Abstract
Background and aims:
The multi-kinase inhibitor sorafenib is a first-line drug for patients with advanced hepatocellular carcinoma (HCC). Treatment options for patients whose disease has progressed on sorafenib are limited. In a recent randomized controlled trial (CELESTIAL trial), patients with advanced HCC who had failed prior systemic therapy had moderate progression-free survival and overall survival advantages when treated with the multi-kinase inhibitor cabozantinib. However, since this treatment is costly and is accompanied by significant adverse events in a large proportion of patients, its cost-effectiveness in these patients should be determined.
Methods:
We developed a Markov model incorporating health outcomes, measured by life-years and quality-adjusted life-years (QALYs) to evaluate the cost-effectiveness of cabozantinib compared with placebo in patients who have failed prior systemic therapy.
Results:
Treatment with cabozantinib results in a mean gain of 11.6 weeks of life (0.22 life-years) as compared with placebo. When quality of life was incorporated, treatment with cabozantinib produced a gain of 0.16 QALYs. The total mean incremental cost of cabozantinib was US$76,406 per patient. The incremental cost-effectiveness ratio for cabozantinib compared with best supportive care was US$469,374/QALY using the recommended dose of 60 mg cabozantinib daily.
Conclusion:
Our results suggest that the use of cabozantinib in patients with advanced HCC who have progressed on prior treatment, results in a modest incremental benefit with high incremental costs, suggesting that it is not cost-effective at conventional willingness to pay thresholds.
Keywords: liver cancer, multi-kinase inhibitor, quality-adjusted life-years, willingness to pay
Introduction
Hepatocellular carcinoma (HCC) is an often fatal cancer with continuously increasing incidence, that predominantly arises in patients with chronic liver disease.1 The current standard of care for patients with an early stage disease, according to the Barcelona Clinic Liver Cancer (BCLC) staging classification, include liver resection, transplantation, or radiofrequency ablation (RFA), all potentially curative modalities.2,3 Indeed, early detection of HCC is associated with improved survival,4 however, only about 25% of patients are detected at an early stage of their disease. For patients whose disease is detected at an advanced stage (BCLC stage C), first-line systemic therapy with either sorafenib5 or lenvatinib6 is currently the standard of care for patients with Child-Pugh A cirrhosis. Recently, based on results of the RESORCE study, treatment with regorafenib was approved as a second-line therapy for patients with advanced HCC who had progressed on sorafenib treatment.7 However, subsequent analyses have called into question the cost-effectiveness of regorafenib treatment for this group of patients.8,9
Cabozantinib is a multi-kinase inhibitor similar in structure to regorafenib, but different in its IC50 for various kinase activities. It is a potent inhibitor of MET and AXL kinases that are implicated in tumor proliferation and that were reported to be involved in acquisition of resistance to antiangiogenic drugs.10 A recent phase III trial (CELESTIAL trial), demonstrated a statistically significant benefit for cabozantinib compared to placebo, in overall survival (OS; median 10.2 versus 8.2 months) and in progression-free survival (PFS; median 5.2 months versus 1.9 months).11,12 The inclusion criteria for the trial were patients with Child-Pugh A cirrhosis and advanced HCC who had failed prior therapy. However, despite the demonstration of a statistically significant survival benefit, the high cost of the drug as well as its substantial side effects, mandate a thorough analysis of its cost-effectiveness. We developed a Markov model to investigate the cost-effectiveness of cabozantinib for patients with Child-Pugh A liver cirrhosis and advanced HCC who had failed prior treatments.
Methods
The data from the CELESTIAL trial were obtained from the published article.11 The IRB committee of the Rabin Medical Center provided the study with an exempt from having a formal IRB approval and the need for receiving informed consent from patients because this study is a cost-effectiveness analysis based on already published data and the data obtained is completely anonymous. We constructed a Markov model according to which the initial decision is to treat patients with cabozantinib or with best supportive care alone. Patients who initially received cabozantinib could stop treatment because of either disease progression or intolerance [grade 3–4 adverse events (AEs)]. Patients who experienced progression after cabozantinib, received best supportive care. Progression to death could occur from each health state (Figure 1). The outputs of the model were life-years (LYs) and quality-adjusted LYs (QALYs), which were further used to calculate the incremental cost-effectiveness ratio (ICER). The Markov model was implemented in TreeAge Pro 2018 software and statistical analyses were performed in MATLAB.
