Abstract
PURPOSE:
Although femtosecond laser-assisted LASIK (laser-assisted in situ keratomileusis). provides a controllable flap size than a mechanical microkeratome, patients still experience dry eye symptoms after LASIK. The purpose of this study is to investigate the effects of different flap sizes on postoperative dry eye syndrome and corneal sensitivity.
PATIENTS AND METHODS:
This is a retrospective comparative study. Fifty-seven consecutive patients (113 eyes) who underwent myopic femtosecond laser-assisted LASIK treatment were recruited. Basic Schirmer's test value, tear breakup time (TBUT), corneal fluorescein staining scores, conjunctival rose bengal staining scores, and corneal sensitivity were measured before surgery, at postoperative 1, 3, and 6 months follow-up.
RESULTS:
When the eyes were grouped by flap diameter sizes (8.7 mm as the cutoff value), there were no significant differences in terms of corneal sensitivity and all dry eye parameters investigated at any time points between the large or small flap diameter groups. However, when the eyes were grouped by the ratio of flap diameter/horizontal corneal white-to-white distance (0.756 as the cutoff value), the larger ratio group showed decreased basic Schirmer's test (7.52 ± 4.43 mm) than the smaller group (12.15 ± 8.14) at 3 months (P = 0.006). Moreover, the group that had larger flap/corneal diameter ratio showed shorter TBUT (4.20 ± 1.73 s) than the smaller group (5.67 ± 1.90 s) at 6 months postoperatively (P = 0.011).
CONCLUSIONS:
LASIK-related dry eye syndrome was associated with flap/corneal diameter ratio, and surgeons should keep this effect in mind when customizing the cornea flap sizes for dry eye patients during LASIK.
Keywords: Corneal sensation, dry eye, femtosecond laser, LASIK
Introduction
Since its introduction in 2002, the use of femtosecond laser for laser-assisted in situ keratomileusis (LASIK) has been steadily increasing. Flap creation by femtosecond laser could be performed easily with fewer complications compared with mechanical microkeratome.[1] The morphology and accuracy of the cuts were very reliable and precise.[2] Furthermore, with the assistance of femtosecond laser, the refractive surgeons are able to accurately adjust the flap dimension in order to create a customized flap based on the needs of the patient and the type of excimer laser in use.
However, dry eye symptoms remain the most common complaint after LASIK surgery, with an incidence rate ranging from 3% to 59%.[3,4,5] Although usually resolved with 3 months, some patients may experience severe symptoms that last longer than 1 year after surgery and negatively influence postoperative visual outcome and patient satisfaction.[6] Proposed risk factors of post-LASIK dry eye include but not limited to damage to corneal nerves during flap creation,[7] suction ring-induced transient elevation of pressure on the conjunctiva,[8,9] degree of preoperative myopia, and presence of preexisting dry eye disease.[10] Whether femtosecond laser-assisted LASIK reduces postoperative dry eye syndrome is still in debate. Our previous study found a higher postoperative tear breakup time (TBUT) when LASIK was performed with femtosecond laser.[7]
Truncation of the sensory nerves during flap creation plays an important role in post-LASIK dry eye syndrome.[11] Mian et al. have shown that when performing LASIK with femtosecond laser, corneal flap hinge position, hinge angle, and thickness had no effects on corneal sensitivity or dry eye syndrome.[12,13] Huang et al.[14] also reported that there is no difference in terms of corneal sensation and dry eye disease with varying superior-hinged and temporal-hinged flap positions. However, the influence of different flap diameters on corneal sensation and dry eye after LASIK remains elusive. Therefore, the purpose of this study is to investigate the effects of flap diameter on corneal sensitivity and dry eye parameters after femtosecond laser assisted-LASIK.
