Abstract
Deficits in semantic memory in individuals with amnestic mild cognitive impairment (aMCI) have been previously reported, but the underlying neurobiological mechanisms remain to be clarified. We examined event-related potentials (ERPs) associated with semantic memory retrieval in 16 individuals with aMCI as compared to 17 normal controls using the Semantic Object Retrieval Task (EEG SORT). In this task, subjects judged whether pairs of words (object features) elicited retrieval of an object (retrieval trials) or not (non-retrieval trials). Behavioral findings revealed that aMCI subjects had lower accuracy scores and marginally longer reaction time compared to controls. We used a multivariate analytical technique (STAT-PCA) to investigate similarities and differences in ERPs between aMCI and control groups. STAT-PCA revealed a left fronto-temporal component starting at around 750 ms post-stimulus in both groups. However, unlike controls, aMCI subjects showed an increase in the frontal-parietal scalp potential that distinguished retrieval from non-retrieval trials between 950 and 1050 ms post-stimulus negatively correlated with the performance on the logical memory subtest of the Wechsler Memory Scale-III. Thus, individuals with aMCI were not only impaired in their behavioral performance on SORT relative to controls, but also displayed alteration in the corresponding ERPs. The altered neural activity in aMCI compared to controls suggests a more sustained and effortful search during object memory retrieval, which may be a potential marker indicating disease processes at the pre-dementia stage.
Keywords: Cognition, electroencephalography, event-related potentials, memory, mild cognitive impairment, semantics
INTRODUCTION
The diagnosis of amnestic mild cognitive impairment (aMCI), a clinical entity proposed to be an intermediate state between normal aging and Alzheimer disease (AD), has been widely used to identify subjects at higher risk of progressing to dementia [1, 2]. Current models of AD suggest that biomarkers associated with AD precede clinically recognizable signs by at least 5 to 10 years [3]. In particular, neuropathological evidence of adaptation and dysfunction of synapses in the medial temporal and neocortical regions has been found in AD patients [4] and is indicative of incipient AD [5, 6]. Advanced neuroimaging techniques have recently played a critical role in characterizing the earliest neurobiological changes that occur in AD [7, 8]. Techniques such as event-related potentials (ERPs), which reflect the summation of post-synaptic potentials that are timed-locked to certain events of interest, are affected by changes in synaptic integrity, and may hence have the potential to detect early disease processes in the AD brain and to monitor disease progression [9].
Although deficits in episodic memory are considered to be the hallmark symptom of AD [10], growing evidence suggests that semantic memory impairment is another key clinical symptom in MCI and early AD [11–13]. While degradation in the neural underpinnings of semantic memory has been suggested in ERP studies of AD showing delayed or diminished/absent semantic ERP components such as in N400 [14–22], fewer ERP studies have examined semantic memory in individuals who are in the pre-dementia phase, or MCI. Using a semantic congruity task with repetition, Olichney et al. found delayed N400 latency in MCI and diminished repetition effects for both congruous (late positive potentials) and incongruous (N400) trials in those MCI patients who converted to AD within 3 years [23, 24]. An ERP study found reduced N400 effect in MCI compared to controls during lexical ambiguity processing [25]. The majority of these studies have used either verification or priming paradigms in the context of word associations and semantic relations, but the extent to which these paradigms directly examine semantic memory retrieval is debatable. In view of the deficits observed in lexico-semantic recall in MCI during category fluency [26, 27], EEG tasks that involve direct lexico-semantic retrieval may advance our understanding of the neurobiological alterations associated with semantic retrieval deficits in individuals with MCI.
The Semantic Object Retrieval Test (SORT) comprises a task that requires recall of a specific concept (e.g., object) upon direct evaluation of semantic feature information [28–30]. In particular, retrieval items (e.g., desert and humps) target active retrieval of objects (e.g., camel) and non-retrieval items (e.g., desert and clock) do not. Semantic memory retrieval dysfunction in SORT will behaviorally manifest as either a retrieval failure (false negative) during the retrieval trials or failure to suppress inappropriate object retrieval (false alarms) during the non-retrieval trials [30, 31]. A previous study using a 32-item SORT showed overall more errors in MCI patients (when errors for both retrieval and non-retrieval items were included). In particular, one-third of the MCI participants performed 2 standard deviations below the normal average [31]. These findings suggest that semantic memory retrieval deficit in MCI on SORT is not homogenously observed and that decrement in SORT performance might be one early predictor of disease progression in some individuals. Given that neural alterations have been found to precede behavioral deficits [3], those MCI subjects who were not evidently impaired based on SORT task performance may have underlying alterations in neural activity during semantic retrieval, which cannot be detected using behavioral paradigms. Thus, objective neuroimaging techniques may help to more fully characterize semantic memory retrieval deficits in individuals with MCI.
Our group developed an EEG version of the SORT (EEG SORT) and has studied neural underpinning related to this task in young and older adults. The first study involving young adults found a left fronto-temporal component (beginning at 750 ms and lasting over 1500 ms post-stimulus) differentiating retrieval from non-retrieval trials [32]. When we examined the effects of normal aging using this same paradigm employing a data-driven approach, we replicated the findings of Brier et al. study. We found that in both younger and older adults differences between retrieval and non-retrieval trials occurred between 750 and 1500 ms. Additionally, we observed a frontal ERP effect (800–1000 ms post-stimulus) which differentiated non-retrieval from retrieval trials in older adults, suggesting a prolonged search process prior to judgment in older adults [30]. It is important to note that this later frontal ERP effect observed in older adults was independent (orthogonal) of the effect that was observed between 750–1500 ms. Intriguingly in our SORT ERP studies, we have not detected N400 effects (250–500 ms post-stimulus), which are typically observed in semantic processing studies [33]. Lack of typical N400 effects in the EEG SORT might be attributed to the fact that retrieval and non-retrieval trials are not analogous to congruous and incongruous trials [also see discussion in 30], which are often carefully and strategically manipulated in paradigms that yield N400 components; thus SORT may involve different and/or additional neural mechanisms [29]. Nevertheless, converging findings from both the young adult study and the aging study show that a left fronto-temporal ERP component corresponds to object memory retrieval in SORT in both young and old.
The primary aim of the current study was to use the EEG SORT to examine semantic object memory retrieval in individuals with aMCI as compared to normal aging controls. We hypothesized to identify altered neural activity associated with the SORT task in individuals with aMCI, as compared to controls, as qualitative differences (e.g., changes in topographical distribution or polarity of scalp potentials) and/or quantitative differences (e.g., reduced or delayed effects but with similar topographical distribution and polarity). A secondary aim of the current study was to look for associations between the ERP effects and neuropsychological measures to more fully address potential functional significance of the ERP effects and its implications [34].
