Abstract
Background:
The aim of this study was to determine whether any clinical factors are independent predictors of positive surgical margins (PSM), and to assess the association of PSM and biochemical recurrence (BR) after robot-assisted radical prostatectomy (RARP).
Methods:
The population included cases with negative surgical margins (control group) and patients with PSM (study group). Tumor grade was evaluated according to the International Society of Urologic Pathology (ISUP) system. A logistic regression model assessed the independent association of factors with the risk of PSM. The risk of BR was assessed by Cox’s multivariate proportional hazards.
Results:
A total of 732 consecutive patients were evaluated. Extend pelvic lymph node dissection (ePLND) was performed in 342 cases (46.7%). Overall, 192 cases (26.3%) had PSM. The risk of PSM was positively associated with the percentage of biopsy positive cores (BPC; odds ratio, OR = 1.012; p = 0.004), extracapsular extension (pT3a; OR=2.702; p < 0.0001), invasion of seminal vesicle (pT3b; OR = 2.889; p < 0.0001), but inversely with body mass index (OR = 0.936; p = 0.021), and high surgeon volume (OR = 0.607; p = 0.006). Independent clinical factors associated with the risk of BR were baseline prostate-specific antigen (PSA; hazard ratio, HR = 1.064; p = 0.004), BPC (HR = 1.015; p = 0.027), ISUP biopsy grade group (BGG) 2/3 (HR = 2.966; p = 0.003), and BGG 4/5 (HR = 3.122; p = 0.022). Pathologic factors associated with the risk of BR were ISUP group 4/5 (HR = 3.257; p = 0.001), pT3b (HR = 2.900; p = 0.003), and PSM (HR = 2.096; p = 0.045).
Conclusions:
In our cohort, features related to host, tumor, and surgeon volume are associated with the risk of PSM, which is also an independent parameter predicting BR after RARP. The surgical volume of the operating surgeon is an independent factor that decreases the risk of PSM, and, as such, the risk of BR.
Keywords: biochemical recurrence, positive surgical margins, prostate cancer, robot-assisted radical prostatectomy, surgical volume
Introduction
Prostate cancer (PCa) is the most common noncutaneous malignancy, and the second leading cause of cancer-related deaths in men.1 Once PCa has been diagnosed and staged, the urologist will council the patient in order to decide the most appropriate management, which includes active surveillance (AS), radical prostatectomy (RP), and radiation therapy (RT).2,3 In developed countries, RP is most frequently performed by the robot-assisted (RARP) approach. An unfavorable outcome after RARP is the detection of positive surgical margins (PSM), which is an important negative prognostic factor for cancer locoregional recurrence.2,3 So far, patients who show unfavorable pathologic outcomes in the surgical specimen, including high-grade tumors with disease extending beyond the prostate, and PSM need accurate counseling for further management options that include immediate RT (after recovery of the urinary function) or close PSA monitoring with salvage RT before PSA approaches values of 0.5 ng/ml.2,3 These issues impair the quality of life of affected patients because of anxiety as well as the toxicities related to adjuvant or salvage treatments, which may include androgen blockade.2,3 PSM after RARP may be related to tumor biology or to the physician’s surgical experience.3–10 In high-volume centers, the rates of PSM were similar among surgeons with similar surgical volumes.9
In a contemporary cohorts of patients, it is important to evaluate factors associated with the risk of PSM after RARP because, when a PSM is detected, the next step is to decide whether adjuvant treatments should be delivered in order to reduce the risk of biochemical recurrence (BR). From this perspective, it is important to evaluate the actual effect of PSM as well as other clinical and pathological parameters on the risk of BR after RARP.
The aim of this study was to test the hypothesis that PSM among other clinical parameters impacts the risk of BR after RARP in a contemporary cohort of patients.
Materials and methods
Study features
The present study is a retrospective analysis of prospectively collected data. It was approved by the Institutional Review Board and included a period ranging from January 2013 to December 2017. Each patient provided informed-signed consent for data collection and analysis. Low, intermediate, and high risk, and locally advanced patients were included in the study if the clinical T stage was ⩽T3b and the prostate volume was ⩽80 cc. Patients with previous surgical prostate treatments, with cT4 stage or metastatic disease or who were under androgen blockade or had prior treatments were excluded. Patients with pT2+ (defined as ‘positive margins in the setting of intra-prostatic or intra-tumoral incision’) according to the Stanford protocol, were excluded.11
Clinical features
Preoperatively, patients were evaluated for age (years) body mass index (BMI; kg/m2) and plasma levels of PSA (ng/ml), which were determined by radioimmunoassay methods. Prostate biopsies had the following features: at least 12–14 cores; reported number of positive cores; measurement of prostate volume (TPV; ml); and cancer grade group classification according to the 2014 International Society of Urologic Pathology (ISUP) system.12 In each case, the percent of positive cores (BPC; percentage) was computed. Patients were clinically staged according the European Society of Urology (EAU) guidelines.2 Tumors were staged by digital rectal exam (DRE) or by multiparametric resonance imaging (mMRI). Pelvic lymph nodes were assessed by computed tomography (CT) or by multiparametric resonance imaging (mpMRI). Enlarged pelvic nodes measuring more than 1 cm in diameter were staged as cN1. The metastatic status was investigated by CT or mMRI as well as by total bone scan. Patients were then classified into risk groups according to the EAU guidelines on PCA.2
Perioperative features
RARP was executed by the da Vinci Robot System (Intuitive Surgical, Inc, Sunnyvale, CA, USA) and performed through the transperitoneal approach with anterograde prostatic dissection.13 The decision to perform an extended lymph node dissection (ePLND) was taken when the risk of lymph node invasion (LNI) was greater than 5%.14 In low-risk patients, the decision to perform an ePLND was based and clinical factors indicating increased risk of tumor upgrading in the surgical specimen.15–19 When indicated, ePLND was performed according to an anatomical template including bilateral external iliac (extending proximally to the crossing of the ureter), obturator, Marcille’s, common iliac, and Cloquet’s nodal stations. The external iliac LN group was dissected laterally to the genitofemoral nerve at the lateral edge of the internal iliac artery and vein from the node of Cloquet to the ureteric crossing of the internal iliac artery, as reported previously.20,21
