Abstract
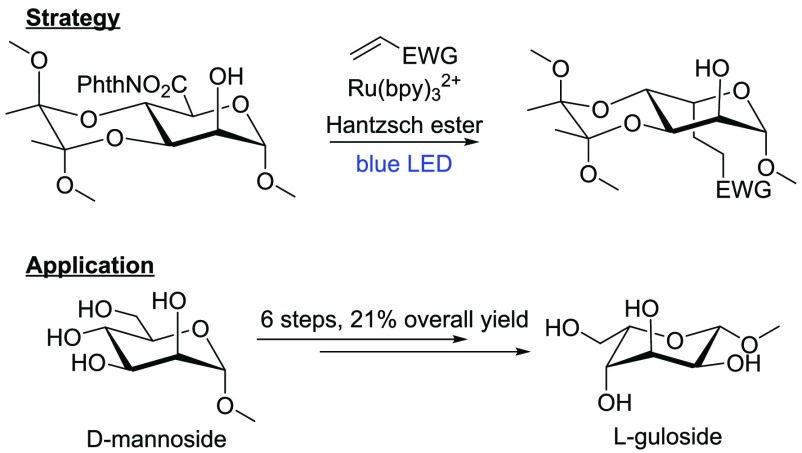
Photodecarboxylation–alkylation of conformationally locked monosaccharides leads to inversion of stereochemistry at C5. This allows the synthesis of l-sugars from their readily available d-counterparts. Via this strategy, methyl l-guloside was synthesized from methyl d-mannoside in 21% yield over six steps.
Modern photoredox catalysis has opened new doors for organic synthesis and has challenged bond disconnection approaches.1 It exploits the reactivity of carbon-centered radicals that are generated either by hydrogen atom transfer (HAT) or via decarboxylation.2,3 Both processes are productive, provided that the resulting radical is stabilized by either orbital overlap of the singly occupied p-orbital with a σ-bond (hyperconjugation)4 or by neighboring heteroatoms with lone pairs (conjugation).5
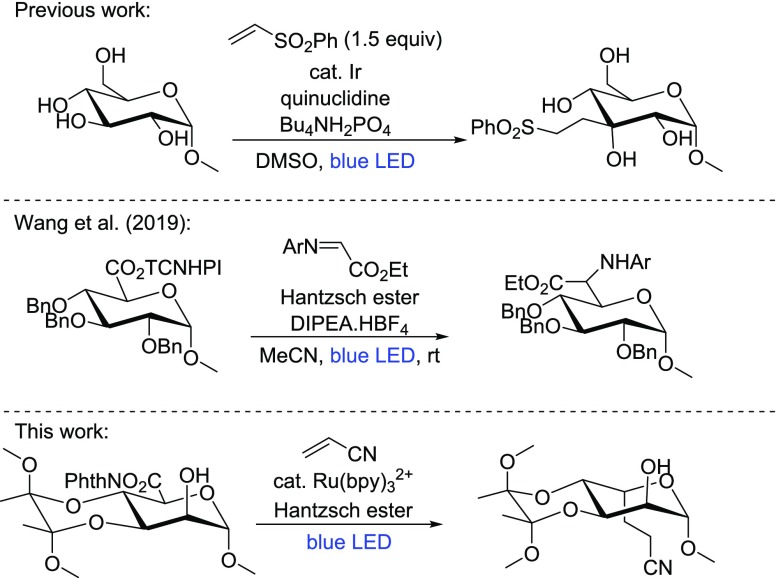
Photoredox catalysis has been utilized in the synthesis of natural products6−8 and even to derivatize complex biomolecules.5,9,10 Its application in the field of carbohydrate chemistry enables the synthesis of derivatives that are difficult to access via existing synthesis routes. We showcased this by employing photocatalytic HAT for the site-selective alkylation of unprotected glucosides.11 C3-alkylated allosides were prepared using this approach. Taylor and co-workers recently demonstrated that, in the presence of diarylborinic acids, the strategy can be extended to differently configured glycosides.12
We subsequently realized that decarboxylative photoalkylation could provide another means to prepare carbohydrate derivatives. If C6 in a hexose is a carboxylic acid, as in uronic acids, it should be amendable to this strategy. In particular, their pyranoside forms should be suitable substrates. After decarboxylation, the resulting radical at C5 is stabilized by the ring oxygen, similar to the classical Barton radical decarboxylation.13−16 The radical has nucleophilic character and can attack electron-poor SOMOphiles, such as Michael acceptors, forming a carbon–carbon bond at the β-position of the SOMOphile.