Figure 1.
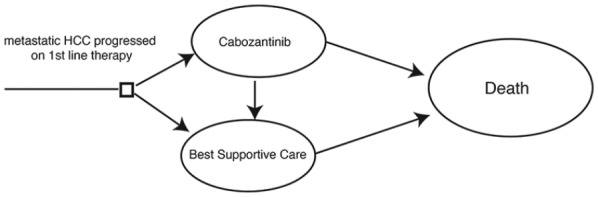
An illustration describing the Markov model used in this cost-effectiveness analysis.
Model survival estimates
We incorporated the PFS and OS data from the CELESTIAL study into the model. We digitized the survival curves for both the experimental arm and control arm using Matlab (Supplementary Figure 1). We fitted these curves to parametric survival models. The most appropriate parametric model was the Weibull model. Digitization and curve fitting can be seen in the supplemental material. We used the PFS curves to estimate the duration of treatment with cabozantinib. We used the OS curves to estimate the progression to death. We used a time horizon of 60 months.
Utility estimates
We used published quality of life data to estimate the utility of patients with metastatic HCC.7,9 Full details of all input variables can be seen in Table 1. In the model, patients receiving cabozantinib were deemed to have a utility of 0.76. Patients who then had progressive disease following cabozantinib were assigned a utility of 0.68. We included grade 3–4 AEs in the model that were noted to occur in a relatively large number of patients included in the trial (roughly, >10% having a particular grade 3–4 AE). Such an approach is recommended by the Institute for Clinical and Economic Review (ICER; https://icer-review.org/methodology/icers-methods/icer_reference_case_july-2018/). We therefore included the following AEs: hand/foot syndrome, fatigue, diarrhea, and hypertension. We assigned disutilities to these AEs based on the published literature.13 We estimated the duration of each AE, and multiplied this by the disutility, with the result then calculated accordingly into the long-term utility. We assumed that hand/foot syndrome would have a disutility of −0.116 lasting 14 days. We assumed that fatigue would have a disutility of −0.115 lasting 10 days. We assumed that diarrhea would have a disutility of −0.103 lasting 5 days. We assumed that hypertension would not have any disutility.
Table 1.
Model parameters: baseline values, ranges, and distributions for Monte Carlo sensitivity analysis.
| Variable | Value | Lower range | Upper range | Study | Distribution |
|---|---|---|---|---|---|
| Age | 64 | 50 | 70 | CELESTIAL | NA |
| Cost diarrhea | 86.02 | 68.82 | 88.8 | GoodRX | gamma |
| Cost hand/foot syndrome | 74 | 59.2 | 88.8 | GoodRX | gamma |
| Cost hypertension | 7 | 5.6 | 8.4 | GoodRX | gamma |
| Duration diarrhea | 5 | 0 | 5 | Estimated | gamma |
| Duration fatigue | 10 | 0 | 10 | Estimated | gamma |
| Duration hand/foot syndrome | 14 | 0 | 14 | Estimated | gamma |
| Duration hypertension | 5 | 0 | 5 | Estimated | gamma |
| Disutility diarrhea | −0.017166667 | −0.0206 | −0.01373 | Lloyd et al.13 | beta |
| Disutility fatigue | −0.037166667 | −0.0446 | −0.02973 | Lloyd et al.13 | beta |
| Disutility hand/foot syndrome | −0.054133333 | −0.0232 | −0.01547 | Lloyd et al.13 | beta |
| Disutility hypertension | 0 | 0 | 0 | – | – |
| γ Placebo progression | 0.860515 | 0.8 | 0.9 | CELESTIAL | Triangular |
| γ Placebo survival | 0.992463 | 0.98 | 1.01 | CELESTIAL | Triangular |
| γ Cabozantinib progression | 0.116877 | 0.1 | 0.15 | CELESTIAL | Triangular |
| γ Cabozantinib survival | 0.992463 | 0.98 | 1.01 | CELESTIAL | Triangular |
| λ Placebo progression | 0.356518 | 0.3 | 0.5 | CELESTIAL | Triangular |
| λ Placebo survival | 0.0855573 | 0.08 | 0.09 | CELESTIAL | Triangular |
| λ Cabozantinib progression | 0.116877 | 0.1 | 0.15 | CELESTIAL | Triangular |
