Abstract
The estrogen signaling pathway via nuclear estrogen receptors (ER) α and β is considered to be the master regulator of the cellular glucose metabolism in the uterus. While in vivo animal studies have demonstrated that 17β-estradiol (E2) treatment increases the expression levels and activities of several glycolytic enzymes in the uterus, the specific ER subtype-dependent regulation of key glycolytic enzymes in the uterus has not been experimentally verified. In this study, the localization of ERα and ERβ in human and mouse endometria were evaluated using immunohistology. Given that ERα and ERβ are not functionally equivalent, ERα, ERβ and ERαβ knockout (ERα-/-, ERβ-/- and ERαβ-/-) mice were utilized to determine the expression pattern of glycolytic enzymes in the uterus. It was found that the level of ERα was higher than that of ERβ in the human and mouse endometrial epithelial and stromal cells, and both receptors were downregulated by E2 treatment in the mouse uterus. The expression of the hexokinase 1 and GAPDH was increased in ERα-/- and ERβ-/- mice compared with wild-type controls. Increased phosphofructokinase expression was observed in ERα-/- and ERαβ-/- mice, whereas increased pyruvate kinase isozyme M2 and pyruvate dehydrogenase expression was observed in ERβ-/- and ERαβ-/- mice. The findings indicated for the first time that while estrogen regulates ERα and ERβ expression in the uterus, ERα and ERβ selectively regulate uterine glycolytic enzyme expression during glycolysis. Additionally, the link between endometrial ER subtypes and glycolysis in women with polycystic ovary syndrome (PCOS) is discussed. The findings suggested that the E2-dependent ER-mediated regulation of glycolysis may be involved in the disturbance of the glucose metabolism in patients with PCOS with endometrial dysfunction.
Keywords: estrogen receptor subtypes, glycolysis, uterus, knockout mice, polycystic ovary syndrome
Introduction
Estrogen elicits many different responses in female reproductive tissues, including the ovary and uterus, as well as in extra-reproductive tissues, such as the brain, adipose tissue and the liver (1,2). It is well known that numerous, but not all, of the concerted actions of estrogen are mediated through binding to two nuclear estrogen receptors (ERs), ERα and ERβ (1), both of which belong to a family of hormone-activated transcription factors and share common structural and functional domains (3). Although there is only ~60% homology in the ligand binding domain between ERα and ERβ, the receptors exhibit a similar binding affinity to endogenous 17β-estradiol (E2) (3). While ERα and ERβ can homo- or heterodimerize in vivo, they are not functionally equivalent, and in vitro experiments show that ERβ functions as a transcriptional inhibitor of ERα when ERα and ERβ are co-expressed (4). Although ERα and ERβ are often co-expressed in estrogen target cells under physiological conditions and although they can act together to regulate gene transcription (1,5), the cellular localization and abundance of the two receptors show distinct patterns in human endometrial epithelial and stromal cells (5). For example, ERα represents the most prominent receptor type in the endometrial epithelial and stromal cells during the menstrual cycle, whereas ERβ is found predominantly in the endometrial stromal cells in the late secretory phase (5). Direct evidence for essential roles of the estrogen signaling pathway in uterine physiology and disease is provided by different ER knockout and mutation studies in mice (1,3) and rats (6). It has been reported that female ERαβ-/- and ERα-/- mice and female ERα-/- rats are insensitive to E2 stimulation and they exhibit uterine hypoplasia and infertility, which is in contrast to loss of ERβ (ERβ-/-) in female mice that leads to subfertility. Moreover, changes in ERα expression levels and the ERα: ERβ ratio are considered to be the main factors behind several gynecological disorders, including impaired fertility and endometrial hyperplasia and carcinoma (5,7).
Polycystic ovary syndrome (PCOS), like numerous complex diseases, has a multifaceted etiology and pathophysiology, and it is associated with hormonal and metabolic impairments, ovarian dysfunction, menstrual irregularity and infertility (8,9). Due to chronic anovulation, patients with PCOS experience sustained and persistent estrogen stimulation but minimal or completely absent progesterone stimulation (10,11), and patients with PCOS with endometrial hyperplasia have a four-fold greater risk of developing endometrial carcinoma than non-PCOS controls (12). Preclinical and clinical studies have provided evidence that the endometrium from PCOS-like rodents and patients with PCOS displays morphologically normal, but structurally and biochemically abnormal responses to hormone stimulation (10,13-17). Although few PCOS endometrial samples have been analyzed, there is some controversial evidence that levels of endometrial ERα and ERβ mRNA and/or protein are higher in patients with PCOS compared with phase-matched non-PCOS controls, regardless of whether endometrial hyperplasia is present or not (18,19). Moreover, studies have previously shown that ERα and ERβ mRNAs are increased in PCOS-like rodent uteri (20,21). These preclinical and clinical findings suggest that altered expression and function of both ERs contribute to endometrial dysfunction in patients with PCOS.
Glycolysis is an energy-producing mechanism that is regulated by different levels and activities of enzymes, such as hexokinase (HK), phosphofructokinase (PFK) and pyruvate kinase (PK) (22). E2 is a master regulator of endometrial cell proliferation (23) and has been shown to increase HK1/2 and PK isozyme M2 (PKM2) activities, as well as glycolytic flux in the rat uterus in vivo (24-26) and in human endometrial stromal cells in vitro (27). Importantly, functional experiments demonstrated that de novo synthesis of E2 in stromal cells facilitates the decidualization process in the mouse uterus, which is a prerequisite for successful implantation and establishment of pregnancy (28). It was reported that the regulation and localization of uterine ERα but not ERβ mRNA was associated with the onset of early implantation in mice (29), and the acceleration of glycolysis is required for endometrial decidualization in humans and mice (30,31). Moreover, suppression of HK2 levels inhibited the proliferation and differentiation of human endometrial stromal cells in vitro (32). Taken together, these in vivo and in vitro studies suggest that it is possible that uterine E2-regulated glycolysis via ERα activation contributes to successful implantation and the establishment of pregnancy. However, whether uterine glycolysis is regulated by E2 in a specific ERα- and/or ERβ-dependent manner remains unclear.
In this study, the localization and regulation of ERα and ERβ in human and mouse endometria was assessed and ER-specific knockout mice that lack ERα and/or ERβ (ERαβ-/-, ERα-/- and ERβ-/-) were used to determine whether the selective contribution of ERα and ERβ results in the differential expression of key glycolytic enzymes in the mouse uterus.