Nerve sparing RP (NSRP) was performed when indicated.15 When the nerve sparing technique was used, clinical stage, cancer localization, and its proximity to the capsule, were recorded. In particular, NSRP surgery was performed by the intrafascial or interfascial technique. Extrafascial dissection was performed when nerve sparing was not indicated.22 Five experienced surgeons performed RARP with a bladder neck sparing technique.23 Surgeon experience was defined according to a previous publication that reported that among surgeons with > 30 RARP procedures, there was no difference in PSM rates.24 All surgeons had completed the RARP learning curve before the beginning of patient enrolment. Our high-volume experienced surgeon had performed more than 500 RARPs; our other four low-volume experienced surgeons had performed between 50 and 60 RARPs. A single high-volume experienced surgeon (WA) performed two-thirds of the procedures in our dataset. Preoperatively, patients were evaluated for surgical risk by the American Anesthesiologists Score (ASA) system.25 Intra-operatively, operating time (OT, minutes) and blood lost (BL, milliliters) were measured. Postoperatively, length of hospital stay (LOHS) was recorded in each patient. Patients were followed for a period of 6 months in order to detect hospital readmission and complications that were classified according to the Clavien–Dindo classification system.26
Pathological features
The dedicated pathologists prepared surgical specimens according to the Stanford protocol.21 Prostate weight (PW, grams) was calculated. Tumors were classified according to the ISUP grade group (PGG) system.12 Nodal packets were grouped according to a standard template and submitted in separate packages. Lymph nodes were assessed for histopathology after hematoxylin and eosin staining. Immunohistochemistry staining was performed when appropriate. In each case, the number of removed and metastatic nodes was computed. Specimens were staged as suggested according to EAU guidelines on PCA.2
Surgical margins were defined as positive when cancer invaded the inked surface of the specimen. Locations were coded as follows: apical, posterior–lateral (left and right), posterior, anterior, and bladder neck. The pathologist evaluated the linear extent of PSM according to a qualitative pattern, which stratified positive surgical margins into two groups as focal and nonfocal. Accordingly, PSM were classified as focal when the linear extent was less than or equal to 1 mm, and nonfocal otherwise. In this report, we did not consider analysis related to stratification of PSM according to linear extent.
Follow up
Follow up, adjuvant treatments, and BR after RARP were evaluated according to EAU standard criteria.2,3 Overall, 580 out of 732 (79%) were available for follow up. Patients with follow up shorter than 4 months were excluded. Overall, BR was evaluated in 458 patients (79%). The median (IQR) follow up was 26 (14–40) months.
Study design
The aim of the study was to verify the hypothesis that qualitative stratification of surgical margins (PSM versus negative) might have different prognostic potential on BR in modern cohorts of patients undergoing RARP. The association of independent clinical parameters with PSM outcome was first evaluated. The association of factors with the risk of BR was then assessed.
Statistical analysis
Factors associated with PSM
Patients were classified into two groups according to PSM (PSM versus control). Summary statistics and distributions of factors between groups were assessed. Data on continuous variables are reported as medians with their respective interquartile ranges (IQRs). Data on categorical variables are presented as frequencies with relative percentages. Associations of factors between groups were analyzed by Mann–Whitney U test for continuous variables and by the Pearson’s chi-squared test or Fisher exact test as appropriate for the categorical ones. Significant factors were entered into the multivariate model. The logistic regression model evaluated the association of factors with the risk of PSM.
Factors associated with BR
Patients were classified into two groups according to BR (BR versus control). Summary statistics and distributions of factors between groups were assessed. Data on continuous variables are reported as medians with their respective IQRs. Data on categorical variables are presented as frequencies with relative percentages. Associations of factors with the risk of BR were first evaluated by univariate Cox proportional hazard model. Significant parameters were entered into the multivariate Cox proportional hazard model in order to detect independent factors associated with the risk of BR.
The software used to run the analysis was IBM-SPSS version 20. All tests were two-sided with a p value < 0.05 indicating statistical significance.
Results
Independent factors associated with the risk PSM
The overall study cohort included 732 patients whose demographics are reported in Table 1. Of the patient population, 34.2% was low risk, 50.1% intermediate risk, and 15.7% high risk or locally advanced according to EAU classification.2 In the surgical specimen, extra prostatic extension was present in 21.9% of cases and showed high-grade disease (PGG 4-5) in 19.5% of subjects. Extend pelvic lymph node dissection was performed in 342 cases; among these, lymph node invasion was detected in 49 cases (14.3%) and 293 (85.7%) were pN0. Among the remaining 390 patients, pathological N stage was not investigated. The median number of dissected nodes was 26. The high-volume surgeon performed 66.1% of the procedures. Nerve sparing surgery was performed in 82% of cases. Major complications (CDS > 2) were detected in 2.9% of cases. Overall, 192 subjects had PSM (26.3%). The association of factors with the risk PSM has been previously reported.27 Table 2 shows independent factors associated with the risk of PSM compared with controls. BMI, BPC, pathologic stage, and high-volume surgeon were independent predictors of PSM; moreover, the association was inverse for BMI (odds ratio, OR = 0.936; p = 0.021) and high-volume surgeon (OR = 0.607; p = 0.006) as well as positive for BPC (OR = 1.012; p = 0.004), pT3a (OR = 2.702; p < 0.0001) and pT3b (OR = 2.889; p < 0.0001).
Table 1.
