4; Figure 6).
1.4. Analysis.
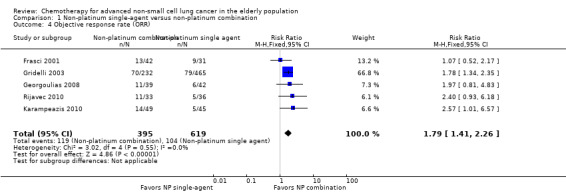
Comparison 1 Non‐platinum single‐agent versus non‐platinum combination, Outcome 4 Objective response rate (ORR).
6.
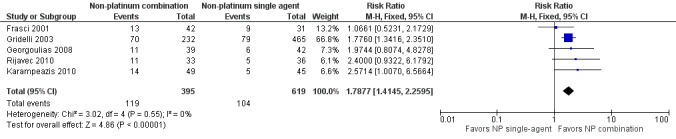
Forest plot of comparison: 1 Non‐platinum single agent vs non‐platinum combination, outcome: 1.6 Overall response rate (ORR).
Gridelli 2003 presented the ORR per treatment arm. Study authors presented planned comparisons between single‐agent arms separately versus the combination arm. For both comparisons, investigators found no statically significant differences in risk ratio (Chi2 = 0.47 for V vs VG arm and 0.18 for G vs VG arm). To avoid unit of analysis errors, we performed a combined analysis of 465 participants assigned to both single‐agent treatment arms. Exclusion of Gridelli 2003 did not change the effects of interventions (RR 1.81, 95% CI 1.19 to 2.76).
Hainsworth 2007 did not report subgroup analysis of elderly participants. In this trial, only 256 of 350 individuals in the ITT population received at least two cycles and were assessable for response, showing no statistically significant differences in RR for the DG arm (RR 25%, 95% CI 18% to 34%) over the D arm (RR 17%, 95% CI 11% to 24%; P value = 0.10).
In the ITT population, Comella 2004 found ORRs of 18% (95% CI 9% to 30%), 13% (95% CI 6% to 24%), 23% (95% CI 13% to 35%), and 32% (95% CI 20% to 45%) for G, P, GV, and GT arms, respectively.
Sensitivity analysis
A sensitivity analysis was performed by excluding Georgoulias 2008 (unplanned elderly subgroup analysis), while maintaining no impact of combination treatment over single‐agent therapy (RR 1.77, 95% CI 1.39 to 2.26). Exclusion of Karampeazis 2010 and Rijavec 2010, from which only unpublished data were available, also did not change effects of the interventions, with risk ratios of 1.73 (95% CI 1.36 to 2.21) and 1.75 (95% CI 1.37 to 2.23), respectively.
Toxicity
Grade 3 or higher hematological adverse events
We found no significant differences in risk of anemia (RR 1.18, 95% CI 0.57 to 2.40; participants = 1064; five studies; I2 = 0%), neutropenia (RR 1.19, 95% CI 0.93 to 1.54; participants = 1064; five studies; I2 = 24%), febrile neutropenia (RR 0.34, 95% CI 0.04 to 3.20; participants = 995; four studies; I2 = 0%), or thrombocytopenia (RR 1.58, 95% CI 0.82 to 3.04; participants = 995; four studies; I2 = 0%) (Analysis 1.5).
1.5. Analysis.
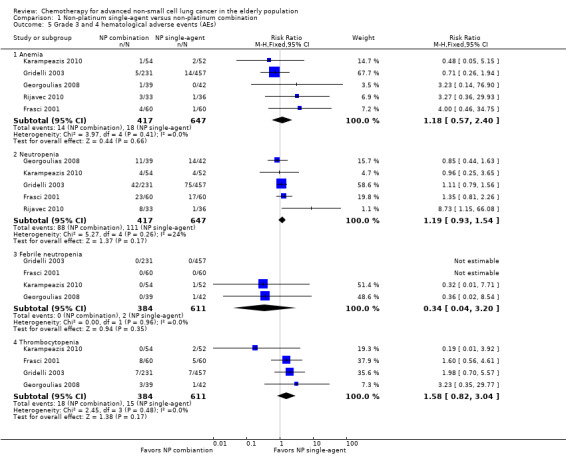
Comparison 1 Non‐platinum single‐agent versus non‐platinum combination, Outcome 5 Grade 3 and 4 hematological adverse events (AEs).
Grade 3 or higher non‐hematological adverse events
We found no significant differences in risk of fatigue (RR 1.16, 95% CI 0.69 to 1.96; participants = 995; four studies; I2 = 0%) or emesis (RR 1.73, 95% CI 0.68 to 4.43; participants = 995; four studies; I2 = 0%). For diarrhea, constipation, and mucositis, few grade 3 or 4 events were observed in all included trials (Analysis 1.6).
1.6. Analysis.
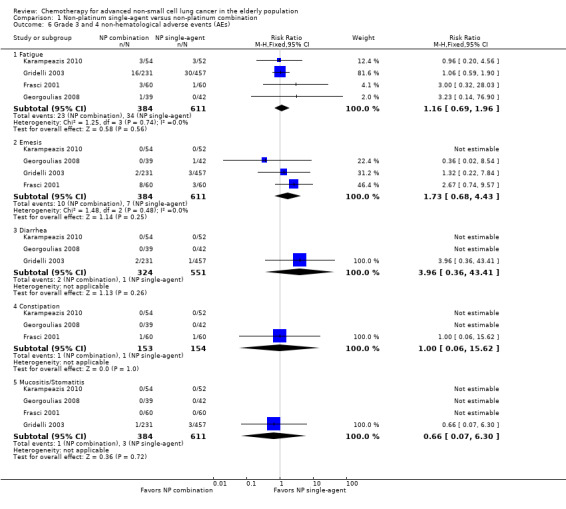
Comparison 1 Non‐platinum single‐agent versus non‐platinum combination, Outcome 6 Grade 3 and 4 non‐hematological adverse events (AEs).
Non‐platinum therapy versus platinum combination therapy
Overall survival (OS)
We included 18 RCTs involving 2309 participants over 70 years of age, which were undertaken for comparison of OS between non‐platinum therapies, given as a single agent or in combination, and platinum combination therapies (Abe 2011; Boni 2012; Chen 2008; Flotten 2012; Georgoulias 2001; Georgoulias 2004; Georgoulias 2005; Kubota 2008; Laack 2004; Lilenbaum 2005; Lou 2010; Quoix 2011b; Sederholm 2005; Treat 2010; Tsukada 2007; Zhang 2006; Zukin 2013; Zwitter 2010). Among these, Sederholm 2005 and Zwitter 2010, involving 168 elderly participants, did not provide a separate analysis on the elderly subgroup. Three RCTs did not provide sufficient data for extraction and inclusion in the meta‐analysis (Lou 2010; Treat 2010; Zhang 2006). However, their results are discussed separately. Chen 2008 and Laack 2004 did not provide HRs and 95% CIs; these data were estimated from Kaplan‐Meier curves (Table 5).
3. Overall survival (OS) and 1‐year survival (1yOS) for platinum combination versus non‐platinum therapy.
| Author, year | Inclusion criteria (age and PS) |
n (elderly) |
N‐based therapy | P‐based combination | HR (95% CI) | P value | ||
| Median OS in months (95% CI) | 1yOS | Median OS in months (95% CI) | 1yOS | |||||
| Zukin 2013 | ≥ 18 yo and PS 2 | 74 | 5.3 (NR) | NR | 9.9 (NR) | NR | 0.49 (0.34‐0.70) | 0.006 |
| Quoix 2011b | ≥ 70 yo | 451 | 6.2 (5.3‐7.3) | 25.4% (19.9‐31.3) | 10.3 (8.3‐12.6) | 44.5% (37.9‐50.9) | 0.64 (0.52‐0.78) | < 0.0001 |
| Abe 2011 | ≥ 70 yo | 233 | 14.8 (11.9‐24.1) | 58.2% (48.3‐66.9) | 13.3 (10.8‐19.4) | 54.5% (44.8‐63.3) | 1.18 (0.83‐1.69) | 0.82 |
| Lou 2010 | ≥ 70 yo | 68 | 9.9 (NR) | 31% (NR) | 9.8 (NR) | 32% (NR) | NR | > 0.05 |
| Chen 2008 | ≥ 70 yo | 65 | 12 (NR) | 50.9% | 11.3 (NR) | 47.2% | 0.65 (0.31‐1.36) | 0.25 |
| Tsukada 2007 | ≥ 70 yo | 112 | 17 (NR) | 66.6% | 10.7 (NR) | 45.2% | 0.69 (0.45‐1.06) | 0.09 |
| Zhang 2006 | ≥ 65 yo | 96 | 8 (NR) | 36.7% (NR) | CisP arm: 9 (NR) | 38.2% (NR) | NR | < 0.05 |
| CarP arm: 10 (NR) | 43.8% (NR) | NR | < 0.05 | |||||
| Lilenbaum 2005 | ≥ 18 yo | 152 | 5.8 (3.8‐9.3) | 31% (22‐43) | 8.0 (5.7‐11) | 35% (26‐48) | 0.84 (0.61‐1.16) | 0.29 |
| Georgoulias 2004 | ≤ 75 yo | 70 | 6.9 (NR) | 38.2% (NR) | 8.0 (NR) | 37.4% (NR) | 0.88 (0.52‐1.48) | 0.63 |
| Boni 2012 | ≥ 70 yo | 101 | NR | NR | NR | NR | 0.76 (0.52‐1.16) | 0.21 |
| Flotten 2012 | No upper age limit | 74* | 4.6 (NR) | 18% | 8.0 (NR) | 28% | 0.59 (0.36‐0.95) | 0.03 |
| Kubota 2008 | NR | 118 | 13.2 (NR) | 56.8% | 13.3 (NR) | 53.6% | 0.99 (0.65‐1.52) | 0.97 |
| Georgoulias 2005 | 18 to 75 yo | 82 | 9.3 (NR) | 33.1% | 8.8 (NR) | 34.3% | 1.05 (0.64‐1.72) | 0.86 |
| Laack 2004 | 18 to 75 yo | 43 | 30.5 (19.4‐61.9) weeks | 30% (10.2‐53.0) | 32.4 (8.4‐80.3) weeks | 34.1% (16.6‐52.5) | 0.87 (0.42‐1.80) | 0.71 |
| Georgoulias 2001 | ≤ 75 yo | 71 | 6.7 (NR) | 33.3% | 7.1 (NR) | 34.3% | 1.08 (0.67‐1.74) | 0.76 |
1yOS: 1‐year survival rate; CarP arm: carboplatin and paclitaxel arm; CI: confidence interval; CispP arm: cisplatin and paclitaxel arm; HR: hazard ratio; NR: not reported; PS: performance status; yo: years old.
The meta‐analysis of 13 RCTs involving 1705 elderly participants showed improvement in OS in favor of platinum combination treatment (HR 0.76, 95% CI 0.69 to 0.85), with moderate heterogeneity observed among trials (I2 = 44%) (Analysis 3.1).
3.1. Analysis.
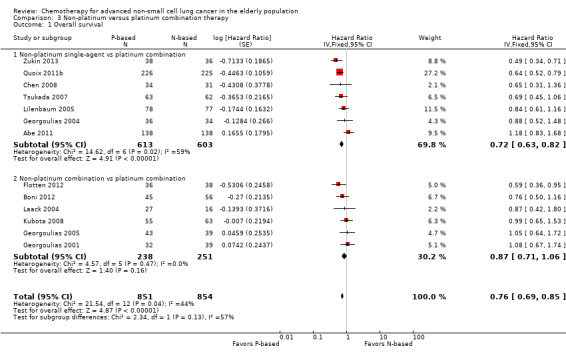
Comparison 3 Non‐platinum versus platinum combination therapy, Outcome 1 Overall survival.
Lou 2010 reported no significant differences in OS between gemcitabine single‐agent therapy and carboplatin‐gemcitabine combination, with median OS of 9.9 months and 9.8 months, respectively. We were not able to extract data for inclusion in the meta‐analysis. Treat 2010 found no statistically significant differences in OS between platinum combination arms and non‐platinum combination arms (GP vs GC arm; P value = 0.585; GP vs PC arm; P value = 0.404) in the ITT population. Ansari 2011 analyzed the elderly subgroup retrospectively, representing 29.78% (338/1135) of the ITT population. A Cox regression model found no statistically significant interactions by treatment arm and by age. We were unable to extract the HR for OS comparisons of GP versus CG and GP versus PC.
Zhang 2006 randomly assigned 96 participants older than 65 years of age and showed a statistically significant advantage of platinum combination over single‐agent therapy. Study authors found a median OS of eight months for the paclitaxel arm versus nine months and 10 months for cisplatin‐paclitaxel and carboplatin‐paclitaxel combinations, respectively. Study authors did not present a separate analysis on participants older than 70 years of age.
OS by non‐platinum therapy
We found no statistically significant differences in the effects of interventions when a non‐platinum control arm was included, with treatment given as single‐agent or combination therapy (test for subgroup differences: Chi² = 2,36, P value = 0.12, I² = 57.6%) (Analysis 3.1).
OS by platinum agent
Exploratory analysis by platinum agent showed improvement in OS for carboplatin combination treatment (HR 0.67, 95% CI 0.59 to 0.78) and no significant differences for cisplatin combination treatment (HR 0.91, 95% CI 0.77 to 1.08) over non‐platinum therapy. Differences between subgroups reached statistical significance (Chi2= 7.16; P value = 0.007; I2 = 86%), suggesting greater benefit of carboplatin over cisplatin regimens when compared with non‐platinum therapy (Analysis 6.1; Figure 7).
6.1. Analysis.
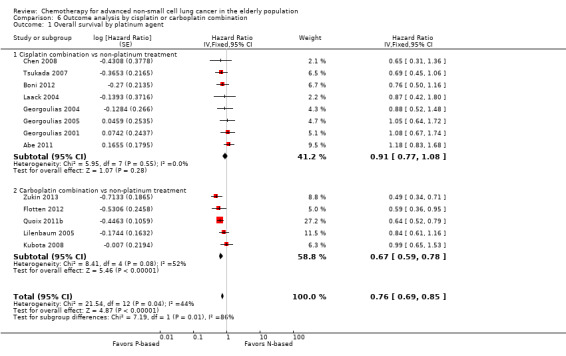
Comparison 6 Outcome analysis by cisplatin or carboplatin combination, Outcome 1 Overall survival by platinum agent.
7.
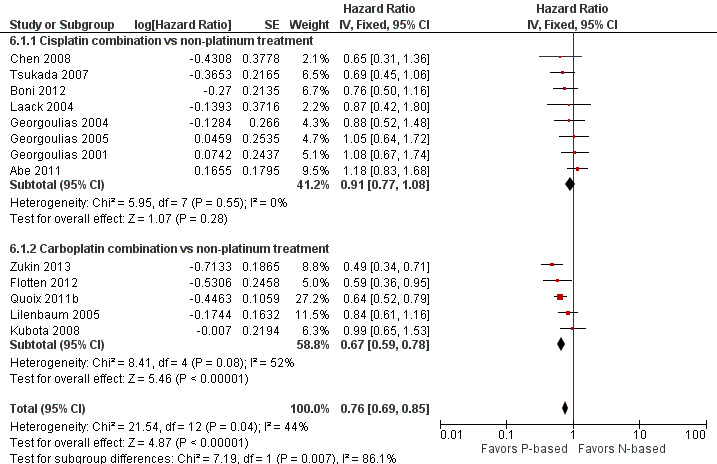
Forest plot of comparison: 3 Overall survival analysis for platinum combination by cisplatin or carboplatin combination, outcome: 3.1 Overall survival by platinum agent.
OS by trial design
We found an advantage for OS in favor of platinum combination in both subgroups of trials (elderly specific (HR 0.74, 95% CI 0.63 to 0.86; four studies; I2 = 66%) and elderly subgroups (HR 0.79, 95% CI 0.68 to 0.91; nine studies; I2 = 35%)), with no statistically significant differences in effects of interventions by type of trial (tests for subgroup differences: Chi² = 0.38; P value = 0.54; I² = 0%) (Analysis 5.1).
5.1. Analysis.

Comparison 5 Outcome analysis by type of trial (elderly specific vs elderly subgroup), Outcome 1 Overall survival.
Sensitivity analysis
We performed a sensitivity analysis by excluding trials prematurely interrupted (Abe 2011; Tsukada 2007), which showed no significant changes in effects of interventions (HR 0.73, 95% CI 0.65 to 0.82), and by excluding unpublished data (Abe 2011; Boni 2012; Georgoulias 2001; Georgoulias 2004; Georgoulias 2005; Kubota 2008; Laack 2004; Tsukada 2007), also with no significant changes in results (HR 0.64, 95% CI 0.56 to 0.75). We did not perform a sensitivity analysis by excluding trials with unclear or high risk of bias, as none were classified as having low risk of bias for all domains.
Quality of life (QoL)
Only five RCTs included QoL assessment. However, we were not able to perform a meta‐analysis of these data because of the paucity of data provided. Findings of each trial are discussed separately.
Kubota 2008 performed a QoL assessment as an additional study (BRI LC03‐01), along with the JMTO LC00‐03, using Functional Assessment of Cancer Therapy‐Lung (FACT‐L), FACT‐Taxane, and Functional Assessment of Chronic Illness Therapy Spiritual Well‐Being Scale (FACIT‐Sp) QoL instruments at baseline and at weeks 6, 12, and 18 from the start of treatment (Kawahara 2011). This study aimed to include 200 participants; however, 109 were screened and 81 enrolled. Only 64 participants were available for QoL analysis; median age (range) was 66 (33 to 75) and 64 (39 to 79) years for the PC and VGD arms, respectively. In the ITT population, study authors found no statistically significant differences between treatment arms for FACT‐L and FACIT‐Sp QoL Instruments. However, a difference in slopes was observed in FACT‐Taxane, with greater decline noted in the PC group. Study authors did not present a separate analysis of the elderly subgroup.
Quoix 2011b assessed QoL by using the EORTC QLQ‐C30 and QLQ‐LC13 at baseline, week 6, and week 18. A total of 94%, 62%, and 49% of participants completed the questionnaire at baseline, week 6, and week 18. The global QoL score was similar between the two arms at all time points evaluated. For specific symptoms, investigators observed more pain (30.2 vs 18.7; P value = 0.003) and dyspnoea (47.4 vs 36.8; P value = 0.014)in the monotherapy arm than in the platinum therapy arm. On the other hand, the score for diarrhea was higher with the platinum combination (18.4 vs 8.8; P value = 0.003). No additional data were available for QoL assessment.
Laack 2004 assessed QoL by using the EORTC QLQ‐C30/LC13 questionnaire. For the ITT population, study authors found no statistically significant differences in any of the domains analyzed. We obtained an elderly subgroup analysis based on only 12 participants upon direct contact with study authors. This limited analysis showed no significant differences in mean scores between treatment arms for all items on the EORTC QLQ‐C30/LC13 questionnaire.
Lou 2010 performed a QoL assessment by using six Lung Cancer Symptom Scale (LCSS) domains (lack of appetite, fatigue, cough, dyspnoea, haemoptysis, and pain) before and three weeks after the last dose of chemotherapy. Study authors included all participants in this analysis. After the last cycle of chemotherapy, study authors found a significant difference in favor of non‐platinum single‐agent treatment for lack of appetite (84 ± 15 vs 71 ± 20; P value = 0.01), fatigue (71 ± 21 vs 55 ± 20; P value = 0.00), and pain (82 ± 17 vs 72 ± 20; P value = 0.03). They observed no significant differences in other symptoms.
Zhang 2006 assessed QoL by looking at changes in Karnofsky Performance Scale (KPS) scores only. Investigators collected no data on specific QoL questionnaire items.
One‐year survival rate (1yOS)
The meta‐analysis of 13 RCTs involving 1695 participants older than 70 years showed improvement in 1yOS for the platinum therapy over non‐platinum therapy (RR 0.89, 95% CI 0.82 to 0.96; I2 = 24%; Analysis 3.2). Inclusion of Zhang 2006, which defined elderly as individuals older than 65 years, did not significantly change effects of the interventions (RR 0.89, 95% CI 0.82 to 0.96; participants = 1791; 14 studies; I2 = 18%; Analysis 4.1).
3.2. Analysis.
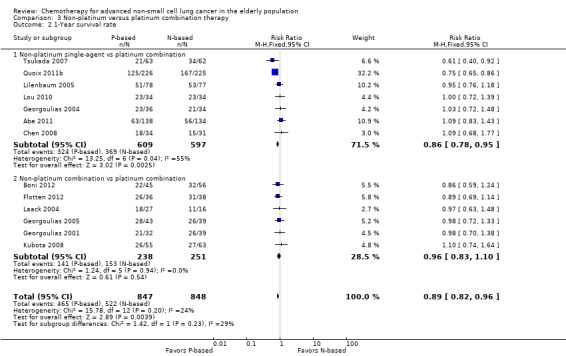
Comparison 3 Non‐platinum versus platinum combination therapy, Outcome 2 1‐Year survival rate.
4.1. Analysis.
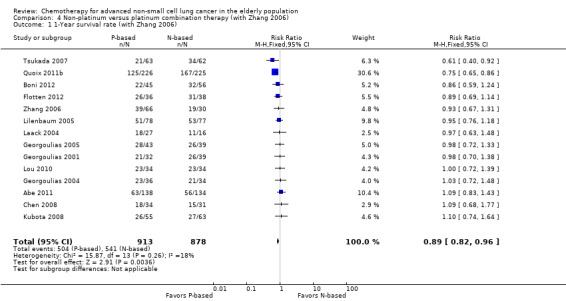
Comparison 4 Non‐platinum versus platinum combination therapy (with Zhang 2006), Outcome 1 1‐Year survival rate (with Zhang 2006).
Sederholm 2005 and Treat 2010 did not perform a separate analysis on the elderly subgroup. Treat 2010, Zukin 2013, and Zwitter 2010 provided insufficient data for inclusion in this analysis.
1yOS by non‐platinum therapy
We found no statistically significant differences in effects of interventions by non‐platinum control arm with treatment given as single‐agent or combination therapy (Chi2 = 1.42; P value = 0.23; I2 = 29.4%) (Analysis 3.2).
1yOS by platinum agent
We found no statistically significant differences in effects of interventions by platinum agent, whether cisplatin‐based or carboplatin‐based (Chi2 = 1.89; P value = 0.17; I2 = 47.0%) (Analysis 6.2).
6.2. Analysis.
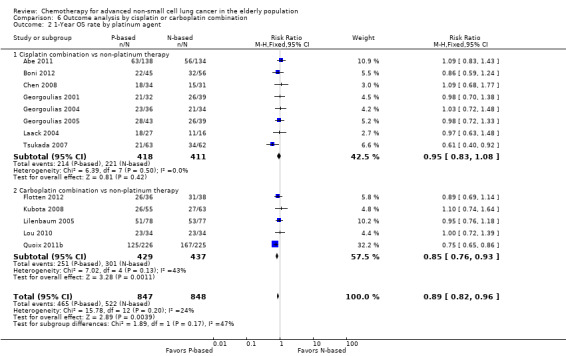
Comparison 6 Outcome analysis by cisplatin or carboplatin combination, Outcome 2 1‐Year OS rate by platinum agent.
1yOS by type of trial design
We found an advantage in favor of platinum combination in both subgroups of trials (elderly specific (RR 0.83, 95% CI 0.75 to 0.93; participants = 981; five studies; I2 = 63%) and elderly subgroups (RR 0.96, 95% CI 0.86 to 1.08; participants = 714; eight studies; I2 = 0%)), with no statistically significant differences in effects of interventions by type of trial (Chi2 = 0.13; P value = 0.08; I2 = 68.1%) (Analysis 5.2).
5.2. Analysis.
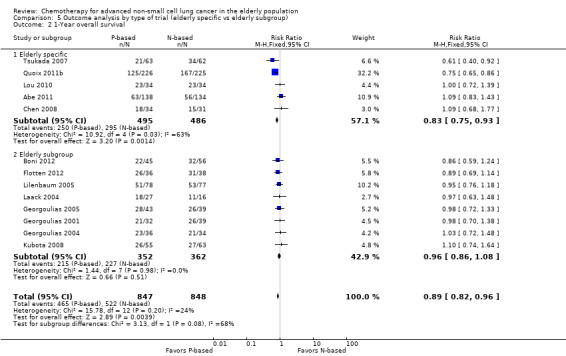
Comparison 5 Outcome analysis by type of trial (elderly specific vs elderly subgroup), Outcome 2 1‐Year overall survival.
Sensitivity analysis
We performed a sensitivity analysis by excluding trials prematurely interrupted (Abe 2011; Tsukada 2007), which showed no significant changes in effects of interventions (RR 0.88, 95% CI 0.81 to 0.96), and by excluding unpublished data (Abe 2011; Boni 2012; Georgoulias 2001; Georgoulias 2004; Georgoulias 2005; Kubota 2008; Laack 2004; Tsukada 2007), without significant changes in the results (RR 0.84, 95% CI 0.76 to 0.93).
Progression‐free survival (PFS)
The meta‐analysis of nine RCTs with 1273 elderly participants showed significant improvement in PFS in favor of platinum combination over non‐platinum therapy (HR 0.70, 95% CI 0.63 to 0.79). In light of the presence of significant heterogeneity (I2 = 63%), we performed an analysis using a random‐effects model, while maintaining a significant difference in PFS in favor of platinum combination (HR 0.76, 95% CI 0.61 to 0.93) (Analysis 3.3). Reasons for heterogeneity among trials are unclear, but it could be explained by performance of unplanned subgroup analyses, small sample size, and prematurely interrupted trials. Exclusion of Quoix 2011b resulted in lower heterogeneity (I2 = 0%) with no changes in results (HR 0.84, 95% CI 0.72 to 0.97) (Table 6).
3.3. Analysis.
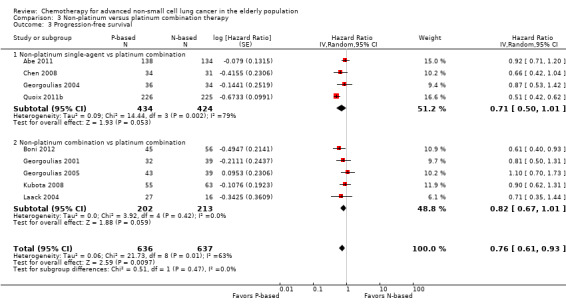
Comparison 3 Non‐platinum versus platinum combination therapy, Outcome 3 Progression‐free survival.
4. Progression‐free survival (PFS) for platinum combination versus non‐platinum therapy.
| Author, year | n (elderly) | Median PFS (95% CI) | HR (95% CI) | P value | |
| Non‐platinum single agent | Platinum‐based combination | ||||
| Quoix 2011b | 451 | 2.8 (2.6‐3.7) months | 6.0 (5.5‐6.8) months | 0.51 (0.42‐0.62) | < 0.0001 |
| Abe 2011 | 233 | 4.4 (3.4‐5.1) months | 4.7 (4.1‐5.8) months | 0.92 (0.71‐1.20) | 0.55 |
| Chen 2008 | 65 | 3.1 (NR) months | 5.2 (NR) months | 0.66 (0.42‐1.04) | 0.07 |
| Georgoulias 2004 | 70 | 2.5 (NR) months | 4.3 months | 0.86 (0.58‐1.29) | 0.48 |
| Boni 2012 | 101 | NR | NR | 0.61 (0.40‐0.93) | 0.02 |
| Kubota 2008 | 118 | 5.8 (MR) months | 5.8 (NR) months | 0.90 (0.62‐1.31) | 0.58 |
| Georgoulias 2005 | 82 | 4.5 (NR) months | 4.6 (NR) months | 1.10 (0.70‐1.73) | 0.68 |
| Laack 2004 | 43 | 10.9 (3.4‐24.9) weeks | 17.1 (8.4‐32.4) weeks | 0.71 (0.35‐1.44) | 0.34 |
| Georgoulias 2001 | 71 | 2.5 (NR) months | 5.1 (NR) | 0.81 (0.50‐1.31) | 0.39 |
95% CI: 95% confidence interval; HR: hazard ratio; NR: not reported; PFS: progression‐free survival.
No data on PFS were available for 1097 elderly participants from nine RCTs. Lou 2010 and Zhang 2006 did not include PFS analysis in the trial design. Seven RCTs did not report a separate PFS for the subgroup of participants over 70 years of age (Flotten 2012; Lilenbaum 2005; Sederholm 2005; Treat 2010; Tsukada 2007; Zukin 2013; Zwitter 2010).
PFS by non‐platinum therapy
We found no statistically significant differences in effects of interventions by using a non‐platinum control arm, with treatment given as single agent or combination (tests for subgroup differences: Chi² = 0.51; P value = 0.47; I² = 0%) (Analysis 3.3).
PFS by platinum agent
We found statistically significant differences in effects of interventions by platinum agent (tests for subgroup differences: Chi² = 0.59; P value = 0.44; I² = 0%) (Analysis 6.3; Figure 8).
6.3. Analysis.
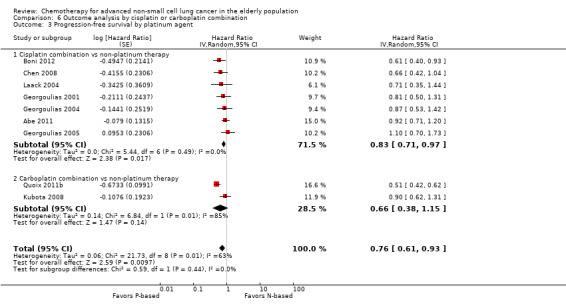
Comparison 6 Outcome analysis by cisplatin or carboplatin combination, Outcome 3 Progression‐free survival by platinum agent.
8.