Patients and Methods
This study was approved by the Chang Gung Medical Foundation Institutional Review Board (Approval No. 103-2554B, Approval Date: September 2, 2014). The board agreed to waive the patient consent. This is a single-center (Department of Ophthalmology, Chang Gung Memorial Hospital, Keelung, Taiwan), nonrandomized, retrospective study. It comprised 113 eyes of 57 patients who had femtosecond laser-assisted LASIK surgery. All surgeries were performed by the same surgeon (Chi-Chin Sun). Indications for myopic LASIK included minimum age of 20 years, normal ophthalmic examination except for refractive errors, stable refraction for at least 6 months, and minimum calculated residual corneal stromal bed thickness >250 μm. Patients with previous eye surgery, topical ocular medications prior to surgery, pregnancy, and specific ocular conditions such as ocular rosacea or autoimmune diseases were excluded from the study. Eyes that had LASIK flap complications or retreatments were also excluded. Fifty-seven patients (112 eyes) were included and grouped either by actual flap size or by ratio to corneal diameter (white-to-white distance).
Preoperatively, the horizontal white-to-white distance of all eyes was measured using a scanning slit topography (Orbscan II, Bausch and Lomb, Rochester, NY, USA). One patient received single-eye LASIK due to anisometropia. The other 56 patients received bilateral simultaneous LASIK using a 60-KHz IntraLase laser (Abbott Medical Optics [AMO], Santa Ana, CA, USA) for flap creation. Laser ablation was performed using the Visx S4 IR (AMO, Santa Ana, CA, USA) laser with an optical zone of 6.5 mm under topical anesthesia. One eye was excluded due to post-LASIK enhancement. The femtosecond laser was preprogrammed for each procedure with a hinge angle of 50° with superior location, raster energy of 1.0 mJ, and side-cut energy of 0.9 mJ. For flap creation, the IntraLase laser delivers 100 nJ pulse energy and 2 MHz repetition rate. The attempted flap thickness was 90 μm in the dominant eye and 120 μm in the nondominant eye. The flap diameter was determined by the operator to match the corneal diameter and the presterilized applanation lens. Postoperatively, all patients were given prednisolone acetate 1% ophthalmic suspension (Pred Forte®, Allergan, NJ, USA) and levofloxacin ophthalmic solution (Cravit®, Santen, Osaka, Japan) to use four times a day for 1 week. Patients were also directed to use artificial tears (Tear Naturale free®, Alcon, Puurs, Belgium) as needed. Every patient had pre- and post-operative (1 week and 1, 3, and 6 months) evaluations in the following sequence: corneal sensitivity (Cochet-Bonnet corneal esthesiometer; Luneau Ophtalmologie, Chartres, France), TBUT, corneal fluorescein staining, conjunctival rose bengal staining, and basic Schirmer's test.
Corneal sensitivity
Corneal sensitivity was measured at the central cornea, and the mean of three measurements was recorded. Cochet–Bonnet esthesiometer is equipped with a 6.0-cm long nylon monofilament that can be adjusted in length. To measure corneal sensation, the nylon monofilament is applanated against the corneal surface and the patient responds when corneal sensation is appreciated. By adjusting the length of the nylon monofilament, pressure gradients can be created ranging from 11 to 200 mg/mm2. Individuals with normal corneal sensation will sense the touch of the monofilament at 6.0 cm. If a patient does not note touch at 6.0 cm, the monofilament is reduced at intervals of 0.5 cm until sensation is perceived. A response was considered positive if the nylon filament was felt at a given length at least two of three times.
Tear breakup time
Fluorescein TBUT is considered a test of tear film stability and is decreased in patients with aqueous tear deficiency, and it can also be decreased in patients with meibomian gland disease and corneal irregularity. TBUT was performed with a fluorescein strip moistened with balanced salt solution (BSS, Alcon, Puurs, Belgium) applied to the inferior cul-de-sac without anesthesia. The patient was asked not to blink, and a stopwatch was used to measure the time from lid opening to tear breakup.
Ocular surface staining
The corneal fluorescein staining scores and conjunctival rose bengal staining scores were measured and graded from 0 (none) to 3 (severe) based on the amount of staining according to the Oxford Scheme of ocular surface staining with minor modification.[7] The conjunctiva was divided into the entire cornea, as well as into superior, inferior, nasal, and temporal quadrants of the bulbar conjunctiva. The cornea was divided into the central cornea, as well as into superior, inferior, nasal, and temporal quadrants as recommended by the National Eye Institute Industry Workshop.
Basic Schirmer's test
After instilling one drop of 0.5% proparacaine (Alcaine®, Alcon, Puurs, Belgium) in each eye and drying the fornix, a sterile standardized Schirmer's test strip (Alcon Laboratories Inc., Fort Worth, TX, USA) was placed at the junction of the lateral and middle-third of the inferior fornix. The length of the wet portion of the strip was measured at 5 min and recorded in millimeters.