| λ Cabozantinib survival | 0.047884 | 0.045 | 0.05 | CELESTIAL | Triangular |
| Incidence diarrhea placebo | 0.02 | 0.014 | 0.016 | CELESTIAL | beta |
| Incidence diarrhea cabozantinib | 0.1 | 0.08 | 0.12 | CELESTIAL | beta |
| Incidence fatigue placebo | 0.04 | 0.032 | 0.048 | CELESTIAL | beta |
| Incidence fatigue cabozantinib | 0.1 | 0.08 | 0.12 | CELESTIAL | beta |
| Incidence hand/foot syndrome placebo | 0 | 0 | 0 | CELESTIAL | beta |
| Incidence hand/foot syndrome cabozantinib | 0.17 | 0.136 | 0.204 | CELESTIAL | beta |
| Incidence hypertension placebo | 0.02 | 0.016 | 0.024 | CELESTIAL | beta |
| Incidence hypertension cabozantinib | 0.16 | 0.128 | 0.192 | CELESTIAL | beta |
| Discount rate (annual) | 0.03 | 0 | 0.05 | – | |
| Utility of base (month) | 0.063333333 | 0.05 | 0.08 | Shlomai et al.9 | Normal |
| Utility of progression (month) | 0.056666667 | 0.04 | 0.07 | Shlomai et al.9 | Normal |
| Cost of cabozantinib 60 mg daily (28 days) | US$15,858 | US$12,686 | US$19,030 | GoodRX | Triangular |
| Cost of cabozantinib 36 mg daily (28 days) | US$9462 | – | – | GoodRX | – |
Cost estimates
Direct medical costs were calculated in US dollars in 2018. In the base case analysis, we applied a dose of cabozantinib of 60 mg daily. This cost US$15,858 per 28-day cycle, based on drug prices taken from GoodRX on 21 October 2018 (https://www.goodrx.com/). In the United States, it is very difficult to know exactly the net price of many drugs, owing to many discounts and rebates that are often not available as public information. We believe that GoodRX provides a close estimate of the actual amount of money that changes hands. In the CELESTIAL trial, the full dose was not received by all patients. We performed an additional analysis using the cost of 36 mg daily that was the median dose received in the trial. Although this dose will not be a true dose used by patients, it provides a good estimate of the cost at a population level.
We estimated the cost of the treatment of the relevant AEs, also using prices from GoodRX. The relevant required treatments were based on established clinical guidelines. We estimated that the treatment of hand/foot syndrome would require 60 g of clobetasol, and 1 tube of lidocaine, at a cost of US$74. We estimated that there would be no financial cost for the treatment of fatigue. We estimated that the treatment of diarrhea would require one physician visit, 60 tablets of lomotil, and 30 tablets of loperamide, at a total cost of US$86.02. We estimated that the treatment of hypertension would require 30 tablets of amlodipine, at a cost of US$7. We performed annual discounting of all costs at a rate of 3%.
Sensitivity analysis
Owing to uncertainties of the model parameters, we performed both univariable and multivariable sensitivity analyses. The univariable sensitivity analysis allows the reader to assess how much affect a specific variable has on the overall results. In the probabilistic sensitivity analysis, the reader can assess the level of certainty there is regarding the overall results, but without specific interrogation of a specific variable. Based on another study,14 for each input variable we assigned a range of ±20%, together with a distribution, as seen in Table 1. In the univariable analysis, we kept all base case values constant. We ran the model multiple times each time varying only one parameter from its upper and lower boundaries of the range. In this way we could establish which individual variables had the highest impact on the ICER.
