Abstract
Background:
The management of patients with metastatic pancreatic cancer (mPC) is challenging, and the optimal treatment strategy is debated among experts. In an attempt to identify treatment decision criteria and to investigate variations in the first-line management of this disease, we performed an analysis of treatment algorithms among experts in the field of pancreatic cancer. The aim of this study was to identify relevant criteria in the complex process of patient selection and decision making for the management of mPC patients.
Methods:
Experts from the ABCSG (Austrian Breast and Colorectal Cancer Study Group) Pancreatic Cancer Club were contacted and agreed to participate in this analysis. Eight experts from seven centers in Austria provided their decision algorithms for the first-line treatment of patients with mPC. Their responses were converted into decision trees based on the objective consensus methodology. The decision trees were used to identify consensus and discrepancies.
Results:
The final treatment algorithms included four decision criteria (performance status, age, comorbidities, and symptomatic disease) and six treatment options: mFOLFIRINOX, gemcitabine + nab-paclitaxel, gemcitabine mono, 5-FU mono, gemcitabine/erlotinib, and best supportive care (BSC).
Conclusions:
We identified consensus for the treatment of young and fit patients with mFOLFIRINOX. With higher age and reduced performance status, gemcitabine + nab-paclitaxel was increasingly used. For patients with Eastern Co-operative Oncology Group Performance Status (ECOG PS) 4, BSC was the treatment of choice. Among experts, different decision criteria and treatment options are implemented in clinical routine. Despite multiple options in current recommendations, a consensus for specific recommendations was identified.
Keywords: decision criteria, decision making, FOLFIRINOX, nab-Paclitaxel, palliative chemotherapy, pancreatic cancer, treatment algorithm
Introduction
In 2018, more than 450,000 cases of pancreatic cancer were registered worldwide, with the highest incidence in Europe and North America.1 Most of these patients die within the 1st year after diagnosis. The median survival for untreated advanced pancreatic cancer is 3.5 months. With active treatment, the median survival rate can be increased to about 8 months. Only a small subset of patients live significantly longer.2 Based on the improved efficacy compared with gemcitabine monotherapy, gemcitabine + nab-paclitaxel (nanoparticle albumin bound paclitaxel) and mFOLFIRINOX [a modified regimen of oxaliplatin, leucovorin, irinotecan, and fluorouracil (5-FU)] are both established standard first-line treatments in patients with metastatic disease.3–5 Since there is no head-to-head comparison between these regimens, multiple treatment options are applicable.6 Additionally, even large phase III trials will not provide information for all possible clinical scenarios or patient and disease characteristics.7 Only selected patients are eligible for clinical trials,8 and restrictions on eligibility cast doubt on the generalizability of trial results.
The goal of the present project was to investigate the first-line treatment strategies for metastatic pancreatic cancer (mPC). Management strategies were converted into decision trees based on the objective consensus methodology,9 and used to identify consensus and discrepancies. It was not the scope of the present analysis to provide treatment recommendations, but rather to illustrate the diversity in decision making and assess patterns of care.
Materials and methods
A decision-making analysis among members of the ABCSG (Austrian Breast & Colorectal Cancer Study Group) Pancreatic Cancer Club was performed. Of 11 members, 8 agreed to participate in this analysis. All these experts are working within a rather homogenous environment (same country, universal health insurance, and uniform chemotherapy availability). Despite these similarities, significant differences in treatment recommendations were identified, although only a few decision criteria were identified as relevant to most experts. Each expert was asked the following question by email: ‘Please describe your strategy for first-line systemic treatment of mPC. Please explain which patient/disease characteristics (and their combinations) lead to which treatment recommendations.’ Participants were asked to describe their individual clinical decision algorithm in the form of free text or diagrams. No specific clinical scenarios, examples, or decision criteria were proposed in order to avoid influencing responses by suggesting specific cut-off values or suggesting that certain criteria should be used in decision making. In an iterative process, any potential gaps in recommendation coverage were addressed between the coordinator (MG) and the individual experts. All information gathered was converted into decision trees as described previously.9 To avoid different subjective interpretation of the other trees based on their origin, the individual decision trees were anonymized, and their origin was not revealed to the participants.