Statistical analysis
All collected data were tabulated in a Microsoft Excel for Windows software version 2003 (Microsoft Corp., Seattle, WA, USA). Data collected were transferred to PASW Statistics (Chicago, IL, USA, version 18) and analyzed. Independent t-test for continuous variables and Chi-square test for categorical variables were used when appropriate. P < 0.05 was considered statistically significant.
Results
The study population comprised 112 eyes from 57 patients with a mean age of 32.79 years. Two methods for grouping flap diameters were used to evaluate the respective effects on dry eye parameters. First, these eyes were grouped by the actual size of the flap diameter with a cutoff value of 8.7 mm, which was the median flap diameter of all study eyes. The demographic data and preoperative and surgical parameters of the group based on actual corneal flap diameter are shown in Table 1. The mean corneal diameter was 8.50 ± 0.21 mm in the group with smaller flap (66 eyes) and 8.90 ± 0.09 mm in group with larger flap (46 eyes). There was no significant difference between the groups in age, sex distribution, preoperative spherical equivalent (SE), astigmatism, mean keratometry, pachymetry, flap thickness, treated SE, and ablation depth [Table 1].
Table 1.
Demographic data apreoperative and surgical parameters of the groups based on the actual size of corneal flap diameter
| Parameters | Flap diameter | t/χ2 P* | |
|---|---|---|---|
| Smaller group (n=66) | Larger group (n=46) | ||
| Mean flap diameter (mm) | 8.51±0.21 | 8.90±0.09 | |
| Age (years) | 31.11±6.85 | 32.76±8.14 | 0.247 |
| Sex | |||
| Male | 13 (19.7) | 10 (21.7) | 0.792 |
| Female | 53 (80.3) | 36 (78.3) | |
| Preoperative SE (D) | −6.83±2.73 | −6.93±2.32 | 0.826 |
| Astigmatism (D) | −1.23±0.96 | −1.47±1.26 | 0.269 |
| Mean keratometry (D) | 43.67±1.72 | 43.94±1.42 | 0.367 |
| Pachymetry (µm) | 548±28.62 | 546.76±25.86 | 0.090 |
| Flap thickness (µm) | 87.24±18.60 | 87.28±21.47 | 0.992 |
| Treated SE (D)** | −6.03±2.05 | −5.99±1.75 | 0.906 |
| Calculated ablation depth (µm) | 107.03±27.36 | 110.22±23.01 | 0.519 |
*Independent sampling t-test or Pearson Chi-square, **Refraction in cornea plane. D=Diopters, SE=Spherical equivalent refraction
Preoperatively, there was no significant difference in corneal sensitivity, basic Schirmer's test, TBUT, corneal fluorescein staining, and conjunctival rose bengal staining between the groups [Figures 1–5]. Slightly decreased corneal sensitivity after surgery was noted in both groups and gradually recovered by 6 months [Figure 1]. In addition, increased corneal fluorescein and conjunctival rose bengal stainings were also noted postoperatively in both groups [Figures 3 and 4]. However, there were no significant differences in corneal sensitivity, TBUT, corneal fluorescein staining scores, conjunctival rose bengal staining scores, and basic Schirmer's test between the groups for a follow-up period of 6 months [Figures 1–5].
Figure 1.
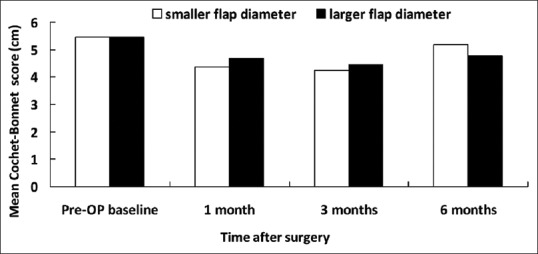
Central Cochet–Bonnet esthesiometry-measured mean corneal sensitivity associated with smaller and larger flap diameters
Figure 5.
