Abstract
Background: The prevalence of neuropathic pain is estimated to be between 7 and 10% in the general population. The efficacy of intravenous (IV) lidocaine has been studied by numerous clinical trials on patients with neuropathic pain. The aim of this systematic review and meta-analysis was to evaluate the efficacy of IV lidocaine compared with a placebo for neuropathic pain and secondly to assess the safety of its administration.
Methods: A literature search on PubMed, Scopus, CENTRAL (Cochrane Central Register of Controlled Trials), and Google scholar databases was performed for relevant studies published up to February 2019. Randomized controlled trials (RCTs) evaluating IV lidocaine treatment for pain relief in patients with neuropathic pain were included.
Results: 26 articles met the inclusion criteria. Patients with varied etiology of neuropathic pain were among the patient samples of these studies. Fifteen articles were included for quantitative analysis. Lidocaine was superior to a placebo in relieving neuropathic pain in the early post-infusion period [Mean Difference (MD) = −11.9; 95% Confidence interval (CI): −16.8 to −7; p < 0.00001]. Multiple infusions of lidocaine over a period of 4 weeks, however, had no significant effect on reliving neuropathic pain (MD = −0.96; 95% CI: −2.02 to 0.11; p = 0.08). IV lidocaine was also associated with a significant number of adverse events compared to a placebo [Odds Ratio (OR) = 7.75; 95% CI: 3.18–18.92; p < 0.00001].
Conclusion: Our study indicates that while IV lidocaine is effective in pain control among patients with neuropathic pain in the immediate post-infusion period, it does not have a long-lasting, persistent effect. IV infusions of the drug are associated with an increased risk of side effects compared to a placebo. However, the risk of serious adverse events is negligible. Further, well-designed RCTs evaluating the effects of various dosages and infusion periods of IV lidocaine are required to provide clear guidelines on its clinical use.
Keywords: neuralgia, causalgia, local anesthetics, pain, adverse events
Introduction
The International Association for the Study of Pain defines neuropathic pain as “pain resulting from damage to the peripheral or central nervous system (1).” Its prevalence has been reported on several countries worldwide, varying from 3.3% in Austria (2) to 6.9% in France (3) and as high as 8% in the UK (4). van Hecke et al. (5), in their systematic review, report the prevalence of neuropathic pain to be between 7 and 10%. Epidemiological surveys show that a large proportion of patients with neuropathic pain do not receive adequate treatment (6). This may be attributable to clinicians' low diagnostic accuracy and by a lack of knowledge of effective drugs and their proper use (7). Neuropathic pain has a significant impact on quality of life and can be responsible for a substantial financial burden on individuals afflicted.
Lidocaine (lignocaine), a widely used local anesthetic, has been used intravenously (IV) as an antiarrhythmic drug. Reports in the 1950s described the effectiveness of IV lidocaine for pain in cancer and post-operative patients (8). Later, in the 1980s, trials suggested that IV lidocaine is also effective for alleviating neuropathic pain (9, 10). Lidocaine is thought to produce analgesia by exerting a blockade of peripheral and central sodium ion gate channels, including those in the spinal dorsal horn. A number of clinical trials have been conducted to date, evaluating the efficacy of IV lidocaine in patients with neuropathic pain (11–14). However, only one systematic review and meta-analysis, which was published in 2005, has evaluated a pooled treatment effect (15). Since 2005, however, a number of new trials on IV lidocaine have been published in the literature (16–19). Therefore, the aim of this systematic review and meta-analysis was to evaluate the efficacy of IV lidocaine compared with a placebo for neuropathic pain and secondly to assess the safety of its administration.
Materials and Methods
Search Strategy
PubMed, Scopus, CENTRAL (Cochrane Central Register of Controlled Trials), and Google scholar databases were searched for relevant studies published up to February 2019. The PICOS (Population, Intervention, Comparison, Outcome, and Study design) outline was used for the electronic search. Keywords used for the patient sample population were: Neuralgia [MeSH] OR neuropathic pain [MeSH] OR pain [MeSH] OR causalgia [MeSH]; for intervention were: lidocaine [MeSH] OR intravenous anesthesia [MeSH] OR lignocaine [MeSH] OR local anesthetics [MeSH] OR intravenous lidocaine [Free text]; for comparison were: saline [MeSH] OR placebo effect [MeSH]; for outcomes were: pain [MeSH] OR analog pain scale [MeSH] OR adverse events [MeSH] OR pain relief [Free text] OR pain assessment [MeSH]. Study designs that were searched were randomized clinical trials (RCTs). We also analyzed references of included studies and pertinent reviews on the topic for the identification of additional studies. Guidelines of the PRISMA Statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses) (20) and the Cochrane Handbook for Systematic Reviews of Intervention (21) were followed during the conduct of the review.
Eligibility Criteria
We included studies conducted on patients (Population) with neuropathic pain from any cause that evaluated the use of intravenous lidocaine (Intervention) for pain relief compared with a placebo (Comparison) and that assessed pain relief and adverse effects (Outcomes). Studies excluded were: non-english language studies, animal studies, retrospective studies, uncontrolled and non-blinded studies and studies comparing lidocaine with an active drug.
Data Collection and Analysis
Two reviewers examined the studies based on the inclusion criteria. The studies were reviewed firstly on their title and abstracts, followed by a full-text review of potentially relevant articles. Any difference in opinion between the reviewers was resolved by discussion. Two reviewers then extracted the following data from the studies: general information on the trial (authors, year of publication, study type), number of patients, etiology of pain, lidocaine dosage, placebo used, outcome assessment scale, pain scores, and follow-up period of outcome measurements and adverse events. Attempts were made as needed to contact the corresponding authors via email for any missing data.
Quality Assessment
The Cochrane Collaboration risk of bias assessment tool for RCTs was used for quality assessment of the included trials (22). Studies were rated as low risk, high risk, or unclear risk of bias for: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Articles were rated on each of the above items as: low risk of bias (score of 2), unclear risk of bias (score of 1), or high risk of bias (score of 0). The overall quality was then categorized as low (score 0–5), medium (score 6–10), or high (score 11–14).
Statistical Analysis
Data collected from the included studies was entered into Review Manager (RevMan, version 5.3; Nordic Cochrane Center [Cochrane Collaboration], Copenhagen, Denmark; 2014) for the meta-analysis. Pain scores reported as mean and standard deviation (SD) were used. If no SD was reported, we calculated it from Standard Error of the Mean (SEM) and sample size. If complete data on pain outcomes was not available from the article, a 2005 meta-analysis (15) on this subject was referred to for missing data. The total number of adverse events, as described in the included studies, was pooled. No distinction was made on the severity of each adverse event. Considering the variation in the studies, a random-effects model was used to calculate pooled effect size. Odds ratios (OR) were calculated for adverse events. The evaluation of heterogeneity was calculated using the I2 statistic. We considered I2 of <40% as unimportant, while that of more than 40% was viewed as moderate to considerable heterogeneity.