MATERIALS AND METHODS
Subjects
We studied 16 aMCI subjects (6F; Mage = 69.7, SD = 7.9 years) and 17 normal control subjects (12F; Mage = 64.9, SD = 6.6 years). Participants (both controls and aMCI) were all Caucasian, 55 years or older, right-handed, and native English speakers. None had a history of learning disability, stroke, major psychiatric illness, alcoholism and substance abuse, uncorrected hearing and vision loss, or elevated depressive symptoms (Beck Depression Inventory-II [35] or Geriatric Depression Scale [36]>10). Controls did not have any subjective memory or cognitive complaints and performed normally on a battery of neuropsychological evaluation (Table 1). All the aMCI subjects met the standard clinical criteria [2] including the following:(a) memory concerns raised by the patient and/or corroborated by a reliable informant, (b) episodic memory impairments (1.5 SD below normal average) verified by objective measures, (c) grossly intact daily functions, and (d) not demented. aMCI subjects were either evaluated at the Alzheimer’s Disease Center at the University of Texas Southwestern Medical Center or by a behavioral neurologist (J.H.) who specializes in memory disorders. Twelve met criteria for single-domain aMCI and four were multiple-domain aMCI. The Clinical Dementia Rating (CDR) [37] was used in all MCI subjects [CDR = 0.5, sum of boxes: 1.57 (mean) ± 1.05 (SD)], and full neuropsychological results are listed in Table 1. Three of the aMCI patients were taking cholinesterase inhibitors when tested (two on donepezil and one on rivastigmine, all on stabilized doses for at least 3 months). Informed consent was obtained from all participants in accordance with the protocols approved by the Institutional Review Boards of The University of Texas at Dallas and of The University of Texas Southwestern Medical Center. Procedures involving experiments on human subjects were done in accord with the ethical standards of the Committee on Human Experimentation of these institutions in which the experiments were done in accord with the Helsinki Declaration of 1975.
Table 1:
Demographic information and results of neuropsychological measures and EEG task performance
| Controls (total n = 17) | aMCI (total n = 16) |
|
|---|---|---|
| Demographics | ||
| Age | 64.9 (6.6) | 69.7 (7.9) |
| Education | 17.2 (1.7) | 16.1 (2.2) |
| Gender | 5M/12F | 10M/6F |
| Neuropsychological measures | ||
| MMSEa | 28.4 (0.9) | 28.3 (1.3) |
| MoCAb | 28.4 (1.6) | — |
| COWAT-letter fluencyc | 50.7 (12.3) | 32.8 (9.2)** |
| Category fluencyc | 22.5 (3.6) | 18.1 (6.5) |
| BNT-30 itemd | 29.0 (1) | 27.8 (1.6)* |
| DS forwardc | 7.9 (0.9) | 6.8 (1.3)* |
| DS backwardc | 6.3 (1.1) | 5 (1.3) |
| Smilaritiese | 27.8 (2.7) | 26.1 (4.6) |
| Digit symbolf | 73.6 (11.3) | 42.6 (10.4)* |
| TMT-Ac Time | 28.2 (5.9) | 38.6 (12.1)* |
| TMT-Bc Time | 53.1 (14) | 101.1 (44.2)** |
| LM immediate | 15.8 (3.2) | 9.3 (2.3)** |
| LM delayed | 14.1 (2.8) | 7.7 (2.5)** |
| EEG SORT task | ||
| R-accuracy (%) | 87.5 (4.6) | 81 (6.1)* |
| NR-accuracy (%) | 93.2 (5.3) | 84.5 (13.4)** |
| R-RT (ms) | 1410 (237) | 1588 (329) |
| NR-RT (ms) | 1689 (404) | 1954 (411)* |
Each represents group mean (standard deviation).
MMSE control n = 5, MCI n = 16.
MoCA control n = 12.
Control n = 15, MCI n = 16.
Control n = 11, MCI n = 16.
Control n = 14, MCI n = 15.
Control n = 13, MCI n = 9.
MMSE, Mini-Mental State Exam [38]; MoCA, Montreal Cognitive Assessment [39]; COWAT, Controlled Oral Word Association Test [40]; Category fluency [41]; BNT, Boston Naming Test-30 items [42]; DS, digit span [43]; TMT, Trail Making Test [44]; LM, logical memory [45]. The numbers in the table are raw test scores. R, retrieval trials; NR, non-retrieval trials. Significance of each test between groups is shown as (*) when p < 0.05 or (**) when p < 0.01 in the aMCI column; otherwise the test is not significant. Tests for neuropsychological measures using raw scores were controlled for age and education.
EEG SORT task and procedure
All participants completed the EEG SORT [30] and the majority performed the EEG task within 1 month of the neuropsychological testing (mean = 41.5 days). The EEG SORT consists of 56 pairs of words in the retrieval condition, with each pair representing features of a particular object. The non-retrieval condition contains 56 pairs derived from the same set of words, but with these words randomly and nonsensically paired. For example, the word pair ‘humps’ and ‘desert’ would facilitate memory retrieval of ‘camel’ (retrieval pair). In contrast, ‘humps’ and ‘monitor’ do not typically lead to memory retrieval of any object (non-retrieval pair). The full sets of retrieval and non-retrieval word pairs were randomly combined into two different sequences, to which subjects were randomly assigned. Word pairs were presented simultaneously for three seconds, with one word above the other. Between each trial, a fixation sign (+) was presented at the center of the screen for three seconds. Participants were asked to push either the ‘yes’ or ‘no’ button under right index or middle finger, respectively, to indicate if a word pair led to object retrieval. The following written instructions were given prior to the EEG task: “You are going to see two words. These represent features that are related to objects. Push the button under your index finger if the two words combine to bring to mind the name of some particular object. If the two words do not combine to bring to mind a particular object, push the button under your middle finger.” In addition, both speed and accuracy of response was emphasized. Response time (RT) and accuracy were recorded for each trial. Responses made outside the window between 300 and 3500 ms were labeled as incorrect. The entire task lasted about 11 minutes. The stimuli were presented on an LCD screen using Stim software (Compumedics Neuroscan, USA) and placed about 46 inches from the participant. The two words in each pair spanned about 5° of both vertical and horizontal visual angle, with the horizontal measure varying slightly by word length. All the words were presented in black color, in lower case Times New Roman font (font size of 72), against a white background.
EEG data acquisition and processing
Continuous EEG was recorded using a 64-electrode elastic cap (Neuroscan Quickcap) through a Neuroscan SynAmps2 amplifier and using Scan 4.5 software (Compumedics Neuroscan, USA; sampling rate: 1 kHz, DC-200 Hz). Electrode impedances were typically below 10 kΩ. The reference electrode was located at midline between Cz and CPz and vertical electroocculogram (VEOG) was recorded at sites above and below left eye. Data were processed off-line using Neuroscan Edit software (Compumedics Neuroscan). Poorly functioning electrodes were identified by visual inspection of the data, and electrodes that had impedance over 20 kΩ were excluded from analysis. No more than 5% of the electrodes were rejected in any single subject (on average, 1.8 % in controls and 2.5 % in aMCI). The continuous EEG data were high-pass filtered at 0.15 Hz and corrected for eye blinks using the spatial filtering function in the Scan 4.5 software. EEG data were then processed using EEGLAB functions [46] in MATLAB (the MathWorks Inc.). The data were first segmented into multiple trial-by-trial EEG epochs (−200 to 2000 ms) and only correct trials were included for analysis. We applied digital low-pass filtering with a cutoff value of 30 Hz (linear finite impulse response function) to minimize high frequency noise, such as muscle activity. Epochs with peak signal amplitude of more than 75 μV were rejected. Bad epochs were identified by visual inspection and by rejection algorithms in EEGLAB (rejecting improbable and abnormally distributed data lying beyond 5 standard deviations from the mean, using pop_jointprob.m and pop_rejkurt.m EEGLAB algorithms, respectively) and were excluded from further analysis. The average retaining rates (good trials / all correct trials) were 82.4% (41.1 trials) and 83.2% (42.7 trials) in the control group, and 82% (37.8 trials) and 82.9% (38.3 trials) in the aMCI group, for retrieval and non-retrieval trials, respectively. The EEG epochs were then re-referenced (global average potential from all electrodes) and baseline corrected (average potential of the pre-stimulus period between −200 and 0 ms). In order to still examine the standard ERPs at individual level, we averaged all the preprocessed EEG epochs to ascertain the quality of the data for each single subject. The trial-by-trial EEG epochs were used for data-driven analysis, i.e., STAT-PCA, which will be described in the next section.
