Abstract
Background:
In order to ensure safer use of non-vitamin K antagonist oral anticoagulants (NOACs), continuously detecting unexpected adverse drug reactions (ADRs) after market approval is necessary.
Methods:
We performed disproportionality analysis to evaluate association between ADRs and NOACs including apixaban, dabigatran, and rivaroxaban using data from the Korea Institute of Drug Safety and Risk Management–Korea Adverse Event Reporting System database (KIDS-KD) between 2012 and 2016. There was no significant signal other than bleeding when considering quantity, signal strength, seriousness, and causality. In order to evaluate the NOAC reports about bleeding, we selected 62 WHO-ART diagnostic codes associated with bleeding. Among the 26 codes that referred to major bleeding, 18 codes referred to gastrointestinal bleeding and 8 were referred to intracranial bleeding. We evaluated the significance of the signals using reporting odds ratios (RORs) adjusted for age and sex.
Results:
Treatments with apixaban, dabigatran, and rivaroxaban were associated with 1989, 1668, and 2960 adverse events, respectively. Any type of bleeding with apixaban, dabigatran, rivaroxaban, and warfarin was reported in 174 (8.8%), 209 (12.5%), 523 (17.8%), and 620 (9.5%) events, respectively. For any bleeding, adjusted RORs of apixaban, dabigatran, and rivaroxaban were 0.99 [95% confidence interval (CI): 0.83–1.17], 1.47 (95% CI: 1.25–1.75), and 2.48 (95% CI: 2.16–2.84), respectively. With respect to major bleeding, the adjusted RORs of apixaban, dabigatran, and rivaroxaban were 1.08 (95% CI: 0.82–1.41), 1.46 (95% CI: 1.10–1.90), and 1.82 (95% CI: 1.43–2.32), respectively.
Conclusion:
Rivaroxaban might have stronger association with bleeding than apixaban and dabigatran.
Keywords: adverse events, bleeding, non-vitamin K antagonist oral anticoagulants, oral anticoagulants
Introduction
Atrial fibrillation (AF) is associated with a four- to five-fold increased risk of stroke and is responsible for approximately 15% of all strokes.1 Oral anticoagulant (OAC) use is well-established for stroke prevention in patients with AF. In recent decades, non-vitamin K antagonist oral anticoagulants (NOACs) have been developed. Randomized controlled trials (RCTs) of NOACs have documented that NOACs have similar efficacy and safety as warfarin, but are more convenient for the patient since NOACs generally do not require periodic checking of the patient’s international normalized ratio.2 Therefore, clinical guidelines for AF suggest that NOACs be included as one of the options or first-line choice for preventing stroke.3,4 The amount of NOACs used to replace warfarin is increasing rapidly.5,6
A risk of bleeding is expected with all anticoagulation therapies, and so prior RCTs and observational studies on adverse events (AEs) of NOACs have mainly focused on rates of bleeding. Some studies and case reports have suggested that NOACs induce other types of AEs such as liver injury, Stevens–Johnson syndrome, and gastroesophageal reflux syndrome.7–9 In order to ensure the safe use of NOACs, information on the occurrence of unexpected adverse drug reactions (ADRs) from studies that use ‘real-world’ healthcare databases is necessary.
We applied signal detection to evaluate the rate of unexpected AEs caused by NOACs through disproportionality analysis of data in spontaneous AE reporting systems.10 The purpose of our study was to detect signals in AE reports of NOACs, and then to evaluate the priority levels of these signals based on the seriousness and causality assessment results. In addition, we compared the reporting trend of each NOACs based on prioritized signals.
Methods
Data source
We used the Korea Institute of Drug Safety and Risk Management (KIDS)–Korea Adverse Event Reporting System (KAERS) database, abbreviated to KIDS-KD, to evaluate AEs related to NOACs. KIDS-KD was established by the Ministry of Food and Drug Safety (MFDS) in 1988. KIDS-KD, managed by the KIDS, contained 1,089,163 reports of AE as of December 2016. More than 90% of reports in KIDS-KD were reported by medical professionals such as doctors, nurses, and pharmacists via 27 regional pharmacovigilance centers.
