Abstract
A 79-year-old female with Sjögren's syndrome (SS) underwent phacoemulsification and lens implantation in both eyes within 2 days. Postoperatively, topical diclofenac 0.1% and tobramycin 0.3% were applied. She presented 10 days later with photophobia, large central corneal melting, and visual acuity of counting finger in both eyes. Diclofenac was discontinued, and systemic doxycycline and steroids were administered. Amniotic membrane transplantation was performed in the left eye with topical steroid and autologous serum 20%. Corneal melting gradually healed in 3 weeks, but the centers of both corneas became thin and opaque. Hyperopic shift and irregular corneal surface were more significant in the right eye than in the left eye. Vision recovered to 0.05 and 0.1 in the right and left eyes, respectively. Topical nonsteroidal anti-inflammatory drugs should be used with caution in cataract surgery in patients with SS.
Keywords: Amniotic membrane, corneal ulcer, nonsteroidal anti-inflammatory agents, phacoemulsification, Sjögren's syndrome
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) can inhibit cyclooxygenases (COXs) and reduce prostaglandin (PG) synthesis to achieve postoperative analgesia and inflammation reduction. Topical NSAIDs are widely used after ocular surgery to reduce pain and inflammation as well as treat cystoid macular edema after cataract surgery.[1] The most common adverse events associated with topical NSAID use include irritation, stinging, impaired corneal sensation, superficial punctate keratitis, and persistent corneal epithelial defect.[1,2,3,4,5] In August 1999, members of the American Society of Cataract and Refractive Surgery reported severe complications of corneal melting, particularly in case of generic diclofenac.[6] Since then, several cases of corneal melting or perforation have been reported after the use of different topical NSAIDs.[7,8,9] The mechanism underlying NSAID-induced corneal melting remains unclear. The occurrence of NSAID-induced corneal melting may be related to NSAID dosage and duration. Other risk factors include dry eye, previous ocular surgery, decreased corneal sensitivity, diabetes mellitus, and autoimmune disease.[10,11] Here, we report a case of bilateral rapid corneal melting after an uneventful cataract surgery and the use of topical diclofenac 0.1% in a patient with Sjögren's syndrome (SS).
Case Report
A 79-year-old female patient with stable and controlled SS was treated with artificial tears. She underwent phacoemulsification and intraocular lens implantation in the left eye first and in the right eye 2 days later. Postoperatively, topical betamethasone 0.1%, tobramycin 0.3%, and diclofenac 0.1% (Winston, Tainan, Taiwan) were applied. However, 2 days later, the patient reported blurred vision and photophobia in both eyes. Topical lubricant eye drops were added every 2 h daily, but they had no effect. She presented 10 days later with marked photophobia, red eyes, and decreased vision in both eyes. She did not have diabetes or other systemic diseases. Visual acuity of counting fingers was noted in both eyes. Slit-lamp examination revealed conjunctival injection, and corneal melting measuring 5 mm × 6 mm and 7 mm × 8 mm was noted in the right and left eyes, respectively [Figure 1a–d]. The centers of the corneas were diffuse edema and thinning without infiltrates. Anterior chamber reaction was trace in both eyes. Posterior chamber intraocular lens was in good centration, and the posterior segment was unremarkable. Schirmer's test after topical anesthesia revealed a value of 1.0 mm in 5 min in both eyes. Diclofenac-associated corneal melting was strongly suspected, and diclofenac 0.1% was immediately discontinued. Preservative-free artificial tears, fluorometholone 0.1%, levofloxacin 0.5% four times a day, and autologous serum 20% hourly were administered. Oral prednisolone 30 mg daily and doxycycline 100 mg two times a day were prescribed. The cornea melting of her left eye was deteriorated and was more severe than the right eye. The patient underwent amniotic membrane transplantation (AMT) in the left eye; she then wore therapeutic contact lenses in both eyes. Cultures of bilateral corneal swabs revealed no growth in 5 days. One week later, corneal melting and edema decreased in both eyes. Oral prednisolone and topical levofloxacin were discontinued. Cornea completely reepithelialized in 2 weeks in the left eye and 3 weeks in the right eye. These topical medications were continued for 2 months. Stromal edema and inflammation resolved gradually. However, a sequela of central thin and opaque cornea occurred in both eyes [Figure 2a and b]. The examination of anterior segment optical coherence tomography demonstrated more hyperreflective anterior stroma in the right cornea than in the left cornea [Figure 2c and d]. The right cornea without AMT was relatively thin. Corneal topography demonstrated more irregular astigmatism in the right cornea than in the left eye. The surface regularity and asymmetric indexes were 2.31 and 3.86 in the right cornea and 1.96 and 2.23 in the left eye, respectively. Finally, visual acuity recovered to 0.05 with +13.5–6.0 × 15 in the right eye and 0.1 with +1.5–10.0 × 174 in the left eye.
