Abstract
Introduction: Pulmonary arterial hypertension (PAH) specific drug therapy using bosentan has significantly improved quality of life and survival, although PAH is still an incurable disease. Recent studies suggest metformin may have additional treatment benefits in PAH. We therefore investigated in vitro pulmonary artery reactivity after combination therapy of bosentan and metformin in PAH patients as compared with bosentan monotherapy in a prospective, randomized study.
Methods: Adult patients with PAH associated with congenital heart defects (PAH-CHD) were randomised to receive bosentan (initially at 62.5 mg twice daily for 4 weeks and then 125 mg twice daily) for 3 months with or without the combination treatment of metformin (500 mg twice daily). Vessel reactivity of isolated pulmonary arteries was examined using a wire myograph.
Results: Phenylephrine (PE)-induced contractions of arteries in patients received combination therapy were significantly attenuated at concentrations of 3 × 10-7 M, 10-6 M and 3 × 10-6 M, compared to those received bosentan monotherapy. After denudation, PE-induced contractions at concentrations of 3 × 10-6 M and 10-5 M were significantly decreased in the combination therapy group. AMP-activated protein kinase (AMPK) inhibitor compound C abrogated the inhibitory effects of metformin on PE-induced contractility. AMPK and eNOS phosphorylation in the pulmonary arteries of patients treated with combination therapy was increased compared to monotherapy (P < 0.05).
Conclusion: Adding metformin to bosentan therapy in patients with PAH-CHD decreased in vitro pulmonary artery contraction induced by PE, which is possibly related to increased AMPK phosphorylation.
Keywords: Bosentan, Metformin, Pulmonary Arterial Hypertension, Congenital Heart Defect, Vessel Reactivity
Introduction
Pulmonary arterial hypertension (PAH) with increased pulmonary vascular resistance (PVR) is a common complication of congenital heart defects (CHD).1 Over 7% of CHD patients have possible PAH and approximately 100 per million general adult population suffer from this disease.2 The development of PAH in CHD patients leads to markedly increased morbidity and mortality.3,4 Currently, there are no curative treatments for PAH associated with CHD (PAH-CHD) other than heart–lung transplantation. Therefore, management focus on slowing down the PAH progress and improving quality of life and heart function before transplantation proceeds. Selected patients may benefit from shunt closure with correctable defects, although this approach is still questionable.5 With the advent of PAH-specific drugs, in particular bosentan-an dual endothelin receptor (ER) antagonist that targets the endothelin (ET) pathway and stimulates vascular smooth muscle cell (VSMC) relaxation, the “treat and repair approach” has been proposed to re-evaluate the operability in PAH-CHD patients, previously thought to be unsuitable candidates for surgery.6 Pulmonary reactivity response to vasodilators, namely acute vasoreactivity testing, remains one of the gold standards to assess prognosis and indication for specific PAH therapy, and to assess the operability of PAH-CHD, given the existence of other more readily attainable prognostic factors.7
Multiple mechanistic pathways, including the ET, nitric oxide (NO) and prostacyclin pathways, are involved in the pathogenesis of PAH. These pathways promote vascular constriction of pulmonary arteries and hence increase vascular resistance and pressure. Combining drugs that target more than one pathway is an attractive option for treatment as additional benefits compared with monotherapy had been observed in previous studies.8-10 As a well-known antihyperglycemic agent, metformin improves insulin resistance in type 2 diabetes, and has been shown to reduce cardiovascular disease risks in those patients.11 Animal studies reveal that metformin reverses the development of experimental PAH in rats,12,13 suggesting that metformin may have additional treatment benefits in PAHs. Indeed, metformin increases the NO concentration and endothelial NO synthase (eNOS) expression and reduces the ET-1 concentration in the insulin-resistant human endothelial cells.14 Through AMP-activated protein kinase (AMPK), metformin not only promotes phosphorylation and activation of eNOS,15,16 but also decreases cytotoxic peroxynitrite, a well-known vasoconstrictor, accompanied by enhanced NO release and reduced nitroxidative stress in obese rats.17 Moreover, metformin-induced AMPK activation suppresses rat VSMC contraction,18 as wells as exaggerates phenylephrine (PE)-induced AMPK phosphorylation and attenuates contractile response in endothelium-denuded rat aorta.19 Song et al also demonstrated that the activity of AMPK negatively suppresses pulmonary VSMC proliferation and has possible utility in modulating pulmonary vascular remodelling.20
We previously found that combination therapy with bosentan and metformin in PAH-CHD patients provides additional improvements in important outcomes such as exercise capacity and pulmonary hemodynamics, compared with bosentan alone.21 Therefore, the current study aimed to investigate the vessel reactivity (induced constriction) of isolated pulmonary arteries from PAH-CHD patients after combination therapy of bosentan and metformin as compared with bosentan monotherapy and explore the possible mechanism of action of metformin in those patients.
