Abstract
Background:
Poor oral hygiene is strongly associated with oral and systemic diseases. Alongside mechanical tooth cleaning, the adjunctive use of mouthrinses has been widely advocated. Although research on the efficacy of various mouthrinse formulations is very active, there are a lack of conclusive data regarding their adverse effects.
Methods:
We undertook a systematic review in accordance wih PRISMA guidelines of electronic databases of clinical trials of any duration with daily home use of mouthwashes, presenting clinical and subjective side effects (PROSPERO registration: CRD42016054037).
Results:
After evaluating 614 titles and abstracts, 154 studies were selected for full-text analysis; 85 final papers were included. Based on the active ingredient in the test product, nine categories were created: cetyl pyridinium chloride, essential oils, chlorhexidine, triclosan, natural products, diclofenac, fluorides, delmopinol, and miscellaneous active substances. Most of the studies were of short duration (less than 6 months) with a defective ‘methods’ description; the reporting of adverse events often being overlooked. Both local morphological (oral mucosa and dental-crown staining, mucosal lesions) and functional (taste modifications, abnormal oral sensation) alterations were reported. Tooth staining was the most commonly listed adverse event, but it was quantitatively assessed only in a very small number of papers; most studies relied on patient reports. Staining was time associated; the longer the study, the higher its reported incidence and severity.
Conclusions:
The reduced report of side effects may partly be due to a lack of an objective measure and lack of general guidelines that demand studies report their adverse events. The most frequently reported adverse effect was teeth staining. As in most studies, the effect was associated with trial duration; clinical trials should be of sufficient duration. New investigations meeting the suggested criteria of a minimal duration of 6 months should be planned.
Keywords: adverse effects, chemical compounds, mouthrinse, oral health, oral staining
Introduction
Oral health is one of the essential components of general health, and is strictly dependent on oral hygiene that can be maintained only through regular, daily, home-based care.1 Good oral hygiene first requires a proper home regimen to maintain plaque-free tooth surfaces, aided occasionally by professional debridement of plaque and calculus. Although mechanical oral hygiene (MOH) at home remains the single, most reliable means of cleaning teeth, use of mouthrinses has been widely advocated as an adjunctive treatment since the bacterial nature of plaque was first understood.2,3 Their use helps to reduce caries, dental plaque and gingivitis with respect to dental, gingival and oral mucosal tissues.4,5 Moreover, they support treating those tooth surfaces that are inaccessible by MOH.2 They also have a high level of acceptance among the public due to their ease of use and breath-freshening effect.6 They are widely used concomitantly with mechanical self-care practices, for both medium- and long-term use.6,7
Mouthrinses are frequently prescribed by dentists.8 However, the market offers a multiplicity of over-the-counter mouthrinses that are freely used by patients without medical supervision.7 As for all substances and practices, patients should be made aware not only of their positive effects but also of the relevant side effects and negative consequences.9
In general, along with a common belief about the effectiveness of most of the current mouthwashes, most of the formulations have some side effects, such as staining, alteration of taste, mucosal desquamation, etc.5 Although research about mouthrinses is very active, with abundant literature being available in the form of systematic reviews and meta-analyses on the efficacy of various formulations,5 there are a lack of conclusive data about their adverse effects, especially when they are used as a complement to MOH in healthy subjects.
Therefore, the purpose of this systematic review was to analyse the evidence regarding the adverse effects of various formulations of home-use mouthrinses to offer a background information for both the choice of the most suitable molecules for each clinical situation, and the possible development of new formulations. In our review, we want to provide information about (PICO questions): systemically healthy patients (Population), who experienced home use of mouthwashes (Intervention) that were compared with a control formulation (Comparison) aiming to detect self-reported or clinically assessed adverse effects (Outcomes).
Material and methods
A detailed protocol was designed and registered in PROSPERO (http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016054037), aiming to answer the following focused PICO question:
(1) Population: systemically healthy patients;
(2) Intervention: home use of mouthwashes;
(3) Comparison: positive or negative control mouthrinse;
(4) Outcomes: adverse effects: self-report or clinical detection of signs or symptoms.
Eligibility criteria
Inclusion criteria
(1) Types of studies and participants: clinical trials of any duration with at least 10 healthy adult patients (aged 18 or older).
(2) Type of intervention: daily home use of mouthwashes alone or versus positive or negative control mouthwashes.
(3) Type of outcome measures:
(a) clinical side effects, such as mucosal irritations, discoloration of tooth and tongue surfaces, calculus development, parotid swelling, increased blood pressure;
(b) subjective reporting of side effects: pain, burning sensation, pruritus, dryness of mouth, taste and sensation disturbances, halitosis;
(4) Language: papers in English, French, Spanish, Italian and Chinese were included.
Exclusion criteria
(1) Patients wearing fixed or removable orthodontic appliances.
(2) Studies assessing abutment teeth of fixed or removable prosthesis.
(3) Reviews (historical or systematic), opinion letters, case reports, case series, congress abstracts.
(4) Studies not reporting adverse or negative side effects.
Information sources and search
Electronic search
Three electronic databases were used in the search for studies satisfying the inclusion criteria for studies published until April 2017: The National Library of Medicine (MEDLINE via Pubmed), Cochrane Central Register of Controlled Trials and Embase. The search was limited to human subjects. The search strategy employed was the following:
((‘Mouthwashes’ [Mesh] OR mouthwash* OR ‘mouth wash*’ OR mouthrinse* OR ‘mouth rinse*’) AND (‘Periodontitis’ [Mesh] OR periodontal OR ‘gingivitis’[MeSH] OR gingival OR ‘mucositis’[MeSH] OR mucosal OR ‘Halitosis’[Mesh] OR ‘oral malodor’ OR ‘peri-implantitis’[MeSH] OR peri-implant) AND (‘Drug-Related Side Effects and Adverse Reactions’[Mesh] OR ‘adverse effects’ [Subheading] OR adverse event* OR side effect*)).
Hand search
A hand search was also conducted spanning the dates January 2001 to April 2017, in the following publications: Journal of Clinical Periodontology, Journal of Periodontology, Journal of Periodontal Research, International Journal of Dental Hygiene, Expert Opinion on Pharmacotherapy, Expert Opinion on Drug Safety and Expert Opinion on Drug Delivery. Cross references were also considered, together with the personal collections of the authors.
Study selection
All the resulting articles were exported to Covidence Systematic review software (Veritas Health Innovation, Melbourne, Australia) and were assessed for eligibility by two reviewers independently (CM, GMT), who screened the titles and abstracts for possible inclusion in the review according to the inclusion criteria listed above. The agreement between the reviewers was also calculated (kappa). Reviewers were first trained and calibrated for study screening against another reviewer (ST) with experience in conducting systematic reviews. Abstracts were excluded if they did not fulfil the inclusion criteria. To avoid the exclusion of potentially relevant articles, abstracts not providing the required information were included in the full-text analysis.
Full texts of potentially relevant studies were independently assessed by the same reviewers. Any disagreement was resolved by discussion between reviewers. Interobserver agreement was assessed by means of kappa scores.
Data extraction
Data were extracted independently by two reviewers. Contents of the data extraction included:
(1) basic information of the paper: title; authors; journal information;
(2) eligibility reassessment: all the items in inclusion criteria; final decision;
(3) study design: methods of randomization; allocation concealment; blinding; funding;
(4) participants’ information: inclusion and exclusion criteria of the trial; demographic characteristics (number, age, sex, etc.); baseline status;
(5) intervention and comparison: intervention and control groups; active principle used in the test group; follow-up period; number of participants lost to follow up and the reasons;
(6) outcome: outcome variables; assessment method; observation time and detailed results;
(7) providing a quantitative description of results, the outcome variables from the various studies were scrutinized to be possibly analysed by a meta-analysis strategy.10
Authors of the primary studies were consulted to obtain any further information not available in the paper. When the study results were published more than once or results were presented in multiple publications, the most complete dataset was identified, and data were included only once.
Due to the high number of studies, they were classified according to the active molecule of the test product. Thus, nine categories were created: cetyl pyridinium chloride (CPC), essential oils (EO), chlorhexidine (CHX), triclosan, natural products, diclofenac, fluorides, delmopinol and miscellanea (active substances used in just one study). Studies sharing the same active principle were analysed together and those studies in which different active molecules were tested were included in more than one category.