Clinical, pathologic and perioperative factors in 732 patients who underwent RARP.
| Clinical Factors | |
|---|---|
| Age, years; median (IQR) | 65 (60–69) |
| BMI, kg/m2; median (IQR) | 25.8 (23.8–28) |
| PSA, ng/ml; median (IQR) | 6.3 (4.9–8.7) |
| TPV, ml; median (IQR) | 39 (30–50) |
| BPC, %; median (IQR) | 29 (17–45.7) |
| cT | |
| cT1c; n (%) | 517 (70.6) |
| cT2; n (%) | 194 (26.5) |
| cT3; n (%) | 21 (2.9) |
| cN | |
| cN0; n (%) | 710 (97) |
| cN1; n (%) | 22 (3) |
| BGG | |
| BGG 1, n (%) | 343 (46.9) |
| BGG 2–3, n (%) | 315 (43) |
| BGG 4–5, n (%) | 74 (10.1) |
| Pathological Factors | |
| PW (g); median (IQR) | 50 (41–63) |
| Dissected nodes; median (IQR) | 26 (21–33) |
| PGG | |
| PGG 1; n (%) | 126 (17.2) |
| PGG 2–3; n (%) | 463 (63.3) |
| PGG 4–5; n (%) | 143 (19.5) |
| pT | |
| pT2; n (%) | 572 (78.1) |
| pT3a; n (%) | 77 (10.5) |
| pT3b; n (%) | 83 (11.4) |
| pN | |
| pN0; n (%) | 293 (40) |
| pNx; n (%) | 390 (53.3) |
| pN1; n (%) | 49 (6.7) |
| PSM | |
| No PSM; n (%) | 540 (73.8) |
| PSM; n (%) | 192 (26.2) |
| Perioperative Factors | |
| OT (min); median (IQR) | 200 (160–240) |
| BL (ml); median (IQR) | 300 (200–500) |
| no ePLND; n (%) | 390 (53.3) |
| ePLND; n (%) | 342 (46.7) |
| NSS | |
| No NSS; n (%) | 87 (11.9) |
| NSS; n (%) | 600 (82) |
| Unknown NSS; n (%) | 45 (6.1) |
| Surgeon | |
| Surgeon low volume; n (%) | 248 (33.9) |
| Surgeon high volume; n (%) | 484 (66.1) |
| ASA score | |
| ASA 1–2; n (%) | 675 (92.2) |
| ASA 3–4; n (%) | 57 (7.8) |
| LOHS | |
| days; median (IQR) | 4 (4–6) |
| CDS | |
| CDS 0; n (%) | 557 (76.1) |
| CDS 1–2; n (%) | 154 (21) |
| CDS > 2; n (%) | 21 (2.9) |
| No readmission; n (%) | 711 (97.1) |
| Readmission; n (%) | 21 (2.9) |
ASA, American Score of Anaestesiologists; BL, blood lost; BGG, biopsy grade group; BMI, body mass index; BPC, biopsy positive cores; CDS, Clavien–Dindo score; cN, clinical nodal stage; cT, clinical tumor stage; ePLND, extended pelvic lymph node dissection; IQR, interquartile range; LOHS, length of hospital stay; NSS, nerve sparing surgery; OT, operating time; PGG, pathology grade group; pN, pathologic nodal stage; PSA, prostate-specific antigen; PSM, positive surgical margins; pT, pathologic tumor stage; PW, prostate weight; RARP, robot-assisted radical prostatectomy; TPV, total prostate volume.
Table 2.
Independent factors associated with the risk of positive surgical margins in 732 patients who underwent robot-assisted radical prostatectomy (RARP).
| Factors | Population | Surgical margins |
Multivariate analysis (*) |
||
|---|---|---|---|---|---|
| negative | positive | OR (95% CI) | p value | ||
| n (%) | 732 | 540 (73.8) | 192 (26.2) | Overall model (**) | |
| BMI, kg/m2; median (IQR) | 25.8 (23.8–28) | 26 (24–28) | 25.2 (23.4–27.8) | 0.936 (0.886–0.990) | 0.021 |
| BPC, %; median (IQR) | 29 (17–45.7) | 28 (17–42) | 33 (21–50) | 1.012 (1.004–1.020) | 0.004 |
| pT2; n (%) | 572 (78.1) | 453 (83.9) | 28 (47.5) | Ref | |
| pT3a; n (%) | 77 (10.5) | 43 (8) | 14 (23.7) | 2.702 (1.631–4.474) | <0.0001 |
| pT3b; n (%) | 83 (11.4) | 44 (8.1) | 17 (28.8) | 2.889 (1.752–4.765) | <0.0001 |
| Surgeon low volume; n (%) | 248 (33.9) | 168 (31.1) | 80 (41.7) | Ref | |
| Surgeon high volume; n (%) | 484 (66.1) | 372 (68.9) | 112 (58.3) | 0.607 (0.425–0.865) | 0.006 |
See Table 1; IQR, interquartile range; OR, odds ratio; CI, confidence interval; (*), overall model of independent factors; (**); adjusted OR.
Independent factors associated with the risk of BR
The study population included 458 patients whose demographic details are reported in Table 3. Median (IQR) follow up was 26 (14–40) months. Risk class distribution was low risk in 158 patients (34.5%), intermediate risk in 228 (49.8%) and high risk/locally advanced in 72 (15.7%). Extended PLND was performed in 217 subjects (47.4%). The median number (IQR) of removed nodes was 26 (21–33). Median (IQR) LOHS was 4 (4–6) days. Hospital readmission was reported in 16 (3.5%) patients. Adjuvant RT was delivered in 31 cases (6.8%) and salvage RT in 9 (2.2%). Androgen deprivation therapy (ADT) was given in 48 cases (10.5%). All patients were alive at time of censoring. Adjuvant RT was more frequently delivered in patients with BR (11 cases; 27.5%) than controls (20 subjects; 4.8%). Adjuvant androgen blockade was more frequently delivered in patients with BR (10 cases; 25%) than controls (23 cases; 5.5%). Adjuvant androgen blockade was administrated alone or combined treatment in 15 cases (37.5%) that recurred. BR was associated with imaging recurrence in 15 patients (37.5%), which included retroperitoneal lymph nodes involvement in 6 patients (40%), bone metastases in 5 patients (33.4%), visceral metastases in 2 patients (13.3%), and bladder neck invasion in 2 patients (13.3%).
Table 3.