In KIDS-KD, drugs were coded according to Anatomical Therapeutic Chemical Classification System (ATC). AEs coded according to the Preferred Terms (PTs) among WHO Adverse Reaction Terminology. KIDS-KD also contained baseline patient characteristics such as age and sex, and seriousness and causality assessment results for reported AEs. Seriousness assessment was determined according to six categories including disability, deformity, life-threatening event, death, hospitalization, and other serious situations. In the causality assessment, the reporter chose one of six categories: ‘certain’, ‘probable’, ‘possible’, ‘unlikely’, ‘unclassified’, and ‘unassessable’. These seriousness and causality assessments were primarily recorded by each pharmacovigilance centers based on the WHO Uppsala Monitoring Center (UMC) criteria.11
We analyzed the KIDS-KD, which included AEs due to antithrombotics (ATC: B01A) and antiarrhythmics (ATC: C01B) from January 2012 to December 2016. We defined the target drugs to be apixaban (ATC: B01AF02), dabigatran (ATC: B01AE07), and rivaroxaban (ATC: B01AF01), in comparison with warfarin (ATC: B01AA03). Since this study was performed using a deidentified secondary database, it was exempted from a review of the institutional review board of Seoul National University College of Medicine/Seoul National University Hospital (IRB No. 1706-112-860).
Signal detection and prioritization
In pharmacovigilance studies, disproportionality analysis, which compares the proportion of AE reports between the target drug and other drugs, is a well-established method for evaluating AE outbreak. When the target drug satisfies the index criteria for a specific AE due to higher disproportionality, the target drug is associated with that AE.12
We applied three indices, including a proportional reporting ratio (PRR), reporting odds ratio (ROR), and information component (IC) for disproportionality analysis (Table 1).13–15 We restricted detected signals to events reported in more than three cases and which satisfied all three criteria indices (described in the following). For signal prioritization, we performed an additional two-step disproportionality analyses. In the first step, in order to reflect seriousness, we calculated the same indices using reports of serious cases including any kinds of six seriousness categories. In the second step, to reflect causality, the same indices were calculated using reports that were assessed to have possible or higher association. We defined a prioritized signal as satisfying quantity, signal strength, seriousness, and causality. Each type of AE that was determined to be a prioritized signal was then reviewed individually.
Table 1.
Indexes for signal detection in pharmacovigilance.
| Index | Calculation | Criteria |
|---|---|---|
| PRR | PRR ⩾ 2 and χ2 ⩾4 | |
| ROR | ROR ⩾ 2 and χ2 ⩾4 | |
| IC | Lower limit of 95% CIs ⩾ 0 |
AE, adverse events; CI, confidence intervals; IC, information component; PRR, proportional reporting ratio; ROR, reporting odds ratio.
Vertical bars in the calculation column mean conditional probability; minus signs in the calculation column mean anything other than the drug or adverse events of interest in the study.
Focused risk quantification
We evaluate the reporting ratio of bleeding for warfarin and each NOAC. In order to select PTs in WHO-ART associated with bleeding, two pharmacoepidemiologists identified the entire PTs and, if the two disagreed, decided whether to include them through consultation. We defined 62 PTs in WHO-ART for any bleeding, and selected 26 of these which referred to major bleeding. Major bleeding was composed of 18 PTs referring to gastrointestinal (GI) bleeding and eight PTs referring to intracranial bleeding. The list of PTs associated with bleeding are supplied in Table S1 in the supplemental material.
We compared ROR adjusted for age and sex (aROR) between each NOAC and warfarin according to the type of bleeding including any bleeding, major bleeding, GI bleeding, and intracranial bleeding. We performed sequential analysis to evaluate the quarterly time trend of aRORs from the first quarter of 2014 to the fourth quarter of 2016. This method was based on analyzing each quarter from the cumulative data, including all the previously reported AEs. We performed a visual inspection to determine whether aRORs for each anticoagulant were persistently stable.
Statistical analysis
We analyzed differences in baseline patient characteristics enrolled in the studies of each NOAC and warfarin by using the χ2 test for categorical variables, and the t test and analysis of variance for continuous variables. PRR, ROR, and IC were calculated according to the formula of Table 1.13–15 We used a multiple logistic regression model to calculate the aROR. All statistical analyses were performed using SAS 9.4 (SAS institute Inc., Cary, NC, USA).