Figure 1.
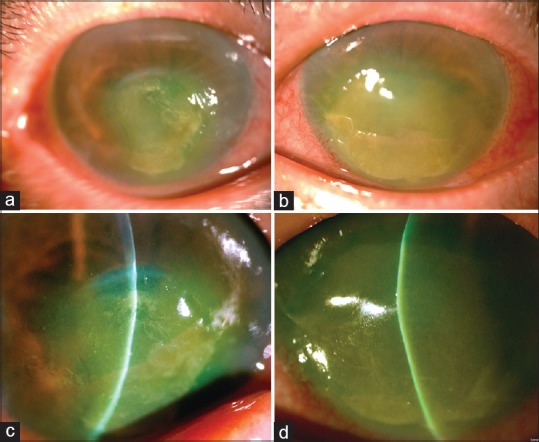
External photography showed bilateral hyperemia, and a large corneal melting measuring 5 mm × 6 mm in the right eye (a) and 7 mm × 8 mm in the left eye (b) Slit-lamp examination revealed central corneal edema, Descemet folds, and corneal thinning at inferior central area in the right (c) and the left (d) eyes
Figure 2.
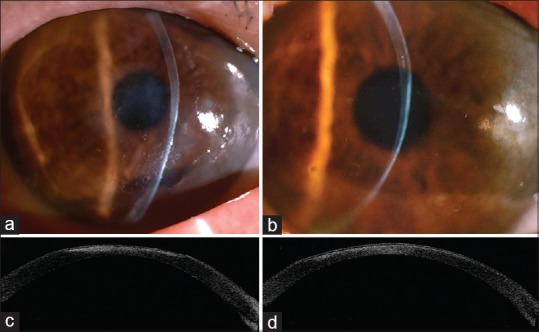
A sequela of a thin and opaque cornea occurred in the right (a) and the left (b) eyes. Anterior segment optical coherence tomography showed a higher hyperreflectivity of anterior stroma in the right cornea (c) than that in the left cornea, which had undergone amniotic membrane transplantation (d)
Discussion
Topical NSAIDs are frequently used to reduce postoperative pain and inflammation after cataract surgery and refractive surgery. NSAIDs-related adverse events include stinging, superficial punctate keratitis, and corneal epithelial defect, but they are infrequent.[1,2,3,4,5] Severe corneal complications of corneal melting and perforation are uncommon.[7,8,9,10,11] Several mechanisms explaining the occurrence of keratolysis have been proposed. First, the selective blockage of the COX pathway by NSAIDs reduces PG synthesis, but shunting of arachidonic acid to the lipoxygenase pathway results in the formation of leukotrienes.[12] Leukotrienes are neutrophil chemoattractants and neutrophil degranulation stimulators. Corneal epithelial cells secrete matrix metallopeptidases (MMPs). Overexpression of MMP-8, a neutrophil collagenase, plays a role in the pathogenesis of keratolysis and corneal melting.[13] Second, NSAIDs can reduce corneal sensitivity.[14] Therefore, neurotrophic epitheliopathy delays corneal wound healing and reepithelialization. Third, NSAIDs may decrease corneal epithelium migration and inhibit keratocyte proliferation, thus delaying wound healing.[15] Most reported cases of corneal melting involved topical diclofenac use.[7,8,9,16] NSAID-induced corneal melting may occur at the incision wound or the central cornea after cataract surgery.[7] In the current case, the bilateral central corneal melting was associated with topical diclofenac use after the cataract surgery.