Associations of factors with the risk of biochemical recurrence after robot-assisted radical prostatectomy in 458 cases.
| Factors | Population | Biochemical recurrence |
Univariate analysis (*) |
Multivariate analysis (*) |
|||
|---|---|---|---|---|---|---|---|
| no | yes | HR (95% CI) | p value | HR (95% CI) | p value | ||
| n (%) | 458 | 418 (91.3) | 40 (8.7%) | ||||
| Clinical Factors | Clinical model | Clinical model | |||||
| Age, years; median (IQR) | 65 (60–69) | 65 (60–69) | 65 (60–69) | 1.023 (0.970–1.079) | 0.405 | ||
| BMI, kg/m 2; median (IQR) | 25.6 (23.5–27.8) | 25.8 (23.8–27.8) | 25.2 (23.5–27.5) | 0.921 (0.826–1.027) | 0.140 | ||
| PSA, ng/mL; median (IQR) | 6.2 (4.7–8.7) | 6.1 (4.7–8.2) | 8.3 (5.2–12.3) | 1.090 (1.050–1.132) | <0.0001 | 1064 (1.020–1.110) | 0,004 |
| TPV, mL; median (IQR) | 39 (30–49.5) | 39 (29.7–50) | 35 (30–44) | 0.984 (0.961–1.008) | 0.984 | ||
| BPC, %; median (IQR) | 29 (17–43) | 28.5 (17–42) | 43 (20–56.7) | 1.021 (1.007–1.035) | 0.003 | 1.015 (1.002–1.029) | 0,027 |
| cT1c; n (%) | 317 (69.2) | 291 (69.6) | 26 (65) | Ref | 0.232 | ||
| cT2; n (%) | 128 (27.9) | 116 (27.8) | 12 (30) | Ref | |||
| cT3; n (%) | 13 (2.8) | 11 (2.6) | 2 (5) | 2.256 (0.663–11.448) | 0.163 | ||
| cN0; n (%) | 444 (96.9) | 404 (96.7) | 40 (100) | Ref | |||
| cN1; n (%) | 14 (3.1) | 14 (3.3) | 0 | 0.447 (0.000–159.073) | 0.462 | ||
| BGG 1, n (%) | 220 (48) | 208 (49.8) | 12 (30) | Ref | Ref | ||
| BGG 2–3, n (%) | 190 (41.5) | 170 (40.6) | 20 (50) | 3.023 (1.473–6.203) | 0.003 | 2.966 (1.441–6.106) | 0,003 |
| BGG 4–5, n (%) | 48 (10.5) | 40 (9.6) | 8 (20) | 5.156 (2.096–12.683) | <0.0001 | 3.122 (1.176–8.289) | 0,022 |
| Pathological Factors | Pathological model | Pathological model | |||||
| PW, gr; median (IQR) | 50 (41–63) | 50 (41–63) | 50.5 (42.5–68.7) | 1.009 (0.991–1.027) | 0.314 | ||
| PGG 1; n (%) | 73 (15.9) | 72 (17.2) | 1 (2.5) | Ref | Ref | ||
| PGG 2-3; n (%) | 296 (64.7) | 277 (66.2) | 19 (47.5) | 5.984 (0.801–44.719) | 0.081 | 4.478 (0.591–33.954) | 0,147 |
| PGG 4-5; n (%) | 89 (19.4) | 69 (16.6) | 20 (50) | 23.740 (3.184–177.025) | 0.002 | 3.257 (1.656–6.406) | 0,001 |
| pT2; n (%) | 359 (78.4) | 341 (81.6) | 18 (45) | Ref | Ref | ||
| pT3a; n (%) | 47 (10.3) | 40 (9.6) | 7 (17.5) | 2.968 (1.239–7.108) | 0.015 | 1.611 (0.647–4.011) | 0,305 |
| pT3b; n (%) | 52 (11.4) | 37 (8.9) | 15 (37.5) | 6.317 (3.163–12.614) | <0.0001 | 2.900 (1.440–5.838) | 0,003 |
| SM negative; n (%) | 344 (75.1) | 321 (76.8) | 23 (57.1) | Ref | Ref | ||
| SM positive; n (%) | 114 (24.9) | 97 (23.2) | 17 (42.5) | 2.041 (1.051–3.963) | 0.035 | 2.287 (1.197–4.370) | 0,012 |
| pN0; n (%) | 188 (41) | 171 (40.9) | 17 (42.5) | Ref | Ref | ||
| pNx; n (%) | 241 (52.6) | 224 (53.6) | 17 (42.5) | Ref | Ref | ||
| pN1; n (%) | 29 (6.3) | 23 (5.5) | 6 (15) | 4.333 (1.809–10.379) | 0.001 | 1.251 (0.479–3.268) | 0,647 |
| Perioperative Factors | Peri-operative model | ||||||
| OT, minutes; median (IQR) | 205 (162.2–240) | 205 (162.2–240) | 210 (161.2–244.5) | 1.005 (0.999–1.011) | 0.080 | ||
| BL, mL; median (IQR) | 300 (200–500) | 300 (200–500) | 325 (150–500) | 0.999 (0.998–1.000) | 0.157 | ||
| No NSS; n (%) | 52 (11.4) | 50 (12) | 2 (5) | Ref | |||
| Unknown NSS; n (%) | 14 (3.1) | 13 (3.1) | 1 (2.5) | Ref | |||
| NSS; n (%) | 392 (85.6) | 355 (84.9) | 37 (92.5) | 1.182 (0.363–3.847) | 0.702 | ||
| Surgeon low volume; n (%) | 151 (33) | 142 (34) | 9 (22.5) | Ref | |||
| Surgeon high volume; n (%) | 307 (67) | 276 (66) | 31 (77.5) | 0.734 (1.542–3.241) | 0.253 | ||
| ASA 1–2; n (%) | 427 (93.3) | 391 (93.5) | 36 (90) | Ref | |||
| ASA 3–4; n (%) | 31 (6.7) | 27 (6.5) | 4 (10) | 1.249 (0.444–3.513) | 0.674 | ||
| CDS 0; n (%) | 352 (76.9) | 322 (77) | 30 (75) | Ref | |||
| CDS 1-2; n (%) | 95 (20.7) | 86 (20.6) | 9 (22.5) | 1.552 (0.735–3.276) | 0.249 | ||
| CDS > 2; n (%) | 11 (2.4) | 10 (2.4) | 1 (2.5) | 1.557 (0.211–11.476) | 0.664 | ||
See Table 1 for abbreviations; IQR, interquartile range; HR, hazard ratio; CI, confidence interval; (*), Cox proportional hazards.