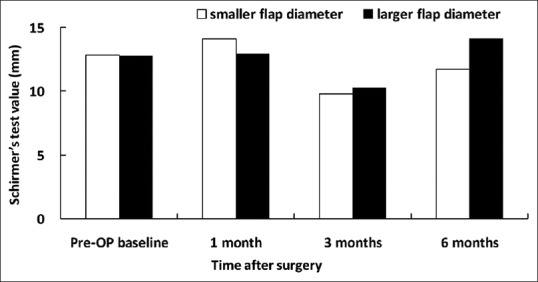
Basic Schirmer's test values in patients associated with smaller and larger flap diameters
Figure 3.
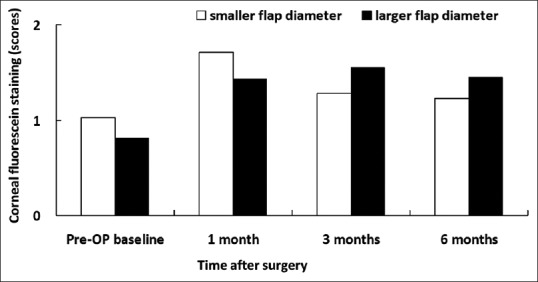
Corneal fluorescein staining in patients with smaller and larger flap diameters
Figure 4.
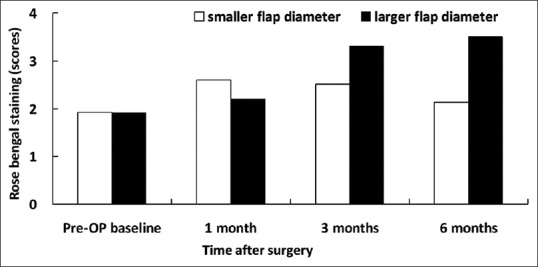
Rose bengal staining scores in patients with smaller and larger flap diameters
Figure 2.
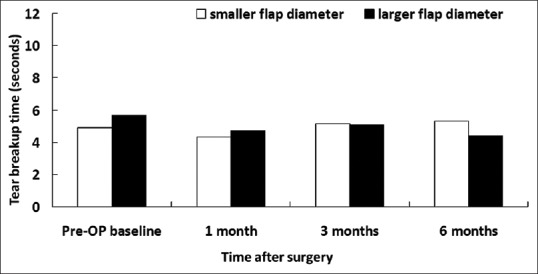
Tear breakup time in patients with smaller and larger flap diameters
Then, the same eyes were grouped by the ratio of flap/corneal diameter (horizontal white-to-white distance). With the cutoff value set at 0.756 (the median value), the group (56 eyes) with smaller flap/corneal ratio had a mean ratio of 0.73 ± 0.02 and the group (56 eyes) with larger flap/corneal ratio had a mean ratio of 0.78 ± 0.02. The mean age was 30.57 ± 6.56 years in the group with smaller flap/corneal ratio and 33.0 ± 8.06 years in the group with larger flap/corneal ratio. There was no significant difference between the groups in age, sex distribution, preoperative SE, astigmatism, mean keratometry, pachymetry, flap thickness, treated SE, and ablation depth [Table 2]. There was also no significant difference in corneal sensitivity, TBUT, corneal fluorescein staining scores, conjunctival staining scores, and basic Schirmer's test between the groups with smaller flap/corneal ratio and larger flap/corneal ratio preoperatively [Figures 6–10].
Table 2.
Demographic data and preoperative and surgical parameters of the groups based on the ratio of flap size to horizontal corneal diameter
| Parameters | Ratio to cornea diameter | t/χ2 P* | |
|---|---|---|---|
| Smaller group (n=56) | Larger group (n=56) | ||
| Mean flap/corneal diameter ratio | 0.73±0.02 | 0.78±0.02 | |
| Age (year) | 30.57±6.56 | 33.00±8.06 | 0.083 |
| Sex, n (%) | |||
| Male | 9 (16.1) | 14 (25.0) | 0.242 |
| Female | 47 (83.9) | 42 (75.0) | |
| Preoperative SE (D) | −6.60±2.14 | −7.14±2.47 | 0.262 |
| Astigmatism (D) | −1.5±1.21 | −1.16±0.94 | 0.100 |
| Mean keratometry (D) | 43.71±1.34 | 43.85±1.84 | 0.639 |
| Pachymetry (µm) | 548.85±31.56 | 547.42±23.65 | 0.186 |
| Flap thickness (µm) | 86.98±19.49 | 87.54±24.98 | 0.883 |
| Treated SE (D)** | −5.76±1.96 | −6.27±1.87 | 0.160 |
| Calculated ablation depth (µm) | 105.32±24.98 | 111.36±26.08 | 0.214 |
*Independent sampling t-test or Pearson Chi-square, **Refraction in cornea plane. D=Diopters, SE=Spherical equivalent refraction
Figure 6.