SS, a chronic inflammatory autoimmune disease with lymphocytic infiltration of the exocrine gland, may lead to dry eyes and dry mouth. External ocular complications of SS include conjunctivitis, scleritis, corneal erosion, haze, and sterile ulcer. However, corneal melting and perforation are infrequent. Corneal melting in SS typically occurs in the central inferior cornea with much thinner at the center of melting area, which is different from the presentations in our case with diffuse edema and thinning. Dry eye and autoimmune diseases may induce decreased corneal sensitivity. SS may be accompanied by decreased corneal sensation and autonomic nervous system dysfunction of ocular surface and therefore influences tear production.[17] Decreased corneal sensitivity could reduce blinking and impair tear reflex. Furthermore, corneal sensitivity significantly dropped after 45–60 min of using topical NSAIDs.[14] Although NSAIDs could effectively control the ocular inflammation and pain, it may be harmful in patients with dry eye or other ocular comorbidities, resulting in further damage of corneal epithelium and delaying corneal wound healing.
Preservatives in topical eye drops can induce inflammation response and damage conjunctival and corneal cells, particularly in patients with dry eye syndrome.[18] An in vitro study reported that tobramycin caused toxicity to rabbit corneal epithelial cells after a 30-min exposure.[19] Concurrent topical diclofenac and tobramycin may have a synergetic effect and cause corneal melting.[20] Cataract surgery can further deteriorate dry eye and cause persistent corneal erosion or corneal perforation in patients with SS.[21] These predisposing factors may have contributed to the corneal melting in the current case.
The autologous serum contains Vitamin A, epidermal growth factor, fibronectin, and transforming growth factor-β, which are crucial for epithelial cell proliferation, differentiation, and migration. Autologous serum can contribute to the healing of corneal epithelial defect; it has been used to treat various ocular surface diseases, including dry eyes, persistent epithelial defects, neurotrophic keratopathy, chemical burn, and toxic keratopathy.[22] The amniotic membrane consists of avascular hypocellular stroma and overlying basement membrane; it also has anti-inflammatory properties and antiangiogenic functions. AMT has been used in ocular surface reconstruction and treatment of many ocular surface diseases because of its cellular proliferation-, migration-, and epithelialization-promoting effect.[23] In the current case, compared with the right eye, the left eye with AMT demonstrated quicker reepithelialization, thicker corneal thickness, less hyperopic shift, more regular corneal surface, and better visual recovery.
Conclusion
Topical NSAIDs can cause severe corneal melting after cataract surgery and should be used with caution, particularly in patients with SS. Early diagnosis and prompt discontinuity of NSAID use are important to manage NSAID-related keratopathy. Autologous serum and AMT can help heal corneal melting. Taken together, in patients with SS, cataract surgery preferably should not be performed in both eyes within a short period.
Declaration of patient consent
The authors certify that they have obtained appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her names and initials will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare that there are no conflicts of interests of this paper.
References
- 1.Kim SJ, Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol. 2010;55:108–33. doi: 10.1016/j.survophthal.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Gills JP. Voltaren associated with medication keratitis. J Cataract Refract Surg. 1994;20:110. doi: 10.1016/s0886-3350(13)80063-6. [DOI] [PubMed] [Google Scholar]
- 3.Singer DD, Kennedy J, Wittpenn JR. Topical NSAIDs effect on corneal sensitivity. Cornea. 2015;34:541–3. doi: 10.1097/ICO.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 4.Probst LE, 5th, Machat JJ. Corneal subepithelial infiltrates following photorefractive keratectomy. J Cataract Refract Surg. 1996;22:281. doi: 10.1016/s0886-3350(96)80233-1. [DOI] [PubMed] [Google Scholar]