In the probability sensitivity analysis, we ran the model 10,000 times, each time randomly selecting different input values for each variable from within their individual ranges. In this way, we were able to see how the results would cluster, despite our uncertainty regarding the input variables.
Results
As outlined in Table 2, the use of cabozantinib, as compared with BSC, results in an incremental gain of 11.65 weeks (0.224 LYs). Upon adjustment for quality of life, the use of cabozantinib results in a gain of 0.163 QALYs. The total incremental cost of cabozantinib treatment, together with treatment of AEs, was between US$47,613 for the 36 mg daily dose and US$76,406 for the 60 mg daily dose. The ICER for cabozantinib treatment as compared with BSC was between US$292,496 and US$469,374 per QALY.
Table 2.
Base case results.
| Strategy | Cost (US$) | Incremental cost (US$) | LY | Incremental LY | QALY | Incremental QALY | ICER (US$/QALY) |
|---|---|---|---|---|---|---|---|
| Placebo | 1 | 1 | 1.00 | – | 0.70 | – | – |
| Cabozantinib 60 mg daily | 76,407 | 76,406 | 1.23 | 0.23 | 0.86 | 0.16 | 469,375 |
| Cabozantinib 36 mg daily | 47,614 | 47,613 | 1.23 | 0.23 | 0.86 | 0.16 | 292,496 |
The results of both the univariable and probabilistic sensitivity analyses can be seen in Figures 2–4. The variables with the greatest effect on the ICER in our model were related to the parameters of the survival curves of both cabozantinib and placebo, with other parameters related to adverse events having only minor influence on the ICER. Across broad variation in the ranges for each parameter, the ICER remained >US$220,000 per QALY (Figure 2). In the probabilistic sensitivity analysis, the cluster of results fell between US$200,000/QALY and US$400,000/QALY when using the median dose of 36 mg daily (Figure 3).
Figure 2.
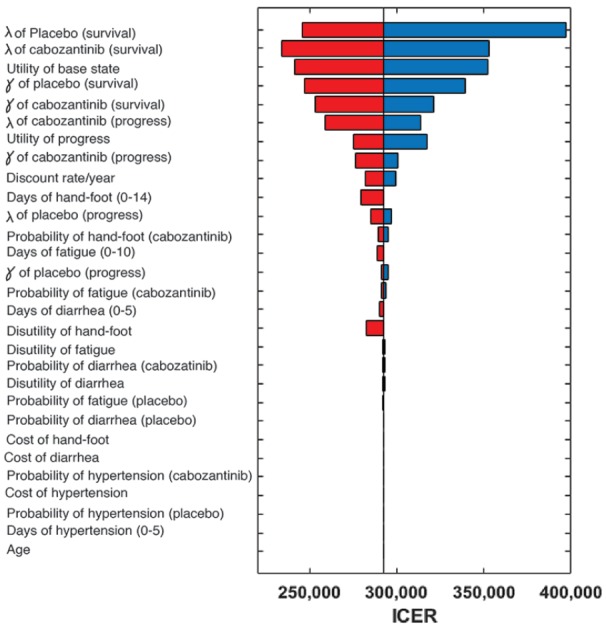
A univariable sensitivity analysis of the ICER for various parameters using median cabozantinib dose of 36 mg daily. The vertical gray line represents the base case ICER value. The width of each bar represents the range of uncertainty associated with each parameter (left of the line: decreased ICER; right of the line: increased ICER). The red and blue segments of each bar represent decreasing or increasing values of the specific parameter, respectively.
ICER, incremental cost-effectiveness ratio.
Figure 3.