Quality assessment (risk of bias in individual studies)
Quality assessment of the included studies was performed according to the Cochrane Handbook for Systematic Reviews of Interventions,10 using the RevMan tool. Six main quality domains were evaluated: random sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); and selective reporting (reporting bias). A seventh field named ‘other sources of bias’ was defined as ‘financial conflict of interests’. Depending on the descriptions given for each individual item, they were rated as: low, unclear or high risk of bias. The item ‘financial conflict of interests’ of those studies funded by the industry was rated as high risk.
Results
Search
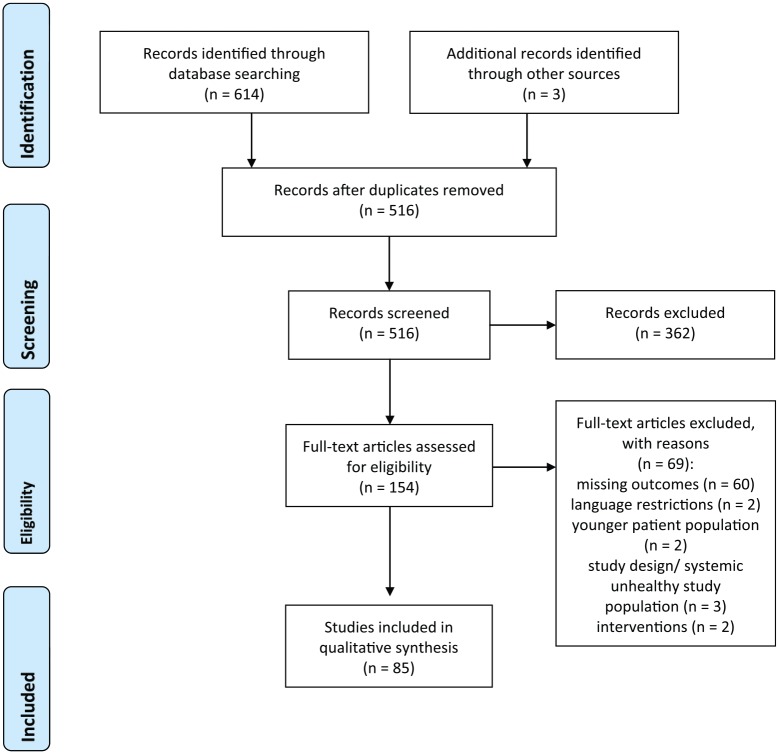
The initial search retrieved 614 titles. After eliminating duplicates, 513 titles remained and 3 articles were found through manual search. After the evaluation of titles and abstracts, 154 studies were selected for full-text analysis (kappa = 0.82). After this analysis, 85 final papers were included (kappa = 0.79). Reasons for exclusion were: missing outcomes (no information on adverse effects provided); language restrictions; younger patient population; study design/systemic unhealthy study population; intervention. Figure 1 depicts the study flow chart.
Figure 1.
Flow diagram (PRISMA format) depicting the screening and selection process.
Although a meta-analysis strategy was planned, the outcome variables found across the different studies did not allow combining them using meta-analysis. Therefore, a qualitative description of the results is presented.
Description of the included studies and the adverse events
Based on the active ingredient in the test product, nine categories were created (Supplementary Table 1). Studies testing more than one active molecule (n = 31) were included in more than one category (27 studies belonged to 2 categories and 4 studies belonged to 3). This is the reason the total number of categorized studies exceeds the number of included studies (n = 85).
Supplementary Table 2 reports a description of the studies (design, details of the studies, intervention and comparators, duration of follow up and numerical results for adverse effects). In most of the investigations (57 out of 85), the subjects performed MOH (toothbrushes, flossing) together with the tested mouthrinses. In 20 studies, only mouthrinses were used, while no specific information was reported in 8 studies. Twenty-four publications reported the relevant intergroup significance of adverse events; the findings are listed in Supplementary Table 3.
CPC
Mouthwashes with CPC were analysed in 20 studies between 1998 and 2014, with more than 750 patients involved. Study duration ranged from 2 weeks to 6 months, with 6 months being the follow-up period in six studies only. In all these last studies, MOH was performed by the subjects (Supplementary Table 2).
Short-term studies caused no teeth staining11,12 or moderate teeth staining13,14 while in long-term studies, staining was observed or reported in CPC users.15,16 Significant (or nearly significant) differences were reported in four studies (Supplementary Table 3).15,17–19
Several studies reported burning and taste alteration in CPC patients,11,13–15,17,20,21 and only two15,20 reported these side effects in placebo groups. Dysguesia and glossodynia were reported in six control-group patients and three CPC group patients (Supplementary Table 2).22–24
In a few studies, mouth ulcers were reported by patients belonging to both CPC11,22 and other mouthrinse groups.11,21,25 Other occasional adverse effects were observed or reported by both the mouthrinse14,15,17,22,25,26 and placebo patients 11,14,15,22 (Supplementary Table 2).
Five studies did not report specific adverse effects;18,28–31 in three of these, no significant differences in adverse effects between groups were reported,18,28,31 while 13 CPC and 14 control patients out of 151 were lost because of adverse effects.29
EO
The effectiveness of EO on periodontal outcomes were analysed in 18 studies between 1991 and 2014, with more than 1000 patients involved (Supplementary Table 2). All studies were a parallel design with a control group, except two that were crossover studies31,32 and one that was without a control group.33 Seven studies compared more than two arms.14,22–24,31,32,34,35
In the majority of studies, MOH was performed in adjunct with mouthrinsing; treatment ranged from 2 weeks to 6 months, and adverse effects were reported already at the 1-month evaluation point or less.14,31,32,34,36–39 Overall, tooth staining, calculus, modifications in taste, altered oral sensation and mucosal lesions were the most frequently reported side effects (Supplementary Table 2).
Intergroup significance of adverse events was reported for tongue alterations and tooth staining: in both occasions, the effects were larger for CHX- than for EO-based mouthwashes (Supplementary Table 3). After 3 months, 35% of the patients analysed by Kerr and colleagues experienced an adverse effect in the group using a nonalcohol EO mouthrinse and 7% in the group using an alcohol EO preparation.40 Adverse effects were reported also for shorter studies: after 2 weeks of treatment, taste problems, mouth ulcer and burning were recorded.14 Moran and colleagues reported occasional complaints of taste in a 3-week study.31 In a study with a similar duration, 21% of 55 patients in the CHX (control) group and 22% in the EO group experienced altered taste; a mild burning sensation was also reported by 14% of patients in the EO group. Generally, 62% of patients experienced at least one adverse effect in the EO group and 32% in the control group.38 In contrast, in a longer, 3-month study, no differences between groups were detected (by evaluation of a questionnaire).41
Macroscopically abnormal, oral, soft-tissue conditions were reported in some studies,22,36,37,39 which were more frequent after 28 days of treatment37 than after 6 months.39 In contrast, Cortelli and colleagues followed up with a study of more than 400 patients over a 6-month period, reporting several oral morphological and functional alterations.22At the same time, in a 6-month study, no cytological changes were induced by two different formulations of EO (with and without alcohol).42
During a 3-month trial, Lauten and coworkers described one case of light-headedness which they thought could possibly be related to the EO mouthrinse.33
CHX
CHX was used as the main ingredient or as a positive control (in approximately three quarters of the studies) in 42 articles published between 1991 and 2014. Duration of the CHX trials ranged from just 4 days41–43 to 6 months.28,44 There were only two studies each that had experimental periods of 3 and 6 months, respectively. Nine trials used a crossover design with the sample size of the included studies ranging from 11 to 366 patients.28,36 Sixteen studies that tested the efficacy of CHX had more than one active ingredient (Supplementary Table 2). Tooth staining was the most commonly assessed and reported adverse event, closely followed by taste alteration. In 12 studies, only mouthrinsing was allowed: most of them lasted 3 weeks or less; only one study lasted longer, at 44 days (Supplementary Table 2).
