Abstract
Treatment with immune checkpoint inhibitors (ICIs) has improved the prognosis of patients with a number of types of cancer, but the frequent development of immune-related adverse effects (irAEs) can worsen the outcome. The most common irAEs involve the gastrointestinal, cutaneous, and endocrine systems, but nephrotoxicity, resulting from damage to the tubule-interstitial compartment, may occur in some patients. The early phases of acute interstitial nephritis (AIN) are characterized by systemic symptoms that indicate a poor clinical state as well as a mild deterioration of renal function. Tubular injury is due to a direct effect mediated by cytotoxic CD8+ T cells, which sustain the local production of pro-inflammatory cytokines that progressively impair renal function. The treatment of AIN is mainly based on high-dose steroids, which in most instances leads to the recovery of renal function. However, the premature discontinuation of ICI therapy may prevent the impact of treatment on the clinical progression of the malignancy. Adequately addressing irAEs requires a standardized therapy that is based on the results of large clinical trials.
Keywords: acute interstitial nephritis, immunotherapy, melanoma, nephrotoxicity
Introduction
The incidence of cutaneous melanoma has steadily risen over the last 30 years, with more than 80,000 new cases were reported in 2017.1 Recent advances in the treatment of melanoma have improved the outlook for many patients in terms of both progression-free survival (PFS) and overall survival (OS). The prognostic benefit has largely been due to the development of tailored therapies targeting BRAF mutations and immune checkpoint inhibitors (ICIs). Immune-modulating agents, including interferons and interleukin (IL)-2, have been used in the treatment of metastatic melanoma but the results in term of clinical benefit and OS have been limited.2 Based on knowledge of the melanoma biology, new therapeutic strategies including the cytotoxic T lymphocyte-associated protein-4 (CTLA-4), the programmed death 1 receptor (PD-1) and PD-ligand 1/2 (PD-L1/2) blockers have been engineered for restraining the molecular signals between melanoma cells and effector immune cells thus achieving great improvement in terms of prognosis and survival.3–5 The CTLA-4 and PD-1 or PD-L1/2 axis are involved in the priming and effector phases of an antitumor immune response. In particular, CTLA-4 is constitutively expressed by T cells and attenuates the immune response competing with CD28 for the binding of costimulatory molecules namely CD80 or CD86 expressed by dendritic cells (DCs), leading to great impact on the stage of T cell activation in the draining lymph nodes and reducing their ability to stimulate tumor-specific T cells. In addition, the interplay between PD-1 and PD-L1/2 ligands that are commonly expressed by DCs, macrophages as well as melanoma cells, inhibits T cell activity to maintain the immune homeostasis and prevent autoimmunity. Therefore, blocking the PD-1/PD-L1/2 axis activates T cells in the tumor microenvironment, releasing inflammatory cytokines and cytotoxic granules to eliminate melanoma cells.
Despite the efficacy of ICIs, their peculiar mechanisms of action are correlated with a new class of side effects called immune-related adverse events (irAEs) that are common, often serious, and characterized by unpredictable development. Their clinical presentation depends on the organ system affected and includes rash, vitiligo, colitis, hypothyroidism, pneumonitis, hypophysitis, and renal failure. Similar occurrences of renal failure were reported in patients with metastatic bladder cancer and Merkel cell carcinoma treated with a monoclonal antibody (MoAb) against PD-L1.6,7 While the majority of irAEs are mild and manageable with moderate doses of steroids, in a limited number of cases, they are severe, although rarely fatal. Many patients recover while continuing therapy whereas in others therapy discontinuation is required, either without subsequent clinical evidence of disease progression or, in a minority of cases, with a rapid progressive disease whose features indicate hyper-progression.8
In previous conventional chemotherapy, nephrotoxicity was a complication frequently limiting life-saving cancer treatment. However, chemotherapy-induced renal damage did not occur in all patients, which suggested the presence of specific factors that enhance individual risk. Thus, apart from the baseline drug toxicity associated with many anti-cancer agents, certain host characteristics, including single-nucleotide polymorphisms in putative genes, microRNAs, chronic inflammatory disorders, and concurrent medications may increase the risk of developing renal failure during treatment.9–12
A relevant issue, therefore, concerns the treatment of renal failure in ICI-treated cancer patients and the strategy to be adopted after discontinuation includes permanent drug cessation and the delay of therapy.13 However, on the basis of the limited amount of available data, renal complication requires further investigations.
Mechanisms of drug-induced interstitial nephritis
Drug-induced acute interstitial nephritis (AIN) is an early but also delayed adverse event that occurs after exposure to the problem drug, and it is the third most common cause of acute kidney injury (AKI).14–16 AIN is mostly due to a drug hypersensitivity reaction (HR), which can be explained by the high rate of renal blood flow, such that antigens are filtered, secreted, and concentrated in large amounts, as well as the continuous contact between tubular cells and the drug or its metabolites.17
The clinical presentation of a drug-induced AIN is similar to that of acute tubular necrosis (ATN). However, AIN is more insidious in onset and is frequently associated with interstitial edema, as well as cellular infiltration, whereas ATN arises from direct tubular epithelial injury and the rapid deterioration of renal function.17 An adverse drug reaction caused by an HR is diagnosed based on the following criteria: the presence of a known immunological manifestation, no other explanation involving the definite pharmacological or idiosyncratic effects of the drug, and a timeline of occurrence within 7–10 days of initial exposure. The mechanisms by which a drug may elicit an HR or an autoimmune response include: the peculiar susceptibility of the host tissue such that it becomes immunogenic after drug exposure, the development of drug-specific antibodies that engage an immune reaction, the intrinsic immunogenicity of the drug in patients with a particular T cell receptor (TCR) or major histocompatibility complex (MHC) profile, metabolism of the drug into a reactive antigen or immunogen that stimulates the innate immune response, for example, cells of the proximal tubule may hydrolyze and metabolize exogenous antigens and then present them through MHC expressed by dendritic cells resident in or recruited to the kidney, and an additional mechanism is haptenization, by which low-molecular-weight compounds irreversibly bind to self-protein, thus creating a hapten that can be trapped in the parenchyma, leading to the impairment of filtration and, eventually, irreversible tubular damage.18
Finally, it has been suggested that a further systemic HR namely type IV, also known as delayed-type or cell-mediated, in which lymphocytes play a major role in the pathogenesis of the drug-induced AIN.17 Although the mechanisms involved in the type IV HR are partially unknown, a critical role appears to be played by direct binding of the drug to the tubular basement membranes, thus the drug may act as a hapten eliciting an immune response.
