Abstract
We sought to describe changes in blood pressure and estimate the effect of HIV on blood pressure (BP) over 4 years of observation in a cohort of 155 HIV‐infected adults (≥40 years) on antiretroviral therapy (ART) and 154 sex‐ and age‐quartile‐matched, population‐based, HIV‐uninfected controls for four years in rural Uganda, we compared changes in blood pressure (BP) by HIV serostatus and tested whether body mass index and inflammation (high‐sensitivity C‐reactive protein and interleukin‐6) and immune activation (sCD14 and sCD163) mediated the effects of HIV on BP using hierarchical multivariate and two‐stage parametric regression models. Overall HIV‐uninfected participants had higher mean BP than HIV‐infected counterparts (differences in trend P < 0.0001 for diastolic BP and P = 0.164 for systolic BP). After initial declines in BP in both groups between years 1 and 2, BP moderately increased in both groups through year 4, with greater change over time observed in the HIV‐uninfected group. Body mass index mediated 72% (95%CI 57, 97) of the association between HIV and systolic BP. We found a minimal mediating effect of sCD14 on the relationship between HIV and SBP (9%, 95% CI 5%, 21%), but found no association between other HIV‐related biomarkers. Over four years of observation, HIV‐infected people in rural Uganda have lower BP than HIV‐uninfected counterparts despite having higher levels of inflammation. BMI, rather than measures of HIV‐associated inflammation, explained a majority of the difference in BP observed.
Keywords: blood pressure trends, effect of HIV on BP, HIV‐infected, HIV‐uninfected, Uganda
1. INTRODUCTION
Cross‐sectional studies report a wide range of effects of HIV and antiretroviral therapy (ART) on blood pressure (BP), with literature comparing estimates between HIV‐infected individuals to HIV‐uninfected individuals reporting higher BP,1 similar BP,2, 3, 4, 5 and lower BP.6, 7 The later studies were conducted in sub‐Saharan Africa (SSA), where concomitant comorbidities for people living with HIV (PLWH) differ from those in high‐income countries,8, 9 and where the disparity in access to care for people with HIV in contrast to uninfected people may be different than in high‐income countries.10
Thus, there is uncertainty regarding (a) whether HIV and its treatment are positively or negatively associated with the differences in BP, and (b) what factors might be responsible for differences in BP observed between those with and without HIV, for example, epidemiology of traditional hypertension risk factors in SSA or HIV‐induced inflammatory states.11 It is plausible that HIV/ART lead to increases in blood pressure via weight gain as a survival benefit of ART and/or HIV‐induced endothelial damage with subsequent accelerated atherosclerosis.12, 13
We assessed (a) blood pressure trajectories by HIV status over 4 years of follow‐up and (b) estimated the differences in blood pressure mediated by HIV‐specific pathways in a mixed cohort of adults aged ≥40 years (HIV‐infected adults stable on ART and HIV‐uninfected, gender‐ and age‐similar controls) in rural Uganda.
2. METHODS
2.1. Study population
The Uganda Non‐Communicable Diseases and Aging Cohort (UGANDAC study ClinicalTrials.gov Identifier: NCT02445079) is a longitudinal cohort of ambulatory HIV‐infected adults stable on antiretroviral therapy (ART) for at least 3 years and matched population‐based, HIV‐uninfected controls in rural southwestern Uganda as described in detail previously.14
Briefly, we first enrolled a convenience sample of HIV‐infected persons aged 40 or greater receiving care at the Mbarara Regional Referral Hospital HIV clinic known as the Immune Suppression Syndrome Clinic. This is a public facility that provides free antiretroviral therapy for a largely rural population. A minimum age of 40 years for this cohort was selected because the study focuses on the non‐communicable risk and comorbidity and because of the low life expectancy at birth in Uganda.
We then used data from a complete population census of a cluster of villages located approximately 20 km from the Mbarara Regional Referral Hospital HIV clinic to identify and conduct home‐based enrollment of HIV‐uninfected controls who were gender‐ and age‐similar (by quartile of the HIV‐infected subgroup). All HIV‐uninfected participants were tested for HIV at all annual study visits. Viral load and CD4 data were abstracted from the HIV clinic electronic medical record database.15
The ethics committees at Mbarara University of Science and Technology (Ref. 06/04‐14) and Partners Healthcare (Ref. 2014P001928/MGH) approved study procedures. Consistent with national guidelines, we received clearance from the Uganda National Council for Science and Technology (Ref. HS1689). All participants provided written informed consent in English or Runyankore/Rukiga, the local language. Participants unable to read had a witness and used an inked fingerprint as signature.