Materials and methods
Animals and tissue collection
Two distinct experiments were performed with the animals. In the first experiment, intact prepubertal female C57BL/6J mice (Taconic Biosciences) at 26 days of age with a body weight (BW) of 13-15 g were used to avoid the complexity of ovarian functions associated with estrous cycles and endogenous surges of gonadotropins (33,34). Animals (n=5/group) were given a subcutaneous injection of 0.5 µg E2/g BW (in 100 µl sesame oil) or vehicle (100 µl sesame oil; Sigma-Aldrich; Merck KGaA) alone for 4 days (35). In the second experiment, homozygous mutant female mice lacking the genes for ERαβ, ERα and ERβ were utilized (age, 60-65 days; weight, 20-25 g); the generation of female ERαβ-/-, ERα-/- and ERβ-/- mice has been previously described (36-38). Scanbur AB bred and provided the different ER knockout mice; animals were inbred on a C57BL/6J background and littermate controls were used in all groups. All adult ER knockout mice were compared to isogenic wild-type (WT) age/weight-matched littermates at the same diestrus stage of the estrous cycle (n=4/group) (39).
Under anesthesia, the uteri were removed and stripped of fat and connective tissue. One side of the uterus in each animal was fixed in 4% formaldehyde neutral-buffered solution for 24 h at 4˚C and then embedded in paraffin for immunohistochemical analysis. The other side was immediately frozen in liquid nitrogen and stored at -70˚C for subsequent western blot analysis. All mice were housed in polycarbonate plastic cages with free access to food pellets and water at the infection-free animal facility of University of Gothenburg under a controlled temperature of 22±2˚C at 55-65% humidity with a 12-h light/dark cycles.
Human endometrial tissue collection
Endometrial tissues were obtained from reproductive-aged women (range, 25-45 years) during the proliferative phase of the menstrual cycle who were undergoing routine gynecological investigation. Tissues were collected in Obstetrics and Gynecology Hospital of Fudan University between March and October 2014. None of selected patients had been exposed to any hormonal or steroidal therapies within three months prior to tissue sampling. Each endometrial sample was diagnosed and staged by routine pathology analysis using standard histological criteria (40). All tissues were fixed in 10% neutral formalin solution for 24 h at 4˚C and embedded in paraffin for immunohistochemical analysis.
The animal study was approved by the Animal Care and Use Committee of the local Ethics Committee of the University of Gothenburg (Sweden) and all animal experiments and care procedures were performed in compliance with the institutional guidelines for the care and use of animals in research (170-2008 and 236-2012). The human study protocol conformed to the principles outlined in the Declaration of Helsinki under approval from the institutional Ethics Review Committee of the Obstetrics and Gynecology Hospital of Fudan University (approval no. OGHFU 2013-23). Appropriate written informed consent was obtained from all patients.
Total protein extraction and western blot analysis
Protein lysates were prepared from mouse uterine tissues using ice-cold RIPA buffer (Sigma-Aldrich; Merck KGaA) supplemented with cOmplete Mini protease inhibitor cocktail tablets (Roche Diagnostics) and PhosSTOP phosphatase inhibitor cocktail tablets (Roche Diagnostics). Protein concentration determination and a western blot analysis protocol were previously described (13,41,42). After determining the total protein concentration by Bradford protein assay (Thermo Fisher Scientific, Inc.), 30-µg protein was resolved on 4-12% Bis-Tris gradient gels (Novex; Thermo Fisher Scientific, Inc.) and transferred to PVDF membranes. The membranes were blocked with 0.01 M Tris-buffered saline supplemented with 0.1% (v/v) Triton X-100 (TBST) containing 5% non-fat dry milk for 1 h at room temperature (RT) and then probed with different primary antibodies in the blocking buffer overnight at 4˚C. The primary antibody details are as follows: HK1 (1:100; cat. no. 2024), HK2 (1:100; cat. no. 2867), PFK (1:100; cat. no. 8164), GAPDH (1:200; cat. no. 5174), PKM2 (1:100; cat. no. 4053), pyruvate dehydrogenase (PDH; 1:100; cat. no. 3205) (all from Cell Signaling Technology, Inc.,) ERα (1:300; cat. no. 6F11; Novocastra Laboratories Ltd.; Leica Biosystems), ERβ (1:1,000; cat. no. 06-629; Upstate Biotechnology, Inc.), progesterone receptor (PR; 1:100; cat. no. sc-538), proliferating cell nuclear antigen (PCNA; 1:100; cat. no. sc-25280) (both from Santa Cruz Biotechnologies, Inc.), total caspase-3 (1:500; cat. no. C92-605; BD Biosciences) and β-actin (1:500; cat. no. A1978; Sigma-Aldrich; Merck KGaA). On day 2, the membranes were washed with TBST followed by either anti-rabbit IgG horseradish peroxidase (HRP)-conjugated goat (1:1,000; cat. no. A0545) or anti-mouse IgG HRP-conjugated goat (1:1,000; cat. no. A2304) secondary antibody (both from Sigma-Aldrich; Merck KGaA) for 1 h at RT. Chemiluminescence signals were detected using SuperSignal West Dura substrate following the manufacturer's instructions (Thermo Fisher Scientific, Inc.). Band densitometry and quantification was performed using Image Laboratory (v5.0; Bio-Rad Laboratories, Inc.) and the protein band densities were normalized to β-actin. To reprobe the membrane with another antibody, the blot was washed with TBST 3x for 10 min at RT and incubated with stripping buffer (65 mM Tris-HCl, 2% SDS and 100 mM β-mercaptoethanol, pH 6.8) at RT for 15 min. Then the steps regarding the washing, blocking and probing of the membrane were repeated.