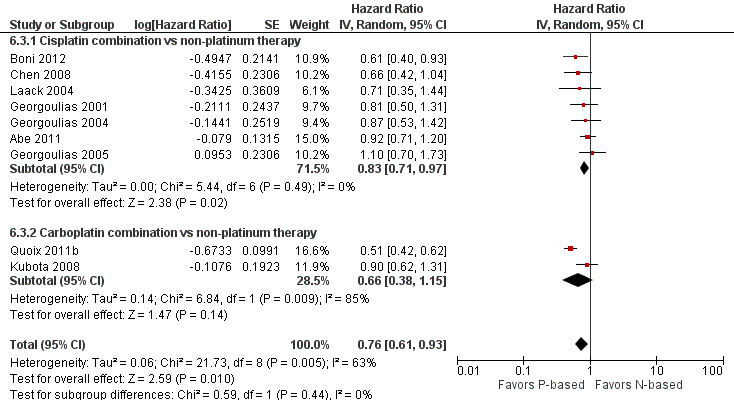
Forest plot of comparison: 3 Outcome analysis for platinum combination by cisplatin or carboplatin combination, outcome: 3.3 Progression‐free survival by platinum agent.
PFS by type of trial design
We found statistically significant differences in effects of interventions by type of trial (tests for subgroup differences: Chi² = 0.77; P value = 0.38; I² = 0%) (Analysis 5.3).
5.3. Analysis.
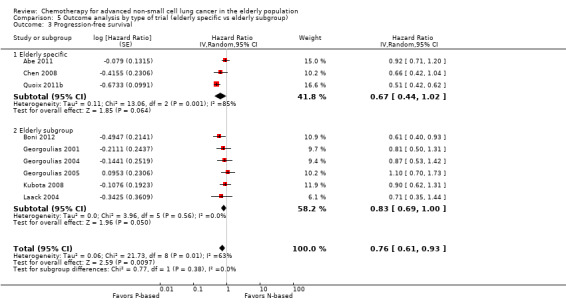
Comparison 5 Outcome analysis by type of trial (elderly specific vs elderly subgroup), Outcome 3 Progression‐free survival.
Sensitivity analysis
We performed a sensitivity analysis by excluding trials prematurely interrupted (Abe 2011), which showed no significant changes in effects of interventions (HR 0.73, 95% CI 0.58 to 0.91), and by excluding unpublished data, which maintained a significant difference in PFS in favor of platinum combination treatment (HR 0.53, 95% CI 0.44 to 0.65). Exclusion of elderly subgroup analysis also did not change the direction and magnitude of treatment effects (HR 0.67, 95% CI 0.44 to 1.02).
Georgoulias 2001, Georgoulias 2004, and Georgoulias 2005 did not report data on PFS, defined as time from randomization until date of disease progression or death. Instead, study authors presented a time‐to‐tumor progression (TTP) analysis, defined as time from randomization until date of disease progression. An exploratory sensitivity analysis performed by excluding these trials did not significantly change results (HR 0.70, 95% CI 0.54 to 0.90; participants = 1050).
Objective response rate (ORR)
The meta‐analysis from 11 RCTs with 1432 elderly participants showed benefit in RR in favor of platinum combination over non‐platinum regimens with low heterogeneity among trials (RR 1.57, 95% CI 1.32 to 1.85; I2 = 24%) (Analysis 3.4).
3.4. Analysis.
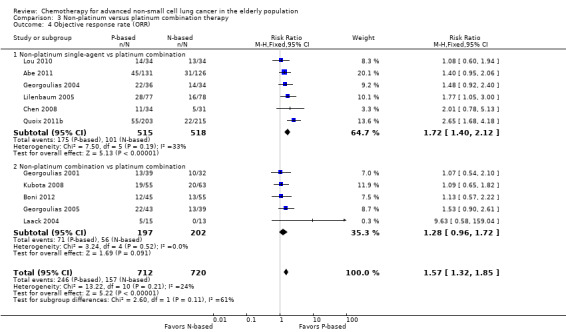
Comparison 3 Non‐platinum versus platinum combination therapy, Outcome 4 Objective response rate (ORR).
Zhang 2006, which defined elderly as individuals older than 65 years of age, did not provide a separate analysis on participants over 70 years of age. Inclusion of this RCT in the meta‐analysis did not change the effects of interventions (RR 1.60, 95% CI 1.36 to 1.88; participants = 1528; 12 studies; I2 = 22%; Analysis 4.2).
4.2. Analysis.
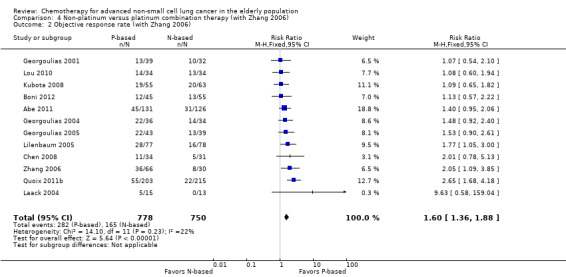
Comparison 4 Non‐platinum versus platinum combination therapy (with Zhang 2006), Outcome 2 Objective response rate (with Zhang 2006).
We performed a sensitivity analysis by excluding all elderly subgroup analyses, while maintaining the benefit of platinum combination treatment (RR 1.80, 95% CI 1.42 to 2.27; participants = 904; five studies; I2 = 47%). Separate exclusion of each elderly subgroup analysis did not influence the results.
No data on RR were provided for 766 participants included in six RCTs (Flotten 2012; Sederholm 2005; Treat 2010; Tsukada 2007; Zukin 2013; Zwitter 2010), which did not report a separate analysis on RR in the elderly subgroup.
ORR by non‐platinum therapy
We found no statistically significant differences in effects of interventions by non‐platinum control arm, with treatment given as single agent or combination (Chi2 = 2.60; P value = 0.11; I2 = 61.5%). We observed a 75% greater chance of ORR for platinum combination over non‐platinum single‐agent treatment (RR 1.75, 95% CI 1.44 to 2.14) compared with a 28% greater chance for platinum combination over non‐platinum combination treatment (RR 1.28, 95% CI 0.96 to 1.72) (Analysis 3.4).
ORR by platinum agent
We found no statistically significant differences in effects of interventions by platinum agent (Chi2 = 1.09; P value = 0.30; I2 = 8.3%) (Analysis 6.4; Figure 9).
6.4. Analysis.
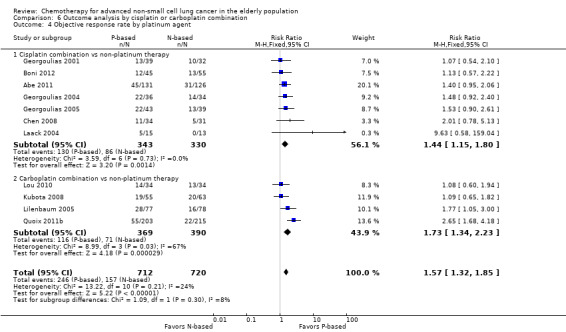
Comparison 6 Outcome analysis by cisplatin or carboplatin combination, Outcome 4 Objective response rate by platinum agent.
9.
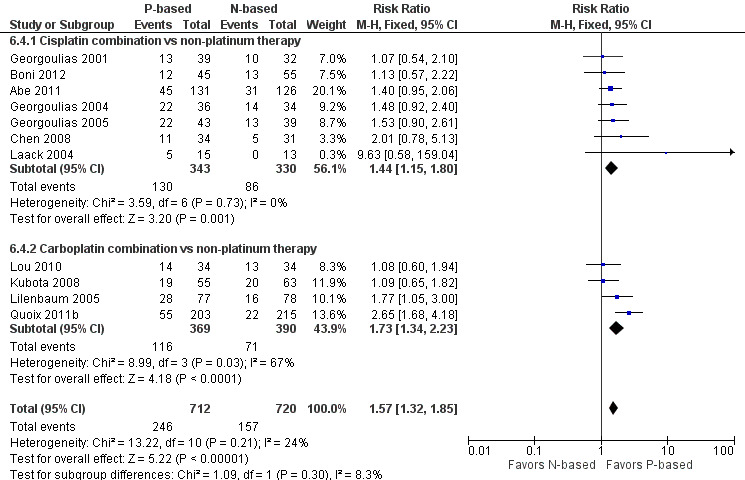
Forest plot of comparison: 3 Outcome analysis for platinum combination by cisplatin or carboplatin combination, outcome: 3.4 Objective response rate by platinum agent.
ORR by type of trial design
We found an advantage in favor of platinum combination in both subgroups of trials (elderly specific (RR 1.76, 95% CI 1.37 to 2.26; participants = 808; four studies; I2 = 58%) and elderly subgroups (RR 1.41, 95% CI 1.12 to 1.76; participants = 624; seven studies; I2 = 0%)), with no statistically significant differences in effects of interventions by type of trial (Chi2 = 1.65; P value = 0.20; I2 = 39.3%) (Analysis 5.4).
5.4. Analysis.
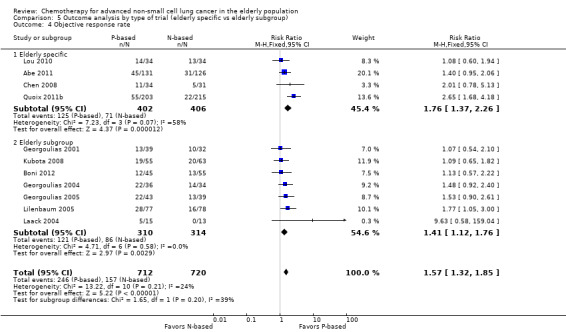
Comparison 5 Outcome analysis by type of trial (elderly specific vs elderly subgroup), Outcome 4 Objective response rate.
Sensitivity analysis
We performed a sensitivity analysis by excluding trials prematurely interrupted (Abe 2011), which showed no significant changes in effects of interventions (RR 1.61, 95% CI 1.33 to 1.94), and by excluding unpublished data (Abe 2011; Boni 2012; Georgoulias 2001; Georgoulias 2004; Georgoulias 2005; Kubota 2008; Laack 2004), with no significant changes in results (RR 1.97, 95% CI 1.48 to 2.61).
Toxicity
Hematological grade 3 or higher adverse events
Using a fixed‐effect model, we found greater risk of anemia (RR 2.53, 95% CI 1.70 to 3.76; participants = 1437; 11 studies; I2 = 23%) and thrombocytopenia (RR 3.59, 95% CI 2.22 to 5.82; participants = 1260; nine studies; I2 = 8%) for platinum combinations. We found no statistically significant differences in risks of neutropenia (RR 1.08, 95% CI 0.94 to 1.25; participants = 1423; 12 studies; I2 = 93%) and febrile neutropenia (RR 1.14, 95% CI 0.74 to 1.75; participants = 1215; eight studies; I2 = 63%), and results for both were associated with high heterogeneity among trials (Analysis 3.5; Figure 10). Using a random‐effects model, we found no significant changes in risk, with risk ratio of 1.49 (95% CI 0.77 to 2.85) for neutropenia (Analysis 3.8) and 1.58 (95% CI 0.56 to 4.50) for febrile neutropenia (Analysis 3.9). Reasons for high heterogeneity with neutropenia could be related to variables associated with chemotherapy regimens used in experimental and control arms, such as non‐platinum single‐agent or combination treatment, usage of granulocyte‐colony stimulating factor (GCSF) as primary prophylaxis, and dosage and regimens of cytotoxic agents. Subgroup analyses showed no statistically significant differences in risk of neutropenia whether non‐platinum was used as single‐agent or combination treatment in the control arm versus platinum regimens (Analysis 3.8). Differences in chemotherapy regimens might also have influenced the results. Abe 2011 showed a lower incidence of neutropenia and febrile neutropenia with the cisplatin‐docetaxel combination, which could be related to the lower dose of cisplatin given and weekly scheduling versus a three‐weekly docetaxel regimen. Exclusion of Abe 2011 resulted in higher risk of neutropenia (RR 1.92, 95% CI 1.16 to 3.18; I2 = 85%) and febrile neutropenia (RR 2.31, 95% CI 1.33 to 4.02; I2 = 0%), along with lower heterogeneity. Georgoulias 2004 used primary prophylaxis for febrile neutropenia only with platinum combination treatment, with no statistically significant differences in febrile neutropenia between treatment arms.
3.5. Analysis.
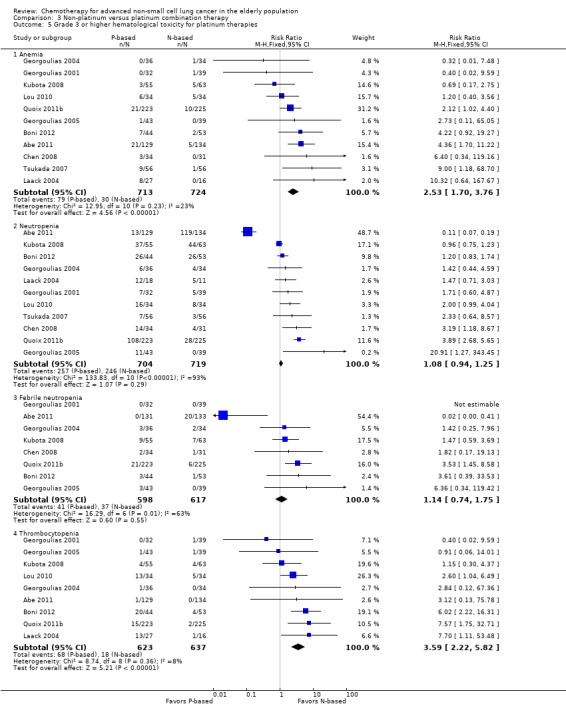
Comparison 3 Non‐platinum versus platinum combination therapy, Outcome 5 Grade 3 or higher hematological toxicity for platinum therapies.
10.
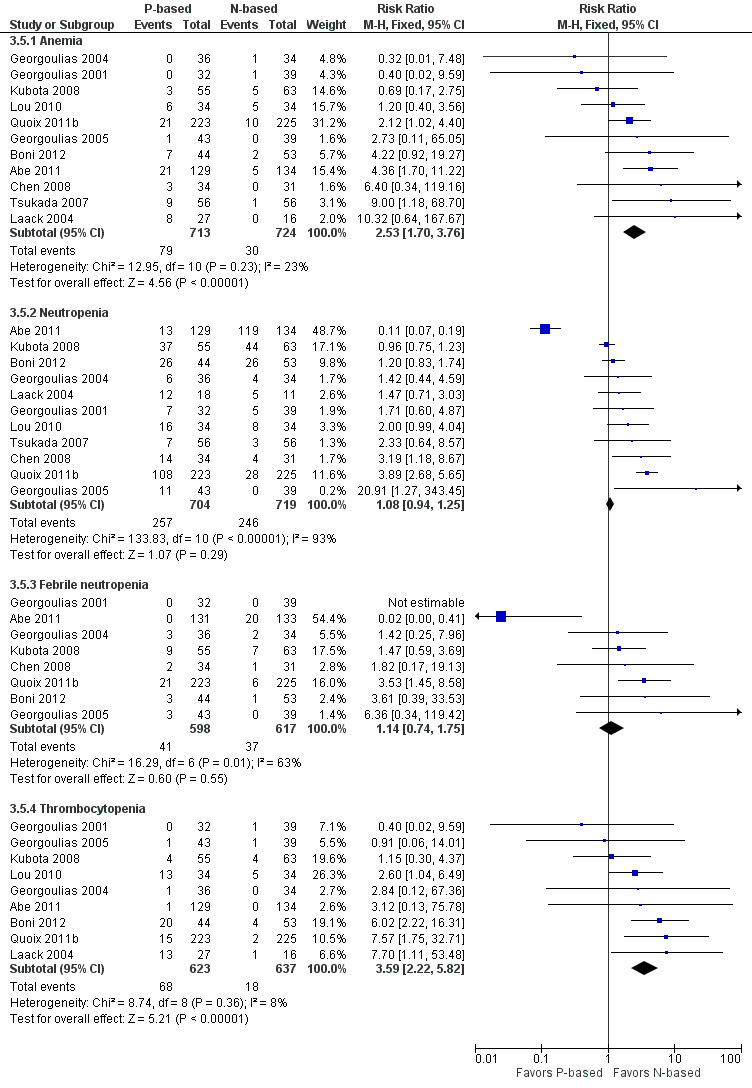
Forest plot of comparison: 4 Non‐platinum vs platinum combination therapy, outcome: 4.6 Grade 3 or higher hematological toxicity for platinum therapies.
3.8. Analysis.
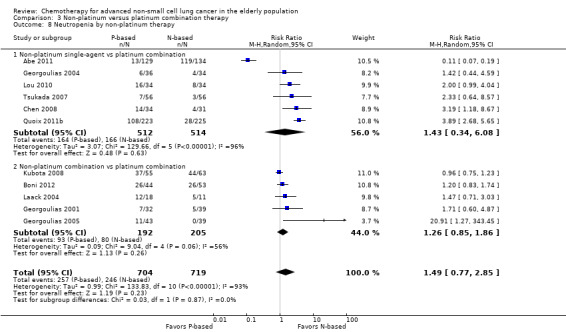
Comparison 3 Non‐platinum versus platinum combination therapy, Outcome 8 Neutropenia by non‐platinum therapy.
3.9. Analysis.
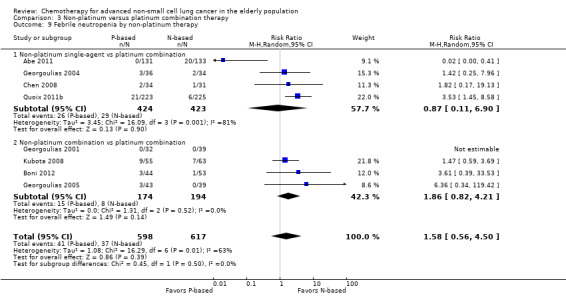
Comparison 3 Non‐platinum versus platinum combination therapy, Outcome 9 Febrile neutropenia by non‐platinum therapy.
Flotten 2012, Sederholm 2005, and Treat 2010 did not perform a separate analysis on the elderly subgroup. Zhang 2006 found no grade 4 adverse events in the safety population. Study authors presented only a combined analysis of grades 1 through 3 hematological toxicity. They did not perform a separate analysis on participants with grade 3 adverse events nor on those older than 70 years of age. Tsukada 2007 did not present data on febrile neutropenia nor on thrombocytopenia.
Toxicity by non‐platinum therapy
We found no differences between subgroups with non‐platinum therapy in the incidence of grade 3 or 4 adverse events for anemia, neutropenia, febrile neutropenia, and thrombocytopenia (Analysis 3.7; Analysis 3.8; Analysis 3.9; Analysis 3.10).
3.7. Analysis.
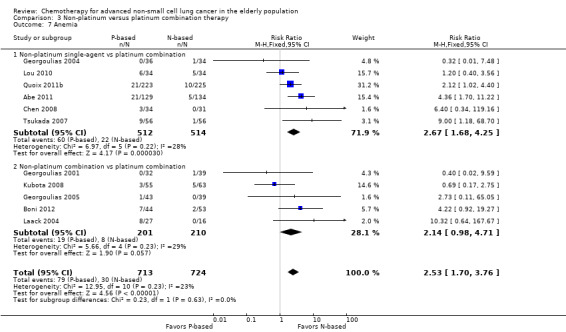
Comparison 3 Non‐platinum versus platinum combination therapy, Outcome 7 Anemia.
3.10. Analysis.
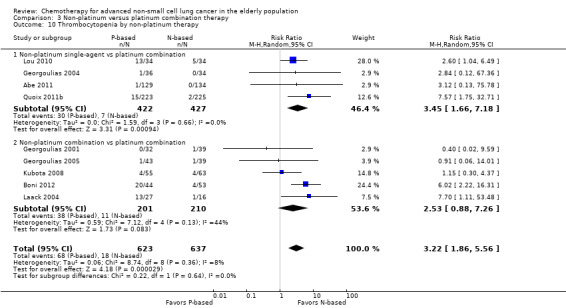
Comparison 3 Non‐platinum versus platinum combination therapy, Outcome 10 Thrombocytopenia by non‐platinum therapy.
Toxicity by platinum agent
We found statistically significant differences between subgroups by platinum agent (Chi2 = 5.44; P value = 0.02; I2 = 81.6%) for anemia. We found a higher incidence of anemia in cisplatin combination versus non‐platinum therapy (RR 4.09, 95% CI 2.22 to 7.55) compared with carboplatin combination versus non‐platinum therapy (RR 1.54, 95% CI 0.90 to 2.66) (Analysis 6.5) and no statistically significant differences among subgroups for neutropenia, febrile neutropenia, and thrombocytopenia (Analysis 6.6; Analysis 6.7; Analysis 6.8).
6.5. Analysis.
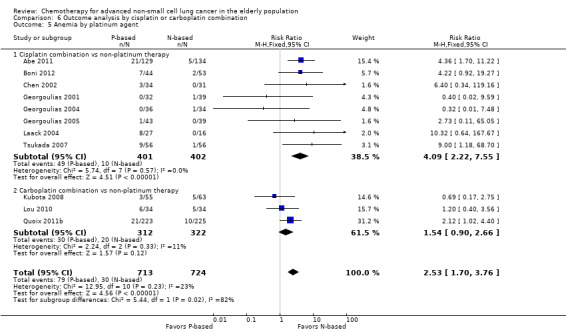
Comparison 6 Outcome analysis by cisplatin or carboplatin combination, Outcome 5 Anemia by platinum agent.
6.6. Analysis.
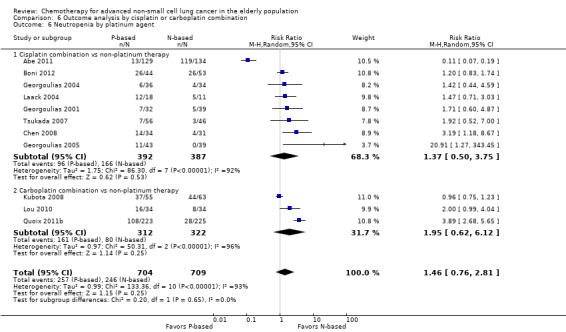
Comparison 6 Outcome analysis by cisplatin or carboplatin combination, Outcome 6 Neutropenia by platinum agent.
6.7. Analysis.

Comparison 6 Outcome analysis by cisplatin or carboplatin combination, Outcome 7 Febrile neutropenia by platinum agent.
6.8. Analysis.
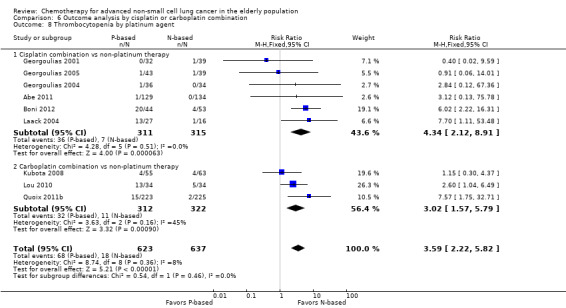
Comparison 6 Outcome analysis by cisplatin or carboplatin combination, Outcome 8 Thrombocytopenia by platinum agent.
Toxicity by type of trial
We found no statistically significant differences in risk of anemia, neutropenia, febrile neutropenia, or thrombocytopenia according to type of trial (Analysis 5.5; Analysis 5.6; Analysis 5.8; Analysis 5.7).
5.5. Analysis.
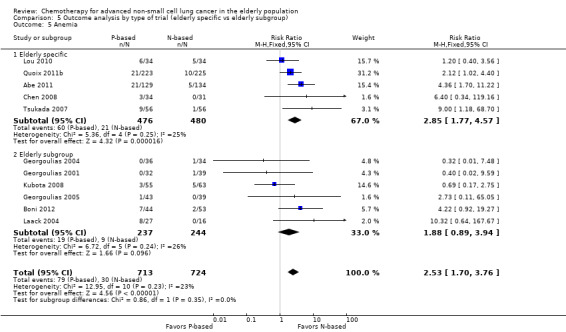
Comparison 5 Outcome analysis by type of trial (elderly specific vs elderly subgroup), Outcome 5 Anemia.
5.6. Analysis.
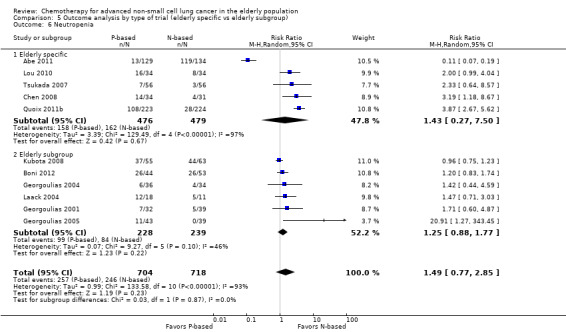
Comparison 5 Outcome analysis by type of trial (elderly specific vs elderly subgroup), Outcome 6 Neutropenia.
5.8. Analysis.
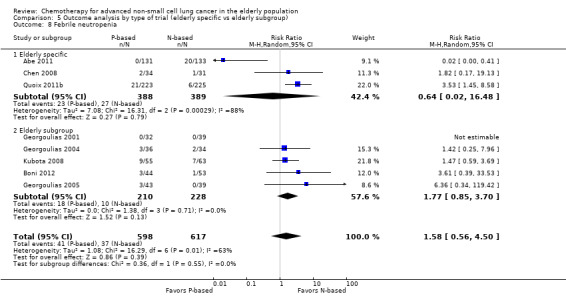
Comparison 5 Outcome analysis by type of trial (elderly specific vs elderly subgroup), Outcome 8 Febrile neutropenia.
5.7. Analysis.
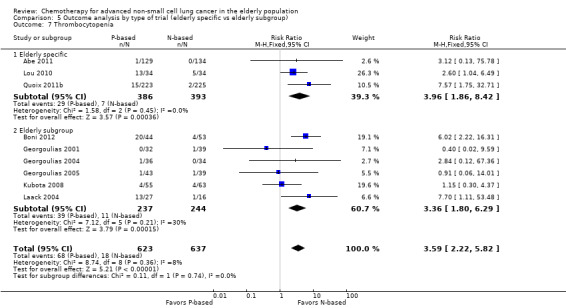
Comparison 5 Outcome analysis by type of trial (elderly specific vs elderly subgroup), Outcome 7 Thrombocytopenia.
Sensitivity analysis
We performed a sensitivity analysis by excluding unpublished data (Abe 2011; Boni 2012;Georgoulias 2001; Georgoulias 2004; Georgoulias 2005; Kubota 2008; Laack 2004; Tsukada 2007) that showed a higher incidence of anemia (RR 1.87, 95% CI 1.03 to 3.39), neutropenia (RR 3.17, 95% CI 2.09 to 4.80), febrile neutropenia (RR 3.29, 95% CI 1.51 to 7.17), and thrombocytopenia (RR 3.83, 95% CI 1.35 to 10.88) for the platinum combination arm.
A sensitivity analysis performed with exclusion of prematurely interrupted trials (Abe 2011; Tsukada 2007) showed a higher incidence of anemia (RR 1.76, 95% CI 1.06 to 2.92), neutropenia (RR 1.89, 95% CI 1.10 to 3.24), febrile neutropenia (RR 2.31, 95% CI 1.33 to 4.02), and thrombocytopenia (RR 3.17, 95% CI 1.72 to 5.85) for the platinum combination arm.
Non‐hematological grade 3 or higher adverse events
We found higher risk of fatigue (RR 1.56, 95% CI 1.02 to 2.38; participants = 1150; seven studies; I2 = 0%), emesis (RR 3.64, 95% CI 1.82 to 7.29), and peripheral neuropathy (RR 7.02, 95% CI 2.42 to 20.41; participants = 776; five studies; I2 = 0%) associated with platinum combination treatment. We found no statistically significant differences in the incidence of diarrhea (RR 1.75, 95% CI 0.91 to 3.38; participants = 1075; seven studies; I2 = 21%) and mucositis (RR 0.93, 95% CI 0.33 to 2.67; participants = 740; five studies; I2 = 0%) (Analysis 3.6).
3.6. Analysis.

Comparison 3 Non‐platinum versus platinum combination therapy, Outcome 6 Grade 3 or higher non‐hematological toxicity for platinum therapies.
Toxicity by non‐platinum therapy
We found no differences in the incidence of grade 3 or 4 fatigue, emesis, diarrhea, mucositis/stomatitis, and peripheral neuropathy according to subgroups based on non‐platinum therapy (Analysis 3.11; Analysis 3.12; Analysis 3.13; Analysis 3.14; Analysis 3.15)
3.11. Analysis.
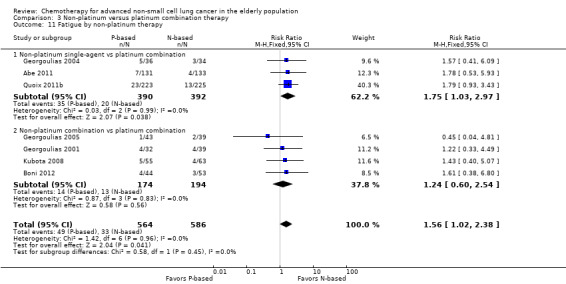
Comparison 3 Non‐platinum versus platinum combination therapy, Outcome 11 Fatigue by non‐platinum therapy.
3.12. Analysis.
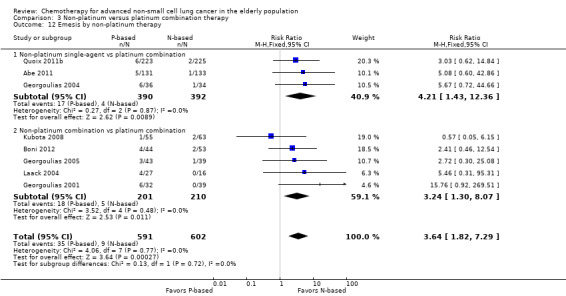
Comparison 3 Non‐platinum versus platinum combination therapy, Outcome 12 Emesis by non‐platinum therapy.
3.13. Analysis.
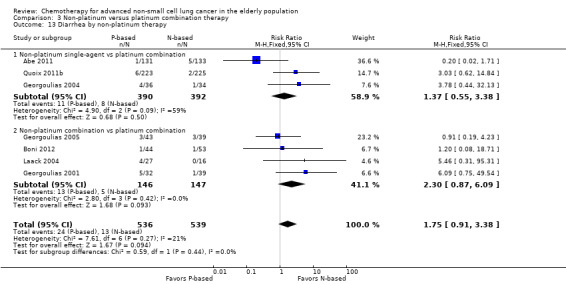
Comparison 3 Non‐platinum versus platinum combination therapy, Outcome 13 Diarrhea by non‐platinum therapy.