To enable cross-comparison of algorithms, compatible criteria are a prerequisite. Therefore, similar criteria were fused into new comprehensive categories to simplify their representation and enable cross-comparability; for example, age, showing various cut-off values by different centers, was translated into the term ‘elderly yes/no’. Similarly, strict definitions for comorbidities were not defined as they represented a range of answers (e.g.: ‘renal failure,’ ‘hepatic disorders,’ or ‘hypertension’). Decision criteria were omitted if used by less than three experts. The preference of some participating centers to treat patients in trials is not represented in the decision trees. General prerequisites, such as specific patient preferences or the capacity to give informed consent, were not evaluated as they were considered universal.
The resulting decision trees were presented to the participants for verification, and corrections were applied if required. The trees were finalized and confirmed by each center by March 2019. The decision trees were then analyzed to determine the majority recommendation for each possible combination of parameters based on the objective consensus methodology.10
Results
Experts from seven Austrian centers (General Hospital/University Hospital of Vienna, Paracelsus Medical University Salzburg, Landesklinikum Wr. Neustadt, Kepler University Hospital of Linz, University Hospital of Graz, Klinikum Klagenfurt, A.ö. Krankenhaus St. Vinzenz Zams) participated and provided written or schematic information of their interdisciplinary local treatment strategy for first-line treatment of mPC (Figure 1). The final treatment algorithms included a total of four decision criteria (Table 1) and six treatment options (Table 2). The parameters considered relevant for the decision were Eastern Cooperative Oncology Group Performance status (ECOG PS), age (elderly, young), comorbidities (significant comorbidities like advanced renal failure, cardiac comorbidities, gastrointestinal diseases, etc., present or not) and symptoms (symptomatic disease with urgent need for therapy or not) (Table 1). Age was simplified into two categories, ‘elderly’ or ‘not elderly.’ While a numeric cut-off was mentioned in individual responses (range 65–75 years of age), the general consensus was that there is no specific age-dependent cut-off point. One decision tree included the criterion ‘bilirubin.’ An increased bilirubin changed the treatment from gemcitabine to capecitabine. Because this was mentioned in only one tree, it was excluded for a simplified overview. One department also used the criterion squamous cell carcinoma (SCC) histology. Because pancreatic SCC is a rare event, accounting for approximately 0.5–2% of all malignant pancreatic carcinomas,11 and because it was used by only one expert, it was not evaluated in the decision tree analysis. Another center used the Hurria score, a prediction tool for chemotherapy toxicity.12 This score was represented by a combination of other implemented criteria.
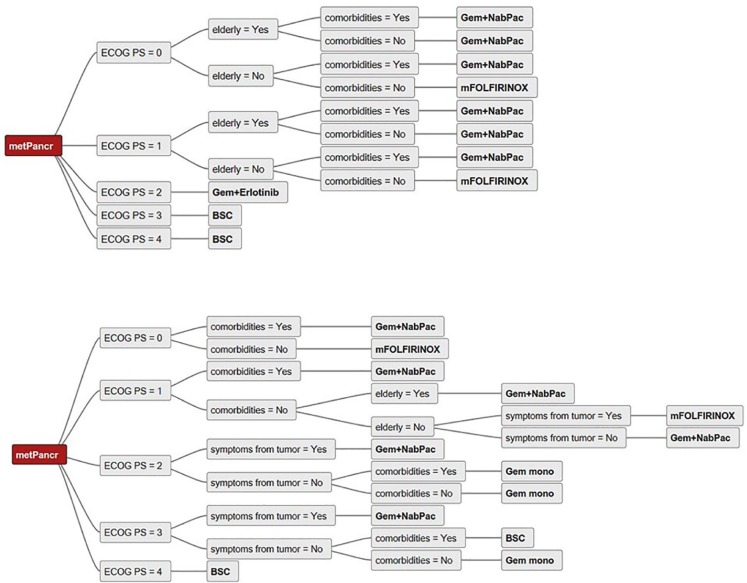
Figure 1.