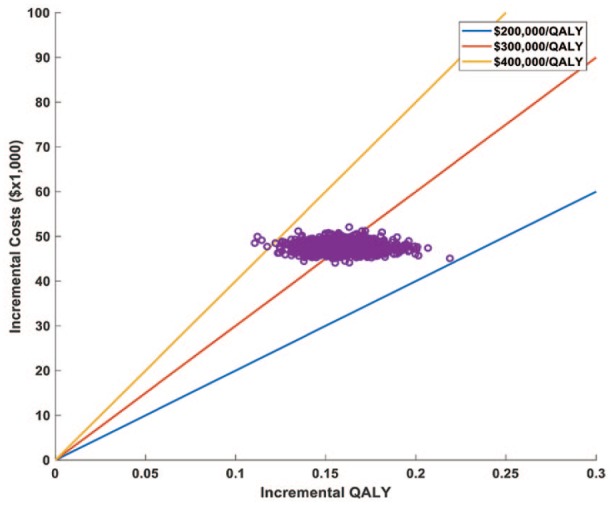
A probabilistic sensitivity analysis using the Monte Carlo simulation plot assuming a median cabozantinib dose of 36 mg daily. Each dot represents a separate run of the model with different input values for each variable randomly selected according to their distribution (see the methods section). The dots cluster between US$200,000 and US$400,000$/QALY.
QALY, quality-adjusted life-years.
Figure 4.
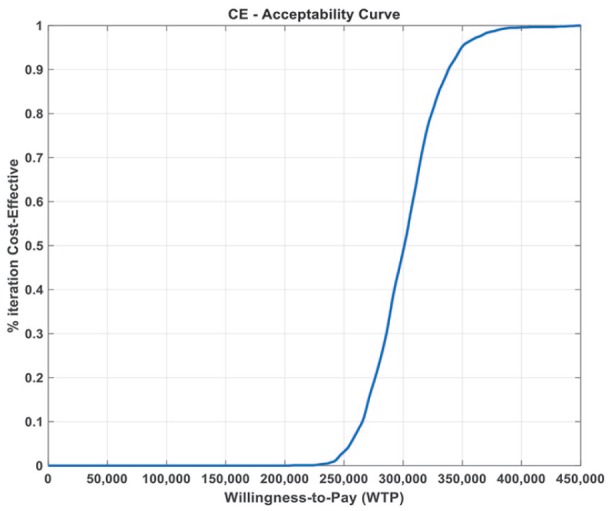
Cost-effectiveness acceptability curve using a median cabozantinib dose of 36 mg daily.
Figure 4 shows the cost-effectiveness acceptability curve, demonstrating 0% likelihood that cabozantinib is cost-effective at willingness-to-pay (WTP) thresholds lower than ~US$230,000 per QALY.
Discussion
Our cost-effectiveness model suggested that the ICER for cabozantinib for treating advanced HCC was approximately US$290,000/QALY, when using the median dose of 36 mg daily. Our model-based sensitivity analyses demonstrated a high level of certainty that this therapy would not be deemed to be cost-effective at conventional willingness to pay thresholds of US$50,000–150,000 per QALY.
The current armamentarium of systemic drugs for advanced HCC is very limited.15 Although cabozantinib has provided hope for patients with this fatal disease, unfortunately the magnitude of benefit is relatively low, and comes with a high financial cost. Healthcare systems around the world will now have to grapple with the challenging question of whether to pay for this therapy. This is a US-based study, using US-based prices, and so the results are relevant only to US-based payers. Currently, reimbursement decisions in the US are not made in connection to cost-effectiveness. However, there is growing concern regarding the cost and cost-effectiveness of many healthcare interventions.16 For example, a recent analysis found that only two thirds of public health interventions are indeed cost effective.17 Therefore, some voices have suggested the use of cost-effectiveness modeling in the early stages of drug development18 as well as in making reimbursement decisions,19 in order to ensure appropriate use of scarce resources. Our current analysis adds to the literature, to provide an example of how such models can be built, to guide decision making. To the best of the authors’ knowledge, it is the first US-based model of this particular drug in this group of patients.