3.14. Analysis.
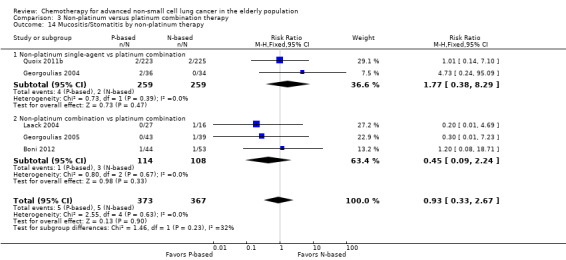
Comparison 3 Non‐platinum versus platinum combination therapy, Outcome 14 Mucositis/Stomatitis by non‐platinum therapy.
3.15. Analysis.
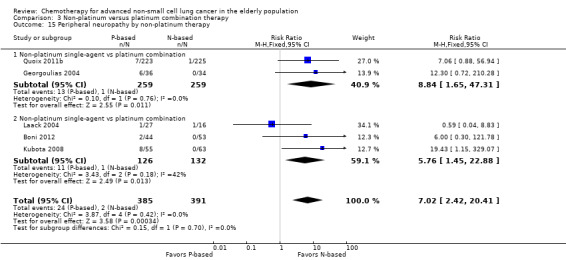
Comparison 3 Non‐platinum versus platinum combination therapy, Outcome 15 Peripheral neuropathy by non‐platinum therapy.
Toxicity by platinum agent
We found no statistically significant differences in risk of fatigue, emesis, or peripheral neuropathy according to subgroups by platinum agent (Analysis 6.9; Analysis 6.10; Analysis 6.11).
6.9. Analysis.
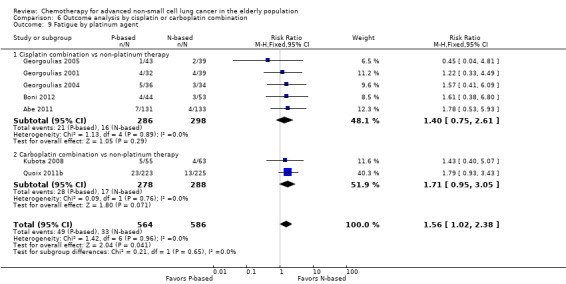
Comparison 6 Outcome analysis by cisplatin or carboplatin combination, Outcome 9 Fatigue by platinum agent.
6.10. Analysis.
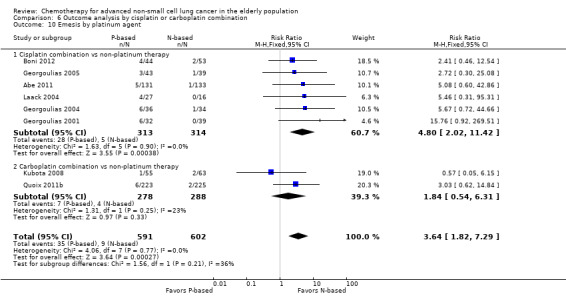
Comparison 6 Outcome analysis by cisplatin or carboplatin combination, Outcome 10 Emesis by platinum agent.
6.11. Analysis.
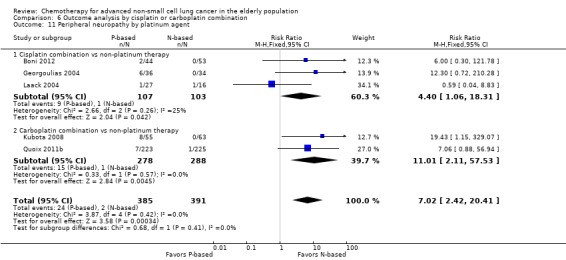
Comparison 6 Outcome analysis by cisplatin or carboplatin combination, Outcome 11 Peripheral neuropathy by platinum agent.
Toxicity by type of trial
We found no differences in the incidence of grade 3 or 4 non‐hematological adverse events for fatigue, emesis, or diarrhea according to subgroups by type of trial (Analysis 5.9; Analysis 5.10; Analysis 5.11).
5.9. Analysis.
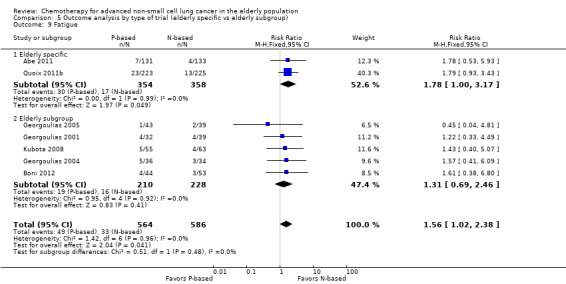
Comparison 5 Outcome analysis by type of trial (elderly specific vs elderly subgroup), Outcome 9 Fatigue.
5.10. Analysis.
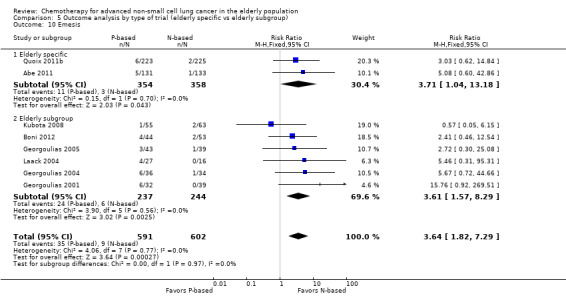
Comparison 5 Outcome analysis by type of trial (elderly specific vs elderly subgroup), Outcome 10 Emesis.
5.11. Analysis.
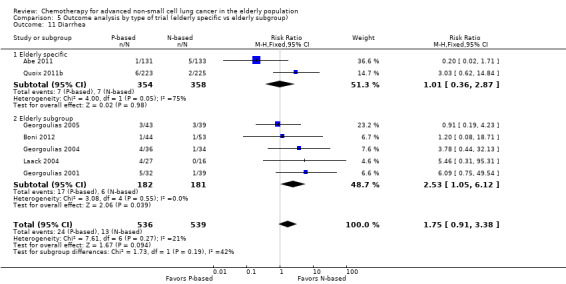
Comparison 5 Outcome analysis by type of trial (elderly specific vs elderly subgroup), Outcome 11 Diarrhea.
Sensitivity analysis
We did not perform a sensitivity analysis by excluding unpublished data, as only Quoix 2011b and Chen 2008 were reported in full‐article form.
A sensitivity analysis performed by excluding prematurely interrupted trials (Abe 2011) showed no impact on risk of fatigue (RR 1.53, 95% CI 0.97 to 2.40) and emesis (RR 3.48, 95% CI 1.67 to 7.26), and a statistically significantly higher risk in the incidence of diarrhea (RR 2.65, 95% CI 1.22 to 5.72).
Discussion
Summary of main results
Despite the fact that more than 50% of patients newly diagnosed with NSCLC are older than 70 years of age, this specific subgroup is still underrepresented in randomized controlled trials (RCTs) that evaluate the role of cytotoxic chemotherapy. Our systematic review supports this fact, finding that 14 RCTs were not included because of absence of elderly participants, and among 51 included trials with 13,103 participants, the elderly represented only 29.3% (n = 3839) of the entire population. Only 10 RCTs were specifically designed for this subgroup, enrolling 2006 elderly participants.
The addition of platinum agent resulted in improvement in overall survival (OS) (hazard ratio (HR) 0.76, 95% confidence interval (CI) 0.69 to 0.85), one‐year overall survival (1yOS) (risk ratio (RR) 0.89, 95% CI 0.82 to 0.96), progression‐free survival (PFS) (HR 0.76, 95% CI 0.61 to 0.93), and overall response rate (ORR) (RR 1.57, 95% CI 1.32 to 1.85) compared with non‐platinum therapy. However, this advantage is associated with greater risk of grade 3 and 4 adverse events compared with non‐platinum therapy, mainly in relation to anemia (RR 2.53, 95% CI 1.70 to 3.76), thrombocytopenia (RR 3.59, 95% CI 2.22 to 5.82), emesis (RR 3.48, 95% CI 1.67 to 7.26), diarrhea (RR 2.65, 95% CI 1.22 to 5.72), and peripheral neuropathy (RR 7.02, 95% CI 2.42 to 20.41). An exploratory subgroup analysis suggests that carboplatin combination should be preferred over cisplatin combination. However, this finding should be interpreted with caution, as it followed a post hoc analysis and was not based on direct comparison.
We found no significant differences in OS (HR 0.92, 95% CI 0.72 to 1.17), 1yOS (RR 0.88, 95% CI 0.73 to 1.07), and PFS (HR 0.94, 95% CI 0.83 to 1.07) between non‐platinum doublets and single‐agent treatments. The non‐platinum combination, however, resulted in an advantage in response rate over single‐agent treatment (RR 1.79, 95% CI 1.41 to 2.26). We found no significant differences in incidence of grade 3 or higher hematological and non‐hematological adverse events. Nevertheless, few events were observed with both treatment regimens.
Despite great clinical relevance, data on patient‐related outcomes such as health‐related quality of life (HRQoL) are scarce in the literature. Few RCTs have adequately incorporated QoL assessment in their trial design, precluding definitive conclusion. For comparison of non‐platinum combination versus non‐platinum single‐agent treatment, data from the largest randomized trial showed no difference in QoL between treatment arms (Gridelli 2003). On the other hand, significant improvement in favor of non‐platinum combination was noted in a smaller, prematurely interrupted randomized trial (Frasci 2001). For comparison of platinum combination versus non‐platinum therapy, only three RCTs provided information on QoL assessment for the elderly; nonetheless, only limited data from the largest trials were presented in full‐text articles (Quoix 2011b). Two remaining trials included only a small number of participants (Laack 2004; Lou 2010).
Overall completeness and applicability of evidence
Elderly patients are a very heterogeneous population in terms of incidence of co‐morbidities, polypharmacy, functional disabilities, and geriatric syndromes such as frailty (Balducci 2010). Strict eligibility criteria applied to clinical trials may, unintentionally, select healthier patients than those treated in the community. Moreover, conventional history and physical examination may fail to detect alterations commonly found in the elderly that might interfere with efficacy and tolerance to different chemotherapy regimens (Wilders 2014). Therefore, our results suggesting the advantage of platinum combination treatment should be interpreted with clinical judgement, as for many elderly patients, poor tolerance to platinum therapy may outweigh the benefits highlighted in this meta‐analysis. Applicability of our results to the growing number of elderly over 80 years of age should be viewed with caution. This group of elderly is underrepresented in clinical trials, and very little information is available on the safety and impact of different treatments for survival.
Combined analysis of different chemotherapy regimens regarding type of cytotoxic agent, dosage, and scheduling does not allow identification of the most appropriate platinum doublet. An exploratory analysis based on type of platinum used suggested better efficacy in the elderly for the carboplatin combination. However, this exploratory analysis must be interpreted with caution, as it was not based on direct comparison of these treatment regimens. For participants who were not candidates for platinum combination, the combination of non‐platinum agents resulted in higher objective response rates with no survival benefit. However, our systematic review could not identify the non‐platinum single agent or combination of choice. Most trials used gemcitabine, vinorelbine, or docetaxel as monotherapy or in combination.
We restricted our systematic review to trials comparing different cytotoxic regimens based on use of platinum or non‐platinum agents as monotherapy or in combination. We excluded trials that used antiangiogenic agents or tyrosine kinase inhibitors. However, patient selection based on predictive molecular biomarkers such as epidermal growth factor receptor (EGFR)‐activating mutations or anaplastic lymphoma kinase (ALK) translocations has changed the traditional approach. For these patients, many RCTs have shown improvement in PFS and ORR, along with a better toxicity profile for EGFR and ALK inhibitors (Lee 2013; Mok 2014). For elderly patients, a similar efficacy and safety profile was suggested in single‐arm phase II trials (Asami 2011; Inoue 2009; Maemondo 2012). Therefore, even outside the scope of this systematic review, we suggest that treatment decisions regarding systemic treatment of elderly patients with advanced NSCLC should be evaluated in the context of these biomarkers. Therefore, we consider the results of these reviews to be most applicable to patients with no activating mutation.
Quality of the evidence
For the non‐platinum single‐agent versus non‐platinum combination comparison, efficacy endpoints (OS, 1yOS, ORR) were considered of low quality. Only one randomized phase III trial completed accrual and was presented in a full‐text article (Gridelli 2003). We considered inclusion of prematurely interrupted RCTs as the result of poor accrual to introduce potential risk of bias (Frasci 2001; Karampeazis 2010). One trial presented unpublished post hoc analyses obtained through direct contact with study authors (Georgoulias 2008). We included two trials in the review but not in the meta‐analysis because data on the elderly subgroup were lacking (Comella 2004; Hainsworth 2007). Investigators allowed inclusion of patients considered elderly or poor candidates for platinum therapy. In both trials, the elderly represented the majority of the participant population. Blinding of assessors was uncommon in the trial designs and was considered to introduce potential risk of bias for PFS, ORR, and toxicity. Toxicity data were classified mostly as of low or very low quality. The small number of events, the wide confidence intervals, differences in reporting, and heterogeneity were responsible for downgrading of the quality of evidence.
Although results suggest convincing benefit for platinum combination over non‐platinum therapy, the quality of evidence for the efficacy endpoints (OS, 1yOS, and ORR) was considered moderate. Only one phase III randomized trial completed accrual and was reported in a full‐text article (Quoix 2011b). Nine trials included for this comparison in the meta‐analysis provided results from the elderly subgroup analysis (Boni 2012; Flotten 2012; Georgoulias 2001; Georgoulias 2005; Georgoulias 2008; Kubota 2008; Laack 2004; Lilenbaum 2005; Zwitter 2010). Six were unpublished post hoc analyses with data obtained through direct contact with study authors, representing 21% (n = 485) of the population included in the platinum combination versus non‐platinum therapy comparison (Boni 2012; Georgoulias 2001; Georgoulias 2005; Georgoulias 2008; Kubota 2008; Laack 2004). Moreover, two unpublished trials were prematurely interrupted after interim analysis ‐ one showing low probability of superiority of the platinum agent (Abe 2011) and the other because it presented a clear advantage of platinum combination treatment in patients between 70 and 75 years old (Tsukada 2007). Other issues affecting the quality of the evidence are related to methodological reasons, as blinding of assessors was uncommon in such trials. The low quality associated with some grade 3 or higher adverse events was also associated with the small number of events observed in both arms, the wide confidence intervals, and differences in reporting between trials.
Potential biases in the review process
To our knowledge, this study represents the largest systematic review and meta‐analysis of chemotherapy for advanced non‐small cell lung cancer in the elderly population. However, we recognize several limitations in our analysis.
Of 51 RCTs fulfilling inclusion criteria for the systematic review, 29 did not provide enough information on the elderly subgroup to allow inclusion of any outcome in the meta‐analysis. These results were treated as missing data. All missing trials consisted of unplanned elderly subgroup analyses of data from trials that evaluated the addition of a platinum agent; for most of them, not even the number of participants over 70 years of age was available.
We have arbitrarily defined elderly as patients over 70 years of age, as data suggest a higher incidence of physiological changes and geriatric syndrome (Balducci 2010). However, we acknowledge the controversy related to the threshold adopted. As expected, other study authors used different definitions for the inclusion criteria in trials designed for the elderly. In the platinum combination versus non‐platinum therapy comparison, Zhang 2006 defined elderly as patients over 65 years of age. We decided to include this trial in the meta‐analysis and to perform an exploratory sensitivity analysis while excluding this trial. All other elderly subgroup analyses, however, were carried out using a threshold of 70 years. For the non‐platinum single‐agent versus non‐platinum combination comparison, two trials included patients older than 65 years or those not considered candidates for platinum combination treatment (Comella 2004; Hainsworth 2007). Neither trial presented a separate analysis on the elderly subgroup. However, older patients represented the majority of the patient population. We included these results in the meta‐analysis and discussed these results separately.
As emphasized previously, a high incidence of co‐morbidities and geriatric syndromes in the elderly may interfere with tolerance and efficacy of cytotoxic agents. Unintentionally, this common group of elderly patients may be excluded from randomized trials, compromising the validation of these results for elderly individuals typically treated in the community. Unfortunately, individual participant characteristics such as age, co‐morbidities, and scores on geriatric scales that could predict poor tolerance to platinum therapy could not be assessed in our study.
Selection of endpoints varies according to study authors. In our systematic review, we allowed inclusion in the meta‐analysis for progression‐free survival trials using time‐to‐progression (TTP) or PFS. TTP was used in three RCTs and was defined as time from randomization to first evidence of progression (Georgoulias 2001; Georgoulias 2004; Georgoulias 2005). PFS was defined as time from randomization to first evidence of progression or death and was adopted in the six remaining RCTs. An exploratory sensitivity analysis was performed to evaluate the robustness of results while excluding trials that used TTP.
Agreements and disagreements with other studies or reviews
Despite the growing number of cancer diagnoses among the elderly, many publications have shown under representation of this subgroup in clinical trials. Hutchins 1999 analyzed the data on 1627 participants enrolled in clinical trials for lung cancer conducted by the Southwestern Oncology Group (SWOG). The elderly represented 39% of the entire participant population, as opposed to 66% found in the community, based on data from the 1990 US Census and the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program. Similar results were reported by Talarico 2004 and the Hellenic Oncology Research Group. Pallis 2011 performed a pooled analysis of five RCTs conducted by this co‐operative group, finding only 23% (n = 424) of the participant population above 70 years of age. In our review, we found that among 6221 adult participants enrolled in 16 RCTs designed for adult patients, the elderly represented 29.4% of the entire study population. Moreover, 26 of the trials included in our systematic review did not provide information about the number of elderly participants included.
This finding raises concerns about the applicability in clinical practice of results from RCTs designed for adults in the elderly subgroup. Many systematic reviews and meta‐analyses have evaluated the role of platinum combination for advanced NSCLC in the adult population. Rajeswaran 2008 performed a meta‐analysis including RCTs that compared platinum versus non‐platinum doublets. Of 18 included trials, we identified 17 through our search strategy. However, only two trials provided sufficient data on the elderly to allow their inclusion in our meta‐analysis (Georgoulias 2001; Georgoulias 2005). The remaining 15 RCTs, despite fulfilling the inclusion criteria for our systematic review, did not provide adequate data on the elderly subgroup. Similarly to our findings in the elderly, however, study authors showed slight improvement in 1yOS (RR 1.08, 95% CI 1.01 to 1.16; P value = 0.03) and ORR (RR 1.11, 95% CI 1.02 to 1.21; P value = 0.02) and higher risk of anemia, emesis, and neurotoxicity. Study authors also performed a subgroup analysis based on type of platinum, and in contrast to our results, they observed benefit in favor of cisplatin but not carboplatin combinations versus non‐platinum therapy. Age differences between participants included in the two meta‐analyses might explain the different results, as carboplatin combinations might be tolerated better than cisplatin combinations by the elderly. D'Addario 2005 also performed a systematic review and meta‐analysis of published RCTs for advanced NSCLC in the adult population. For this analysis, review authors allowed inclusion of RCTs that compared platinum doublets or triplets versus non‐platinum agents of second or third generation, given as single‐agent treatment or in combination. Review authors included 37 RCTs with 7633 participants that showed improvement in 1yOS and ORR in favor of the platinum combination, with odds ratios of 1.21 (95% CI 1.09 to 1.35) and 1.62 (95% CI 1.48 to 1.8), respectively. Greater risks of anemia (Peto odds ratio (OR) 1.54, 95% CI 1.08 to 2.2), neutropenia (Peto OR 1.23, 95% CI 1.01 to 1.49), thrombocytopenia (Peto OR 3.1, 95% CI 2.29 to 4.19), and emesis (Peto OR 1.92, 95% CI 1.35 to 2.73) were also associated with platinum combinations. The systematic review and meta‐analysis conducted by Hotta 2004 also showed advantages in OS and ORR in favor of the platinum combination (HR 0.87, 95% CI 0.80 to 0.94; OR 2.32, 95% CI 1.68 to 3.20, respectively). Review authors included eight RCTs comparing platinum doublets versus non‐platinum single agents. Despite differences in participant age between our systematic review and those previously mentioned, our results for OS, 1yOS, and ORR for platinum combination in the elderly are comparable with those found in a meta‐analysis for the adult population. This finding is also supported by the work of Pallis 2011 and Langer 2002, who found similar benefit for survival, response rate, and time‐to‐progression for younger and older participants. However, we recognize limitations in external validation of these results for the elderly in clinical practice, as patients enrolled in clinical trials are likely to be healthier than those typically treated in the community.
Qi 2012 performed a systematic review and meta‐analysis to evaluate the role of different cytotoxic regimens for advanced NSCLC in the elderly, defined as individuals older than 65 years of age. Investigators allowed the inclusion only of RCTs that compared doublet cytotoxic agents versus single‐agent third‐generation cytotoxic agents. In nine included RCTs, investigators found no significant differences in OS between doublets and single‐agent therapy (HR 0.84, 95% CI 0.71 to 1.00) with significant heterogeneity (I2 = 76.6%). Subgroup analysis based on platinum doublets versus non‐platinum single agents found no statistically significant differences in OS between treatment strategies (HR 0.68, 95% CI 0.41 to 1.14). Our systematic review involved a larger number of trials, allowing inclusion of RCTs that compared not only platinum combination versus single‐agent treatment but also non‐platinum doublets and triplets. Our results showed a statistically significant advantage for OS of platinum combination over non‐platinum therapy (HR 0.76, 95% CI 0.69 to 0.85). Similar to our findings, Qi 2012 showed improvement in favor of platinum combination for 1ysOS (RR 1.40, 95% CI 1.09 to 1.81, two studies) and ORR (RR 1.64, 95% CI 1.38 to 1.96; four studies). Toxicity data showed greater risks of anemia, neutropenia, thrombocytopenia, and neurotoxicity associated with doublet chemotherapy; however, investigators did not perform a subgroup analysis by platinum combination.
Authors' conclusions
Implications for practice.
Our assessment of treatment effect supports the use of platinum combination for fit elderly patients with advanced NSCLC, with advantages for survival (number needed to treat for an additional beneficial outcome (NNTB) for 1yOS 12.6, 95% CI 7.8 to 34.5) and response rate (NNTB for ORR 8.0, 95% CI 5.0 to 14.3). Nonetheless, such treatment is also associated with greater risk of grade 3 or 4 hematological (number needed to treat for an additional harmful outcome (NNTH) for anemia 15.6, 95% CI 8.7 to 34.5; NNTH for thrombocytopenia 13.7, 95% CI 7.4 to 28.6) and non‐hematological adverse events (NNTH for peripheral neuropathy 32.3, 95% CI 10.1 to 142.9). Exploratory analysis also suggests that carboplatin combinations should be preferred over cisplatin combinations; however, this finding should be interpreted with caution, as it was not based on a direct comparison between cisplatin and carboplatin combinations. For patients who are not candidates for platinum treatment (unfit), our findings suggest an increase in response rate in favor of non‐platinum doublets, with similar efficacy for survival. Unfortunately, we also found scarce evidence on the impact of different treatment regimens on quality of life, challenging the process of decision making.
Implications for research.
For many decades, elderly patients were treated with non‐platinum single‐agent regimens based on improvement in quality of life and survival, with manageable toxicity (Gridelli 2001). Research seeking more efficacious and tolerable treatment regimens failed to show significant improvement with non‐platinum combination over single‐agent treatment. Meanwhile, few elderly patients were included in trials with platinum combinations. Therefore, the role of such regimens in this patient population remains controversial.
Although our results suggest a significant advantage of platinum combination, co‐morbidities, low performance status, and geriatric syndromes commonly noted in elderly patients may compromise external validation of the results found of our meta‐analysis. Even in patients fulfilling eligibility criteria, comprehensive geriatric assessment (CGA) may further identify subtle alterations and geriatric syndromes not captured through standard history and physical examination (Wilders 2014). Therefore, adequate tools that identify elderly patients at higher risk for major toxicity associated with platinum combination are needed and require validation in randomized trials (Extermann 2012; Hurria 2011). Trials specifically designed for the elderly are feasible and permit the use of geriatric scales as potential predictive tools of treatment‐related adverse events. However, formal geriatric assessment has seldom been incorporated in clinical trials, and future studies should consider incorporating such tools. We believe that traditional eligibility criteria based on chronological age, performance status, and co‐morbidities might be insufficient to capture the complexities of elderly patient care. Thus, we strongly encourage future clinical trials dedicated to the elderly in which new instruments of prediction and patient selection can be developed and the impact of different treatment strategies for this group of patients can be evaluated.
For fit elderly patients, our data suggest that carboplatin combination should be preferred over cisplatin combination as standard treatment. However, the reasons for this difference are unclear, and we acknowledge the weakness of such findings. de Castria 2013 performed a Cochrane systematic review to compare cisplatin versus carboplatin associated with a third‐generation drug for advanced non‐small cell lung cancer. Results showed no significant differences in survival and response rate between the two regimens but higher risk of emesis with cisplatin regimens and higher risks of neurotoxicity and thrombocytopenia with carboplatin regimens. This review involved the general adult population, and review authors performed no subgroup analyses based on age. Whether such findings can be extrapolated to the elderly population is unclear. On the other hand, some study authors suggest that less toxicity is associated with carboplatin than with cisplatin in the elderly, and this could explain our findings (Ezer 2014). Today, no RCT directly addresses this question for the elderly population, precluding definitive conclusions. Thus, we encourage enrolment of fit elderly patients in clinical trials of platinum regimens versus single‐agent treatment (Gridelli 2014; NCT01593293; NCT01405586; NCT01656551). We suggest that investigational approaches for this patient population should be similar to those used for the general adult population. For those not considered candidates for platinum regimens, non‐platinum single‐agent treatment might be considered as standard.
Although survival improvement is the primary endpoint for many trials, it might not reflect the real need for this patient population. For older patients, the impact of treatment on active life expectancy is as important and informative as extension of life itself. In this review, we found little evidence to show how quality of life was affected by different treatments. We encourage incorporation of patient‐related outcomes and functional assessments in randomized trials of intervention.
Notes
The current review is not compliant with Cocharne Commercial Sponsorship policy. It will be updated within 6 months. The update will have a majority of authors and lead author free of conflicts.
Acknowledgements
Review authors would like to thank Cesare Gridelli, Fergus Macbeth, Kwun Fong, Elisabeth Quoix, and Virginie Westeel for their comments on the review; Corynne Marchal (Managing Editor of the Cochrane Lung Cancer Review Group, France) for editorial support; Lars Lidgard for feedback provided as a Consumer of the Lung Cancer Group; and the Brazilan Cochrane Centre.
We also would like to thank the following study authors, who responded to our request for additional data: Vassilis Georgoulias, Athanasios Karampeazis, Kaoru Kubota, Eckart Laack, and Luca Boni.
Appendices
Appendix 1. Search strategy for CENTRAL (via Ovid)
1. exp Lung Neoplasms/
2. exp Carcinoma, Non‐Small‐Cell Lung/
3. nsclc.tw.
4. (lung$ or pulmonary or bronchus or bronchogenic or bronchial or bronchoalveolar or alveolar).tw.
5. ("non small cell" or "non‐oat cell").tw.
6. (cancer or carcinoma$ or neoplasm$ or malignan$ or tumo?r).tw.
7. 4 and 5 and 6
8. 1 or 2 or 3 or 7
9. exp Platinum Compounds/
10. $platinum.tw.
11. exp Cisplatin/
12. cisplatin.tw.
13. platinol.tw.
14. carboplatin.tw.
15. oxaliplatin.tw.
16. (vinorelbine or vimblastine or vindesine).tw.
17. paclitaxel.tw.
18. docetaxel.tw.
19. ifosfamide.tw.
20. mitomycin.tw.
21. etoposide.tw.
22. gemcitabine.tw.
23. uracil‐tegafur.tw.
24. pemetrexed.tw.
25. capecitabine.tw.
26. ironotecan.tw.
27. Topotecan.tw.
28. paraplatin.tw.
29. eloxatin$.tw.
30. chemotherap$.tw.
31. or/9‐30
32. exp palliative care/
33. exp terminal care/
34. exp quality of life/
35. (pleural enffusion$ or pericardial effusion$).mp.
36. (palliat$ adj3 (intent$ or manag$ or symtom$)).mp.
37. (stage IIIb or stage IIIb‐IV or stage IV or stage III‐IV).mp.
38. or/32‐37
39. 8 and 31 and 38
Appendix 2. Search strategy for MEDLINE (via Ovid)
1. randomi?ed controlled trial.pt.
2. controlled clinical trial.pt.
3. (randomised or randomised).ab,ti.
4. placebo.ab,ti.
5. drug therapy.fs.
6. randomly.ab,ti.
7. trial.ab,ti.
8. groups.ab,ti.
9. or/1‐8
10. (animals not (humans and animals)).sh.
11. 9 not 10
12. exp Lung Neoplasms/
13. exp Carcinoma, Non‐Small‐Cell Lung/
14. nsclc.tw.
15. (lung$ or pulmonary or bronchus or bronchogenic or bronchial or bronchoalveolar or alveolar).tw.
16. ("non small cell" or "non‐oat cell").tw.
17. (cancer or carcinoma$ or neoplasm$ or malignan$ or tumo?r).tw.
18. 15 and 16 and 17
19. 12 or 13 or 14 or 18
20. exp Platinum Compounds/
21. $platinum.tw.
22. exp Cisplatin/
23. cisplatin.tw.
24. platinol.tw.
25. carboplatin.tw.
26. oxaliplatin.tw.
27. (vinorelbine or vimblastine or vindesine).tw.
28. paclitaxel.tw.
29. docetaxel.tw.
30. ifosfamide.tw.
31. mitomycin.tw.
32. etoposide.tw.
33. gemcitabine.tw.
34. uracil‐tegafur.tw.
35. pemetrexed.tw.
36. capecitabine.tw.
37. irinotecan.tw.
38. topotecan.tw.
39. paraplatin.tw.
40. eloxatin$.tw.
41. chemotherap$.tw.