Sample decision trees illustrating the input from two centers for the analysis.
BSC, Best supportive care; ECOG PS, Eastern Co-operative Oncology Group Performance Status; Gem, Gemcitabine; metPancr, metastatic pancreatic cancer; mFOLFIRINOX, modified regimen of oxaliplatin, leucovorin, irinotecan, and fluorouracil (5-FU); NabPac, nanoparticle albumin bound paclitaxel.
Table 1.
Decision criteria implemented per center.
| A | B | C | D | E | F | G | H | |
|---|---|---|---|---|---|---|---|---|
| ECOG PS |

|
|||||||
| Age | ||||||||
| Symptoms | ||||||||
| Comorbidities | ||||||||
ECOG PS, Eastern Co-operative Oncology Group Performance Status.
Table 2.
Treatment options implemented per center.
| A | B | C | D | E | F | G | H | |
|---|---|---|---|---|---|---|---|---|
| Gem/Erlotinib |
|
|||||||
| Gem/nabPac | ||||||||
| mFOLFIRINOX | ||||||||
| Gem mono | ||||||||
| 5-FU mono | ||||||||
| BSC | ||||||||
5-FU, 5-Fluorouracil; BSC, Best supportive care; Gem, Gemcitabine; nabPac, nanoparticle albumin bound paclitaxel.
The final treatment options analyzed were mFOLFIRINOX, gemcitabine + nab-paclitaxel (gem + nabpac), gemcitabine (gem) mono, 5-fluorouracil (5-FU) mono, gemcitabine + erlotinib, and best supportive care (BSC).
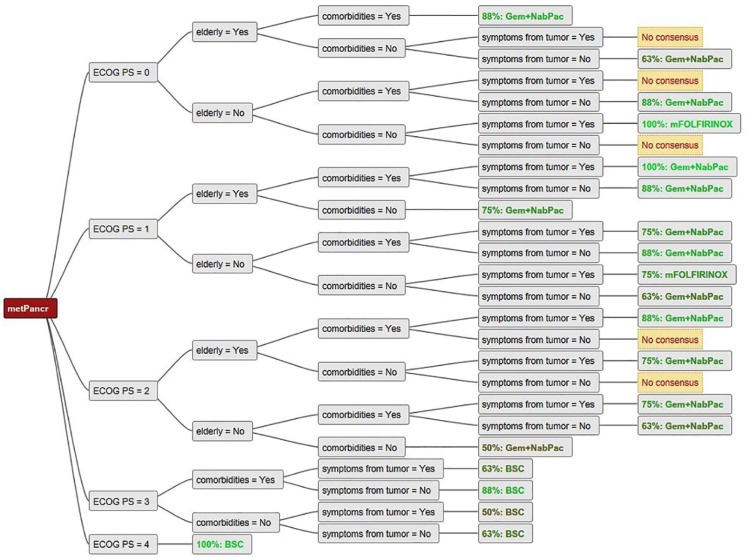
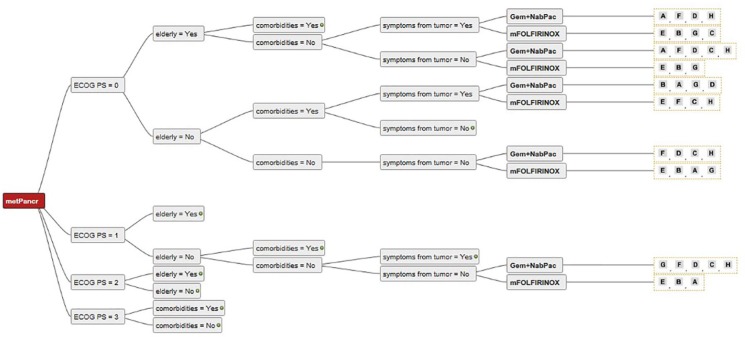
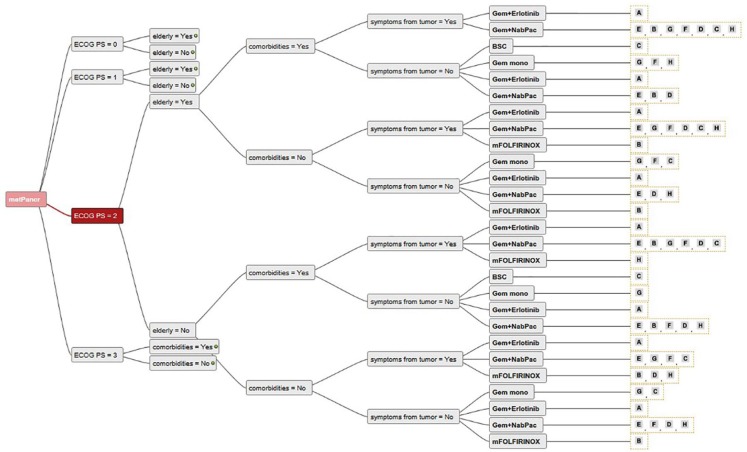
Consensus among the seven centers was found for the use of mFOLFIRINOX in young and fit patients without comorbidities (Figures 2 and 3). There was also a trend towards the use of gem + nabpac in elderly patients with mPC (Figures 2 and 3). In patients with poor performance status (ECOG PS 3 and 4), the large majority of experts believed BSC to be the preferred treatment choice (Figures 2 and 3). For fit but elderly patients, or those with comorbidities (Figure 4), there was no clear consensus, and the use of mFOLFIRINOX and gem/nabpac was balanced among the experts. For patients with ECOG PS 2 (Figure 5), there was a broad