Immunohistochemical analyses and microscopy
Immunohistochemistry and dual-immunofluorescence were performed according to previously described methods (20,42,43). Human endometria and mouse uterine and ovarian tissues were fixed in 4% formaldehyde neutral-buffered solution for 24 h at 4˚C, paraffin-embedded and 5 µm sections were obtained. Two sections per sample were stained using standard hematoxylin and eosin methods (13). After deparaffinization (xylene, 10 min at RT) and rehydration (100, 90 and 70% ethanol, each 10 min at RT), the sections were immersed in epitope retrieval buffer (10 mM sodium citrate buffer, pH 6.0) and heated in a 700 W microwave for 15 min. Sections were subsequently rinsed twice with deionized H2O and once with TBST, each 5 min at RT. Endogenous peroxidase was removed and non-specific binding was blocked by incubation with 3% H2O2 for 10 min at RT and then with 10% normal goat serum for 1 h at RT. After incubation with primary antibody overnight at 4˚C in a humidified chamber, same sections were incubated with secondary antibodies (30 min; RT) and stain from the avidin-biotinylated-peroxidase ABC kit according to the manufacturer's instructions (Vector Laboratories, Inc.; Maravai LifeSciences) followed by a 5-min treatment with 3,3'-diaminobenzidine (SK-4100; Vector Laboratories, Inc.; Maravai LifeSciences) at RT. All sections were incubated with DAB for the same length of time so that comparisons could be made between individual samples and all slides were stained in a single run to eliminate inter-experiment variations in staining intensity. Digital images of stained sections were obtained with a Nikon E-1000 microscope (Nikon Corporation) using bright-field optics (magnification, x2, x10 and x40) and photomicrographed using Easy Image 1 (Bergström Instrument AB). Primary antibodies for immunohistochemistry included: ERα (1:50; cat. no. MC-20; Santa Cruz Biotechnologies Inc.), ERβ (1:300; cat. no. 06-629; Upstate Biotechnology, Inc.), ERβ1 (1:100; cat. no. PPG5/10), ERβ2 (1:100; cat. no. 57/3) (both from AbD Serotec; Bio-Rad Laboratories, Inc.), cytokeratin 8 (1:200; cat. no. C5301; Sigma-Aldrich; Merck KGaA) and Ki-67 (1:100; cat. no. 9027; Cell Signaling Technology, Inc.).
Human and mouse endometrial tissue sections were blocked in PBS containing 1% BSA and 3% fat-free milk for 1 h at room temperature. Sections were incubated with the anti-ERα (1:50; cat. no. MC-20; Santa Cruz Biotechnologies for human tissues; and 1:100; cat. no. 6F11; Novocastra Laboratories for mouse tissues), anti-ERβ1 (1:100; cat. no. PPG5/10; AbD Serotec for human tissues), anti-ERβ2 (1:100; cat. no. 57/3; AbD Serotec for human tissues) or anti-ERβ (1:300; cat. no. 06-629; Upstate Biotechnology for mouse tissues) antibody in PBS supplemented with 0.1% (v/v) Triton X-100 (PBST) containing 1% BSA and 3% fat-free milk overnight at 4˚C. After washing with PBST three times for 5 min each, sections were incubated with Alexa Fluor 594-conjugated goat polyclonal anti-rabbit IgG (1:250; cat. no. A11037), Alexa Fluor 488-conjugated goat polyclonal anti-rabbit IgG (1:250; cat. no. A11008) or Alexa Fluor 488-conjugated goat polyclonal anti-mouse IgG (1:250; cat. no. A11039) (all from Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at RT. After the sections were washed with PBST, they were examined under an Axiovert 200 confocal microscope (magnification, x20 and x60; Zeiss GmbH) equipped with a laser-scanning confocal imaging LSM 510 META system (Carl Zeiss AG) and were photomicrographed. Background settings were adjusted from the examination of negative control specimen; different controls for non-specific staining have been described previously (43).
Statistical analysis
For all experiments, n represents the numbers of individual animals. Data are presented as the mean ± SEM (n=4/group). Statistical analyses were performed using the SPSS version 24.0 (IMB Corp.). The normal distribution of the data was tested by Shapiro-Wilk test. Differences between groups were analyzed by one-way ANOVA followed by Bonferroni's post hoc test for normally distributed data or by the Kruskal-Wallis test followed by Mann-Whitney U test for skewed data. P<0.05 was considered to indicate a statistically significant difference.
Results and Discussion
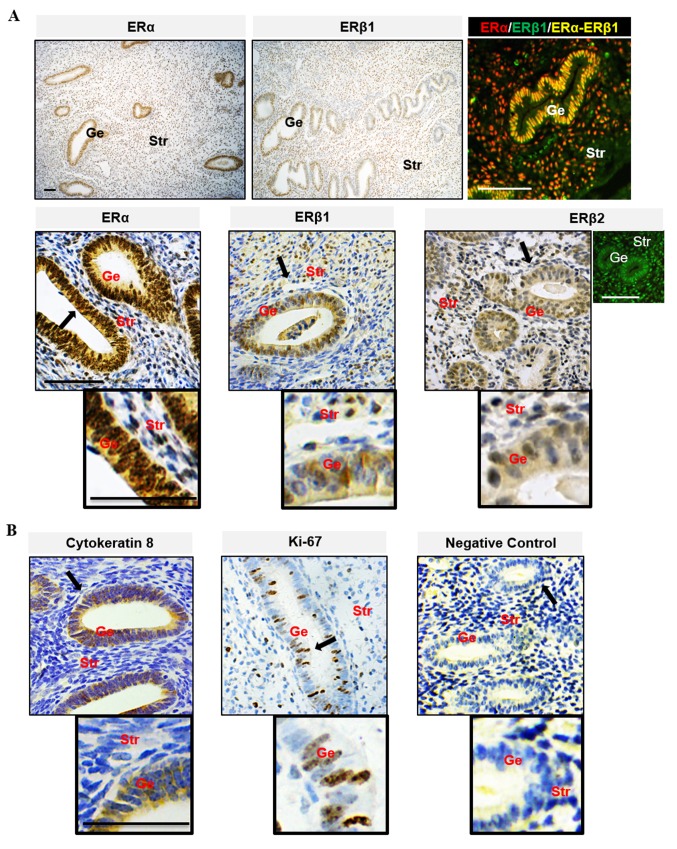
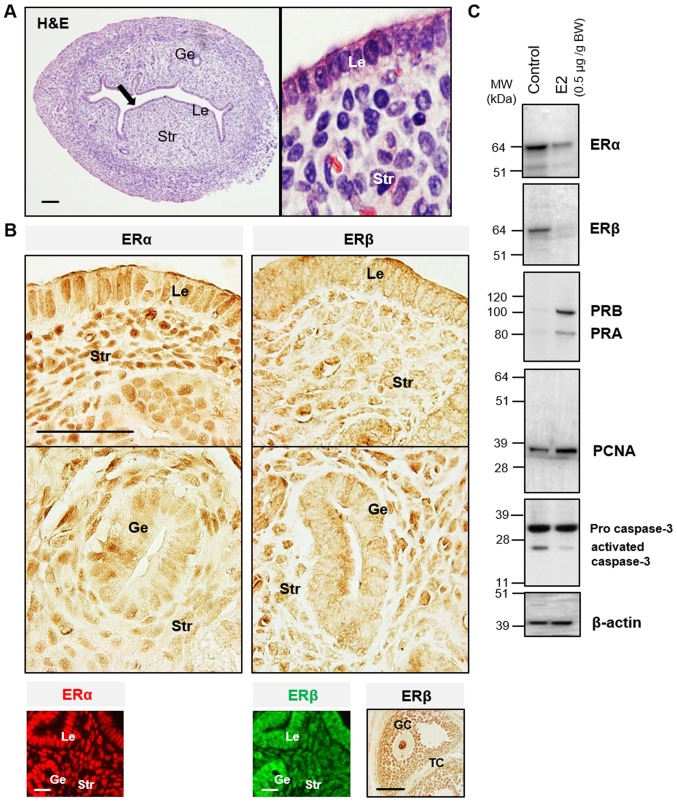
The endometrium is composed of a lining of surface epithelium and associated glands, and a stroma composed of connective tissue (5). During each reproductive cycle, the endometrial epithelial and stromal cells display distinct and well-defined patterns of functional differentiation under the cyclic influence of estrogen and progesterone (23). Increasing evidence suggests that the differential effects of estrogen on endometrial cells likely depend on the total amount of cellular ERs and/or the ratio of ERα to ERβ (5,7). In this study, it was found that the level of ERα was higher than ERβ in human (Fig. 1) and mouse (Fig. 2A and B) endometrial epithelial and stromal cells. This suggested that ERα was the predominant ER expressed in the uterus. Immunohistochemical analysis of tissues from women during the estrogen-dominant proliferative phase showed strong positive nuclear staining for ERα in epithelial and stromal cells