42. or/20‐41
43. exp palliative care/
44. exp terminal care/
45. exp quality of life/
46. (pleural enffusion$ or pericardial effusion$).mp.
47. (palliat$ adj3 (intent$ or manag$ or symtom$).mp.
48. (stage IIIb or stage IIIb‐IV or stage IV or stage III‐IV).mp.
49. or/43‐48
50. 19 and 42 and 49
51. 11 and 50
Appendix 3. Search strategy for EMBASE (via Elsevier)
#1 random*:ab,ti OR placebo*:ab,ti
#2 single*:ab,ti OR double*:ab,ti OR triple*:ab,ti OR treble*:ab,ti AND (blind*:ab,ti OR mask*:ab,ti)
#3 controlled AND clinical AND trial*:ab,ti
#4 'retracted article':de
#5 #1 OR #2 OR #3 OR #4
#6 animal*:de NOT human*:de
#7 #5 NOT #6
#8 'lung tumor'/exp
#9 'lung non small cell cancer'/exp
#10 nsclc:ab,ti
#11 lung*:ab,ti OR pulmonary:ab,ti OR bronchus:ab,ti OR bronchogenic:ab,ti OR bronchial:ab,ti OR bronchoalveolar:ab,ti OR alveolar:ab,ti
#12 'non small cell':ab,ti OR 'non‐oat cell':ab,ti
#13 cancer:ab,ti OR carcinoma*:ab,ti OR neoplasm*:ab,ti OR malignan*:ab,ti OR tumo?r:ab,ti
#14 #11 AND #12 AND #13
#15 #8 OR #9 OR #10 OR #14
#16 'platinum derivative'/exp
#17 platinum:ab,ti
#18 'cisplatin'/exp
#19 cisplatin:ab,ti
#20 platinol:ab,ti
#21 carboplatin:ab,ti
#22 oxaliplatin:ab,ti
#23 vinorelbine:ab,ti OR vimblastine:ab,ti OR vindesine:ab,ti
#24 paclitaxel:ab,ti
#25 docetaxel:ab,ti
#26 ifosfamide:ab,ti
#27 mitomycin:ab,ti
#28 etoposide:ab,ti
#29 gemcitabine:ab,ti
#30 'uracil tegafur':ab,ti
#31 pemetrexed:ab,ti
#32 capecitabine:ab,ti
#33 irinotecan:ab,ti
#34 topotecan:ab,ti
#35 paraplatin:ab,ti
#36 eloxatin*:ab,ti
#37 chemotherap*:ab,ti
#38 #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37
#39 'palliative therapy'/exp
#40 'terminal care'/exp
#41 'quality of life'/exp
#42 'pleural enffusion':ab,ti OR 'pleural enffusions':ab,ti OR 'pericardial effusion':ab,ti
#43 palliat* NEAR/3 (intent* OR manag* OR symtom*)
#44 'stage iiib' OR 'stage iiib‐iv' OR 'stage iv' OR 'stage iii‐iv'
#45 #39 OR #40 OR #41 OR #42 OR #43 OR #44
#46 #15 AND #38 AND #45
#47 #7 AND #46
#48 [medline]/lim
#49 [embase]/lim
#50 #49 NOT #48
#51 #47 AND #50
Appendix 4. Search strategy for LILACS
((TW:Lung Neoplasms) OR (TW:Neoplasias Pulmonares) OR (TW:Neoplasias Pulmonares) OR (MH: C04.588.894.797.520) OR (MH:C08.381.540) OR (MH:C08.785.520) OR (TW:Carcinoma, Non‐Small‐Cell Lung) OR (TW: Carcinoma de Pulmón de Células no Pequeñas) OR (TW:Carcinoma Pulmonar de Células não Pequenas) OR (MH:C04.588.894.797.520.109.220.249) OR (MH:C08.381.540.140.500) OR (MH:C08.785.520.100.220.500) OR (((TW:nsclc) OR (TW:lung$) OR (TW:pulmonary) OR (TW:bronchus) OR (TW:bronchogenic) OR (TW:bronchial) OR (TW:bronchoalveolar) OR (TW:alveolar)) AND ((TW: non small cell)) AND ((TW: cancer) OR (TW: carcinoma$) OR (TW: neoplasm$) OR (TW: malignan$) OR (TW: tumo?r)))) AND ((TW:Platinum Compounds) OR (TW:Compuestos de Platino) OR (TW:Compostos de Platina) OR (MH:D01.710) OR (TW:platinum) OR (MH:D01.210.375) OR (MH:D01.625.125) OR (MH:D01.710.100) OR (TW:cisplatin) OR (TW:cisplatino) OR (TW:platinol) OR (TW:carboplatin) OR (TW:oxaliplatin) OR (TW:vinorelbine) OR (TW:vimblastine) OR (TW:vindesine) OR (TW:paclitaxel) OR (TW: docetaxel) OR (TW:ifosfamide) OR (TW:mitomycin) OR (TW:etoposide) OR (TW:gemcitabine) OR (TW:uracil‐tegafur) OR (TW:pemetrexed) OR (TW:capecitabine) OR (TW:ironotecan) OR (TW:Topotecan) OR (TW:paraplatin) OR (TW:eloxatin$) OR (TW:chemotherap$)) AND ((TW:Palliative Care) OR (TW:Atención Paliativa) OR (TW:Assistência Paliativa) OR (MH:E02.760.666) OR (MH:N02.421.585.666) OR (TW:Terminal Care) OR (TW:Cuidado Terminal) OR (TW:Assistência Terminal) OR (MH:E02.760.905) OR (MH:N02.421.585.905) OR (TW:pleural enffusion$) OR (TW:pericardial effusion$) OR (TW:palliative intent$) OR (TW:palliative manag$) OR (TW:palliative symtom$) OR (TW:stage IIIb) OR (TW:stage IIIb‐IV) OR (TW:stage IV) OR (TW:stage III‐IV))
Data and analyses
Comparison 1. Non‐platinum single‐agent versus non‐platinum combination.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival (OS) | 5 | Hazard Ratio (Random, 95% CI) | 0.92 [0.72, 1.17] | |
| 2 1‐Year survival rate (OS1y) | 4 | 993 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.73, 1.07] |
| 3 Progression‐free survival | 4 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.83, 1.07] | |
| 4 Objective response rate (ORR) | 5 | 1014 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.41, 2.26] |
| 5 Grade 3 and 4 hematological adverse events (AEs) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Anemia | 5 | 1064 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.57, 2.40] |
| 5.2 Neutropenia | 5 | 1064 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.93, 1.54] |
| 5.3 Febrile neutropenia | 4 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.20] |
| 5.4 Thrombocytopenia | 4 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.82, 3.04] |
| 6 Grade 3 and 4 non‐hematological adverse events (AEs) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Fatigue | 4 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.69, 1.96] |
| 6.2 Emesis | 4 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.68, 4.43] |
| 6.3 Diarrhea | 3 | 875 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.96 [0.36, 43.41] |
| 6.4 Constipation | 3 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.62] |
| 6.5 Mucositis/Stomatitis | 4 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.07, 6.30] |
Comparison 2. Non‐platinum single‐agent versus non‐platinum combination (with Comella 2004).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival (with Comella 2004) | 6 | Hazard Ratio (Random, 95% CI) | 0.88 [0.70, 1.11] | |
| 2 1‐Year survival rate | 5 | 1257 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.02] |
Comparison 3. Non‐platinum versus platinum combination therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 13 | 1705 | Hazard Ratio (Fixed, 95% CI) | 0.76 [0.69, 0.85] |
| 1.1 Non‐platinum single‐agent vs platinum combination | 7 | 1216 | Hazard Ratio (Fixed, 95% CI) | 0.72 [0.63, 0.82] |
| 1.2 Non‐platinum combination vs platinum combination | 6 | 489 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.71, 1.06] |
| 2 1‐Year survival rate | 13 | 1695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.82, 0.96] |
| 2.1 Non‐platinum single‐agent vs platinum combination | 7 | 1206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.78, 0.95] |
| 2.2 Non‐platinum combination vs platinum combination | 6 | 489 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.10] |
| 3 Progression‐free survival | 9 | 1273 | Hazard Ratio (Random, 95% CI) | 0.76 [0.61, 0.93] |
| 3.1 Non‐platinum single‐agent vs platinum combination | 4 | 858 | Hazard Ratio (Random, 95% CI) | 0.71 [0.50, 1.01] |
| 3.2 Non‐platinum combination vs platinum combination | 5 | 415 | Hazard Ratio (Random, 95% CI) | 0.82 [0.67, 1.01] |
| 4 Objective response rate (ORR) | 11 | 1432 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.32, 1.85] |
| 4.1 Non‐platinum single‐agent vs platinum combination | 6 | 1033 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.40, 2.12] |
| 4.2 Non‐platinum combination vs platinum combination | 5 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.96, 1.72] |
| 5 Grade 3 or higher hematological toxicity for platinum therapies | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Anemia | 11 | 1437 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.70, 3.76] |
| 5.2 Neutropenia | 11 | 1423 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.25] |
| 5.3 Febrile neutropenia | 8 | 1215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.74, 1.75] |
| 5.4 Thrombocytopenia | 9 | 1260 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.59 [2.22, 5.82] |
| 6 Grade 3 or higher non‐hematological toxicity for platinum therapies | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Fatigue | 7 | 1150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.02, 2.38] |
| 6.2 Emesis | 8 | 1193 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.64 [1.82, 7.29] |
| 6.3 Diarrhea | 7 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.91, 3.38] |
| 6.4 Constipation | 3 | 609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.25, 2.62] |
| 6.5 Mucositis/Stomatitis | 5 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.33, 2.67] |
| 6.6 Peripheral neuropathy | 5 | 776 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.02 [2.42, 20.41] |
| 7 Anemia | 11 | 1437 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.70, 3.76] |
| 7.1 Non‐platinum single‐agent vs platinum combination | 6 | 1026 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.68, 4.25] |
| 7.2 Non‐platinum combination vs platinum combination | 5 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.98, 4.71] |
| 8 Neutropenia by non‐platinum therapy | 11 | 1423 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.77, 2.85] |
| 8.1 Non‐platinum single‐agent vs platinum combination | 6 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.34, 6.08] |
| 8.2 Non‐platinum combination vs platinum combination | 5 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.85, 1.86] |
| 9 Febrile neutropenia by non‐platinum therapy | 8 | 1215 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.56, 4.50] |
| 9.1 Non‐platinum single‐agent vs platinum combination | 4 | 847 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.11, 6.90] |
| 9.2 Non‐platinum combination vs platinum combination | 4 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.82, 4.21] |
| 10 Thrombocytopenia by non‐platinum therapy | 9 | 1260 | Risk Ratio (M‐H, Random, 95% CI) | 3.22 [1.86, 5.56] |
| 10.1 Non‐platinum single‐agent vs platinum combination | 4 | 849 | Risk Ratio (M‐H, Random, 95% CI) | 3.45 [1.66, 7.18] |
| 10.2 Non‐platinum combination vs platinum combination | 5 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [0.88, 7.26] |
| 11 Fatigue by non‐platinum therapy | 7 | 1150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.02, 2.38] |
| 11.1 Non‐platinum single‐agent vs platinum combination | 3 | 782 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.03, 2.97] |
| 11.2 Non‐platinum combination vs platinum combination | 4 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.60, 2.54] |
| 12 Emesis by non‐platinum therapy | 8 | 1193 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.64 [1.82, 7.29] |
| 12.1 Non‐platinum single‐agent vs platinum combination | 3 | 782 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.21 [1.43, 12.36] |
| 12.2 Non‐platinum combination vs platinum combination | 5 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [1.30, 8.07] |
| 13 Diarrhea by non‐platinum therapy | 7 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.91, 3.38] |
| 13.1 Non‐platinum single‐agent vs platinum combination | 3 | 782 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.55, 3.38] |
| 13.2 Non‐platinum combination vs platinum combination | 4 | 293 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.87, 6.09] |
| 14 Mucositis/Stomatitis by non‐platinum therapy | 5 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.33, 2.67] |
| 14.1 Non‐platinum single‐agent vs platinum combination | 2 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.38, 8.29] |
| 14.2 Non‐platinum combination vs platinum combination | 3 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.09, 2.24] |
| 15 Peripheral neuropathy by non‐platinum therapy | 5 | 776 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.02 [2.42, 20.41] |
| 15.1 Non‐platinum single‐agent vs platinum combination | 2 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.84 [1.65, 47.31] |
| 15.2 Non‐platinum single‐agent vs platinum combination | 3 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.76 [1.45, 22.88] |
Comparison 4. Non‐platinum versus platinum combination therapy (with Zhang 2006).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 1‐Year survival rate (with Zhang 2006) | 14 | 1791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.82, 0.96] |
| 2 Objective response rate (with Zhang 2006) | 12 | 1528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.36, 1.88] |
Comparison 5. Outcome analysis by type of trial (elderly specific vs elderly subgroup).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 13 | Hazard Ratio (Fixed, 95% CI) | 0.76 [0.69, 0.85] | |
| 1.1 Elderly specific | 4 | Hazard Ratio (Fixed, 95% CI) | 0.74 [0.63, 0.86] | |
| 1.2 Elderly subgroup analysis | 9 | Hazard Ratio (Fixed, 95% CI) | 0.79 [0.68, 0.91] | |
| 2 1‐Year overall survival | 13 | 1695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.82, 0.96] |
| 2.1 Elderly specific | 5 | 981 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.75, 0.93] |
| 2.2 Elderly subgroup | 8 | 714 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.08] |
| 3 Progression‐free survival | 9 | Hazard Ratio (Random, 95% CI) | 0.76 [0.61, 0.93] | |
| 3.1 Elderly specific | 3 | Hazard Ratio (Random, 95% CI) | 0.67 [0.44, 1.02] | |
| 3.2 Elderly subgroup | 6 | Hazard Ratio (Random, 95% CI) | 0.83 [0.69, 1.00] | |
| 4 Objective response rate | 11 | 1432 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.32, 1.85] |
| 4.1 Elderly specific | 4 | 808 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.37, 2.26] |
| 4.2 Elderly subgroup | 7 | 624 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.12, 1.76] |
| 5 Anemia | 11 | 1437 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.70, 3.76] |
| 5.1 Elderly specific | 5 | 956 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [1.77, 4.57] |
| 5.2 Elderly subgroup | 6 | 481 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.89, 3.94] |
| 6 Neutropenia | 11 | 1422 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.77, 2.85] |
| 6.1 Elderly specific | 5 | 955 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.27, 7.50] |
| 6.2 Elderly subgroup | 6 | 467 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.88, 1.77] |
| 7 Thrombocytopenia | 9 | 1260 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.59 [2.22, 5.82] |
| 7.1 Elderly specific | 3 | 779 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.96 [1.86, 8.42] |
| 7.2 Elderly subgroup | 6 | 481 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.36 [1.80, 6.29] |
| 8 Febrile neutropenia | 8 | 1215 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.56, 4.50] |
| 8.1 Elderly specific | 3 | 777 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.02, 16.48] |
| 8.2 Elderly subgroup | 5 | 438 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.85, 3.70] |
| 9 Fatigue | 7 | 1150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.02, 2.38] |
| 9.1 Elderly specific | 2 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.00, 3.17] |
| 9.2 Elderly subgroup | 5 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.69, 2.46] |
| 10 Emesis | 8 | 1193 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.64 [1.82, 7.29] |
| 10.1 Elderly specific | 2 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.71 [1.04, 13.18] |
| 10.2 Elderly subgroup | 6 | 481 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.61 [1.57, 8.29] |
| 11 Diarrhea | 7 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.91, 3.38] |
| 11.1 Elderly specific | 2 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.36, 2.87] |
| 11.2 Elderly subgroup | 5 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.05, 6.12] |
Comparison 6. Outcome analysis by cisplatin or carboplatin combination.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival by platinum agent | 13 | Hazard Ratio (Fixed, 95% CI) | 0.76 [0.69, 0.85] | |
| 1.1 Cisplatin combination vs non‐platinum treatment | 8 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.77, 1.08] | |
| 1.2 Carboplatin combination vs non‐platinum treatment | 5 | Hazard Ratio (Fixed, 95% CI) | 0.67 [0.59, 0.78] | |
| 2 1‐Year OS rate by platinum agent | 13 | 1695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.82, 0.96] |
| 2.1 Cisplatin combination vs non‐platinum therapy | 8 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.83, 1.08] |
| 2.2 Carboplatin combination vs non‐platinum therapy | 5 | 866 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.76, 0.93] |
| 3 Progression‐free survival by platinum agent | 9 | Hazard Ratio (Random, 95% CI) | 0.76 [0.61, 0.93] | |
| 3.1 Cisplatin combination vs non‐platinum therapy | 7 | Hazard Ratio (Random, 95% CI) | 0.83 [0.71, 0.97] | |
| 3.2 Carboplatin combination vs non‐platinum therapy | 2 | Hazard Ratio (Random, 95% CI) | 0.66 [0.38, 1.15] | |
| 4 Objective response rate by platinum agent | 11 | 1432 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.32, 1.85] |
| 4.1 Cisplatin combination vs non‐platinum therapy | 7 | 673 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.15, 1.80] |
| 4.2 Carboplatin combination vs non‐platinum therapy | 4 | 759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.34, 2.23] |
| 5 Anemia by platinum agent | 11 | 1437 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.70, 3.76] |
| 5.1 Cisplatin combination vs non‐platinum therapy | 8 | 803 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.09 [2.22, 7.55] |
| 5.2 Carboplatin combination vs non‐platinum therapy | 3 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.90, 2.66] |
| 6 Neutropenia by platinum agent | 11 | 1413 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.76, 2.81] |
| 6.1 Cisplatin combination vs non‐platinum therapy | 8 | 779 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.50, 3.75] |
| 6.2 Carboplatin combination vs non‐platinum therapy | 3 | 634 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.62, 6.12] |
| 7 Febrile neutropenia by platinum agent | 8 | 1215 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.56, 4.50] |
| 7.1 Cisplatin combination vs non‐platinum therapy | 6 | 649 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.17, 7.67] |
| 7.2 Carboplatin combination vs non‐platinum therapy | 2 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [0.97, 5.46] |
| 8 Thrombocytopenia by platinum agent | 9 | 1260 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.59 [2.22, 5.82] |
| 8.1 Cisplatin combination vs non‐platinum therapy | 6 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.34 [2.12, 8.91] |
| 8.2 Carboplatin combination vs non‐platinum therapy | 3 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.02 [1.57, 5.79] |
| 9 Fatigue by platinum agent | 7 | 1150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.02, 2.38] |
| 9.1 Cisplatin combination vs non‐platinum therapy | 5 | 584 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.75, 2.61] |
| 9.2 Carboplatin combination vs non‐platinum therapy | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.95, 3.05] |
| 10 Emesis by platinum agent | 8 | 1193 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.64 [1.82, 7.29] |
| 10.1 Cisplatin combination vs non‐platinum therapy | 6 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.80 [2.02, 11.42] |
| 10.2 Carboplatin combination vs non‐platinum therapy | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.54, 6.31] |
| 11 Peripheral neuropathy by platinum agent | 5 | 776 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.02 [2.42, 20.41] |
| 11.1 Cisplatin combination vs non‐platinum therapy | 3 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.40 [1.06, 18.31] |
| 11.2 Carboplatin combination vs non‐platinum therapy | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.01 [2.11, 57.53] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abe 2011.
| Methods | Randomized phase III trial Eligible patients
Exlcusion criteria not presented |
|
| Participants | D arm: 137 elderly participants randomly assigned/108 assessable participants for interim analysis/median age at baseline: 76 (range 70 to 87) years DP arm: 139 elderly participants randomly assigned/113 assessable participants for interim analysis/median age at baseline: 76 (range 70 to 86) years Demographics for study population: < 75/≥ 75 years of age: 22%/78%; male/female: 70%/30%; PS 0/1: 35%/65%; stage III/IV or relapsed: 32%/68% |
|
| Interventions | D arm: docetaxel 60 mg/m2 i.v. infusion on day 1 every 3 weeks DP arm: docetaxel 20 mg/m2 i.v. infusion and cisplatin 25 mg/m2 i.v. infusion on days 1, 8, and 15 every 4 weeks |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Results presented in abstract form only. Trial prematurely stopped after first interim analysis showed that predictive probability that DP would be superior to D was 0.996% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by minimization method. Stratification factors: age and stage. No further information available |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding of outcome assessment for OS and 1yOS rate but considered to have low impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | Unclear risk | No information on blinding of outcome assessment for other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on incomplete outcome data analysis |
| Selective reporting (reporting bias) | High risk | Results presented in abstract form at ASCO meeting |
| Other bias | High risk | Study prematurely stopped after first interim analysis because of low chance to achieve primary endpoint |
Alberola 2003.
| Methods | Randomized multi‐center phase III trial Inclusion criteria
Exclusion criteria
|
|
| Participants | CV‐VI arm: 187 (ITT population) ‐ median age (range): 60 (33 to 76) years/number of elderly participants not reported CG arm: 182 (ITT population) ‐ median age (range): 59 (33 to 75) years/number of elderly participants not reported CVG arm: 188 (ITT population) ‐ median age (range): 59 (33 to 75) years/number of elderly participants not reported |
|
| Interventions | GV‐VI arm: gemcitabine 1000 mg/m2 and vinorelbine 30 mg/m2 on days 1 and 8 for 3 cycles, followed by vinorelbine 30 mg/m2 on days 1 and 8 plus ifosfamide 3 g/m2 on day 1, for 3 cycles CG arm: cisplatin 100 mg/m2 on day 1 plus gemcitabine 1250 mg/m2 on days 1 and 8, every 21 days CGV arm: cisplatin 100 mg/m2 on day 1 plus gemcitabine 1000 mg/m2 and vinorelbine 25 mg/m2 on days 1 and 8, every 21 days |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | No information on number of elderly participants nor on specific subgroup analysis performed despite multiple attempts to contact study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation process was performed centrally by random permutated blocks within strata methods. Patients were stratified according to disease stage (IIIB v IV), baseline PS (0 to 1 v 2), and centre" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding of outcome assessment |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "From September 1998 to July 2000, 570 patients were enrolled in the study. Thirteen patients were ineligible because of unconfirmed histology (three patients), inadequate stage (four patients), prior chemotherapy (two patients), previous diagnosis of cancer (two patients), withdrawal of consent (one patient), and concurrent acute complication before chemotherapy (one patient)" |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | No separate elderly subgroup analysis |
Berghmans 2013.
| Methods | Randomized multi‐center phase III trial Eligible patients
Exclusion criteria
|
|
| Participants | GIP arm: 231 (ITT population) ‐ median age (range): 58 (29 to 78) years/number of elderly participants not reported DP arm: 233 (ITT population) ‐ median age (range): 58 (28 to 81) years/number of elderly participants not reported IG arm: 229 (ITT population) ‐ median age (range): 59 (30 to 84) years/number of elderly participants not reported |
|
| Interventions | GIP arm: gemcitabine 1000 mg/m2 on days 1 and 8 + ifosfamide 3000 mg/m2 on day 1 + cisplatin 50 mg/m2 on day 1 DP arm: docetaxel 75 mg/m2 + cisplatin 50 mg/m2 on day 1 IG arm: ifosfamide 3000 mg/m2 + gemcitabine 1000 mg/m2 on days 1 and 8 |
|
| Outcomes | Primary endpoint
Secondary endpoints
|
|
| Notes | No information on number of elderly participants included nor on specific subgroup analysis performed, despite multiple attempts to contact study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Central randomisation using a minimization algorithm was performed by calling the ELCWP central office" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment analysis |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Study not blinded |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Study not blinded. "Each patient record will be evaluated for evaluation and response in regular meetings of the Group. Patient's original record and radiological documents have to be available at this time" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "707 patients were randomised, out of whom 14 were ineligible" |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | No separate elderly subgroup analysis |
Boni 2012.
| Methods | Randomized multi‐center phase III trial Eligible patients
Exclusion criteria
|
|
| Participants | GP arm: 106 (ITT population)/20 elderly participants GN arm: 106 (ITT population)/29 elderly participants GIP arm: 110 (ITT population)/25 elderly participants GIN arm: 111 (ITT population)/27 elderly participants Median age:72.63 years (range 70 to 79) for entire cohort, 85.15% male, 51.49% PS 0, 42.57% PS 1, 47.52% adenocarcinoma, 26.73% squamous cell carcinoma, 25.74% other histologies |
|
| Interventions | GP arm: gemcitabine 1250 mg/m2 on days 1 and 8 plus cisplatin 80 mg/m2 on day 1, every 3 weeks GIP arm: gemcitabine 1000 mg/m2 on days 1 and 8 plus ifosfamide 2 g/m2 with mesna 1200 mg as bolus i.v. infusion before ifosfamide and after 4 hours and 8 hours plus cisplatin 80 mg/m2 on day 1, every 3 weeks GN arm: gemcitabine 1250 mg/m2 on days 1 and 8 plus vinorelbine 25 mg/m2 on days 1 and 8, every 8 weeks GIN arm: gemcitabine 1000 mg/m2 on days 1 and 8 plus ifosfamide 3 g/m2 with mesna 1600 mg as a bolus i.v. infusion before ifosfamide and after 4 hours and 8 hours plus vinorelbine 25 mg/m2 on days 1 and 8, every 3 weeks |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Trial was designed as a factorial trial to compare (1) effectiveness of 2 different treatment strategies, 1 containing cisplatin and 1 containing vinorelbine instead of cisplatin, and (2) 1 regimen with 2 and 1 with 3 drugs for the addition of ifosfamide Subgroup analysis of participants ≥ 70 years of age not planned as part of the original protocol; done at request of study authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment centrally performed by fax at Trial Unit of the National Institute for Cancer Reseach of Geneva with use of permuted blocks of variable sizes. Elderly subgroup not planned |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment analysis |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear influence on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For elderly population, 4 participants never treated and excluded from safety population; included in ITT population for other efficacy data analyses |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias. Participant characteristics and summary data provided by study author after direct contact |
| Other bias | High risk | Study designed for general population; elderly subgroup analysis not planned. Data for participants ≥ 70 years of ageobtained after request to study authors |
Buccheri 1997.
| Methods | Randomized phase III trial Eligible patients
Exclusion criteria
|
|
| Participants | MACC arm: 78 ITT population ‐ median age 64 years/no information on inclusion of elderly patients MVP arm: 78 ITT population ‐ median age 65 years/no information on inclusion of elderly patients |
|
| Interventions | MACC arm: methotrexate 40 mg/m2; doxorubicin 40 mg/m2 i.v.; cyclophosphamide 400 mg/m2 i.v. infusion, and lomustine 30 mg/m2 per os on day 1, every 3 weeks MVP arm: mitomycin C 10 mg/m2; vinblastine 6 mg/m2, and cisplatin 40 mg/m2 i.v. on day 1, every 3 weeks |
|
| Outcomes | Outcomes measured (no definition of which are primary or secondary outcomes)
|
|
| Notes | No information on numbers of elderly participants nor on specific subgroup analysis performed, despite multiple attempts to contact study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central office stratified participants according to stage of disease and ECOG, to ensure balanced distribution between treatment groups, then randomly assigned participants within each stratum |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment analysis |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study, considered to have unclear influence on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 45, 58, and 29 participants completed QoL instrument at 6, 12, and 18 weeks |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | High risk | Study closed to further accrual before target sample of 183 enrolled (with 156 patients) was reached, as 125 patients had already died Study designed for general population; elderly subgroup analysis not planned |
Chen 2002.
| Methods | Randomized phase II trial Eligible patients
Exclusion criteria
|
|
| Participants | PG arm: 45 ITT population ‐ median age (range): 67 (35 to 80) years/number of elderly participants not reported CP arm: 45 ITT population ‐ median age (range): 64 (37 to 77) years/number of elderly participants not reported |
|
| Interventions | PG arm: paclitaxel 175 mg/m2 over 3‐hour i.v. infusion on day 1 and gemcitabine 1000 mg/m2 over 30‐minute i.v. infusion on days 1 and 8, every 3 weeks CP arm: paclitaxel 175 mg/m2 over 3‐hour i.v. infusion on day 1 and carboplatin AUC7 (predicted using measured clearances and the Calvert formula) over 1‐hour i.v. infusion on day 1, every 3 weeks All participants received dexamethasone (10 mg i.v. at –12 and –6 hours), cimetidine (300 mg i.v.), and diphenhydramine (50 mg i.v.) before paclitaxel administration Metoclopramide (40 mg i.v.) given before paclitaxel plus carboplatin or gemcitabine as antiemetic prophylaxis. Dexamethasone (10 mg i.v.) and metoclopramide (20 mg i.v.) given before gemcitabine treatment (day 8) as antiemetic prophylaxis |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | No information on inclusion of elderly patients nor on specific subgroup analysis, despite multiple attempts to contact study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligile participants randomly assigned to paclitaxel plus carboplatin regimen or paclitaxel plus gemcitabine regimen by a statistical office not involved in the trial with use of computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment analysis |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear influence on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | High risk | Study designed for general population; elderly subgroup analysis not planned. Neither numbers nor outcomes available for this subgroup |
Chen 2008.
| Methods | Randomized phase II trial Eligible patients
Exclusion criteria
|
|
| Participants | V arm: 31 elderly participants ‐ median age (range): 76.5 (70 to 82) years/PS 2: 16/stage IV: 29 CV arm: 35 elderly participants ‐ median age (range): 75.6 (70 to 83) years/PS 2:16/stage IV: 27 |
|
| Interventions | V arm: vinorelbine 25 mg/m2 over 10‐minute i.v. infusion on days 1 and 8, every 3 weeks CV: cisplatin 50 mg/m2 over 6‐hour i.v. infusion on day 1 and vinorelbine 22.5 mg/m2 over 10‐minute i.v. infusion on days 1 and 8, every 3 weeks Planned maximum number of cycles: 6 for responding participants and 4 for those with stable disease |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised into the vinorelbine (V) or vinorelbine plus cisplatin (VP) treatment arm by an outside centre not involved in the study" ‐ no further information regarding randomization process |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment analysis |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear influence on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | Small phase II trial, designed to evaluate differences in response rate between treatment arms |
Comella 2004.