Our findings of a lack of cost-effectiveness are comparable with the finding for regorafenib in the same disease setting.9 There are additional novel agents that have been tested in the setting of advanced HCC, such as nivolumab and pembrolizumab, however we are unaware of published cost-effectiveness analyses of these agents from a US payers’ perspective. In general, however, many treatments in this disease setting add a small incremental clinical benefit, at a high cost, thus are often not considered to be cost-effective.20
The cost-effectiveness of cabozantinib would most probably be improved if there were reliable biomarkers to select only those patients most likely to benefit from the therapy. In the CELESTIAL trial, there is some suggestion that patients who had received one prior line of therapy are more likely to benefit from cabozantinib than patients who had received two prior lines of therapy. This suggestion is based on observations from the forest plot demonstrating a hazard ratio of 0.74 for one prior line compared with a hazard ratio of 0.90 for two prior lines. However, such an observation can be considered only as hypothesis generating as opposed to practice changing as this was not a preplanned analysis.
As with all research, this model has several limitations. It was a trial-based study, using data from the CELESTIAL trial.11 All such studies are flawed from the outset, as the trial data may not appropriately correlate to the real-world data. In particular, patients recruited into the trial may not appropriately represent real-world patients receiving this therapy. The trial was limited for patients with Child-Pugh A cirrhosis and advanced HCC who had previously failed therapy with sorafenib. Patients were also predominantly ECOG performance status less than or equal to 1. It is reasonable to assume that in the real world, patients receiving this therapy may have a poorer performance status and poorer liver function. This may impact the survival results, as well as the duration of treatment, with an expected impact on cost. In addition, understanding the net price of drugs is difficult due to secret rebates and negotiations, thus our cost estimations may be inaccurate. The actual dose used in the real world is also uncertain. Ideally, this study would be performed with input data from real-world studies. However, this is not yet possible, as there is not yet enough drug usage, in order to have available data. The very nature of a cost-effectiveness analysis to guide reimbursement decision making, means that real-world data will usually not be available.
A couple of additional issues implicated in inherent limitations of this study need attention. First, adverse events, such as hand/foot syndrome, may sometimes be managed differently by different clinicians. Some clinicians may use urea cream, which has a comparable cost to clobetasol, and is therefore unlikely to impact the overall results, as demonstrated in the tornado presentation of the univariate sensitivity analysis. In addition, some patients will receive a dose reduction following hand/foot syndrome. Such dose reductions are accounted for in our structural sensitivity analysis of 36 mg daily instead of 60 mg daily.
Second, our model is structured in a way that all patients are considered to receive full supportive care, irrespective of whether they are receiving cabozantinib or not, or whether they have indeed progressed on cabozantinib, and are receiving supportive care alone. Given that all patients receive supportive care at all times that they are alive, it is not expected that there should be any difference in costs between arms of the model, therefore they were not added. There is of course some level of uncertainty regarding this issue, which is incorporated overall in the probabilistic sensitivity analysis.
By large, the quality of life estimates are specific for this intervention (treatment with cabozantinib). Other interventions may have much higher levels of efficacy, and thus may have an improved impact on quality of life as the patients respond to treatment. Conversely, other therapies may cause more side effects and decrease quality of life more substantially. To the best of the authors’ knowledge, there is no published data regarding quality of life in the CELESTIAL trial. We therefore had to perform an estimation for this in the model. The inclusion/exclusion criteria for patients in the RESORCE trial were very similar to those in the CELESTIAL trial, and patients received a drug with a very similar toxicity profile. We therefore felt that it was justified to use the quality of life data from the RESORCE trial for patients in our model of cabozantinib, although it is possible that this approach adds some uncertainty to the results.
Last, there are now a number of different treatment options in advanced HCC. Ideally, we would analyze all possible treatment options in a single model, however this is not possible due to the lack of available data on which to base such a model. As the evidence for different treatments are from different trials, it would be inappropriate to use different trials as the basis for a model, as this may introduce bias into the model results.
However, despite these limitations, we believe that our sensitivity analyses account for the uncertainty, and produce a relatively reliable range for the final results of the model.
In summary, cabozantinib provides only a modest survival benefit, at a high cost, for patients with advanced HCC, refractory to sorafenib with Child-Pugh A cirrhosis. Our model suggests that this is not a cost-effective healthcare intervention, and provides an example of how cost-effectiveness analyses can be used in the USA to make reimbursement decisions.