Material and Methods
In accordance with the Declaration of Helsinki, all study protocols were approved by the Institutional ethics committee of Nanchang University on human research, and written informed consent was obtained from all patients for the use of their tissue. Between May 2016 and December 2017, 93 adult patients (18-65 years) firstly diagnosed with PAH-CHD at our hospital were enrolled. The inclusion and exclusion criteria have been described previously.21
Treatment
All enrolled patients were randomised to receive bosentan (initially at 62.5 mg twice daily for 4 weeks and then 125 mg twice daily) for 3 months with or without the combination treatment of metformin (500 mg twice daily). At 3 months follow-up, right heart catheterization and acute vasoreactivity testing were performed to assess the eligibility for shunt closure.22,23 Selected patients, assessed by our heart surgery team, underwent operations with biventricular circulation.
Wire myography
Lung tissue biopsy was taken from the left upper lobe before cardiopulmonary bypass intraoperatively and placed in ice-cold physiological saline solution (PSS; mmol/L; 119 NaCl, 25 NaHCO3, 4.69 KCl, 2.4 MgSO4, 1.6 CaCl2, 1.18 KH2PO4, 5 glucose, 0.034 EDTA; pH 7.4). The small pulmonary arteries (internal diameter ≈250 µm) were dissected and carefully cleaned of adherent connective tissue and cut into 2 mm segments. 16 artery segments (8 of them had mechanical removal of endothelium using the steel wire) from each patient were mounted onto an isometric wire myograph system (610M wire myography; Danish Myotechniques, Aarhaus, Denmark). The vessels were bathed in PSS, gassed with 5% CO2/95% air, maintained at a temperature of 37°C, and allowed to equilibrate for 30 min before normalization to an internal diameter of 0.9 of L3.67kPa using normalization software (Myodata, Danish Myotechnologies, Aarhus, Denmark), which has been shown to be optimal in our preliminary study using the method described previously.24 Vessel viability was assessed by exposure to high-potassium PSS (KPSS, mmol/L;12.45 NaCl, 25 NaHCO3, 120 KCl, 2.4 MgSO4, 1.6 CaCl2, 1.18 KH2PO4, 5 glucose, 0.034 EDTA; pH 7.4), followed, after washout with PSS, by 1 µM PE. The constriction by PE was allowed to plateau, then 1 μM the endothelial dependent vasodilator acetylcholine (ACh) was added to test the integrity of the endothelium or ensuring complete denudation of artery rings as described previously.25 A concentration-response curve to PE or ET1 (0.1 nM to 10 μM) was performed. A NOS inhibitor, Nω-nitro-L-arginine methyl ester (L-NAME; Abcam, UK; 40 µM), was used to blocking NOS in the endothelium. An AMPK inhibitor, compound C (Abcam, UK; 40 µM), was used to test the effect of AMPK activation by metformin on vessel tone.19 Arteries were preincubated with either L-NAME or compound C for 30 min before a second concentration–response curve was performed. Contractile responses to PE or ET1 are shown as a percentage of the contraction induced by KPSS.
Western blotting
Whole-cell lysates were made from homogenized isolated pulmonary artery samples with radio-immunoprecipitation assay buffer (Abcam, UK) containing a protease inhibitor cocktail (Roche, UK). Protein concentrations were determined through a Direct Detect Spectrometer (Merck, Germany). 20 µg protein from each sample was resolved by SDS-PAGE and transferred to nitrocellulose membranes for Western blotting with antibodies specifically recognizing human AMPK and phosphorylated AMPKα (p-AMPK) (Cell Signaling Technology, US), as well as eNOS, phosphorylated eNOS (p-eNOS), ERA, ERB and GAPDH (Abcam, UK). Immune complexes were visualized using horseradish peroxidase-conjugated secondary antibody with enhanced chemiluminescence on a BioRad Chemidoc MP system.
Statistical analysis
Data are expressed as means ± SEM and were compared using one-way ANOVA or two-way repeated-measures ANOVA with Bonferroni post hoc testing where appropriate. Sigmoidal curve fitting was performed on wire myography concentration-response curve data using GraphPad Prism software 7.0 (San Diego, CA). A p-value of < 0.05 was accepted as statistically significant.
Results
After 3 months of treatments, 20 of 28 patients with bosentan monotherapy and 18 of 24 patients with combination therapy were eligible for operations with biventricular circulation. 7 patients with monotherapy and 3 patients with combination therapy were excluded from the statistical analysis due to either unsuccessful dissection of suitable pulmonary arteries or poor response of isolated arteries to vessel constrictor or dilator.