Only six studies did not assess or report tooth staining as an adverse event.21,28,36,45–47 Among the 35 remaining studies that reported dental staining, 25 used a validated index, most commonly the Lobene index. In general, subjects using CHX reported more staining on their teeth than subjects using other molecules or placebo; rinses of different CHX concentrations did not change staining intensity.27,48–51 However, the addition of an antidiscoloration system to CHX was found to be effective in reducing teeth staining without altering its antigingivitis effectiveness.52–55 A similar effect was observed for the addition of PVP (polyvinylpyrrolidone); the staining decreased with the increase in PVP concentration.43 In contrast, the addition of either CPC or sodium fluoride (NaF) to CHX had unfavourable effects on plaque accumulation, with a significantly larger number of participants reporting tongue staining and increases in supragingival calculus (Supplementary Table 3).11
Brecx and colleagues found more staining with CHX rinses than with an amine/stannous fluoride solution (Meridol®) and a placebo, but CHX was more effective on other clinical indices than Meridol.56 Similar observations were made in studies comparing CHX with EO,30,35,57 hexatidine,58,59 natural compounds,60–62 triclosan35,63 and delmopinol.60,64 In contrast, no differences in staining caused by CHX in comparison with placebo18 nor AmF/SnF2 (americium fluoride/stannous fluoride)65 were reported. Kim and coworkers described more staining in DXAMase (glucanhydrolase–dextranase + amylase) users than in CHX users (Supplementary Table 2).66
In those studies where CHX was compared for its effectiveness versus placebo, CHX users reported more teeth staining. Only after 7 days of use, more tooth staining, tongue discoloration and taste alteration were observed in subjects using CHX; alterations increased with the duration of CHX use, while no changes occurred in the placebo group.17,67–69 Leyes Borrajo and colleagues and Zimmer and colleagues compared the effectiveness of two CHX mouthrinses (with and without alcohol) with placebo; more people belonging to the CHX categories reported teeth staining than those using placebo.19,70 No differences in staining were reported by Lorenz and coworkers.71 Joyston-Bechal and Hernaman found a decrease in staining after 8 weeks of CHX use, but the variation was significant only in the placebo group.72
In addition to those studies that used validated staining indices, some used patient self-reports, and reported greater staining in CHX users than those belonging to the comparison group.73,74 Other sporadic adverse events of CHX were apthous ulcers,21 tongue lesions and discoloration,28,36,46 taste disturbances, glossodynia and oral paraesthesia,21,28,45,62 and dry mouth.45Zimmer and colleagues also observed some gastrointestinal symptoms in CHX users.19
Triclosan
Seven studies were included published in the last 3 decades. These studies involved 200 patients (cumulative) who used mouthrinses with triclosan as the active ingredient. Its activity was compared with either placebo,75–77 CHX34,46,63 or EO.32 In four studies, lasting 4 to 21 days, the patients did not perform MOH (Supplementary Table 2). Only one study reported on the long-term (6 months) effects of triclosan, with a variety of adverse events described by the study participants.76 In the other investigations, mucosal lesions similar to apthous ulcers, leucoplakia and poorly defined tongue lesions were consistently reported. In a report by Waaler and coworkers, the majority of the test particpants’ complaints consisted of severe-to-mild adverse reactions from the combination of triclosan and sodium lauryl sulphate, although they did demonstrate this formulation was highly efficacious in controlling dental plaque.77
Natural products
In order to avoid the adverse side effects of CHX, the World Health Organization advised researchers to investigate natural products such as herb and plant extracts (NP) for efficacy as inhibitors of supragingival plaque formation and the development of gingivitis. In response to this call, seven studies published during the last 15 years investigated various mouthrinses containing NPs focusing on antimicrobial efficacy and related side effects in a total population of approximately 300 patients.
Five studies combined mouthwashes and MOH (Supplementary Table 1). None of the formulations tested were of 6 months’ duration. The longest study lasted for 14 weeks, investigating the active ingredient sanguinarine.78 The studies either used CHX, EO or CPC as control active ingredients. Two studies lasting 4 weeks each described comparable levels of teeth staining, as reported with CHX,48,73 while a 3-month investigation did not describe teeth staining with the test formulation.62 Taste disturbances were reported by Bhat and coworkers in 9% of their 24 patients.73 Other vaguely described altered oral sensations were reported by subjects using herbal mouthwashes.14 In all cases, the reported side effects were significantly less frequent than those observed in the control groups.
Diclofenac
Only two studies tested a mouthwash containing diclofenac, in two small groups of patients who underwent oral or periodontal surgery.79,80 After 7 days of treatment, some patients in one of the studies reported mild oral burning.79 No information about oral hygiene was reported.
Fluorides
Literature investigating the side effects of fluorides are much more recent: out of the 10 retrieved papers, half were published in the current century.11,63,65,81,82 Overall, the observation times ranged from 3 weeks (one study) to 7 months (one 6 and one 7 months), and around 300 individuals were involved in the studies; in all investigations but one, the mouthrinse use was paired with MOH (Supplementary Table 2). In this last study, a group of healthy volunteers refrained from brushing for 3 weeks and used a mouthwash with NaF and CHX: their tongue staining was found to significantly increase (Supplementary Table 3).11
Patients using NaF and tin fluoride (SnF) mouthrinses had a significantly lower tooth-staining level than patients using CHX.56,63 In contrast, Horwitz and colleagues did not report significant differences between CHX or AmF/SnF.65 According to Ciancio and coworkers, the use of SnF did not significantly increase dental staining.83 In contrast, staining was a side effect described for an AmF/SnF-containing mouthrinse used twice daily.81 After a 6-month observation period, Hasturk and colleagues did not report adverse effects during the use of a fluoridated hydrogen-peroxide-based mouthrinse; however, staining of teeth or tissues increased during the experiment.82
In other investigations, oral soreness, aphthous ulcers and calculus63 were listed together with dental discoloration.25Additionally, Zimmermann and coworkers reported ulcerations in 4–8% of their 120 patients with chronic gingivitis.84
Delmopinol
A handful of relatively old studies assessed the effect of delmopinol on plaque formation, comparing its activity to either CHX44,64,74,85 or placebo.85–87 In these studies, treatment duration ranged from 2 weeks to 6 months (three studies), with approximately 300 individuals in total. Two 14-day studies used only the mouthwashes for oral hygiene, while the longest studies included also MOH (Supplementary Table 2). In comparison with placebo, a greater number of delmopinol mouthrinse users reported paraesthesia and altered taste sensations in the oral mucosa, together with staining. In the longest studies, some reduction in side effects during the 3- to 6-month visit was reported.44,87 The same trend was not confirmed by Hase and colleagues.64 However, as a confounder, the particpants in this last paper were about 10 years younger (on average) than those of the other two studies.
Miscellanea
Some active ingredients were investigated in only one study, with a range of adverse effects being reported. The duration of these eight studies, investigating eight separate active ingredients, ranged from 3 to 14 weeks, with one study assessing the immediate effect of different concentrations of alcohol on oral pain.88 The participants in four investigations performed MOH together with mouthwash use in their 3- to 8-weeks trials, while other two studies limited the intervention to mouthwash use only (Supplementary Table 2),
In a short-term trial, probiotics at different concentrations resulted in high levels of both local and general adverse effects (30–50%).89 The use of a mouthwash formula with hydrogen peroxide showed high levels of tongue alterations.90 In a 4-day plaque regrowth study, Lorenz and coworkers reported discoloration of the tongue and unpleasant taste after the use of N-chlorotaurine (NCT).47
Scully and colleagues tested a special population positive for oral candidiasis, reporting an unacceptable level of mucosal irritation; although plaque reduction and absence of tooth staining were also recorded.91 Oral mucosal lesions, as well as relatively disparate general adverse effects, were reported by other studies.66,92,93
Risk of bias in individual studies
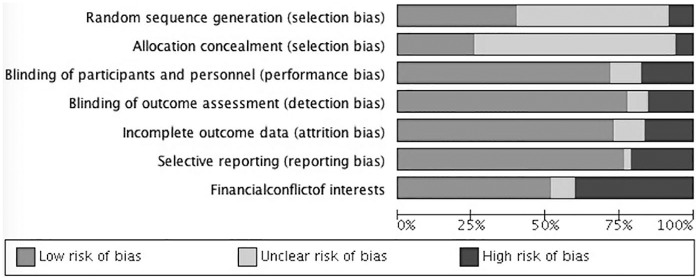
Figure 2 is the risk-of-bias graph depicting percentages of authors’ judgements regarding each risk-of-bias item across all the 85 included studies. When analysing each study individually, only 1 out of 85 had a low risk of bias for all the 7 fields. However, seven studies had a low risk of bias for the six main criteria (except other sources of bias: financial conflict of interest). Three more studies had low risk of bias in six out of seven criteria. The remaining studies (n = 74) had a high or unclear risk of bias in two or more fields. Supplementary Figure 1 shows the risk-of-bias summary for each included study.