Modification, including homologation, of the C6 hydroxyl group in readily available d-sugars, such as glucose, mannose, galactose, and N-acetylglucosamine, has been extensively studied and is well-developed.17−21 Nonetheless, the decarboxylative photoalkylation would provide a unique opportunity to invert the stereochemistry at C5, which leads to the corresponding C6 functionalized l-sugars and sugar derivatives. In contrast to the commonly found C6-deoxy sugars l-rhamnose and l-fucose, l-sugars oxidized at C6 are not readily available. Therefore, the latter have to be prepared either from C6-deoxy sugars via C–H activation22 or via epimerization protocols that are mostly lengthy.23 As such, the decarboxylative photoalkylation would fill an unmet need in the synthesis of l-sugar derivatives, which are a rare but integral part of biology.24 The challenge in this strategy is the control of stereochemistry at the (re)formed C5 stereocenter. It seemed most productive to rely on substrate control, in this case control over the conformation of the six-membered ring upon formation of the radical. Inspired by the work of the Overman group,25 we decided to adopt their method for the activation of the carboxylic acid at C6—using the N-hydroxyphthalimide ester (NHP ester) as the redox active group (Figure 1).
Figure 1.
Our previous work on glucoside C–H activation and new approaches to C5 activation via photodecarboxylation.
While performing our studies, the Wang group published their results on the decarboxylative photoalkylation of furanoses and pyranoses.26 Their results showed that the alkylation of benzyl and benzoyl-protected glycuronides led to retention of configuration at C5. Here, we present an approach in a complementary vein, leading, in contrast, to an inversion of configuration at C5. To illustrate the scope and utility of our method, we demonstrate how methyl l-guloside is prepared from methyl d-mannoside in six steps and an overall yield of 21%.
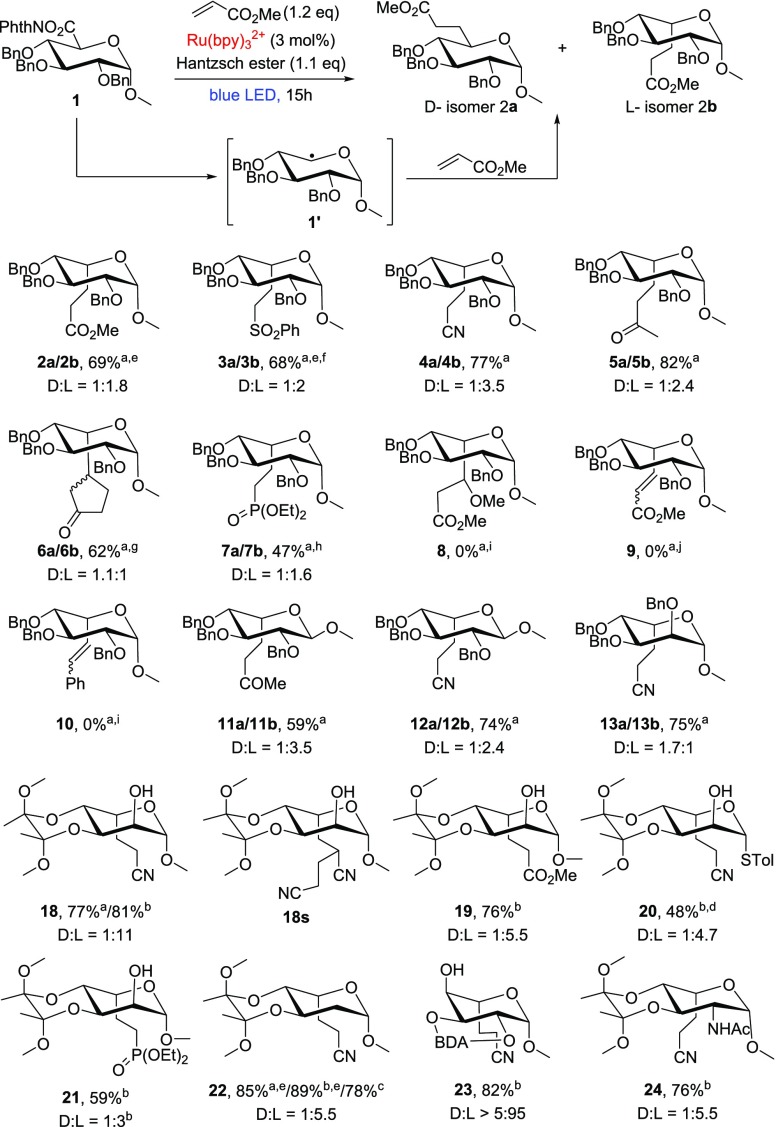
We initiated our investigation with the hypothesis that radical 1′, generated from the NHP ester 1, would add to a SOMOphile, e.g., a Michael acceptor, to give the photoalkylation products (see Scheme 1). To indicate the stereochemistry at C5 throughout this paper, regardless the exact nature of the substituent, and relate this to accepted nomenclature in carbohydrate chemistry, we denote products with retention of stereochemistry as “d” and those with inversion as “l”. Initial success was obtained with methyl acrylate under the reaction conditions proposed by Overman, leading to the separable diastereomers 2a and 2b in 24% and 45% yield, respectively. Other SOMOphiles, such as phenyl vinyl sulfone, acrylonitrile, and methyl vinyl ketone worked as well with comparable yields and again with a slight preference for the l-isomer (Scheme 1, products 3, 4, and 5). Cyclopentenone gave somewhat lower yields (6), because of a troublesome purification. Diethyl vinylphosphate as a SOMOphile caused problems in purification and multiple addition but still afforded the desired product (7). Use of the less-polarized SOMOphile 3-methoxy methyl acrylate gave the corresponding xyloside, rather than the desired product (8). Reduction of the substrate is an expected side reaction, also observed by Okada in the original report of the reaction associated with NHP esters.27 Alkynes were not suitable as SOMOphiles; methyl propiolate provided a mixture of uncharacterized products, whereas phenyl acetylene yielded the xyloside. For both alkynes, the desired products (9 and 10) were not obtained.