Adding metformin to bosentan therapy attenuates in vitro PE-induced contractility in pulmonary arteries from PAH-CHD patients
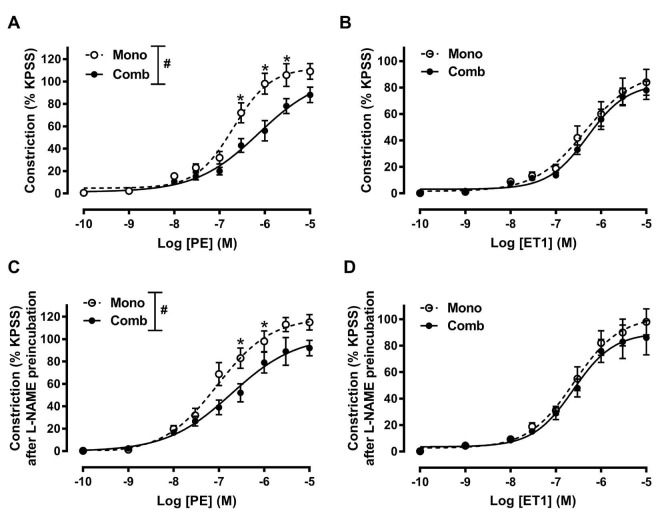
Both PE and ET1 induced dose-dependent constrictions in isolated pulmonary arteries with intact endothelium. These arteries were examined following prior treatments with either bosentan monotherapy or bosentan with metformin as combination therapy (Figure 1A and B). PE-induced contractions of arteries in patients received combination therapy were significantly attenuated at concentrations of 3 × 10-7 M, 10-6 M and 3 × 10-6 M, compared to those received monotherapy (Figure 1A). Combination treatment had no effects on ET1-induced contractions (Figure 1B).
Figure 1.
The reactivity of pulmonary artery with intact endothelium after bosentan monotherapy and bosentan/metformin combination therapy for 3 months. (A) and (B) Concentration-response curves for PE or ET-1 induced constriction of isolated pulmonary arteries. (C) and (D) Concentration-response curves for PE or ET-1 induced constriction of isolated pulmonary arteries after 30 min L-NAME preincubation. Constriction of pulmonary arteries in response to PE was significantly lower in combination therapy compared with monotherapy before or after L-NAME preincubation. Data are expressed as means ± SEM. Mono, bosentan monotherapy, n = 13; Comb, bosentan/metformin combination therapy, n = 15; # P < 0.05; two-way ANOVA; * P < 0.05; two-way ANOVA with Bonferroni post hoc test.
To determine the potential mechanisms of metformin-improved NO production on pulmonary artery response, vessels were preincubated with L-NAME for 30 min to block the endothelial NOS activity. We found that L-NAME preincubation increased the contractions to PE or ET1 by decreasing the NO bioavailability. However, blocking eNOS by L-NAME did not alter differential responses to PE between arteries in patients with monotherapy or combination therapy (Figure 1C).
Compound C prevents the inhibitory effect of metformin on PE-induced contractility
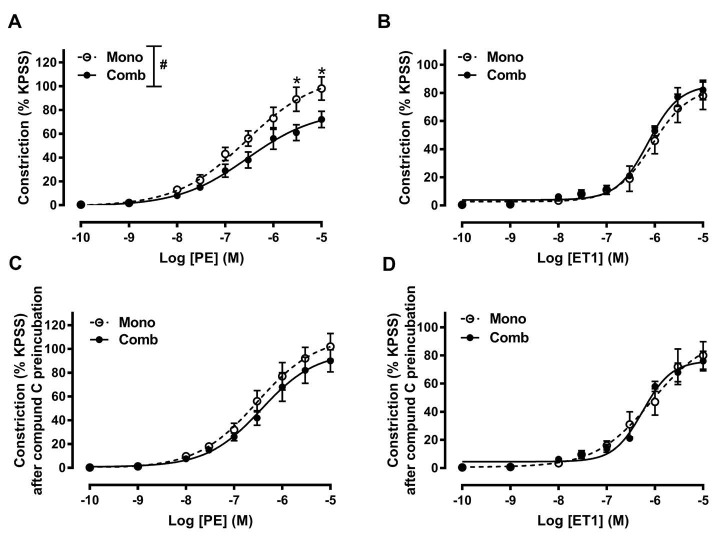
To determine the direct effect of metformin on VSMCs, we tested the vascular response to vessel constrictors on endothelium-denuded pulmonary arteries. Both PE and ET1 induced dose-dependent constrictions on endothelium-denuded pulmonary arteries (Figure 2). After denudation, PE-induced pulmonary artery contractions at concentrations of 3 × 10-6 M and 10-5 M were significantly decreased in the combination therapy group, compared to the monotherapy groups (Figure 2A). There were no significant differences in ET1-induced contractions (Figure 2B). Interestingly, preincubating the rings with compound C abrogated the inhibitory effect of metformin on PE induced contractility (Figure 2C).
Figure 2.
The reactivity of endothelium-denuded pulmonary artery after bosentan monotherapy and bosentan/metformin combination therapy for 3 months. (A) and (B) Concentration-response curves for PE or ET-1 induced constriction of isolated pulmonary arteries. (C) and (D) Concentration-response curves for PE or ET-1 induced constriction of isolated pulmonary arteries after 30 min compound C preincubation. Constriction of pulmonary arteries in response to PE was significantly lower in combination therapy compared with monotherapy before compound C preincubation. Compound C abrogated the inhibitory effect of metformin on PE induced contractility. Data are expressed as means ± SEM. Mono, bosentan monotherapy n = 13; Comb, bosentan/metformin combination therapy, n = 15; #P < 0.05; two-way ANOVA; * P < 0.05; two-way ANOVA with Bonferroni post hoc test.