Results
Search Results
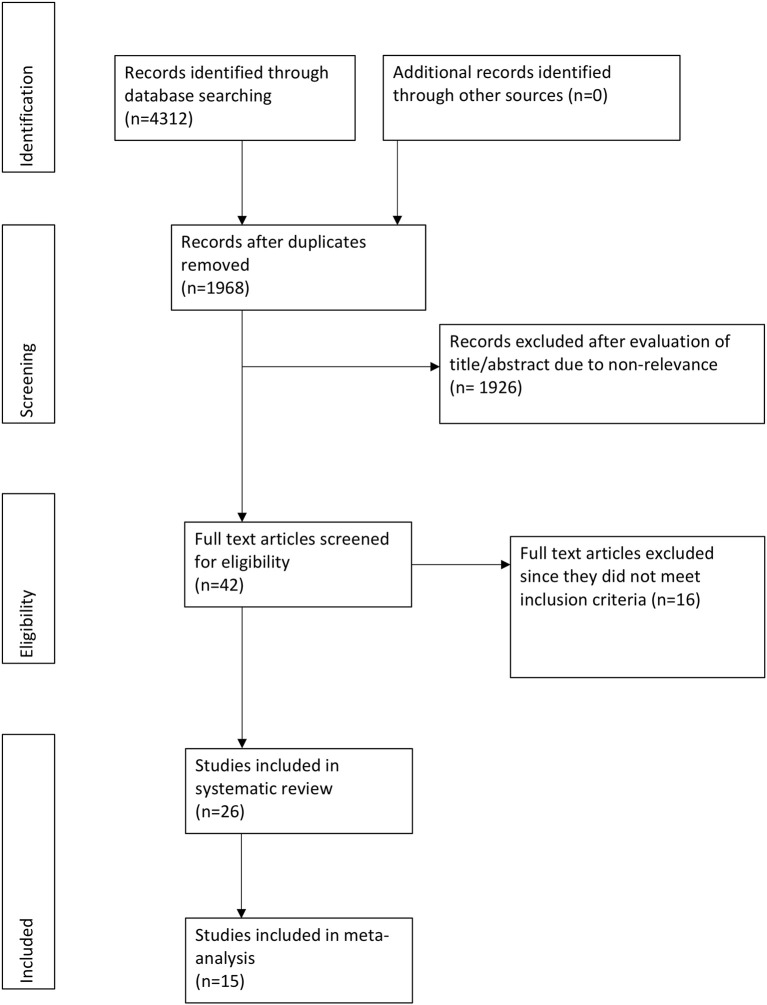
Four thousand three hundred twenty-one records were found after the initial search (Figure 1). Full texts of 42 studies were reviewed, of which 16 studies were excluded: 2 were duplicate publications (23, 24), 4 were non-randomized (25–28), 2 were non-blinded (29, 30), 2 were retrospective studies (31, 32), 2 had used lignocaine for prevention of post-herpetic neuralgia (phn) (33, 34), 3 did not use a placebo (35–37), while the remaining study did not use any controls (38). A total of 26 articles were included in the systematic review (11–14, 16–19, 39–56).
Figure 1.
Flowchart of study.
Quality Assessment
The risk of bias summary is presented in Figure 2. Methods of randomization were clearly described in 9 studies (16–18, 42, 43, 52–54, 56), allocation concealment was described in eight studies (16, 18, 39, 41–43, 52, 54) and blinding of outcome assessment was clearly mentioned in 6 studies (17, 18, 42, 51, 52, 56). Ten of the studies were categorized as medium quality, while the remaining were rated as high quality trials.
Figure 2.
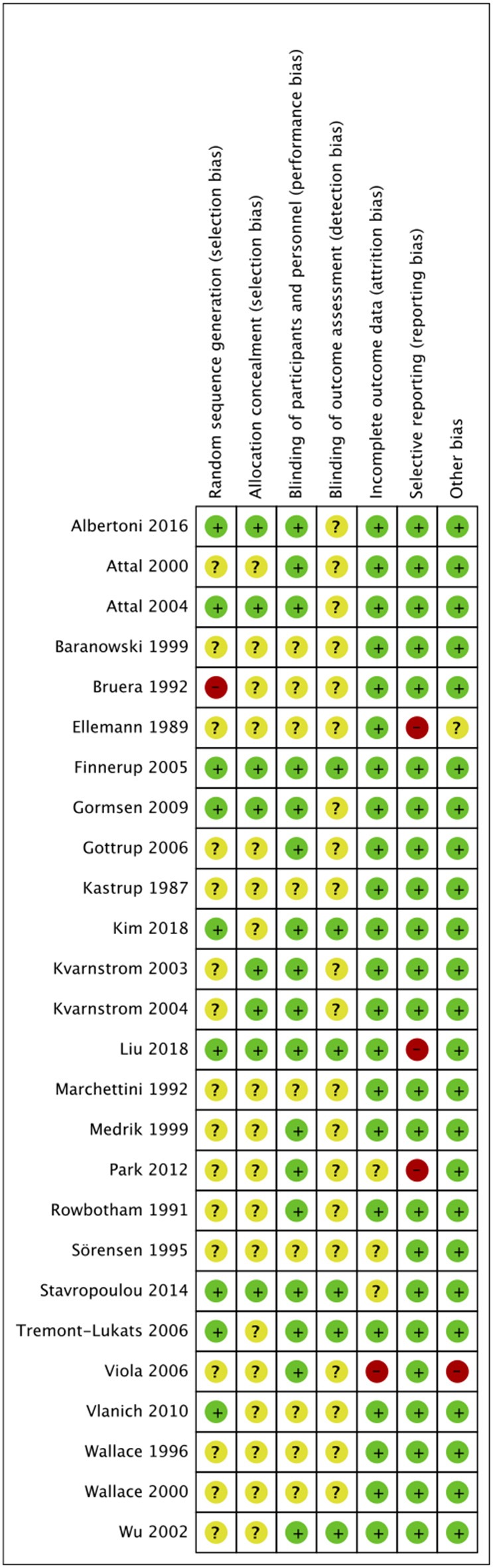
Risk of bias summary.