In contrast, the pathogenesis of immune-related AIN is considered similar to that of autoimmune diseases, where activated lymphocytes target self-antigens. Therefore, dual PD-1/CTLA-4 blockade synergistically breaks the tolerance by unleashing quiescent tissue-specific self-reactive T cells, which express high levels of PD-1 receptor. Of interest, the AIN pattern injury demonstrates pathological findings indistinguishable from other drug-induced AIN, which are commonly characterized by T cell-predominant infiltration of the renal interstitium associated with eosinophils and plasma cells.
A drug-induced AIN, therefore, mostly depends on a delayed T cell-mediated HR whose pathogenesis is based on three sequential steps: the antigen recognition and presentation phase followed by the regulatory and effector phases.19,20 During the antigen recognition and presentation phase, haptens are endocytosed by resident interstitial cells or tubular epithelial cells and are thus able to present antigen to the dendritic cells located near the basolateral aspect of tubular epithelial cells. Activated by antigenic signals, these dendritic cells migrate throughout the kidney lymphatics to regional nodes, where the antigens are presented to naïve T cells. In addition, the renal interstitium is infiltrated by dormant macrophages and fibroblasts that become activated and subsequently participate in enhancing inflammation, by recruiting cytokines, soluble factors, and neutrophils. During the regulatory and effector phases, both the local production of ILs and cell-to-cell contact mediate bidirectional crosstalk between recruited inflammatory cells and the kidney tissue.21 The release of collagenases, elastases, and reactive oxygen species produced by resident macrophages provoke renal damage but this may be limited by protective events that include the down-regulation of MHC-II and the activation of suppressor T cells. Other determinants of the severity of renal damage, fibrosis, and irreversible impairment are the nature of the antigenic exposure, as well as the degree of inflammatory and immune cell activation. According to the clinical presentation of patients with drug-induced renal damage,22 the Common Terminology Criteria for Adverse Events (CTCAEv40) recognizes five different grades of renal injury, based on creatinine levels (grades 1 to 3), dialysis requirement (grade 4) and fatal complications (grade 5) (Table 1). The Kidney Disease Improving Global Outcomes (KDIGO) criteria are used to classify the severity of tubular damage based on an increased creatinine level and glomerular filtration rate, although a definite diagnosis of AIN requires renal biopsy. With the rapid expansion of the indications for the use of ICIs in the treatment of cancer, new adverse effects, including AIN, are emerging and must be considered in the administration of these drugs.
Table 1.
KDIGO clinical practice guidelines for the management of AKI and common terminology criteria for adverse events v4.0 (CTCAE).
| RIFLE AKI criteria | |||
|---|---|---|---|
| Serum creatinine* | Glomerular filtration | Urine output | |
| Risk | ×1.5 | Reduced (>25%) | <0.5 ml/kg/h for 6 h |
| Injury | ×2.0 | Reduced (>50%) | <0.5 ml/kg/h for 12 h |
| Failure | ×3.0 or higher | Reduced (>75%) | <0.3 ml/kg/h for 24 h or anuria for 12 h |
| Loss of kidney function | Complete loss of function >4 weeks | ||
| End-stage Kidney Disease | Complete loss of function >3 months | ||
| Related AKI criteria | |||
| Serum Creatinine | Urine Output | ||
| Stage I | ×1.5–1.9 | <0.5 ml/kg/h for 6 h | |
| Stage II | ×2.0–2.9 | <0.5 ml/kg/h for 12 h | |
| Sage III | ×3.0 or higher | <0.3 ml/kg/h for 24 h or anuria for 12 h | |
| CTCAE v4.0 | |||
| Serum Creatinine | Recommendations | ||
| Grade 1 | ×1.5–2.0 | Intervention not indicated | |
| Grade 2 | ×2.0–3.0 | Minimal, local noninvasive intervention indicated | |
| Grade 3 | ×3.0 or 4.0 mg/dl | Hospitalization indicated | |
| Grade 4 | Life threatening consequences: dialysis indicated | Life threatening consequences: urgent intervention indicated | |
| Grade 5 | Death | Death related to adverse event | |
Increase of creatinine values with respect to baseline.
AKI, acute kidney injury; CTCAE, Common Terminology Criteria for Adverse Events; KDIGO, Kidney Disease Improving Global Outcomes.