variation of treatment options (mFOLFIRINOX, gem + nabpac, gem mono, gem + erlotinib or BSC) in Austria, even for the same patient. For example, for younger patients with ECOG PS 2, without symptoms or comorbidities, four experts recommended gem + nabpac, two recommended gem mono, one gem + erlotinib, and one mFOLFIRINOX.
Figure 2.
Decision tree showing 100% agreement for a specific treatment option.
BSC, Best supportive care; ECOG PS, Eastern Co-operative Oncology Group Performance Status; Gem, Gemcitabine; metPancr, metastatic pancreatic cancer; mFOLFIRINOX, modified regimen of oxaliplatin, leucovorin, irinotecan, and fluorouracil (5-FU); NabPac, nanoparticle albumin bound paclitaxel.
Figure 3.
Comparison of all decision trees showing consensus/no consensus for a certain treatment option for patients with ECOG 0–4.
BSC, Best supportive care; ECOG PS, Eastern Co-operative Oncology Group Performance Status; Gem, Gemcitabine; metPancr, metastatic pancreatic cancer; mFOLFIRINOX, modified regimen of oxaliplatin, leucovorin, irinotecan, and fluorouracil (5-FU); NabPac, nanoparticle albumin bound paclitaxel.
No consensus: no majority was identified.
Figure 4.
Decision tree showing different treatment options recommended by different centers for the same patients (ECOG 0–1).
ECOG, Eastern Co-operative Oncology Group Performance status; Gem, Gemcitabine; metPancr, metastatic pancreatic cancer; mFOLFIRINOX, modified regimen of oxaliplatin, leucovorin, irinotecan, and fluorouracil (5-FU); NabPac, nanoparticle albumin bound paclitaxel.
Individual experts are represented with letters “A” to “H”.
Figure 5.
Decision tree showing different treatment options recommended by different centers for the same patients (ECOG 2).
ECOG, Eastern Co-operative Oncology Group Performance status; Gem, Gemcitabine; metPancr, metastatic pancreatic cancer; mFOLFIRINOX, modified regimen of oxaliplatin, leucovorin, irinotecan, and fluorouracil (5-FU); NabPac, nanoparticle albumin bound paclitaxel.