Scheme 1. Scope of the Decarboxylative Photoalkylation Reaction.
Only the l-products are shown. aRu(bpy)3Cl2·6H2O. Solvent: 7:3 THF:water. bRu(bpy)3(PF6)2. Solvent: dry THF. cTCNHPI ester was used instead. dYield for the l-product. Products are separable by column chromatography, but the d-product was impure. d:l ratio determined by HPLC. eYield adjusted for co-eluting phthalimide. fYield calculated after subsequent deprotection. gMixture of diastereomers. hd-product contaminated with coeluting unknown. iReduction to xyloside. jIntractable mixture.
The study proceeded with the NHP esters of methyl 2,3,4-O-tribenzyl-β-glucuronide and methyl 2,3,4-O-tribenzyl-α-mannuronide (34 and 35; see the Supporting Information). The yields and d:l ratios for β-glucuronide products 11 and 12 were comparable to those of α-glucuronide 2 and 4. We obtained the products of the α-mannuronide 13 as an inseparable mixture of the expected diastereomers with, in this case, a slight preference for d-isomer 13a.
At this point, it was clear that, although the reaction protocol was fine, the stereochemistry of the product was not fully under control. In the literature, the stereoselectivity of radical glycosylation at C1, a related process, has been well-studied. Protected glucosides give α-C-glycosides via a radical intermediate that adopts a boat conformation so that the C2 acyl/alkoxy substituent is axial, maximizing overlap of the lone pair on the ring oxygen, the radical at C1, and the σ*CO orbital at C2.28,29 Under similar conditions, xylosides yield mainly β-C-glycosides, presumably via the inverted 1C4 chair intermediate, because of its stability, relative to the boat conformer.30 Moreover, the reactivity of the SOMOphile has an effect on the stereoselectivity.31 We concluded that the fluxional nature of the glycosyl radical was the reason for the poor stereoselectivities observed with perbenzylglycuronides.
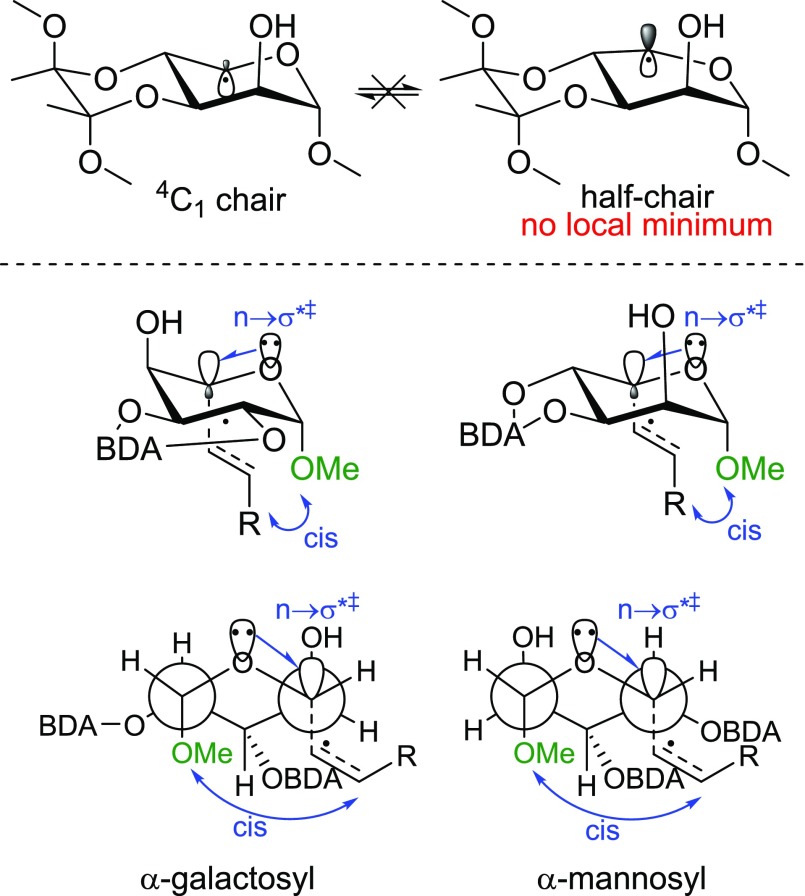
The Matsuda group showed that the stereochemical outcome of radical glycosylations can be controlled by locking the substrate either in the 4C1 conformation using the butane diacetal (BDA) protecting group or in the 1C4 conformation, using a boronate ester.32 They revealed that conformationally restricted C1 radicals are predominantly attacked from the axial direction, because of the overlap in the transition state of the σ*‡ orbital of the forming C–C bond with the lone pair of the ring oxygen. This special case of the anomeric effect determines the outcome of the reaction. Approach from the top face, although less hindered, disrupts this favorable overlap, leading to a less-stable transition state.