but weak to moderate positive nuclear staining for ERβ1 and ERβ2, and ERα immunoreactivity was more abundant in the nucleus than in the cytoplasm in epithelial cells. ERα and ERβ1 were heterogeneously co-localized in the nucleus of epithelial and stromal cells. Compared with ERβ1, the ERβ2 immunoreactivity was evenly detected in the nuclei and cytoplasm of epithelial and stromal cells (Fig. 1A). These observations of endometrial cellular ERα and ERβ localization were broadly in agreement with previous human studies (5). As shown in Fig. 2B, ERα immunoreactivity was detected in the nuclei of epithelial and stromal cells, whereas ERβ immunoreactivity was detected mainly in the nuclei of stromal cells only. It was found that prepubescent mice treated chronically with E2 had decreased ERα and ERβ protein expression and increased PR isoform protein expression compared with the vehicle-treated controls (Fig. 2C). In the same experimental mouse uterus, increased PCNA, a cellular marker for proliferation, was associated with decreased activated caspase-3, a marker for cell apoptosis (Fig. 2C). These finding confirmed that E2 contributed to normal endometrial growth through the direct regulation of uterine ERα and ERβ in vivo.
Figure 1.
Localization of ER subtypes in the human endometria. (A) The localization of ERα (red) and ERβ (green). (B) Cellular marker proteins of cytokeratin 8 and Ki 67 in human endometria during the estrogen-dominant proliferative phase was assessed using immunohistology. Sections exposed to human endometrial tissues (the proliferative phase) were used as negative controls. Brown spots were observed using 3,3'-diaminobenzidine as the chromogen. Black arrows indicate areas shown at higher magnification; scale bar, 100 µm. Ge, glandular epithelial cells; Str, stromal cells; ER, estrogen receptor.
Figure 2.
Localization of ER subtypes in the mouse uterus. (A) Uterine tissue sections stained with hematoxylin and eosin staining. (B) Immunohistological localization of ERα and ERβ in the mouse uterus. A mouse ovarian tissue section was used as the positive control for the anti-ERβ antibody specificity. Immunohistochemistry was performed using 3,3'-diaminobenzidine (brown). Immunofluorescence detection shows ERα (red) and ERβ (green). Black arrows indicate areas shown at higher magnification; scale bar, 100 µm. (C) Western blot analysis of ER subtypes, proliferation and apoptosis markers in the prepubescent mouse uterus. E2, 17β-estradiol; BW, body weight; MW, molecular weight; Le, luminal epithelial cells; Ge, glandular epithelial cells; Str, stromal cells; GC, granulosa cells; TC, thecal cells; ER, estrogen receptor; PR, progesterone receptor; proliferating cell nuclear antigen.
There is increasing clinical and experimental evidence suggesting that aberrant regulation of ERα and ERβ expression is involved in the development and progression of several reproductive and metabolic diseases (1,2). For example, one female patient with a homozygous ERα mutation and female mice and rats lacking ERα display similar polycystic ovary phenotypes and infertility (6,44,45) as observed in patients with PCOS. Moreover, an ERβ polymorphism (+1730 G/A) has been implicated in susceptibility to the development of PCOS in humans (46). While the exact mechanisms of the pathogenesis of PCOS remains unknown, increasing evidence suggests that PCOS is a clinically heterogeneous and multifactorial disorder (8,9). Taken together, these findings indicate that multiple cellular and molecular signaling pathways are likely to be involved in its pathogenesis.
Glycolysis is the splitting apart of a glucose molecule in the cytosol by a sequence of enzymatic reactions, and its efficient operation requires adequate glucose uptake mediated by a number of glucose transporters (GLUTs) (47). Among the GLUTs, GLUT1 has been identified as the most prominent in endometrial tissues in vivo (48). Thus, it is thought that GLUT1 is responsible for the basal level of glucose uptake needed for normal glucose utilization in the uterus. It has been reported that E2 decreases glucose uptake in association with decreased GLUT1 expression in human and mouse endometrial stromal cells in vitro (49). Although there is no cyclical fluctuation of insulin-sensitive GLUT4 expression observed in human endometrium (50), it was previously shown that GLUT4 mRNA and protein expression are decreased in patients with PCOS compared with non-PCOS controls (50-52) and a similar observation has been made in the PCOS-like rat uterus (13,51). An analysis of gene expression in endometrial tissues found significantly reduced levels of key glycolytic genes in patients with PCOS compared with non-PCOS controls (53). Reproductive dysfunction and infertility are common in patients with PCOS (11,54), who often display E2-mediated endometrial hyperproliferation (55). Further studies have demonstrated that several proteins involved in cytosolic glycolysis, such as PKM2, are impaired in the endometrium of patients with PCOS and in PCOS-like animals with an endometrial hypoplasia phenotype (13,56). These findings support the notion that dysregulation of E2-mediated glycolysis is, at least partially, involved in the endometrial dysfunction in patients with PCOS with endometrial hyperplasia.