- 5.Shimazaki J, Saito H, Yang HY, Toda I, Fujishima H, Tsubota K. Persistent epithelial defect following penetrating keratoplasty: An adverse effect of diclofenac eyedrops. Cornea. 1995;14:623–7. [PubMed] [Google Scholar]
- 6.NSAID Adverse Reaction Report. American Society of Cataract and Refractive Surgery. 1999 Aug 3; [Google Scholar]
- 7.Lin JC, Rapuano CJ, Laibson PR, Eagle RC, Jr, Cohen EJ. Corneal melting associated with use of topical nonsteroidal anti-inflammatory drugs after ocular surgery. Arch Ophthalmol. 2000;118:1129–32. [PubMed] [Google Scholar]
- 8.Flach AJ. Corneal melts associated with topically applied nonsteroidal anti-inflammatory drugs. Trans Am Ophthalmol Soc. 2001;99:205–10. [PMC free article] [PubMed] [Google Scholar]
- 9.Guidera AC, Luchs JI, Udell IJ. Keratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugs. Ophthalmology. 2001;108:936–44. doi: 10.1016/s0161-6420(00)00538-8. [DOI] [PubMed] [Google Scholar]
- 10.Isawi H, Dhaliwal DK. Corneal melting and perforation in Stevens Johnson syndrome following topical bromfenac use. J Cataract Refract Surg. 2007;33:1644–6. doi: 10.1016/j.jcrs.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 11.Zanini M, Savini G, Barboni P. Corneal melting associated with topical diclofenac use after laser-assisted subepithelial keratectomy. J Cataract Refract Surg. 2006;32:1570–2. doi: 10.1016/j.jcrs.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 12.Rao P, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci. 2008;11:81s–110s. doi: 10.18433/j3t886. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien TP, Li QJ, Sauerburger F, Reviglio VE, Rana T, Ashraf MF, et al. The role of matrix metalloproteinases in ulcerative keratolysis associated with perioperative diclofenac use. Ophthalmology. 2001;108:656–9. doi: 10.1016/s0161-6420(00)00590-x. [DOI] [PubMed] [Google Scholar]
- 14.Szerenyi K, Sorken K, Garbus JJ, Lee M, McDonnell PJ. Decrease in normal human corneal sensitivity with topical diclofenac sodium. Am J Ophthalmol. 1994;118:312–5. doi: 10.1016/s0002-9394(14)72954-x. [DOI] [PubMed] [Google Scholar]
- 15.Hashizume N, Saika S, Okada Y, Miyamoto T, Shimizu K, Ohnishi Y. Effects of antiinflammatory drugs on migration of the rabbit corneal epithelium. J Cataract Refract Surg. 2001;27:1499–502. doi: 10.1016/s0886-3350(01)00866-5. [DOI] [PubMed] [Google Scholar]
- 16.Congdon NG, Schein OD, von Kulajta P, Lubomski LH, Gilbert D, Katz J. Corneal complications associated with topical ophthalmic use of nonsteroidal antiinflammatory drugs. J Cataract Refract Surg. 2001;27:622–31. doi: 10.1016/s0886-3350(01)00801-x. [DOI] [PubMed] [Google Scholar]
- 17.Han SB, Hyon JY, Wee WR, Lee JH, Lee YJ, Yun PY. Reduced corneal sensitivity in patients with primary Sjögren's syndrome. Acta Ophthalmol. 2010;88:e277–8. doi: 10.1111/j.1755-3768.2009.01693.x. [DOI] [PubMed] [Google Scholar]
- 18.Jee D, Park SH, Kim MS, Kim EC. Antioxidant and inflammatory cytokine in tears of patients with dry eye syndrome treated with preservative-free versus preserved eye drops. Invest Ophthalmol Vis Sci. 2014;55:5081–9. doi: 10.1167/iovs.14-14483. [DOI] [PubMed] [Google Scholar]
- 19.Lass JH, Mack RJ, Imperia PS, Mallick K, Lazarus HM. An in vitro analysis of aminoglycoside corneal epithelial toxicity. Curr Eye Res. 1989;8:299–304. doi: 10.3109/02713688908997572. [DOI] [PubMed] [Google Scholar]
- 20.Ornek K, Yalçindaǧ FN, Ozdemir O. Corneal melting associated with a fixed-dose combination of diclofenac 0.1% plus tobramycin 0.3% following cataract surgery. J Cataract Refract Surg. 2008;34:1417. doi: 10.1016/j.jcrs.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 21.Cohen K. Sterile comeal perforation after cataract surgery in Sjögren's syndrome. Br J Ophthalmol. 1982;66:179–82. doi: 10.1136/bjo.66.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geerling G, Maclennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004;88:1467–74. doi: 10.1136/bjo.2004.044347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prabhasawat P, Tesavibul N, Komolsuradej W. Single and multilayer amniotic membrane transplantation for persistent corneal epithelial defect with and without stromal thinning and perforation. Br J Ophthalmol. 2001;85:1455–63. doi: 10.1136/bjo.85.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]