We realized that a similar approach could be used to enhance the l-selectivity of the decarboxylative photoalkylation of glycuronides. The rigid 6,6-trans-fused bicyclic system that is formed upon protection of a 1,2-trans diol with the BDA group33−36 should restrict the conformational freedom of the glycosyl radical. This reasoning is supported by our DFT calculation (ZORA-BLYP-D3(BJ)/TZ2P) of the BDA-mannosyl, BDA-galactosyl, and BDA-2-deoxyglucosyl C5 radical. The 4C1conformer is, by far, the most stable conformer. (See the Supporting Information.) This is consistent with the ab initio calculations of Matsuda et al. on the conformers of a C1 radical.30 As in the case of a C1 radical, axial attack of the C5 radical should be favored, leading to the l-product (see Figure 2).
Figure 2.
(Top) Results of DFT geometry optimization of the mannosyl radical for both the chair and the half-chair conformer. (Bottom) Prediction of the stereochemical outcome of the C5 alkylation in both the α-galactosyl and α-mannosyl radical modeled after Matsuda et al. The chair conformation and Newman projection viewed from the ring oxygen are depicted.
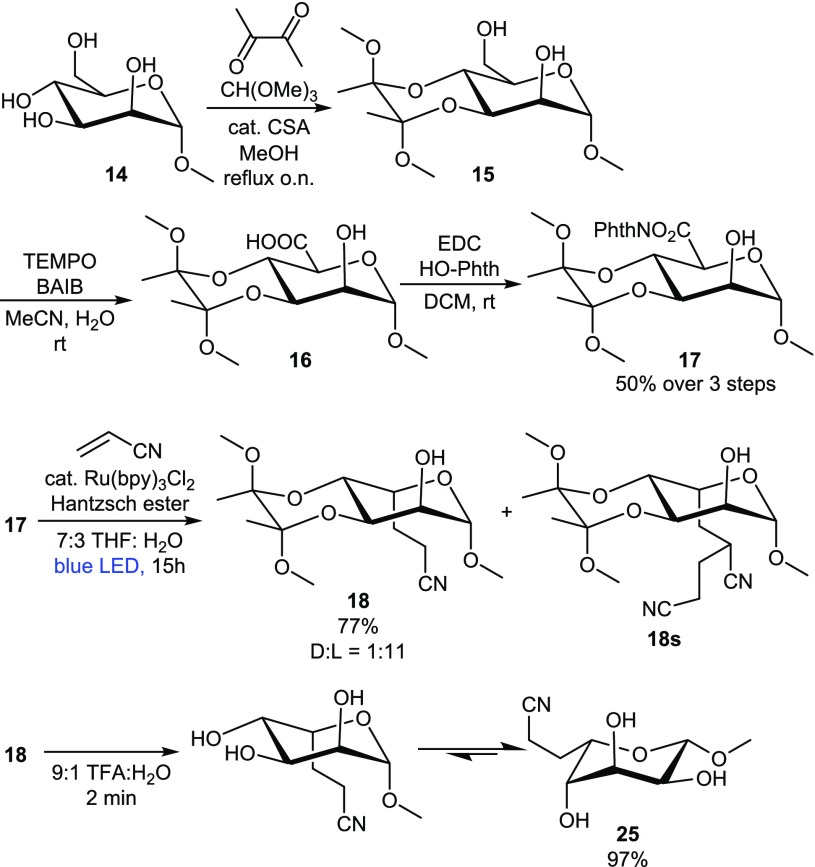
Therefore, we embarked on the synthesis of the BDA-locked NHP-esters of glycuronides. Mannuronide 17 was prepared in 50% yield over three steps without intermediate purification by reacting the C3-OH and C4-OH in 14 with butanedione, oxidizing the primary OH with TEMPO/BAIB37 and esterifying the resulting acid with N-hydroxyphthalimide (see Scheme 2).