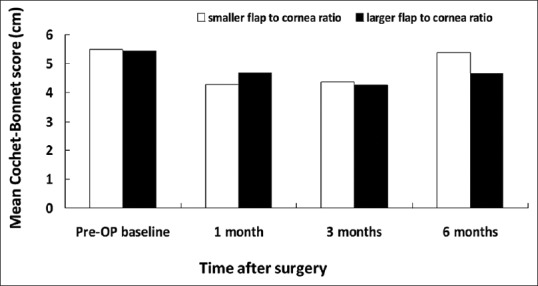
Central Cochet–Bonnet esthesiometry-measured mean corneal sensitivity associated with smaller and larger flap/horizontal corneal diameter ratio
Figure 10.
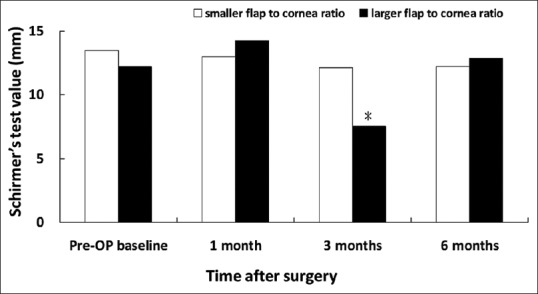
Basic Schirmer's test values in patients associated with smaller and larger flap/horizontal corneal diameter ratio. *P < 0.05 compared with the group of smaller flap/corneal diameter ratio
Despite that there was no significant between-group difference in corneal sensitivity, corneal fluorescein staining and rose bengal conjunctival staining for a follow-up period of 6 months; however, there was significant between-group difference in basic Schirmer's test score for 3-month follow up. The larger ratio group showed decreased tear secretion in Schirmer's test (7.52 ± 4.43 mm) than the smaller group (12.15 ± 8.14) at 3 months (P = 0.006) [Figure 10]. Furthermore, the group with larger flap/corneal diameter ratio showed shorter TBUT (4.20 ± 1.73 s) than the smaller ratio group (5.67 ± 1.90 s) at 6 months (P = 0.011) [Figure 7].
Figure 7.
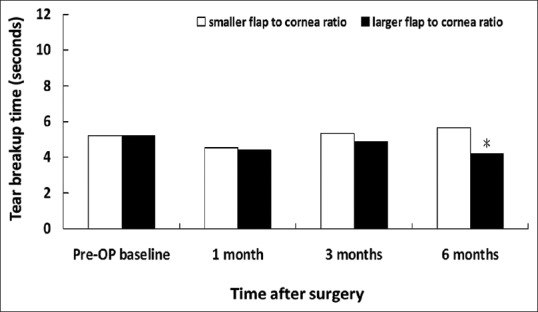
Tear breakup time in patients associated with smaller and larger flap/horizontal corneal diameter ratio. *P < 0.05 compared with the group of smaller flap/corneal diameter ratio
Discussion
Although accumulating studies have documented the safety and effectiveness of LASIK surgery,[15,16] some patients still report post-LASIK dry eye symptoms. A large body of evidence has shown that femtosecond laser-assisted LASIK decreased dry eye signs and symptoms after surgery.[7,17] However, the incidence of postflap LASIK dry eye disease is higher and the duration of symptoms persists longer compared to small incision lenticule extraction (SMILE) procedure, so-called SMILE surgery.[18] There are multiple theories as to how LASIK contributes to the pathophysiology of dry eyes.[7,8,9,10] Among them, the wildly accepted concept is iatrogenic corneal nerve damage during flap creation and subsequent excimer laser ablation.[19]
It was previously believed that the corneal nerves predominantly enter the cornea at the 3- and 9-o’clock positions.[20,21] However, Müller et al.[22] performed an elegant histopathologic analysis in the human corneas and showed conclusively that no larger nerve trunks were present at 3- and 9-o’clock in the human cornea. Other studies[23,24,25] also confirmed the equal distribution of nerve trunks around the circumference of the cornea. Since the cornea was innervated equally, a larger flap may damage a greater percentage of the corneal neural architecture than a smaller flap during LASIK surgery. Hypothetically, the greater the loss of corneal sensation, the greater the risk for post-LASIK dry eye syndrome will be. To our knowledge, there is no published paper mentioned about the correlation between flap diameter and the effect on dry eye syndrome. In the current study, patients with different sizes of corneal flap did not show any statistical significance among corneal sensitivity and dry eye-related parameters between the groups at any time points. However, when taking the effect of corneal diameter into account, the group with larger flap/corneal diameter ratio showed significant lower basic Schirmer's test value at 3 months and shorter TBUT at 6 months compared to smaller flap/corneal diameter ratio group. These results confirm our hypothesis and emphasize the importance and necessity of considering the flap size relative to the corneal diameter while planning a LASIK surgery.