Characteristics of Included Studies and Data Analysis
Details of the included studies are presented in Table 1. The trial design was parallel in five studies (16, 17, 52, 53, 56), while all the remaining studies were cross-over trials. Patients with various causes of neuropathic pain were studied in the included articles. In one trial of post-amputation pain where phantom and stump pain were studied, only scores of stump pain were included (51). In another trial where spontaneous and evoked pain was studied, we included only the spontaneous pain group (42). The sample size of included studies varied from 10 to 30 participants per group, with the exception of one trial that had a large sample size of 90 patients in the intervention group. However, pain scores in the trial were not reported as mean and SD. The dosage and infusion times of lidocaine also varied across the studies. The duration of lidocaine infusion ranged from 30 min to 6 h. One study (43) used lidocaine injection given over just 1 min. A 10-point or 100-point Visual analog scale (VAS) was used by all studies to assess pain, except for one trial which used the McGill Pain Questionnaire (53). In the majority of studies, pain was assessed in the immediate post-infusion period, i.e., from just after infusion to up to 1–3 days post-infusion. Data from these studies was pooled to evaluate the early effect of lidocaine on neuropathic pain. In three trials (16, 17, 53), lidocaine was infused in 4 sessions over a period of 4 weeks and pain was assessed after the 4th infusion. These three studies were pooled together to evaluate the effect of multiple lidocaine infusions on neuropathic pain.
Table 1.
Characteristics of included studies.
| References | Methodology | Cause of pain | Evaluable participants | Lidocaine dosage | Placebo | Pain scale used | Time of measurement post infusion | Pain values as Mean (SD) | |
|---|---|---|---|---|---|---|---|---|---|
| Lidocaine | Placebo | ||||||||
| Kastrup et al. (14) | Crossover 5 weeks washout | Painful diabetic neuropathy | 15 | Lidocaine 5 mg/kg ×30 min | 0.9% saline | 100-VAS | 1–3 days | 27.27 (24.53) | 35.4 (29.33) |
| Ellemann et al. (13) | Crossover 1 week washout | Neuropathic cancer pain | 10 | Lidocaine 5 mg/kg x 30 min | 0.9% saline | 10-VAS | Immediately after & at 1 h | NA | NA |
| Rowbotham et al. (11) | Crossover 2 days washout | PHN | 19 | Lidocaine 5 mg/kg x 1 h | 0.9% saline | 100-VAS | Up to 60 min | 29.8 (24.5) | 43.6 (29.3) |
| Bruera et al. (12) | Crossover 2 days washout | Neuropathic pain from cancer | 10 | Lidocaine 5 mg/kg x 30 min | 0.9% saline | 100-VAS | Immediately after & up to 2 days | 36.9 (26) | 34.1 (29.8) |
| Marchettini et al. (45) | Crossover washout not reported | Peripheral neuropathic pain | 10 | Lidocaine 1.5 mg/kg over 1 min | 0.9% saline | 100-VAS | At 35 min | 59.3 (25) | 65 (14) |
| Sörensen et al. (44) | Crossover 1 week washout | Fibromyalgia | 12 | Lidocaine 5 mg/kg x 30 min | 0.9% saline | 100-VAS | Up to 60 min | NA | NA |
| Wallace et al. (49) | Crossover 1 week washout | Neuropathic pain from peripheral nerve injury | 11 | Lidocaine IV infusions targeted to deliver plasma concentrations of 0.5, 1.0, 1.5, 2.0, and 2.5 mcg/ml | 0.9% saline | 100-VAS | Till post infusion | 24.82 (19.61) | 55.1 (36.66) |
| Baranowski et al. (50) | Crossover 1 week washout | PHN | 24 | Lidocaine IV at 1 and 5 mg/kg x 2 h | 0.9% saline | 100-VAS | Till post infusion | 17.5 (31.35) | 10.08 (27.24) |
| Medrik et al. (47) | Crossover 2–7 day washout | Painful lumbosacral radiculopathy | 30 | Lidocaine 5 mg/kg IV x 1–2 h | 0.9% saline | 100-VAS | Up to 1 h | 31 (27.39) | 38 (27.39) |
| Attal et al. (48) | Crossover 3 weeks washout | Neuropathic pain from stroke and spinal cord injury | 16 | Lidocaine 5 mg/kg x 30 min | 0.9% saline | 100-VAS | Till post infusion | 31 (28) | 46 (24) |
| Wallace et al. (40) | Crossover 1 week washout | Complex regional pain syndrome | 16 | Lidocaine IV infusions targeted to deliver plasma concentrations of 0.5, 1.0, 1.5, 2.0, and 3 mcg/ml | Diphenhydramine 70–80 mg | 100-VAS | At 20 min | NA | NA |
| Wu et al. (51) | Crossover 1 day washout | Postamputation pain, stump pain | 22 | Lidocaine 1 mg/kg bolus and a 4 mg/kg iv infusion for 40 min | Diphenhydramine, 10 mg bolus iv + 40 mg infusion | 100-VAS | Up to 30 min | 36.5 (23.5) | 50.1 (25.5) |
| Kvarnström et al. (39) | Crossover 1 week washout | Peripheral neuropathic pain (trauma, surgery, compression) | 12 | Lidocaine 1.0 mg/kg for 10 min and then 1.5 mg/kg for 30 min | 0.9% saline | 10-VAS | Up to 150 min | NA | NA |