Increased AMPK and eNOS phosphorylation after adding metformin to bosentan therapy
To confirm the activity of AMPK and eNOS after metformin treatment, western blotting was performed. Western blotting revealed that increased levels of p-AMPK and p-eNOS in the pulmonary arteries of patients treated with combination therapy, compared to bosentan monotherapy (Figure 3A and B). However, there were no significant differences in the pulmonary protein levels of AMPK, eNOS, ERA and ERB between the two treatment regimens (Figure 3A and B).
Figure 3.
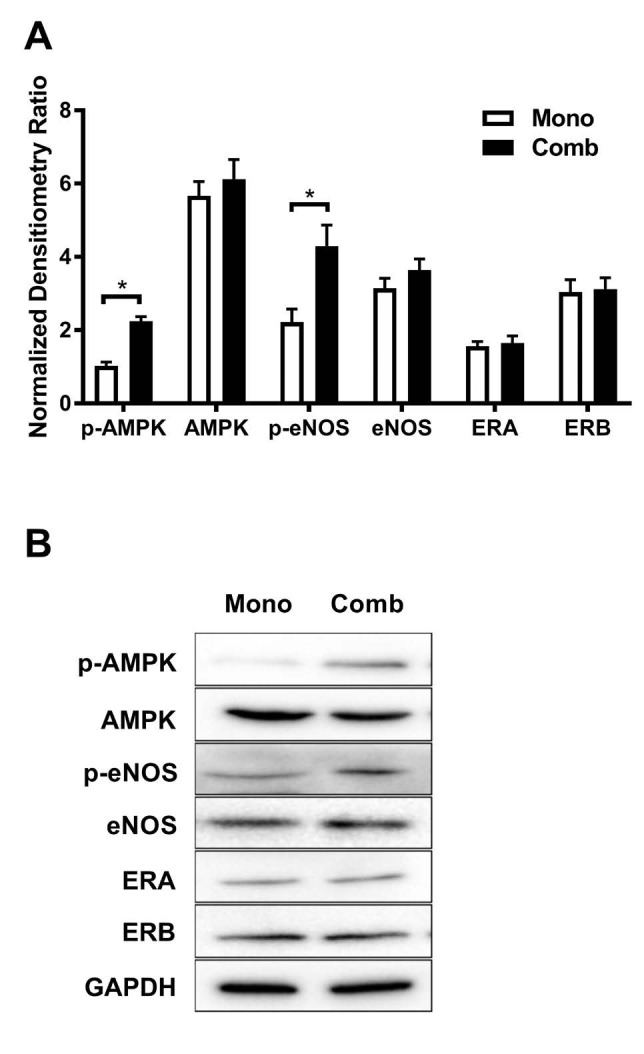
Pulmonary artery protein expressions after bosentan monotherapy and bosentan/metformin combination therapy for 3 months. (A) Protein levels of p-AMPK, AMPK, p-eNOS, eNOS, ERA and ERB. There were increased levels of p-AMPK and p-eNOS in the pulmonary arteries of patients treated with combination therapy, compared to bosentan monotherapy. (B) representative immunoblots of the corresponding proteins. Data are expressed as means ± SEM. Mono, bosentan monotherapy; Comb, bosentan/metformin combination therapy; * P < 0.05; one-way ANOVA.
Discussion
In the present study, we compared the pulmonary artery response after 3 months treatment of bosentan monotherapy vs combination bosentan/metformin therapy and found that pulmonary artery contraction induced by PE was decreased after adding metformin to bosentan therapy in PAH-CHD patients. This is significant because PAH is characterized by progressive inflammation and vessel wall remodelling leading to increased vasoconstriction and increased pulmonary artery resistance.26,27
The beneficial effects of metformin on the vascular function has been suggested in clinical observations,28 although the molecular mechanisms are still unclear. In rats, oral administration of metformin diminished vascular reactivity to catecholamine constrictor both with and without the endothelium.29,30 In humans, metformin improves vascular function in patients presented with insulin resistance.31,32 We found that increased phosphorylation of AMPK and eNOS after chronic metformin treatment, which is consistent with results from previous translational studies with metformin treatments.33-35 The AMPK-eNOS-NO pathway is therefore considered to be the main pathway attributing to the regulative effect of metformin on vascular function.36 AMPK is a heterotrimetric enzyme comprising a catalytic subunit and two regulatory subunits. The catalytic subunit consists of an N-terminalcatalytic kinase domain and a C-terminal regulatory domain. The phosphorylation of the kinase domain by upstream kinases is required for AMPK activation.37 AMPK not only is involved in the regulation of cellular and organ metabolism,38 but also plays a regulatory role over vascular structure and function and is essential for the maintenance of cardiovascular health.39 Activation of AMPK by metformin stimulates NO synthesis in vascular endothelial cells by increasing phosphorylation and activation of eNOS15,40 and reduces mitochondrial reactive oxygen species, which diminish the bioavailability of NO.41 However, blocking the eNOS or removing the endothelium did not fully reverse the inhibitory effect on vessel constriction by metformin in our study. A direct effect of metformin on VSMCs is suspected. Indeed, it is reported that the activation of AMPK by metformin suppresses VSMC contraction by inhibiting myosin light chain (MLC) kinases and MLC phosphorylation in rats,18 reduces VSMC proliferation and migration,42 and attenuates elevation of intracellular Ca2+ levels in VSMCs.43,44 A direct effect via AMPK on smooth muscle cell wall of the artery is supported in our study, where we found that the AMPK inhibitor compound C reversed the inhibitory effect on PE-induced contraction by metformin on endothelium-denuded pulmonary arteries. Consistent with this, metformin increases AMPK phosphorylation and attenuates contractile responses in endothelium-denuded rat aorta,19 suggesting a potential role of AMPK as an intermediary signalling component for metformin action in pulmonary artery response.