Scheme 2. L-Selective Decarboxylative Alkylation of Methyl-α-Mannoside 14.
NHP-ester 17 was subjected to the photoalkylation reaction with acrylonitrile to give 18 in 77% yield, with a rewarding d:l ratio of 1:11, overwhelmingly favoring the l-isomer. The presence of the d-isomer was confirmed after quantitative removal of the BDA group.33 A small amount of double addition product was also isolated (18s). In an attempt to minimize the formation of 18s, the amount of acrylonitrile and Hantzsch ester was varied, but this did not result in a significantly improved yield. Compound 25 adopts the 1C4 conformer, as judged from the coupling constants in the variable temperature 1H NMR spectra (J1,2 = 1.5 Hz in 18 and 8.2 Hz in 25).
To assess the generality of the approach, the methyl glycosides of N-acetylglucosamine, 2-deoxyglucose, and galactose were similarly converted to the corresponding NHP esters and subjected to decarboxylative photoalkylation with various SOMOphiles (see Scheme 1, 18–24). The mannuronides and galacturonides provided the l-product with high selectivity upon alkylation with acrylonitrile (18 and 24), while the NHP esters of N-acetylglucosaminuronide and 2-deoxyglucuronide showed a somewhat lower l-selectivity upon alkylation (22 and 24). The stereoselectivity was sensitive for the SOMOphile used (18, 19 and 21). Nevertheless, the l-product was always favored. This scope demonstrated the functional group tolerance of the current strategy as well, as free hydroxyl groups and amides were tolerated. During the course of the investigation, the NHP ester of methyl galacturonide was found to be susceptible to hydrolysis, and, therefore, the reaction was performed in anhydrous tetrahydrofuran (THF) with the organic soluble Ru(bpy)3(PF6)2. Yields and selectivities were comparable, as expected. The procedure was further fine-tuned by switching N-hydroxyphthalimide to N-hydroxytetrachlorophthalimide (TCNHPI), the latter pioneered by Baran and co-workers as a redox-active group.38,39 This avoided coelution of the byproduct phthalimide. To demonstrate the utility of the methodology for oligosaccharide synthesis, l-thio-guloside 20, a donor in glycosylation reactions, was prepared in 48% yield.
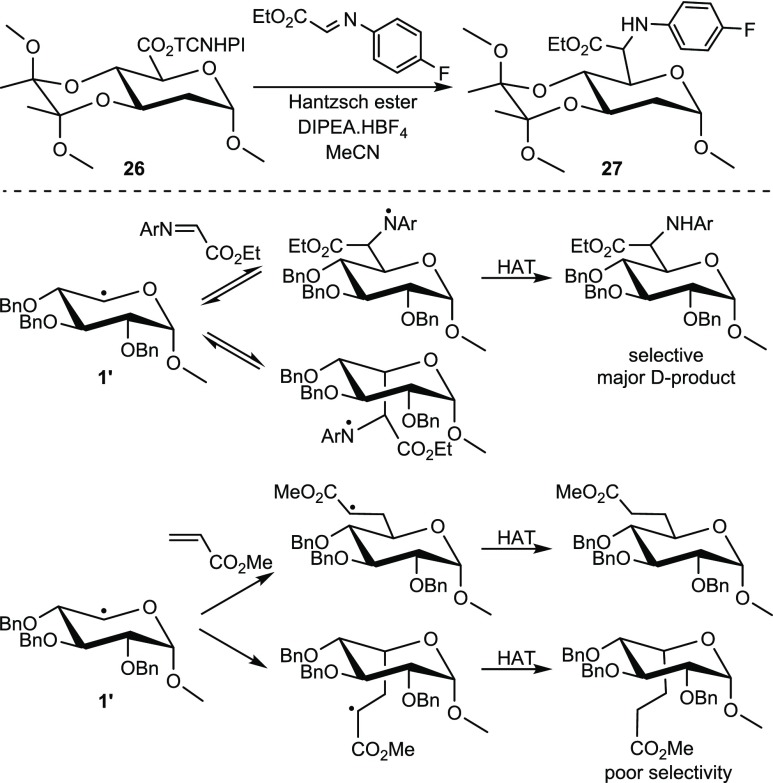
To compare our results with those of Wang et al., the TCNHPI -ester of methyl 2-deoxyglucuronide 26 was used in their benchmark reaction with the p-fluoroaniline imine of ethyl glyoxylate (Scheme 3). Contrary to the aforementioned SOMOphiles, the isolated product 27 had the d-configuration. Combining this result with the previously observed low selectivity with the NHP esters of the perbenzyl glycosides, we hypothesize that the addition of radical 1′ to the imine is reversible, leading to the thermodynamic product, whereas the addition to a Michael acceptor is irreversible, leading to a mixture of d- and l-products. This also explains the poor selectivity observed in the reaction of NHP-glycoside esters without conformational lock (see Scheme 3, bottom).