| Attal et al. (43) | Crossover with 2 weeks washout | Trauma, PHN | 22 | Lidocaine 5 mg/kg x 30 min | 0.9% saline | 100-VAS | At 60 min | 19 (22) | 38 (22) |
| Kvarnström et al. (41) | Crossover 4 days washout | Traumatic spinal cord injury | 10 | Lidocaine 1.0 mg/kg for 10 min and then 1.5 mg/kg for 30 min | 0.9% saline | 10-VAS | Up to 150 min | NA | NA |
| Finnerup et al. (42) | Crossover 6 days washout | Trauma or disease of the spinal cord or cauda equina | 12 | Lidocaine 5 mg/kg x 30 min | 0.9% saline | 100-VAS | At 35 min | 42.67 (28.86) | 59.42 (18.6) |
| Gottrup et al. (46) | Crossover 2 days washout | Peripheral neuropathic pain | 19 | Lidocaine 5 mg/kg x 30 min | 0.9% saline | 100-VAS | Up to 40 min | 45 (29) | 49 (25) |
| Tremont-Lukats et al. (56) | Parallel | Peripheral neuropathic pain | 31 | Lidocaine at 1, 3, and 5 mg/kg/h as 6 h infusion | 0.9% saline | 100-VAS | Up to 10 h | NA | NA |
| Viola et al. (55) | Crossover 4 weeks washout | Painful diabetic neuropathy | 15 | Lignocaine 5 and 7.5 mg/kg over 4 h for 4 weeks | 0.9% saline | MPQ | At day 14 | NA | NA |
| Gormsen et al. (54) | Crossover 3–11 days washout | Peripheral nerve injury | 13 | Lidocaine 5 mg/kg ×30 min | 0.9% saline + vitamin B | 100-VAS | Up to 24 h | NA | NA |
| Vlainich et al. (53) | Parallel | Fibromyalgia | 30 (15 each group) | Lidocaine 240 mg diluted in 125 mL infused over a period of 1 h, once a week, for 4 weeks | 0.9% saline | 10-VAS | At 4 weeks | 4.1 (2.3) | 4 (2.1) |
| Park et al. (19) | Crossover 2 weeks washout | Neuropathic pain of failed back surgery syndrome | 18 | Lidociane 1 mg/kg and 5 mg/kg at 60 ml/h | 0.9% saline | 100-VAS | Up to 60 min | NA | NA |
| Stavropoulou et al. (18) | Crossover 2 days washout | Trigeminal neuralgia | 20 (n = 40)* | Lidocaine 5 mg/kg ×1 h in two sessions | 0.9% saline | 10-VAS | Till post infusion | 1.46 (1.37) | 3.33 (2.02) |
| Albertoni et al. (16) | Parallel | Fibromyalgia | 38 (19 each group) | Lidocaine 240 mg diluted in 125 mL infused over a period of 1 h, once a week, for 4 weeks | 0.9% saline | 10-VAS | At 4 weeks | 3.3 (1.6) | 4.4 (2.7) |
| Kim et al. (17) | Parallel | PHN or Complex regional pain syndrome type II | 42 (21 each group) | Lidocaine 3 mg/kg infused over a period of 1 h, once a week, for 4 weeks | 0.9% saline | 10-VAS | At 4 weeks | 2.9 (2.53) | 4.74 (2.67) |
| Liu et al. (52) | Parallel | PHN | 183 | Lidocaine 5 mg/kg ×1.5 h | 0.9% saline | 10-VAS | Up to 4 weeks | NA | NA |
SD, Standard Deviation; VAS, Visual analog scale; PHN, Post herpetic neuralgia; h, Hours; NA, Not available; wk, week; mins, minutes; MPQ, McGill Pain Questionnaire.
Each participant underwent two sessions of treatment and placebo.
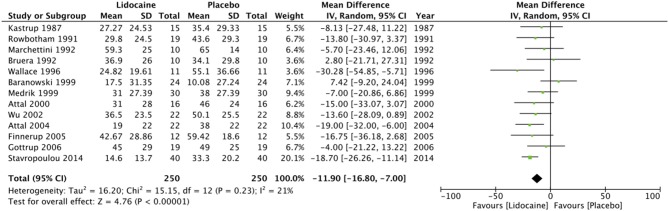
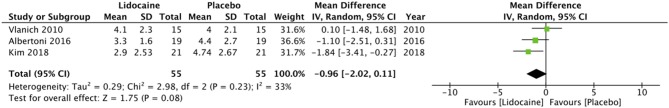
Pain scores from the immediate post-infusion period were available from 13 studies (11, 12, 14, 18, 42, 43, 45–51). Data on 250 patients from these studies was pooled to estimate the effect size. Our analysis indicates that lidocaine is superior to placebo in relieving neuropathic pain in the early post-infusion period (MD = −11.9; 95% CI: −16.8 to −7; p < 0.00001). Heterogeneity was non-significant (I2 = 21%, p = 0.23) (Figure 3). Multiple infusions of lidocaine over a period of 4 weeks, however, has no significant effect on relieving neuropathic pain (MD = −0.96; 95% CI: −2.02 to 0.11; p = 0.08) (Figure 4).
Figure 3.
Forrest plot of IV lidocaine vs. placebo for pain in the immediate post-transfusion period.
Figure 4.
Forrest plot of IV lidocaine vs. placebo for pain persistent pain relief.
Adverse Events
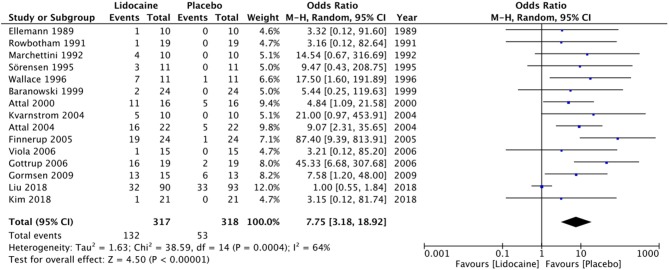
Lightheadedness, somnolence, peri-oral paresthesia, nausea, headache, dysarthria, dry mouth, and metallic taste were some of the most common side effects noted by the trials (Table 2). Data was available from 15 studies (11, 13, 17, 42–46, 49, 50, 52, 54, 55) for a meta-analysis on adverse events from IV lidocaine usage. Three hundred seventeen patients received lidocaine, while 318 patients received a placebo. One hundred thirty-two patients (41.64%) in the lidocaine group experienced adverse events, compared to 53 patients (16.66%) in the placebo group. Our analysis shows that IV lidocaine is associated with a significant number of adverse events, compared to a placebo (OR = 7.75; 95% CI: 3.18–18.92; p < 0.00001) (Figure 5).
Table 2.
Adverse events reported in included trials.