Incidence, clinical and pathological features of ICI-induced nephrotoxicity
Reviews of clinical trials of immunotherapy have reported an overall incidence of renal failure >3%.10 Grade III–IV AKI, or the need for dialysis, occurred in only 1–2% of patients treated with a single agent, although the rate increased up to 5% in those receiving a combination of ipilimumab and nivolumab,23 while the time for the development of this complication results are variable. According to the KDIGO guidelines,24,25 AKI stage I–II is more common than stage III (Table 1). While fatigue, hematuria, eosinophilia, and worsening hypertension were noted, in the majority of patients an increased serum creatinine level and pyuria were the only clinical indications. The majority of patients show a typical tubule-interstitial presentation, that is, normal urinary output, glomerular casts, aseptic leukocyturia, and low-grade or the absence of proteinuria while nephrotic syndrome has been occasionally reported in those receiving ipilimumab.10,26–28 Thus, despite the durable clinical benefits of ICI therapy with ipilimumab, nivolumab, and pembrolizumab in terms of a progressive improvement in the objective response rate (50–60%), the permanent discontinuation of immunotherapy is mandatory in patients who develop an irAE, including AIN.23
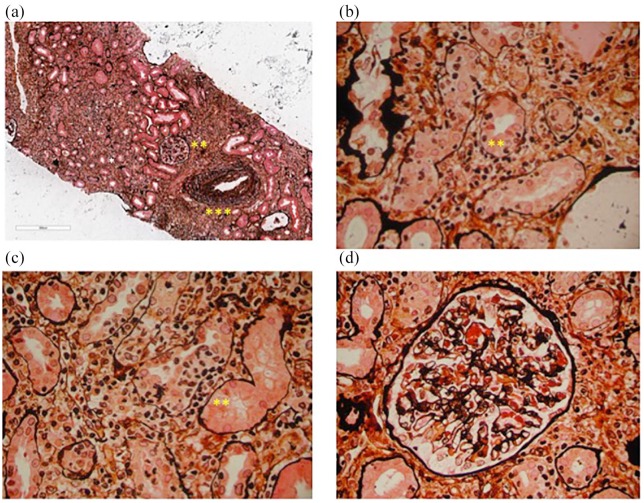
Renal biopsy reveals that in the majority of patients tubular rather than glomerular injury and the most frequent pathological manifestations induced by ICIs consist of edema, interstitial inflammation, and tubulitis. These pathological features are seen in Figure 1, which shows the renal biopsy results from a patient treated with anti-PD-1. The interstitial infiltrates consist of inflammatory and immune cells, including CD4+ and CD8+ lymphocytes as well as eosinophils, whose presence in the vicinity of the tubular basement membrane is a hallmark of AIN. Table 2 shows the most relevant features of inflammatory infiltrate revealed by biopsy in reported cases in the literature. Tubulitis is characterized by tubular dilation, cytoplasmic vacuolization, prominent nucleoli, an altered brush border, and interstitial edema. In the absence of adequate treatment, AIN may evolve into a chronic phase characterized by interstitial fibrosis associated with tubular atrophy, the glomeruli, and blood vessels that are usually not involved. Neither immunofluorescence nor electron microscopy allows a conclusive diagnosis of AIN because the findings are often negative for the deposition of immunoglobulin or complement fractions,29–32 while electron microscopy is useful to reveal AIN associated with minimal change disease.33 Moreover, granulomatosis interstitial nephritis is a rare histologic variant characterized by the infiltration of histiocytes and macrophages, monocytes, plasma cells, and lymphocytes that surround tubular and glomerular structures, and an accumulation of eosinophils is highly atypical.34,35
Figure 1.
The hallmark of acute interstitial nephritis induced by an anti-PD-1 monoclonal antibody. (a) A representative renal biopsy from a patient treated with anti-PD-1 who developed severe tubulitis (*). The diffuse infiltrate mostly consists of lymphocytes and plasma cells. The glomerular morphology (**) is almost normal but a mild to moderate intimal fibrosis is seen in an interlobular artery. (b) and (c) Diffuse severe tubular inflammation (*) without findings associated with atrophy, including lymphocyte infiltration and interstitial edema (**). (d) Mild-moderate capillary congestion in a glomerulus surrounded by an interstitial infiltrate but without any other significant abnormalities. Silver methenamine staining of renal tissues visualized by optical microscopy (50× and 400× magnification).
Table 2.
Clinical presentation and characteristics of the inflammatory infiltrate in reported cases of ICI-associated AIN.
| ICI administered | AIN n |
Clinical presentation | Histologic examination (inflammatory infiltrate) |
|||||
|---|---|---|---|---|---|---|---|---|
| Lymphocytes | Plasma cells | Eosinophils | Macrophages | Granuloma | Reference | |||
| Anti-PD-1 | 2 | Creatinine increase, Hyperkaliemia. metabolic acidosis |
p | p | a | a | a | * |
| Anti-PD-1 | 1 | Creatinine increase, microhematuria no fever, rash, nor eosinophilia |
p | a | p | p | a | Wanchoo et al.14 |
| Anti-CTLA-4 (5) Anti-PD-1 (3) Anti-CTLA-4+Anti-PD-1 (4) |
12 | Pyuria (7) hematuria (2) hypertension (2) eosinophilia (1) Oliguria (1) |
p | p | p | a | p | Cortazar et al.10 |
| Anti-PD-1 | 6 | Creatinine increase (6) bilateral lower extremity edema (2) eosinophilia (2) pyuria (2) Hypertension (2) Rash, fever, oliguria (1) |
p | a | p | a | a | Shirali et al.26 |
| Anti-CTLA-4 | 1 | Creatinine increase, Rash, eosinophilia |
p | p | p | a | p | Thajudeen et al.35 |
| Anti-CTLA-4 | 2 | Creatinine increase (2) pyuria (2) rash, fatigue, anorexia, fever (1) eosinophilia (1) hematuria (1) proteinuria (1) |
p | a | a | a | p | Izzedine et al.28 |
| Anti-CTLA-4 | 1 | Creatinine increase, metabolic acidosis oliguria |
Kidney biopsy not completed | Forde et al.27 | ||||
a, absent; AIN, acute interstitial nephritis; ICI, immune checkpoint inhibitors; n, number; p, present.