Figure 2.
Risk-of-bias graph: review authors’ judgements about each risk-of-bias item presented as percentages across all included studies.
CPC
Only 1 out of 20 studies in this category showed low risk of bias for all the seven domains considered.25 Four studies showed low risk of bias for the six main domains, except for the seventh field ‘financial conflict of interest’.12,16,17,21 Three studies showed high risk of bias in four categories.22–24
EO
Only 1 out of the 18 studies in this category showed low risk of bias for the six domains, except for the second field ‘allocation concealment’.34 Three studies showed low risk of bias for five domains except for the first two.33,37,42 Four studies showed high risk of bias in four categories.22–24,41
CHX
Out of the 42 studies included in this category, four studies showed low risk of bias for six of the seven domains.17,19,21,47Five studies showed high risk of bias in three categories.36,45,60,64,67
Triclosan
Out of the seven studies belonging to this category, one showed low risk of bias for six of the seven domains46 and two studies showed low risk of bias in five domains.62,63 The worst rated was one study with four domains evaluated as high risk of bias.76
Natural products
Among seven studies included in this category, one showed low risk of bias for six of the seven domains73 and three studies showed low risk of bias in five domains.14,62,78 The remaining three studies showed only three categories with low risk.48,60,61
Diclofenac
Among the only two studies included in this category, one demonstrated low risk of bias for five of the seven domains80 and the other showed a low risk of bias in three domains and unclear risk of bias for the remaining fields.79
Fluorides
One study out of 10 in this category was found to have a low risk of bias for all the assessed domains.25 Four studies showed low risk of bias for five domains,63,65,82,84 while one showed high risk of bias in two categories.81
Delmopinol
One out of seven studies in this group demonstrated low risk of bias for the six main domains, except for the seventh field ‘financial conflict of interest’.84 One study was found to have low risk of bias for five fields and unclear bias for the remaining two.94 Three studies showed high risk of bias in three categories.64,85,86
Miscellanea
Eight studies belonged to this category. One showed low risk of bias for the six main domains, except for the seventh field ‘financial conflict of interest’.91 Another study showed low risk of bias for six domains, as well.47 Two studies showed high risk of bias in four categories.89,95
Discussion
Principal findings
Regular oral hygiene self-care practices are the cornerstone for the maintenance of oral health (World Health Organization Global Oral Health Programme, http://www.who.int/oral_health/objectives/en/), and mouthrinses are commonly used as an adjuvant to mechanical tooth brushing. This systematic review was a compilation and analysis of the adverse events commonly reported as a result of the use of mouthrinses containing various active ingredients. The review was limited to the documented adverse effects reported in clinical trials investigating daily home use of mouthrinses.
In synthesis, the most frequently reported adverse events were local morphological (oral mucosa and dental-crown staining, mucosal lesions) and functional (taste modifications, abnormal oral sensation) alterations. In particular, staining seemed to be time associated; the longer the study, the higher the reported incidence and severity of this adverse event. This was particularly true for both CHX and CPC.
Indeed, CHX is the most widely used mouthrinse, followed by EO and CPC. CHX was synthesized for the first time in the 1950s and, despite advances in biochemistry and dental technology, it remains the active ingredient with the best antiplaque effect.9 It is often considered the standard for maintenance of oral hygiene. The most commonly assessed and reported adverse event was tooth staining, followed by taste and mucosal alterations. Adverse effects with the use of CHX were usually proportional to the duration of treatment.8 Of interest, some recent trials tested the addition of an antidiscoloration system to CHX, finding an effective reduction of dental staining without altering its clinical effect.11,52–55 A similar antistaining effect was reported for the combination of CHX and PVP at high concentrations.43
Staining was also reported in 6-month trials using mouthwashes containing CPC, while the effect was not detected in shorter studies. Oral burning, taste alterations, ulcers and stomatitis were also reported for this molecule.29 A similar set of adverse events followed the use of EO-based mouthwashes, and patients using diclofenac complained of oral burning.
Taste alterations and mucosal irritations were frequent side effects of triclosan. Indeed, this molecule has been recently removed from use in the EU and USA.96,97 Similar adverse events were listed for the natural products, but the longest study lasted only for 14 weeks. Taste disturbances, altered oral sensations, feeling of sickness were all reported with a significantly less frequent occurrence than in the control groups using either CHX, or CPC and EO.
The use of fluoride-based mouthwashes was associated with a variety of oral alterations, both mucosal (ulcerations) and dental (discoloration), while staining, paraesthesia and altered taste sensations were reported after delmopinol use. The active ingredients of the miscellanea group (investigated in just one study) were also associated with both local and systemic side effects.
Although research about the efficacy of various mouthwash formulations is very active, conclusive data about their adverse effects are still missing. When planning this review, we made a preliminary PubMed search using the words ‘mouthwash’ and ‘side effects’. A total of 2794 full-text papers, published between 1956 and 2016, were found (https://www.ncbi.nlm.nih.gov/pubmed/). The majority of them were published in the last 8–9 years; 50% of them appeared in 2008 and subsequent years. Nonetheless, less than 15% of them were actually analysed in this review, mostly because of insufficient descriptions of experimental and clinical procedures. One example is the detailed reporting of all the interventions of oral care: eight of the analysed studies did not inform about the use of MOH, together with the mouthrinse preparations, thus making a specific analysis difficult. In general, longer studies combined chemical and mechanical oral care, while in the shorter ones, both protocols were reported, with a clear prevalence of mixed practices.
Therefore, literature has not provided a shared consensus about the best active substances that may be continually used to maintain oral health without significant local or general adverse effects.
Indeed, together with the variety of proposed molecules, there are no actual systematic reviews dealing with the side effects of mouthrinses available on the market, leaving both dentists and patients alike, unaware of the scientifically proven risks of these medicaments.
Review limitations
One major concern is that the majority of studies were of short duration, with few studies lasting for more than 6 months, the minimal duration suggested by the Council of Dental Therapeutics criteria.98
Among the studies included in the current review, only mouthrinses containing CPC (6 studies out of 20), EO (4 studies out of 18), CHX (2 studies out of 42), delmopinol (3 studies out of 7), triclosan (1 study out of 7) and fluorides (1 study that lasted 6 and 1 for 7 months out of 10) had at least 1 investigation of recommended study duration (6 months). None of the tested formulations containing natural products, diclofenac or miscellanea (active substances with only one study) met the criteria for appropriate follow-up length.
We retrieved the investigations from a selected set of internationally recognized databases, as well as from peer-reviewed journals devoted to research in the periodontal and related fields. Even if we also scrutinized our personal collections of papers, no systematic search was performed on grey literature, and this should be acknowledged as a study limitation.
Another concern is the risk of bias in the study protocols; the main problem found in the analysed papers is a defective methods description that impedes the actual estimation of all the domains suggested by the existing literature.10 Notwithstanding the existence of several controlled studies about the use of mouthwashes, a correct and complete report of the relevant adverse effects is still missing. This is despite the fact that the CONSORT harm-reporting guidelines99 have been published since 2004; none of the articles included in this review made a reference to, or used, these guidelines. Further, the reporting of adverse events was often overlooked and it was very difficult to summarize the side effects of the included studies due to an overall lack of uniform reporting criteria. This might also partly be explained by a lack of objective measures.99 Indeed, very few studies performed an objective assessment of the patients’ oral cavity to examine and report on signs of adverse reactions; most studies relied on patient reports. Even tooth staining, the most commonly listed adverse event, was assessed by a quantitative clinical index (Lobene index) only in a very small number of papers.15,48,57,83
Indeed, although we planned to perform a meta-analysis, the different studies presented a variety of outcome variables that could not be combined in a quantitative synthesis; results were described from a qualitative point of view.
Among all the included studies, only one study had a low risk of bias for all of the seven analysed categories;25 a majority of the studies had unclear, undetailed experimental procedures, and in most instances, the selection bias for random sequence generation and allocation concealment could not be estimated. Another related limitation is the risk of bias due to possible financial conflict of interests: around 40% of studies had a high risk of bias in this domain. At the same time, the risks of performance, detection, attrition and reporting biases were judged low for most of the papers.