Differences between groups are detailed in Table 3. As shown, BR occurred in 40 patients (8.7%). The distribution of risk classification between groups was significant and was as follows: low risk 7 (17.5%) versus 151 (36.1%), intermediate risk 21 (52.5%) versus 207 (49.5%), and high risk/locally advanced risk 12 (30%) versus 60 (14.4%) for BR versus control groups, respectively (data not shown). Patients who had BR had higher rates of aggressive disease than controls who had higher rates of low risk disease. Extended PLND was performed in 23 (57.5%) patients with BR and in 194 (46.4%) cases in the control group, but the difference was not significant (p = 0.180), and neither was the median number of removed nodes (p = 0.095).
On univariate analysis, clinical factors associated with the risk of BR were prostate-specific antigen (PSA; hazard ratio, HR = 1.090; p < 0.0001), BPC (HR = 1.021; p = 0.003), BGG 2/3 (HR = 3.023; p = 0.003), and BGG 4/5 (HR = 5.156; p < 0.001). Pathological factors associated with the risk of BR were PGG 4/5 (HR = 23.740; p = 0.002), pT3a (HR = 2.968; p = 0.015), pT3b (HR = 6.317; p < 0.0001), PSM (HR = 2.041; p = 0.035), and pN1 (HR = 4.333; p = 0.001). Perioperative parameters did not show any significant association.
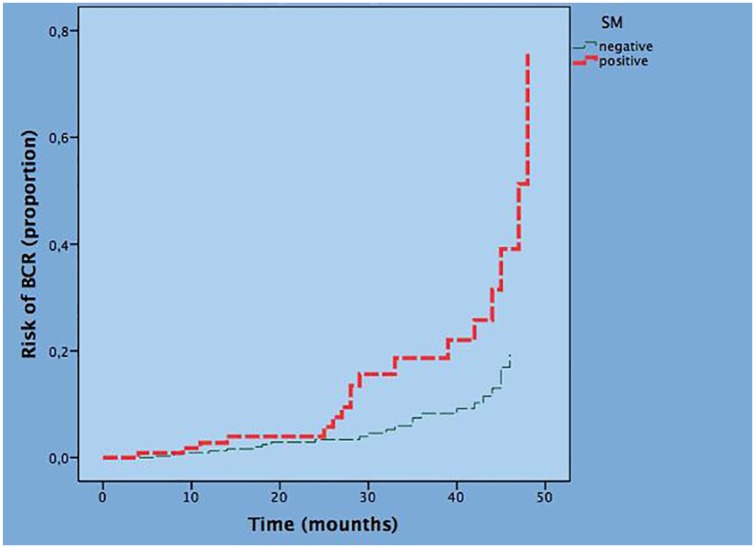
Multivariate analysis confirmed PSA, BPC, BGG 2/3 and BGG 4/5 as independent predictors of BR for clinical parameters as well as PGG 4/5, pT3b and PSM for pathological parameters while PGG 2/3, pT3a and pN1 lost significance. The final multivariate model of clinical and pathological factors associated with the risk of BR with adjusted HR is reported in Table 4. Considering clinical parameters, PSA (HR = 1.064; p = 0.004), BPC (HR = 1.015; p = 0.027), BGG 2/3 (HR = 2.966; p = 0.003), and BGG 4/5 (HR = 3.122; p = 0.022) are independent predictors of the risk of BR. Considering pathological parameters, PGG 4/5 (HR = 3.257; p = 0.001), pT3b (HR = 2.900; p = 0.003), and PSM (HR = 2.096; p = 0.045) were independent predictors of the risk of BR. Figure 1 depicts the risk curves of BR stratified by PSM; as shown, the risk of BR is increased by the presence of PSM.
Table 4.
Final multivariate models of factors associated with the risk of biochemical recurrence after robot-assisted radical prostatectomy in 458 cases.
| Factors | Multivariate analysis (Cox proportional hazards) |
||
|---|---|---|---|
| HR | 95%CI | p-value | |
| Clinical model | |||
| PSA | 1.064 | 1.020–1.110 | 0.004 |
| BPC | 1.015 | 1.002–1.029 | 0.027 |
| BGG 1 | Ref | ||
| BGG 2-3 | 2.966 | 1.441–6.106 | 0.003 |
| BGG 4-5 | 3.122 | 1.176–8.289 | 0.022 |
| Pathological model | |||
| PGG 1 | Ref | ||
| PGG 2-3 | Ref | ||
| PGG 4-5 | 3.194 | 1.575–6.058 | 0.001 |
| pT2 | Ref | ||
| pT3a | Ref | ||
| pT3b | 3.091 | 1.575–6.058 | 0.001 |
| Negative surgical margin | Ref | ||
| Positive surgical margin | 2.287 | 1.197–4.370 | 0.012 |
| PSA-Pathological combined model | |||
| PSA | 1.058 | 1.015–1.102 | 0.007 |
| PGG 1-3 | Ref | ||
| PGG 4-5 | 1.961 | 0.911–4.222 | 0.085 |
| pT2 | Ref | ||
| pT3a | Ref | ||
| pT3b | 2.631 | 1.210–5.719 | 0.015 |
| Negative surgical margin | Ref | ||
| Positive surgical margin | 2.401 | 1.119–5.018 | 0.020 |
| Final PSA-Pathological combined model (*) | |||
| PSA | 1.066 | 1.024–1.109 | 0.002 |
| pT2-3a | Ref | ||
| pT3b | 3.053 | 1.428–6.525 | 0.004 |
| Negative surgical margin | Ref | ||
| Positive surgical margin | 2.680 | 1.312–5.476 | 0.007 |
See Table 1 for abbreviations; (*) adjusted HR.
Figure 1.
Risk curve of BR by surgical margins status.
BR, biochemical recurrence.