personal cases from the Medical Oncology Unit of the University of Bari
Nephrotoxicity induced by ICIs
The efficiency of immune editing and thus of tumor cell escape is controlled by the enhancement or suppression of effector CD8+ T cells by stimulatory or inhibitory molecules that modulate their signals, thus providing ‘checkpoints’ for immune system regulation. The efficiency of T cell responses mostly depends on activation of the CD28 receptor, which interacts with the B7 ligand expressed by dendritic cells after the engagement of the TCR by MHC-II.36–38 The intracellular signals driving the differentiation of resting lymphocytes into effector T cells can be efficiently restrained by specific immune checkpoints, including those targeting both CTLA-4 and PD-1 receptors.39 The CTLA-4 receptor is expressed abundantly by T cells and regulates the amplitude of the early stages of T cell activation while competing with CD28 to bind B7, thereby reducing the efficacy of co-stimulation.36 CTLA-4 arrests cell cycle progression, promotes apoptosis and renders newly antigen-specific T cells anergic. The inhibitory signals driven by the CTLA-4 pathway are mostly activated in effector T cells and T regulatory cells, thus leading to a suppressive milieu that restrains immune surveillance. The trans-membrane PD-1 receptor, which binds both PD-L1 and PD-L2, is expressed by the majority of immune cells.40 Following the binding of these ligands to the receptor, its cytoplasmic domain containing an immunoreceptor tyrosine-based inhibitory motif (ITIM) and the immunoreceptor tyrosine-based switch motif that is mainly implicated in driving the receptor-mediated immune suppressive signals. The blockade of the CTLA-4 and PD-1 receptors by their ligands partly restores the T cell activity, restrains the production of suppressive cytokines and limits the major mechanisms that negatively influence the melanoma microenvironment.41,42
However, the mechanisms activated by the blockade of immune checkpoint driving the acute renal damage are largely unknown although but generally attributable to: direct immunogenicity of the drug that consists of the direct binding of ICIs to target molecules expressed on nonimmune system cells (off-target effect), for example, intra-parenchymal renal cells express high levels of PD-L1 and PD-L2, which may be the targets of anti-PD-1 agents that directly promote renal injury,18,42 and the effect of ICIs on the immune system mediated by an autoimmune phenomena induced by loss of peripheral tolerance to self-reactive T cells, the re-activation of drug-specific T cells through ICI-induced loss of tolerance;26,43–46 the migration and activation of effector T cells associated with pro-inflammatory cytokines release, and the generation of autoantibodies that directly drive renal damage.
In addition to melanoma, recent clinical trials have identified over 30 cancer histotypes with sensitivity to anti-PD(L)-1. These ‘PD-Lomas’47 include microsatellite instability (MSI)-high solid tumors, Hodgkin and non-Hodgkin lymphoma, Merkel cell carcinoma, non-small cell lung cancer (NSCLC), renal, urothelial, triple-negative breast, and head and neck cancers.48,49 Encouraging results have been obtained by targeting PDL-1 in other cancer types.6 However, despite anti-PDL-1-related improvements in PFS and OS, a variety of irAEs have been reported in treated patients, including cutaneous manifestations, colitis, pneumonitis, endocrinopathies, and AIN whose incidence ranges from 3%–20% depending on the clinical setting and inhibitor type.50
The mechanisms inducing CTLA-4 and PD-1 nephrotoxicity are different, as are the patterns of renal damage induced by the anti-CTLA-4 MoAbs Ipilimumab® and Tremelimumab®.51 These patterns consist of: a lupus-like glomerulonephritis with anti-double-stranded DNA antibody production and either class-G immunoglobulin or C3 deposits within the glomeruli, 26 and a tubular-interstitial nephritis that usually resembles the hypersensitivity seen in AIN.52 Previous reports demonstrated the up-regulation of PD-1 in renal allograft kidneys as well as during acute vascular rejection, which suggests a negative role for PD-1 in alloreactive T cell responses but also the protection of tubular cells from T cell-mediated injury during acute allograft rejection. In a study based on a model of ischemia-reperfusion-induced inflammation, T regulatory cells were shown to express large amounts of PD-1, which limited their interaction with both tubular cells and other T cells, thus favoring renal cell cytotoxicity.53 In addition, the development of spontaneous lupus-like glomerulonephritis and arthritis in PD-1 deficient mice has been reported.54 Furthermore, the ICI-induced loss of tolerance has been demonstrated in murine models showing that PD-1 signaling is critical for supporting the peripheral tolerance of self-antigens by restraining self-reactive T cells and stimulating tolerogenic DCs. In this context, PD-1 signals limit CD8+ T cell-induced inflammation and PD-1 knockout mice spontaneously developing glomerular damage.54,55