As stated before, the use of standardized reports may help in a better description of the experiments, decreasing the ‘unclear’ risk category.99 Most importantly, standardized guidelines can help researchers in better planning and execution of their trials.
Meaning of the study and implications for clinicians, patients and policymakers
As summarized before, the most frequently reported adverse effect was staining of teeth and oral cavity: in most studies using CHX and CPC, the effect depended on the duration of the trial. This finding stresses the necessity of clinical trials of sufficient duration, as short-term trials may miss this side effect. New investigations meeting the suggested criteria of a minimal duration of 6 months should be planned.98 Some interesting developments for research are focusing on combinations of chemicals active against plaque and gingivitis but with a reduced staining.11,43,52–55
At the same time, actual duration of mouthrinse use in daily practice is an important factor influencing the extent of adverse events.5,8 Consequently, CHX is recommended for acute or short-term use while mouthrinses containing EOs and CPC are used for long-term or maintenance treatment,5 in spite of having reduced efficacy in comparison with CHX.9
Indeed, as staining is mostly an aesthetical side effect, some mixtures of active ingredients that combine effective antiplaque and antigingivitis actions with a reduced oral discoloration need to the investigated more in detail and with longer studies.11,43,52–55
Unanswered questions and future research
We reiterate that the current review focused on studies performed in systemically healthy adults. Papers about the adverse effects in patients with major systemic diseases, in special-needs groups, in children and adolescents, during pregnancy and lactation were not selected, and the outcomes may be different from the current ones. That may be the topic of future investigations.
In conclusion, further in vivo investigations should be planned, especially for the long-term use of these preparations. In general, there is a need for establishment of a system for reporting adverse events associated with the use of mouthrinses in an objective manner. Although guidelines exist for the need to report adverse events in the use of therapeutic devices or products (e.g. Australia https://www.tga.gov.au/reporting-adverse-events and Italy http://www.aifa.gov.it/content/come-segnalare-una-sospetta-reazione-avversa), they are seldom followed. Additionally, considering the large amount of over-the-counter preparations and the general unawareness of their belonging to the ‘drugs’ category, it is very important that patients report adverse events to national agencies or to their dentists. At the same time, there were many studies that have not reported or addressed anything about adverse events, while some choose to limit the reporting to a single statement (‘no adverse events reported’). There is a need for journals to encourage authors to conform to CONSORT harm-reporting guidelines.
Supplemental Material
Supplemental material, review_MW_Supplementary_Table_1_final for Adverse events associated with home use of mouthrinses: a systematic review by Gianluca M. Tartaglia, Santosh Kumar Tadakamadla, Stephen Thaddeus Connelly, Chiarella Sforza and Conchita Martín in Therapeutic Advances in Drug Safety
Supplemental Material
Supplemental material, review_MW_Supplementary_Table_2_final for Adverse events associated with home use of mouthrinses: a systematic review by Gianluca M. Tartaglia, Santosh Kumar Tadakamadla, Stephen Thaddeus Connelly, Chiarella Sforza and Conchita Martín in Therapeutic Advances in Drug Safety
Supplemental Material
Supplemental material, review_MW_Supplementary_Table_3_final for Adverse events associated with home use of mouthrinses: a systematic review by Gianluca M. Tartaglia, Santosh Kumar Tadakamadla, Stephen Thaddeus Connelly, Chiarella Sforza and Conchita Martín in Therapeutic Advances in Drug Safety
Supplemental Material
Supplemental material, Supplemen_Fig_1_2 for Adverse events associated with home use of mouthrinses: a systematic review by Gianluca M. Tartaglia, Santosh Kumar Tadakamadla, Stephen Thaddeus Connelly, Chiarella Sforza and Conchita Martín in Therapeutic Advances in Drug Safety
Acknowledgments
The study was made by researchers working in Italy, Spain, USA and Australia.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Gianluca M. Tartaglia  https://orcid.org/0000-0001-7062-5143
https://orcid.org/0000-0001-7062-5143
Contributor Information
Gianluca M. Tartaglia, Department of Biomedical Sciences for Health, Functional Anatomy Research Centre (FARC), Università degli Studi di Milano, Via Luigi Mangiagalli 31, Milano, MI 20133, Italy Private Practice, SST Dental Clinic, Via Martiri della Libertà 58, 20090 Segrate, MI, Italy.
Santosh Kumar Tadakamadla, Menzies Health Institute Queensland, Griffith University, Nathan, QLD, Australia.
Stephen Thaddeus Connelly, San Francisco Veterans Affairs Health System, University of California San Francisco, San Francisco, CA, USA.
Chiarella Sforza, Department of Biomedical Sciences for Health, Università degli Studi di Milano, Milano, Italy.
Conchita Martín, Facultad de Odontología, Universidad Complutense de Madrid, Madrid, Spain.
References
- 1. Lockhart PB, Brennan MT, Thornhill M, et al. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc 2009; 140: 1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gurenlian JR. The role of dental plaque biofilm in oral health. J Dent Hyg 2007; 81: 1–11. [Google Scholar]
- 3. Loe H. Oral hygiene in the prevention of caries and periodontal disease. Int Dent J 2000; 50: 129–139. [DOI] [PubMed] [Google Scholar]
- 4. Shahid M. Regular supervised fluoride mouthrinse use by children and adolescents associated with caries reduction. Evid Based Dent 2017; 18: 11–12. [DOI] [PubMed] [Google Scholar]
- 5. Tartaglia GM, Kumar S, Fornari CD, et al. Mouthwashes in the 21st century: a narrative review about active molecules and effectiveness on the periodontal outcomes. Expert Opin Drug Deliv 2017; 14: 973–982. [DOI] [PubMed] [Google Scholar]
- 6. Van der Weijden FA, Van der Sluijs E, Ciancio SG, et al. Can chemical mouthwash agents achieve plaque/gingivitis control? Dent Clin North Am 2015; 59: 799–829. [DOI] [PubMed] [Google Scholar]
- 7. Wilder RS, Bray KS. Improving periodontal outcomes: merging clinical and behavioral science. Periodontol 2000 2016; 71: 65–81. [DOI] [PubMed] [Google Scholar]
- 8. Manipal S, Hussain S, Wadgave U, et al. The mouthwash war chlorhexidine vs. herbal mouth rinses: a meta-analysis. J Clin Diagn Res 2016; 10: ZC81–ZC83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Serrano J, Escribano M, Roldán S, et al. Efficacy of adjunctive anti-plaque chemical agents in managing gingivitis: a systematic review and meta-analysis. J Clin Periodontol 2015; 42: S106–S138. [DOI] [PubMed] [Google Scholar]
- 10. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. London: The Cochrane Collaboration, 2011. http://handbook.cochrane.org. [Google Scholar]
- 11. Bascones A, Morante S, Mateos L, et al. Influence of additional active ingredients on the effectiveness of non-alcoholic chlorhexidine mouthwashes: a randomized controlled trial. J Periodontol 2005; 76: 1469–1475. [DOI] [PubMed] [Google Scholar]