It is worthy to note that compound C is the primary reagent used as an AMPK inhibitor and has been widely used in biochemical in vitro and some in vivo experiments. However, Compound C unfortunately inhibits several other kinases much more potently than AMPK and is therefore highly non-specific, and its inhibitory effects seem to be dose-dependent.45,46 Furthermore, ET1 is a strong vasoconstrictor and is one of key mediators of PAH development.47 It is reported that the activation of AMPK by metformin suppresses ET1 induced pulmonary artery VSMC proliferation in rats,48 and inhibits ET1 expression at the transcriptional and translational level in the aorta.49 Additional effects of metformin on the ET pathway and/or other pathways may also contribute to the differential vascular responses we observed.
Conclusions
In summary, our findings suggest that adding metformin to bosentan therapy in patients with PAH-CHD decreased in vitro pulmonary artery contraction induced by PE, which is possibly related to increased AMPK phosphorylation. Our findings demonstrate the efficacy of metformin clinically in diseased pulmonary arteries.
Competing interests
The authors report no relationships that could be construed as a conflict of interest.
Ethical approval
In accordance with the Declaration of Helsinki, all study protocols were approved by the Institutional ethics committee of Nanchang University on human research, and written informed consent was obtained from all patients for the use of their tissue.
Acknowledgments
The authors gratefully acknowledge the financial support from The Limingzhang Sciences Foundation (2014-778903). Shutan Liao is funded under an NHMRC Development Grant.
Please cite this article as: Liao S, Li D, Hui Z, McLachlan CS, Zhang Y. Chronic dosing with metformin plus bosentan decreases in vitro pulmonary artery contraction from isolated arteries in adults with pulmonary hypertension. J Cardiovasc Thorac Res 2019;11(3):189-195. doi: 10.15171/jcvtr.2019.32.
References
- 1.Gatzoulis Ma, Alonso-Gonzalez R, Beghetti M. Pulmonary Arterial Hypertension In Paediatric And Adult Patients With Congenital Heart Disease. Eur Respir Rev. 2009;18(113):154–61. doi: 10.1183/09059180.00003309. [DOI] [PubMed] [Google Scholar]
- 2.Van Riel Ac, Schuuring Mj, Van Hessen Id, Zwinderman Ah, Cozijnsen L, Reichert Cl, Et Al. Contemporary Prevalence Of Pulmonary Arterial Hypertension In Adult Congenital Heart Disease Following The Updated Clinical Classification. Int J Cardiol. 2014;174(2):299–305. doi: 10.1016/J.ijcard.2014.04.072. [DOI] [PubMed] [Google Scholar]
- 3.Barst Rj, Ivy Dd, Foreman Aj, Mcgoon Md, Rosenzweig Eb. Four- And Seven-Year Outcomes Of Patients With Congenital Heart Disease-Associated Pulmonary Arterial Hypertension (From The Reveal Registry) Am J Cardiol. 2014;113(1):147–55. doi: 10.1016/J.amjcard.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Kaemmerer H, Gorenflo M, Hoeper M, Huscher D, Ewert P, Pittrow D. [Pulmonary Arterial Hypertension In Patients With Congenital Heart Disease: Current Issues And Health Care Situation] Dtsch Med Wochenschr. 2013;138(23):1247–52. doi: 10.1055/S-0033-1343189. [DOI] [PubMed] [Google Scholar]
- 5.Gatzoulis Ma, Beghetti M, Landzberg Mj, Galie N. Pulmonary Arterial Hypertension Associated With Congenital Heart Disease: Recent Advances And Future Directions. Int J Cardiol. 2014;177(2):340–7. doi: 10.1016/J.ijcard.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Hu Z, Xie B, Zhai X, Liu J, Gu J, Wang X, Et Al. Midterm Results Of “Treat And Repair” For Adults With Non-Restrictive Ventricular Septal Defect And Severe Pulmonary Hypertension. J Thorac Dis. 2015;7(7):1165–73. doi: 10.3978/J.issn.2072-1439.2015.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apitz C, Hansmann G, Schranz D. Hemodynamic Assessment And Acute Pulmonary Vasoreactivity Testing In The Evaluation Of Children With Pulmonary Vascular Disease Expert Consensus Statement On The Diagnosis And Treatment Of Paediatric Pulmonary Hypertension The European Paediatric Pulmonary Vascular Disease Network, Endorsed By Ishlt And Dgpk. Heart. 2016;102 Suppl 2:Ii23–9. doi: 10.1136/Heartjnl-2014-307340. [DOI] [PubMed] [Google Scholar]