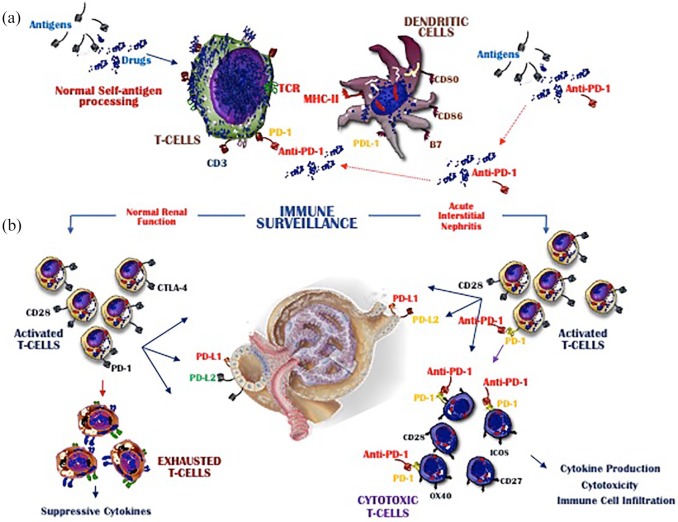
The effect of the PD-1 receptor in cancer reflects its interaction with its two major ligands: PDL-1, expressed by immune and nonimmune cells, and PDL-2, expressed on the surface of dendritic cells and macrophages.56 Murine models have shown that PD-1 signaling is essential to the peripheral tolerance of self-antigens as it restrains the expansion of self-reactive T cells and stimulates tolerogenic DCs.46 Both the PD-1/PDL-1 and the PD-1/PDL-2 axes are critical mediators of immune control in the kidney, because they limit CD8+ T cell-mediated inflammatory injury, as evidenced by the observation that PD-1 knockout mice spontaneously develop glomerulonephritis.54,57 As shown in Figure 2, PD-1 inhibitors reactivate exhausted T cells primed by the exposure to pathogenetic stimuli but subsequently inhibited by PD-1 signaling.26,46,55 However, both scenarios may enhance T cell migration to the kidney and the cytotoxic activity of these cells. In conclusion, the restrained engagement of PDL-1 expressed by renal cells with PD-1 on T cells following PD-1 inhibitor treatment may allow the stimulation proliferation of T cell, leading to cytotoxicity, cytokine over production, and the infiltration of circulating immune cells, including immature and functionally defective subsets of plasmacytoid DCs.58 This large-scale recruitment of inflammatory and immune cells is a prerequisite for the development of AIN in patients receiving immunotherapy for advanced cancer.
Figure 2.
Mechanisms of renal damage induced by PD-1 inhibitors. (a) shows the mechanisms regulating the immune surveillance in melanoma and the basic events activated during the T cell interplay with DCs. This crosstalk is the consequence of antigen/drug processing and mostly occurs via TCR-MHC engagement. (b) The resulting T cell activation is followed by migration toward lymphoid and nonlymphoid tissues, including the kidney. T cells expressing PD-1 may bind PD-L1 expressed on renal cells, which generates inhibitory signals driving T cell exhaustion (left). In contrast, T cells in which PD-1 is blocked by ICIs (right) migrate toward the kidney, where they may cause cytotoxicity by the local over production of nephritogenic cytokines. This event may result in irreversible damage to the tubules and the progressive deterioration of kidney function.
Prognostic biomarkers and treatment
The improved survival of patients with melanoma and NSCLC treated with ICIs has encouraged studies aimed at the identification of either potential predictive or prognostic markers of responsiveness to immunotherapy.59,60 In contrast, biomarkers of toxicity or irAEs have been less thoroughly investigated. Sarcopenia and low muscle mass were shown to be associated with the occurrence of irAEs but other potential baseline risk factors include previous autoimmune disorders, tumor infiltration, and viral infections.61 While the risk of an irAE in patients receiving anti-CTLA-4 inhibitors is dose-dependent, cumulative toxicity induced by anti-PD-1 MoAbs has not been demonstrated.52,62 However, the prognostic applicability of these observations in the clinical setting is limited, such that the research focus has shifted to the T cell repertoire, IL-17 levels and, recently, to circulating B cells. The latter was shown to be numerically impaired in patients receiving ICIs. Parallel findings include enrichment of peripheral plasmablasts and the CD21low PD-1+ memory B cell subset. Measurements of transcriptional activity in this cell population prior to and after ICIs revealed the increased transcription of genes associated with cell activation and cytokine production. Additional features of the CD21low population are the ability to traffic toward nonlymphoid tissues and actively participate in inflammatory events involved in autoimmunity. Thus, changes in the frequency of CD21low cells may be predictive of irAEs.39 While a putative biomarker of responsiveness to ipilimumab with clinical applicability has yet to be identified,60 adequate PD-L1 expression by tumor cells is a prerequisite in the selection of patients with metastatic NSCLC or advanced urothelial carcinoma who are candidates for anti-PD-1 MoAb (pembrolizumab) therapy.63,64 However, the relevance of clinical data validating the use of immunotherapy in patients with melanoma and NSCLC, and the early identification of irAEs remains challenging. An intriguing paradox, however, is the unexpected positive association between irAEs and survival and specifically between melanoma and the development of rash and vitiligo observed in dedicated clinical trials65 and explained as a consequence of immune activation.