Supplemental Material
Supplemental material, Supp._Fig_1 for Cabozantinib for patients with advanced hepatocellular carcinoma: a cost-effectiveness analysis by Amir Shlomai, Moshe Leshno and Daniel A. Goldstein in Therapeutic Advances in Gastroenterology
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Amir Shlomai  https://orcid.org/0000-0001-7437-9381
https://orcid.org/0000-0001-7437-9381
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Amir Shlomai, Department of Medicine D and the Liver Institute, Rabin Medical Center, Beilinson hospital, 39 Jabotinsky street, Petach-Tikva, 49100, Israel; The Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Moshe Leshno, Coller School of Management, Tel Aviv University, Tel Aviv, Israel.
Daniel A. Goldstein, Institute of Oncology, Davidoff Cancer Center, Rabin Medical Center, Petach-Tikva, Israel The Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
References
- 1. El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011; 365: 1118–1127. [DOI] [PubMed] [Google Scholar]
- 2. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farinati F, Sergio A, Baldan A, et al. Early and very early hepatocellular carcinoma: when and how much do staging and choice of treatment really matter? A multi-center study. BMC cancer 2009; 9: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014; 11: e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 6. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 7. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 389: 56–66. [DOI] [PubMed] [Google Scholar]
- 8. Parikh Neehar D, Singal Amit G, Hutton David W. Cost effectiveness of regorafenib as second-line therapy for patients with advanced hepatocellular carcinoma. Cancer 2017; 123: 3725–3731. [DOI] [PubMed] [Google Scholar]
- 9. Shlomai A, Leshno M, Goldstein DA. Regorafenib treatment for patients with hepatocellular carcinoma who progressed on sorafenib—a cost-effectiveness analysis. PLoS One 2018; 13: e0207132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xiang Q, Chen W, Ren M, et al. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin Cancer Res 2014; 20: 2959. [DOI] [PubMed] [Google Scholar]
- 11. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018; 379: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kudo M. Cabozantinib as a second-line agent in advanced hepatocellular carcinoma. Liver Cancer 2018; 7: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lloyd A, Nafees B, Narewska J, et al. Health state utilities for metastatic breast cancer. Br J Cancer 2006; 95: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goulart B, Ramsey S. A trial-based assessment of the cost-utility of bevacizumab and chemotherapy versus chemotherapy alone for advanced non-small cell lung cancer. Value Health 2011; 14: 836–845. [DOI] [PubMed] [Google Scholar]
- 15. Pinter M, Peck-Radosavljevic M. Review article: systemic treatment of hepatocellular carcinoma. Aliment Pharmacol Ther 2018; 48: 598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shepard DS. Cost-effectiveness in health and medicine. By Gold M.R., Siegel J.E, Russell L.B., Weinstein M.C. (eds). New York: Oxford University Press, 1996. J Ment Health Pol Econ 1999; 2: 91–92. [Google Scholar]
- 17. Owen L, Pennington B, Fischer A, et al. The cost-effectiveness of public health interventions examined by NICE from 2011 to 2016. J Public Health (Oxf) 2018; 40: 557–566. [DOI] [PubMed] [Google Scholar]
- 18. Jönsson B. Bringing in health technology assessment and cost-effectiveness considerations at an early stage of drug development. Mol Oncol 2015; 9: 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garrison LP, Jr, Neumann PJ, Willke RJ, et al. A health economics approach to US value assessment frameworks—summary and recommendations of the ISPOR special task force report [7]. Value Health 2018; 21: 161–165. [DOI] [PubMed] [Google Scholar]
- 20. Zhang P, Yang Y, Wen F, et al. Cost-effectiveness of sorafenib as a first-line treatment for advanced hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2015; 27: 853–859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supp._Fig_1 for Cabozantinib for patients with advanced hepatocellular carcinoma: a cost-effectiveness analysis by Amir Shlomai, Moshe Leshno and Daniel A. Goldstein in Therapeutic Advances in Gastroenterology