Statistical analysis
A standard general linear model (GLM) was applied to measures of RT and accuracy to assess the effects of group, condition, and group by condition interaction. We analyzed pre-processed EEG epochs using STAT-PCA, a procedure that utilizes statistical inference at each temporal and spatial unit (STAT) followed by a principal component (factoring) analysis (PCA) to isolate the most salient temporal and spatial properties of the ERPs [47].
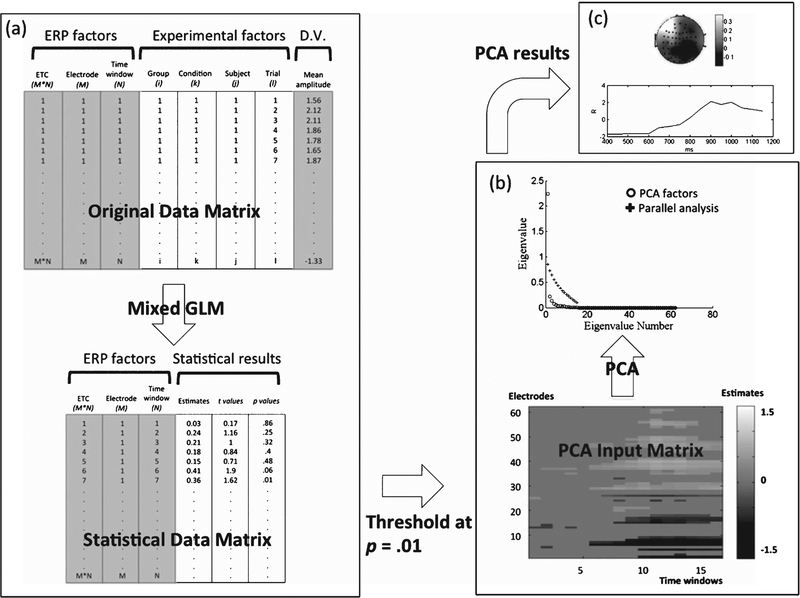
In the first step of STAT-PCA, all the trial-by-trial EEG epochs are converted into an overall data matrix, coded by a number of ERP factors and experimental factors (Fig. 1a). ERP factors included a column for coding electrode-time combination (ETC) and two columns for electrode and time window, respectively. For the current study, we had 62 electrodes and 16 time windows (50-ms time windows between 400 and 1200 ms post-stimulus), resulting in 62 × 16 = 992 electrode-time combinations in total. In a previous study of SORT ERP [30], we determined that 50-ms time windows maintained an appropriate effective temporal resolution. Smaller fractionated windows (15 to 30 ms) only added to the multiple-testing burden without measurably improving the signal-to-noise ratio or the temporal resolution of mean amplitude data. We focused on the later time windows based on previous findings showing that SORT ERP effects arise later than 400 ms post-stimulus [30, 32]. However, we tested the time window between 0 and 400 ms using the same analysis to examine any potential effects. The experimental factors included columns for group (i = 2), condition (k = 2), subject (j = 17 and 16 in normals and MCIs, respectively), and trials (l varied across individuals and conditions). The dependent variable (D.V.) was the mean amplitude within each 50-ms time window for each combination of the above factors (Fig. 1a).
Fig. 1.
Schematic flow chart of the STAT-PCA analysis. STAT-PCA is composed of two main steps: statistical analysis (a) followed by principal component analysis (b). The final results can be represented using both spatial and temporal information (c). Refer to the Methods section for detailed, step-wise descriptions. ETC, electrode-time combination; D.V., dependent variable; GLM, generalized linear modeling.
STAT-PCA retains EEG trial-by-trial information in the data structure, which allows flexible modeling of experimental effects (e.g., within-subject and between-subject effects) by accounting for both intra-subject and inter-subject variance components [47]. In addition, it allows an automatic weighting of subject-level averages because trial numbers retained for analysis vary from subjects to subjects due to varied accuracy and data quality among different subjects. To clarify, the STAT-PCA approach performs tests on the means of EEG epochs (hence averaging like ERP) for each level of the experimental factors so in essence the method is still predicated on ERP. The inclusion of subjects as a random variable in the statistical model, rather than in the PCA, makes results less sensitive to the deletion or addition of a single subject and makes the statistical inference generalizable to the population from which the subjects were sampled. We implemented a linear mixed model for each one of the 992 electrode-time combinations
to examine the effects of group (λi), condition (Γk) and the interaction between group and condition (λΓ)ik, on the scalp potential (mean amplitude) Yijkl, where each subscript indexes group, subject, condition and trial, respectively (Fig. 1a). The variances associated with subject variability, bj(i), and trial variability, εijkl, were estimated by default residual maximum likelihood estimation of variance components. The outputs were the statistical results, including estimates, t and p values, as depicted in the data matrix in Fig. 1a. Three such data matrices were generated (one matrix each for the effects of condition, group, and condition x group). In these matrices, only those estimates surviving an alpha threshold of 0.01 were passed on to the PCA step (Fig. 1). The average potential of each of these three effects (estimates) of interest was retained if the corresponding test (p value) was below threshold; otherwise, they were set to zero. We chose this alpha level for the significance mask as a compromise between false positives and statistical power in our specific multiple-testing context. Additionally, this thresholding approach reduced the noise fed to the PCA to isolate only the most salient effects [47]. We were also guided to some extent by a priori hypotheses of both timing and spatial location based on knowledge of previous findings using EEG SORT [30, 32].
We then applied PCA on the spatial factor (electrode) to the 3 matrices of thresholded average potentials, and retained the main spatial components by parallel analysis based on eigenvalues [48] (as represented in Fig. 1b). For each retained spatial component, the temporal components were simply the resulting PCA scores. We depicted the retained spatial/temporal components by plotting spatial PCA loadings in topographical distribution across scalp and temporal PCA scores as a time-series (Fig. 1c). The GLMs for both task performance and ERP data were implemented in SAS (Cary, NC), using the mixed model procedure, with the Kenward-Roger degree-of-freedom method to adjust the downward bias in variances when testing for fixed effects in mixed models [47].
In order to examine the relationships between ERP effects and behavioral measures, we performed linear regression analyses (IBM SPSS Statistics 21) combining data from both groups to test how well individual STAT-PCA effect scores would predict task performance (RT and accuracy averaged across retrieval and non-retrieval conditions) and neuropsychological measures (including logical memory immediate and delayed recall, letter fluency, category fluency, BNT, digit span forward and backward, digit symbol, and trail-making tests A & B, controlling for age and education). Assumptions of linearity, normally distributed errors, and uncorrelated errors were checked and met. Each individual STAT-PCA effect score was calculated as the matrix product of the ERP mean amplitude matrix (16 × 62 matrix), the PCA loading vector (62 × 1 matrix), and the PCA score vector (1 × 16 matrix), collapsing both spatial and temporal dimensions and calculating one single effect score (1 × 1 matrix) for each subject. The ERP mean amplitude matrix was derived from both retrieval and non-retrieval trials, separately, to account for their distinctive contributions to the object memory retrieval process.