The impact of steroids on the outcome of treatment in cancer patients who develop an irAE in response to ICI therapy is thus far unclear. Contradictory results were obtained in two retrospective studies that examined the utility of steroids in the management of adverse events, including renal failure.66,67 A phase II trial of ipilimumab demonstrated that the benefit of steroids in terms of irAEs does not extend to either PFS or OS. Although data from patients receiving anti-PD-1 MoAbs is limited, a clinical deterioration of efficacy was not experienced by the majority of patients who, additionally, received steroids. Given the conflicting data, the modest information obtained by trials investigating ICIs, and the lack of randomized prospective trials in this field, the benefit of steroids in the treatment of irAEs still needs to be demonstrated. However, according to results from single institutions, steroids are effective in limiting renal toxicity in the majority of patients. In addition, it is unclear whether steroids specifically dampen the efficacy of anti-PD-1 treatment, since the rates of disease progression and stable disease were similar in patients forced to stop treatment and in patients who resumed treatment after a period of discontinuation.14 However, patients refractory to steroids may be treated with other immunomodulatory medications that include infliximab, an anti-tumor necrosis factor (TNF)-α MoAb, the anti-metabolite mycophenolate mofetil, the calcineurin inhibitors tacrolimus, and cyclosporine. Other strategies proposed for patients not eligible to receive infliximab and mostly affected by gastrointestinal complications include vedolizumab, an anti-α4β7 MoAb.13
Conclusions
Despite the encouraging results obtained in trials of immunotherapeutic agents, these drugs may induce nonspecific immunological activation leading to adverse effects that may necessitate the discontinuation of therapy. Therefore, identification of the molecular mechanisms underlying irAEs is required to optimize therapeutic strategy planning. For example, drawing on systematic reviews, meta-analyses, randomized controlled trials, and case series, a multidisciplinary panel of experts has developed clinical practice guidelines for the management of adverse events associated with ICI immunotherapy.22,68
While the clinical management of an irAE depends on the organ injured, ICI treatment should be continued in patients who develop grade 1 toxicities, with the exception of neurologic, hematologic, and cardiac complications. ICI treatment may need to be discontinued in most patients with grade 2 adverse events, with the resumption of treatment when the symptoms revert to grade 1 or less. Corticosteroids may be administered without contraindications.69 Grade 3 toxicities generally warrant the suspension of ICI therapy and these patients should receive high-dose corticosteroids (prednisone 1–2 mg/kg/day or methylprednisolone 1–2 mg/kg/day), which can be tapered within 4–6 weeks. Patients with steroid-refractory irAEs may require additional immunosuppressive agents, including mycophenolate.70 In general, the definite discontinuation of ICIs is recommended in patients with refractory grade 3 and 4 toxicities, with the exception of those with endocrine disorders that have been controlled by hormone replacement. In conclusion, while the potential influence of other medications on renal function in patients receiving ICIs is still unclear, drugs exerting a direct nephrotoxic effect should probably be avoided during immunotherapy. At present, to the best of the authors’ knowledge, no data is available in the medical literature on the relationship between the incidence of renal irAEs and the efficacy of immunotherapy in terms of response rates in different cancer types.
Previous studies based on experimental models of both cancer and autoimmune disorders demonstrated the immunomodulatory effect of vitamin D, which was attributed to its direct role on Th17 cells.71,72 Therefore, the administration of vitamin D during ICI therapy could theoretically prevent or ameliorate irAEs.73,74 Finally, other reports have demonstrated that the gut microbiota modulates the clinical response to cancer therapy, including the onset of irAEs, and that variations in the gut flora influence the efficacy of ICIs. In a murine model, mice receiving CTLA4 antagonists developed T cell-mediated damage of the duodenal mucosa and a parallel dysregulation of the gut microbiota.75 The inflammatory microenvironment in the intestine may induce the expansion of Th17 cells and increases the risk of irAEs. In addition, retrospective clinical data in melanoma differentiated a favorable from unfavorable gut microbiota in relation to a potential predictive role in patients receiving anti-PD-1 MoAbs. Other reports proved that the abundance of Akkermansia muciniphila predicts the resistance to PD-1 blockers.76–78 Moreover, genitourinary cancer harbors high neoantigen load as well as high tumor mutational burden. Therefore, urothelial cancer responds to ICIs while resident urinary microbiota should be explored as a hidden factor implicated in cancer progression.79,80 Further studies of the molecular mechanisms that are activated in the development of AIN are required. Their results will facilitate the planning of adequate preventive strategies that will avoid the need for treatment discontinuation.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by a grant (# 173536) from the Italian Association for Cancer Research.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Tucci Marco  https://orcid.org/0000-0003-4008-4897
https://orcid.org/0000-0003-4008-4897
Contributor Information
Tucci Marco, DIMO, Department of Biomedical Sciences and Clinical Oncology, University of Bari ‘Aldo Moro’, Section of Internal Medicine and Oncology, P.za Giulio Cesare, 11 - 70124 BARI, Italy.
Passarelli Anna, DIMO, Department of Biomedical Sciences and Clinical Oncology, University of Bari, ‘Aldo Moro’ Italy.
Todisco Annalisa, DIMO, Department of Biomedical Sciences and Clinical Oncology, University of Bari, ‘Aldo Moro’ Italy.
Mannavola Francesco, DIMO, Department of Biomedical Sciences and Clinical Oncology, University of Bari, ‘Aldo Moro’ Italy.
Stucci Luigia Stefania, DIMO, Department of Biomedical Sciences and Clinical Oncology, University of Bari, ‘Aldo Moro’ Italy.
D’Oronzo Stella, DIMO, Department of Biomedical Sciences and Clinical Oncology, University of Bari, ‘Aldo Moro’ Italy.
Rossini Michele, DETO, Department of Emergency and Organ Transplantation, University of Bari, ‘Aldo Moro’ Italy.
Taurisano Marco, DETO, Department of Emergency and Organ Transplantation, University of Bari, ‘Aldo Moro’ Italy.
Gesualdo Loreto, DETO, Department of Emergency and Organ Transplantation, University of Bari, ‘Aldo Moro’ Italy.