We also evaluated the association between PSA and pathological factors in the prediction of BR and we found that PSA (HR = 1.058; p = 0.007), PSM (HR = 2.401, p = 0.020), and pT3b (HR = 2.631, p = 0.015) were independent predictors of BR. In addition, when the statistically significant factors were compared, all remained significant (see Table 4 ‘final PSA-pathological factors combined model’).
Discussion
Factors associated with the risk of PSM
In large contemporary series, PSM rates after RARP range from 15% to 29.5%.28–34 Surgery and tumor biology are factors that are associated with a PSM; the former is related to technique and surgeon’s experience, while the latter depends on the stage and grade of the tumor.3–10 The risk of PSM after RARP has been associated with clinical and pathological factors.28–30
In our study, PSM rates were 26.2%, which confirmed findings in the literature; moreover, similar clinical and pathological predictors of PSM were also reported by other studies. In our previous experience, we found that higher preoperative total testosterone serum levels were predictive of positive surgical margins after RP.35 However, unusual factors, including BMI and operative load of experienced surgeons, emerged as independent parameters associated with the risk of PSM. These findings represent a novelty and need to be explained. The influence of BMI during RARP is unclear, controversial, and the subject has been investigated to show that the association might be absent or positive.32,36–38 We previously found that BMI is associated with major postoperative complications after RARP.19 Patel and associates suggest that the positive association between BMI and PSM might be related to both reduced vision and angle movements during RARP in obese patients.29 The present study shows that higher BMI is an independent factor that is associated with a reduced risk of PSM. This might be explained by periprostatic fat tissue thickness, which is more represented in obese patients, who are then less likely to have focal PSM during RARP. Although this hypothesis needs to be verified, it is supported by a study showing a significant correlation between BMI and periprostatic fat thickness (r = 0.37), which was measured by CT scans.39 Our study has shown that, in a high-volume center, the high-volume experienced surgeon specifically and independently decreased the risk of PSM. The operating load of the experienced surgeon is an important parameter, which is ongoing and being amplified in robotic surgery. Indeed, a systematic review of the literature concerning the volume–outcome relationship for RP has studied the subject dealing with surgeon volume and oncological outcomes.40 The review has shown that overall oncological outcomes are improved by increasing surgeon volume. Hu and associates have shown that patients operated by high-volume surgeons were less likely to undergo salvage therapy after RARP.41 Moreover, Steinsvik and associates demonstrated that high-volume surgeons reduced overall risk of PSM after RARP.42 The identification of high-volume surgeons in high-volume centers might be a point to consider when counseling patients before RARP. With both BMI and high-load experienced surgeon emerging as independent factors together with other known parameters representing a novel finding, this association needs to be confirmed by further studies.
Factors associated with the risk of BR
When RARP is performed with radical intent, PSA levels are thought to decrease to undetectable levels according to EAU guidelines on PCA.2,3 However, although PSA levels may decline to undetectable levels, unfavorable pathological outcomes after RARP, including extracapsular extension, seminal vesicle invasion, high PGG, and PSM, may surface.2,3 Indeed, all these parameters are associated with an increased risk of BR.3–10 On the other hand, the detection of PSM with or without other pathological features in combination with detectable PSA levels after surgery is an even more pertinent issue because further treatments are mandatory.2,3
Considering modern cohorts of patients who underwent RARP, few studies consider specifically the role of PSM as one of the several parameters able to predict BR after undetectable PSA.32–34 Rajan and colleagues reported PSM rates of 23.1% with BR occurring in 18.9% of cases; however, factors predicting PSM were not assessed, and parameters associated with the risk of BR were evaluated instead.32 In this study, the authors found that independent parameters associated with the risk of BR were baseline PSA > 10 ng/ml and BGG 1>1 for clinical factors as well as pT3a, pT3b, and PSM > 3 mm or multifocal for pathological factors. Jo and coworkers reported PSM rates of 20.5%, with BR detected in 18.7% of patients; as in the previous study, factors predicting PSM were not evaluated but factors predicting BR were assessed instead.34 The authors reported independent factors associated with the risk of BR were age, cT > 2, PSA > 10 ng/ml, BGG > 1, BPC > 50% among clinical factors as well as extracapsular extension, seminal vesicle invasion, and PSM at the apex among pathological factors in the study. So far, in these two studies, the assessment of PSM was one of the independent pathological factors predicting the risk of BR. In our study, we detected BR rates of 8.7% with basal PSA, BPC, BGG 2/3, and BGG 4/5 as independent clinical predictors as well as extracapsular extension, seminal vesicle invasion, and PSM as pathological independent predictors of BR. Further, when we combined PSA with pathological factors, PSA, PSM, and pT3b persisted as independent predictors of BR. BMI and high-volume experienced surgeon did not predict the occurrence of BR, probably because they were not directly associated with such risk, but indirectly instead by lowering the rates of PSM, which were independently and directly associated with BR, as previously shown.
The main features that differentiate our study from the two previously mentioned studies include the contemporaneous evaluation of factors associated with the risk of both PSM and BR. Our study shows that the high-volume experienced surgeons can reduce the risk of PSM after RARP in high-volume centers, and thus avoid treatments related to managing this unfavorable event. This information is important when counseling patients who are specifically concerned about their surgeon’s experience and operative volume and how it relates to their oncologic outcomes as well as PSM rates. Overall, these results represent a new way to approach robotic surgery in PCA patients, and, as such, it is a novelty, which differentiates our study from other contemporary series. However, further confirmatory studies are required.
Strengths and limitations and of the study
While our study has strengths, it also presents several limitations. First, although data was collected prospectively, it is retrospective and thus suffers from all the limitations related to this type of study. Second, follow-up was limited. Third, 152 (21%) of patients were lost during follow up because many patients traveled to our tertiary center from long distances and some patients chose to continue their follow up with their local physician. Despite multiple attempts to contact them, many remained unreachable. Third, prostate biopsies performed at outside institutions were not re-evaluated; however, their features had good standard quality to support their analysis. However, beyond these limits, our study has also many strengths, which include the large contemporary cohort of patients in a high-volume center, and all specimens being evaluated by a dedicated pathologist.