| References | Number of adverse events | Adverse events reported | |
|---|---|---|---|
| Lidocaine (n/N) | Placebo(n/N) | ||
| Kastrup et al. (14) | 0 | 0 | None |
| Ellemann et al. (13) | 1/10 | 0/10 | Transient drowsiness |
| Rowbotham et al. (11) | 1/19 | 0/19 | Nausea & Lightheadedness (1) |
| Bruera et al. (12) | 0 | 0 | None |
| Marchettini et al. (45) | 4/10 | 0/10 | Lightheadedness (4) |
| Sörensen et al. (44) | 3/11 | 0/11 | Nausea & perioral numbness (2); drowsiness, dysarthria, & tremor (1) |
| Wallace et al. (49) | 7/11 | 1/11 | Lightheadedness (6), Nausea (1) |
| Baranowski et al. (50) | 2/24 | 0/24 | Circumoral paresthesia (2) |
| Medrik et al. (47) | NA | NA | Dizziness, nausea, drowsiness, paresthesia, weakness, headache, palpitation |
| Attal et al. (48) | 11/16 | 5/16 | Lightheadedness/dizziness (7), somnolence (5), nausea/vomiting (3), dysarthria/garbled speech (3), malaise (2), headache (1), tinnitus (1), blurred vision (1), palpitation (1), facial numbness (1), dry mouth (1) |
| Wallace et al. (40) | NA | NA | Lightheadedness, Sedation, and dry mouth |
| Wu et al. (51) | 0 | 0 | None |
| Kvarnström et al. (39) | NA | NA | Somnolence, lightheadedness, out-of-body sensation changes in hearing/vision, nausea, itching, unpleasant experience, paresthesia |
| Attal et al. (43) | 16/22 | 5/22 | Lightheadedness, perioral numbness, and garbled speech |
| Kvarnström et al. (41) | 5/10 | 0/10 | Somnolence, perioral numbness (2) |
| Finnerup et al. (42) | 19/24 | 1/24 | Somnolence (11), dizziness (7), dysarthria (7), lightheadedness (7), blurred vision (3) |
| Gottrup et al. (46) | 16/19 | 2/19 | Tiredness (7), Nausea (4), Feeling drunk (3), Paresthesia (3), Blurred vision (3), Dizziness (2), Changed taste (3), Dysarthria (3), Headache (2), Dry mouth (2) |
| Tremont-Lukats et al. (56) | NA | NA | Lightheadedness (10), perioral numbness & headaches (6); nausea (4), diplopia (3), incoordination (3), throat tightness (3) |
| Viola et al. (55) | 1/15 | 0/15 | Lightheadedness (1) |
| Gormsen et al. (54) | 13/15 | 6/13 | Headache (2), Oral paresthesia (3), Dizziness (3), Somnolence (2), Memory impairment (3), Discomfort in the head (2), Fatigue (5), Feeling abnormal (2), Dry mouth (8), Nausea (3), Muscle spasms (2) |
| Vlainich et al. (53) | 0 | 0 | Not reported |
| Park et al. (19) | 0 | 0 | Not reported |
| Stavropoulou et al. (18) | NA | NA | Somnolence (13), Dry mouth (5), Dizziness (5), Headache (3), Feeling flushed (2), Confusion (1), Dysarthria (1), Tinnitus (1) |
| Albertoni et al. (16) | NA | NA | Nausea, vomiting, dizziness, drowsiness, paraesthesia, constipation, and dry mouth |
| Kim et al. (17) | 1/21 | 0/21 | Chest discomfort (1) |
| Liu et al. (52) | 32/90 | 33/93 | Drowsiness (5), Headache (6), Dizziness (19), Vomiting (2), Dry mouth (14), Metallic taste (2), Numbness (3) |
n/N, number of events/total number of participants; NA, not available; figures in parenthesis indicate number of patients reporting the side-effect.
Figure 5.
Forrest plot of adverse events with IV lidocaine vs. placebo.
Discussion
Chronic pain can be broadly classified into three categories of causation: (1). Due to tissue disease or damage (nociceptive pain), (2). due to somatosensory system disease or damage (neuropathic pain), or (3). a combination of nociceptive and neuropathic pain (mixed pain). Clinically, characteristics of neuropathic pain include the presence of burning/shooting/crawling/electric type of pain, abnormal sensation, or hypersensitivity (allodynia or hyperalgesia), and paraesthesia. Patients usually complain of spontaneous pain but some might also report evoked pain (57). These characteristics are not diagnostic for neuropathic pain, but indicate a strong possibility for it. While research indicates that there is damage of neuronal pathways in neuropathic pain, there are several mechanisms involved in its genesis. These mechanisms are independent of disease etiology, as the same mechanism can be seen in different diseases (57). In consideration of this theory, a large number of studies with different etiologies of pain were included in our review.
Regarding the actual mechanisms of pain initiation, research suggests that nerve injury results in an abnormal rate of proliferation and activation of sodium channels. Sodium channels produce uncontrolled persistent discharges resulting in a central hyperexcitable state. Such ectopic discharges can be initiated along the injured nerve, in the dorsal root ganglion, and in the peripheral neuromata (58, 59). The mechanism of action of IV lidocaine involves the alteration in the activation of sodium channels leading to a modification in pain response. Due to its sodium channel blocking action, lidocaine decreases peripheral nociceptor sensitization, and central hyperexcitability (60). The anti-inflammatory property of lidocaine also reduces circulating inflammatory cytokines, which are involved in the processes of secondary hyperalgesia and central sensitization. These actions of IV lidocaine occur at levels much lower than those required to produce a nerve conduction blockade (61).
According to our systematic review, a large number of clinical trials have tested IV lidocaine for neuropathic pain. However, the majority of these studies enrolled few patients (<30) and reported the use of a diverse range of lidocaine dosages and infusion times. Studies also assessed pain scored after various time periods. To consolidate data for the purposes of a quantitative analysis, we divided the studies into two groups. The first group consisted of studies reporting the efficacy of IV lidocaine in the immediate post-transfusion period while the second group consisted of studies where lidocaine was transfused over a period of 4 weeks to study its long-term, persistent effect. In the most recent systematic review of 2005 (15), data on 187 patents in the lidocaine group and 186 patients in the placebo group was analyzed and lidocaine was found to be superior to a placebo in the treatment of neuropathic pain (p = 0.003). From our systematic literature search, we found 11 new studies published after 2005 which were included in this review. Three (16, 17, 53) out of these 11 studies were classified into group two for the quantitative analysis of a long-term, persistent effect of IV lidocaine. Of the eight remaining studies, pain data was extractable as mean and SD from three of the studies. These were included in the meta-analysis of pain relief in the immediate post-transfusion period. Our results based on the data on 250 patients in each group shows that IV lidocaine is more effective than a placebo in relieving neuropathic pain in the immediate post-infusion period (p < 0.00001). From our analysis of data on 55 patients in lidocaine and placebo groups, we found that IV lidocaine does not have a long-term, persistent effect after repeated weekly infusions over a period of 4 weeks (p = 0.08). Although animal studies have reported that systemic lidocaine has long-term benefits for pain relief (62), our analysis suggests that the effect of lidocaine in humans is transient and does not last over a long period of time. This may be explained by the pharmacokinetics of the drug. The onset of action of lidocaine is between 30 and 60 min and the effects can last from 2 to 6 h after the end of the infusion, following which the analgesic effect rapidly wears off (17).