RESULTS
EEG SORT task performance
Behavioral measures included RT and accuracy (Table 1). For RT, there was a significant main effect of condition, F(1,31) = 49.24, p < 0.001, showing longer responses for non-retrieval trials (1817 ± 423 ms) than for retrieval trials (1496 ± 295 ms), and a trend in the main effect of group, F(1,31) = 3.81, p = 0.06, with aMCI patients (1771 ± 411 ms) having longer response times than did controls (1549 ± 355 ms). The interaction between condition and group was not significant (p = 0.35). For accuracy, the main effect of group was significant, F(1,31) = 13.19, p = 0.001, showing aMCI subjects (82.8 ± 10.4%) performed less well than did controls (90.3 ± 5.7%). The main effect of condition was also± significant, showing lower accuracy during retrieval trials (84.4 ± 6.2%) compared to non-retrieval trials (90 ± 10.8 %), F(1,31) = 5.91, p = 0.021. The interaction between condition and group was not significant (p = 0.58). To examine the relations between RT and accuracy, correlation analyses were done combining both groups. Findings showed that during retrieval trials the longer the RT, the less accurate the responses, R = −0.388, p = 0.026; in contrast, there was no significant association between RT and accuracy in non-retrieval trials (p > 0.1, after excluding two extreme MCI outliers in the analysis). When both retrieval and non-retrieval trials were pooled together, no significant correlation was found (p > 0.1, after excluding two extreme MCI outliers in the analysis). Also, no significant correlations were found for either condition when the two groups were tested separately (p > 0.1).
STAT-PCA results
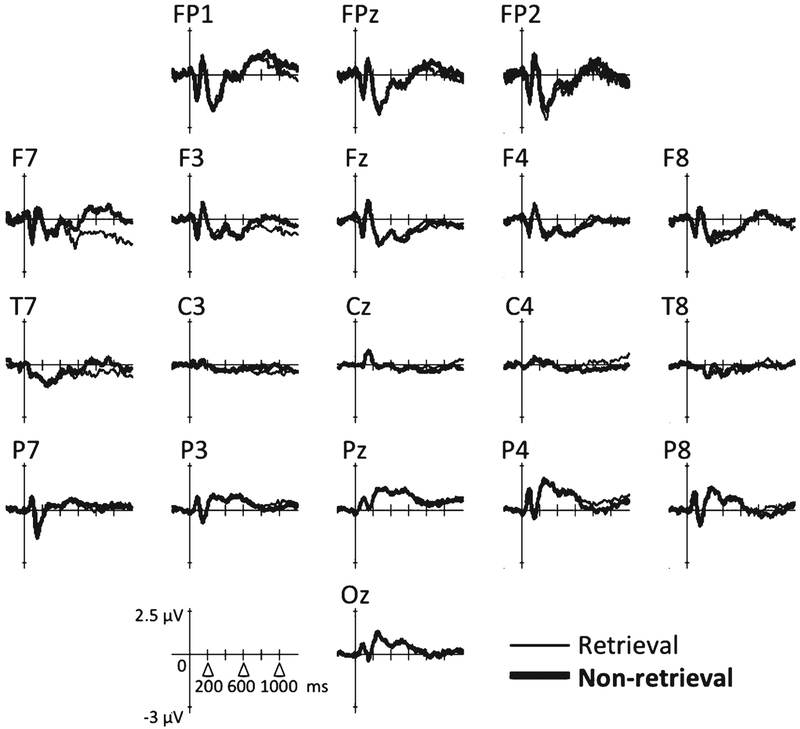
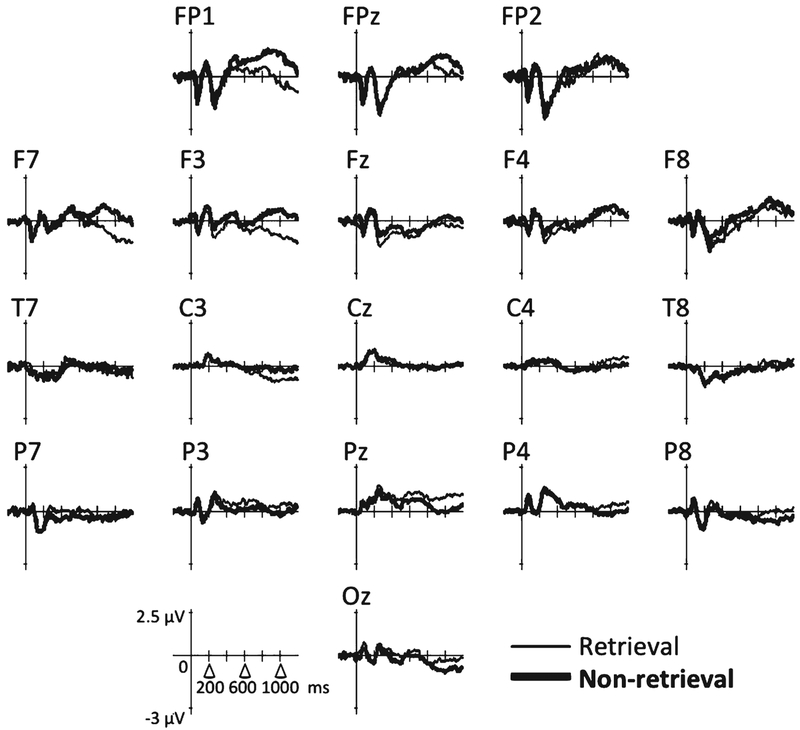
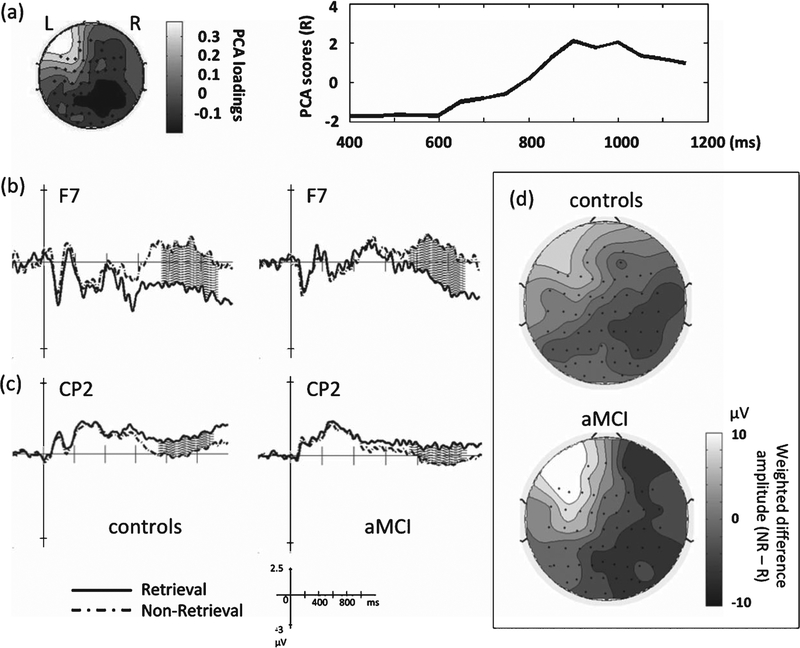
Group ERPs are shown in Figs. 2 and 3; topographical representations of the group ERPs over time (averaged across 100-ms time windows) are shown in Fig. 4. For condition differences (retrieval versus non-retrieval), one spatial PCA factor was detected (77.6% of total variance). The factor was loaded over the left fronto-temporal region (F7, FT7) starting at around 750 ms post-stimulus, with a smaller amplitude component near the right centro-parietal region (CP2, CP4) (Fig. 5a). In the left fronto-temporal region, the retrieval condition was associated with larger negative potentials relative to the non-retrieval condition in both groups (Fig. 5b); conversely, in the right centro-parietal region, the non-retrieval condition was associated with larger negative potentials relative to the retrieval condition (Fig. 5c).
Fig. 2.
Group ERPs for the control group.
Fig. 3.
Group ERPs for the aMCI group.