Results
Between 2012 and 2016, KIDS-KD contained 58,834 reports of AEs referring to the investigated drug classes, thereof 54,964 occurred after the use of antithrombotics and 3870 occurred after the use of antiarrhythmics. Among the 58,834 reports, 1989 (3.4%), 1668 (2.8%), 2960 (5.0%), and 6550 (11.1%) cases included the use of apixaban, dabigatran, rivaroxaban, and warfarin, respectively (Table 2). Since NOACs are the only newly approved drugs, more than 90% of reports with NOACs were reported after 2014. All the baseline characteristics were significantly different between each anticoagulant, and NOAC cases were older than warfarin users.
Table 2.
Baseline characteristics of apixaban, dabigatran, rivaroxaban, and warfarin in KIDS-KD 2012–2016.
| Characteristics | Apixaban (%) | Dabigatran (%) | Rivaroxaban (%) | Warfarin (%) | p value |
|---|---|---|---|---|---|
| Sex | <0.001 | ||||
| Male | 984 (49.5) | 860 (51.6) | 1393 (47.1) | 3125 (47.7) | |
| Female | 952 (47.9) | 722 (43.3) | 1493 (50.4) | 3382 (51.6) | |
| Unknown | 53 (2.7) | 86 (5.2) | 74 (2.5) | 43 (0.7) | |
| Age (Mean ± SD) | 73.3 ± 9.6 | 70.7 ± 10.4 | 67.8 ± 13.0 | 62.3 ± 15.9 | <0.001 |
| 0–19 | 0 (0.0) | 0 (0.0) | 2 (0.1) | 13 (0.2) | |
| 20–29 | 0 (0.0) | 0 (0.0) | 21 (0.7) | 49 (0.8) | |
| 30–39 | 1 (0.1) | 16 (1.0) | 49 (1.7) | 829 (12.7) | |
| 40–49 | 48 (2.4) | 30 (1.8) | 61 (2.1) | 342 (5.2) | |
| 50–59 | 109 (5.5) | 156 (9.4) | 206 (7.0) | 990 (15.1) | |
| 60–69 | 313 (15.7) | 298 (17.9) | 380 (12.8) | 1362 (20.8) | |
| 70–79 | 741 (37.3) | 595 (35.7) | 643 (21.7) | 1773 (27.1) | |
| 80+ | 446 (22.4) | 233 (14.0) | 229 (7.7) | 635 (9.7) | |
| Unknown | 331 (16.6) | 340 (20.4) | 1369 (46.3) | 557 (8.5) | |
| Reported year | <0.001 | ||||
| 2012 | 0 (0.0) | 56 (3.4) | 62 (2.1) | 432 (6.6) | |
| 2013 | 0 (0.0) | 194 (11.6) | 146 (4.9) | 1039 (15.9) | |
| 2014 | 60 (3.0) | 206 (12.4) | 549 (18.6) | 1076 (16.4) | |
| 2015 | 462 (23.2) | 431 (25.8) | 1219 (41.2) | 1436 (21.9) | |
| 2016 | 1467 (73.8) | 781 (46.8) | 984 (33.2) | 2567 (39.2) | |
| Serious events a | 1581 (79.5) | 369 (22.1) | 1300 (43.9) | 2187 (33.4) | <0.001 |
| Disability | 7 (0.4) | 10 (0.6) | 13 (0.4) | 19 (0.3) | |
| Life threat | 10 (0.5) | 8 (0.5) | 19 (0.6) | 31 (0.5) | |
| Death | 103 (5.2) | 26 (1.6) | 225 (7.6) | 118 (1.8) | |
| Hospitalization | 1045 (52.5) | 294 (17.6) | 959 (32.4) | 1770 (27.0) | |
| Others | 1203 (60.5) | 63 (3.8) | 290 (9.8) | 904 (13.8) | |
| Causality a | 123 (6.2) | 503 (30.2) | 936 (31.6) | 2680 (40.9) | <0.001 |
| Certain | 4 (0.2) | 14 (0.8) | 18 (0.6) | 839 (12.8) | |
| Probable | 28 (1.4) | 162 (9.7) | 126 (4.3) | 969 (14.8) | |
| possible | 91 (4.6) | 327 (19.6) | 792 (26.8) | 872 (13.3) | |
| Total reports | 1989 (100.0) | 1668 (100.0) | 2960 (100.0) | 6550 (100.0) |
KIDS-KD, Korea Institute of Drug Safety and Risk Management (KIDS)-Korea Adverse Event Reporting System (KAERS) database.
Assessed by reporter.