Scheme 3. (Top) Photoalkylation of 26 with an Imine SOMOphile, According to Wang et al. (Bottom) An Explanation of the Observed d-Selectivity in the Case of Imine Addition.
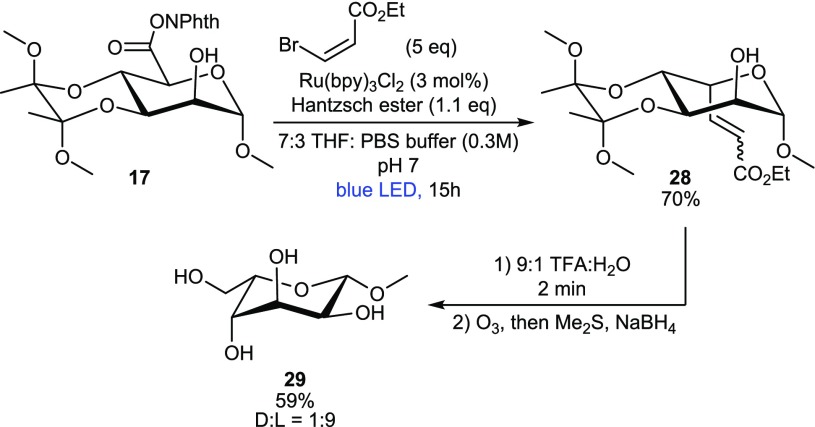
With these results in hand, we decided to apply our methodology to the synthesis of l-gulose from d-mannose. l-Gulose is a rare sugar that has been synthesized previously via different routes40−43 and is part of the important anticancer drug bleomycin A2.44l-Guluronic acid forms, together with d-mannuronic acid, the biopolymer alginic acid, which is widely found in the cell walls of brown algae and the pathogenic bacterium P. aeruginosa.45 A strategy to introduce the required hydroxymethylene unit was found using ethyl (Z)-β-bromoacrylate as the SOMOphile, which eliminates HBr after photoalkylation to produce the corresponding alkene 28 in 70% yield (Scheme 4). We noted that ozonolysis, followed by reductive workup, invariably led to epimerization of the axial C5 substituent. Therefore, the BDA group was removed first, allowing ring flip, so that the C5 substituent would be equatorial. In the event, ozonolysis, followed by reductive workup using NaBH4, afforded methyl l-guloside 29 in 59% yield with retention of stereochemistry.
Scheme 4. Synthesis of Methyl β-L-Guloside 29.
In this investigation, we have synthesized alkylated glycosides from their corresponding NHP esters via decarboxylative photoalkylation. The stereochemical outcome of the reaction could be controlled by locking the substrate in a 4C1 chair conformation via its butane diacetal derivative. This strategy provides the products with inversion of stereochemistry at C5, when Michael acceptors are used as SOMOphiles. Compared to most of the previous strategies to prepare l-hexoses, the current strategy has the advantage that the pyranose connectivity is preserved. This is important, since most synthetic manipulations of monosaccharides rely heavily on the substrate control provided by the rigid pyranose form.46
Acknowledgments
We thank P. van der Meulen, Dr. J. Kemmink, R. Sneep, and T. Tiemersma (University of Groningen) for assistance on analyses. The Dutch Science Foundation NWO is acknowledged for funding.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.9b03016.