| Methods | Randomized phase III trial Eligibility criteria
Exclusion criteria
|
|
| Participants | P arm: 68 randomly assigned and analyzed participants ‐ median age (range): 75 (49 to 86) years; PS 2: 19; stage III: 24/59 elderly G arm: 63 randomly assigned and analyzed participants ‐ median age (range): 72 (50 to 81) years; PS 2: 22; stage III: 16/50 elderly GV arm: 68 randomly assigned and analyzed participants ‐ median age (range): 72 (42 to 82) years; PS 2: 21; stage III: 28/55 elderly GP arm: 65 randomly assigned and analyzed participants ‐ median age (range): 73 (53 to 83) years; PS 2: 15; stage III: 25/56 elderly |
|
| Interventions | P arm: paclitaxel 100 mg/m2 over 1‐hour i.v. infusion on days 1, 8, and 15, every 4 weeks. Doses could be increased to 120 mg/m2 on second cycle and to 140 mg/m2 on third cycle, according to tolerance G arm: gemcitabine 1200 mg/m2 over 30‐minute i.v. infusion on days 1, 8, and 15, every 4 weeks. Doses could be increased to 1400 mg/m2 on second cycle and to 1600 mg/m2 on third cycle, according to tolerance GV arm: gemcitabine 1000 mg/m2 over 30‐minute i.v. infusion and vinorelbine 25 mg/m2 over 15‐minute i.v. infusion on days 1 and 8, every 4 weeks. Doses could be increased to gemcitabine 1200 mg/m2 on second cycle and to vinorelbine 30 mg/m2 on third cycle, according to tolerance GP arm: gemcitabine 1000 mg/m2 over 30‐minute i.v. infusion and paclitaxel 80 mg/m2 over 1‐hour i.v. infusion on days 1 and 8 every 4 weeks. Doses could be increased to gemcitabine 1200 mg/m2 on second cycle and to paclitaxel 100 mg/m2 on third cycle, according to tolerance For all arms, in the absence of grade 2 or higher WHO toxicity, intrapatient dose escalation over first 3 cycles was planned and used thereafter |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Study prematurely stopped because of poor accrual after results of Gridelli 2003, in which no benefit was observed in favor of gemcitabine‐vinorelbine combination over each drug given as single agent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were registered by fax at the coordinating centre. After verifying the eligibility criteria, patients were stratified according to stage (IIIB vs IV), PS (0‐1 vs 2) and Charlson index score (0‐2 vs 3‐4) and allocated using a computer‐generated random list one of four arms: gemcitabine (GEM), paclitaxel (PTX), gemcitabine plus paclitaxel (GT) or gemcitabine plus vinorelbine (GV)" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment analysis |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear influence on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | Unclear risk | No information on blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants analyzed according to intention‐to‐treat principle. 16 participants among whole population not treated because of withdrawal of participant consent (5 cases) or physician's decision (11 cases). These participants not considered for activity and toxicity analyses but included in survival analysis |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | High risk | No planned elderly subgroup analysis (participants > 70 years of age) for any outcome. HR for OS reported for ITT population (doublet vs single agent) |
Depierre 1994.
| Methods | Randomized phase III trial, multi‐center Eligibility criteria
Exclusion criteria
|
|
| Participants | PV arm: 121 ITT population ‐ median age (range): 59.2 years (not reported)/number of elderly participants not reported V arm: 119 ITT population ‐ median age (range): 58.8 years (not reported)/number of elderly participants not reported |
|
| Interventions | PV arm: cisplatin 80 mg/m2 i.v. infusion every 3 weeks and vinorelbine 30 mg/m2 weekly. Hydration with 2000 mL of 5% dextrose solution administered 30 minutes before cisplatin infusion. Administration of methylprednisolone (120 mg) and of metoclopramide recommended to prevent nausea and vomiting V arm: vinorelbine 30 mg/m2 i.v. infusion, weekly |
|
| Outcomes | Overall survival (OS) defined as interval from randomization to death from any cause Time‐to‐progression (TTP) defined as interval from randomization to progressive disease Response rate Toxicity according to WHO criteria |
|
| Notes | No planned elderly subgroup analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No evidence of selection bias. "Randomization was performed through a centralized blind telephone assignment procedure, with stratification by centre and stage" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment analysis |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding of assessors |
| Blinding of outcome assessment (detection bias) Other outcomes | Unclear risk | No information on blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on judgment of attrition bias for the elderly |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | Study designed for general adult population; no elderly subgroup analysis planned |
Flotten 2012.
| Methods | Randomized multi‐center phase III trial Inclusion criteria
Exclusion criteria
|
|
| Participants | VG arm: 215 (ITT population)/38 (participants ≥ 75 years old) VC arm: 222 (ITT population)/36 (participants ≥ 75 years old) |
|
| Interventions | VG arm: vinorelbine 60 mg/m2 orally plus gemcitabine 1000 mg/m2 on days 1 and 8, every 3 weeks for planned 3 cycles VC arm: vinorelbine 60 mg/m2 orally on days 1 and 8 plus carboplatin AUC5 on day 1, every 3 weeks for planned 3 cycles Participants ≥ 75 years of age had doses reduced by 25% Both groups received prophylactic antiemetics with an i.v. glucocorticoid and a 5‐HT3 antagonist on day 1, and VG participants also on day 8. VC participants received oral 5‐HT3 antagonist twice daily on day 8 |
|
| Outcomes | Primary outcome
Seconday outcomes
|
|
| Notes | Participants underwent chest X‐ray and CT scan of thorax and upper abdomen before randomization and chest X‐ray at week 9 and every 8 weeks thereafter Further imaging to determine disease progression performed at treating physician's discretion. Study not designed to assess response rate nor time‐to‐progression Disease progression, unacceptable toxicity, and participant request were reasons for discontinuation of study treatment Despite contact with study authors, we retrieved no data related to outcomes among the elderly population |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After participants had signed the informed consent form and had completed the baseline HRQoL form, they were randomly assigned by phone to the central study office at Haukeland University Hospital, Bergen, Norway. Randomization was stratified by WHO PS 0 to 1 vs 2, stage IIB vs IV, and < 75 vs ≥ 75 years of age |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment analysis |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear influence on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Seven participants excluded from all analyses ‐ 'six because of ineligibility and one because of administration of wrong study therapy.' Three participants received no study treatment. No information on number of randomly assigned elderly who were not assessable |
| Selective reporting (reporting bias) | High risk | Study not designed to assess response or time‐to‐progression. Disease progression, unacceptable toxicity, and participant request were reasons for discontinuation of study treatment. Imaging for disease progression analysis done at treating physician's discretion. HRQoL analysis not reported for the elderly subgroup. Only OS data available for the elderly |
| Other bias | Unclear risk | Study designed for general population; elderly subgroup analysis planned |
Frasci 2001.
| Methods | Randomized phase II trial Inclusion criteria
Exclusion criterion
|
|
| Participants | V arm: 60/median age of included participants: 74 (range 71 to 81) years; 13% ECOG 2; 16.7% Charlson score > 2 VG arm: 60/median age of included participants: 75 (range 71 to 83) years; 16% ECOG 2; 20% Charlson score > 2 |
|
| Interventions | V arm: vinorelbine 30 mg/m2 i.v. infusion on days 1 and 8, every 3 weeks for maximum of 6 cycles VG arm: vinorelbine 30 mg/m2 i.v. infusion plus gemcitabine 1200 mg/m2 i.v. infusion on days 1 and 8, every 3 weeks for maximum of 6 cycles All participants received antiemetic prophylaxis consisting of HT3‐receptor antagonist |
|
| Outcomes | Primary outcomes
|
|
| Notes | Study prematurely stopped after first interim analysis showed increased risk of death for participants in V arm Evaluation of co‐morbidities using Charlson score before start of treatment. Quality of life assessment using Lung Cancer Symptom Scale (LCSS) questionnaire at diagnosis, after third and sixth cycles of chemotherapy. Response rate and toxicity classified according to World Health Organization (WHO) criteria |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed centrally at the Division of Medical Oncology of the National Tumor Institute of Naples". "(...) procedure that used the centre, stage IIIB vs IV and performance (ECOG 0 or 1 vs 2) as stratifying variables". "Patients were assigned to one of the two arms by computer‐driven minimization" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment analysis |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear influence on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Five patients excluded because no information sent to co‐ordination center. After early trial interruption, additional 21 patients included and outcome data not reported |
| Selective reporting (reporting bias) | High risk | Study not planned for PFS analysis. Therefore, no data on this outcome. Absence of important outcome associated with reporting bias, despite lack of inclusion in study design |
| Other bias | High risk | Study closed prematurely because planned interim analysis showed that magnitude of survival gain achieved by combination therapy met chosen criteria for early discontinuation. However, additional 21 participants included after that and outcome data not reported |
Georgoulias 2001.
| Methods | Randomized phase III trial, multi‐center Inclusion criteria
Exclusion criteria
|
|
| Participants | CD arm: 205 (ITT population)/32 elderly participants ‐ median age (range): 71 (70 to 76) years; PS 1: 62.5%; stage IV: 59.4% DG arm: 201 (ITT population)/39 elderly participants ‐ median age (range): 72 (70 to 76) years; PS 1: 71.8%; stage IV: 69.2% |
|
| Interventions | CD arm: docetaxel 100 mg/m2 over 1‐hour i.v. infusion plus cisplatin 80 mg/m2 i.v. infusion on day 2 after adequate hydralazine. Antiemetic treatment of ondansetron 4 mg i.v. on day 1, and 8 mg i.v. on day 2, plus dexamethasone 4 mg i.v. before administration of cisplatin, followed by ondansetron 8 mg 3 times daily for 3 days. rhG‐CSF 150 µg/m2 from day 3 to day 9 as primary prophylaxis for neutropenia or until absolute granulocyte count ≥ 1200 µg/L on 2 consecutive measurements, after nadir DG arm: gemcitabine 1100 mg/m2 over 30‐minute i.v. infusion on days 1 and 8 plus docetaxel 100 mg/m2 over 1‐hour i.v. infusion on day 8, after administration of gemcitabine Standard antiemetic treatment of 4 mg ondansetron given intravenously before administration of chemotherapy (days 1 and 8) and 8 mg ondansetron thrice daily for 2 to 3 days. rhG‐CSF 150 µg/m2 from day 9 to day 15 for all participants as primary prophylaxis for neutropenia Participants with stable disease treated for maximum of 6 cycles; those with complete or partial response treated until disease progression or intolerable toxicity |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | Data on unplanned elderly subgroup attained through direct contact with study authors Planned subgroup analysis according to stage, participant performance status, and histology "The study was designed to have 80% power to detect a 12% improvement in the overall response rate with the group 1 regimen at the one‐sided 5% level of statistical significance" "We aimed to recruit 412 patients (206 patients in each group) to achieve the statistical requirements of the fixed sample‐size design" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were centrally randomised by computer at a one‐to‐one ratio to receive either cisplatin and docetaxel (group 1) or gemcitabine and docetaxel (group 2). The allocation to either regimen was done by stratified randomisation according to age, performance status and stage of disease" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment analysis |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear influence on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In group 1, 14 participants, and in group 2, 21not evaluable for cisplatin. 20 participants not treated, 3 of whom died from disease within 3 weeks of enrollment, with 1 refusing further treatment after first cycle, and 5 not following the protocol. No data available for evaluation of attrition bias for unplanned elderly subgroup analysis |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias. Study authors provided data for all outcomes in the unplanned elderly subgroup analysis |
| Other bias | High risk | Study designed for general population; elderly subgroup analysis not planned |
Georgoulias 2004.
| Methods | Randomized phase III trial, multi‐center Inclusion criteria
Exclusion criteria
|
|
| Participants | D arm: 152 (ITT population)/34 elderly participants ‐ median age (range): 72.5 (70 to 77) years CD arm: 167 (ITT population)/36 elderly participants ‐ median age (range): 72.5 (70 to 76) years Median ages at baseline: 72.5 (range 70 to 77) years and 72.5 (range 70 to 76) years for D and CD arm, respectively. 8 participants (9.6%) with ECOG PS of 2 |
|
| Interventions | D arm: docetaxel 100 mg/m2 on day 1, every 3 weeks, without rhG‐CSF support, on outpatient basis Antiemetic support: 8 mg ondansetron i.v. before treatment and 8 mg oral ondansetron 3 times a day, for 2 to 3 days CD arm: docetaxel 100 mg/m2 on day 1 and cisplatin 80 mg/m2 on day 2 after appropriate hydration; rhG‐CSF given prophylactically (150 µg/m2/d subcutaneously from day 3 to day 9, or until AGC ≥ 1200 µL on 2 consecutive measurements after nadir). Cycles repeated every 3 weeks Antiemetic support: 8 mg ondansetron i.v. infusion on day 1, before administration of docetaxel, and 8 mg ondansetron plus 4 mg dexamethasone i.v. on day 2, before administration of cisplatin, followed by 8 mg oral ondansetron 3 times a day for 3 days. Regimen required 24‐hour hospitalization for hydration All participants received standard before and after medication with oral dexamethasone |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | "The study was designed to have 90% power to detect a difference in median survival of 7 months for single‐agent D versus 12 months for DC at the statistically significant level of 5%. To achieve these statistical requirements, 150 patients/arm were needed" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned patients were stratified to age, PS and stage of the disease. Patients were centrally randomly assigned in a 1:1 ratio to receive either DC or D" Elderly subgroup analysis not planned |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment analysis |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear influence on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Nine (5%) DC and 10 (6%) D participants lost to follow‐up and considered non‐responders. No available data for attrition bias analysis on elderly subgroup |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias. Additional data provided by study authors |
| Other bias | High risk | Study designed for general population; elderly subgroup analysis not planned |
Georgoulias 2005.
| Methods | Randomized phase III trial, multi‐center Inclusion criteria
Exclusion criteria
|
|
| Participants | VC arm: 204 (ITT population)/43 elderly participants DG arm: 209 (ITT population)/39 elderly participants |
|
| Interventions | VC arm: vinorelbine 30 mg/m2 over 30‐minute i.v. infusion on days 1 and 8 plus cisplatin 80 mg/m2 on day 8. rhG‐CSF 150 µg/m2/d subcutaneously, given prophylactically to all participants on days 9 through 15. Cycles repeated every 3 weeks DG arm: gemcitabine 1000 mg/m2 over 30‐minute i.v. infusion on days 1 and 8 plus docetaxel 100 mg/m2 over i.v. infusion on day 8. rhG‐CSF 150 µg/m2/d subcutaneously given prophylactically to all participants on days 9 through 15. Cycles repeated every 3 weeks All participants given ondansetron and those receiving cisplatin also administered 4 mg dexamethasone, adequate hydration, and forced diuresis. DG regimen administered on outpatient basis, whereas most VC participants admitted overnight for hydration |
|
| Outcomes | Primary outcome
Secondary endpoints
|
|
| Notes | Data on elderly subgroup achieved through direct contact with study authors Planned subgroup analysis: disease stage, PS, and histology "The study was designed to detect a 4‐month difference of overall survival with an 80% power at a significance level of .05. Three hundred sixty‐two patients (181 per arm) were required in order to achieve the statistical hypothesis" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were centrally registered and stratified according to age, PS and stage of the disease" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment analysis |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear influence on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants died as a result of progressive disease before chemotherapy administration, whereas 5 refused treatment and 4 did not meet entry criteria in the DG group. In addition, 5 participants refused treatment and 7 did not meet entry criteria in the VG group. All participants were included in response analysis |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias. Additional data provided by study authors |
| Other bias | High risk | Study designed for general population; elderly subgroup analysis not planned |
Georgoulias 2008.
| Methods | Randomized phase III trial Inclusion criteria
Exclusion criteria
|
|
| Participants | DG arm: 157 participants/39 elderly/median age: 73 (range 70 to 78) years. One participant (2.4%) in the D arm had ECOG PS of 2 D arm: 155 participants/42 elderly/median age: 72 (range 70 to 78) years/6 participants (15.4%) in DG arm had ECOG PS of 2 We found imbalance between treatment arms for PS (Fisher exact test, P value = 0.04; Table 13) |
|
| Interventions | DG arm: gemcitabine 1100 mg/m2 over 30‐minute intravenous (i.v.) infusion on days 1 and 8, and docetaxel 75 mg/m2 over 1‐hour i.v. infusion on day 1, repeated every 3 weeks for 6 cycles, without rhG‐CSF D arm: docetaxel 100 mg/m2 over 1‐hour i.v. infusion on day 1, every 3 weeks for 6 cycles, without rhG‐CSF For both arms, treatment administered until disease progression, unacceptable toxicity, or consent withdrawal |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | All analyses of elderly subgroups retrieved through direct contact with study author Responses analyzed on intention‐to‐treat basis Participants given ≥ 1 cycle of chemotherapy assessed for toxicity Study was prematurely closed because of predefined OS significant difference in favor of DG arm over D arm (for general population) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were centrally randomised and stratified according to age, PS (older than 65 year vs younger) PS (2 vs 0‐1) and stage of disease (IIIB vs IV)" Elderly subgroup not planned |
| Allocation concealment (selection bias) | Unclear risk | No information for allocation concealment analysis |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear influence on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 10 participants not evaluable because of treatment administration (consent withdrawn; n = 7 participants), violation of entry criteria (n = 2 participants), and misdiagnosis (n = 1 participant). No data provided for elderly subgroup |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias. Additional data provided by study authors |
| Other bias | High risk | Study designed for general population; elderly subgroup analysis not planned. Imbalance detected for PS between treatment arms for the elderly subgroup |
5. Participant characteristics in unplanned elderly subgroup (Georgoulias 2008).
| Treatment arm | DG arm | D arm | |||
| Number of participants | 39 | 42 | |||
| N | % | N | % | P value (Fisher's exact test) |
|
| Age | |||||
| Median | 72 | 73 | |||
| Min‐Max | 70‐78 | 70‐78 | |||
| Sex | 0.233 | ||||
| Male | 34 | 87.2 | 40 | 95.2 | |
| Female | 5 | 12.8 | 2 | 4.8 | |
| Performance status | 0.04 | ||||
| 0 | 13 | 33.3 | 23 | 54.8 | |
| 1 | 20 | 51.3 | 18 | 42.9 | |
| 2 | 6 | 15.4 | 1 | 2.4 | |
| Histological subtypes | 0.5476 | ||||
| Squamous | 10 | 25.6 | 15 | 35.7 | |
| Adenocarcinoma | 17 | 43.6 | 14 | 33.3 | |
| Other | 12 | 30.8 | 13 | 31 | |
| Stage | 0.6196 | ||||
| IIIB | 9 | 23.1 | 12 | 28.6 | |
| IV | 30 | 76.9 | 30 | 71.4 | |
| Number of organs Involved | 0.06 | ||||
| 1 | 9 | 23.1 | 14 | 33.3 | |
| 2 | 14 | 35.9 | 21 | 50 | |
| ≥ 3 | 16 | 41 | 7 | 16.7 | |
Gricorescu 2007.
| Methods | Randomized phase II trial Eligible patients
Exclusion criteria
|
|
| Participants | GV/GI arm: 50 (ITT population) ‐ median age: 59 years/elderly participants not reported GP arm: 52 (ITT population) ‐ median age: 56 years/elderly participants not reported |
|
| Interventions | GV/GI arm: gemcitabine 1000 mg/m2 plus vinorelbine 25 mg/m2 on days 1 and 8 for 2 cycles, followed by gemcitabine 1000 mg/m2 on days 1 and 8 plus ifosfamide 2000 mg/m2 on day 1, for 2 cycles. To prevent hemorrhagic cystitis, mesna was administrated at a dose equivalent to 20% of ifosfamide dose GP arm: gemcitabine 1250 mg/m2 on days 1 and 8 with cisplatin 70 mg/m2 given on day 1, for 4 cycles |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | No information on inclusion of elderly participants nor on specific subgroup analysis, despite multiple attempts to contact study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear influence on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | Unclear risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | Study designed for general population; elderly subgroup analysis not planned |
Gridelli 2003.
| Methods | Randomized phase III open‐label trial Inclusion criteria
Exclusion criteria
|
|
| Participants | V arm: 233/median age at baseline: 74 (range 63 to 85) years; 19% ECOG 2 G arm: 233/median age at baseline: 74 (range 70 to 86) years; 18% ECOG 2 GV arm: 232/median age at baseline: 74 (range 69 to 84) years; 20% ECOG 2 > 70% of entire population had ADL score of 6; 50% had IADL score > 75% |
|
| Interventions | V arm: vinorelbine 30 mg/m2 i.v. infusion on days 1 and 8, every 3 weeks for maximum of 6 cycles G arm: gemcitabine 1200 mg/m2 i.v. infusion on days 1 and 8, every 3 weeks for maximum of 6 cycles GV arm: gemcitabine 1000 mg/m2 i.v. infusion on days 1 and 8 plus vinorelbine 25 mg/m2 i.v. infusion on days 1 and 8, every 3 weeks for maximum of 6 cycles |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Statistical analysis planned to test whether GV was superior to each single agent separately (estimated 370 events to detect improvement from 27 weeks to 36 weeks on overall survival corresponding to HR 0.75, with 1‐tailed alpha error of 5%, power 0.87) Two geriatric scales (Active Daily Life (ADL) and Intrumental Active Daily Life (IADL)) completed by investigators at baseline and after third and sixth cycles Quality of life assessed by European Organisation for Research and Treatment of Cancer (EORTC) core questionnaire (QLQ‐C30) and Lung Cancer‐Specific Module (QLC‐LC13). Response and toxicity assessed by WHO criteria |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed centrally at the Clinical Trials Office National Cancer Institute (Naples, Italy), using a computer‐generated procedure of minimization. Patients were stratified according to institution, ECOG, performance status (0, 1 or 2) and disease stage (IIIB versus IV)" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Hainsworth 2007.
| Methods | Randomized multi‐center phase III trial Eligible patients
Exclusion criteria
|
|
| Participants | D arm: 171 ITT population/115 elderly population DG arm: 174 ITT population/117 elderly population |
|
| Interventions | D arm: docetaxel 36 mg/m2 over 30‐minute i.v. infusion on days 1, 8 and 15, and every 4 weeks DG arm: gemcitabine 800 mg/m2, over 30‐minute i.v. infusion, followed by docetaxel 30 mg/m2, over 30‐minute i.v. infusion; both drugs administered on days 1, 8, and 15, every 4 weeks Standard hypersensitivity and antiemetic prophylaxis administered before each dose of chemotherapy |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | No information on subgroup analysis of elderly participants, despite multiple attempts to contact study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participant enrollment in this multi‐center, randomized, phase III study was initiated in August 2001. Random sequence generation was not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study assigned unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study authors reported insufficient data to allow attrition bias analysis in the elderly subgroup |
| Selective reporting (reporting bias) | Low risk | No outcome data were provided for participants older than 70 years. Study authors presented a separate analysisfor OS only in participants with good PS, including those older than 65 years. No other outcomes were reported for the elderly subgroup. However, the trial was not planned for elderly subgroup analysis, and we considered it to have low risk of reporting bias |
| Other bias | High risk | No data were provided on unplanned elderly subgroup analysis. OS analysis was performed only for the subgroup of participants with good PS, which included only participants older than 65 years |
Hara 1990.
| Methods | Randomized trial Eligible patients
|
|
| Participants | MCT arm: 68 participants (ITT population) ‐ median age (range): 63 (37 to 75) ‐ number of elderly not reported CAPM arm: 58 participants (ITT population) ‐ median age (range): 60 (27 to 75) ‐ number of elderly not reported |
|
| Interventions | MCT arm: mitomycin C 4 mg/body i.v. infusion and cytosine arabinoside 30 mg/body on days 1, 4, 14, 21, and 28, and tegafur 600 mg orally every day CAPM arm: cyclophosphamide 400 mg/m2 i.v. infusion on day 1, Adriamycin 30 mg/m2 i.v. infusion on day 1, cisplatin 60 mg/m2 i.v. infusion on day 1, and mitomycin C 3 mg/m2 i.v. infusion on day 1 |
|
| Outcomes | Neither primary nor secondary outcomes defined | |
| Notes | No contact established with study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were divided at the time of registration into those with stage III and those with stage IV disease, according to the criteria set by the American Joint Commission on Staging, and then randomised to receive either CAPM or MCT regimens" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding of outcome assessment, although study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | Unclear risk | No information on blinding of assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 17 participants not evaluated: 14 received only 1 cycle or refused treatment; 3 were lost to follow‐up. No information provided for the elderly subgroup |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | No information on elderly subgroup planned; none could be retrieved |
Hsu 2008.
| Methods | Randomized phase II trial Inclusion criteria
Exclusion criteria
|
|
| Participants | GE arm: 43 participants (ITT population) ‐ median age: 62.3 years (33.9 to 78.6)/elderly participants not reported GP arm: 42 participants (ITT population) ‐ median age: 60.9 years (37.6 to 76)/elderly participants not reported |
|
| Interventions | GE arm: gemcitabine 1000 mg/m2 over 30‐minute i.v. infusion on days 1, 8, and 15 plus epirubicin 70 mg/m2 on day 15 GP arm: gemcitabine 1000 mg/m2 on days 1, 8, and 15 plus cisplatin 100 mg/m2 over 3‐hour i.v. infusion on day 15. Dose of cisplatin reduced to 80 mg/m2 after first 10 participants randomly assigned because of toxicity |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Despite multiple attempts, we could not contact study authors to retrieve data on the elderly population | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed by an independent statistical office at the clinical trial centre of National Taiwan University Hospital, using a computer‐generated randomisation allocation sequence. The sequence was concealed until the treatment arms were assigned by the statistical office. EligIble patients were randomised in 1:1 ratio to two treatment arms, namely gemcitabine plus conventional‐dose epirubicin (GE) or gemcitabine—cisplatin (GC) arms" |
| Allocation concealment (selection bias) | Low risk | Sequence concealed until treatment arms assigned by the statistical office |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant refused randomization; 5 participants refused protocol treatment after randomization (1 in GC arm and 4 in GE arm) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Study designed for general population; elderly subgroup analysis not planned nor available |
Jeremic 1997.
| Methods | Prospective randomized trial, open‐label Inclusion criteria
Exclusion criteria
|
|
| Participants | E arm: 59 participants/22 participants ≥ 60 years/number of elderly participants not informed CE arm: 58 participants/18 participants ≥ 60 years/number of elderly participants not informed |
|
| Interventions | E arm: etoposide 50 mg/m2/d p.o. on days 1 through 21 every 28 days CE arm: carboplatin 400 mg/m2 over 30‐minute i.v. infusion on day 1 and etoposide 50 mg/m2/d p.o. on days 1 through 21 every 28 days |
|
| Outcomes | Response rate (according to WHO response criteria) Toxicity according to ECOG criteria Overall survival |
|
| Notes | Study prematurely interrupted because chief investigator left the department | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding of outcome assessment for OS |
| Blinding of outcome assessment (detection bias) Other outcomes | Unclear risk | No information on blinding of outcome assessment for PFS, ORR, and toxicity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | High risk | Trial prematurely stopped because of personnel problems (chief investigator had to leave department). No information provided for elderly subgroup |
Karampeazis 2010.
| Methods | Randomized phase III trial Eligible patients
No further eligibility nor exclusion criteria presented |
|
| Participants | G arm: 45 participants ‐ median age (range): 78 (70 to 92) years; 11 (21.2%) PS 2, 40 (76.9%) stage IV DG arm: 49 participants ‐ median age (range): 74 (70 to 84) years; 7 (13%) PS 2; 37 (68.5%) stage IV Imbalance between older participants in D arm vs DG arm (P value < 0.001). No imbalance observed for other participant characteristics. For the ITT population, 30 (55.5%) participants classified as fit in CGA, 18 (33.3%) as vulnerable, and 6 (11.1%) as frail. All participants scored 6 on ADL scale; 68% scored 7 to 8 on IADL scale |
|
| Interventions | G arm: gemcitabine 1200 mg/m2 on days 1 and 8 every 3 weeks DG arm: docetaxel 30 mg/m2 and gemcitabine 900 mg/m2 on days 1 and 8 every 3 weeks No further information available from abstract |
|
| Outcomes | Study authors did no report primary and secondary outcomes Comphrensive Geriatric Assessment (CGA) based on ADL, IADL, MMSE, CIRS‐G, and body mass index data collected |
|
| Notes | Study available in abstract form. Study authors provided additional unpublished data after direct contact. Characteristics of study population regarding age, performance status, stage, histology, and CGA provided, as well as summary data for OS, 1yOS, PFS, RR, and toxicity with longer follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Chemotherapy‐naïve patients >70y old with stage IIIB/IV NSCLC and performance status (PS) 0‐2 were stratified according to stage and PS and randomised to either D 30 mg/m2 plus G 900mg/m2 (days 1 and 8) or G 1,200mg/m2 (days 1 and 8) every 21 days" ‐ no further information available on randomization process |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding of assessors for OS and 1yOS; study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | Unclear risk | No information on blinding of assessors for other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias. Additional data provided by study authors |
| Other bias | High risk | Study prematurely stopped for poor accrual. Data available from unpublished data; imbalance for age observed between treatment arms |
Katakami 2006.