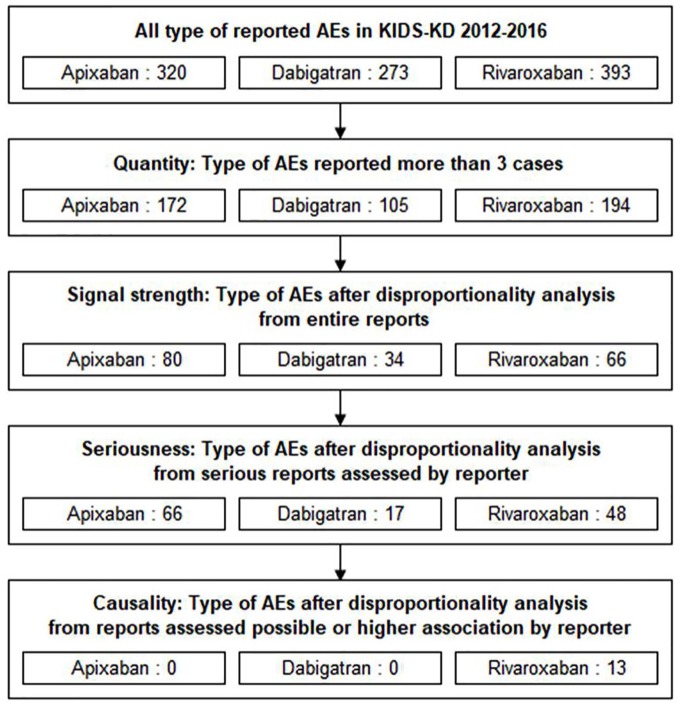
There were 320, 273, and 393 types of AE in KIDS-KD connected to apixaban, dabigatran, and rivaroxaban, respectively. After calculating the PRR, ROR, and IC from the full reports, 80, 34, and 66 signals were detected for apixaban, dabigatran, and rivaroxaban, respectively (data not shown). From serious cases, the signals of apixaban, dabigatran, and rivaroxaban were reduced to 66, 17, and 48, respectively. Finally, 13 rivaroxaban signals remained after signal prioritization (Figure 1). Ten of these prioritized signals were bleeding diagnoses, and other signals, which included anemia, hypochromic anemia, and increased prothrombin time, were explained as laboratory results associated with bleeding.
Figure 1.
Flow chart of signal detection and prioritization in KIDS-KD 2012–2016.
AE, adverse event; KIDS-KD, Korea Institute of Drug Safety and Risk Management (KIDS)-Korea Adverse Event Reporting System (KAERS) database.
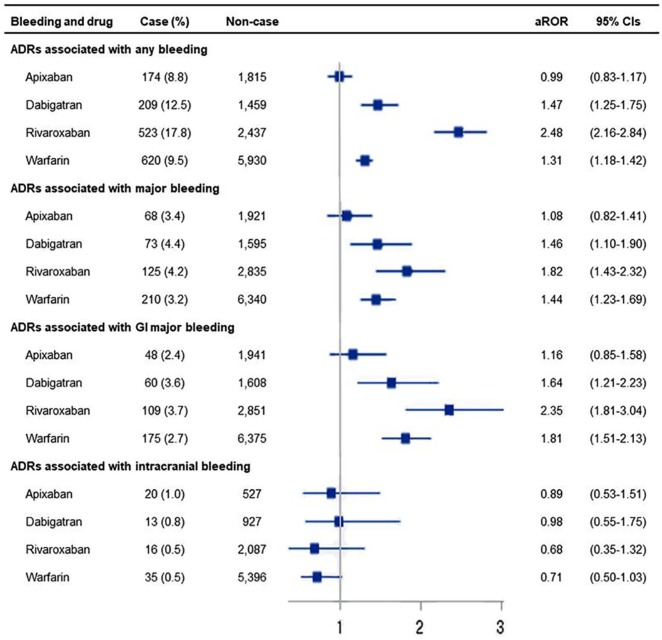
Any type of bleeding with apixaban, dabigatran, rivaroxaban, and warfarin was reported in 174 (8.8%), 209 (12.5%), 523 (17.8%), and 620 (9.5%) cases, respectively (Figure 2). Rivaroxaban had a higher aROR (2.48, 95% CI: 2.16–2.84) related to any bleeding than apixaban (0.99, 95% CI: 0.83–1.17), dabigatran (1.47, 95% CI: 1.25–1.75), and warfarin (1.30, 95% CI: 1.18–1.42). With respect to major bleeding, the aROR of rivaroxaban (1.82, 95% CI: 1.43–2.32) was not statistically higher than that of dabigatran (1.46, 95% CI: 1.10–1.90) or warfarin (1.44, 95% CI: 1.23–1.69), but it was statistically higher than that of apixaban (1.08, 95% CI: 0.82–1.41). Adjusted ROR patterns for GI bleeding were similar to that for major bleeding, but comparatively clear differences were seen between the patterns for each OAC. Specifically, rivaroxaban (2.35, 95% CI: 1.81–3.04) had a statistically higher aROR than apixaban (1.08, 95% CI: 0.82–1.41).