- 12. Rioboo M, García V, Serrano J, et al. Clinical and microbiological efficacy of an antimicrobial mouth rinse containing 0.05% cetylpyridinium chloride in patients with gingivitis. Int J Dent Hyg 2012; 10: 98–106. [DOI] [PubMed] [Google Scholar]
- 13. Hu CZ, Jin HL, Liang JP, et al. Analysis for clinical effect of a rinse containing cetylpyridinium chloride in treatment of gingivitis and periodontitis. Shanghai Kou Qiang Yi Xue 2003; 12: 414–418. [PubMed] [Google Scholar]
- 14. Samuels N, Grbic JT, Saffer AJ, et al. Effect of an herbal mouth rinse in preventing periodontal inflammation in an experimental gingivitis model: a pilot study. Compend Contin Educ Dent 2012; 33: 204–206, 208–211. [PubMed] [Google Scholar]
- 15. Costa X, Laguna E, Herrera D, et al. Efficacy of a new mouth rinse formulation based on 0.07% cetylpyridinium chloride in the control of plaque and gingivitis: a 6-month randomized clinical trial. J Clin Periodontol 2013; 40: 1007–1015. [DOI] [PubMed] [Google Scholar]
- 16. Van Leeuwen MP, Rosema NA, Versteeg PA, et al. Long-term efficacy of a 0.07% cetylpyridinium chloride mouth rinse in relation to plaque and gingivitis: a 6-month randomized, vehicle-controlled clinical trial. Int J Dent Hyg 2015; 13: 93–103. [DOI] [PubMed] [Google Scholar]
- 17. Santos S, Herrera D, Lopez E, et al. A randomized clinical trial on the short-term clinical and microbiological effects of the adjunctive use of a 0.05% chlorhexidine mouth rinse for patients in supportive periodontal care. J Clin Periodontol 2004; 31: 45–51. [DOI] [PubMed] [Google Scholar]
- 18. Escribano M, Herrera D, Morante S, et al. Efficacy of a low-concentration chlorhexidine mouth rinse in non-compliant periodontitis patients attending a supportive periodontal care programme: a randomized clinical trial. J Clin Periodontol 2010; 37: 266–275. [DOI] [PubMed] [Google Scholar]
- 19. Zimmer S, Korte P, Verde P, et al. Randomized controlled trial on the efficacy of new alcohol-free chlorhexidine mouthrinses after 8 weeks. Int J Dent Hyg 2015; 13: 110–116. [DOI] [PubMed] [Google Scholar]
- 20. Herrera D, Santos S, Ferrus J, et al. Efficacy of a 0.15% benzydamine hydrochloride and 0.05% cetylpyridinium chloride mouth rinse on 4-day de novo plaque formation. J Clin Periodontol 2005; 32: 595–603. [DOI] [PubMed] [Google Scholar]
- 21. Hu D, Li X, Sreenivasan PK, et al. A randomized, double-blind clinical study to assess the antimicrobial effects of a cetylpyridinium chloride mouth rinse on dental plaque bacteria. Clin Ther 2009; 31: 2540–2548. [DOI] [PubMed] [Google Scholar]
- 22. Cortelli SC, Cortelli JR, Wu MM, et al. Comparative antiplaque and antigingivitis efficacy of a multipurpose essential oil-containing mouthrinse and a cetylpyridinium chloride-containing mouthrinse: a 6-month randomized clinical trial. Quintessence Int 2012; 43: e82–e94. [PubMed] [Google Scholar]
- 23. Cortelli SC, Cortelli JR, Shang H, et al. Long-term management of plaque and gingivitis using an alcohol-free essential oil containing mouthrinse: a 6-month randomized clinical trial. Am J Dent 2013; 26: 149–155. [PubMed] [Google Scholar]
- 24. Cortelli SC, Cortelli JR, Shang H, et al. Gingival health benefits of essential-oil and cetylpyridinium chloride mouthrinses: a 6-month randomized clinical study. Am J Dent 2014; 27: 119–126. [PubMed] [Google Scholar]
- 25. Yates R, West N, Addy M, et al. The effects of a potassium citrate, cetylpyridinium chloride, sodium fluoride mouthrinse on dentine hypersensitivity, plaque and gingivitis. A placebo-controlled study. J Clin Periodontol 1998; 25: 813–820. [DOI] [PubMed] [Google Scholar]
- 26. Mankodi S, Bauroth K, Witt JJ, et al. A 6-month clinical trial to study the effects of a cetylpyridinium chloride mouthrinse on gingivitis and plaque. Am J Dent 2005; 18: 9A–14A. [PubMed] [Google Scholar]
- 27. Quirynen M, Avontroodt P, Peeters W, et al. Effect of different chlorhexidine formulations in mouthrinses on de novo plaque formation. J Clin Periodontol 2001; 28: 1127–1136. [DOI] [PubMed] [Google Scholar]
- 28. Stookey GK, Beiswanger B, Mau M, et al. A 6-month clinical study assessing the safety and efficacy of two cetylpyridinium chloride mouthrinses. Am J Dent 2005; 18: 24A–28A. [PubMed] [Google Scholar]
- 29. Albert-Kiszely A, Pjetursson BE, Salvi GE, et al. Comparison of the effects of cetylpyridinium chloride with an essential oil mouth rinse on dental plaque and gingivitis - a six-month randomized controlled clinical trial. J Clin Periodontol 2007; 34: 658–667. [DOI] [PubMed] [Google Scholar]
- 30. Witt JJ, Walters P, Bsoul S, et al. Comparative clinical trial of two antigingivitis mouthrinses. Am J Dent 2005; 18: 15A–17A. [PubMed] [Google Scholar]
- 31. Moran J, Pal D, Newcombe R, et al. Comparison of a phenolic and a 0.2% chlorhexidine mouthwash on the development of plaque and gingivitis. Clin Prevent Dent 1991; 13: 31–35. [PubMed] [Google Scholar]
- 32. Moran J, Addy M, Newcombe R. A 4-day plaque regrowth study comparing an essential oil mouthrinse with a triclosan mouthrinse. J Clin Periodontol 1997; 24: 636–639. [DOI] [PubMed] [Google Scholar]
- 33. Lauten J D, Boyd L, Hanson MB, et al. A clinical study: melaleuca, manuka, calendula and green tea mouth rinse. Phytother Res 2005; 19: 951–957. [DOI] [PubMed] [Google Scholar]
- 34. Ros-Llor I, Lopez-Jornet P. Cytogenetic analysis of oral mucosa cells, induced by chlorhexidine, essential oils in ethanolic solution and triclosan mouthwashes. Environ Res 2014; 132: 140–145. [DOI] [PubMed] [Google Scholar]
- 35. Parikh-Das AM, Sharma NC, Du Q, et al. Superiority of essential oils versus 0.075% CPC-containing mouthrinse: a two-week randomized clinical trial. J Clin Dent 2013; 24: 94–99. [PubMed] [Google Scholar]
- 36. Eldridge KR, Finnie SF, Stephens JA, et al. Efficacy of an alcohol-free chlorhexidine mouthrinse as an antimicrobial agent. J Prosthet Dent 1998; 80: 685–690. [DOI] [PubMed] [Google Scholar]
- 37. Borden LC, Chaves ES, Bowman JP, et al. The effect of four mouthrinses on oral malodor. Compend Contin Educ Dent 2002; 23: 531–6, 538, 540 passim; quiz 548. [PubMed] [Google Scholar]
- 38. Botelho MA, Dos Santos RA, Martins JG, et al. Comparative effect of an essential oil mouthrinse on plaque, gingivitis and salivary Streptococcus mutans levels: a double blind randomized study. Phytother Res 2009; 23: 1214–1219. [DOI] [PubMed] [Google Scholar]
- 39. Charles CH, Sharma NC, Galustians HJ, et al. Comparative efficacy of an antiseptic mouthrinse and an antiplaque/antigingivitis dentifrice. A six-month clinical trial. J Am Dent Assoc 2001; 132: 670–675. [DOI] [PubMed] [Google Scholar]
- 40. Kerr AR, Corby PM, Kalliontzi K, et al. Comparison of two mouthrinses in relation to salivary flow and perceived dryness. Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 119: 59–64. [DOI] [PubMed] [Google Scholar]
- 41. Cosyn J, Princen K, Miremadi R, et al. A double-blind randomized placebo-controlled study on the clinical and microbial effects of an essential oil mouth rinse used by patients in supportive periodontal care. Int J Dent Hyg 2013; 11: 53–61. [DOI] [PubMed] [Google Scholar]
- 42. Bagan JV, Vera-Sempere F, Marzal C, et al. Cytological changes in the oral mucosa after use of a mouth rinse with alcohol: a prospective double blind control study. Med Oral Patol Oral Cir Bucal 2012; 17: e956–e961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Claydon N, Addy M, Jackson R, et al. Studies on the effect of polyvinyl pyrrolidone on the activity of chlorhexidine mouthrinses: plaque and stain. J Clin Periodontol 2001; 28: 558–564. [DOI] [PubMed] [Google Scholar]