| Methods | Randomized phase II trial Eligibility criteria
Exclusion criteria
|
|
| Participants | CD arm: 68 participants (ITT population) – median age (range): 65 (31 to 75)/number of elderly not reported DG arm: 63 participants (ITT population) – median age (range): 61 (40 to 75)/number of elderly nor reported |
|
| Interventions | DG arm: docetaxel 60 mg/m2 over 1‐hour i.v. infusion on day 1 and gemcitabine 800 mg/m2 over 30‐minute i.v. infusion on days 1 and 8, every 3 weeks CD arm: cisplatin 80 mg/m2 over 1‐hour i.v. infusion and docetaxel 60 mg/m2 over 1‐hour i.v. infusion on day 1, every 3 weeks. |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated to receive DC or DG stratified by study centre, disease stage (IIIB or IV) and sex" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding of assessors for OS and 1yOS but study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding of outcome assessors for other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two patients in the DG arm did not receive any protocol treatment. One patient suffered from uncontrollable atrial fibrillation, and the investigator decided against this patient receiving protocol treatment. The other patient had a massive hematemesis from a gastric cancer that was discovered after enrolment (second primary). Because two patients were deemed ineligible, 131 patients were evaluated for survival, response, and toxicity" |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Study was stopped because of toxicity before target population was enrolled. "The planned patient number was 150 (75 in each arm). However, an unexpected high incidence of grade 3 interstitial lung disease (ILD) was identified exclusively in DG arm by the Adverse Event Reporting system. The principal investigator stopped the enrolment into the trial on September 30, 2003. The Safety Committee reviewed the investigator’s report and recommended that the Japan Lung Cancer Cooperative Clinical Study Group terminate the study immediately because of lung injury in the DG arm" No data provided for the elderly subgroup |
Kubota 2008.
| Methods | Randomized phase III trial, open‐label, multi‐center Inclusion criteria
Exclusion criteria
|
|
| Participants | VGD arm: 196 (ITT population)/63 elderly participants CP arm: 197 (ITT population)/55 elderly participants Median age at baseline: 73 (range 70 to 81) years for both groups; 79% and 85% were male, 60% and 65% had adenocarcinoma histology, 78% and 89% had stage IV disease, and 70% and 58% had ECOG PS of 1, for non‐platinum combination and platinum combination, respectively |
|
| Interventions | VGD arm: vinorelbine 25 mg/m2 and gemcitabine 1000 mg/m2 on days 1 and 8 every 3 weeks for 3 cycles. Single‐agent docetaxel (60 mg/m2) was subsequently given i.v. on day 1, every 3 weeks for a further 3 cycles. Premedications, such as antiemetic agents or corticosteroids, were given as needed. All participants were assigned 8 mg of dexamethasone orally before docetaxel administration (experimental arm) CP arm: paclitaxel 225 mg/m2 plus carboplatin AUC6 for 3 hours on day 1, every 3 weeks for 6 cycles. Participants were assigned premedication with dexamethasone, diphenhydramine, and ranitidine or cimetidine Erythropoietin‐stimulating agents were not used. G‐CSF was permitted at any time during the study, except for prophylactic use, in both groups (control arm) |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | "We calculated that we would need 200 patients per group to detect such a difference, with a power of 0·85 using a two‐sided Log‐rank test at a significance level of 0·05" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to the experimental regimen or the standard regimen (Figure 1). After providing written informed consent, participants were registered via fax and, if eligibility was confirmed, were allocated to one of the treatment groups by computer |
| Allocation concealment (selection bias) | Unclear risk | "Central randomisation to each group was applied by use of a dynamic balancing algorithm to obtain a good balance between groups in terms of the stratified factors. Randomisation was done centrally by members of the Japan Multi‐National Trial Organisation (JMTO) data centre at the Translational Research Informatics Centre, Kobe, Hyogo, Japan" |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | "Neither patients nor physicians were blinded to allocated treatment." We considered the study to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | "Neither patients nor physicians were blinded to allocated treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Eight patients (2·0%) were ineligible for analysis: five withdrew informed consent, two had other malignancies, and one had stage IIIB disease without pleural effusion" No information provided for the elderly subgroup |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. Additional data provided by study authors |
| Other bias | High risk | Study designed for the general population; elderly subgroup analysis not planned |
Laack 2004.
| Methods | Randomized multi‐center phase III trial Eligible patients
Exclusion criteria
|
|
| Participants | GV arm: 143 participants (ITT population) ‐ median age (range): 60.8 (41 to 75.9) years/16 elderly participants with the following characteristics: stage IIB: 2, and stage IV: 14; male: 14, and female: 2; KPS: 100%: 2, 90%: 3, 80%: 8, and 70%: 3 GVP arm: 144 participants (ITT population) ‐ median age (range): 61.1 (40.6 to 75.9)/27 elderly participants with the following characteristics: stage IIIB: 2, and stage IV: 26; male: 22, and female: 5; KPS: 100%: 4, 90%: 10, 80%: 8, and 70%: 5 |
|
| Interventions | GV arm: gemcitabine 1000 mg/m2 over 30‐minute i.v. infusion and vinorelbine 25 mg/m2 over 15‐minute i.v. infusion on days 1 and 8, every 3 weeks GVP arm: gemcitabine 1000 mg/m2 over 30‐minute i.v. infusion and vinorelbine 25 mg/m2 over 15‐minute i.v. infusion on days 1 and 8 and cisplatin 75 mg/m2 on day 2 over 1‐hour infusion with standard pre‐hydration and post‐hydration, every 3 weeks Obs: prophylaxis for febrile neutropenia with use of granulocyte‐colony stimulating factor at physician's decision on an individual basis |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Elderly subgroup analysis not planned in the original protocol. Unpublished post hoc analysis of participants older than 70 years obtained upon direct request to study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to receive either GV or GVP. Before random assignment, patients were stratified according to participating centre. Randomization (stratified block randomisation with enclosed block length) was performed centrally by the Department of Biostatistics of the German Cancer Research Center (Heidelberg, Germany) using facsimile forms" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Thirteen randomly assigned patients (4.3%) did not fulfil the eligibility criteria and were excluded from the full analysis set. Four patients had a stage IIIB disease without malignant pleural effusion, one patient was staged as stage IIIA disease, two patients had brain metastases, two patients did not have NSCLC (one patient had small cell lung cancer and one patient a malignant melanoma), two patients revealed a Karnofsky performance status of lower than 70%, one patient did not fulfil the eligibility criteria concerning tumor size, and one patient refused treatment after randomisation" No further details on attrition bias provided for elderly subgroup |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | High risk | Study designed for the general population; elderly subgroup analysis not planned. Unpublished post hoc elderly subgroup analysis obtained upon direct request to study authors |
Le Chevalier 1994.
| Methods | Randomized multi‐center phase III trial Eligible patients
Exclusion criteria
|
|
| Participants | VNR arm: 206 participants (ITT population) ‐ median age (range): 60 (NR) years/elderly participants not reported C‐VNR arm: 206 participants (ITT population) ‐ median age (range): 59 (NR) years/elderly participants not reported C‐VDS arm: 200 participants (ITT population) ‐ median age (range): 59 (NR) years/elderly participants not reported |
|
| Interventions | VNR arm: vinorelbine 30 mg/m2 over 20‐minute i.v. infusion weekly C‐VNR arm: cisplatin 120 mg/m2 over 1‐hour i.v. infusion on days 1 and 29, then every 6 weeks, plus vinorelbine 30 mg/m2 weekly C‐VDS arm: cisplatin 120 mg/m2 over 1‐hour i.v. infusion on days 1 and 29, then every 6 weeks, plus vindesine 3 mg/m2 weekly for 6 weeks, then every 2 weeks |
|
| Outcomes | Primary endpoint
Secondary endpoints
|
|
| Notes | No additional data retrieved from the study. Data no longer accessible to study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was centralized and treatment arms were allocated using a computer‐generated list stratified by centre and stage (stage IIIA and local recurrence v stage IIIB and metastatic disease to avoid an imbalance in the random allocation of potentially curable patients)" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding of outcome assessors for OS and 1yOS but study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Twenty‐four patients (4%) were deemed ineligible: five had cerebral metastasis at the time of inclusion, two had a previous malignancy, two had errors in diagnosis, five had a PS 3, and 10 had no measurable lesion. They were distributed as follows: nine to NVB‐ P, 11 to VDS‐P, and four to NVB" No information provided for elderly subgroup |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | Elderly subgroup analysis not planned nor performed |
Lilenbaum 2005.
| Methods | Randomized phase III trial, open‐label Inclusion criteria
Exclusion criteria
|
|
| Participants | P arm: 277 participants (ITT population)/78 elderly participants CP arm: 284 participants (ITT population)/77 elderly participants |
|
| Interventions | P arm: paclitaxel 225 mg/m2 over 3‐hour i.v. infusion, every 3 weeks for maximum of 6 cycles CP arm: carboplatin AUC6 over 30‐minute i.v. infusion plus paclitaxel 225 mg/m2 over 3‐hour i.v. infusion, every 3 weeks for maximum of 6 cycles Secondary prophylaxis with filgrastim used for participants who developed febrile neutropenia or grade 4 neutropenia lasting > 5 days for all subsequent cycles |
|
| Outcomes | Primary outcome
Secondary outcome
|
|
| Notes | Study planned for adult population and elderly subgroup | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was centralized at the CALGB data management centre in Durham, NC. Patient randomisation was stratified by stage (IIIB v IV v recurrent), PS (0 to 1 v 2), and age (< 70 v ≥70 years of age)" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Twenty‐three patients (3.9%) either withdrew from the study before receiving protocol therapy or were later found to be ineligible" |
| Selective reporting (reporting bias) | High risk | PFS and toxicity data not reported for the elderly subgroup |
| Other bias | Unclear risk | Study designed for the general population; elderly subgroup analysis planned |
Lilenbaum 2005b.
| Methods | Randomized phase II trial Inclusion criteria
Exclusion criteria
|
|
| Participants | GV arm: 82 participants (ITT population) ‐ median age (range): 66 (42 to 86) years/number of elderly participants not reported CP arm: 83 participants (ITT population) ‐ median age (range): 63 (38 to 86) years/number of elderly participants not reported |
|
| Interventions | GV arm: vinorelbine 25 mg/m2 i.v. infusion plus gemcitabine 1000 mg/m2 i.v. infusion, both given on days 1 and 8, every 3 weeks up to 6 cycles CP arm: paclitaxel 200 mg/m2 i.v. infusion plus carboplatin AUC6 according to Calvert formula, both administered on day 1 every 3 weeks up to 6 cycles |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | No additional data accessible, despite contact with study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised to receive vinorelbine(...)" Random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | Elderly subgroup analysis neither planned nor performed. Unplanned subgroup analysis not retrieved |
Lou 2010.
| Methods | Randomized trial Eligible patients
No exclusion criteria reported |
|
| Participants | G arm: 34 participants ‐ median age (range): 72 (70 to 80) years; 3 participants with PS of 0, 28 with PS of 1, and 3 with PS of 2 CG arm: 34 participants ‐ median age (range): 72 (70 to 77) years; 1 participant with PS of 0, 30 with PS of 1, and 3 with PS of 2 |
|
| Interventions | G arm: gemcitabine 1000 mg/m2 i.v. infusion on days 1 and 8, every 3 weeks CG arm: carboplatin AUC5 i.v. infusion on day 2 and gemcitabine 1000 mg/m2 i.v. infusion on days 1 and 8, every 3 weeks |
|
| Outcomes | Outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding of outcome assessors for OS and 1yOS. Study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding of outcome assessors for other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | Low risk | No evidence of other bias |
Manegold 1998.
| Methods | Two randomized phase II trials conducted in Europe and Taiwan Eligible patients for the 2 trials ‐ almost identical
Exclusion criteria
|
|
| Participants | G arm (Europe): 71 participants (ITT population) ‐ median age (range): 59 (32 to 80) years/elderly participants not reported G arm (Taiwan): 27 participants (ITT population) ‐ median age (range): 63 (36 to 75) years/elderly participants not reported EP arm (Europe): 75 participants (ITT population) ‐ median age (range): 59 (33 to 78) years/elderly participants not reported EP arm (Taiwan): 26 participants (ITT population) ‐ median age (range): 60 (35 to 75) years/elderly participants not reported |
|
| Interventions | G arm: gemcitabine 1000 mg/m2 over 30‐minute i.v. infusion on days 1, 8, and 15 every 4 weeks (Europe)/gemcitabine 1250 mg/m2 over 30‐minute i.v. infusion on days 1, 8, and 15, every 4 weeks (Taiwan) EP arm: cisplatin 100 mg/m2 i.v. infusion on day 1 and etoposide 100 mg/m2 i.v. infusion on days 1, 2, and 3, every 4 weeks (Europe)/cisplatin 80 mg/m2 i.v. infusion on day 1 and etoposide 80 mg/m2 i.v. infusion on days 1, 2, and 3, every 4 weeks (Taiwan) |
|
| Outcomes | Neither primary nor secondary outcomes reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomise to receive either..." No further information on randomization process provided |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding of outcome assessment |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | No separate elderly subgroup analysis |
Mok 2005.
| Methods | Randomized phase II trial, conducted at a single center (Department of Clinical Oncology of the Chinese University of Hong Kong) Inclusion criteria
Exclusion criteria
|
|
| Participants | GE arm: 45 participants (ITT population) ‐ median age (range): 61 (38 to 70) years/number of elderly not reported CP arm: 44 participants (ITT population) ‐ median age (range): 56 (23 to 72) years/number of elderly not reported |
|
| Interventions | GE arm: gemcitabine 1000 mg/m2 over 30‐minute i.v. infusion on days 1, 8, and 15 plus etoposide 50 mg/m2 p.o. days 1 through 14, every 4 weeks GP arm: cisplatin 75 mg/m2 over 1‐hour i.v. infusion on day 1 plus gemcitabine 1000 mg/m2 over 30‐minute i.v. infusion on days 1, 8, and 15, every 4 weeks |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients who had signed informed consent were randomly assigned to the GE or the GP arm. Randomization was stratified according to disease stage (stage IIIb vs IV) and was independently performed by a Comprehensive Cancer Trial Unit at the Chinese University of Hong Kong |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding of assessment |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding of assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | No separate elderly subgroup analysis |
Perng 1997.
| Methods | Randomized multi‐centerphase III trial Eligible patients were
Exclusion criteria
|
|
| Participants | G arm: 27 participants (ITT population) ‐ median age (range): 63 (36 to 75) years/number of elderly participants not reported EP arm: 26 participants (ITT population) ‐ median age (range): 60 (35 to 75) years/number of elderly participants not reported |
|
| Interventions | G arm: gemcitabine 1250 mg/m2 over 30‐minute i.v. infusion on days 1, 8, and 15, every 4 weeks. Dexamethasone and metoclopramide were given before gemcitabine infusion as antiemetic prophylaxis EP arm: cisplatin 80 mg/m2 over 1‐hour i.v. infusion on day 1 and etoposide 80 mg/m2 over 60‐minute i.v. infusion on days 1, 2, and 3 of each 28‐day cycle. In EP arm, chemotherapy administered after hospitalization. Granisetron, dexamethasone, metoclopramide, and lorazepam given before cisplatin as antiemetic prophylaxis. Dexamethasone and metoclopramide used on days 2 and 3 as antiemetics |
|
| Outcomes | Neither planned primary nor secondary outcomes reported | |
| Notes | No information on inclusion of elderly patients nor specific subgroup analysis was obtained, despite multiple attempts to contact study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants randomly assigned to GEM and EP regimens by a statistical office not involved in the trial, using a computer‐ generated list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two patients in the EP arm were excluded from analysis due to protocol violation. One patient suffered grade 3 hearing impairment before entering the trial and the other patient was found to have brain metastases on the second day of the first cycle of treatment" |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | No separate elderly subgroup analysis |
Pujol 2005.
| Methods | Randomized multi‐center phase III trial Eligible patients
Exclusion criteria
|
|
| Participants | GD arm: 155 participants (ITT population) ‐ median age (range): 60 (37 to 75) years/elderly participants not reported CV arm: 156 participants (ITT population) ‐ median age (range): 57 (39 to 74) years/elderly participants not reported |
|
| Interventions | GD arm: gemcitabone 1000 mg/m2 over 30‐minute i.v. infusion on days 1 and 8 plus docetaxel 85 mg/m2 i.v. infusion on day 8 before gemcitabine, every 3 weeks up to 8 cycles CV arm: cisplatin 1000 mg/m2 over 60 to 120‐min i.v. infusion plus vinorelbine 30 mg/m2 over 10 to 20‐minute i.v. infusion on days 1, 8, 15, and 22, every 4 weeks up to 6 cycles No prophylactic G‐CSF allowed. Participants with prolonged aplasia treated with G‐CSF |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Despite multiple attempts, we could not contact study authors to retrieve data on the elderly population | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was centralized by an independent academic research institute (Institut Universitaire de la Recherche Clinique, Montpellier, France) according to computer‐generated lists. A stratification by centre was done" |
| Allocation concealment (selection bias) | Unclear risk | No information no allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | Study designed for the general population; elderly subgroup analysis not planned |
Quoix 2011b.
| Methods | Randomized phase III trial, multi‐center, open‐label Inclusion criteria
Exclusion criteria
|
|
| Participants | Monotherpy arm: 226 Doublet chemotherapy arm: 255 Baseline characteristics for intention‐to‐treat population: median age: 77.1 (range 70.0 to 88.8) years; 27.3% (123) ECOG of 2, 20.1% (88) with ADL score < 6, 15.2% (67) with MMSE < 23, 24.4% (110) with Charlson index > 2 |
|
| Interventions | Monotherapy arm: vinorelbine 25 mg/m2 i.v. infusion on days 1 and 8 or gemcitabine 1150 mg/m2 on days 1 and 8, every 3 weeks for maximum of 5 cycles Doublet chemotherapy arm: carboplatin AUC6 i.v. infusion on day 1 plus paclitaxel 90 mg/m2 i.v. infusion on days 1, 8, and 15, every 4 weeks for maximum of 4 cycles Primary prophylaxis with growth factor support not recommended, but secondary prophylaxis allowed for participants who developed grade 3 or 4 neutropenia in previous cycles |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was done centrally by computer. We used the minimisation method and stratified patients by study centre, WHO performance status score (0–1 vs 2), stage (III vs IV), and age (≤̀80 vs >80 years)" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Response reviewed through investigator panels. No information on toxicity and quality of life assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Before the start of treatment, 1 participant excluded from the monotherapy and 2 from doublet chemotherapy group |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | Low risk | No evidence of other bias |
Rijavec 2010.
| Methods | Randomized phase II trial Eligible patients
Exclusion criteria not presented |
|
| Participants | D arm: not informed DG arm: not informed Demographics for study population: 75 randomly assigned, 69 participants (ITT population), median age 75 years (range 70 to 82) |
|
| Interventions | D arm: docetaxel 35 mg/m2 i.v. infusion on days 1, 8, and 15, every 4 weeks DG arm: docetaxel 35 mg/m2 i.v. infusion and gemcitabine 800 mg/m2 i.v. infusion on days 1, 8, and 15, every 4 weeks |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Study prematurely stopped because of slow accrual | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding of outcome assessors. Study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | Unclear risk | No information on blinding of assessors for other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information for attrition bias analysis |
| Selective reporting (reporting bias) | High risk | Results presented in abstract form only. Characteristics of included patients and toxicity partially presented |
| Other bias | High risk | Trial was stopped prematurely because of slow accrual |
Rosso 1988.
| Methods | Randomized multi‐center trial Eligible patients
Exclusion criteria
|
|
| Participants | E arm: 113 participants (ITT population) ‐ median age (range): not reported/56 participants > 60 years of age/elderly population not reported EP arm: 103 participants (ITT population) ‐ median age (range): not reported/59 participants > 60 years of age/elderly population not reported |
|
| Interventions | E arm: etoposide 120 mg/m2 i.v. infusion on days 1, 2, and 3, every 3 weeks EP am: cisplatin 60 mg/m2 on days 1 and 2 plus etoposide 120 mg/m2 i.v. infusion on days 1, 2, and 3, every 3 weeks for maximum of 6 cycles |
|
| Outcomes | Neither primary nor secondary outcomes reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised to be treated...." No further information no random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Of 216 patients accrued into the study, 23 were not evaluable..." |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | No separate elderly subgroup analysis |
Saito 2012.
| Methods | Randomized phase II trial Inclusion criteria
Exclusion criteria
|
|
| Participants | GV arm: 43 participants (ITT population) ‐ median age (range): 67 (34 to 76) years/number of elderly not reported CP arm: 41 participants (ITT population) ‐ median age (range): 65 (20 to 77) years/number of elderly not reported |
|
| Interventions | CP arm: carboplatin AUC6 over 60‐minute i.v. infusion and paclitaxel 200 mg/m2 over 3‐hour i.v. infusion on day 1, every 3 weeks GV arm: gemcitabine 1000 mg/m2 over 30‐minute i.v. infusion and vinorelbine 25 mg/m2 over 6 to 10‐minute i.v. infusion on days 1 and 8, every 3 weeks |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were randomly assigned to 1 of the 2 treatment arms by a minimization method with disease stage (IIIB vs. IV) and body weight loss in the previous 6 months (<5% vs. >5%) as stratifying variables. Randomization was performed at the West Japan Thoracic Oncology Group (now known as the West Japan Oncology Group) Data Center" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two patients were subsequently considered to be ineligible and 3 did not receive the protocol treatment" |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | No separate elderly subgroup analysis |
Sculier 2002.
| Methods | Randomized multi‐center phase III trial Eligible patients
Exclusion criteria
|
|
| Participants | CCI arm: 94 participants (ITTpopulation) ‐ 45 participants > 60 years of age CCG arm: 92 participants (ITT population) ‐ 52 participants > 60 years of age IG arm: 94 participants (ITT population) ‐ 46 participants > 60 years of age |
|
| Interventions | CCI arm: cisplatin 60 mg/m2 over 60‐minute i.v. infusion and carboplatin AUC3 over 30‐minute i.v. infusion and Ifosfamide 4500 mg/m2 over 18‐hour i.v. infusion on day 1, every 4 weeks CCG arm: cisplatin 60 mg/m2 over 60‐minute i.v. infusion on day 1 and carboplatin AUC3 over 30‐minute i.v. infusion on day 1 and gemcitabine 1000 mg/m2 as on days 1, 8, and 15, every 4 weeks IG arm: ifosfamide 4500 mg/m2 over 18‐hour i.v. infusion on day 1 and gemcitabine 1000 mg/m2 on days 1, 8, and 15, every 4 weeks Responding participants given additional courses until best response, disease progression, or major toxicity |
|
| Outcomes | Primary outcome
|
|
| Notes | Despite multiple attempts to contact study authors, no data related to elderly subgroup analysis obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed centrally using the minimisation technique and stratified according to centre, Karnofsky PS, presence of brain metastases and prior chest irradiation" |
| Allocation concealment (selection bias) | Unclear risk | "Treatment allocation was obtained by calling the ELCWP (European Lung Cancer Working Party) data centre" |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Four (1.4%) were ineligible for the study (three in the CCG arm and one in the IG arm) for the following reasons: small‐cell lung cancer histology, prior chemotherapy administration, increased bilirubinaemia prior to randomisation, absence of informed consent" |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | No separate elderly subgroup analysis |
Sederholm 2005.
| Methods | Randomized multi‐center phase III trial Eligible patients
Exclusion criteria
|
|
| Participants | G arm: 170 participants (ITT population)/34 elderly participants GC arm: 164 participants (ITT population)/41 elderly participants |
|
| Interventions | G arm: gemcitabine 1250 mg/m2 over 30 to 60‐minute i.v. infusion on days 1 and 8, every 3 weeks for maximum of 6 cycles, unless disease progression or intolerable toxicity. GC arm: carboplatin AUC5 over 30 to 60‐minute i.v. infusion on day 1 and gemcitabine 1250 mg/m2 over 30 to 60‐minute i.v. infusion on days 1 and 8, every 3 weeks |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | No information on subgroup analysis of elderly participants, despite multiple attempts to contact study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to receive either..." |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | Study designed for general population; elderly subgroup analysis not planned |
Smit 2003.
| Methods | Randomized multi‐center, phase III trial Eligible patients
|
|
| Participants | CP arm: 159 participants (ITT population) ‐ median age (range): 57 (27 to 75) years/elderly participants not reported CG arm: 160 participants (ITT population) ‐ median age (range): 57 (28 to 75) years/elderly participants not reported GP arm: 161 participants (ITT population) ‐ median age (range): 56 (31 to 75) years/elderly participants not reported |
|
| Interventions | CP arm: cisplatin 80 mg/m2 i.v. infusion on day 1 plus paclitaxel 175 mg/m2 over 3‐hour i.v. infusion on day 1, every 3 weeks CG arm: cisplatin 80 mg/m2 i.v. infusion on day 1 plus gemcitabine 1250 mg/m2 over 30‐minute i.v. infusion on days 1 and 8, every 3 weeks GP arm: paclitaxel 175 mg/m2 over 3‐hour i.v. infusion on day 1 plus gemcitabine 1250 mg/m2 over 30‐minute i.v. infusion on days 1 and 8, every 3 weeks Prophylactic antiemetics during and after cisplatin administration, typically ondansetron and dexamethasone. Responding participants received maximum of 6 cycles |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | No information on inclusion of elderly patients nor on specific subgroup analysis, despite multiple attempts to contact study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed centrally by the EORTC Data Center after stratification for PS (0 to 1 v 2), stage of disease (IIIB v IV), and institute, using the minimization technique" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | Study designed for the general population; elderly subgroup analysis not planned |
Stathopoulos 2004.
| Methods | Randomized multi‐center phase III trial Eligible patients
Exclusion criteria
|
|
| Participants | CP arm: 185 participants (ITT population) ‐ median age (range): 65 (30 to 83) years/elderly participants not reported PV arm: 175 participants (ITT population) ‐ median age (range): 65 (36 to 84) years/elderly participants not reported |
|
| Interventions | CP arm: carboplatin AUC6 plus paclitaxel 175 mg/m2 over 3‐hour i.v. infusion on day 1, every 3 weeks up to 6 cycles PV arm: paclitaxel 135 mg/m2 plus vinorelbine 25 mg/m2 on day 1, every 2 weeks up to 9 cycles |
|
| Outcomes | Primary outcomes
|
|
| Notes | No information on number of elderly participants nor specific subgroup analysis obtained, despite multiple attempts to contact study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation was performed centrally and patients were stratified by three prognostic variables: disease stage (locally advanced versus metastatic disease), performance status (ECOG performance status of 0–2) and investigational site" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Twelve patients, four in arm A and eight in arm B, did not undergo any treatment: some refused and others had renal or heart abnormalities" |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | Study designed for general population; elderly subgroup analysis not planned |
Tan 2005.
| Methods | Randomized multi‐center international trial Eligible patients
Exclusion criteria
|
|
| Participants | GV arm: 157 participants (ITT population) ‐ median age (range): 57 (29 to 74) years/elderly subgroup not reported CV arm: 159 participants (ITT population) ‐ median age (range): 60 (30 to 75) years/elderly subgroup not reported |
|
| Interventions | GV arm: gemcitabine 1000 mg/m2 i.v. infusion plus vinorelbine 25 mg/m2 i.v. infusion on days 1 and 8, every 3 weeks up to 6 cycles CV arm: carboplatin AUC5 on day 1 plus vinorelbine 30 mg/m2 i.v. infusion on days 1 and 8, every 3 weeks up to 6 cycles |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Despite multiple attempts, we could not contact study authors to retrieve data on elderly population | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were randomised centrally and stratified by centre and by disease stage, IIIB/IV or relapsing" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | No separate elderly subgroup analysis |
Treat 2010.
| Methods | Randomized multi‐center phase III trial Inclusion criteria
Exclusion criteria
|
|
| Participants | PG arm: 377 participants (ITT population)/112 elderly participants CG arm: 379 participants (ITT population)/119 elderly participants CP arm: 379 participants (ITT population)/107 elderly participants |
|
| Interventions | PG arm: paclitaxel 200 mg/m2 over 3‐hour i.v. infusion on day 1 plus gemcitabine 1000 mg/m2 over 30‐minute i.v. infusion on days 1 and 8, every 3 weeks CG arm: carboplatin AUC5.5 over 15 to 30‐minute i.v. infusion on day 1 plus gemcitabine 1000 mg/m2 over 30‐minute i.v. infusion on days 1 and 8, every 3 weeks ‐ participants with ≥ 20% of bone marrow previously irradiated received a reduced dose of carboplatin AUC5 CP arm: carboplatin AUC6 over 15 to 30‐minute i.v. infusion on day 1 plus paclitaxel 225 mg/m2 over 3‐hour i.v. infusion on day 1, every 3 weeks In the PG and CP arms, participants received prophylactic treatment with dexamethasone 20 mg orally 12 and 6 hours before paclitaxel infusion, diphenhydramine 50 mg i.v. ≤ 1 hour before paclitaxel infusion, and cimetidine 300 mg i.v. (or equivalent) ≤ 1 hour before paclitaxel Granulocyte colony‐stimulating factor (G‐CSF) allowed only for secondary prophylaxis in case of persistent neutropenia despite dose modifications |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Trial designed for the following pair‐wise comparison
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients with stage IIIB (with pleural or pericardial effusion), stage IV, or recurrent NSCLC who met all eligibility criteria were randomly allocated to receive one of the three treatment regimens as summarized in Figure 1. Patient stratification by baseline weight loss (< 5% versus ≥ 5% in previous 6 months), stage of disease (IIIB with effusion versus IV), and brain metastasis (presence versus absence) took place at the time of randomisation to ensure balance across treatment arms with respect to these characteristics" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study authors presented ITT analysis for survival and TTP outcomes. 23, 22, and 13 participants did not start treatment in CG, GP, and CP arms, respectively. They were not included in the ITT analysis. No information on elderly outcomes |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | Unclear risk | Outcomes for elderly population not fully reported. Study designed for the general population; elderly subgroup analysis not planned |
Tsukada 2007.
| Methods | Randomized phase III trial Eligible patients
Exlcusion criteria not presented |
|
| Participants | D arm: 63 elderly participants randomly assigned/56 assessable participants for interim analysis DP arm: 63 elderly participants randomly assigned/56 assessable participants for interim analysis Demographics for study population: median age: 76 years, < 75 years/≥ 75 years: 39%/61%; male/female: 77%/23%; PS 0/1: 39%/61%; stage III/IV or relapsed: 30%/70% |
|
| Interventions | D arm: docetaxel 25 mg/m2 .i.v. infusion on days 1, 8, and 15, every 4 weeks DP arm: docetaxel 20 mg/m2 i.v. infusion and cisplatin 25 mg/m2 i.v. infusion on days 1, 8, and 15, every 4 weeks |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Study prematurely stopped after first interim analysis showed strong interaction in favor of DP arm in participants between 70 and 74 years of age | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding of assessor but study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | Unclear risk | No information on blinding of assessors for other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information for attrition bias analysis |
| Selective reporting (reporting bias) | High risk | Results presented in abstract form only. OS reported only for a subgroup of participants between 70 and 74 years of age. ITT analysis retrieved in slide from ASCO meeting presentations. Only limited data available. No other outcomes reported |
| Other bias | High risk | Study prematurely stopped after first interim analysis showed strong interaction in favor of DP arm for participants between 70 and 74 years of age |
Vansteenkiste 2001.