Silvestris Franco, DIMO, Department of Biomedical Sciences and Clinical Oncology, University of Bari, ‘Aldo Moro’ Italy.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Arenas-Ramirez N, Woytschak J, Boyman O. Interleukin-2: biology, design and application. Trends Immunol 2015; 36: 763–777. [DOI] [PubMed] [Google Scholar]
- 3. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372: 320–330. [DOI] [PubMed] [Google Scholar]
- 4. Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014; 384: 1109–1117. [DOI] [PubMed] [Google Scholar]
- 5. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cattrini C, Boccardo F. Atezolizumab and bladder cancer: facing a complex disease. Lancet 2018; 391: 305–306. [DOI] [PubMed] [Google Scholar]
- 7. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 2016; 17: 1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017; 23: 1920–1928. [DOI] [PubMed] [Google Scholar]
- 9. Mannavola F, Tucci M, Felici C, et al. miRNAs in melanoma: a defined role in tumor progression and metastasis. Expert Rev Clin Immunol 2015; 12: 79–89. [DOI] [PubMed] [Google Scholar]
- 10. Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016; 90: 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perazella MA, Rosner MH. Acute kidney injury in patients with cancer. Oncology (Williston Park) 2018; 32: 351–359. [PubMed] [Google Scholar]
- 12. Perazella MA, Izzedine H. New drug toxicities in the onco-nephrology world. Kidney Int 2015; 87: 909–917. [DOI] [PubMed] [Google Scholar]
- 13. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 2016; 44: 51–60. [DOI] [PubMed] [Google Scholar]
- 14. Wanchoo R, Karam S, Uppal NN, et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol 2017; 45: 160–169. [DOI] [PubMed] [Google Scholar]
- 15. Weber JS, Dummer R, de Pril V, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 2013; 119: 1675–1682. [DOI] [PubMed] [Google Scholar]
- 16. Clive DM, Stoff JS. Renal syndromes associated with nonsteroidal anti-inflammatory drugs. N Engl J Med 1984; 310: 563–572. [DOI] [PubMed] [Google Scholar]
- 17. Paueksakon P, Fogo AB. Drug-induced nephropathies. Histopathology 2017; 70: 94–108. [DOI] [PubMed] [Google Scholar]
- 18. Raghavan R, Shawar S. Mechanisms of drug-induced interstitial nephritis. Adv Chronic Kidney Dis 2017; 24: 64–71. [DOI] [PubMed] [Google Scholar]
- 19. Airy M, Raghavan R, Truong LD, et al. Tubulointerstitial nephritis and cancer chemotherapy: update on a neglected clinical entity. Nephrol Dial Transplant 2013; 28: 2502–2509. [DOI] [PubMed] [Google Scholar]
- 20. Raghavan R, Eknoyan G. Acute interstitial nephritis - a reappraisal and update. Clin Nephrol 2014; 82: 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neilson EG. Pathogenesis and therapy of interstitial nephritis. Kidney Int 1989; 35: 1257–1270. [DOI] [PubMed] [Google Scholar]
- 22. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 2018; 36: 1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sznol M, Ferrucci PF, Hogg D, et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol 2017; 35: 3815–3822. [DOI] [PubMed] [Google Scholar]
- 24. Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J 2013; 6: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kellum JA. Diagnostic criteria for acute kidney injury: present and future. Crit Care Clin 2015; 31: 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shirali AC, Perazella MA, Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 2016; 68: 287–291. [DOI] [PubMed] [Google Scholar]
- 27. Forde PM, Rock K, Wilson G, et al. Ipilimumab-induced immune-related renal failure–a case report. Anticancer Res 2012; 32: 4607–4608. [PubMed] [Google Scholar]
- 28. Izzedine H, Gueutin V, Gharbi C, et al. Kidney injuries related to ipilimumab. Invest New Drugs 2014; 32: 769–773. [DOI] [PubMed] [Google Scholar]
- 29. Rossert J. Drug-induced acute interstitial nephritis. Kidney Int 2001; 60: 804–817. [DOI] [PubMed] [Google Scholar]
- 30. Alexopoulos E. Drug-induced acute interstitial nephritis. Ren Fail 1998; 20: 809–819. [DOI] [PubMed] [Google Scholar]
- 31. Kleinknecht D. Interstitial nephritis, the nephrotic syndrome, and chronic renal failure secondary to nonsteroidal anti-inflammatory drugs. Semin Nephrol 1995; 15: 228–235. [PubMed] [Google Scholar]
- 32. Cornell LD. IgG4-related tubulointerstitial nephritis. Kidney Int 2010; 78: 951–953. [DOI] [PubMed] [Google Scholar]
- 33. Gao B, Lin N, Wang S, et al. Minimal change disease associated with anti-PD1 immunotherapy: a case report. BMC Nephrol 2018; 19: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kleinknecht D, Vanhille P, Druet P. Acute granulomatous interstitial nephritis of drug origin. Presse Med 1988; 17: 201–205. [PubMed] [Google Scholar]
- 35. Thajudeen B, Madhrira M, Bracamonte E, et al. Ipilimumab granulomatous interstitial nephritis. Am J Ther 2015; 22: e84–e87. [DOI] [PubMed] [Google Scholar]
- 36. Gardner D, Jeffery LE, Sansom DM. Understanding the CD28/CTLA-4 (CD152) pathway and its implications for costimulatory blockade. Am J Transplant 2014; 14: 1985–1991. [DOI] [PubMed] [Google Scholar]
- 37. Passarelli A, Mannavola F, Stucci LS, et al. Immune system and melanoma biology: a balance between immunosurveillance and immune escape. Oncotarget 2017; 8: 106132–106142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tucci M, Stucci S, Passarelli A, et al. The immune escape in melanoma: role of the impaired dendritic cell function. Expert Rev Clin Immunol 2014; 10: 1395–1404. [DOI] [PubMed] [Google Scholar]
- 39. Das R, Bar N, Ferreira M, et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest 2018; 128: 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 2018; 18: 153–167. [DOI] [PubMed] [Google Scholar]