Figure 2.
aRORs according to type of bleeding in apixaban, dabigatran, rivaroxaban, and warfarin in KIDS-KD 2012–2016.
Adjusted for age and gender; horizontal bars represent 95% CIs.
ADR, adverse drug reaction; aROR, adjusted reporting odds ratio; CI, confidence interval; GI, gastrointestinal; KIDS-KD, Korea Institute of Drug Safety and Risk Management (KIDS)-Korea Adverse Event Reporting System (KAERS) database.
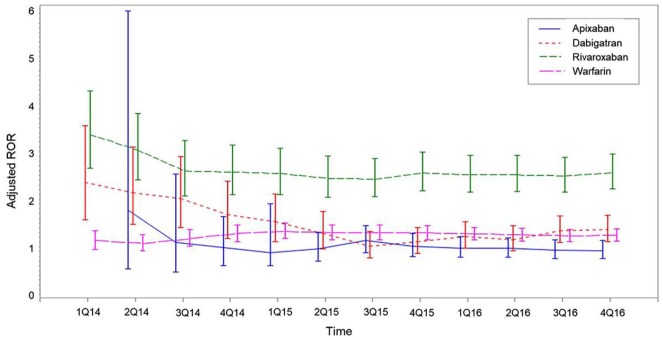
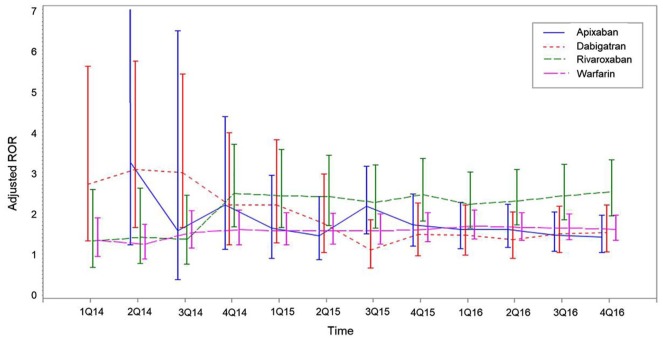
Of all 58,834 reports, 13,302 (22.6%) that did not contain dates of AE occurrence were excluded from the sequential analysis. The time trend for aROR for any bleeding due to NOACs fluctuated and had wide 95% CIs in 2014, but became stable after the middle of 2015. Of the aRORs for any bleeding, rivaroxaban had persistently higher aRORs than other OACs from the second quarter of 2015 (Figure 3). For GI bleeding, 95% CIs of adjusted rivaroxaban RORs overlapped with those for apixaban, dabigatran, and warfarin (Figure 4).
Figure 3.
Sequential analyses of aROR of any bleeding in apixaban, dabigatran, rivaroxaban, and warfarin in KIDS-KD 2012–2016.
Adjusted for age and gender; vertical bars represent 95% Cis.
CIs, confidence intervals; KIDS-KD, Korea Institute of Drug Safety and Risk Management (KIDS)-Korea Adverse Event Reporting System (KAERS) database; ROR, reporting odds ratios.
Figure 4.
Sequential analyses of aROR of GI bleeding in apixaban, dabigatran, rivaroxaban, and warfarin in KIDS-KD 2012–2016.
Adjusted for age and gender; Vertical bars represent 95% CIs.
aROR, adjusted reporting odds ratio; CI, confidence interval; GI, gastrointestinal; KIDS-KD, Korea Institute of Drug Safety and Risk Management (KIDS)-Korea Adverse Event Reporting System (KAERS) database.