The correct dosage needed for pain relief with IV lidocaine is debatable. While some studies have recorded pain relief after 1 mg/kg (50) and 2 mg/kg (35) infusions, others have reported no significant pain relief from lower doses of lidocaine (56). Tremont-Lukats et al. (56), in a trial comparing 3 doses of IV lidocaine (1, 3, and 5 mg/kg), concluded that lidocaine infused at 1 and 3 mg/kg/h was no better than a placebo in relieving neuropathic pain. Another debatable subject is the optimal rate of lidocaine administration. The majority of studies (13, 14, 44, 48) have used a high infusion rate of 167 μg/kg/min (5 mg/kg over 30 min); however, an infusion rate >50 μg/kg/min may lead to adverse cardiovascular events (17). Conversely, other studies (56) that used a very low infusion rate of 14 μg/kg/min over 6 h found the treatment was effective. However, in a clinical outpatient setting, a 6-h infusion is not practical. The lack of clear guidelines on dosage and infusion rates can be attributed to the complex nature of neuropathic pain and the methodologic variability of the clinical trials conducted on IV lidocaine infusion. Considering the heterogeneity in past literature, further evidence in the form of well-controlled homogenous RCTs are required to provide clarity on dosage and infusion rates of IV lidocaine.
All studies included in our review used IV lidocaine in patients with normal conduction as demonstrated by electrocardiography (ECG) and normal serum electrolyte concentrations, as IV lidocaine can cause serious adverse events, such as cardiac arrhythmias and hemodynamic instability. The participants were monitored for changes in ECG and blood pressure throughout the infusion period. Side effects reported were mostly minor in nature, such as lightheadedness, somnolence, peri-oral paresthesia, nausea, headache, dysarthria, dry mouth, and metallic taste. Our analysis suggests that patients receiving IV lidocaine are more prone to adverse events compared to a placebo. However, it is notable that no serious adverse events were reported by any of the trials.
Some limitations of our review need to be mentioned. Firstly, not all studies included in the review were suitable for quantitative analysis. This was mainly due to a lack of clear presentation of the data by the trials. Secondly, there was a lack of standardization of lidocaine dosages and infusion rates across studies. Thirdly, not all studies included were high quality trials. Ten of the studies were rated as “medium quality” based on the quality assessment scale. Fourthly, it was not possible to conduct a subgroup analysis based on the specific etiology of neuropathic pain considering the limited number of studies available and the wide range of etiologies reported.
Nevertheless, our review is the first update conducted since the 2005 meta-analysis (15) on the use of IV lidocaine for neuropathic pain. Our study indicates that while IV lidocaine is effective in pain control in patients with neuropathic pain in the immediate post-transfusion period, it does not have a long-lasting, persistent effect. IV infusions of the drug are associated with an increased risk of side effects compared to a placebo. However, the risk of serious adverse events is negligible. Further, well-designed RCTs evaluating the effects of various dosages and infusion periods of IV lidocaine are required to provide clear guidelines on its clinical use.
Data Availability
All datasets analyzed for this study are included in the manuscript/supplementary files.
Author Contributions
BZ and KL conceived and designed the study. BZ, XZ, HW, and SW were involved in literature search and data collection. QZ analyzed the data. BZ wrote the paper. KL reviewed and edited the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Merskey H, Bogduk N. Classification of Chronic Pain: Description of Chronic Pain Syndromes and Definitions of Pain Terms, 2nd edn. Seattle: IASP Press; (1994). [Google Scholar]
- 2.Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, Rieder A. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiol Scand. (2008) 52:132–6. 10.1111/j.1399-6576.2007.01486.x [DOI] [PubMed] [Google Scholar]
- 3.Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. (2008) 136:380–7. 10.1016/j.pain.2007.08.013 [DOI] [PubMed] [Google Scholar]
- 4.Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. (2006) 7:281–9. 10.1016/j.jpain.2005.11.008 [DOI] [PubMed] [Google Scholar]
- 5.van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. (2014) 155:654–62. 10.1016/j.pain.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 6.Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. (2011) 152:2836–43. 10.1016/j.pain.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 7.Finnerup NB, Attal N, Haroutounian S, Mcnicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. (2015) 14:162–73. 10.1016/S1474-4422(14)70251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert CRA, Hanson IR, Brown AB, Hingson RA. Intravenous use of xylocaine. Curr Res Anesth Analg. (1951) 30:301–13. 10.1213/00000539-195101000-00057 [DOI] [PubMed] [Google Scholar]
- 9.Boas RA, Covino BG, Shahnarian A. Analgesic responses to i.v. lignocaine. Br J Anaesth. (1982) 54:501–5. 10.1093/bja/54.5.501 [DOI] [PubMed] [Google Scholar]
- 10.Lindblom U. Reduction of hyperpathia indicates pain relief. Pain. (1984) 18:322. 10.1016/0304-3959(84)90828-5 [DOI] [PubMed] [Google Scholar]
- 11.Rowbotham MC, Reisner-Keller LA, Fields HL. Both intravenous lidocaine and morphine reduce the pain of postherpetic neuralgia. Neurology. (1991) 41:1024–8. 10.1212/WNL.41.7.1024 [DOI] [PubMed] [Google Scholar]
- 12.Bruera E, Ripamonti C, Brenneis C, Macmillan K, Hanson J. A randomized double-blind crossover trial of intravenous lidocaine in the treatment of neuropathic cancer pain. J Pain Symptom Manage. (1992) 7:138–40. 10.1016/S0885-3924(06)80004-7 [DOI] [PubMed] [Google Scholar]
- 13.Ellemann K, Sjogren P, Banning AM, Jensen TS, Smith S, Geertsen P. Trial of intravenous lidocaine on painful neuropathy in cancer patients. Clin J Pain. (1989) 5:291–4. 10.1097/00002508-198912000-00003 [DOI] [PubMed] [Google Scholar]
- 14.Kastrup J, Petersen P, Dejgård A, Angelo HR, Hilsted J. Intravenous lidocaine infusion—a new treatment of chronic painful diabetic neuropathy? Pain. (1987) 28:69–75. 10.1016/0304-3959(87)91061-X [DOI] [PubMed] [Google Scholar]
- 15.Challapalli V, Tremont-Lukats IW, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetic agents to relieve neuropathic pain. Cochrane Database Syst Rev. (2005) CD003345 10.1002/14651858.CD003345.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albertoni Giraldes A, Salomão R, Leal P, Brunialti M, Sakata R. Effect of intravenous lidocaine combined with amitriptyline on pain intensity, clinical manifestations and the concentrations of IL-1, IL-6, and IL-8 in patients with fibromyalgia: a randomized double-blind study. Int J Rheum Dis. (2016) 19:946–53. 10.1111/1756-185X.12904 [DOI] [PubMed] [Google Scholar]
- 17.Kim Y-C, Castañeda AM, Lee C, Jin H-S, Park KS, Moon JY. Efficacy and safety of lidocaine infusion treatment for neuropathic pain. Reg Anesth Pain Med. (2018) 43:415–24. 10.1097/AAP.0000000000000741 [DOI] [PubMed] [Google Scholar]
- 18.Stavropoulou E, Argyra E, Zis P, Vadalouca A, Siafaka I. The effect of intravenous lidocaine on trigeminal neuralgia: a randomized double blind placebo controlled trial. ISRN Pain. (2014) 2014:1–5. 10.1155/2014/853826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park CH, Jung SH, Han CG. Effect of intravenous lidocaine on the neuropathic pain of failed back surgery syndrome. Kor J Pain. (2012) 25:94–8. 10.3344/kjp.2012.25.2.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA group. preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins J, Green S. Cochrane Handbook for Systemic Reviews of Interventions. Version 5. The Cochrane Collaboration (2011).