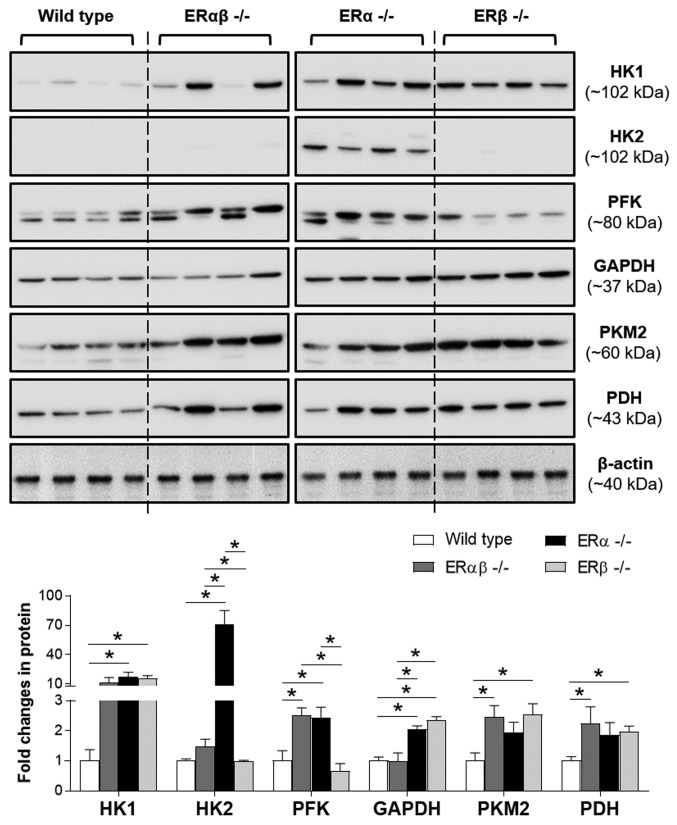
Changes in the glucose metabolism are a fundamental part of many biological processes (22). However, at present there is limited knowledge as to whether the estrogenic regulation of the uterine glucose metabolism depends on different ER subtypes. In this study, the expression pattern of uterine glycolytic enzymes in ERαβ-/-, ERα-/- and ERβ-/- mice was compared with WT controls (Fig. 3). Using western blot analysis, it was found that HK1 and GAPDH expression was significantly increased in ERα-/- and ERβ-/- mice compared with the WT controls. Moreover, significantly increased HK2 and PFK expression was observed in ERα-/- mice compared with the WT controls, whereas significantly increased PKM2 and PDH expression was observed for ERβ-/- mice compared with the WT controls. As indicated in the expression pattern of glycolytic enzymes, disruption of ERα and ERβ (ERαβ-/-) resulted in significantly increased PFK, PKM2 and PDH expression compared with the WT controls. This suggested that although ERα is the predominant ER expressed in the uterus, ERβ may partially compensate for the loss of ERα by increasing the expression of certain glycolytic enzymes in the uterus. Furthermore, the significantly increased uterine PDH expression in ERβ-/- but not ERα-/- animals compared with the WT controls suggested that the cell's mitochondria contained primarily ERβ and not ERα (5). It is noteworthy that the estrogen responsiveness becomes more complex because human and rodent reproductive tissues contain splice variants of ERα and ERβ (5,35,57), and the two subtypes form heterodimers with in vivo (3). It was previously shown that ERα-/- mouse uteri, similar to ERαβ-/- mouse uteri, remain to have one ERα splice form (35) and the levels of estrogen-regulated ERα protein are positively associated with endometrial hyperplasia in patients with PCOS (56). To better understand the role of estrogen-regulated glycolysis in the endometria of patients with PCOS, further studies are needed to determine whether ERα splice variants are differentially regulated by E2 using well-controlled endometrial tissue samples collected from patients with PCOS with various phenotypes.
Figure 3.
Expression of glycolytic enzymes in ER knockout mouse uteri. Protein levels of glycolytic enzymes were determined by western blot and are presented relative to β-actin (n=4/group). Data are expressed as the mean ± SEM. *P<0.05. HK, hexokinase; PFK, phosphofructokinase; PKM2, pyruvate kinase isoform M2; PDH, pyruvate dehydrogenase.
Furthermore, the western blot analysis demonstrated that two distinct forms of PFK were present in the mouse uterus (Fig. 3). PFK is synthesized as an unstable inactive monomer, which associates rapidly to form minimally active dimers essential for maintaining the tertiary structure of the enzyme (58). Several studies have shown that PFK has three isoforms (M, P and L) and differentially expresses in various mammalian tissues in vivo (59,60). It is hypothesized that the PFK antibody used in this study was able to detect two different isoforms of PFK; however, which PFK isoform is expressed in the mouse uterus remains to be determined. As the varying ratio of PFK isoforms may determine the glycolytic rate in a tissue-specific manner (61), further work is needed to determine which PFK isoforms contribute to the uterine glycolytic rates in mice.
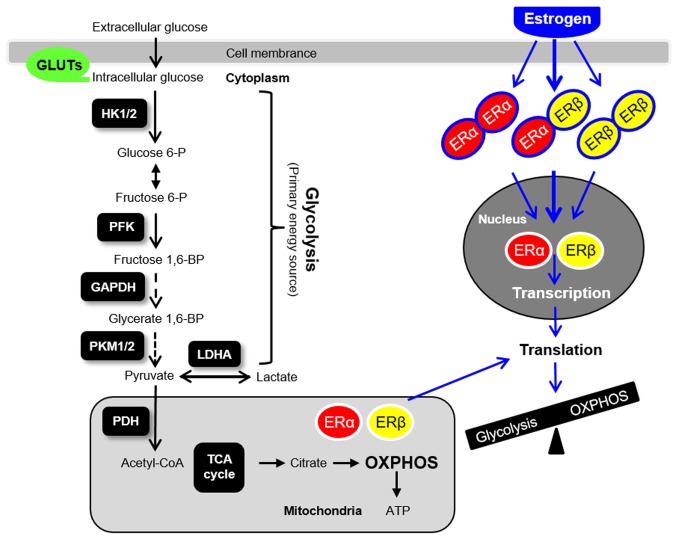
The role of aberrant glucose metabolism in the development of hormone-related diseases has become a topic of great interest. In addition to hyperandrogenism, numerous patients with PCOS also exhibit core metabolic manifestations, including peripheral insulin resistance (8,9). Of interest, female ERα-/- but not ERβ-/- mice develop obesity and insulin resistance (2). Hulchiy et al (62) have reported that endometrial ERα but not ERβ mRNA is decreased in overweight/obese patients with PCOS compared with controls, which is in contrast to the increased endometrial ERα and ERβ mRNA and/or protein expression observed in patients with hyperandrogenic PCOS (18,19). Thus, it remains unclear how insulin resistance and hyperandrogenism differentially affect ER subtype-mediated regulation of glycolysis in the endometrium in patients with PCOS. Based on a growing number of preclinical and clinical studies (13,18,41,42,63,64), it is hypothesized that abnormal steroid hormone responsiveness, such as hyperandrogenism, metabolic dysfunction, such as insulin resistance, molecular aberrations in the endometrium, such as glycolysis (Fig. 4), oxidative stress, immune factors and inflammatory uterine environments are all potential to be involved in the endometrial dysfunction observed in patients with PCOS (42). Further investigations are required to elucidate the crosstalk between these possible mechanisms in the uterus under both physiological and pathological conditions.