Discussion
NOACs and bleeding risk
In our study, rivaroxaban had a higher aROR for any bleeding than other OACs, including apixaban, dabigatran, and warfarin. A higher aROR indicated that rivaroxaban was more frequently reported as being connected to any bleeding than other AEs were reported as being connected to other anticoagulants, and this reporting tendency was preserved from the third quarter of 2015 onwards. For major bleeding, the aROR of rivaroxaban was higher than that of apixaban, but similar to those of dabigatran and warfarin, and this was affected by reports of GI bleeding rather than intracranial bleeding.
These outcomes were similar to previous results of RCTs and observational studies. The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonist for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF) trial reported that the major bleeding rate of rivaroxaban was 3.60 per 100 patient-years (PYs).16 This rate was relatively higher than the 2.13 per 100 PYs of apixaban in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial and 3.11 per 100 PYs of dabigatran, 150 mg, in The Randomized Evaluation of Long-term Anti-coagulant Therapy (RE-LY) trial.17,18 In post-approval observational studies, the major bleeding rate attributed to each NOAC was similar to the results of the RCTs: 2.29–2.38 per 100 PYs, 2.04–3.60 per 100 PYs, and 2.90–6.00 per 100 PYs for apixaban, dabigatran, and rivaroxaban, respectively.19–22 In pairwise comparisons among NOACs, the major bleeding rates attributed to apixaban, dabigatran, and rivaroxaban were the lowest, intermediate, and the highest, respectively. In the subgroup analyses, intracranial bleeding rates attributed to all three NOACs were lower than that of warfarin.21–23 For GI bleeding, the incidence rate of rivaroxaban was higher than that of warfarin, though apixaban and dabigatran had similar incidences as warfarin.22,24
According to the results of previous studies, rivaroxaban was associated with a significantly higher risk of minor bleeding and GI bleeding than apixaban or dabigatran. Some researchers suggested that the conventional dose of rivaroxaban was related to a higher bleeding risk. Larsen et al. and Nielsen et al. reported, based on database from a Danish prescription registry, that rivaroxaban had a relatively higher hazard ratio (HR) for any bleeding and major bleeding than apixaban or dabigatran at conventional doses.21 However, a reduced dose of rivaroxaban, 20 mg once daily or 15 mg once daily, caused a similar HR of any bleeding and major bleeding compared with apixaban or dabigatran.25 Since the bleeding risk could be explained as the additive and opposing effects aimed at preventing strokes in patients with AF, it would be desirable that the use of a risk-adjusted dose of rivaroxaban be recommended in order to maintain a balance between bleeding risk and effects of preventing the stroke.
Another potential hypothesis was that physicians tended to prescribe rivaroxaban to older and higher-risk patients.26 When choosing between NOACs, physicians may decide to prescribe a particular NOAC to patients who are similar to those included in the pivotal RCT, and the RCT of rivaroxaban included older and higher-risk participants than the other RCTs. However, in our study, we could not confirm this finding. Moreover, patients who reported AEs with rivaroxaban were younger on average than that of patients who reported AEs with apixaban or dabigatran.
Disproportionality analysis in the spontaneous AE reporting system
This study aimed to broadly examine the disproportionality of AEs with each NOAC. For newly approved drugs such as NOACs, the clinical trial provides the most valuable scientific evidence for effectiveness and safety. Recently, many epidemiologic studies, including both nationwide claims-based or registry-based studies, have been conducted. Research using spontaneous AE reporting system cannot provide strong evidence for safety, but can provide highly sensitive and timely evidence of unexpected safety issues.8 Research with this method is usually performed to detect unexpected ADRs that are not found in the list of well-documented ADRs found on the drug label information.27–29 Therefore, continuous monitoring is needed along with cooperation with other epidemiologic studies on drug safety.
In our study, 34, 66, and 80 signals for dabigatran, rivaroxaban, and apixaban, respectively, were detected using pharmacovigilance disproportionality analysis from full AE reports. Because there were too many signals to evaluate, signal prioritization was needed. Therefore, we set up more specific criteria including the results of seriousness and causality assessments performed by the reporters. After prioritization, the remaining signals were more specific and included relatively serious issues associated with bleeding and NOACs. However, this method for prioritization could not detect unexpected ADRs, since the assessments of causality were performed by primary practitioners based on well-established safety information. Therefore, we are planning to apply more loose criteria of prioritization to increase the sensitivity of detecting unexpected ADRs in future studies.