- 44. Lang NP, Hase JC, Grassi M, et al. Plaque formation and gingivitis after supervised mouthrinsing with 0.2% delmopinol hydrochloride, 0.2% chlorhexidine digluconate and placebo for 6 months. Oral Dis 1998; 4: 105–113. [DOI] [PubMed] [Google Scholar]
- 45. Jose A, Butler A, Payne D, et al. A randomised clinical study to evaluate the efficacy of alcohol-free or alcohol-containing mouthrinses with chlorhexidine on gingival bleeding. Br Dent J 2015; 219: 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lang N, Sander L, Barlow A, et al. Experimental gingivitis studies: effects of triclosan and triclosan-containing dentifrices on dental plaque and gingivitis in three-week randomized controlled clinical trials. J Clin Dent 2002; 13: 158–166. [PubMed] [Google Scholar]
- 47. Lorenz K, Mayer D, Bruhn G, et al. Effect of N-chlorotaurine mouth rinses on plaque regrowth and plaque vitality. Clin Oral Investig 2009; 13: 9–14. [DOI] [PubMed] [Google Scholar]
- 48. Duss C, Lang NP, Cosyn J, et al. A randomized, controlled clinical trial on the clinical, microbiological, and staining effects of a novel 0.05% chlorhexidine/herbal extract and a 0.1% chlorhexidine mouthrinse adjunct to periodontal surgery. J Clin Periodontol 2010; 37: 988–997. [DOI] [PubMed] [Google Scholar]
- 49. Ernst CP, Prockl K, Willershausen B. The effectiveness and side effects of 0.1% and 0.2% chlorhexidine mouthrinses: a clinical study. Quintessence Int 1998; 29: 443–448. [PubMed] [Google Scholar]
- 50. Olsson H, Asklow B, Johansson E, et al. Rinsing with alcohol-free or alcohol-based chlorhexidine solutions after periodontal surgery. A double-blind, randomized, cross-over, pilot study. Swed Dent J 2012; 36: 91–99. [PubMed] [Google Scholar]
- 51. Santhosh K, Surbhi L, Harish T, et al. Do active ingredients in non alcoholic chlorhexidine mouth wash provide added effectiveness? Observations from a randomized controlled trial. Odontostomatol Trop 2012; 33: 26–34. [PubMed] [Google Scholar]
- 52. Bernardi F, Pincelli MR, Carloni S, et al. Chlorhexidine with an antidiscoloration system. A comparative study. Int J Dent Hyg 2004; 2: 122–126. [DOI] [PubMed] [Google Scholar]
- 53. Cortellini P, Labriola A, Zambelli R, et al. Chlorhexidine with an antidiscoloration system after periodontal flap surgery: a cross-over, randomized, triple-blind clinical trial. J Clin Periodontol 2008; 35: 614–620. [DOI] [PubMed] [Google Scholar]
- 54. Graziani F, Gabriele M, D’Aiuto F, et al. Dental plaque, gingival inflammation and tooth -discolouration with different commercial - formulations of 0.2% chlorhexidine rinse: a double-blind randomised controlled clinical trial. Oral Health Prev Dent 2015; 13: 101–111. [DOI] [PubMed] [Google Scholar]
- 55. Solis C, Santos A, Nart J, et al. 0.2% chlorhexidine mouthwash with an antidiscoloration system versus 0.2% chlorhexidine mouthwash: a prospective clinical comparative study. J Periodontol 2011; 82: 80–85. [DOI] [PubMed] [Google Scholar]
- 56. Brecx M, Macdonald LL, Legary K, et al. Long-term effects of Meridol and chlorhexidine mouthrinses on plaque, gingivitis, staining, and bacterial vitality. J Dent Res 1993; 72: 1194–1197. [DOI] [PubMed] [Google Scholar]
- 57. Charles CH, Mostler KM, Bartels LL, et al. Comparative antiplaque and antigingivitis effectiveness of a chlorhexidine and an essential oil mouthrinse: 6-month clinical trial. J Clin Periodontol 2004; 31: 878–884. [DOI] [PubMed] [Google Scholar]
- 58. Ernst CP, Canbek K, Dillenburger A, et al. Clinical study on the effectiveness and side effects of hexetidine and chlorhexidine mouthrinses versus a negative control. Quintessence Int 2005; 36: 641–652. [PubMed] [Google Scholar]
- 59. Sharma NC, Galustians HJ, Qaqish J, et al. Antiplaque and antigingivitis effectiveness of a hexetidine mouthwash. J Clin Periodontol 2003; 30: 590–594. [DOI] [PubMed] [Google Scholar]
- 60. Gupta D, Bhaskar DJ, Gupta RK, et al. Effect of Terminalia chebula extract and chlorhexidine on salivary pH and periodontal health: 2 weeks randomized control trial. Phytother Res 2014; 28: 992–998. [DOI] [PubMed] [Google Scholar]
- 61. Gupta RK, Gupta D, Bhaskar DJ, et al. Preliminary antiplaque efficacy of aloe vera mouthwash on 4 day plaque re-growth model: randomized control trial. Ethiop J Health Sci 2014; 24: 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pereira SLDS, De Oliveira JWG, Ângelo KKSV, et al. Clinical effect of a mouth rinse containing Ocimum gratissimum on plaque and gingivitis control. J Contemp Dent Pract 2011; 12: 350–355. [PubMed] [Google Scholar]
- 63. Kumar S, Patel S, Tadakamadla J, et al. Effectiveness of a mouthrinse containing active ingredients in addition to chlorhexidine and triclosan compared with chlorhexidine and triclosan rinses on plaque, gingivitis, supragingival calculus and extrinsic staining. Int J Dent Hyg 2013; 11: 35–40. [DOI] [PubMed] [Google Scholar]
- 64. Hase JC, Attstrom R, Edwardsson S, et al. 6-month use of 0.2% delmopinol hydrochloride in comparison with 0.2% chlorhexidine digluconate and placebo. (I). Effect on plaque formation and gingivitis. J Clin Periodontol 1998; 25: 746–753. [DOI] [PubMed] [Google Scholar]
- 65. Horwitz J, Machtei EE, Peled M, et al. Amine fluoride/stannous fluoride and chlorhexidine mouthwashes as adjuncts to surgical periodontal therapy: a comparative study. J Periodontol 2000; 71: 1601–1606. [DOI] [PubMed] [Google Scholar]
- 66. Kim D, Ryu SJ, Son EJ, et al. Glucanhydrolase from Lipomyces starkeyi KSM 22 as potential mouthwash ingredient. J Microbiol Biotechnol 2002; 12: 993–997. [Google Scholar]
- 67. Gurgan CA, Zaim E, Bakirsoy I, et al. Short-term side effects of 0.2% alcohol-free chlorhexidine mouthrinse used as an adjunct to non-surgical periodontal treatment: a double-blind clinical study. J Periodontol 2006; 77: 370–384. [DOI] [PubMed] [Google Scholar]
- 68. Türkoǧlu O, Becerik S, Emingil G, et al. The effect of adjunctive chlorhexidine mouthrinse on clinical parameters and gingival crevicular fluid cytokine levels in untreated plaque-associated gingivitis. Inflam Res 2009; 58: 277–283. [DOI] [PubMed] [Google Scholar]
- 69. Jenkins S, Addy M, Newcombe R. Evaluation of a mouthrinse containing chlorhexidine and fluoride as an adjunct to oral hygiene. J Clin Periodontol 1993; 20: 20–25. [DOI] [PubMed] [Google Scholar]
- 70. Leyes Borrajo JL, Garcia VL, Lopez CG, et al. Efficacy of chlorhexidine mouthrinses with and without alcohol: a clinical study. J Periodontol 2002; 73: 317–321. [DOI] [PubMed] [Google Scholar]
- 71. Lorenz K, Bruhn G, Heumann C, et al. Effect of two new chlorhexidine mouthrinses on the development of dental plaque, gingivitis, and discolouration. A randomized, investigator-blind, placebo-controlled, 3-week experimental gingivitis study. J Clin Periodontol 2006; 33: 561–567. [DOI] [PubMed] [Google Scholar]
- 72. Joyston-Bechal S, Hernaman N. The effect of a mouthrinse containing chlorhexidine and fluoride on plaque and gingival bleeding. J Clin Periodontol 1993: 20: 49–53. [DOI] [PubMed] [Google Scholar]
- 73. Bhat N, Mitra R, Oza S, et al. The antiplaque effect of herbal mouthwash in comparison to chlorhexidine in human gingival disease: a randomized placebo controlled clinical trial. J Complement Integr Med 2014; 11: 129–137. [DOI] [PubMed] [Google Scholar]