2.2. Data collection
Between December 2013 and May 2018, trained study nurses conducted interviews to capture household asset ownership, smoking history, history of diagnosis, and/or management of cardiovascular disease and its risk factors (hypertension, diabetes mellitus, and dyslipidemia), current medications, and physical activity using a validated physical activity questionnaire for sub‐Saharan Africa.16
Study nurses then measured weight using standardized scales (seca 762, Hanover, USA) and height using a roll‐up measuring stadiometers (seca 206, Hanover, USA). Height was measured to the nearest 0.1 cm, and weight was measured to the nearest kilogram. We used height and weights to calculate body mass (BMI) as weight (in kilograms) divided by the square of height (in meters) and categorized BMI as underweight (<18.5 kg/m2), normal weight (18.5‐24.9 kg/m2), overweight (25‐29.9 kg/m2), or obese (>30 kg/m2).
For our analytic outcome, we measured bilateral, resting blood pressure using automated digital upper arm sphygmomanometers (Omron Healthcare Inc, Bannockburn, USA) with small (<21 cm), normal (22‐32 cm), and large cuffs (35‐44 cm). Participants were seated in a chair and allowed to rest for 5 minutes before six measurements, 3 per arm, were performed with 3‐minute intervals. We used the average of the second and third measurements from both arms to determine the blood pressure of each participant. We set a range of 70‐270 mm Hg as plausible values for systolic blood pressure (SBP) and 30‐150 mm Hg for diastolic blood pressure (DBP). For this analysis, blood pressure was the outcome of interest, specified as a continuous variable.
A trained laboratory technician collected approximately 40 mL of venous blood into acid citrate dextrose (ACD) and serum separators tubes. Specimens were brought to the Mbarara Clinical and Research Laboratory within one hour of collection for centrifugation and separation into serum and plasma components, respectively. Blood samples were centrifuged, and derivative plasma aliquots were stored at −80°C until shipment to the United States for testing at the Laboratory for Clinical Biochemistry Research at the University of Vermont. For this analysis, we used results of thawed cryopreserved specimens for serum albumin and four biomarkers, that is, serum high‐sensitivity C‐reactive protein (hs‐CRP) assayed using latex immunoturbidimetry (LabCorp); plasma levels of IL‐6 (MesoScale Discovery); plasma levels of soluble CD14 (sCD14, R&D Systems); and plasma levels of soluble CD163 (sCD163, R&D Systems).
2.3. Statistical analysis
We described the cohort characteristics at enrollment and compared the distributions between HIV‐infected and matched HIV‐uninfected participants in bivariate analyses using t tests for continuous variables, chi‐square tests for categorical variables, and trend test using median values of categories for ordinal variables (Table 1).
Table 1.
Baseline (at enrollment) characteristics, UGANDAC study 2019
| Characteristic | HIV‐uninfected, n = 154 | HIV‐infected, n = 155 | P‐value |
|---|---|---|---|
| Demographics | |||
| Age (y), mean (SD) | 51.5 (7.7) | 51.2 (6.6) | 0.695 |
| Women, n (%) | 77 (50) | 74 (47.7) | 0.733 |
| Risk factors | |||
| Smoking, n (%) | <0.001† | ||
| Never | 72 (46.7) | 91 (58.7) | |
| Current | 32 (20.8) | 9 (5.8) | |
| Former | 50 (32.5) | 55 (35.5) | |
| Physical activity (METS h/wk), n (%) | 0.001† | ||
| Low‐intensity activity (<3 METS h/wk) | 3 (2.0) | 13 (8.4) | |
| Moderate activity (3 to <6 METS h/wk) | 1 (0.7) | 9 (5.8) | |
| Vigorous activity (≥6 METS h/wk) | 147 (95.5) | 126 (81.3) | |
| Diabetes mellitus (HbA1c >7% or treatment, n (%) | 9 (5.8) | 9 (5.8) | 0.590a |
| Measurements | |||
| Body mass index category, n (%) | 0.001† | ||
| Underweight (<18.5 kg/m2) | 27 (17.5) | 13 (8.4) | |
| Normal (18.5 to < 25 kg/m2) | 90 (58.4) | 99 (63.9) | |
| Overweight/obese (>25 kg/m2) | 37 (23.0) | 43 (27.7) | |
| Systolic blood pressure (mm Hg), mean (SD)b | 120 (17.9) | 114 (19.4) | 0.015 |
| Diastolic blood pressure (mm Hg), mean (SD)b | 76 (11.5) | 72 (11.8) | 0.0004 |
| eGFR, mL/min/1.73m2, mean (SD)c | 113 (13) | 112 (15) | 0.801 |
| Current CD4 (cells/mL), median (IQR) | ‐ | 433 (336, 559)‡ | ‐ |
| Nadir CD4 (cells/mL), median (IQR) | ‐ | 20 (20, 550)‡ | ‐ |
| Duration of ART (y), median (IQR) | ‐ | 10 (9, 10)‡ | ‐ |
| HIV viral load, mean (SD) | ‐ | 189,988 (296,848)‡ | ‐ |
| ART regimen, n (%) | |||
| Protease inhibitor based | ‐ | 19 (12.3)‡ | |
| Non‐protease inhibitor based | ‐ | 136 (87.7)‡ | |
Low‐intensity activity (activity with <3 metabolic equivalents [MET] h/wk; moderate activity (activity with 3 to <6 MET h/wk); vigorous activity (activity with ≥6 METS h/wk). A total 10 (3 HIV‐uninfected and 7 HIV‐infected) were missing physical activity assessment.