- 8.Kemp K, Savale L, O’callaghan Ds, Jais X, Montani D, Humbert M, Et Al. Usefulness Of First-Line Combination Therapy With Epoprostenol And Bosentan In Pulmonary Arterial Hypertension: An Observational Study. J Heart Lung Transplant. 2012;31(2):150–8. doi: 10.1016/J.healun.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Sitbon O, Jais X, Savale L, Cottin V, Bergot E, Macari Ea, Et Al. Upfront Triple Combination Therapy In Pulmonary Arterial Hypertension: A Pilot Study. Eur Respir J. 2014;43(6):1691–7. doi: 10.1183/09031936.00116313. [DOI] [PubMed] [Google Scholar]
- 10.Humbert M, Barst Rj, Robbins Im, Channick Rn, Galie N, Boonstra A, Et Al. Combination Of Bosentan With Epoprostenol In Pulmonary Arterial Hypertension: Breathe-2. Eur Respir J. 2004;24(3):353–9. doi: 10.1183/09031936.04.00028404. [DOI] [PubMed] [Google Scholar]
- 11.Abbasi F, Chu Jw, Mclaughlin T, Lamendola C, Leary Et, Reaven Gm. Effect Of Metformin Treatment On Multiple Cardiovascular Disease Risk Factors In Patients With Type 2 Diabetes Mellitus. Metabolism. 2004;53(2):159–64. doi: 10.1016/j.metabol.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Agard C, Rolli-Derkinderen M, Dumas-De-La-Roque E, Rio M, Sagan C, Savineau Jp, Et Al. Protective Role Of The Antidiabetic Drug Metformin Against Chronic Experimental Pulmonary Hypertension. Br J Pharmacol. 2009;158(5):1285–94. doi: 10.1111/J.1476-5381.2009.00445.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean A, Nilsen M, Loughlin L, Salt Ip, Maclean Mr. Metformin Reverses Development Of Pulmonary Hypertension Via Aromatase Inhibition. Hypertension. 2016;68(2):446–54. doi: 10.1161/Hypertensionaha.116.07353. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Li J, Yang O, Kong J, Lin G. Effect Of Metformin On Insulin-Resistant Endothelial Cell Function. Oncol Lett. 2015;9(3):1149–53. doi: 10.3892/Ol.2015.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrow Va, Foufelle F, Connell Jm, Petrie Jr, Gould Gw, Salt Ip. Direct Activation Of Amp-Activated Protein Kinase Stimulates Nitric-Oxide Synthesis In Human Aortic Endothelial Cells. J Biol Chem. 2003;278(34):31629–39. doi: 10.1074/Jbc.m212831200. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Peng Ic, Sun W, Su Mi, Hsu Ph, Fu Y, Et Al. Amp-Activated Protein Kinase Functionally Phosphorylates Endothelial Nitric Oxide Synthase Ser633. Circ Res. 2009;104(4):496–505. doi: 10.1161/Circresaha.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambe T, Mason Rp, Dawoud H, Bhatt Dl, Malinski T. Metformin Treatment Decreases Nitroxidative Stress, Restores Nitric Oxide Bioavailability And Endothelial Function Beyond Glucose Control. Biomed Pharmacother. 2018;98:149–56. doi: 10.1016/J.biopha.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 18.Sung Jy, Choi Hc. Metformin-Induced Amp-Activated Protein Kinase Activation Regulates Phenylephrine-Mediated Contraction Of Rat Aorta. Biochem Biophys Res Commun. 2012;421(3):599–604. doi: 10.1016/J.bbrc.2012.04.052. [DOI] [PubMed] [Google Scholar]
- 19.Pyla R, Osman I, Pichavaram P, Hansen P, Segar L. Metformin Exaggerates Phenylephrine-Induced Ampk Phosphorylation Independent Of Camkkbeta And Attenuates Contractile Response In Endothelium-Denuded Rat Aorta. Biochem Pharmacol. 2014;92(2):266–79. doi: 10.1016/J.bcp.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song Y, Wu Y, Su X, Zhu Y, Liu L, Pan Y, Et Al. Activation Of Ampk Inhibits Pdgf-Induced Pulmonary Arterial Smooth Muscle Cells Proliferation And Its Potential Mechanisms. Pharmacol Res. 2016;107:117–24. doi: 10.1016/J.phrs.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Liao S, Li D, Hui Z, Mclachlan Cs, Zhang Y. Metformin Added To Bosentan Therapy In Patients With Pulmonary Arterial Hypertension Associated With Congenital Heart Defects: A Pilot Study. Erj Open Research. 2018;4(3) doi: 10.1183/23120541.00060-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopes Aa, O’leary Pw. Measurement, Interpretation And Use Of Haemodynamic Parameters In Pulmonary Hypertension Associated With Congenital Cardiac Disease. Cardiol Young. 2009;19(5):431–5. doi: 10.1017/S1047951109990771. [DOI] [PubMed] [Google Scholar]