| Methods | Randomized multi‐center phase III trial Eligible patients
Exclusion criteria
|
|
| Participants | G arm: 84 participants (ITT population) ‐ median age (SD): 63.7 (±8.2) years/elderly subgroup not reported PV arm: 85 participants (ITT population) ‐ median age (SD): 63.1 (±8.6) years/elderly subgroup not reported |
|
| Interventions | G arm: gemcitabine 1000 mg/m2 over 30‐minute i.v. infusion days 1, 8, and 15, every 28‐day cycle without standard use of antiemetics (after first cycle prophylactic non‐5‐HT3‐ antagonist antiemetics allowed at discretion of the investigator) PV arm: cisplatin 100 mg/m2 over 1 to 4‐hour i.v. infusion on day 1 and vindesine 3 mg/m2 (maximum 5 mg) on days 1 and 15, every 28‐day cycle with standard pre‐hydration, forced diuresis, and use of 5‐HT3‐antagonists |
|
| Outcomes | Outcomes
Definitions of primary or secondary outcomes |
|
| Notes | Despite multiple attempts to contact study authors, no further data on elderly subgroup provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After this, and after a thorough eligibility check, randomisation between a treatment with GEM or PV was carried out by fax at a central location for all sites" Randomization performed according to CONSORT guidelines |
| Allocation concealment (selection bias) | Low risk | "Each patient's study drug regimen was unknown until the time of randomisation" |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Trial designed as open‐label |
| Blinding of outcome assessment (detection bias) Other outcomes | Low risk | "All claimed responses and stable diseases were to be reviewed by a panel including at least two oncologists (never reviewing their own patients), one research nurse, and one independent external radiologist blinded to treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | Study designed for the general population; elderly subgroup analysis not planned |
Wachters 2003.
| Methods | Randomized multi‐center phase III trial Eligible patients
Exclusion criteria
|
|
| Participants | GE arm: 121 participants (ITT population) ‐ median age (range): 60 (32 to 76) years/elderly subgroup not reported GC arm: 119 participants (ITT population) ‐ median age (range): 60 (29 to 80) years/elderly subgroup not reported |
|
| Interventions | GE arm: gemcitabine 1125 mg/m2 over 30‐minute i.v. infusion on days 1 and 8 plus epirubicin 100 mg/m2 over 5‐minute bolus i.v. infusion on day 1, every 3 weeks GC arm: gemcitabine 1125 mg/m2 over 30‐minute i.v. infusion on days 1 and 8 plus cisplatin 80 mg/m2 over 3‐hour i.v. infusion on day 2. For pre‐hydration, participants in GC arm admitted to hospital for 2 days, every 3 weeks Treatment plan consisted of 5 cycles for each treatment arm, with interruptions due to tumor progression, intolerable toxicity, or participant preference. No primary prophylaxis with G‐CSF |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | No information on inclusion of elderly participants nor on specific subgroup analysis, despite multiple attempts to contact study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were randomised by telephone to receive either cisplatin or epirubicin both with gemcitabine" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Low risk | "After treatment, tumour responses were evaluated by an independent observer" |
| Blinding of outcome assessment (detection bias) Other outcomes | Low risk | "After treatment, tumour responses were evaluated by an independent observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Three randomised patients did not receive chemotherapy because of rapidly deteriorating performance status due to progression of disease before treatment initiation. These patients were included in all analyses" |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | No separate elderly subgroup analysis |
Yamamoto 2004.
| Methods | Randomized phase II trial Eligible patients
Exclusion criteria
|
|
| Participants | DI arm: 57 participants (ITT population) ‐ median age (range): 57 (42 to 77) years/elderly participants included not reported DC arm: 51 participants (ITT population) ‐ median age (range): 62 (39 to 74) years/elderly participants included not reported |
|
| Interventions | DC arm: docetaxel 60 mg/m2 i.v. infusion and cisplatin 80 mg/m2 i.v. infusion on day 1, every 3 weeks DI arm: docetaxel 60 mg/m2 i.v. infusion on day 8 and irinotecan 60 mg/m2 i.v. infusion on days 1 and 8, every 3 weeks |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Despite multiple attempts to contact study authors, no data related to elderly subgroup analysis were obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to receive the DC regimen or the DI regimen by a minimisation method using stage (IIIB/IV) and treatment institution" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All patients were included in the survival evaluation, and all were assessable for antitumoural efficacy and toxicity" |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | No separate elderly subgroup analysis |
Yamamoto 2006.
| Methods | Randomized phase II trial Eligible patients
Exclusion criteria
|
|
| Participants | GV arm: 64 participants (ITT population) ‐ median age (range): 62 (36 to 74) years/elderly participants included not reported CG arm: 64 participants (ITT population) ‐ median age (range): 60 (30 to 74) years/elderly participants included not reported |
|
| Interventions | GV arm: gemcitabine 1000 mg/m2 in 100 mL of normal saline solution as 30‐minute i.v. infusion and vinorelbine 25 mg/m2 in 20 mL of normal saline solution as 5‐minute i.v. infusion on days 1 and 8, every 3 weeks GC arm: gemcitabine given at a dose of 1000 mg/m2 in 100 mL of normal saline solution as 30‐minute i.v. infusion on days 1 and 8. Carboplatin administered at area under the curve (AUC) of 5 in 500 mL of normal saline solution as 60‐minute i.v. infusion on day 1 only, every 3 weeks. Antiemetics (5HT‐3 antagonists and dexamethasone) permitted as prophylaxis for nausea and vomiting. GCSF allowed for participants with grade 4 leukopenia, grade 4 neutropenia, or febrile neutropenia, according to investigator decision |
|
| Outcomes | Primary outcome
Secondary outcome
|
|
| Notes | Despite multiple attempts to contact study authors, no data related to elderly subgroup analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned randomly to receive the GC regimen or the GV regimen and were stratified by disease stage (Stage IIIB vs. Stage IV), prior treatment (yes vs. no), and institution" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "In the GV arm, 2 patients did not receive trial therapy because of deterioration in their condition. These 2 patients were excluded from the analysis of toxicity, response, and progression‐free survival" |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | No separate elderly subgroup analysis |
Zhang 2006.
| Methods | Randomized trial Eligible patients
|
|
| Participants | P arm: 30 participants CisP arm: 34 participants CarP arm: 32 participants Median age of study population: 70 years; 51 with stage IIIB and 45 stage IV disease. PS at baseline not informed. No geriatric data scales collected |
|
| Interventions | P arm: paclitaxel 60 mg/m2 i.v. infusion on days 1, 8, and 15, every 4 weeks CisP arm: cisplatin 30 mg/m2 i.v. infusion on days 2 to 4 and paclitaxel 60 mg/m2 i.v. infusion on days 1, 8, and 15, every 4 weeks CarP arm: carboplatin AUC5 i.v. infusion on day 2 and paclitaxel 60 mg/m2 i.v. infusion on days 1, 8, and 15, every 4 weeks |
|
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | No information on blinding of assessors. Study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | No information on blinding of assessors for other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | No separate analysis on participants > 70 years |
Zukin 2013.
| Methods | Randomized multi‐center phase III trial Inclusion criteria
Exclusion criteria
|
|
| Participants | P arm: 109 participants (ITT population)/36 elderly participants CP arm: 108 participants (ITT population)/38 elderly participants |
|
| Interventions | P arm: pemetrexed 500 mg/m2 i.v. on day 1, every 21 days for up to 4 cycles CP arm: carboplatin AUC5 and pemetrexed 500 mg/m2, both administered i.v. on day 1, every 21 days for up to 4 cycles All participants received premedications with dexamethasone, vitamin B12, and folic acid according to the pemetrexed label. Maintenance therapy was not allowed |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | "The study was designed with 80% power and a two‐sided type I error of 0.05, assuming that pemetrexed plus carboplatin would result in a median survival of at least 4.3 months and pemetrexed alone would result in a median survival of at least 2.9 months (hazard ratio [HR], 0.674) Despite multiple attempts to contact study authors, no information about subgroup analysis of the elderly was retrieved |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random assignment was performed by an independent provider not involved in the study and stratified by stage (IIIB v IV), weight loss (< 5% v ≥ 5%), and age (< 70 v ≥70 years)" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Twelve patients—seven in the P arm and five in the CP arm—were deemed ineligible because of stage IIIB disease without a malignant pleural effusion (n = 4), uncontrolled CNS disease (n = 2), non measurable disease (n = 1), glomerular filtration rate < 45 mL/min (n = 2), transaminases > 5x the upper limit of normal range (n = 2), and prior chemotherapy" No information about number of elderly participants excluded |
| Selective reporting (reporting bias) | High risk | Data for the elderly provided only for OS outcome |
| Other bias | High risk | Elderly subgroup analysis not planned. Study authors presented a post hoc analysis for OS only |
Zwitter 2010.
| Methods | Randomized phase II trial Eligible patients
Exclusion criteria not presented |
|
| Participants | G arm: 57 participants (ITT population)/24 elderly CG arm: 55 participants (ITT population)/18 elderly |
|
| Interventions | G arm: gemcitabine 1250 mg/m2 over 20 to 30‐minute i.v. infusion on days 1 and 8, every 3 weeks for maximum of 6 cycles CG arm: gemcitabine 800 mg/m2 over 6‐hour i.v. infusion on day 1 and cisplatin 60 mg/m2 i.v. infusion on day 2, every 3 weeks for maximum of 6 cycles |
|
| Outcomes | Overall survival Progression‐free survival No information on which outcome was defined as primary or secondary |
|
| Notes | No data related to elderly subgroup analysis obtained, despite contact with study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were registered for the trial by e‐mail to the data manager of the unit of clinical research. Randomization between the arms A (gemcitabine as monotherapy) and B (low‐dose gemcitabine in long infusion and cisplatin at reduced dose), 1:1, was done using a computer‐generated sequence of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of outcome assessment (detection bias) OS and 1y OS rate outcome | Unclear risk | Open‐label study considered to have unclear impact on mortality outcomes |
| Blinding of outcome assessment (detection bias) Other outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "During the interval between the patient’s registration for the trial and the actual start of chemotherapy, the general condition of nine patients (four randomised into arm A and five into arm B) deteriorated to such a degree that they received supportive treatment only. These patients are not included in the statistics of the response to treatment, toxicity, quality of life, and time to progression, but remain in the trial for survival as the primary endpoint" |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | Unclear risk | No data available for elderly subgroup |
1yOS: one‐year survival rate; ADL: activities of daily living; AGC: absolute granulocyte count; AJCC: American Joint Committee on Cancer; ALT: amino alanine transferase; ASCO: American Society of Clinical Oncology; AST: aspartate amino transferase; AUC: area under the curve; CGA: Comprehensive Geriatric Assessment; CIRS‐G: Cumulative Illness Rating Scale for Geriatrics; CNS: central nervous system; CONSORT: Consolidated Standards of Reporting Trials; ECOG: Eastern Cooperative Oncology Group; ELCWP: European Lung Cancer Working Party; EORTC: European Organization for Research and Treatment of Cancer; G‐CSF: granulocyte‐colony stimulating factor; Gy: Gray; HRQoL: health‐related quality of life; IADL: instrumental activities of daily living; ITT: intention‐to‐treat; i.v.: intravenously; LVEF: left ventricle ejection fraction; MMSE: Mini‐Mental State Examination; MUGA: multi‐gated acquisition; NCI‐CTAE: National Cancer Institute ‐ Common Terminology Criteria for Adverse Events; NSCLC: non‐small cell lung cancer; OS: overall survival; PFS: progression‐free survival; PS: performance status; QoL: quality of life; RECIST: Response Evaluation Criteria in Solid Tumors; rhG‐CSF: recombinant human granulocyte colony‐stimulating factor; RR: response rate; TTP: time‐to‐progression; UNL: upper normal limit; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Binder 2007 | Unpublished data related to elderly subgroup could not be retrieved, despite contact with study author |
| Colucci 1997 | Study did not allow inclusion of patients ≥ 70 years of age |
| Comella 2007 | Study did not allow inclusion of patients ≥ 70 years of age |
| De Marinis 1999 | Study was designed to evaluate the role of vindesine and lonidamine through 4‐arm randomization to (1) V arm; (2) L arm; (3) VL arm, and (4) BSC arm. Investigators performed a factorial 2 × 2 analysis of arms (1) and (4) vs arms (2) and (3) (effect of lonidamine) or (2) and (4) vs arms (1) and (3). Therefore, we considered that no information was available about analysis of non‐platinum single‐agent vs non‐platinum combination. We also considered uncertain the activity of lonidamine in the treatment of advanced non‐small cell lung cancer |
| Gebbia 2003 | Trial was designed to compare 4 different strategies vs platinum combinations as follows: (1) gemcitabine‐Ifosfamide for 2 cycles followed by cisplatin‐vinorelbine; (2) cisplatin‐vinorelbine for 2 cycles followed by gemcitabine‐ifosfamide; (3) vinorelbine‐cisplatin; and (4) cisplatin‐gemcitabine |
| Greco 2002 | Elderly subgroup represents only a small minority |
| Gridelli 1996 | Study did not allow inclusion of patients ≥ 70 years of age |
| Gridelli 2003b | Participants were eligible if they had histological or cytological proof of non‐small cell lung cancer (NSCLC) and were younger than 70 years of age |
| Gridelli 2007 | Participants were randomly assigned to (1) pemetrexed, or (2) sequential pemetrexed and gemcitabine. We have not considered sequential therapy as a non‐platinum combination. Eligibility criteria included patients older than 70 years of age, or younger but considered poor candidates for platinum therapy |
| Morabito 2013 | Inclusion criteria did not allow enrolment of patients older than 70 years |
| Novello 2009 | Study did not allow inclusion of patients ≥ 70 years of age |
| Rocha Lima 2004 | Participants were randomly assigned to 2 non‐platinum combinations: (1) gemcitabine‐irinotecan combination, or (2) gemcitabine‐docetaxel combination |
| Rubio 2009 | Elderly patients were not included in the trial, even though the protocol allowed inclusion of patients from 18 to 75 years old |
| Zatloukal 2008 | Phase II trial in which 62 participants were randomly assigned to (1) cisplatin 75 mg/m2 and larotaxel 50 mg/m2 on day 1, or (2) gemcitabine 800 mg/m2 on days 1 and 8 plus larotaxel 50 mg/m2 on day 8. We considered larotaxel an investigational drug, whose activity is not well established. Therefore, we excluded this RCT from our review |
Characteristics of ongoing studies [ordered by study ID]
NCT01405586.
| Trial name or title | MILES‐3: Cisplatin in Combination With Gemcitabine for Elderly Patients With Lung Cancer |
| Methods | Multi‐center randomized controlled trial, open‐label |
| Participants | Eligibility criteria Inclusion criteria
Exclusion criteria
|
| Interventions | Control arm: gemcitabine 1200 mg/m2 days 1 and 8, every 3 weeks for 6 cycles Experimental arm: cisplatin 60 mg/m2 day 1, every 3 weeks + gemcitabine 1000 mg/m2 days 1 and 8, every 3 weeks for 6 cycles |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | March 2011 |
| Contact information | Francesco Perrone, M.D., Ph.D.; +39 081 5903571; email: francesco.perrone@usc‐intnapoli.net Maria Carmela Piccirillo, M.D.; +39 081 5903615; email: marilina.piccirillo@usc‐intnapoli.net |
| Notes |
NCT01593293.
| Trial name or title | A randomized, Open‐Label, Phase III Study Comparing Pemetrexed With and Without Carboplatin in Elderly Patients With Advanced Non‐Squamous Non‐Small Cell Lung Cancer |
| Methods | Randomized multi‐center open‐label |
| Participants | Eligibility criteria Inclusion criteria
Exclusion criteria
|
| Interventions | Control arm: pemetrexed 500 mg/m2 on day 1, every 3 weeks until progression or unacceptable toxicity Experimental arm: pemetrexed 500 mg/m2 on day 1 plus carboplatin AUC5 i.v. on day 1, every 3 weeks for 4 cycles, followed by pemetrexed 500 mg/m2 on day 1, every 3 weeks for maintenance therapy |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | March 2012 |
| Contact information | Sang‐We Kim, M.D.; 82‐2‐3010‐3215; email: swkim@amc.seoul.kr |
| Notes |
NCT01656551.
| Trial name or title | A Factorial Study Comparing Pemetrexed With Gemcitabine and Testing the Efficacy of the Addition of Cisplatin in Elderly Patients With Non‐Squamous Advanced, Metastatic, or Recurrent NSCLC |
| Methods | Controlled randomized trial, open‐label, multi‐center. This study used a factorial design that allowed 2 comparisons: single‐agent therapy vs chemotherapy plus cisplatin (arms A + C vs arms B + D), and gemcitabine vs pemetrexed (arms A + B vs arms C + D) |
| Participants | Eligibility criteria Inclusion criteria
Exclusion criteria
|
| Interventions | Arm A (active comparator): gemcitabine 1200 mg/m2 days 1 and 8, every 3 weeks Arm B (experimental arm): cisplatin 60 mg/m2 day 1 + gemcitabine 1000 mg/m2 days 1 and 8, every 3 weeks Arm C (active comparator): pemetrexed 500 mg/m2 i.v. day 1, every 3 weeks Arm D (experimental arm): cisplatin 60 mg/m2 day 1 + pemetrexed 500 mg/m2 day 1, every 3 weeks |
| Outcomes | Primary outcome
Secondary outcomes
Other outcome measures
|
| Starting date | July 2012 |
| Contact information | Francesco Perrone, M.D., Ph.D.; +39 081 5903571; email: francesco.perrone@usc‐intnapoli.net Maria Carmela Piccirillo, M.D.; +39 081 5903681; email: marilina.piccirillo@usc‐intnapoli.net |
| Notes |
Differences between protocol and review
For time‐to‐events outcomes, we combined data using the generic inverse variance method, and we presented measurements of treatment effect as hazard ratios (HRs) and 95% confidence intervals (CIs). For dichotomous data, we used the Mantel‐Haenszel method, and we presented measurements of treatment effect as risk ratios (RRs) with 95% CIs. We decided to combine the two comparisons of non‐platinum single‐agent versus platinum combination treatment and non‐platinum combination versus platinum combination in non‐platinum therapy versus platinum combination therapy. We explored the non‐platinum regimen (given as single‐agent or combination treatment) in between‐trial subgroup analyses. We also performed exploratory between‐trial subgroup analyses based on type of platinum agent used (cisplatin or carboplatin) and type of trial conducted (specifically designed for the elderly or elderly subgroup analysis from an adult intention‐to‐treat population). A planned subgroup analysis based on age, performance status, histology, and smoking history could not be performed because data were insufficient. We also performed unplanned sensitivity analyses while excluding unpublished or prematurely interrupted trials as representative of grey literature.
Contributions of authors
FNS ‐ background, objectives, and outcomes definitions and protocol organization in RevMan 5.3, study selection, data extraction, data analysis, and discussion.
TBdC ‐ data extraction, data analysis, and discussion.
MRSC ‐ study selection, data extraction, data analysis, and discussion.
RR ‐ methodological topics and critical appraisal of the last protocol version.
Sources of support
Internal sources
Brazilian Cochrane Center, Brazil.
External sources
No sources of support supplied
Declarations of interest
Tiago B de Castria, Marcelo RS Cruz and Rachel Riera declare no conflicts of interest.
Fabio N Santos: has recieved payment from Roche, Merck‐Sharp Dome and Bristol‐Meyers Squibb for lectures on new systemic treatment for advanced melanoma.
He has received support with travel, accommodations and subscription for ESMO 2014 Congress at Madrid, Spain from Pfizer, support with travel, accommodations and subscription for ASCO 2013 at Chicago, United States of America from Bayer and Support with travel, accommodations and subscription for Hot Topics in Melanoma Meeting 2015 at New York, NY, United States of America from Merck‐Sharp Dome.
Edited (no change to conclusions)
References
References to studies included in this review
Abe 2011 {unpublished data only}
- Abe T, Yokoyama A, Takeda K, Ohe S, Kudoh S, Ichinose Y, et al. Randomized phase III trial comparing weekly docetaxel (D)‐cisplatin (P) combination with tri weekly D alone in elderly patients (pts) with advanced non‐small cell lung cancer (NSCLC): an intergroup trial of JCOG0803/WJOG4307L. Journal of Clinical Oncology 2011;29(suppl;abstr 7509):.. [Google Scholar]
Alberola 2003 {published data only (unpublished sought but not used)}
- Alberola V, Camps C, Provencio M, Isla D, Rosell R, Vadell C, et al. Cisplatin plus gemcitabine versus a cisplatin‐based triplet versus non platinum sequential doublets in advanced non‐small cell lung cancer: a Spanish lung cancer group phase III randomized trial. Journal of Clinical Oncology 2003;21:3207‐13. [DOI] [PubMed] [Google Scholar]
Berghmans 2013 {published data only}
- Berghmans T, Lafitte JJ, Scherpereel A, Paesmans M, Lecomte J, Marco VG, et al. An ELCWP phase III trial comparing ifosfamide and cisplatin regimens in advanced NSCLC. Anticancer Research 2013;33(12):5477‐82. [PubMed] [Google Scholar]
Boni 2012 {published and unpublished data}
- Boni C, Tiseo M, Boni L, Baldini E, Recchia F, Barone C, et al. Triples versus doublets with or without cisplatin in the first‐line treatment of stage IIIB, IV non‐small cell lung cancer (NSCLC) patients: a multicenter randomised trial (FAST). British Journal of Cancer 2012;106:658‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buccheri 1997 {published data only (unpublished sought but not used)}
- Buccheri G, Ferrigno D. Efficacy of platinum‐based regimens in non‐small cell lung cancer. A negative report from the Cuneo Lung Cancer Study Group. Lung Cancer 1997;18:57‐70. [DOI] [PubMed] [Google Scholar]
Chen 2002 {published data only (unpublished sought but not used)}
- Chen YM, Perng RP, Lee YC, Shih JF, Lee CS, Tsai CM, et al. Paclitaxel plus carboplatin, compared with paclitaxel plus gemcitabine, shows similar efficacy while more cost‐effective: a randomized phase II study of combination chemotherapy against inoperable non‐small‐cell lung cancer previously untreated. Annals of Oncology 2002;13:108‐15. [DOI] [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen YM, Perng RP, Shih JF, Whang‐Peng J. A phase II randomized study of vinorelbine alone or with cisplatin against chemo‐naive inoperable non‐small cell lung cancer in the elderly. Lung Cancer 2008;61:214‐9. [DOI: 10.1016/j.lungcan.2007.12.009] [DOI] [PubMed] [Google Scholar]
Comella 2004 {published data only (unpublished sought but not used)}
- Comella P, Frasci G, Carnicelli P, Massidda B, Buzzi F, Filipelli G, et al. Gemcitabine with either paclitaxel or vinorelbine vs paclitaxel or gemcitabine alone for elderly or unfit advanced non‐small‐cell lung cancer patients. British Journal of Cancer 2004;91:489‐97. [DOI: 10.1039/sj.bjc.6602011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Depierre 1994 {published data only}
- Depierre A, Chastang C, Quoix E, Lebeau B, Blanchon F, Paillot N at al. Vinorelbine versus vinorelbine plus cisplatin in advanced non‐small cell lung cancer: a randomized trial.. Annals of Oncology 1994;5(1):37‐42. [DOI] [PubMed] [Google Scholar]
Flotten 2012 {published data only (unpublished sought but not used)}
- Flotten O, Gronberg BH, Bremnes R, Amundsen T, Sundstrom S, Rolke H, et al. Vinorelbine and gemcitabine vs vinorelbine and carboplatin as first‐line treatment of advanced NSCLC. A phase III randomised controlled trial by the Norwegian Lung Cancer Study Group. British Journal of Cancer 2012;107:442‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frasci 2001 {published data only}
- Frasci G, Lorusso V, Panza N, Comella P, Nicolella G, Bianco A, et al. Gemcitabine plus vinorelbine versus vinorelbine alone in elderly patients with advanced non‐small‐cell lung cancer. Journal of Clinical Oncology 2000;18(13):2529‐36. [DOI] [PubMed] [Google Scholar]
- Frasci G, Lorusso V, Panza N, Comella P, Nicolella G, Bianco A, et al. Gemcitabine plus vinorelbine yields better survival outcome than vinorelbine alone in elderly patients with advanced non‐small‐cell lung cancer. A Southern Italy Cooperative Oncology Group (SICOG) phase III. Lung Cancer 2001;34:S65‐9. [DOI] [PubMed] [Google Scholar]
Georgoulias 2001 {published and unpublished data}
- Georgoulias V, Papadakis E, Alexopoulos A, Tsiafaki X, Rapti A, Veslemes M, et al. Platinum‐based and non‐platinum‐based chemotherapy in advanced non‐small‐cell lung cancer: a randomised multicentre trial. Lancet 2001;357:1478‐84. [DOI] [PubMed] [Google Scholar]
Georgoulias 2004 {published and unpublished data}
- Georgoulias V, Ardavanis A, Agelidou A, Agelidou M, Chandrinos V, Tsaroucha E, et al. Docetaxel versus docetaxel plus cisplatin as front‐line treatment of patients with advanced non‐small‐cell lung cancer: a randomized, multicenter phase III trial. Journal of Clinical Oncology 2004;22(13):2602‐9. [DOI: 10.1200/JCO.2004.11.004] [DOI] [PubMed] [Google Scholar]
Georgoulias 2005 {published and unpublished data}
- Georgoulias V, Ardavanis A, Tsifaki X, Agelidou A, Mixalopoulou P, Anagnostopoulou O, et al. Vinorelbine plus cisplatin versus docetaxel plus gemcitabine in advanced non‐small cell lung cancer: a phase III randomized trial. Journal of Clinical Oncology 2005;23(13):2937‐45. [DOI] [PubMed] [Google Scholar]
Georgoulias 2008 {published and unpublished data}
- Georgoulias V, Androuakis N, Kotsakis A, Hatzidaki D, Syrios K, Polyzos A, et al. Docetaxel versus docetaxel plus gemcitabine as front‐line treatment of patients with advanced non‐small cell lung cancer: a randomized multicenter phase III trial. Lung Cancer 2008;59:57‐63. [DOI] [PubMed] [Google Scholar]
Gricorescu 2007 {published data only (unpublished sought but not used)}
- Grigorescu A, Ciuleanu T, Firoiu E, Muresan D, Teodorescu G, Basson BR. A randomized phase II trial of sequential gemcitabine plus vinorelbine followed by gemcitabine plus ifosfamide versus gemcitabine plus cisplatin in the treatment of chemo‐naive patients with stages III and IV non‐small cell lung cancer (NSCLC). Lung Cancer 2007;57:168‐74. [DOI] [PubMed] [Google Scholar]
Gridelli 2003 {published data only}
- Gridelli C, Perrone F, Gallo C, Cigolari S, Rossi A, Piantedosi F, et al. Chemotherapy for elderly patients with advanced non‐small cell lung cancer: the multicenter Italian Lung Cancer in the Elderly study (MILES): phase III randomized trial. Journal of the National Cancer Institute 2003;95(5):362‐72. [DOI] [PubMed] [Google Scholar]
- Maione P, Perrone F, Gallo C, Manzione L, Piantedosi FV, Barbera S, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly with advanced non‐small cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian Lung Cancer in the Elderly study. Journal of Clinical Oncology 2005;23(28):6865‐72. [DOI] [PubMed] [Google Scholar]
Hainsworth 2007 {published data only (unpublished sought but not used)}
- Hainsworth JD, Spigel DR, Farley C, Shipley DL, Bearden JD, Gandhi J, et al. Weekly docetaxel versus docetaxel/gemcitabine in the treatment of elderly or poor performance status patients with advanced non‐small cell lung cancer. A randomized phase 3 trial of the Minnie Pearl Cancer Research network. Cancer 2007;110:2027‐34. [DOI] [PubMed] [Google Scholar]
Hara 1990 {published data only (unpublished sought but not used)}
- Hara N, Ohta M, Ichikawa Y, Kanda T, Shima K, Tamura K, et al. The benefit of cisplatin‐based polychemotherapy for adenocarcinoma of the lung. The Kyushu Lung Cancer Chemotherapy Study Group. Cancer Chemotherapy and Pharmacology 1990;26:47‐51. [DOI] [PubMed] [Google Scholar]