The Schirmer's test allows quantitative analysis of tear volume but is marred by low specificity and sensitivity. The TBUT evaluates the integrity of precorneal tear film and disruption of any component of the tear may affect its stability and decrease TBUT. Although we did not find any significant difference on corneal sensitivity between larger flap/corneal diameter ratio and smaller flap/corneal diameter ratio, the corneal sensitivity in the larger ratio group was significantly decreased (0.008) at 3 months compared to preoperative corneal sensitivity. It has been proposed that decreased corneal sensation may negatively affect the lacrimal function unit and corneal-blinking reflex loops, which in turn may further decrease tear secretion and blinking.[6] As meibum is controlled by blinking, a reduced blinking time may prevent adequate meibomian gland secretion and leads to rapid tear evaporation. Therefore, it is reasonable to explain why we found lower basic Schirmer's test value at 3 months and shorter TBUT at 6 months in the larger flap/corneal diameter ratio group. Moreover, not only did we find a statistically significantly lower TBUT at 6 months in the larger flap-to-cornea diameter group, but we also noted a trend toward significance in corneal sensitivity [Figure 6] and ocular surface staining scores at 3 and 6 months postoperatively [Figures 8 and 9], too. These findings may, in part, explain the effect of TBUT observed at 6 months.
Figure 8.
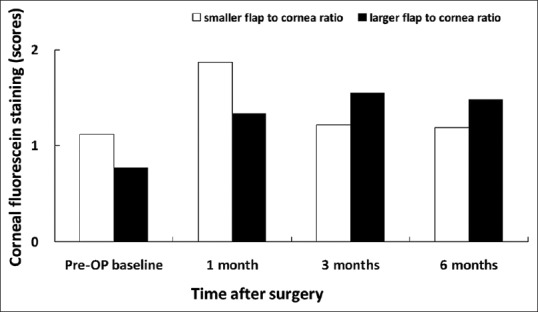
Corneal fluorescein staining in patients associated with smaller and larger flap/horizontal corneal diameter ratio
Figure 9.
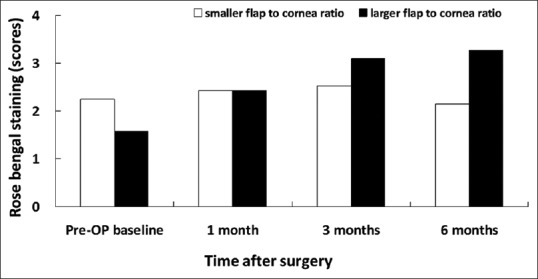
Rose bengal staining in patients associated with smaller and larger flap/horizontal corneal diameter ratio
Another interesting finding is that the group with larger flap/corneal diameter ratio showed shorter TBUT at 6 months compared to the group with smaller flap/corneal diameter ratio instead of early postoperative period. In general, the normal TBUT is longer than 10 s. However, the average preoperative TBUT in the group with larger flap/corneal diameter ratio is around 5 s, indicating a higher proportion of patients experience dry eye syndrome before surgery. Accumulating evidence also demonstrates that the presence of preoperative dry eye disease will significantly influence the occurrence of chronic dry eye after LASIK.[11,26] In fact, slightly decreased TBUT was noted in the group with larger flap/corneal diameter ratio throughout 6 months postoperatively, though not reaching significant difference compared to baseline TBUT [Figure 7]. Moreover, the so-called “LASIK-induced neurotrophic epitheliopathy”[11] caused by a neurotrophic effect on the cornea, is more likely to occur in the group with larger flap/corneal diameter ratio. Taken these factors together, we believe that dry eye parameters in eyes with a larger flap/corneal diameter ratio may need a longer time period to recover to their baseline levels. However, this study is limited to a relatively small case number. Further large-scale study is mandatory to validate our findings.