Trend test: blood pressure.
Average of second and third same sitting left and right arm blood pressure measurements: SD; standard deviation.
eGFR; estimated glomerular filtration rate estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation for black race stratified by gender (mL/min/1.73 m2).
[Corrections added on July 26, 2019, after first online publication: †p‐values have each been moved up by one row, so that they now align with the row headings “Smoking,” “Physical activity,” and “Body mass index category”; ‡the entries have been moved one column to the right, so they now appear under the column heading “HIV‐infected.”]
For all of our models, HIV serostatus was the primary exposure of interest, blood pressure was the outcome of interest, and BMI and biomarkers of HIV inflammation were mediators. We used a priori knowledge to select potential confounders that are postulated to affect the association between HIV and BP (Figure 1) including age, gender, smoking, and physical activity. There is evidence that untreated and early treated HIV is associated with weight loss and reduced BMI17, 18 and also that BMI is directly correlated with BP.17 Therefore, BMI was treated as a mediator of the effect of HIV on BP and not a confounder as is in most analyses18, 19, 20 to avoid collider stratification bias.21
Figure 1.
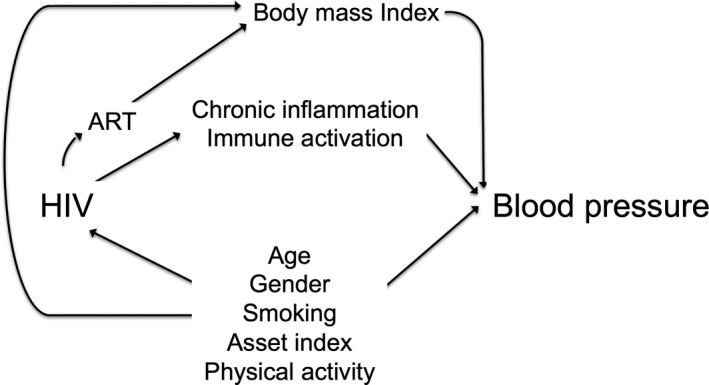
Directed acyclic graph for the relationship between HIV and blood pressure, UGANDAC study 2019
Observations were left‐truncated at the dates of enrollment into the UGANDAC study (baseline date) and right‐censored at the earliest of the participant's date of death, or date of emigration, or May 31, 2018 (the latest date of study observation). Time was expressed as the years from the date of first BP measurement.
We fitted hierarchical multivariable regression models using restricted maximum‐likelihood estimation to obtain participant‐specific, averaged coefficients to test for an association between HIV status and BP trajectories (SBP and DBP separately). All models included HIV, follow‐up time, a HIV × follow‐up time product term, and covariates as described above (Table 2). We repeated analyses stratified by sex. We also examined whether hypertension treatment status modified the effect of HIV on the slopes of BP trajectories with inclusion of a hypertension × HIV × follow‐up time product term. The parameter estimates from the subgroup analyses limited to those without hypertension were similar to the parameter estimates from the analysis of the entire sample (data not shown), so we present the results from the total sample.
Table 2.