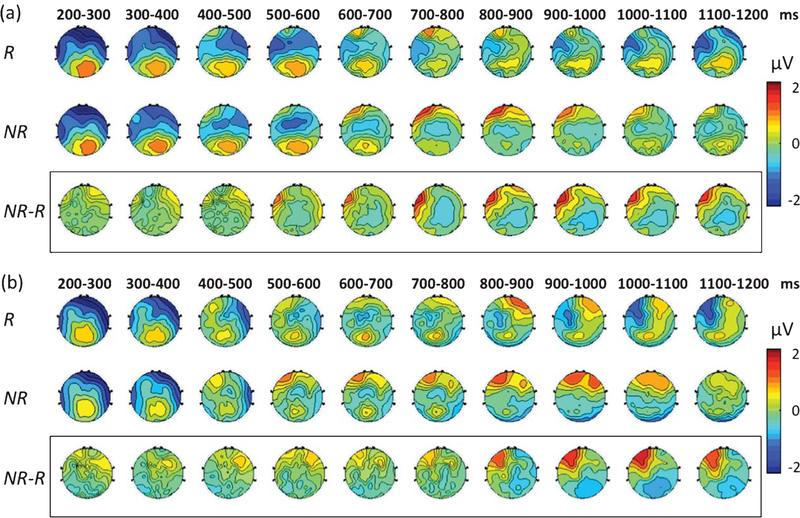
Fig. 4.
Group-level topographical representations of ERP over time. Group level ERPs are illustrated in a topographical fashion for the control(a) and the aMCI group (b) between 200 and 1200 ms post-stimulus. Each scalp map is representative of the mean potentials averaged across each 100-ms time window. R, retrieval trials; NR, non-retrieval trials. NR-R represents the difference ERP.
Fig. 5.
Results of the condition effect. The condition effect (retrieval versus non-retrieval trials) examined by STAT-PCA was found to localize to the left fronto-temporal region, starting from 750 ms post-stimulus (a). Electrode F7 shows the condition effect was attributable to greater negativity in retrieval than non-retrieval trials (a), while electrode CP2 shows a reverse pattern (c), observed in both groups (shaded areas: 750–1100 ms). Topographical representations at the group level of the scalp potentials (difference amplitude of NR minus R) weighted by the PCA scores derived from the condition effect (d).
To represent topographical patterns of the group level results based on the temporal PCA scores, we calculated the matrix product of each subject’s mean ERP data matrix (62 × 16 matrix) and the PCA scores (16 × 1 matrix), averaged them within each group, and plotted the weighted data matrix (62 × 1 matrix) overlying the scalp (as shown in Fig. 5d). Both groups showed that the left fronto-temporal and right centro-parietal effects were modulated by condition. For group differences (normal versus aMCI), there were no significant effects prior to 1000 ms post-stimulus onset.
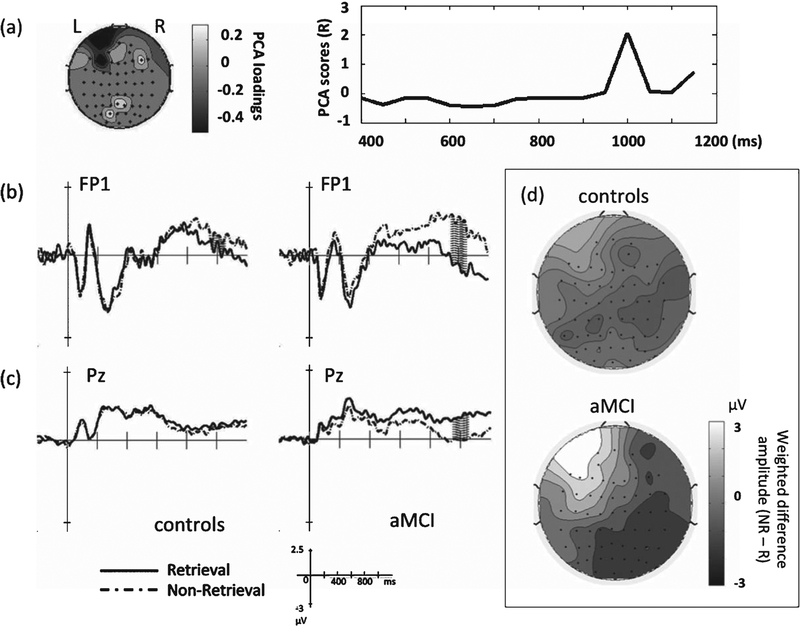
For the group by condition interaction, one spatial PCA factor emerged (43.6% of total variance) in the frontal (FP1, F3) and parietal electrodes (Pz, P2), maximal between 950 and 1050 ms post-stimulus (Fig. 6a). This interaction was mostly a result of the condition effect in the aMCI group, with a more positive frontal potential in non-retrieval than retrieval trials (Fig. 6b) and, conversely, a more positive parietal potential in retrieval trials than non-retrieval trials (Fig. 6c). Topographical representations based on the temporal PCA scores for each separate group (Fig. 6d) showed that only aMCI subjects had this fronto-parietal effect modulated by condition, but controls did not. Finally, the same sets of analyses that were performed on the time window between 0 and 400 ms did not reveal any significant results or any PCA components using the same threshold (p = 0.01) in STAT-PCA.
Fig. 6.
Results of the group by condition interaction. The interaction effect localizes to the frontal and parietal regions, peaking between 950 and 1050 ms post-stimulus (a). Electrodes FP1 (b) and Pz (c) show the interaction effect reflects greater positivity in non-retrieval than retrieval trials in the frontal area but greater positivity in retrieval than non-retrieval trials in the parietal area, observed mainly in the aMCI group (shaded areas: 950–1050 ms). Topographical representations at the group level of the scalp potentials (difference amplitude of NR minus R) weighted by the PCA scores derived from the group by condition interaction (d).
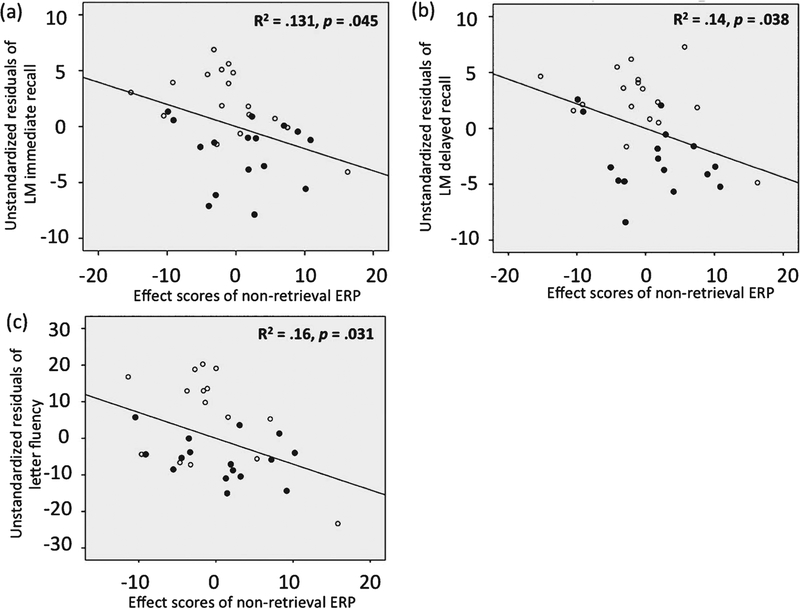
In terms of regression analyses using ERP effects to predict behavioral and neuropsychological measures, the PCA loading and PCA score vectors were derived from the retained PCA factors for condition (Fig. 5a) and group/condition interaction (Fig. 6a). Each subject’s STAT-PCA effect score represents the degree to which each subject’s ERP pattern corresponding to each condition (separately for retrieval and non-retrieval trials) conforms to the STAT-PCA results. Thus, the larger an individual effect score, the more consistent an individual subject’s ERP pattern is to the STAT-PCA result. The results are shown in Table 2. The late fronto-parietal ERP effect captured by non-retrieval trials was larger in those subjects with lower scores in logical memory, including both immediate, R = −0.363, p = 0.045, and delayed recall, R = −0.374, p = 0.038, and letter fluency, R = −0.4, p = 0.031 (scatter plots shown in Fig. 7). No other correlations were found to be significant between the front-parietal ERP effect and other neuropsychological measures (p > 0.05). Same tests for retrieval trials did not show any significant correlations with any behavioral measures including logical memory and letter fluency (Table 2).