Individual experts are represented with letters “A” to “H”.
Discussion
This is the first in-depth decision making analysis of expert-defined decision criteria in the setting of mPC using the objective consensus methodology. We performed an analysis among medical oncology experts in the field of PC from different centers within a rather homogenous environment, comparable to other developed countries in Europe. We obtained six different treatment options for the first-line treatment of mPC, and selected four criteria for treatment choice.
Collecting decision-making patterns directly from clinical experts provides valuable insights into decision making, especially in determining which decision criteria are most relevant in clinical practice, as these may not be recorded in clinical trials, databases or health records.7,13,14
Although we found consensus for the use of mFOLFIRINOX in young and fit patients without comorbidities, many of our experts alternatively discuss the use of gem + nabPac with their patients in this situation. The lack of a head-to-head comparison between these two regimens, and the findings of a retrospective analysis comparing the efficacy of gem + nabPac & mFOLFIRINOX revealing no difference,5 justify this discussion. Notably, most mPC patients are older than 65 years.
In agreement with published data,4,15,16 in patients with ECOG PS 2, gem + nabPac was considered by most experts, though, especially for younger patients with ECOG PS 2, heterogeneous treatment options were recommended (mFOLFIRINOX, gem + nabPac, gem + erlotinib or gem mono). In patients with a worse performance status (ECOG PS 3), the large majority of experts believed BSC to be the preferred treatment choice, but in some instances systemic therapy was still recommended, especially in symptomatic patients. In this scenario, gemcitabine monotherapy would have been chosen. Interestingly, but in view of the modest gain in median survival, understandably,17 gemcitabine + erlotinib was offered as treatment choice by only one out of eight experts. For patients with ECOG 4, all of our experts recommended BSC.
We also compared the treatment recommendations of academic centers and community hospitals. There was no trend toward special treatment recommendations. The treatment options were not significantly different, nor were the decision criteria.
The expert group size and composition are debatable; however, consensus was identified and it is unlikely that including more experts would have led to different results. Patient complexity and heterogeneity, as well as their treatment preferences, were not addressed, and therefore this analysis represents an abstract representation. We used a simplified and reduced number of decision criteria for better visualization of decision trees, potentially ignoring other minor and subconscious factors.7,18 We also cannot be sure that the experts would treat every single patient according to the provided algorithms. There was agreement among the experts, that while consensus was identified for many instances, treatment options, if available, should be discussed with the patients.
Conclusion
The aim of this study was to assess criteria in the complex process of patient selection and decision-making for the management of mPC patients. This study was conducted because there is no conclusive evidence to aid patient selection. The application of a decision tree analysis was able to identify decision criteria that were relevant for all participating centers. ECOG PS and age were the most relevant factors in decision making for the management of mPC patients. We found consensus for treating young and fit patients with mFOLFIRINOX. Higher age and worse performance status were associated with a consensus to use gem + nab-paclitaxel. For patients with ECOG PS 3 or higher, the large majority of experts recommended BSC. Despite multiple options in current recommendations, a consensus for specific recommendations was identified. This survey is only a surrogate, but provides information on which criteria treatment decisions are made.
Acknowledgments
We thank AstraZeneca for financial support.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: AstraZeneca.
Conflict of interest statement: Paul Martin Putora received financial support by AstraZeneca. All other authors have no conflict of interest.
Ethics approval/informed consent: Ethics approval was not applicable as no patient or confidential data was used in this analysis. No humans or animals were used for this research (https://www.swissethics.ch/).
ORCID iD: Markus Glatzer  https://orcid.org/0000-0003-2277-7577
https://orcid.org/0000-0003-2277-7577
Contributor Information
Werner Scheithauer, Division of Oncology, Department of Internal Medicine I, General Hospital of Vienna, Vienna, Austria.
Paul Martin Putora, Department of Radiation Oncology, Kantonsspital St. Gallen, Switzerland; Department of Radiation Oncology, University of Bern, Switzerland.