Conclusion
In high-volume centers, features related to host, tumor, and experienced surgeon volume are pivotal factors associated with the risk of PSM, which is also an independent parameter predicting BR after RARP. A high-volume experienced surgeon is an independent factor that decreases the risk of PSM and therefore the risk of BR. This issue is pivotal when counseling patients who elect to undergo robotic surgery as primary active treatment for PCA.
Acknowledgments
Antonio Benito Porcaro and Alessandro Tafuri contributed equally.
Footnotes
Author contributions: ABP performed project development, data Analysis and interpretation, and manuscript writing. AT performed project development, data collection, data analysis and interpretation, and manuscript writing. MS, MP, TP, NA, RR, PC, LT, and CC performed data collection. AS performed data collection, English language and critical revision, and manuscript writing. FM, GN, MB, VDM, SS, and WA performed supervision and critical revision.
Funding: The author(s) received no financial support for the research, authorship, and publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
ORCID iDs: Antonio Benito Porcaro  https://orcid.org/0000-0002-7890-040X
https://orcid.org/0000-0002-7890-040X
Alessandro Tafuri  https://orcid.org/0000-0003-1404-2925
https://orcid.org/0000-0003-1404-2925
Contributor Information
Antonio Benito Porcaro, Department of Urology Azienda Ospedaliera Universitaria Integrata Verona, Piazzale Stefani 1, Verona, 37126, Italy.
Alessandro Tafuri, Department of Urology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy; USC Institute of Urology, and Catherine & Joseph Aresty Department of Urology, University of Southern California, Los Angeles, CA, USA.
Marco Sebben, Department of Urology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
Nelia Amigoni, Department of Urology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
Tania Processali, Department of Urology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
Marco Pirozzi, Department of Urology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
Riccardo Rizzetto, Department of Urology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
Aliasger Shakir, USC Institute of Urology, and Catherine & Joseph Aresty Department of Urology, University of Southern California, Los Angeles, CA, USA.
Paolo Corsi, Department of Urology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
Leone Tiso, Department of Urology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
Clara Cerrato, Department of Urology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
Filippo Migliorini, Department of Urology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
Giovanni Novella, Department of Urology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
Matteo Brunelli, Department of Pathology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
Riccardo Bernasconi, Department of Pathology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
Vincenzo De Marco, Department of Urology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
Salvatore Siracusano, Department of Urology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
Walter Artibani, Department of Urology, University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Mottet N, Bellmunt J, Bolla M, et al. Cornford, EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2017; 71: 618–629. [DOI] [PubMed] [Google Scholar]
- 3. Artibani W, Porcaro AB, De Marco V, et al. Management of biochemical recurrence after primary curative treatment for prostate cancer: a review. Urol Int 2017; 100: 251–262. [DOI] [PubMed] [Google Scholar]
- 4. Yossepowitch O, Bjartell A, Eastham JA, et al. Positive surgical margins in radical prostatectomy: outlining the problem and its long-term consequences. Eur Urol 2009; 55: 87–99. [DOI] [PubMed] [Google Scholar]
- 5. Yossepowitch O, Briganti A, Eastham JA, et al. Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol 2014; 65: 303–313. [DOI] [PubMed] [Google Scholar]
- 6. Sooriakumaran P, Dev HS, Skarecky D, et al. The importance of surgical margins in prostate cancer. J Surg Oncol 2016; 113: 310–315. [DOI] [PubMed] [Google Scholar]
- 7. Fleshner NE, Evans A, Chadwick K, et al. Clinical significance of the positive surgical margin based upon location, grade, and stage. Urol Oncol 2010; 28: 197–204. [DOI] [PubMed] [Google Scholar]
- 8. Fontenot PA, Mansour AM. Reporting positive surgical margins after radical prostatectomy: time for standardization. BJU Int 2013; 111: E290–E299. [DOI] [PubMed] [Google Scholar]
- 9. Weizer AZ, Strope S, Wood DP. Margin control in robotic and laparoscopic prostatectomy: what are the REAL outcomes? Urol Oncol 2010; 28: 210–214. [DOI] [PubMed] [Google Scholar]
- 10. Meeks JJ, Eastham JA. Radical prostatectomy: positive surgical margins matter. Urol Oncol 2013; 31: 974–979. [DOI] [PubMed] [Google Scholar]
- 11. Srigley JR, Humphrey PA, Amin MB, et al. ; Members of the Cancer Committee, College of American Pathologists. Protocol for the examination of specimens from patients with carcinoma of the prostate gland. Arch Pathol Lab Med 2009; 133: 1568–1576. [DOI] [PubMed] [Google Scholar]
- 12. Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016; 40: 244–252. [DOI] [PubMed] [Google Scholar]
- 13. Menon M, Tewari A, Peabody J; VIP Team. Vattikuti institute prostatectomy: technique. J Urol 2003; 169: 2289–2292. [DOI] [PubMed] [Google Scholar]
- 14. Briganti A, Larcher A, Abdollah F, et al. Updated nomogram predicting lymph mode invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol 2012; 61: 480–487. [DOI] [PubMed] [Google Scholar]
- 15. Porcaro AB, Siracusano S, de Luyk N, et al. Low-risk prostate cancer and tumor upgrading in the surgical specimen: analysis of clinical factors predicting tumor upgrading in a contemporary series of patients who were evaluated according to the modified Gleason score grading system. Curr Urol 2017; 10: 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Porcaro AB, de Luyk N, Corsi P, et al. Bilateral lymph node micrometastases and seminal vesicle invasion associated with same clinical predictors in localized prostate cancer. Tumori 2017; 103: 299–306. [DOI] [PubMed] [Google Scholar]