Table 2:
Regression analysis between behavioral measures and STAT-PCA effect scores (based on group by condition interaction)
| Retrieval | Non-retrieval | |
|---|---|---|
| SORT-RT | R = −0.044 | R = 0.277 |
| SORT-accuracy | R = −0.042 | R = −0.149 |
| LM-immediate | R = −0.143 | R = −0.363* |
| LM-delayed | R = −0.192 | R = −0.374* |
| Letter fluency | R = −0.113 | R = −0.4* |
p < 0.05. Analyses for LM (logical memory) and letter fluency were controlled for age and education.
Fig. 7.
Scatter plots of the significant regression analysis results using STAT-PCA effect scores and neuropsychological measures. Scatter plots based on the regression analysis using individual STAT-PCA effect scores (based on the group by condition interaction) in non-retrieval trials to predict LM immediate recall (a), LM delayed recall (b), and letter fluency (c). The unstandardized residuals were derived after the variances accounted for by age and education were removed in the regression analysis. LM: logical memory. Unfilled dots indicate normal subjects and filled dots indicate aMCI subjects.
DISCUSSION
Overall, we found that aMCI patients performed less accurately and more slowly during the EEG SORT compared to controls. While both control and aMCI groups demonstrated a left fronto-temporal ERP component starting around 750 ms post-stimulus, the MCI patients compared to controls, showed greater ERP difference between retrieval and non-retrieval trials occurring at around 950–1050 ms post-stimulus mainly in the fronto-parietal regions.
The lower accuracy in aMCI patients during SORT may be attributed to lexical or semantic processing deficits which have been documented widely using measures such as verbal fluency, BNT, and other tasks probing various aspects of knowledge about objects, famous people, buildings, and events [49–53]. On the other hand, slower RT could be due to slower processing speed in motor and cognitive functions, since aMCI patients performed worse than normal controls in speed-based tasks including trail making tests A & B and digit symbol. Regardless of group differences, RT was longer in non-retrieval trials compared to retrieval trials in both groups. We posit that arriving at a response in non-retrieval trials takes longer and requires more cognitive effort to search for any potential answers. Not until all the possibilities that initially come to mind are dismissed would someone make a negative decision/response. Here RT-accuracy tradeoff is less likely, since our analysis at the group level (including both groups) showed that accuracy was in fact worse when RT was longer in retrieval trials (R = −0.388), as opposed to better as would have been if an RT-accuracy tradeoff was a prominent factor.
With regard to the ERP findings, an ERP effect of comparable magnitude was observed in both controls and aMCI patients in the left fronto-temporal region. This ERP component, reflecting a more negative potential in retrieval than non-retrieval trials starting around 750 ms, was also reported in previous studies on EEG SORT involving young and normal aging adults [30, 32]. This ERP component has been posited to indicate earlier stages of activation of semantic object memory during retrieval process [29]. That both controls and aMCI patients showed this effect suggests that earlier stages in semantic object memory retrieval in aMCI patients may be relatively intact. That is, the activation of object memory representation initially appears to be comparable in aMCI and controls, prior to other delayed differential responses potentially at a later semantic or post-semantic stage that might more substantially impact their performance in retrieving object memory during the SORT.
What differed between aMCI and controls was an increased fronto-parietal scalp potential between 950 and 1050 ms that differentiated retrieval from non-retrieval trials in aMCI patients but not in controls. In Chiang et al. [30], the fronto-parietal effect was found in healthy older adults, but at an earlier time frame between 800 and 1000 ms (but no such effect in young adults) and was posited to relate to a more extensive search during non-retrieval trials. It is plausible that aMCI patients require more effort in searching for a semantic link between the two features above and beyond what is needed by those who are normally aging, which is indexed neurally by this more positive potential in the non-retrieval compared to the retrieval condition in the frontal electrodes. The disease process appears to affect the neural substrates that are critical for semantic object retrieval [28, 29] and thus leads to the delayed activity in scalp potentials. Other alternative cognitive processes to which this effect may be linked include a late decision making process that could be more demanding later during non-retrieval trials and the updating of working memory, as other late or delayed ERP components have suggested in AD/MCI [19, 23, 54]. Given that the fronto-parietal delayed response (between 950 and 1050 ms) occurred at least 600 ms earlier than the average RT (1588 and 1954 ms for retrieval and non-retrieval trials, respectively) and that there was no correlation between RT and this component, it is unlikely that this reflects prominent differences in motor responses, or lateralized readiness potential (LRP, normally occurs 100–200 ms before a motor response).
The fronto-parietal distribution of the late differential ERP effect indicates significant correlation in time between frontal and parietal potentials though in opposite polarities. At the neural level, it could be that in one condition, different cellular populations are being synaptically impinged relative to the other condition. Those different neural impingements could be happening within the same regions of brain, thus causing activation of distinct neural generators projecting to opposite polarities at different sites on the scalp. Alternatively, the activity could be in different brain regions altogether, with consequent opposite-polarity across the scalp. However, it would be overly speculative to propose a detailed mechanism for how this front-parietal distribution on the scalp potential reflects either activity in individual underlying neural generators or the summation of potentials from different generators, given the complexities associated with EEG source identification [55]. The question warrants future studies using EEG source localization methods, or neuroimaging methods such as functional magnetic resonance imaging that afford higher spatial resolution.
The association between the late fronto-parietal effect during semantic object memory retrieval and episodic memory performance further suggests that altered neurophysiological activity on SORT is associated with the hallmark cognitive impairment in AD [10, 12], as we found that the worse a subject performed on logical memory (both immediate and delayed recall), the more the subject demonstrated delayed fronto-parietal ERP effect during non-retrieval trials. Studies have shown that over the course of MCI and AD, semantic memory dysfunction is associated with progressive reduction in brain volume and regional blood flow in a number of brain regions [12]. These brain regions include not only neocortical structures (e.g., inferior frontal gyrus and anterior temporal lobe) that are central to semantic-related functions [51, 56, 57], but also medial temporal lobe structures that normally display signs of neural degeneration and closely associated with episodic memory deficits [58, 59]. More specifically, regions in the frontal and temporal lobes and other subcortical regions (thalamus and caudate) have been associated with SORT performance in a number of neuroimaging and neuropsychological studies [28, 60–62], and these regions could also be susceptible to AD pathology [63–65]. Furthermore, the strong negative correlation between the fronto-parietal effects and letter fluency performance suggests that there is some degree of deficits in output lexical processing that could be also related to this late differential activity.