Experimental details; DFT calculations; experimental procedures; NMR and HRMS spectra of the new compounds (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- McAtee R. C.; McClain E. J.; Stephenson C. R. J. Trends Chem. 2019, 1 (1), 111–125. 10.1016/j.trechm.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero N. A.; Nicewicz D. A. Chem. Rev. 2016, 116 (17), 10075–10166. 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- Twilton J.; Le C.; Zhang P.; Shaw M. H.; Evans R. W.; MacMillan D. W. C. Nat. Rev. Chem. 2017, 1, 0052. 10.1038/s41570-017-0052. [DOI] [Google Scholar]
- Nawrat C. C.; Jamison C. R.; Slutskyy Y.; MacMillan D. W. C.; Overman L. E. J. Am. Chem. Soc. 2015, 137 (35), 11270–11273. 10.1021/jacs.5b07678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarver S. J.; Qiao J. X.; Carpenter J.; Borzilleri R. M.; Poss M. A.; Eastgate M. D.; Miller M. M.; MacMillan D. W. C. Angew. Chem., Int. Ed. 2017, 56 (3), 728–732. 10.1002/anie.201608207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao D. J.; Slutskyy Y.; Muuronen M.; Le A.; Kohler P.; Overman L. E. J. Am. Chem. Soc. 2018, 140 (8), 3091–3102. 10.1021/jacs.7b13799. [DOI] [PubMed] [Google Scholar]
- Garnsey M. R.; Slutskyy Y.; Jamison C. R.; Zhao P.; Lee J.; Rhee Y. H.; Overman L. E. J. Org. Chem. 2018, 83 (13), 6958–6976. 10.1021/acs.joc.7b02458. [DOI] [PubMed] [Google Scholar]
- Pitre S. P.; Weires N. A.; Overman L. E. J. Am. Chem. Soc. 2019, 141 (7), 2800–2813. 10.1021/jacs.8b11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S.; Liu C.; Kölmel D. K.; Qiao J. X.; Zhang Y.; Poss M. A.; Ewing W. R.; Macmillan D. W. C. Nat. Chem. 2018, 10 (2), 205–211. 10.1038/nchem.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y.; Kimura A.; Osawa T.; Hari Y. J. Org. Chem. 2018, 83 (18), 10701–10708. 10.1021/acs.joc.8b00637. [DOI] [PubMed] [Google Scholar]
- Wan I. C.; Witte M. D.; Minnaard A. J. Chem. Commun. 2017, 53 (36), 4926–4929. 10.1039/C7CC01416C. [DOI] [PubMed] [Google Scholar]
- Dimakos V.; Su H. Y.; Garrett G. E.; Taylor M. S. J. Am. Chem. Soc. 2019, 141 (13), 5149–5153. 10.1021/jacs.9b01531. [DOI] [PubMed] [Google Scholar]
- Jin J.; MacMillan D. W. C. Angew. Chem., Int. Ed. 2015, 54 (5), 1565–1569. 10.1002/anie.201410432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble A.; McCarver S. J.; MacMillan D. W. C. J. Am. Chem. Soc. 2015, 137 (2), 624–627. 10.1021/ja511913h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton D. H. R.; Crich D.; Motherwell W. B. J. Chem. Soc., Chem. Commun. 1983, 53 (17), 939. 10.1039/c39830000939. [DOI] [Google Scholar]
- Barton D. H. R.; Crich D.; Motherwell W. B. Tetrahedron 1985, 41 (19), 3901–3924. 10.1016/S0040-4020(01)97173-X. [DOI] [Google Scholar]
- Jäger M.; Minnaard A. J. Chem. Commun. 2016, 52, 656–664. 10.1039/C5CC08199H. [DOI] [PubMed] [Google Scholar]
- Descotes G.; Martin J.-C.; Tachi-Dung Carbohydr. Res. 1978, 62 (1), 61–71. 10.1016/S0008-6215(00)83378-9. [DOI] [Google Scholar]
- Ogawa S.; Funaki Y.; Iwata K.; Suami T. Bull. Chem. Soc. Jpn. 1976, 49 (7), 1975–1979. 10.1246/bcsj.49.1975. [DOI] [Google Scholar]
- Durka M.; Tikad A.; Périon R.; Bosco M.; Andaloussi M.; Floquet S.; Malacain E.; Moreau F.; Oxoby M.; Gerusz V.; Vincent S. P. Chem. - Eur. J. 2011, 17 (40), 11305–11313. 10.1002/chem.201100396. [DOI] [PubMed] [Google Scholar]
- Horneman A. M.; Lundt I. J. Carbohydr. Chem. 1995, 14 (1), 1–8. 10.1080/07328309508006432. [DOI] [Google Scholar]
- Frihed T. G.; Pedersen C. M.; Bols M. Angew. Chem., Int. Ed. 2014, 53 (50), 13889–13893. 10.1002/anie.201408209. [DOI] [PubMed] [Google Scholar]