Hsu 2008 {published data only (unpublished sought but not used)}
- Hsu C, Kuo SH, Hu FC, Cheng AL, Shih JY, Yu CHJ, et al. Gemcitabine plus conventional‐dose epirubicin versus gemcitabine plus cisplatin as first‐line chemotherapy for stage IIIB/IV non‐small cell lung cancer ‐ a randomized phase II trial. Lung Cancer 2008;62:334‐43. [DOI] [PubMed] [Google Scholar]
Jeremic 1997 {published data only (unpublished sought but not used)}
- Jeremic B, Shibamoto Y, Acimovic L, Milicic B, Milisavljevic S, Nikolic N. Prolonged administration of oral etoposide alone or with intravenous carboplatin in stage IV non‐small cell lung cancer: a randomized trial. Lung Cancer 1997;18:179‐88. [DOI] [PubMed] [Google Scholar]
Karampeazis 2010 {unpublished data only}
- Karampeazis A, Vamvakas L, Pallis AG, Christophyllakis C, Kentepozidis NK, Chandrinos V, et al. Docetaxel (D) plus gemcitabine (G) compared with G in elderly patients with advanced non‐small cell lung cancer (NSCLC): preliminary results of a randomized phase III Hellenic Oncology Research Group trial. Journal of Clinical Oncology 2010;28:abstr 7605. [Google Scholar]
Katakami 2006 {published data only (unpublished sought but not used)}
- Katakami N, Takiguchi Y, Yoshimori K, Isobe H, Bessho A, Yoshimura A, et al. Docetaxel in combination with either cisplatin or gemcitabine in unresectable non‐small cell lung carcinoma: a randomized phase II study by the Japan Lung Cancer Cooperative Clinical Study Group. Journal of Thoracic Oncology 2006;1(5):447‐53. [PubMed] [Google Scholar]
Kubota 2008 {published and unpublished data}
- Kawahara M, Tada H, Tokoro A, Teramukai S, Origasa H, Kubota K, et al. Quality‐of‐life evaluation for advanced non‐small‐cell lung cancer: a comparison between vinorelbine plus gemcitabine followed by docetaxel versus paclitaxel plus carboplatin regimens in a randomized trial: Japan Multinational Trial Organization LC00‐03 (BRI LC03‐01). BMC Cancer 2011;11:356‐63. [DOI: 10.1186/1471-2407-11-356] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K, Kawahara M, Ogawara M, Nishiwaki Y, Komuta K, Minato K, et al. Vinorelbine plus gemcitabine followed by docetaxel versus carboplatin plus paclitaxel in advanced non‐small cell lung cancer: a randomised, open‐label, phase III study. Lancet Oncology 2008;9:1135‐42. [DOI] [PubMed] [Google Scholar]
Laack 2004 {published and unpublished data}
- Laack E, Dickgreber N, Muller T, Knuth A, Benk J, Lorenz C, et al. Randomized phase III study of gemcitabine, vinorelbine and cisplatin in the treatment of advanced non‐small cell lung cancer: from the German and Swiss Lung Cancer Study Group. Journal of Clinical Oncology 2004;22(12):2348‐56. [DOI] [PubMed] [Google Scholar]
Le Chevalier 1994 {published data only (unpublished sought but not used)}
- Chevalier T, Brisgand D, Douillard JY, Pujol JL, Alberola V, Monnier A, et al. Randomized study of vinorelbine and cisplatin versus vindesine and cisplatin versus vinorelbine alone in advanced non‐small‐cell lung cancer: results of a European multicenter trial including 612 patients. Journal of Clinical Oncology 1994;12:360‐7. [DOI] [PubMed] [Google Scholar]
- Soria JC, Brisgand D, Chevalier T. Do all patients with advanced non‐small‐cell lung cancer benefit from cisplatin‐based combination therapy?. Annals of Oncology 2001;12:1667‐70. [DOI] [PubMed] [Google Scholar]
Lilenbaum 2005 {published data only (unpublished sought but not used)}
- Lilenbaun RC, Herndon JE, List MA, Desch C, Watson DM, Miller AA, et al. Single‐agent versus combination chemotherapy in advanced non‐small cell lung cancer: the Cancer and Leukemia group B (study 9730). Journal of Clinical Oncology 2005;23(1):190‐6. [DOI] [PubMed] [Google Scholar]
Lilenbaum 2005b {published data only (unpublished sought but not used)}
- Lilenbaum RC, Chen CS, Chidiac T, Schwarzenberger PO, Thant M, Versola M, et al. Phase II randomized trial of vinorelbine and gemcitabine versus carboplatin and paclitaxel in advanced non‐small cell lung cancer. Annals of Oncology 2005;16:97‐101. [DOI] [PubMed] [Google Scholar]
Lou 2010 {published data only}
- Lou GY, Li T, Gu CP, Hong D, Zhang YP. Efficacy study of single‐agent gemcitabine versus gemcitabine plus carboplatin in untreated elderly patients with stage IIIb/IV non‐small‐cell lung cancer. Zhonghua Yi Xue Za Zhi 2010;90(2):100‐2. [PubMed] [Google Scholar]
Manegold 1998 {published data only (unpublished sought but not used)}
- Manegold C. Single‐agent gemcitabine versus cisplatin/etoposide in patients with inoperable, locally advanced, or metastatic non‐small cell lung cancer. Seminars in Oncology 1998;25(Suppl 9):18‐22. [PubMed] [Google Scholar]
Mok 2005 {published data only (unpublished sought but not used)}
- Mok TS, Lam KC, Lee C, Zhang L, Wong H, Chan AT, et al. Phase II randomized study comparing the toxicity profile of gemcitabine plus cisplatin with gemcitabine plus oral etoposide in the treatment of advanced non‐small cell lung cancer. Oncology 2005;68(4‐6):485‐92. [DOI] [PubMed] [Google Scholar]
Perng 1997 {published data only (unpublished sought but not used)}
- Perng RP, Chen YM, Ming‐Liu J, Tsai CM, Lin WC, Yang KY, et al. Gemcitabine versus the combination of cisplatin and etoposide in patients with inoperable non‐small‐cell lung cancer in a phase II randomized study. Journal of Clinical Oncology 1997;15(5):2097‐102. [DOI] [PubMed] [Google Scholar]
Pujol 2005 {published data only (unpublished sought but not used)}
- Pujol JL, Breton JL, Gervais R, Rebattu P, Depierre A, Morere JF, et al. Gemcitabine‐docetaxel versus cisplatin‐vinorelbine in advanced or metastatic non‐small‐cell lung cancer: a phase III study addressing the case for cisplatin. Annals of Oncology 2005;16:602‐10. [DOI] [PubMed] [Google Scholar]
Quoix 2011b {published data only}
- Quoix E, Zalcman G, Oster JP, Westeel V, Pichon E, Lavolé A, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non‐small cell lung cancer: IFCT‐0501 randomized, phase 3 trial. Lancet Oncology 2011;378:1079‐88. [DOI] [PubMed] [Google Scholar]
Rijavec 2010 {unpublished data only}
- Rijavec E, Belvedere O, Aita M, Rossetto C, Follador A, Sacco C, et al. Docetaxel (D) versus docetaxel/gemcitabine (D&G) in the treatment of older patients with advanced non‐small cell lung cancer (NSCLC): an Alpe Adria Thoracic Oncology Multidisciplinary Group randomized phase II trial (ATOM 017). Journal of Clinical Oncology 2010;28(suppl;abstract e18028):.. [Google Scholar]
Rosso 1988 {published data only (unpublished sought but not used)}
- Rosso R, Ardizzoni A, Salvati F, Gallo‐Curcio C, Rubagotti A, Fusco V, et al. Etoposide v etoposide and cisplatin in the treatment of advanced non‐small cell lung cancer: a FONICAP randomized study. Seminars in Oncology 1988;15(6 Suppl 7):49‐51. [PubMed] [Google Scholar]
Saito 2012 {published data only (unpublished sought but not used)}
- Saito H, Nakagawa K, Takeda K, Iwamoto Y, Ando M, Maeda M, et al. Randomized phase II study of carboplatin‐paclitaxel or gemcitabine‐vinorelbine in patients with advanced non‐small‐cell lung cancer and a performance status of 2: West Japan Thoracic Oncology Group 0004. American Journal of Clinical Oncology 2012;35(1):58‐63. [DOI] [PubMed] [Google Scholar]
Sculier 2002 {published data only}
- Sculier JP, Lafitte JJ, Lecomte J, Berghmans T, Thiriaux J, Florin MC, et al. A three‐arm phase III randomised trial comparing combinations of platinum derivates, ifosfamide and/or gemcitabine in stage IV non‐small cell lung cancer. Annals of Oncology 2002;13:874‐82. [DOI] [PubMed] [Google Scholar]
Sederholm 2005 {published data only (unpublished sought but not used)}
- Sederholm C, Hillerdal G, Lamberg K, Kolbeck K, Dufmats M, Westberg R, et al. Phase III trial of gemcitabine plus carboplatin versus single agent gemcitabine in the treatment of locally advanced or metastatic non‐small cell lung cancer: the Swedish Lung Cancer Study Group. Journal of Clinical Oncology 2005;23(33):8380‐8. [DOI] [PubMed] [Google Scholar]
Smit 2003 {published data only (unpublished sought but not used)}
- Giaccone G, European Organization for Research and Treatment of Cancer Group. Early results of a randomized phase III trial of platinum‐containing doublets versus a non‐platinum doublet in the treatment of advanced non‐small cell lung cancer: European Organization for Research and Treatment of Cancer 08975. Seminars in Oncology 2002;29(3 Suppl 9):47‐9. [DOI] [PubMed] [Google Scholar]
- Smit EF, Meerbeeck JPAM, Lianes P, Debruyne C, Legrand C, Schramel F, et al. Three‐arm randomized study of two cisplatin‐based regimens and paclitaxel plus gemcitabine in advanced non‐small‐cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group ‐ EORTC 08975. Journal of Clinical Oncology 2003;21:3909‐17. [DOI] [PubMed] [Google Scholar]
Stathopoulos 2004 {published data only (unpublished sought but not used)}
- Staphoupoulos GP, Veslemes M, Georgatou N, Antoniou D, Giamboudakis P, Katis K, et al. Front‐line paclitaxel‐vinorelbine verus paclitaxel‐carboplatin in patients with advanced non‐small cell lung cancer: a randomized phase III trial. Annals of Oncology 2004;15:1048‐55. [DOI] [PubMed] [Google Scholar]
Tan 2005 {published data only (unpublished sought but not used)}
- Tan EH, Szczesna A, Krzakowski M, Macha HN, Gatzemeier U, Mattson K, et al. Randomized study of vinorelbine‐gemcitabine versus vinorelbine‐carboplatin in patients with advanced non‐small cell lung cancer. Lung Cancer 2005;49:233‐40. [DOI] [PubMed] [Google Scholar]
Treat 2010 {published data only (unpublished sought but not used)}
- Ansari RH, Socinski MA, Edelman MJ, Belani CP, Gonin R, Catalano RB, et al. A retrospective analysis of outcomes by age in a three‐arm phase III trial of gemcitabine in combination with carboplatin or paclitaxel vs. paclitaxel plus carboplatin for advanced non‐small cell lung cancer. Critical Reviews in Oncology/Hematology 2011;78(2):162–71. [DOI] [PubMed] [Google Scholar]
- Treat JA, Gonin R, Socinski MA, Edelman MJ, Catalano RB, Marinucci DM, et al. A randomized, phase III multicenter trial of gemcitabine in combination with carboplatin or paclitaxel versus paclitaxel plus carboplatin in patients with advanced or metastatic non‐small cell lung cancer. Annals of Oncology 2010;21:540‐7. [DOI] [PubMed] [Google Scholar]
Tsukada 2007 {unpublished data only}
- Tsukada H, Yokoyama A, Nishiwaki Y, Shinkai T, Harada M, Ando M, et al. Randomized controlled trial comparing docetaxel (D)‐cisplatin (P) combination with D alone in elderly patients (pts) with advanced non‐small cell lung cancer (NSCLC): JCOG0207. Journal of Clinical Oncology 2005;25(suppl abstract 7629):.. [Google Scholar]
Vansteenkiste 2001 {published data only (unpublished sought but not used)}
- Vansteenkiste J, Vandebroek J, Nackaerts K, Dooms C, Galdermans D, Bosquée L, et al. Influence of cisplatin‐use, age, performance status and duration of chemotherapy on symptom control in advanced non‐small cell lung cancer: detailed symptom analysis of a randomised study comparing cisplatin‐vindesine to gemcitabine. Lung Cancer 2003;40:191‐9. [DOI] [PubMed] [Google Scholar]
- Vansteenkiste JF, Vandebroek JE, Nackaerts KL, Weynants P, Valcke YJ, Verresen DA, et al. Clinical‐benefit response in advanced non‐small‐cell lung cancer: a multicentre prospective randomised phase III study of single agent gemcitabine versus cisplatin‐ vindesine. Annals of Oncology 2001;12(9):1221‐30. [DOI] [PubMed] [Google Scholar]
Wachters 2003 {published data only (unpublished sought but not used)}
- Wachetrs FM, Putten JWG, Kramer H, Erjavec Z, Eppinga Z, Strijbos H, et al. First‐line gemcitabine with cisplatin or epirubicin in advanced non‐small‐cell lung cancer: a phase III. British Journal of Cancer 2003;89:1192‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamamoto 2004 {published data only (unpublished sought but not used)}
- Yamamoto N, Fukuoka M, Negoro SI, Nakagawa K, Saito H, Matsui K, et al. Randomised phase II study of docetaxel/cisplatin vs docetaxel/irinotecan in advanced non‐small‐cell lung cancer: a West Japan Thoracic Oncology Group (WJTOG9803). British Journal of Cancer 2004;90:87‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamamoto 2006 {published data only (unpublished sought but not used)}
- Yamamoto N, Nakagawa K, Uejima H, Sugiura T, Takada Y, Negoro S, et al. Randomized phase II study of carboplatin/gemcitabine versus vinorelbine/gemcitabine in patients with advanced non small cell lung cancer. West Japan Thoracic Oncology Group (WJTOG) 0104. Cancer 2006;107:599‐605. [DOI] [PubMed] [Google Scholar]
Zhang 2006 {published data only (unpublished sought but not used)}
- Zhang K, Hong J, Xie G. Weekly single‐agent versus combination chemotherapy for elderly patients with advanced non‐small cell lung cancer. Chinese Journal of Clinical Oncology 2006;33(10):583‐5. [Google Scholar]
Zukin 2013 {published data only (unpublished sought but not used)}
- Zukin M, Barrios CH, Pereira JR, Ribeiro RA, Beato CAM, Nascimento YN, et al. Randomized phase III trial of single‐agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non‐small cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. Journal of Clinical Oncology 2013;31(23):2849‐53. [DOI] [PubMed] [Google Scholar]
Zwitter 2010 {published and unpublished data}
- Zwitter M, Kovac V, Rajer M, Vrankar M, Smrdel U. Two schedules of chemotherapy for patients with non‐small cell lung cancer in poor performance status: a phase II randomized trial. Anticancer Drugs 2010;21(6):662–8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Binder 2007 {published and unpublished data}
- Binder D, Schweisfurth H, Grah C, Schaper C, Temmesfeld B, Siebert G, et al. Docetaxel/gemcitabine or cisplatin/gemcitabine followed by docetaxel in the first‐line treatment of patients with metastatic non‐small cell lung cancer (NSCLC): results of a multicentre randomized phase II. Cancer Chemotherapy and Pharmacology 2007;60:143‐50. [DOI] [PubMed] [Google Scholar]
Colucci 1997 {published data only}
- Colucci G, Gebbia V, Galetta D, Riccardi F, Cariello S, Gebbia N, et al. Cisplatin and vinorelbine followed by ifosfamide plus epirubicin vs the opposite sequence in advanced unresectable stage III and metastatic stage IV non‐small‐cell lung cancer: a prospective randomized study of the Southern Italy Oncology Group (GOIM). British Journal of Cancer 1997;76(11):1509‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Comella 2007 {published data only}
- Comella P, Filippelli G, Cataldis G, Massidda B, Frasci G, Maiorino L, et al. Efficacy of the combination of cisplatin with either gemcitabine and vinorelbine or gemcitabine and paclitaxel in the treatment of locally advanced or metastatic non‐small‐cell lung cancer: a phase III randomised trial of the Southern Italy Cooperative Oncology Group (SICOG 0101). Annals of Oncology 2007;18:324‐30. [DOI] [PubMed] [Google Scholar]
De Marinis 1999 {published data only}
- Marinis F, Rinaldi M, Ardizzoni A, Bruzzi P, Pennucci MC, Portalone L, et al. The role of vindesine and lonidamine in the treatment of elderly patients with advanced non‐small cell lung cancer: a phase III randomized FONICAP trial. Italian Lung Cancer Task Force. Tumori 1999;85:177‐82. [DOI] [PubMed] [Google Scholar]
Gebbia 2003 {published data only}
- Gebbia V, Galetta D, Caruso M, Verderame F, Pezzella G, Valdesi M, et al. Gemcitabine and cisplatin versus vinorelbine and cisplatin versus ifosfamide+gemcitabine followed by vinorelbine and cisplatin versus vinorelbine and cisplatin followed by ifosfamide and gemcitabine in stage IIIB‐IV non small cell lung carcinoma: a prospective randomized phase III trial of the Gruppo Oncologico Italia Meridionale. Lung Cancer 2003;39:179‐89. [DOI] [PubMed] [Google Scholar]
Greco 2002 {published and unpublished data}
- Greco FA, Gray JR, Thompson DS, Burris HA III, Erland JB, Barton JH, et al. Prospective randomized study of four novel chemotherapy regimens in patients with advanced non small cell lung carcinoma. A Minnie Pearl Cancer Research Network Trial. Cancer 2002;95:1279‐85. [DOI: 10.1002/cncr.10810] [DOI] [PubMed] [Google Scholar]
Gridelli 1996 {published data only}
- Gridelli C, Perrone F, Palmeri S, D'Aprile M, Cognetti F, Rossi A, et al. Mitomycin C plus vindesine plus etoposide (MEV) versus mitomycin C plus vindesine plus cisplatin (MVP) in stage IV non‐small‐cell lung cancer: a phase III multicentre randomised trial. The "Gruppo Oncologico Centro‐Sud‐Isole' (G.O.C.S.I.). Annals of Oncology 1996;7:821‐6. [DOI] [PubMed] [Google Scholar]
Gridelli 2003b {published data only}
- Gridelli C, Gallo C, Shepherd FA, Illiano A, Piantedosi F, Robbiati SF, et al. Gemcitabine plus vinorelbine compared with cisplatin plus vinorelbine or cisplatin plus gemcitabine for advanced non‐small‐cell lung cancer: a phase III trial of the Italian GEMVIN Investigators and the National Cancer Institute of Canada Clinical Trials Group. Journal of Clinical Oncology 2003;21:3025‐34. [DOI] [PubMed] [Google Scholar]
Gridelli 2007 {published data only}
- Gridelli C, Kaukel E, Gregorc V, Migliorino MR, Muller TR, Manegold C, et al. Single agent pemetrexed or sequential pemetrexed/gemcitabine as front‐line treatment of advanced non‐small‐cell lung cancer in the elderly patients or patients ineligible for platinum‐based chemotherapy: a multicenter, randomized, phase II trial. Journal of Thoracic Oncology 2007;2(3):221‐9. [DOI] [PubMed] [Google Scholar]
Morabito 2013 {published data only}
- Morabito A, Gebbia V, Maio M, Cinieri S, Vigano MG, Bianco R, et al. Randomized phase III trial of gemcitabine and cisplatin vs. gemcitabine alone in patients with advanced non‐small cell lung cancer and a performance status of 2: the CAPPA‐2 study. Lung Cancer 2013;81(1):77‐83. [DOI] [PubMed] [Google Scholar]
Novello 2009 {published data only}
- Novello S, Falcone A, Crino L, Rinaldi M, Nardi M, Marinis F, et al. Randomised multicenter phase II study of two schedules of docetaxel and gemcitabine or cisplatin/gemcitabine followed by docetaxel as first line treatment for advanced non‐small cell lung cancer. Lung Cancer 2009;66:327‐32. [DOI] [PubMed] [Google Scholar]
Rocha Lima 2004 {published data only}
- Rocha Lima CM, Rizvi NA, Zhang C, Herndon JE 2nd, Crawford J, Govindan R, et al. Randomized phase II trial of gemcitabine plus irinotecan or docetaxel in stage IIIB or stage IV NSCLC. Annals of Oncology 2004;15:410‐8. [DOI] [PubMed] [Google Scholar]
Rubio 2009 {published data only}
- Rubio JC, Vazquez S, Vazquez F, Amenedo M, Firvida JL, Mel JR, et al. A phase II randomized trial of gemcitabine‐docetaxel versus gemcitabine‐cisplatin in patients with advanced non‐small cell lung carcinoma. Cancer Chemotherapy and Pharmacology 2009;64:379‐84. [DOI] [PubMed] [Google Scholar]
Zatloukal 2008 {published data only}
- Zatloukal P, Gervais R, Vansteenkiste J, Bosquee L, Sessa C, Brain E, et al. Randomized multicenter phase II study of larotaxel (XRP9881) in combination with cisplatin or gemcitabine as first‐line chemotherapy in non irradiable stage IIIB or stage IV non‐small cell lung cancer. Journal of Thoracic Oncology 2008;3(8):894‐901. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01405586 {published data only}
- NCT01405586. Cisplatin in combination with gemcitabine for elderly patients with lung cancer. https://clinicaltrials.gov/ct2/show/NCT01405586?term=mile‐3&rank=1 (accessed 19 July 2015).
NCT01593293 {published data only}
- NCT01593293. Pemetrexed with or without carboplatin for elderly non‐squamous non‐small cell lung cancer (ACE). https://clinicaltrials.gov/ct2/show/NCT01593293 (accessed 19 July 2015).
NCT01656551 {published data only}
- NCT01656551. MILES‐4: study comparing gemcitabine and pemetrexed, with or without cisplatin, in patients with nonsquamous lung cancer. https://clinicaltrials.gov/ct2/show/NCT01656551?term=NCT01656551&rank=1 (accessed 19 July 2015).
Additional references
American Cancer Society 2011
- American Cancer Society. Global Cancer Facts & Figures. 2nd edition. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc‐027766.pdf (accessed 1 May 2012).
Ansari 2011
- Ansari RH, Socinski MA, Edelman MJ, Belani CP, Gonin R, Catalano RB, et al. A retrospective analysis of outcomes by age in a three‐arm phase III trial of gemcitabine in combination with carboplatin or paclitaxel vs. paclitaxel plus carboplatin for advanced non‐small cell lung cancer. Critical Reviews in Oncology/Hematology 2011;78(2):162–71. [DOI] [PubMed] [Google Scholar]
Asami 2011
- Asami K, Koizumi T, Hirai K, Ameshima S, Tsukadaira A, Catalano RB, et al. Gefitinib as first‐line treatment in elderly epidermal growth factor receptor‐mutated patients with advanced lung adenocarcinoma: results of a Nagano Lung Cancer Research Group study. Clinical Lung Cancer 2011;12(6):387‐92. [DOI] [PubMed] [Google Scholar]
Balducci 2010
- Balducci L. ESH‐SIOG International Conference on Haematological Malignancies in the Elderly. Experts Review of Hematology 2010;3:675‐7. [DOI] [PubMed] [Google Scholar]
D'Addario 2005
- D'Addario G, Pintilie M, Leighl NB, Feld R, Cerny T, Shepherd FA. Platinum‐based versus non‐platinum‐based chemotherapy in advanced non‐small‐cell lung cancer: a meta‐analysis of the published literature. Journal of Clinical Oncology 2005;23(13):2926‐36. [DOI] [PubMed] [Google Scholar]
Davidoff 2010
- Davidoff AJ, Ang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non‐small cell lung cancer. Journal of Clinical Oncology 2010;28:2191‐7. [DOI] [PubMed] [Google Scholar]
de Castria 2013
- Castria TB, Silva EMK, Gois AFT, Riera R. Cisplatin versus carboplatin in combination with third‐generation drugs for advanced non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD009256] [DOI] [PubMed] [Google Scholar]
Extermann 2012
- Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer 2012;118:3377‐86. [DOI] [PubMed] [Google Scholar]
Ezer 2014
- Ezer N, Smith CB, Galsky MD, Mhango G, Gu F, Gomez J, et al. Cisplatin vs. carboplatin‐based chemoradiotherapy in patients >65 years of age with stage III non‐small cell lung cancer. Radiotherapy and Oncology 2014;112(2):272‐8. [DOI: 10.1016/j.radonc.2015.06.001] [DOI] [PubMed] [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. British Medical Journal 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gridelli 2001
- Gridelli C. The ELVIS trial: a phase III study of single‐agent vinorelbine as first‐line treatment in elderly patients with advanced non‐small cell lung cancer. Elderly Lung Cancer Vinorelbine Italian Study. The Oncologist 2001;Suppl 1:4‐7. [DOI] [PubMed] [Google Scholar]
Gridelli 2014
- Gridelli C, Rossi A, Maio M, Leo S, Filipazzi V, Favaretto AG, et al. Rationale and design of MILES‐3 and MILES‐4 studies: two randomized phase 3 trials comparing single‐agent chemotherapy versus cisplatin‐based doublets in elderly patients with advanced non‐small‐cell lung cancer. Clinical Lung Cancer 2014;2:166‐70. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hotta 2004
- Hotta K, Matsuo K, Ueoka H, Kiura K, Tobata M, Tonimoto M. Addition of platinum compounds to a new agent in patients with advanced non‐small‐cell lung cancer: a literature based meta‐analysis of randomised trials. Annals of Oncology 2004;15:1782‐9. [DOI] [PubMed] [Google Scholar]
Hurria 2011
- Hurria A, Togawa K, Mohile SG, Owusu C, Keplin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. Journal of Clinical Oncology 2011;29:3457‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutchins 1999
- Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Albain KS. Underrepresentation of patients 65 years of age or older in cancer‐treatment trials. The New England Journal of Medicine 1999;341(27):2061–7. [DOI] [PubMed] [Google Scholar]
Inoue 2009
- Inoue A, Kobayashi K, Usui K, Maemondo K, Okinaga S, Mikani I, et al. First‐line gefitinib for patients with advanced non–small‐cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. Journal of Clinical Oncology 2009;27:1394‐400. [DOI] [PubMed] [Google Scholar]
Kawahara 2011
- Kawahara M, Tada H, Tokoro A, Teramukai S, Origasa H, Kubota K, et al. Quality‐of‐life evaluation for advanced non‐small‐cell lung cancer: a comparison between vinorelbine plus gemcitabine followed by docetaxel versus paclitaxel plus carboplatin regimens in a randomized trial: Japan Multinational Trial Organization LC00‐03 (BRI LC03‐01). BMC Cancer 2011;11:356‐63. [DOI: 10.1186/1471-2407-11-356] [DOI] [PMC free article] [PubMed] [Google Scholar]
Langer 2002
- Langer CJ, Manola J, Bernardo P, Kugler JW, Bonomi P, Cella D, et al. Cisplatin‐based therapy for the elderly with advanced non‐small‐cell lung cancer: implications of Eastern Cooperative Oncology Group 5592, a randomized trial. Journal of the National Cancer Institute 2002;94(3):173‐81. [DOI] [PubMed] [Google Scholar]
Lee 2013
- Lee CK, Brown C, Gralla RJ, Hirsh V, Thongprasert S, Tsai CM, et al. Impact of EGFR inhibitor in non‐small cell lung cancer on progression‐free and overall survival: a meta‐analysis. Journal of the National Cancer Institute 2013;105(9):595‐605. [DOI] [PubMed] [Google Scholar]
Maemondo 2012
- Maemondo M, Minegishi Y, Inoue A, Kobayashi K, Harada M, Okinaga S, et al. First‐line gefitinib in patients aged 75 or older with advanced non‐small cell lung cancer harbouring epidermal growth factor receptor mutations: NEJ 003 study. Journal of Thoracic Oncology 2012;7(9):1417‐22. [DOI] [PubMed] [Google Scholar]
Maione 2005
- Maione P, Perrone F, Gallo C, Manzione L, Piantedosi FV, Barbera S, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly with advanced non‐small cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. Journal of Clinical Oncology 2005;23(28):6865‐72. [DOI] [PubMed] [Google Scholar]
Mok 2014
- Mok T, Kim DW, Wu YL, Solomon BJ, Nakagawa K, Mekhailet T, et al. First‐line crizotinib versus pemetrexed–cisplatin or pemetrexed–carboplatin in patients (pts) with advanced ALK‐positive non‐squamous non‐small cell lung cancer (NSCLC): results of a phase III study (PROFILE 1014). Journal of Clinical Oncology 2014;32:5s (suppl;abstract 8002). [Google Scholar]
NCI Common Toxicity Criteria
- National Cancer Institute. Common Toxicity Criteria. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010‐06‐14_QuickReference_8.5x11.pdf (accessed 1 May 2012).
NSCLC Collaborative Group 2010
- Non‐Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD007309.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NSCLC Meta‐Analyses Collaborative Group 2008
- NSCLC Meta‐Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non‐small‐cell lung cancer: a systematic review and meta‐analysis of individual patient data from 16 randomized controlled trials. Journal of Clinical Oncology 2008;26(28):4617‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pallis 2011
- Pallis AG, Karampeazis A, Vamvakas L, Vardakis N, Kotsakis A, Bozionelou V, et al. Efficacy and treatment tolerance in older patients with NSCLC: a meta‐analysis of five phase III randomized trials conducted by the Hellenic Onocology Research Group. Annals of Oncology 2011;22:2448‐55. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Qi 2012
- Qi WX, Tang L, He A, Shen Z, Lin F, Yao Y. Doublet versus single cytotoxic agent as first line treatment for elderly patients with advanced non‐small‐cell lung cancer: a systematic review and meta‐analysis. Lung 2012;190:477‐85. [DOI] [PubMed] [Google Scholar]
Quoix 2011a
- Quoix E, Westeel V, Zalcman G, Milleron B. Chemotherapy in elderly patients with advanced non‐small cell lung cancer. Lung Cancer 2011;74(3):364‐8. [DOI] [PubMed] [Google Scholar]
Rajeswaran 2008
- Rajeswaran A, Trojan A, Burnand B, Giannelli M. Efficacy and side effects of cisplatin‐ and carboplatin‐based doublet chemotherapeutic regimens versus non‐platinum‐based doublet chemotherapeutic regimens as first line treatment of metastatic non‐small cell lung carcinoma: a systematic review of randomised controlled trials. Lung Cancer 2008;59:1‐11. [DOI] [PubMed] [Google Scholar]
Review Manager [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager. Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Talarico 2004
- Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7‐year experience by the US Food and Drug Administration. Journal of Clinical Oncology 2004;22(22):4626‐31. [DOI: 10.1200/JCO.2004.02.175] [DOI] [PubMed] [Google Scholar]
Therasse 2000
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;93(2):205‐16. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8(16):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilders 2014
- Wildiers H, Heeren P, Puts M, Topinkova E, Janssen‐Heijnen MLG, Extermann M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. Journal of Clinical Oncology 2014;32(24):2595‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]