Strengths and limitations
This study has several strengths. First, KIDS-KD was a suitable database for detecting signals as we applied disproportional analysis. This database contained all AE reports from 27 regional pharmacovigilance centers composed of 25 general hospitals covering all provinces in South Korea, the National Medical Center that responded to AE reports from nationwide public health care institutions, and the Korean Pharmaceutical Association that received AE reports from nationwide pharmacists. Furthermore, KIDS-KD is the most active spontaneous adverse event reporting system in the world. According to the UMC reports in 2015, South Korea was the third-highest ranked country for both total number of AE reports and reports per million inhabitants, preceded by the United States and Singapore, respectively.30 Second, we analyzed the trend of the strength of association between drug and AE on a quarterly basis. aRORs for NOACs stabilized from the second quarter of 2015 onwards and were persistently higher for rivaroxaban than for apixaban or dabigatran. This consistent aROR supports the association between bleeding risk and class of NOACs.
This study has several limitations. First, a spontaneous AE reporting system does not have denominator data. In order to estimate AE incidence, the number of AE occurrences per total amount of consumed drugs is needed. Therefore, we applied disproportionality analysis to measure different occurrence rates in the same drug classes. Second, spontaneous AE reporting systems have inadequate information on individual cases including indicated disease, comorbidities, and regimen of drugs taken. Disproportionality analysis was not performed to confirm causality assessment, but for screening the occurrence of AEs. Third, since practitioners are concerned about the safety issues of newly approved drugs, AEs of these drugs are reported relatively more frequently than those of other drugs. It is possible that AEs of newly approved drugs were reported more frequently than overall reporting of AEs. Therefore, we additionally analyzed the change in reporting rate between different NOACs, which is similar in the timing of approval and interest of practitioners. Furthermore, we performed sequential analyses to evaluate the persistency of AE reporting. Since the middle of 2015, the reporting rate of bleeding stabilized.
Conclusion
The risk of bleeding, a well-recognized AE of NOACs, was found to be similar to the results of previous studies in that rivaroxaban showed a higher risk of any bleeding than apixaban or dabigatran. This finding was persistently maintained in various databases and research methods. We have added to the evidence about bleeding risk of NOACs by disproportionality analysis using the Korean spontaneous AE databases. Further well-designed pharmacoepidemiologic studies are needed to evaluate the causality of the unexpected AEs of NOACs other than bleeding.
Supplemental Material
Supplemental material, NOAC_signal_evaluation_tables_S1_180807 for Comparison of bleeding risks among non-vitamin K antagonist oral anticoagulants using the Korea adverse event reporting system database by Young-Jin Ko, Seonji Kim, Kyounghoon Park, Minsuk Kim, Bo Ram Yang, Joongyub Lee, Mi-Sook Kim and Byung-Joo Park in Therapeutic Advances in Drug Safety
Acknowledgments
The authors greatly appreciate KIDS for providing KIDS-KD for this study.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Korea Medical Institute (KMI) Grant Program in 2017.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Young-Jin Ko  https://orcid.org/0000-0001-8473-3217
https://orcid.org/0000-0001-8473-3217
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Young-Jin Ko, Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea.
Seonji Kim, Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea.
Kyounghoon Park, Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea.
Minsuk Kim, Division of Cardiology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
Bo Ram Yang, Medical Research Collaborating Center, Seoul National University College of Medicine/Seoul National University Hospital, Seoul, Korea.
Joongyub Lee, Prevention and Management Center, Incheon Regional Cardiocerebrovascular center, Inha University Hospital, Incheon, Korea.
Mi-Sook Kim, Medical Research Collaborating Center, Seoul National University College of Medicine/Seoul National University Hospital, Seoul, Korea.
Byung-Joo Park, Department of Preventive Medicine, Seoul National University College of Medicine, 103 Daehak-ro, Jongno-gu, Seoul, 03080, Korea.