Figure 4.
Glycolysis, mitochondria-mediated energy metabolism and ER-mediated genomic actions in the uterus. GLUT, glucose transporter; HK, hexokinase; PFK, phosphofructokinase; PKM, pyruvate kinase isoform; LDHA, lactate dehydrogenase A; PDH, pyruvate dehydrogenase; OXPHOS, oxidative phosphorylation; TCA, tricarboxylic acid; ER, estrogen receptor; P, phosphate; BP, bisphosphate.
Acknowledgements
Not applicable.
Funding
This study was funded by grants from the Swedish Medical Research Council (grant no. 10380), the Swedish state under the ALF agreement between the Swedish government and the county councils (grant no. ALFGBG-147791), the Jane and Dan Olsson's Foundation, the Knut and Alice Wallenbergs Foundation, and the Adlerbert Research Foundation to HB and LRS, as well as the Guangzhou Medical University High-level University Construction Talents Fund (grant no. B185006010046) and the National Natural Science Foundation of China (grant no. 81774136).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LRS conceptualized the experiments, supervised the study and provided key research direction. MH, YZ, EE, XL and LRS performed the experiments. MH and LRS took responsibility for the integrity of the data analysis. LRS wrote and revised the manuscript. HB interpreted data and provided the critical comments on the manuscript. LRS and HB provided scientific oversight and guidance. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The animal study was approved by the Animal Care and Use Committee of the local Ethics Committee of the University of Gothenburg (Sweden) and all animal experiments and care procedures were performed in compliance with the institutional guidelines for the care and use of animals in research (170-2008 and 236-2012). The human study protocol conformed to the principles outlined in the Declaration of Helsinki under approval from the institutional Ethics Review Committee of the Obstetrics and Gynecology Hospital of Fudan University (approval no. OGHFU 2013-23). Appropriate written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hamilton KJ, Hewitt SC, Arao Y, Korach KS. Estrogen hormone biology. Curr Top Dev Biol. 2017;125:109–146. doi: 10.1016/bs.ctdb.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barros RP, Gustafsson JÅ. Estrogen receptors and the metabolic network. Cell Metab. 2011;14:289–299. doi: 10.1016/j.cmet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Hewitt SC, Winuthayanon W, Korach KS. What's new in estrogen receptor action in the female reproductive tract. J Mol Endocrinol. 2016;56:R55–R71. doi: 10.1530/JME-15-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews J, Wihlén B, Tujague M, Wan J, Strom A, Gustafsson JA. Estrogen receptor (ER) beta modulates ERalpha-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol Endocrinol. 2006;20:534–543. doi: 10.1210/me.2005-0140. [DOI] [PubMed] [Google Scholar]
- 5.Hapangama DK, Kamal AM, Bulmer JN. Estrogen receptor β: The guardian of the endometrium. Hum Reprod Update. 2015;21:174–193. doi: 10.1093/humupd/dmu053. [DOI] [PubMed] [Google Scholar]
- 6.Rumi MA, Dhakal P, Kubota K, Chakraborty D, Lei T, Larson MA, Wolfe MW, Roby KF, Vivian JL, Soares MJ. Generation of Esr1-knockout rats using zinc finger nuclease-mediated genome editing. Endocrinology. 2014;155:1991–1999. doi: 10.1210/en.2013-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez AC, Blanchard Z, Maurer KA, Gertz J. Estrogen signaling in endometrial cancer: A key oncogenic pathway with several open questions. Horm Cancer. 2019;10:51–63. doi: 10.1007/s12672-019-0358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2(16057) doi: 10.1038/nrdp.2016.57. [DOI] [PubMed] [Google Scholar]
- 9.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Feng Y, Lin JF, Billig H, Shao R. Endometrial progesterone resistance and PCOS. J Biomed Sci. 2014;21(2) doi: 10.1186/1423-0127-21-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Shao R. PCOS and obesity: Insulin resistance might be a common etiology for the development of type I endometrial carcinoma. Am J Cancer Res. 2014;4:73–79. [PMC free article] [PubMed] [Google Scholar]
- 12.Fearnley EJ, Marquart L, Spurdle AB, Weinstein P, Webb PM. Australian Ovarian Cancer Study Group and Australian National Endometrial Cancer Study Group: Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: An Australian case-control study. Cancer Causes Control. 2010;21:2303–2308. doi: 10.1007/s10552-010-9658-7. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Sun X, Sun X, Meng F, Hu M, Li X, Li W, Wu XK, Brännström M, Shao R, Billig H. Molecular characterization of insulin resistance and glycolytic metabolism in the rat uterus. Sci Rep. 2016;6(30679) doi: 10.1038/srep30679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuyucu Y, Çelik LS, Kendirlinan Ö, Tap Ö, Mete UÖ. Investigation of the uterine structural changes in the experimental model with polycystic ovary syndrome and effects of vitamin D treatment: An ultrastructural and immunohistochemical study. Rep Biol. 2018;18:53–59. doi: 10.1016/j.repbio.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Brosens I, Benagiano G. Menstrual preconditioning for the prevention of major obstetrical syndromes in polycystic ovary syndrome. Am J Obstet Gynecol. 2015;213:488–493. doi: 10.1016/j.ajog.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Leonhardt H, Gull B, Kishimoto K, Kataoka M, Nilsson L, Janson PO, Stener-Victorin E, Hellström M. Uterine morphology and peristalsis in women with polycystic ovary syndrome. Acta Radiol. 2012;53:1195–1201. doi: 10.1258/ar.2012.120384. [DOI] [PubMed] [Google Scholar]