Conclusions
The latest tendency for LASIK flap creation has been to try to create flaps with the largest possible diameters in order to compensate for any decentration.[27] However, our study showed that larger flap to cornea ratio but not actual flap diameter might be prone to develop postoperative dry eye syndrome. Therefore, while deciding flap size before LASIK surgery, one should take the patient's corneal diameter into consideration.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare that there are no conflicts of interests of this paper.
References
- 1.Nordan LT, Slade SG, Baker RN, Suarez C, Juhasz T, Kurtz R, et al. Femtosecond laser flap creation for laser in situ keratomileusis: Six-month follow-up of initial U.S. Clinical series. J Refract Surg. 2003;19:8–14. doi: 10.3928/1081-597X-20030101-03. [DOI] [PubMed] [Google Scholar]
- 2.Khoramnia R, Salgado JP, Lohmann CP, Kobuch KA, von Mohrenfels CW. Precision, morphology, and histology of corneal flap cuts using a 200-kHz femtosecond laser. Eur J Ophthalmol. 2012;22:161–7. doi: 10.5301/EJO.2011.8376. [DOI] [PubMed] [Google Scholar]
- 3.Jabbur NS, Sakatani K, O’Brien TP. Survey of complications and recommendations for management in dissatisfied patients seeking a consultation after refractive surgery. J Cataract Refract Surg. 2004;30:1867–74. doi: 10.1016/j.jcrs.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Vroman DT, Sandoval HP, Fernández de Castro LE, Kasper TJ, Holzer MP, Solomon KD, et al. Effect of hinge location on corneal sensation and dry eye after laser in situ keratomileusis for myopia. J Cataract Refract Surg. 2005;31:1881–7. doi: 10.1016/j.jcrs.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 5.Yu EY, Leung A, Rao S, Lam DS. Effect of laser in situ keratomileusis on tear stability. Ophthalmology. 2000;107:2131–5. doi: 10.1016/s0161-6420(00)00388-2. [DOI] [PubMed] [Google Scholar]
- 6.Toda I. Dry eye after LASIK. Invest Ophthalmol Vis Sci. 2018;59:DES109–15. doi: 10.1167/iovs.17-23538. [DOI] [PubMed] [Google Scholar]
- 7.Sun CC, Chang CK, Ma DH, Lin YF, Chen KJ, Sun MH, et al. Dry eye after LASIK with a femtosecond laser or a mechanical microkeratome. Optom Vis Sci. 2013;90:1048–56. doi: 10.1097/OPX.0b013e31829d9905. [DOI] [PubMed] [Google Scholar]
- 8.Konomi K, Chen LL, Tarko RS, Scally A, Schaumberg DA, Azar D, et al. Preoperative characteristics and a potential mechanism of chronic dry eye after LASIK. Invest Ophthalmol Vis Sci. 2008;49:168–74. doi: 10.1167/iovs.07-0337. [DOI] [PubMed] [Google Scholar]
- 9.Shin SY, Lee YJ. Conjunctival changes induced by LASIK suction ring in a rabbit model. Ophthalmic Res. 2006;38:343–9. doi: 10.1159/000096229. [DOI] [PubMed] [Google Scholar]
- 10.Toda I, Asano-Kato N, Hori-Komai Y, Tsubota K. Laser-assisted in situ keratomileusis for patients with dry eye. Arch Ophthalmol. 2002;120:1024–8. doi: 10.1001/archopht.120.8.1024. [DOI] [PubMed] [Google Scholar]
- 11.Ambrósio R, Jr, Tervo T, Wilson SE. LASIK-associated dry eye and neurotrophic epitheliopathy: Pathophysiology and strategies for prevention and treatment. J Refract Surg. 2008;24:396–407. doi: 10.3928/1081597X-20080401-14. [DOI] [PubMed] [Google Scholar]