- 23.Kozlik-Feldmann R, Hansmann G, Bonnet D, Schranz D, Apitz C, Michel-Behnke I. Pulmonary Hypertension In Children With Congenital Heart Disease (Pah-Chd, Pphvd-Chd) Expert Consensus Statement On The Diagnosis And Treatment Of Paediatric Pulmonary Hypertension The European Paediatric Pulmonary Vascular Disease Network, Endorsed By Ishlt And Dgpk. Heart. 2016;102(Suppl 2):Ii42–8. doi: 10.1136/Heartjnl-2015-308378. [DOI] [PubMed] [Google Scholar]
- 24.Angus Ja, Wright Ce. Techniques To Study The Pharmacodynamics Of Isolated Large And Small Blood Vessels. J Pharmacol Toxicol Methods. 2000;44(2):395–407. doi: 10.1016/s1056-8719(00)00121-0. [DOI] [PubMed] [Google Scholar]
- 25.Baranowska-Kuczko M, Kozlowska H, Kozlowski M, Schlicker E, Kloza M, Surazynski A, Et Al. Mechanisms Of Endothelium-Dependent Relaxation Evoked By Anandamide In Isolated Human Pulmonary Arteries. Naunyn Schmiedebergs Arch Pharmacol. 2014;387(5):477–86. doi: 10.1007/S00210-014-0961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low A, George S, Howard L, Bell N, Millar A, Tulloh Rmr. Lung Function, Inflammation, And Endothelin-1 In Congenital Heart Disease-Associated Pulmonary Arterial Hypertension. J Am Heart Assoc. 2018;7(4) doi: 10.1161/Jaha.117.007249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuder Rm. Pulmonary Vascular Remodeling In Pulmonary Hypertension. Cell Tissue Res. 2017;367(3):643–9. doi: 10.1007/S00441-016-2539-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant Pj. Beneficial Effects Of Metformin On Haemostasis And Vascular Function In Man. Diabetes Metab. 2003;29(4 Pt 2):6S44–52. doi: 10.1016/s1262-3636(03)72787-6. [DOI] [PubMed] [Google Scholar]
- 29.Verma S, Bhanot S, Mcneill Jh. Decreased Vascular Reactivity In Metformin-Treated Fructose-Hypertensive Rats. Metabolism. 1996;45(9):1053–5. doi: 10.1016/s0026-0495(96)90000-1. [DOI] [PubMed] [Google Scholar]
- 30.Lobato Ns, Filgueira Fp, Hagihara Gn, Akamine Eh, Pariz Jr, Tostes Rc, Et Al. Improvement Of Metabolic Parameters And Vascular Function By Metformin In Obese Non-Diabetic Rats. Life Sci. 2012;90(5-6):228–35. doi: 10.1016/J.lfs.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Joya-Galeana J, Fernandez M, Cervera A, Reyna S, Ghosh S, Triplitt C, Et Al. Effects Of Insulin And Oral Anti-Diabetic Agents On Glucose Metabolism, Vascular Dysfunction And Skeletal Muscle Inflammation In Type 2 Diabetic Subjects. Diabetes Metab Res Rev. 2011;27(4):373–82. doi: 10.1002/Dmrr.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romualdi D, Costantini B, Selvaggi L, Giuliani M, Cristello F, Macri F, Et Al. Metformin Improves Endothelial Function In Normoinsulinemic Pcos Patients: A New Prospective. Hum Reprod. 2008;23(9):2127–33. doi: 10.1093/Humrep/Den230. [DOI] [PubMed] [Google Scholar]
- 33.Cao X, Li H, Tao H, Wu N, Yu L, Zhang D, Et Al. Metformin Inhibits Vascular Calcification In Female Rat Aortic Smooth Muscle Cells Via The Ampk-Enos-No Pathway. Endocrinology. 2013;154(10):3680–9. doi: 10.1210/En.2013-1002. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Cx, Pan Sn, Meng Rs, Peng Cq, Xiong Zj, Chen Bl, Et Al. Metformin Attenuates Ventricular Hypertrophy By Activating The Amp-Activated Protein Kinase-Endothelial Nitric Oxide Synthase Pathway In Rats. Clin Exp Pharmacol Physiol. 2011;38(1):55–62. doi: 10.1111/J.1440-1681.2010.05461.X. [DOI] [PubMed] [Google Scholar]