- 17.Villavicencio A, Bacallao K, Gabler F, Fuentes A, Albornoz J, Casals A, Vega M. Deregulation of tissue homeostasis in endometria from patients with polycystic ovarian syndrome with and without endometrial hyperplasia. Gynecol Oncol. 2007;104:290–295. doi: 10.1016/j.ygyno.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Piltonen TT. Polycystic ovary syndrome: Endometrial markers. Best Pract Res Clin Obstet Gynaecol. 2016;37:66–79. doi: 10.1016/j.bpobgyn.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Baracat MC, Serafini PC, Simões Rdos S, Maciel GA, Soares JM Jr, Baracat EC. Systematic review of cell adhesion molecules and estrogen receptor expression in the endometrium of patients with polycystic ovary syndrome. Int J Gynaecol Obstet. 2015;129:1–4. doi: 10.1016/j.ijgo.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Hu M, Zhang Y, Feng J, Xu X, Zhang J, Zhao W, Guo X, Li J, Vestin E, Cui P, et al. Uterine progesterone signaling is a target for metformin therapy in PCOS-like rats. J Endocrinol. 2018;237:123–137. doi: 10.1530/JOE-18-0086. [DOI] [PubMed] [Google Scholar]
- 21.Li SY, Song Z, Song MJ, Qin JW, Zhao ML, Yang ZM. Impaired receptivity and decidualization in DHEA-induced PCOS mice. Sci Rep. 2016;6(38134) doi: 10.1038/srep38134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burns JS, Manda G. Metabolic pathways of the warburg effect in health and disease: Perspectives of choice, Chain or chance. Int J Mol Sci. 2017;18(E2755) doi: 10.3390/ijms18122755. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35:851–905. doi: 10.1210/er.2014-1045. [DOI] [PubMed] [Google Scholar]
- 24.Baquer NZ, McLean P. The effect of oestradiol on the profile of constant and specific proportion groups of enzymes in rat uterus. Biochem Biophys Res Commun. 1972;48:729–734. doi: 10.1016/0006-291x(72)90667-5. [DOI] [PubMed] [Google Scholar]
- 25.Baquer NZ, Sochor M, Kunjara S, McLean P. Effect of oestradiol on the carbohydrate metabolism of immature rat uterus: The role of fructose-2, 6-bis-phosphate and of phosphoribosyl pyrophosphate. Biochem Mol Biol Int. 1993;31:509–519. [PubMed] [Google Scholar]
- 26.Reiss NA. Ontogeny and estrogen responsiveness of creatine kinase and glycolytic enzymes in brain and uterus of rat. Neurosci Lett. 1988;84:197–202. doi: 10.1016/0304-3940(88)90407-7. [DOI] [PubMed] [Google Scholar]
- 27.Singhal RL, Valadares JR. Estrogenic regulation of uterine pyruvate kinase. Am J Physiol. 1970;218:321–327. doi: 10.1152/ajplegacy.1970.218.2.321. [DOI] [PubMed] [Google Scholar]
- 28.Das A, Mantena SR, Kannan A, Evans DB, Bagchi MK, Bagchi IC. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc Natl Acad Sci USA. 2009;106:12542–12547. doi: 10.1073/pnas.0901647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–5321. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuo RJ, Gu XW, Qi QR, Wang TS, Zhao XY, Liu JL, Yang ZM. Warburg-like glycolysis and lactate shuttle in mouse decidua during early pregnancy. J Biol Chem. 2015;290:21280–21291. doi: 10.1074/jbc.M115.656629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kommagani R, Szwarc MM, Kovanci E, Gibbons WE, Putluri N, Maity S, Creighton CJ, Sreekumar A, DeMayo FJ, Lydon JP, O'Malley BW. Acceleration of the glycolytic flux by steroid receptor coactivator-2 is essential for endometrial decidualization. PLoS Genet. 2013;9(e1003900) doi: 10.1371/journal.pgen.1003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lv H, Tong J, Yang J, Lv S, Li WP, Zhang C, Chen ZJ. Dysregulated pseudogene HK2P1 may contribute to preeclampsia as a competing endogenous RNA for hexokinase 2 by impairing decidualization. Hypertension. 2018;71:648–658. doi: 10.1161/HYPERTENSIONAHA.117.10084. [DOI] [PubMed] [Google Scholar]
- 33.Shao R, Markström E, Friberg PA, Johansson M, Billig H. Expression of progesterone receptor (PR) A and B isoforms in mouse granulosa cells: Stage-dependent PR-mediated regulation of apoptosis and cell proliferation. Biol Reprod. 2003;68:914–921. doi: 10.1095/biolreprod.102.009035. [DOI] [PubMed] [Google Scholar]
- 34.Shao R, Zhang FP, Rung E, Palvimo JJ, Huhtaniemi I, Billig H. Inhibition of small ubiquitin-related modifier-1 expression by luteinizing hormone receptor stimulation is linked to induction of progesterone receptor during ovulation in mouse granulosa cells. Endocrinology. 2004;145:384–392. doi: 10.1210/en.2003-0527. [DOI] [PubMed] [Google Scholar]
- 35.Shao R, Egecioglu E, Weijdegård B, Kopchick JJ, Fernandez-Rodriguez J, Andersson N, Billig H. Dynamic regulation of estrogen receptor-alpha isoform expression in the mouse fallopian tube: Mechanistic insight into estrogen-dependent production and secretion of insulin-like growth factors. Am J Physiol Endocrinol Metab. 2007;293:E1430–E1442. doi: 10.1152/ajpendo.00384.2007. [DOI] [PubMed] [Google Scholar]
- 36.Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- 37.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng Y, Johansson J, Shao R, Mannerås L, Fernandez-Rodriguez J, Billig H, Stener-Victorin E. Hypothalamic neuroendocrine functions in rats with dihydrotestosterone-induced polycystic ovary syndrome: Effects of low-frequency electro-acupuncture. PLoS One. 2009;4(e6638) doi: 10.1371/journal.pone.0006638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Hu M, Meng F, Sun X, Xu H, Zhang J, Cui P, Morina N, Li X, Li W, et al. Metformin ameliorates uterine defects in a rat model of polycystic ovary syndrome. EBioMedicine. 2017;18:157–170. doi: 10.1016/j.ebiom.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu M, Zhang Y, Guo X, Jia W, Liu G, Zhang J, Li J, Cui P, Sferruzzi-Perri AN, Han Y, et al. Hyperandrogenism and insulin resistance induce gravid uterine defects in association with mitochondrial dysfunction and aberrant reactive oxygen species production. Am J Physiol Endocrinol Metab. 2019;316:E794–E809. doi: 10.1152/ajpendo.00359.2018. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Pishdari B, Cui P, Hu M, Yang HP, Guo YR, Jiang HY, Feng Y, Billig H, Shao R. Regulation of androgen receptor expression alters AMPK phosphorylation in the endometrium: In vivo and in vitro studies in women with polycystic ovary syndrome. Int J Bio Sci. 2015;11:1376–1389. doi: 10.7150/ijbs.13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quaynor SD, Stradtman EW Jr, Kim HG, Shen Y, Chorich LP, Schreihofer DA, Layman LC. Delayed puberty and estrogen resistance in a woman with estrogen receptor alpha variant. N Engl J Med. 2013;369:164–171. doi: 10.1056/NEJMoa1303611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamilton KJ, Arao Y, Korach KS. Estrogen hormone physiology: Reproductive findings from estrogen receptor mutant mice. Reprod Biol. 2014;14:3–8. doi: 10.1016/j.repbio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JJ, Choi YM, Choung SH, Yoon SH, Lee GH, Moon SY. Estrogen receptor beta gene +1730 G/A polymorphism in women with polycystic ovary syndrome. Fertil Steril. 2010;93:1942–1947. doi: 10.1016/j.fertnstert.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 47.Frolova AI, Moley KH. Glucose transporters in the uterus: An analysis of tissue distribution and proposed physiological roles. Reproduction. 2011;142:211–220. doi: 10.1530/REP-11-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frolova AI, Moley KH. Quantitative analysis of glucose transporter mRNAs in endometrial stromal cells reveals critical role of GLUT1 in uterine receptivity. Endocrinology. 2011;152:2123–2128. doi: 10.1210/en.2010-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frolova A, Flessner L, Chi M, Kim ST, Foyouzi-Yousefi N, Moley KH. Facilitative glucose transporter type 1 is differentially regulated by progesterone and estrogen in murine and human endometrial stromal cells. Endocrinology. 2009;150:1512–1520. doi: 10.1210/en.2008-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui P, Li X, Wang X, Feng Y, Lin JF, Billig H, Shao R. Lack of cyclical fluctuations of endometrial GLUT4 expression in women with polycystic ovary syndrome: Evidence for direct regulation of GLUT4 by steroid hormones. BBA Clin. 2015;4:85–91. doi: 10.1016/j.bbacli.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Cui P, Jiang HY, Guo YR, Pishdari B, Hu M, Feng Y, Billig H, Shao R. Reversing the reduced level of endometrial GLUT4 expression in polycystic ovary syndrome: A mechanistic study of metformin action. Am J Transl Res. 2015;7:574–586. [PMC free article] [PubMed] [Google Scholar]
- 52.Orostica L, Astorga I, Plaza-Parrochia F, Vera C, Garcia V, Carvajal R, Gabler F, Romero C, Vega M. Proinflammatory environment and role of TNF-α in endometrial function of obese women having polycystic ovarian syndrome. Int J Obes (Lond) 2016;40:1715–1722. doi: 10.1038/ijo.2016.154. [DOI] [PubMed] [Google Scholar]
- 53.Kim JY, Song H, Kim H, Kang HJ, Jun JH, Hong SR, Koong MK, Kim IS. Transcriptional profiling with a pathway-oriented analysis identifies dysregulated molecular phenotypes in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:1416–1426. doi: 10.1210/jc.2008-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evans J, Salamonsen LA, Winship A, Menkhorst E, Nie G, Gargett CE, Dimitriadis E. Fertile ground: Human endometrial programming and lessons in health and disease. Nat Rev Endocrinol. 2016;12:654–667. doi: 10.1038/nrendo.2016.116. [DOI] [PubMed] [Google Scholar]
- 55.Hardiman P, Pillay OC, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet. 2003;361:1810–1812. doi: 10.1016/s0140-6736(03)13409-5. [DOI] [PubMed] [Google Scholar]
- 56.Wang T, Zhang J, Hu M, Zhang Y, Cui P, Li X, Li J, Vestin E, Brännström M, Shao LR, Billig H. Differential expression patterns of glycolytic enzymes and mitochondria-dependent apoptosis in PCOS patients with endometrial hyperplasia, an early hallmark of endometrial cancer, in vivo and the impact of metformin in vitro. Int J Biol Sci. 2019;15:714–725. doi: 10.7150/ijbs.31425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 58.Schoneberg T, Kloos M, Brüser A, Kirchberger J, Sträter N. Structure and allosteric regulation of eukaryotic 6-phosphofructokinases. Biol Chem. 2013;394:977–993. doi: 10.1515/hsz-2013-0130. [DOI] [PubMed] [Google Scholar]
- 59.Eto K, Sakura H, Yasuda K, Hayakawa T, Kawasaki E, Moriuchi R, Nagataki S, Yazaki Y, Kadowaki T. Cloning of a complete protein-coding sequence of human platelet-type phosphofructokinase isozyme from pancreatic islet. Biochem Biophys Res Commun. 1994;198:990–998. doi: 10.1006/bbrc.1994.1141. [DOI] [PubMed] [Google Scholar]
- 60.Hannemann A, Jandrig B, Gaunitz F, Eschrich K, Bigl M. Characterization of the human P-type 6-phosphofructo-1-kinase gene promoter in neural cell lines. Gene. 2005;345:237–247. doi: 10.1016/j.gene.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 61.Al Hasawi N, Alkandari MF, Luqmani YA. Phosphofructokinase: A mediator of glycolytic flux in cancer progression. Crit Rev Oncol Hematol. 2014;92:312–321. doi: 10.1016/j.critrevonc.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Hulchiy M, Nybacka Å, Sahlin L, Hirschberg AL. Endometrial expression of estrogen receptors and the androgen receptor in women with polycystic ovary syndrome: A lifestyle intervention study. J Clin Endocrinol Metab. 2016;101:561–571. doi: 10.1210/jc.2015-3803. [DOI] [PubMed] [Google Scholar]
- 63.Shang K, Jia X, Qiao J, Kang J, Guan Y. Endometrial abnormality in women with polycystic ovary syndrome. Reprod Sci. 2012;19:674–683. doi: 10.1177/1933719111430993. [DOI] [PubMed] [Google Scholar]
- 64.Hu M, Li J, Zhang Y, Li X, Brännström M, Shao LR, Billig H. Endometrial progesterone receptor isoforms in women with polycystic ovary syndrome. Am J Transl Res. 2018;10:2696–2705. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.