- 17. Porcaro AB, de Luyk N, Corsi P, et al. Clinical factors predicting and stratifying the risk of lymph node invasion in localized prostate cancer. Urol Int 2017; 99: 207–214. [DOI] [PubMed] [Google Scholar]
- 18. Porcaro AB, Inverardi D, Corsi P, et al. Prostate specific antigen levels and proportion of biopsy positive cores are independent predictors of upgrading patterns in low risk prostate cancer. Minerva Urol Nefrol. Epub ahead of print 3 October 2018. DOI: 10.23736/S0393-2249.18.03172-7 [DOI] [PubMed] [Google Scholar]
- 19. Porcaro AB, Siracusano S, Luyk N, et al. Clinical factors stratifying the risk of tumor upgrading to high-grade disease in low-risk prostate cancer. Tumori 2018; 104: 111–115. [DOI] [PubMed] [Google Scholar]
- 20. Porcaro AB, Cacciamani GE, Sebben M, et al. Lymph nodes invasion of Marcille’s Fossa Associates with high metastatic load in prostate cancer patients undergoing extended pelvic lymph node dissection: the role of ‘marcillectomy’. Urol Int 2019; 1–8. [DOI] [PubMed] [Google Scholar]
- 21. Cacciamani GE, Porcaro AB, Sebben M, et al. Extended pelvic lymphadenectomy for prostate cancer: should the Cloquet’s nodes dissection be considered only an option? Minerva Urol Nefrol 2019; 71: 136–145. [DOI] [PubMed] [Google Scholar]
- 22. Kumar A, Tandon S, Samavedi S, et al. Current status of various neurovascular bundle-sparing techniques in robot-assisted radical prostatectomy. J Robotic Surg 2016; 10: 187–200. [DOI] [PubMed] [Google Scholar]
- 23. Freire MP, Weinberg AC, Lei Y, et al. Anatomic bladder neck preservation during robotic-assisted laparoscopic radical prostatectomy: description of technique and outcomes. Eur Urol 2009; 56: 972–980. [DOI] [PubMed] [Google Scholar]
- 24. Atug F, Castle EP, Srivastav SK, et al. Positive surgical margins in robotic-assisted radical prostatectomy: impact of learning curve on oncologic outcomes. Eur Urol 2006; 49: 866–871; discussion 871–2. [DOI] [PubMed] [Google Scholar]
- 25. Dripps RD. The role of anesthesia in surgical mortality. JAMA 1961; 178: 261. [DOI] [PubMed] [Google Scholar]
- 26. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Porcaro AB, Sebben M, Corsi P, et al. Risk factors of positive surgical margins after robot-assisted radical prostatectomy in high-volume center: results in 732 cases. J Robot Surg. Epub ahead of print 5 April 2019. DOI: 10.1007/s11701-019-00954-x [DOI] [PubMed] [Google Scholar]
- 28. Ficarra V, Novara G, Secco S, et al. Predictors of positive surgical margins after laparoscopic robot assisted radical prostatectomy. J Urol 2009; 182: 2682–2688. [DOI] [PubMed] [Google Scholar]
- 29. Patel VR, Coelho RF, Rocco B, et al. Positive surgical margins after robotic assisted radical prostatectomy: a multi-institutional study. J Urol 2011; 186: 511–517. [DOI] [PubMed] [Google Scholar]
- 30. Coelho RF, Chauhan S, Orvieto MA, et al. Predictive factors for positive surgical margins and their locations after robot-assisted laparoscopic radical prostatectomy. Eur Urol 2010; 57: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 31. Tholomier C, Bienz M, Hueber PA, et al. Oncological and functional outcomes of 722 robot-assisted radical prostatectomy (RARP) cases: the largest Canadian 5-year experience. Can Urol Assoc J 2014; 8: 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rajan P, Hagman A, Sooriakumaran P, et al. Oncologic outcomes after robot-assisted radical prostatectomy: a large European single-centre cohort with median 10-year follow-up. Eur Urol Focus 2018; 4: 351–359. [DOI] [PubMed] [Google Scholar]
- 33. Antonelli A, Sodano M, Peroni A, et al. Positive surgical margins and early oncological outcomes of robotic vs open radical prostatectomy at a medium case-load institution. Minerva Urol Nefrol 2017; 69: 63–68. [DOI] [PubMed] [Google Scholar]
- 34. Jo JK, Hong SK, Byun SS, et al. Positive surgical margin in robot-assisted radical prostatectomy: correlation with pathology findings and risk of biochemical recurrence. Minerva Urol Nefrol 2017; 69: 493–500. [DOI] [PubMed] [Google Scholar]
- 35. Porcaro AB, Tafuri A, Sebben M, et al. Positive Association between preoperative total testosterone levels and risk of positive surgical margins by prostate cancer: results in 476 consecutive patients treated only by radical prostatectomy. Urol Int 2018; 101: 38–46. [DOI] [PubMed] [Google Scholar]
- 36. Moskovic DJ, Lavery HJ, Rehman J, et al. High body mass index does not affect outcomes following robotic assisted laparoscopic prostatectomy. Can J Urol 2010; 17: 5291–5298. [PubMed] [Google Scholar]
- 37. Wiltz AL, Shikanov S, Eggener SE, et al. Robotic radical prostatectomy in overweight and obese patients: oncological and validated-functional outcomes. Urology 2009; 73: 316–322. [DOI] [PubMed] [Google Scholar]
- 38. Castle EP, Atug F, Woods M, et al. Impact of body mass index on outcomes after robot assisted radical prostatectomy. World J Urol 2007; 26: 91–95. [DOI] [PubMed] [Google Scholar]
- 39. van Roermund JG, Bol GH, Witjes JA, et al. Periprostatic fat measured on computed tomography as a marker for prostate cancer aggressiveness. World J Urol 2009; 28: 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leow JJ, Leong EK, Serrell EC, et al. Systematic review of the volume–outcome relationship for radical prostatectomy. Eur Urol Focus 2018; 4: 775–789. [DOI] [PubMed] [Google Scholar]
- 41. Hu JC, Wang Q, Pashos CL, et al. Utilization and outcomes of minimally invasive radical prostatectomy. J Clin Oncol 2008; 26: 2278–2284. [DOI] [PubMed] [Google Scholar]
- 42. Steinsvik EA, Axcrona K, Angelsen A, et al. Does a surgeon’s annual radical prostatectomy volume predict the risk of positive surgical margins and urinary incontinence at one-year follow-up? Findings from a prospective national study. Scand J Urol 2013; 47: 92–100. [DOI] [PubMed] [Google Scholar]