- 74. Collaert B, Attstrom R, De Bruyn H, et al. The effect of delmopinol rinsing on dental plaque formation and gingivitis healing. J Clin Periodontol 1992; 19: 274–280. [DOI] [PubMed] [Google Scholar]
- 75. Almerich JM., Cabedo B, Ortola JC, et al. Influence of alcohol in mouthwashes containing triclosan and zinc: an experimental gingivitis study. J Clin Periodontol, 2005; 32: 539–544. [DOI] [PubMed] [Google Scholar]
- 76. Schaeken MJ, Van der Hoeven JS, Saxton CA, et al. The effect of mouthrinses containing zinc and triclosan on plaque accumulation, development of gingivitis and formation of calculus in a 28-week clinical test. J Clin Periodontol 1996; 23: 465–470. [DOI] [PubMed] [Google Scholar]
- 77. Waaler SM, Rolla G, Skjorland KK, et al. Effects of oral rinsing with triclosan and sodium lauryl sulfate on dental plaque formation: a pilot study. Scand J Dent Res 1993; 101: 192–195. [DOI] [PubMed] [Google Scholar]
- 78. Tenenbaum H, Dahan M, Soell M. Effectiveness of a sanguinarine regimen after scaling and root planing. J Periodontol 1999; 70: 307–311. [DOI] [PubMed] [Google Scholar]
- 79. Agarwal S, Mathur S, Kothiwale S, et al. Efficacy and acceptability of 0.074% diclofenac-containing mouthwash after periodontal surgery: a clinical study. Indian J Dent Res 2010; 21: 408–412. [DOI] [PubMed] [Google Scholar]
- 80. Weinstein RL. Double blind placebo-controlled study on efficacy, acceptability and safety of mouthwash diclofenac in oral or periodontal postoperative period. Minerva Stomatol 2001; 50: 315–319. [PubMed] [Google Scholar]
- 81. Guarnelli ME, Zangari F, Manfrini R, et al. Evaluation of additional amine fluoride/stannous fluoride-containing mouthrinse during supportive therapy in patients with generalized aggressive periodontitis. A randomized, crossover, double-blind, controlled trial. J Clin Periodontol 2004; 31: 742–748. [DOI] [PubMed] [Google Scholar]
- 82. Hasturk H, Nunn M, Warbington M, et al. Efficacy of a fluoridated hydrogen peroxide-based mouthrinse for the treatment of gingivitis: a randomized clinical trial. J Periodontol 2004; 75: 57–65. [DOI] [PubMed] [Google Scholar]
- 83. Ciancio SG, Shibly O, Mather ML, et al. Clinical effects of a stannous fluoride mouthrinse on plaque. Clin Prevent Dent 1992; 14: 27–30. [PubMed] [Google Scholar]
- 84. Zimmermann A, Flores-de-Jacoby L, Pan P, et al. Gingivitis, plaque accumulation and plaque composition under long-term use of Meridol. J Clin Periodontol 1993; 20: 346–351. [DOI] [PubMed] [Google Scholar]
- 85. Hase JC, Ainamo J, Etemadzadeh H, et al. Plaque formation and gingivitis after mouthrinsing with 0.2% delmopinol hydrochloride, 0.2% chlorhexidine digluconate and placebo for 4 weeks, following an initial professional tooth cleaning. J Clin Periodontol 1995; 22: 533–539. [DOI] [PubMed] [Google Scholar]
- 86. Hase J, Soder PO, Soder B, et al. Development of plaque and gingivitis after mouthrinsing with 0.2% delmopinol hydrochloride. Eur J Oral Sci 1995; 103: 172–178. [DOI] [PubMed] [Google Scholar]
- 87. Claydon N, Hunter L, Moran J, et al. A 6-month home-usage trial of 0.1% and 0.2% delmopinol mouthwashes (I). Effects on plaque, gingivitis, supragingival calculus and tooth staining. J Clin Periodontol 1996; 23: 220–228. [DOI] [PubMed] [Google Scholar]
- 88. Bolanowski SJ, Gescheider GA, Sutton SV. Relationship between oral pain and ethanol concentration in mouthrinses. J Periodont Res 1995; 30: 192–197. [DOI] [PubMed] [Google Scholar]
- 89. Zahradnik RT, Magnusson I, Walker C, et al. Preliminary assessment of safety and effectiveness in humans of ProBiora3™ a probiotic mouthwash. J Appl Microbiol 2009; 107: 682–690. [DOI] [PubMed] [Google Scholar]
- 90. Tombes MB, Gallucci B. The effects of hydrogen peroxide rinses on the normal oral mucosa. Nursing Res 1993; 42: 332–337. [PubMed] [Google Scholar]
- 91. Scully C, El-Kabir M, Greenman J, et al. The effects of mouth rinses and dentifrice-containing magnesium monoperoxyphthalate (MMPP) on oral microflora, plaque reduction, and mucosa. J Clin Periodontol 1999; 26: 234–238. [DOI] [PubMed] [Google Scholar]
- 92. Preshaw PM, Lauffart B, Brown P, et al. Effects of ketorolac tromethamine mouthrinse (0.1%) on crevicular fluid prostaglandin E2 concentrations in untreated chronic periodontitis. J Periodontol 1998; 69: 777–783. [DOI] [PubMed] [Google Scholar]
- 93. Van Dyke T, Paquette D, Grossi S, et al. Clinical and microbial evaluation of a histatin-containing mouthrinse in humans with experimental gingivitis: a phase-2 multi-center study. J Clin Periodontol 2002; 29: 168–176. [DOI] [PubMed] [Google Scholar]
- 94. Yeung S, Groenlund C, Chapple C, et al. The efficacy of Decapinol mouthwash 2 mg/mL in preventing gingivitis. Aust Dent J 1995; 40: 220–225. [DOI] [PubMed] [Google Scholar]
- 95. De Nardo R, Chiappe V, Gomez M, et al. Effects of 0.05% sodium hypochlorite oral rinse on supragingival biofilm and gingival inflammation. Int Dent J 2012; 62: 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Commission Regulation (EU) No 2016/110 of 27 January 2016 [Internet]. Official Journal of the European Union. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2016.021.01.0086.01.ENG; https://www.federalregister.gov/documents/2017/12/20/2017–27317/safety-and-effectiveness-of-health-care-antiseptics-topical-antimicrobial-drug-products-for (2016, accessed 18 March 2018). [Google Scholar]
- 97. US Food and Drug Administration. Safety and effectiveness of health care antiseptics; topical antimicrobial drug products for over-the-counter human use. https://www.federalregister.gov/documents/2017/12/20/2017–27317/safety-and-effectiveness-of-health-care-antiseptics-topical-antimicrobial-drug-products-for (2017, accessed 18 March 2018). [PubMed]
- 98. Institute of Medicine (US) Council on Health Care Technology; Goodman C, editor. Medical technology assessment directory: a pilot reference to organizations, assessments, and information resources. Washington, DC: National Academies Press; American Dental Association Council on Dental Therapeutics, 1988. https://www.ncbi.nlm.nih.gov/books/NBK218341/ [PubMed] [Google Scholar]
- 99. Ioannidis JP, Evans SJ, Gøtzsche PC, et al. CONSORT Group. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med 2004; 141: 781–788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, review_MW_Supplementary_Table_1_final for Adverse events associated with home use of mouthrinses: a systematic review by Gianluca M. Tartaglia, Santosh Kumar Tadakamadla, Stephen Thaddeus Connelly, Chiarella Sforza and Conchita Martín in Therapeutic Advances in Drug Safety
Supplemental material, review_MW_Supplementary_Table_2_final for Adverse events associated with home use of mouthrinses: a systematic review by Gianluca M. Tartaglia, Santosh Kumar Tadakamadla, Stephen Thaddeus Connelly, Chiarella Sforza and Conchita Martín in Therapeutic Advances in Drug Safety
Supplemental material, review_MW_Supplementary_Table_3_final for Adverse events associated with home use of mouthrinses: a systematic review by Gianluca M. Tartaglia, Santosh Kumar Tadakamadla, Stephen Thaddeus Connelly, Chiarella Sforza and Conchita Martín in Therapeutic Advances in Drug Safety
Supplemental material, Supplemen_Fig_1_2 for Adverse events associated with home use of mouthrinses: a systematic review by Gianluca M. Tartaglia, Santosh Kumar Tadakamadla, Stephen Thaddeus Connelly, Chiarella Sforza and Conchita Martín in Therapeutic Advances in Drug Safety