References
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22: 983–988. [DOI] [PubMed] [Google Scholar]
- 2. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383: 955–962. [DOI] [PubMed] [Google Scholar]
- 3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130: e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33: 2719–2747. [DOI] [PubMed] [Google Scholar]
- 5. Ko YJ, Kim S, Park K, et al. Impact of the health insurance coverage policy on oral anticoagulant prescription among patients with atrial fibrillation in Korea from 2014 to 2016. J Korean Med Sci 2018; 33: e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kjerpeseth LJ, Ellekjaer H, Selmer R, et al. Trends in use of warfarin and direct oral anticoagulants in atrial fibrillation in Norway, 2010 to 2015. Eur J Clin Pharmacol. Epub ahead of print 25 July 2017. DOI: 10.1007/s00228-017-2296-1. [DOI] [PubMed] [Google Scholar]
- 7. Liakoni E, Ratz Bravo AE, Terracciano L, et al. Symptomatic hepatocellular liver injury with hyperbilirubinemia in two patients treated with rivaroxaban. JAMA Intern Med 2014; 174: 1683–1686. [DOI] [PubMed] [Google Scholar]
- 8. O’Dea D, Whetteckey J, Ting N. A prospective, randomized, open-label study to evaluate two management strategies for gastrointestinal symptoms in patients newly on treatment with dabigatran. Cardiol Ther 2016; 5: 187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barrett P, Vuppalanchi R, Masuoka H, et al. Severe drug-induced skin and liver injury from rivaroxaban. Dig Dis Sci 2015; 60: 1856–1858. [DOI] [PubMed] [Google Scholar]
- 10. Montastruc JL, Sommet A, Bagheri H, et al. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol 2011; 72: 905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meyboom RH, Hekster YA, Egberts AC, et al. Causal or casual? The role of causality assessment in pharmacovigilance. Drug Saf 1997; 17: 374–389. [DOI] [PubMed] [Google Scholar]
- 12. Meyboom RH, Egberts AC, Edwards IR, et al. Principles of signal detection in pharmacovigilance. Drug Saf 1997; 16: 355–365. [DOI] [PubMed] [Google Scholar]
- 13. Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf 2001; 10: 483–486. [DOI] [PubMed] [Google Scholar]
- 14. Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf 2004; 13: 519–523. [DOI] [PubMed] [Google Scholar]
- 15. Bate A, Lindquist M, Edwards IR, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol 1998; 54: 315–321. [DOI] [PubMed] [Google Scholar]
- 16. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 17. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 18. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 19. Potpara TS, Lip GY. Postapproval Observational studies of non-vitamin K antagonist oral anticoagulants in atrial fibrillation. JAMA 2017; 317: 1115–1116. [DOI] [PubMed] [Google Scholar]
- 20. Lip GY, Keshishian A, Kamble S, et al. Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin. A propensity score matched analysis. Thromb Haemost 2016; 116: 975–986. [DOI] [PubMed] [Google Scholar]
- 21. Larsen TB, Skjoth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ 2016; 353: i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yao X, Abraham NS, Sangaralingham LR, et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc 2016; 5. pii: e003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Staerk L, Fosbol EL, Lip GYH, et al. Ischaemic and haemorrhagic stroke associated with non-vitamin K antagonist oral anticoagulants and warfarin use in patients with atrial fibrillation: a nationwide cohort study. Eur Heart J 2017; 38: 907–915. [DOI] [PubMed] [Google Scholar]
- 24. Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med 2016; 176: 1662–1671. [DOI] [PubMed] [Google Scholar]
- 25. Nielsen PB, Skjoth F, Sogaard M, et al. Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ 2017; 356: j510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noseworthy PA, Yao X, Abraham NS, et al. Direct comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in nonvalvular atrial fibrillation. Chest 2016; 150: 1302–1312. [DOI] [PubMed] [Google Scholar]
- 27. Park K, Soukavong M, Kim J, et al. Signal detection of imipenem compared to other drugs from Korea adverse event reporting system database. Yonsei Med J 2017; 58: 564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim S, Park K, Kim MS, et al. Data-mining for detecting signals of adverse drug reactions of fluoxetine using the Korea adverse event reporting system (KAERS) database. Psychiatry Res 2017; 256: 237–242. [DOI] [PubMed] [Google Scholar]
- 29. Soukavong M, Kim J, Park K, et al. Signal detection of adverse drug reaction of amoxicillin using the Korea adverse event reporting system database. J Korean Med Sci 2016; 31: 1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lundin T. Positive trends for Vigibase: 12 million reports & counting. Uppsala Rep 2016; 72: 14–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, NOAC_signal_evaluation_tables_S1_180807 for Comparison of bleeding risks among non-vitamin K antagonist oral anticoagulants using the Korea adverse event reporting system database by Young-Jin Ko, Seonji Kim, Kyounghoon Park, Minsuk Kim, Bo Ram Yang, Joongyub Lee, Mi-Sook Kim and Byung-Joo Park in Therapeutic Advances in Drug Safety