Comparison of inflammatory markers at baseline categorized by sex and HIV serostatus, UGANDAC study 2019
| Inflammatory marker |
Total Cohort n = 309 |
HIV‐Uninfected men n = 77 |
HIV‐Infected men n = 81 |
P‐value |
HIV‐Uninfected women n = 77 |
HIV‐Infected women n = 74 |
P‐value |
P‐value‡ Comparing all subgroups |
|---|---|---|---|---|---|---|---|---|
| hs‐CRP (μg/mL), median (IQR) | 0.9 (0.4, 2.4) | 0.3 (0.2, 0.8) | 0.9 (0.5, 2.6) | <0.001 | 1.1 (0.5, 2.5) | 1.7 (0.9, 3.9) | 0.002 | <0.001 |
| IL‐6 (pg/mL), median (IQR) | 0.4 (0.3, 0.6) | 0.40 (0.3, 0.5) | 0.39 (0.3, 0.6) | 0.740 | 0.5 (0.3, 0.7) | 0.4 (0.3, 0.7) | 0.620 | 0.320 |
| sCD14 (pg/mL), median (IQR) | 1320 (1074, 1640) | 1140 (976, 1356) | 1361 (1112, 1649) | 0.005 | 1214 (1006, 1405) | 1576 (1335, 1837) | <0.001 | <0.001 |
| sCD163 (pg/mL), median (IQR) | 486 (374, 647) | 493 (358, 665) | 443 (328, 575) | 0.05 | 487 (362, 621) | 518 (374, 647) | 0.940 | 0.110 |
Abbreviations: hs‐CRP, high‐sensitivity C‐reactive protein; IL‐6, interleukin‐6; IQR, interquartile range; sCD14, soluble CD 14; sCD163, soluble CD 163.
P‐values in the final column reflect Kruskal‐Wallis non‐parametric testing to assess equivalence across the four sex and serostatus subgroups.
To estimate the extent to which biomarkers of immune activation, biomarkers of inflammation, and BMI mediate the association between HIV and blood pressure, we fit parametric models for each putative mediator (specified as log‐transformed variables and on the continuous scale), including product terms for HIV serostatus, follow‐up time, and each biomarker. We performed sensitivity analyses to (a) evaluate the extent to which the direct and indirect effects are robust to violations of interaction and no omitted variable bias assumptions and (b) quantify the extent to which the unmeasured confounding variable would have to be to invalidate inferences about the direct and indirect effects.22 We used bootstrapping to compute bias‐corrected confidence intervals.23 Statistical significance for all analyses was set as P < 0.05 (2‐sided). We performed all analyses using Stata version 15 (Stata Corporation).
3. RESULTS
A total of 309 individuals enrolled in UGANDAC study, had annual blood pressure measurements, and provided blood for marker testing. Baseline cohort characteristics of UGANDAC have been previously described.24 Briefly, the cohort is evenly divided by HIV serostatus (155 [50.2%] HIV‐uninfected vs 154 [49.8] HIV‐infected) and gender (50% HIV‐uninfected women vs 48% HIV‐infected women). Mean age at enrollment was 51.5 (SD 7.7) years in HIV‐uninfected and 51.2 (6.6) in HIV‐infected participants. The HIV‐infected participants had taken ART for a median 10 years (IQR 9, 10). (Table 1) HIV‐infected participants had higher median (IQR) levels of markers of inflammation and immune activation except IL‐6 (Table 2).
Median follow‐up time was 3.1 years (IQR 2.1, 3.2) for the HIV‐infected participants and 2.3 years (2.0, 3.1) for HIV‐uninfected participants (P = 0.06). Of note, 8 died and 18 were lost to follow‐up (LTFU) over the 4 years of follow‐up. We found participants with diagnosed hypertension constituted: 8.4% (26/309) at enrollment, 9.7% (30/308) in year 2, 11.3% (31/274) in year 3, and 11.4% (20/175) in year 4. Those with hypertension were more likely to be older (mean age 51 vs 54 years, P = 0.025), overweight/obese (23% vs 54%, P = 0.002), and had lower mean estimated glomerular filtration rate (eGFR; 112 vs 103, P = 0.001; Table 3).
Table 3.