- Frihed T. G.; Bols M.; Pedersen C. M. Chem. Rev. 2015, 115 (9), 3615–3676. 10.1021/acs.chemrev.5b00104. [DOI] [PubMed] [Google Scholar]
- de Leder Kremer R. M.; Gallo-Rodriguez C. Adv. Carbohydr. Chem. Biochem. 2004, 59, 9–67. 10.1016/S0065-2318(04)59002-9. [DOI] [PubMed] [Google Scholar]
- Pratsch G.; Lackner G. L.; Overman L. E. J. Org. Chem. 2015, 80 (12), 6025–6036. 10.1021/acs.joc.5b00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji P.; Zhang Y.; Wei Y.; Huang H.; Hu W.; Mariano P. A.; Wang W. Org. Lett. 2019, 21 (9), 3086–3092. 10.1021/acs.orglett.9b00724. [DOI] [PubMed] [Google Scholar]
- Okada K.; Okamoto K.; Morita N.; Okubo K.; Oda M. J. Am. Chem. Soc. 1991, 113 (24), 9401–9402. 10.1021/ja00024a074. [DOI] [Google Scholar]
- Korth H.; Sustmann R.; Dupuis J.; Giese B. J. Chem. Soc., Perkin Trans. 2 1986, (9), 1453–1459. 10.1039/p29860001453. [DOI] [Google Scholar]
- Gong H.; Gagné M. R. J. Am. Chem. Soc. 2008, 130 (36), 12177–12183. 10.1021/ja8041564. [DOI] [PubMed] [Google Scholar]
- Abe H.; Shuto S.; Matsuda A. J. Am. Chem. Soc. 2001, 123 (48), 11870–11882. 10.1021/ja011321t. [DOI] [PubMed] [Google Scholar]
- Kiya N.; Hidaka Y.; Usui K.; Hirai G. Org. Lett. 2019, 21 (6), 1588–1592. 10.1021/acs.orglett.9b00133. [DOI] [PubMed] [Google Scholar]
- Oestreich M. Angew. Chem., Int. Ed. 2014, 53 (9), 2282–2285. 10.1002/anie.201310585. [DOI] [PubMed] [Google Scholar]
- Hense A.; Ley S. V.; Osborn H. M. I.; Owen D. R.; Poisson J.; Warriner S. L.; Wesson K. E. J. Chem. Soc., Perkin Trans. 1 1997, (14), 2023–2032. 10.1039/a702497e. [DOI] [Google Scholar]
- Douglas N. L.; Ley S. V.; Lücking U.; Warriner S. L. J. Chem. Soc., Perkin Trans. 1 1998, (1), 51–66. 10.1039/a705275h. [DOI] [Google Scholar]
- Ley S. V.; Priepke H. W. M. Angew. Chem., Int. Ed. Engl. 1994, 33 (22), 2292–2294. 10.1002/anie.199422921. [DOI] [Google Scholar]
- Baeschlin D. K.; Chaperon A. R.; Green L. G.; Hahn M. G.; Ince S. J.; Ley S. V. Chem. - Eur. J. 2000, 6 (1), 172–186. . [DOI] [PubMed] [Google Scholar]
- Walvoort M. T. C.; Sail D.; van der Marel G. A.; Codée J. D. C. In Carbohydrate Chemistry: Proven Synthetic Methods, Vol. 1; Kováč P., Ed.; CRC Press, 2016; pp 99–105. [Google Scholar]
- Qin T.; Cornella J.; Li C.; Malins L. R.; Edwards J. T.; Kawamura S.; Maxwell B. D.; Eastgate M. D.; Baran P. S. Science 2016, 352 (6287), 801–805. 10.1126/science.aaf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGruyter J. N.; Malins L. R.; Wimmer L.; Clay K. J.; Lopez-Ogalla J.; Qin T.; Cornella J.; Liu Z.; Che G.; Bao D.; Stevens J. M.; Qiao J. X.; Allen M. P.; Poss M. A.; Baran P. S. Org. Lett. 2017, 19 (22), 6196–6199. 10.1021/acs.orglett.7b03121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. M.; Keranen M. D.; O’Doherty G. A. J. Org. Chem. 1999, 64 (9), 2982–2983. 10.1021/jo990410+. [DOI] [PubMed] [Google Scholar]
- Haukaas M. H.; O’Doherty G. A. Org. Lett. 2001, 3 (24), 3899–3902. 10.1021/ol016743m. [DOI] [PubMed] [Google Scholar]
- Harris J. M.; Keränen M. D.; Nguyen H.; Young V. G.; O’Doherty G. A. Carbohydr. Res. 2000, 328 (1), 17–36. 10.1016/S0008-6215(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Wang H.-Y. L.; O’Doherty G. A. Chem. Commun. 2011, 47 (37), 10251. 10.1039/c1cc13837e. [DOI] [PubMed] [Google Scholar]
- Umezawa H. Prog. Biochem. Pharmacol. 1976, 11, 18–27. [PubMed] [Google Scholar]
- Davies J. C. Paediatr. Respir. Rev. 2002, 3 (2), 128–134. 10.1016/S1526-0550(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Wang T.; Demchenko A. V. Org. Biomol. Chem. 2019, 17 (20), 4934–4950. 10.1039/C9OB00573K. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.