Birgit Grünberger, Department of Internal Medicine, Haematology and Oncology, Landesklinikum Wiener Neustadt, Austria.
Wolfgang Eisterer, Department of Internal Medicine and Oncology, Klinikum Klagenfurt, Austria.
Ewald Wöll, Department of Internal Medicine, Krankenhaus St.Vinzenz Zams, Austria.
Gerald Prager, Division of Oncology, Department of Internal Medicine I, General Hospital of Vienna, Vienna, Austria.
Renate Schaberl-Moser, Division of Oncology, Department of Internal Medicine, Medical University of Graz, Austria.
Richard Greil, Division of Oncology, Department of Internal Medicine, Paracelsus Medical University Medical University of Salzburg, Salzburg Cancer Research Institute-SCRI-LIMCR, and Cancer Cluster, Austria.
Markus Glatzer, Department of Radiation Oncology, Kantonsspital St. Gallen, Rorschacher Strasse 95, St. Gallen, 9007, Switzerland.
References
- 1. Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 2019; 10: 10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Uson Junior PL, Franca MS, Rodrigues HV, et al. Higher overall survival in metastatic pancreatic cancer: the impact of where and how treatment is delivered. Einstein (Sao Paulo) 2015; 13: 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 4. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kang J, Hwang I, Yoo C, et al. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: retrospective analysis. Invest New Drugs 2018; 36: 732–741. [DOI] [PubMed] [Google Scholar]
- 6. Panje CM, Glatzer M, Siren C, et al. Treatment options in oncology. JCO Clin Cancer Inform 2018; 2: 1–10. [DOI] [PubMed] [Google Scholar]
- 7. Glatzer M, Panje CM, Siren C, et al. Decision making criteria in oncology. Oncology. Epub ahead of print 18 September 2018. DOI: 10.1159/000492272. [DOI] [PubMed] [Google Scholar]
- 8. George SL. Reducing patient eligibility criteria in cancer clinical trials. J Clin Oncol 1996; 14: 1364–1370. [DOI] [PubMed] [Google Scholar]
- 9. Panje CM, Glatzer M, von Rappard J, et al. Applied Swarm-based medicine: collecting decision trees for patterns of algorithms analysis. BMC Med Res Methodol 2017; 17: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Putora PM, Panje CM, Papachristofilou A, et al. Objective consensus from decision trees. Radiat Oncol 2014; 9: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schultheis AM, Nguyen GP, Ortmann M, et al. Squamous cell carcinoma of the pancreas in a patient with germline BRCA2 mutation-response to neoadjuvant radiochemotherapy. Case Rep Oncol Med 2014; 2014: 860532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol 2016; 34: 2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rothermundt C, Bailey A, Cerbone L, et al. Algorithms in the first-line treatment of metastatic clear cell renal cell carcinoma–analysis using diagnostic nodes. Oncologist 2015; 20: 1028–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rothermundt C, Fischer GF, Bauer S, et al. Pre- and postoperative chemotherapy in localized extremity soft tissue sarcoma: a European organization for research and treatment of cancer expert survey. Oncologist 2018; 23: 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Macarulla T, Pazo-Cid R, Guillen-Ponce C, et al. Phase I/II trial to evaluate the efficacy and safety of nanoparticle albumin-bound paclitaxel in combination with gemcitabine in patients with pancreatic cancer and an ECOG performance status of 2. J Clin Oncol 2019; 37: 230–238. [DOI] [PubMed] [Google Scholar]
- 16. Martin AJ, Alfonso PG, Ruperez AB, et al. Nab-paclitaxel plus gemcitabine as first-line palliative chemotherapy in a patient with metastatic pancreatic cancer with Eastern Cooperative Oncology Group performance status of 2. Oncol Lett 2016; 12: 727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25: 1960–1966. [DOI] [PubMed] [Google Scholar]
- 18. Ozdemir S, Finkelstein EA. Cognitive bias: the downside of shared decision making. JCO Clin Cancer Inform 2018; 2: 1–10. [DOI] [PubMed] [Google Scholar]