- 12.Mian SI, Shtein RM, Nelson A, Musch DC. Effect of hinge position on corneal sensation and dry eye after laser in situ keratomileusis using a femtosecond laser. J Cataract Refract Surg. 2007;33:1190–4. doi: 10.1016/j.jcrs.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 13.Mian SI, Li AY, Dutta S, Musch DC, Shtein RM. Dry eyes and corneal sensation after laser in situ keratomileusis with femtosecond laser flap creation effect of hinge position, hinge angle, and flap thickness. J Cataract Refract Surg. 2009;35:2092–8. doi: 10.1016/j.jcrs.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Huang JC, Sun CC, Chang CK, Ma DH, Lin YF. Effect of hinge position on corneal sensation and dry eye parameters after femtosecond laser-assisted LASIK. J Refract Surg. 2012;28:625–31. doi: 10.3928/1081597X-20120815-07. [DOI] [PubMed] [Google Scholar]
- 15.Tanzer DJ, Brunstetter T, Zeber R, Hofmeister E, Kaupp S, Kelly N, et al. Laser in situ keratomileusis in United States naval aviators. J Cataract Refract Surg. 2013;39:1047–58. doi: 10.1016/j.jcrs.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 16.Schallhorn SC, Farjo AA, Huang D, Boxer Wachler BS, Trattler WB, Tanzer DJ, et al. Wavefront-guided LASIK for the correction of primary myopia and astigmatism a report by the American Academy of OPhthalmology. Ophthalmology. 2008;115:1249–61. doi: 10.1016/j.ophtha.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Salomão MQ, Ambrósio R, Jr, Wilson SE. Dry eye associated with laser in situ keratomileusis: Mechanical microkeratome versus femtosecond laser. J Cataract Refract Surg. 2009;35:1756–60. doi: 10.1016/j.jcrs.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elmohamady MN, Abdelghaffar W, Daifalla A, Salem T. Evaluation of femtosecond laser in flap and cap creation in corneal refractive surgery for myopia: A 3-year follow-up. Clin Ophthalmol. 2018;12:935–42. doi: 10.2147/OPTH.S164570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon R, Donnenfeld ED, Perry HD. The effects of LASIK on the ocular surface. Ocul Surf. 2004;2:34–44. doi: 10.1016/s1542-0124(12)70022-8. [DOI] [PubMed] [Google Scholar]
- 20.Müller LJ, Vrensen GF, Pels L, Cardozo BN, Willekens B. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci. 1997;38:985–94. [PubMed] [Google Scholar]
- 21.Auran JD, Koester CJ, Kleiman NJ, Rapaport R, Bomann JS, Wirotsko BM, et al. Scanning slit confocal microscopic observation of cell morphology and movement within the normal human anterior cornea. Ophthalmology. 1995;102:33–41. doi: 10.1016/s0161-6420(95)31057-3. [DOI] [PubMed] [Google Scholar]
- 22.Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: Structure, contents and function. Exp Eye Res. 2003;76:521–42. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 23.Al-Aqaba MA, Fares U, Suleman H, Lowe J, Dua HS. Architecture and distribution of human corneal nerves. Br J Ophthalmol. 2010;94:784–9. doi: 10.1136/bjo.2009.173799. [DOI] [PubMed] [Google Scholar]
- 24.Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90:478–92. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 25.He J, Bazan NG, Bazan HE. Mapping the entire human corneal nerve architecture. Exp Eye Res. 2010;91:513–23. doi: 10.1016/j.exer.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bower KS, Sia RK, Ryan DS, Mines MJ, Dartt DA. Chronic dry eye in photorefractive keratectomy and laser in situ keratomileusis: Manifestations, incidence, and predictive factors. J Cataract Refract Surg. 2015;41:2624–34. doi: 10.1016/j.jcrs.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slade SG. The use of the femtosecond laser in the customization of corneal flaps in laser in situ keratomileusis. Curr Opin Ophthalmol. 2007;18:314–7. doi: 10.1097/ICU.0b013e3281bd88a0. [DOI] [PubMed] [Google Scholar]