- 22.Higgins J, Altman D, Sterne J. Cochrane statistical methods group and the cochrane bias methods group. chapter 8: assessing risk of bias in included studies. In: Cochrane Handbook for Systemic Reviews of Interventions, The Cochrane Collaboration.
- 23.Kastrup J, Angelo H, Petersen P, Dejgård A, Hilsted J. Treatment of chronic painful diabetic neuropathy with intravenous lidocaine infusion. Br Med J. (1986) 292:173. 10.1136/bmj.292.6514.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlainich R, Issy AMH, Sakata RK. Effect of intravenous lidocaine associated with amitriptyline on pain relief and plasma serotonin, norepinephrine, and dopamine concentrations in fibromyalgia. Clin J Pain. (2011) 27:285–8. 10.1097/AJP.0b013e3181ffbfde [DOI] [PubMed] [Google Scholar]
- 25.Carroll I, Younger J, MAckey S. Pain quality predicts lidocaine analgesia among patients with suspected neuropathic pain. Pain Med. (2010) 11:617–21. 10.1111/j.1526-4637.2010.00807.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schafranski MD, Malucelli T, Machado F, Takeshi H, Kaiber F, Schmidt C, et al. Intravenous lidocaine for fibromyalgia syndrome: an open trial. Clin Rheumatol. (2009) 28:853–5. 10.1007/s10067-009-1137-8 [DOI] [PubMed] [Google Scholar]
- 27.Bach FW, Jensen TS, Kastrup J, Stigsby B, Dejgård A. The effect of intravenous lidocaine on nociceptive processing in diabetic neuropathy. Pain. (1990) 40:29–34. 10.1016/0304-3959(90)91047-M [DOI] [PubMed] [Google Scholar]
- 28.Sakurai M, Kanazawa I. Positive symptoms in multiple sclerosis: their treatment with sodium channel blockers, lidocaine, and mexiletine. J Neurol Sci. (1999) 162:162–8. 10.1016/S0022-510X(98)00322-0 [DOI] [PubMed] [Google Scholar]
- 29.Posner IA. Treatment of fibromyalgia syndrome with intravenous lidocaine. J Musculoskelet Pain. (1994) 2:55–65. 10.1300/J094v02n04_05 [DOI] [Google Scholar]
- 30.Catala E, Ferrandiz M, Aliaga L, Serra R, Castro MA, Villar Landeira JM. Intravenous lidocaine compared with sympathetic blocks as treatment for post-herpetic neuralgia. A 1-year survey. Pain Clin. (1994) 7:205–10. [Google Scholar]
- 31.Schwartzman RJ, Patel M, Grothusen JR, Alexander GM. Efficacy of 5-day continuous lidocaine infusion for the treatment of refractory complex regional pain syndrome. Pain Med. (2009) 10:401–12. 10.1111/j.1526-4637.2009.00573.x [DOI] [PubMed] [Google Scholar]
- 32.Przeklasa-Muszynska A, Kocot-Kepska M, Dobrogowski J, Wiatr M, Mika J. Intravenous lidocaine infusions in a multidirectional model of treatment of neuropathic pain patients. Pharmacol Rep. (2016) 68:1069–75. 10.1016/j.pharep.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 33.Xǔ G, Xu S, Tang WZ, Xú G, Cheng C, Xu J. Local injection of methylcobalamin combined with Lidocaine for acute herpetic neuralgia. Pain Med. (2016) 17:572–81. 10.1093/pm/pnv005 [DOI] [PubMed] [Google Scholar]
- 34.Ni J, Wang X, Tang Y, Yang L, Zeng Y, Guo Y. Subcutaneous injection of triamcinolone and lidocaine to prevent postherpetic neuralgia. Pain Physician. (2017) 20:397–403. [PubMed] [Google Scholar]
- 35.Galer BS, Harle J, Rowbotham MC. Response to intravenous lidocaine infusion predicts subsequent response to oral mexiletine: a prospective study. J Pain Symptom Manage. (1996) 12:161–7. 10.1016/0885-3924(96)00126-1 [DOI] [PubMed] [Google Scholar]
- 36.Hayashida M, Fukuda KI, Fukunaga A, Meno A, Sato K, Tarui K, et al. Analgesic effect of intravenous ATP on postherpetic neuralgia in comparison with responses to intravenous ketamine and lidocaine. J Anesth. (2005) 19:31–5. 10.1007/s00540-004-0273-1 [DOI] [PubMed] [Google Scholar]
- 37.Tanen DA, Shimada M, Danish DC, Santos Dos F, Makela M, Riffenburgh RH. Intravenous lidocaine for the Emergency Department treatment of acute radicular low back pain, a randomized controlled trial. J Emerg Med. (2014) 47:119–24. 10.1016/j.jemermed.2012.12.014 [DOI] [PubMed] [Google Scholar]
- 38.Heuvel van den SAS, Wal van der SEI, Smedes LA, Radema SA, Alfen van N, Vissers KCP, et al. Intravenous lidocaine: old-school drug, new purpose—reduction of intractable pain in patients with chemotherapy induced peripheral neuropathy. Pain Res Manag. (2017) 2017:1–9. 10.1155/2017/8053474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kvarnström A, Karlsten R, Quiding H, Emanuelsson BM, Gordh T. The effectiveness of intravenous ketamine and lidocaine on peripheral neuropathic pain. Acta Anaesthesiol Scand. (2003) 47:868–77. 10.1034/j.1399-6576.2003.00187.x [DOI] [PubMed] [Google Scholar]
- 40.Wallace MS, Ridgeway BM, Leung AY, Gerayli A, Yaksh TL. Concentration-effect relationship of intravenous lidocaine on the allodynia of complex regional pain syndrome types I and II. Anesthesiology. (2000) 92:75–83. 10.1097/00000542-200001000-00017 [DOI] [PubMed] [Google Scholar]
- 41.Kvarnström A, Karlsten R, Quiding H, Gordh T. The analgesic effect of intravenous ketamine and lidocaine on pain after spinal cord injury. Acta Anaesthesiol Scand. (2004) 48:498–506. 10.1111/j.1399-6576.2003.00330.x [DOI] [PubMed] [Google Scholar]