- 41. Perazella MA. Checkmate: kidney injury associated with targeted cancer immunotherapy. Kidney Int 2016; 90: 474–476. [DOI] [PubMed] [Google Scholar]
- 42. Perazella MA, Shirali AC. Nephrotoxicity of cancer immunotherapies: past, present and future. J Am Soc Nephrol 2018; 29: 2039–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chae YK, Galvez C, Anker JF, et al. Cancer immunotherapy in a neglected population: the current use and future of T-cell-mediated checkpoint inhibitors in organ transplant patients. Cancer Treat Rev 2018; 63: 116–121. [DOI] [PubMed] [Google Scholar]
- 44. Caturegli P, Di Dalmazi G, Lombardi M, et al. Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol 2016; 186: 3225–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016; 375: 1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010; 236: 219–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hirsch L, Zitvogel L, Eggermont A, et al. PD-Loma: a cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br J Cancer 2019; 120: 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018; 359: 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moskovitz J, Moy J, Ferris RL. Immunotherapy for head and neck squamous cell carcinoma. Curr Oncol Rep 2018; 20: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Axelrod ML, Johnson DB, Balko JM. Emerging biomarkers for cancer immunotherapy in melanoma. Semin Cancer Biol 2018; 52: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 2013; 31: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016; 54: 139–148. [DOI] [PubMed] [Google Scholar]
- 53. Jaworska K, Ratajczak J, Huang L, et al. Both PD-1 ligands protect the kidney from ischemia reperfusion injury. J Immunol 2015; 194: 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999; 11: 141–151. [DOI] [PubMed] [Google Scholar]
- 55. Waeckerle-Men Y, Starke A, Wüthrich RP. PD-L1 partially protects renal tubular epithelial cells from the attack of CD8+ cytotoxic T cells. Nephrol Dial Transplant 2007; 22: 1527–1536. [DOI] [PubMed] [Google Scholar]
- 56. Bordon Y. T cell responses: a dendritic cell designed for two. Nat Rev Immunol 2013; 13: 844–845. [DOI] [PubMed] [Google Scholar]
- 57. Fadel F, Karoui El K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med 2009; 361: 211–212. [DOI] [PubMed] [Google Scholar]
- 58. Tucci M, Quatraro C, Lombardi L, et al. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum 2008; 58: 251–262. [DOI] [PubMed] [Google Scholar]
- 59. Chae YK, Arya A, Iams W, et al. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC). J Immunother Cancer 2018; 6: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tucci M, Passarelli A, Mannavola F, et al. Serum exosomes as predictors of clinical response to ipilimumab in metastatic melanoma. OncoImmunology 2018; 7: e1387706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stucci S, Palmirotta R, Passarelli A, et al. Immune-related adverse events during anticancer immunotherapy: pathogenesis and management. Oncol Lett 2017; 14: 5671–5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010; 11: 155–164. [DOI] [PubMed] [Google Scholar]
- 63. Reck M. Pembrolizumab as first-line therapy for metastatic non-small-cell lung cancer. Immunotherapy 2018; 10: 93–105. [DOI] [PubMed] [Google Scholar]
- 64. Bellmunt J, Bajorin DF. Pembrolizumab for advanced urothelial carcinoma. N Engl J Med 2017; 376: 2304–2304. [DOI] [PubMed] [Google Scholar]
- 65. Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 2016; 22: 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gonzalez E, Gutiérrez E, Galeano C, et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int 2008; 73: 940–946. [DOI] [PubMed] [Google Scholar]
- 67. Clarkson MR, Giblin L, O’Connell FP, et al. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant 2004; 19: 2778–2783. [DOI] [PubMed] [Google Scholar]
- 68. Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J Immunother Cancer 2017; 5: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 2016; 27: 559–574. [DOI] [PubMed] [Google Scholar]
- 70. Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29: iv264–iv266. [DOI] [PubMed] [Google Scholar]
- 71. Cafforio P, D’Oronzo S, Felici C, et al. 1,25(OH)2 vitamin D(3) contributes to osteoclast-like trans-differentiation of malignant plasma cells. Exp Cell Res 2017; 358: 260–268. [DOI] [PubMed] [Google Scholar]
- 72. Cafforio P, Savonarola A, Stucci S, et al. PTHrP produced by myeloma plasma cells regulates their survival and pro-osteoclast activity for bone disease progression. J Bone Miner Res 2014; 29: 55–66. [DOI] [PubMed] [Google Scholar]
- 73. Stucci LS, D’Oronzo S, Tucci M, et al. Vitamin D in melanoma: controversies and potential role in combination with immune check-point inhibitors. Cancer Treat Rev 2018; 69: 21–28. [DOI] [PubMed] [Google Scholar]
- 74. Gonzalez E. Vitamin D receptor ligand therapy in chronic kidney disease. Clin Nephrol 2008; 70: 271–283. [PubMed] [Google Scholar]
- 75. Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 2016; 7: 10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018; 359: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018; 359: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359: 91–97. [DOI] [PubMed] [Google Scholar]
- 79. Shrestha E, White JR, Yu SH, et al. Profiling the urinary microbiome in men with positive versus negative biopsies for prostate cancer. J Urol 2018; 199: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Atkins MB, Clark JI, Quinn DI. Immune checkpoint inhibitors in advanced renal cell carcinoma: experience to date and future directions. Ann Oncol 2017; 28: 1484–1494. [DOI] [PubMed] [Google Scholar]