In conclusion, aMCI patients demonstrated worse performance than controls in a semantic object memory retrieval task. Neural responses at the earlier stage of semantic retrieval process show little impact of aMCI. However, the difficulty in retrieving and searching for semantic object memory appears to be better indexed by the delayed accentuated scalp potential in aMCI as compared to normals, suggesting a more effortful search to bind object features. The delayed neural activity negatively associated with episodic memory measures may index the severity of the disease state. Our findings support the view that ERP data complement behavioral measures and help to characterize transitions from normal to pathological aging.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute of Health (RC1-AG035954, P30AG12300), the RGK foundation, Alzheimer’s Association New Investigator Grant (NIRG-11–173815), the Berman Research Initiative at the Center for BrainHealth, and the Friends of BrainHealth New Scientist Award. The authors thank Dr. Elizabeth K. Bartz, Rajen Patel, Monique Salinas, Erin Venza, Audette Rackley, Molly Keebler, and Claire Gardner for their invaluable assistance in data collection.
Footnotes
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/14-2781r2).
REFERENCES
- [1].Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, Smith GE, Jack CRJ (2009) Mild cognitive impairment: Ten years later. Arch Neurol 66, 1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L (2014) Mild cognitive impairment: A concept in evolution. J Intern Med 275, 214–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ (2013) Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol 12, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Masliah E, Crews L, Hansen L (2006) Synaptic remodeling during aging and in Alzheimer’s disease. J Alzheimers Dis 9, 91–99. [DOI] [PubMed] [Google Scholar]
- [5].Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298, 789–791. [DOI] [PubMed] [Google Scholar]
- [6].Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL (2004) Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci 24, 10191–10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Prvulovic D, Bokde ALW, Faltraco F, Hampel H (2011) Functional magnetic resonance imaging as a dynamic candidate biomarker for Alzheimer’s disease. Prog Neurobiol 95, 557–569. [DOI] [PubMed] [Google Scholar]
- [8].Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack C, Jagust W, Trojanowski JQ, Toga AW, Beckett L (2005) The Alzheimer’s disease neuroimaging initiative. Neuroimaging Clin N Am 15, 869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Olichney JM, Yang J, Taylor J, Kutas M (2011) Cognitive event-related potentials: Biomarkers of synaptic dysfunction across the stages of Alzheimer’s disease. J Alzheimers Dis 26(Suppl 3), 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CRJ, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Salmon DP, Butters N, Chan AS (1999) The deterioration of semantic memory in Alzheimer’s disease. Can J Exp Psychol 53, 108–117. [DOI] [PubMed] [Google Scholar]
- [12].Gainotti G, Quaranta D, Vita MG, Marra C (2014) Neuropsychological predictors of conversion from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis 38, 481–495. [DOI] [PubMed] [Google Scholar]
- [13].Kraut MA, Cherry B, Pitcock JA, Vestal L, Henderson VW, Hart J Jr (2006) The Semantic Object Retrieval Test (SORT) in normal aging and Alzheimer disease. Cogn Behav Neurol 19, 177–184. [DOI] [PubMed] [Google Scholar]
- [14].Iragui V, Kutas M, Salmon DP (1996) Event-related brain potentials during semantic categorization in normal aging and senile dementia of the Alzheimer’s type. Electroencephalogr Clin Neurophysiol 100, 392–406. [PubMed] [Google Scholar]
- [15].Ostrosky-Solís F, Castañeda M, Pérez M, Castillo G, Bobes MA (1998) Cognitive brain activity in Alzheimer’s disease: Electrophysiological response during picture semantic categorization. J Int Neuropsychol Soc 4, 415–425. [DOI] [PubMed] [Google Scholar]
- [16].Olichney JM, Iragui VJ, Salmon DP, Riggins BR, Morris SK, Kutas M (2006) Absent event-related potential (ERP) word repetition effects in mild Alzheimer’s disease. Clin Neurophysiol 117, 1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Revonsuo A, Portin R, Juottonen K, Rinne JO (1998) Semantic processing of spoken words in Alzheimer’s disease: An electrophysiological study. J Cogn Neurosci 10, 408–420. [DOI] [PubMed] [Google Scholar]
- [18].Auchterlonie S, Phillips NA, Chertkow H (2002) Behavioral and electrical brain measures of semantic priming in patients with Alzheimer’s disease: Implications for access failure versus deterioration hypotheses. Brain Cogn 48, 264–267. [PubMed] [Google Scholar]
- [19].Schwartz TJ, Federmeier KD, Van Pettern C, Salmon DP, Kutas M (2003) Electrophysiological analysis of context effects in Alzheimer’s disease. Neuropsychology 17, 187–201. [DOI] [PubMed] [Google Scholar]
- [20].Ford JM, Askari N, Gabrieli JDE, Mathalon DH, Tinklenberg JR, Menon V, Yesavage J (2001) Event-related brain potential evidence of spared knowledge in Alzheimer’s disease. Psychol Aging 16, 161–176. [DOI] [PubMed] [Google Scholar]
- [21].Spironelli C, Bergamaschi S, Mondini S, Villani D, Angrilli A (2013) Functional plasticity in Alzheimer’s disease: Effect of cognitive training on language-related ERP components. Neuropsychologia 51, 1638–1648. [DOI] [PubMed] [Google Scholar]
- [22].Grieder M, Crinelli RM, Jann K, Federspiel A, Wirth M, Koenig T, Stein M, Wahlund L, Dierks T (2013) Correlation between topographic N400 anomalies and reduced cerebral blood flow in the anterior temporal lobes of patients with dementia. J Alzheimers Dis 36, 711–731. [DOI] [PubMed] [Google Scholar]
- [23].Olichney JM, Morris SK, Ochoa C, Salmon DP, Thal LJ, Kutas M, Iragui VJ (2002) Abnormal verbal event related potentials in mild cognitive impairment and incipient Alzheimer’s disease. J Neurol Neurosurg Psychiatry 73, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Olichney JM, Taylor JR, Gatherwright J, Salmon DP, Bressler AJ, Kutas M, Iragui-Madoz V (2008) Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology 70, 1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Taler V, Klepousniotou E, Phillips NA (2009) Comprehension of lexical ambiguity in healthy aging, mild cognitive impairment, and mild Alzheimer’s disease. Neuropsychologia 47, 1332–1343. [DOI] [PubMed] [Google Scholar]
- [26].Verma M, Howard RJ (2012) Semantic memory and language dysfunction in early Alzheimer’s disease: A review. Int J Geriatr Psychiatry 27, 1209–1217. [DOI] [PubMed] [Google Scholar]
- [27].Duong A, Whitehead V, Hanratty K, Chertkow H (2006) The nature of lexico-semantic processing deficits in mild cognitive impairment. Neuropsychologia 44, 1928–1935. [DOI] [PubMed] [Google Scholar]
- [28].Kraut MA, Kremen S, Segal JB, Calhoun V, Moo LR, Hart JJ (2002) Object activation from features in the semantic system. J Cogn Neurosci 14, 24–36. [DOI] [PubMed] [Google Scholar]
- [29].Hart JJ, Maguire MJ, Motes M, Mudar RA, Chiang H, Womack KB, Kraut MA (2013) Semantic memory retrieval circuit: Role of pre-SMA, caudate, and thalamus. Brain Lang 126, 89–98. [DOI] [PubMed] [Google Scholar]