- 42.Finnerup NB, Biering-Sørensen F, Johannesen IL, Terkelsen AJ, Juhl GI, Kristensen AD, et al. Intravenous lidocaine relieves spinal cord injury pain: a randomized controlled trial. Anesthesiology. (2005) 102:1023–30. 10.1097/00000542-200505000-00023 [DOI] [PubMed] [Google Scholar]
- 43.Attal N, Rouaud J, Brasseur L, Chauvin M, Bouhassira D. Systemic lidocaine in pain due to peripheral nerve injury and predictors of response. Neurology. (2004) 62:218–25. 10.1212/01.WNL.0000103237.62009.77 [DOI] [PubMed] [Google Scholar]
- 44.Sorensen J, Bengtsson A, Backman E, Henriksson K, Bengtsson M. Pain analysis in patients with fibromyalgia. Effects of intravenous morphine, lidocaine, and ketamine. Scand J Rheumatol. (1995) 24:360–5. 10.3109/03009749509095181 [DOI] [PubMed] [Google Scholar]
- 45.Marchettini P, Lacerenza M, Marangoni C, Pellegata G, Sotgiu ML, Smirne S. Lidocaine test in neuralgia. Pain. (1992) 48:377–82. 10.1016/0304-3959(92)90087-R [DOI] [PubMed] [Google Scholar]
- 46.Gottrup H, Bach FW, Juhl G, Jensen TS. Differential effect of ketamine and lidocaine on spontaneous and mechanical evoked pain in patients with nerve injury pain. Anesthesiology. (2006) 104:527–36. 10.1097/00000542-200603000-00021 [DOI] [PubMed] [Google Scholar]
- 47.Medrik-Goldberg T, Lifschitz D, Pud D, Adler R, Eisenberg E. Intravenous lidocaine, amantadine, and placebo in the treatment of sciatica: a double-blind, randomized, controlled study. Reg Anesth Pain Med. (1999) 24:534–40. 10.1097/00115550-199924060-00011 [DOI] [PubMed] [Google Scholar]
- 48.Attal N, Gaude V, Brasseur L, Dupuy M, Guirimand F, Parker F, et al. Intravenous lidocaine in central pain A double-blind, placebo-controlled, psychophysical study. Neurology. (2000) 54:564–74. 10.1212/WNL.54.3.564 [DOI] [PubMed] [Google Scholar]
- 49.Wallace MS, Dyck JB, Rossi SS, Yaksh TL. Computer-controlled lidocaine infusion for the evaluation of neuropathic pain after peripheral nerve injury. Pain. (1996) 66:69–77. 10.1016/0304-3959(96)02980-6 [DOI] [PubMed] [Google Scholar]
- 50.Baranowski AP, De Courcey J, Bonello E. A trial of intravenous lidocaine on the pain and allodynia of postherpetic neuralgia. J Pain Symptom Manage. (1999) 17:429–33. 10.1016/S0885-3924(99)00032-9 [DOI] [PubMed] [Google Scholar]
- 51.Wu C, Tella P, Staats P, Vaslav R, Kazim D, Wesselmann U, et al. Analgesic effects of intravenous lidocaine and morphine on postamputation pain. Anesthesiology. (2002) 96:841–8. 10.1097/00000542-200204000-00010 [DOI] [PubMed] [Google Scholar]
- 52.Liu H, Lu F, Zhou D, Yin Y, Li J, Yang B, et al. The analgesic and emotional response to intravenous lidocaine infusion in the treatment of postherpetic neuralgia: a randomized, double-blinded, placebo-controlled study. Clin J Pain. (2018) 34:1025–31. 10.1097/AJP.0000000000000623 [DOI] [PubMed] [Google Scholar]
- 53.Vlainich R, Issy AM, Gerola LR, Sakata RK. Effect of intravenous lidocaine on manifestations of fibromyalgia. Pain Pract. (2010) 10:301–5. 10.1111/j.1533-2500.2010.00362.x [DOI] [PubMed] [Google Scholar]
- 54.Gormsen L, Finnerup NB, Almqvist PM, Jensen TS. The efficacy of the AMPA receptor antagonist NS1209 and lidocaine in nerve injury pain: a randomized, double-blind, placebo-controlled, three-way crossover study. Anesth Analg. (2009) 108:1311–9. 10.1213/ane.0b013e318198317b [DOI] [PubMed] [Google Scholar]
- 55.Viola V, Newnham HH, Simpson RW. Treatment of intractable painful diabetic neuropathy with intravenous lignocaine. J Diabetes Complications. (2006) 20:34–9. 10.1016/j.jdiacomp.2005.05.007 [DOI] [PubMed] [Google Scholar]
- 56.Tremont-lukats IW, Hutson PR, Backonja M. A randomized, double-masked, placebo-controlled pilot trial of extended IV lidocaine infusion for relief of ongoing neuropathic pain. Clin J Pain. (2006) 22:266–71. 10.1097/01.ajp.0000169673.57062.40 [DOI] [PubMed] [Google Scholar]
- 57.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. (2010) 9:807–19. 10.1016/S1474-4422(10)70143-5 [DOI] [PubMed] [Google Scholar]
- 58.Chabal C, Russell LC, Burchiel KJ. The effect of intravenous lidocaine, tocainide, and mexiletine on spontaneously active fibers originating in rat sciatic neuromas. Pain. (1989) 38:333–8. 10.1016/0304-3959(89)90220-0 [DOI] [PubMed] [Google Scholar]
- 59.Kajander KC, Wakisaka S, Bennett GJ. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci Lett. (1992) 138:225–8. 10.1016/0304-3940(92)90920-3 [DOI] [PubMed] [Google Scholar]
- 60.Rogers M, Tang L, Madge DJ, Stevens EB. The role of sodium channels in neuropathic pain. Semin Cell Dev Biol. (2006) 17:571–81. 10.1016/j.semcdb.2006.10.009 [DOI] [PubMed] [Google Scholar]
- 61.Kandil E, Melikman E, Adinoff B. Lidocaine infusion: a promising therapeutic approach for chronic pain. J Anesth Clin Res. (2017) 8:1–14. 10.4172/2155-6148.1000697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaplan SR, Bach FW, Shafer SL, Yaksh TL. Prolonged alleviation of tactile allodynia by intravenous lidocaine in neuropathic rats. Anesthesiology. (1995) 83:775–85. 10.1097/00000542-199510000-00017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets analyzed for this study are included in the manuscript/supplementary files.