- 35.Yu Jw, Deng Yp, Han X, Ren Gf, Cai J, Jiang Gj. Metformin Improves The Angiogenic Functions Of Endothelial Progenitor Cells Via Activating Ampk/Enos Pathway In Diabetic Mice. Cardiovasc Diabetol. 2016;15:88. doi: 10.1186/S12933-016-0408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salt Ip, Hardie Dg. Amp-Activated Protein Kinase: An Ubiquitous Signaling Pathway With Key Roles In The Cardiovascular System. Circ Res. 2017;120(11):1825–41. doi: 10.1161/Circresaha.117.309633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawley Sa, Davison M, Woods A, Davies Sp, Beri Rk, Carling D, Et Al. Characterization Of The Amp-Activated Protein Kinase Kinase From Rat Liver And Identification Of Threonine 172 As The Major Site At Which It Phosphorylates Amp-Activated Protein Kinase. J Biol Chem. 1996;271(44):27879–87. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 38.Hardie Dg. Ampk: Positive And Negative Regulation, And Its Role In Whole-Body Energy Homeostasis. Curr Opin Cell Biol. 2015;33:1–7. doi: 10.1016/J.ceb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Ewart Ma, Kennedy S. Ampk And Vasculoprotection. Pharmacol Ther. 2011;131(2):242–53. doi: 10.1016/J.pharmthera.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Davis Bj, Xie Z, Viollet B, Zou Mh. Activation Of The Amp-Activated Kinase By Antidiabetes Drug Metformin Stimulates Nitric Oxide Synthesis In Vivo By Promoting The Association Of Heat Shock Protein 90 And Endothelial Nitric Oxide Synthase. Diabetes. 2006;55(2):496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 41.Zou Mh, Kirkpatrick Ss, Davis Bj, Nelson Js, Wiles Wgt, Schlattner U, Et Al. Activation Of The Amp-Activated Protein Kinase By The Anti-Diabetic Drug Metformin In Vivo Role Of Mitochondrial Reactive Nitrogen Species. J Biol Chem. 2004;279(42):43940–51. doi: 10.1074/Jbc.m404421200. [DOI] [PubMed] [Google Scholar]
- 42.Vigetti D, Clerici M, Deleonibus S, Karousou E, Viola M, Moretto P, Et Al. Hyaluronan Synthesis Is Inhibited By Adenosine Monophosphate-Activated Protein Kinase Through The Regulation Of Has2 Activity In Human Aortic Smooth Muscle Cells. J Biol Chem. 2011;286(10):7917–24. doi: 10.1074/Jbc.m110.193656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma Rv, Bhalla Rc. Metformin Attenuates Agonist-Stimulated Calcium Transients In Vascular Smooth Muscle Cells. Clin Exp Hypertens. 1995;17(6):913–29. doi: 10.3109/10641969509033643. [DOI] [PubMed] [Google Scholar]
- 44.Chen Xl, Panek K, Rembold Cm. Metformin Relaxes Rat Tail Artery By Repolarization And Resultant Decreases In Ca2+ Influx And Intracellular [Ca2+] J Hypertens. 1997;15(3):269–74. doi: 10.1097/00004872-199715030-00008. [DOI] [PubMed] [Google Scholar]
- 45.Dasgupta B, Seibel W. Compound C/Dorsomorphin: Its Use And Misuse As An Ampk Inhibitor. Methods Mol Biol. 2018;1732:195–202. doi: 10.1007/978-1-4939-7598-3_12. [DOI] [PubMed] [Google Scholar]
- 46.Bain J, Plater L, Elliott M, Shpiro N, Hastie Cj, Mclauchlan H, Et Al. The Selectivity Of Protein Kinase Inhibitors: A Further Update. Biochem J. 2007;408(3):297–315. doi: 10.1042/Bj20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guignabert C, Tu L, Girerd B, Ricard N, Huertas A, Montani D, Et Al. New Molecular Targets Of Pulmonary Vascular Remodeling In Pulmonary Arterial Hypertension: Importance Of Endothelial Communication. Chest. 2015;147(2):529–37. doi: 10.1378/Chest.14-0862. [DOI] [PubMed] [Google Scholar]
- 48.Wu Y, Liu L, Zhang Y, Wang G, Han D, Ke R, Et Al. Activation Of Ampk Inhibits Pulmonary Arterial Smooth Muscle Cells Proliferation. Exp Lung Res. 2014;40(5):251–8. doi: 10.3109/01902148.2014.913092. [DOI] [PubMed] [Google Scholar]
- 49.Tang St, Su H, Zhang Q, Tang Hq, Wang Cj, Zhou Q, Et Al. Sitagliptin Inhibits Endothelin-1 Expression In The Aortic Endothelium Of Rats With Streptozotocin-Induced Diabetes By Suppressing The Nuclear Factor-Kappab/Ikappabalpha System Through The Activation Of Amp-Activated Protein Kinase. Int J Mol Med. 2016;37(6):1558–66. doi: 10.3892/Ijmm.2016.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]