- [30].Chiang HS, Mudar RA, Spence JS, Pudhiyidath A, Eroh J, DeLaRosa B, Kraut MA, Hart J Jr (2014) Age-related changes in feature-based object memory retrieval as measured by event-related potentials. Biol Psychol 100, 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kraut MA, Cherry B, Pitcock JA, Anand R, Li J, Vestal L, Henderson VW, Hart JJ (2007) The Semantic Object Retrieval Test (SORT) in amnestic mild cognitive impairment. Cogn Behav Neurol 20, 62–67. [DOI] [PubMed] [Google Scholar]
- [32].Brier MR, Maguire MJ, Tillman GD, Hart JJ, Kraut MA (2008) Event-related potentials in semantic memory retrieval. J Int Neuropsychol Soc 14, 815–822. [DOI] [PubMed] [Google Scholar]
- [33].Kutas M, Federmeier KD (2000) Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn Sci (Regul Ed) 4, 463–470. [DOI] [PubMed] [Google Scholar]
- [34].Drago V, Babiloni C, Bartrés-Faz D, Caroli A, Bosch B, Hensch T, Didic M, Klafki H, Pievani M, Jovicich J, Venturi L, Spitzer P, Vecchio F, Schoenknecht P, Wiltfang J, Redolfi A, Forloni G, Blin O, Irving E, Davis C, Hårdemark H, Frisoni GB (2011) Disease tracking markers for Alzheimer’s disease at the prodromal (MCI) stage. J Alzheimers Dis 26Suppl 3, 159–199. [DOI] [PubMed] [Google Scholar]
- [35].Beck AT, Steer RA, Brown GK. (1996) Manual for the Beck Depression Inventory-II, Psychological Corp, San Antonio, TX. [Google Scholar]
- [36].Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982) Development and validation of a Geriatric Depression Screening Scale: A preliminary report. J Psychiatr Res 17, 37–49. [DOI] [PubMed] [Google Scholar]
- [37].Morris JC (1993) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43, 2412–2414. [DOI] [PubMed] [Google Scholar]
- [38].Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [39].Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- [40].Benton AL, Hamsher K. (1976) Multilingual Aphasia Examinatioin 2nd ed, AJA Associates, Iowa City, IA. [Google Scholar]
- [41].Goodglass H, Kaplan E, Barresi B. (2001) Boston Diagnostic Aphasia Examination 3rd ed, Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- [42].Williams BW, Mack W, Henderson VW (1989) Boston Naming Test in Alzheimer’s disease. Neuropsychologia 27, 1073–1079. [DOI] [PubMed] [Google Scholar]
- [43].Wechsler D (2008) Wechsler Adult Intelligence Scale–Fourth Edition: Technical and Interpretive Manual, Pearson, San Antonio, TX. [Google Scholar]
- [44].Reitan RM (1958) Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 8, 271–276. [Google Scholar]
- [45].Wechsler D (1997) Wechsler Memory Scale- Third Edition, the Psychological Corporation, San Antonio, TX. [Google Scholar]
- [46].Delorme A, Makeig S (2004) EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134, 9. [DOI] [PubMed] [Google Scholar]
- [47].Spence JS, Brier MR, Hart JJ, Ferree TC (2013) Removing an intersubject variance component in a general linear model improves multiway factoring of event-related spectral perturbations in group EEG studies. Hum Brain Mapp 34, 651–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Horn JL (1965) A rationale and test for the number of factors in factor analysis. Psychometrika 30, 179–185. [DOI] [PubMed] [Google Scholar]
- [49].Seindenberg M, Guidotti L, Nielson KA, Woodard JL, Durgerian S, Hang Q, Gander A, Antuono P, Rao SM (2009) Semantic knowledge for famous names in mild cognitive impairment. J Int Neuropsychol Soc 15, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Leyhe T, Müller S, Eschweiler GW, Saur R (2010) Deterioration of the memory for historic events in patients with mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia 48, 4093–4101. [DOI] [PubMed] [Google Scholar]
- [51].Joubert S, Brambati SM, Ansado J, Barbeau EJ, Felician O, Didic M, Lacombe J, Goldstein R, Chayer C, Kergoat M (2010) The cognitive and neural expression of semantic memory impairment in mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia 48, 978–988. [DOI] [PubMed] [Google Scholar]
- [52].Ahmed S, Arnold R, Thompson SA, Graham KS, Hodges JR (2008) Naming of objects, faces and buildings in mild cognitive impairment. Cortex 44, 746–752. [DOI] [PubMed] [Google Scholar]
- [53].Adlam AR, Bozeat S, Arnold R, Watson P, Hodges JR (2006) Semantic knowledge in mild cognitive impairment and mild Alzheimer’s disease. Cortex 42, 675–684. [DOI] [PubMed] [Google Scholar]
- [54].Taylor JR, Olichney JM (2007) From amnesia to dementia: ERP studies of memory and language. Clin EEG Neurosci 38, 8–17. [DOI] [PubMed] [Google Scholar]
- [55].Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave dP (2004) EEG source imaging. Clin Neurophysiol 115, 2195–2222. [DOI] [PubMed] [Google Scholar]
- [56].Apostolova LG, Lu P, Rogers S, Dutton RA, Hayashi KM, Toga AW, Cummings JL, Thompson PM (2008) 3D mapping of language networks in clinical and pre-clinical Alzheimer’s disease. Brain Lang 104, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].McDonald CR, Gharapetian L, McEvoy LK, Fennema-Notestine C, Hagler DJJ, Holland D, Dale AM (2012) Relationship between regional atrophy rates and cognitive decline in mild cognitive impairment. Neurobiol Aging 33, 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Venneri A, McGeown WJ, Hietanen HM, Guerrini C, Ellis AW, Shanks MF (2008) The anatomical bases of semantic retrieval deficits in early Alzheimer’s disease. Neuropsychologia 46, 497–510. [DOI] [PubMed] [Google Scholar]
- [59].Barbeau EJ, Didic M, Joubert S, Guedj E, Koric L, Felician O, Ranjeva J, Cozzone P, Ceccaldi M (2012) Extent and neural basis of semantic memory impairment in mild cognitive impairment. J Alzheimers Dis 28, 823–837. [DOI] [PubMed] [Google Scholar]
- [60].Assaf M, Calhoun VD, Kuzu CH, Kraut MA, Rivkin PR, Hart JJ, Pearlson GD (2006) Neural correlates of the object-recall process in semantic memory. Psychiatry Res 147, 115–126. [DOI] [PubMed] [Google Scholar]
- [61].Pergola G, Bellebaum C, Gehlhaar B, Koch B, Schwarz M, Daum I, Suchan B (2013) The involvement of the thalamus in semantic retrieval: A clinical group study. J Cogn Neurosci 25, 872–886. [DOI] [PubMed] [Google Scholar]
- [62].Slotnick SD, Moo LR, Kraut MA, Lesser RP, Hart J Jr (2002) Interactions between thalamic and cortical rhythms during semantic memory recall in human. Proc Natl Acad Sci U S A 99, 6440–6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Braak H, Braak E (1991) Alzheimer’s disease affects limbic nuclei of the thalamus. Acta Neuropathol 81, 261–268. [DOI] [PubMed] [Google Scholar]
- [64].Cash DM, Ridgway GR, Liang Y, Ryan NS, Kinnunen KM, Yeatman T, Malone IB, Benzinger TLS, Jack CRJ, Thompson PM, Ghetti BF, Saykin AJ, Masters CL, Ringman JM, Salloway SP, Schofield PR, Sperling RA, Cairns NJ, Marcus DS, Xiong C, Bateman RJ, Morris JC, Rossor MN, Ourselin S, Fox NC (2013) The pattern of atrophy in familial Alzheimer disease: Volumetric MRI results from the DIAN study. Neurology 81, 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ryan NS, Keihaninejad S, Shakespeare TJ, Lehmann M, Crutch SJ, Malone IB, Thornton JS, Mancini L, Hyare H, Yousry T, Ridgway GR, Zhang H, Modat M, Alexander DC, Rossor MN, Ourselin S, Fox NC (2013) Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer’s disease. Brain 136, 1399–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]