Comparison of baseline characteristics of normotensive and hypertensive participants, UGANDAC study 2019
| Characteristic | Normotensive n = 283 | Hypertensive n = 26 | P‐value |
|---|---|---|---|
| Age, mean (SD) | 51 (6.9) | 54 (9.1) | 0.025 |
| Women, n (%) | 137 (48.4) | 14 (53.9) | 0.684 |
| Smoking, n (%) | |||
| Never | 147 (51.9) | 16 (61.5) | 0.078 |
| Former | 95 (14.5) | 0 (0) | |
| Current | 41 (33.6) | 10 (38.5) | |
| METS h/wk, n (%)a | |||
| Low‐intensity activity (activity with MET value of <3 | 14 (4.9) | 2 (7.7) | 0.770 |
| Moderate activity (activity with MET value of 3 to <6) | 10 (3.5) | 0 (0) | |
| Vigorous activity (activity with MET value of ≥6) | 249 (88.0) | 24 (92.3) | |
| Body mass index category, n (%) | |||
| Underweight (<18.5) | 40 (14.1) | 0 (0) | 0.002 |
| Normal (18.5 to <25) | 177 (62.5) | 12 (46.1) | |
| Overweight/Obese (25 to <30) | 66 (23.3) | 14 (53.9) | |
| Systolic blood pressure, mean (SD)^ | 114.52 (16.2) | 143.8 (24.4) | <0.0001 |
| Diastolic blood pressure, mean (SD)^ | 72.7 (10.8) | 88.4 (13.9) | <0.0001 |
| HIV‐infected | 141 (49.8) | 14 (53.9) | 0.838 |
| Diabetes mellitus (HbA1c >7% or on treatment) | 16 (5.7) | 2 (7.7) | 0.459 |
| Glomerular filtration rate (eGFR), mean (SD)# | 112.57 (14.0) | 103.1 (19.1) | 0.001 |
Abbreviation: METS, metabolic equivalent. ^Average of second and third same sitting left and right arm blood pressure measurements: #eGFR; Estimated glomerular filtration rate estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation for black race stratified by gender (mL/min/1.73 m2)
10 participants were missing physical activity assessment.
3.1. Predictors of absolute changes in mean blood pressure
In multivariable models assessing predictors of absolute changes in mean blood pressures (with HIV status and year product terms), we found each year increase in age and each 1 g/dL increase in serum albumin were associated with higher SBP whereas being a woman and current smoking compared to never smoking predicted decreases in mean systolic blood pressure (Table 4).
Table 4.
Adjusted mixed‐effects regression models for absolute changes in mean systolic blood pressures, UGANDAC study 2019
|
Model 1 Coeff (95% CI) |
Model 2 Coeff (95% CI) |
Model 3 Coeff (95% CI) |
Model 4 Coeff (95% CI) |
|
|---|---|---|---|---|
| Model parameters | ||||
| AIC (df) | 7581.9 (16) | 7572.2 (18) | 7379.307 (20) | 4849.07 (17) |
| BIC | 7659.8 | 7659.8 | 7476.2 | 4924.18 |
| β0 coefficient | 106.6 (96.1, 117.1)*** | 108.3 (97.8, 118.8)*** | 108.4 (96.4, 120.3)*** | 85.13 (66.80, 103.46)*** |
| Characteristic | ||||
| Age (y) | 0.2 (0.1, 0.4)* | 0.3 (0.1, 0.5)** | 0.3 (0.1, 0.4)* | 0.300 (0.09, 0.51)** |
| Men | Ref | Ref | Ref | Ref |
| Women | −3.1 (−6.1, −0.2)* | −4.4 (−7.41, −1.3)** | −4.5 (−7.5, −1.4)** | −3.79 (−7.13, −0.45)* |
| HIV serostatus and year | ||||
| HIV‐uninfected year 0 | Ref | Ref | Ref | Ref |
| HIV‐uninfected year 1 | −5.7 (−8.0, −3.5)*** | −5.8 (−8.1, −3.6)*** | −6.0 (−8.2, −3.7)*** | −6.46 (−9.17, −3.75)*** |
| HIV‐uninfected year 2 | −5.5 (−8.0, −2.9)*** | −5.6 (−8.1, −3.1)*** | −5.8 (−8.4, −3.2)*** | −5.56 (−8.94, −2.17)** |
| HIV‐uninfected year 3 | −4.1 (−7.4, −0.8)* | −4.6 (−7.9, −1.3)** | −4.6 (−7.5, −1.3)** | |
| HIV‐uninfected year 4 | 0.9 (−7.0, 8.7) | −0.1 (−8.0, 7.8) | −0.2 (−8.1, 7.7) | |
| HIV‐infected Year 0 | −6.7 (−10.3, −3.1)*** | −7.5 (−11.1, −3.9)*** | −8.0 (−11.6, −4.3)*** | −7.82 (−11.47, −4.17)*** |
| HIV‐infected year 1 | −7.0 (−10.6, −3.5)*** | −7.9 (−11.4, −4.3)*** | −8.4 (−12.0, −4.8)*** | −7.88 (−11.50, −4.26)*** |
| HIV‐infected year 2 | −6.7 (−10.3, −3.1)*** | −7.5 (−11.1, −3.8)*** | −7.6 (−11.3, −4.0)*** | −8.68 (−12.49, −4.88)*** |
| HIV‐infected year 3 | −4.2 (−8.2, −0.3)* | −5.2 (−9.2, −1.1)* | −5.4 (−9.4, −1.3)** | |
| HIV‐infected year 4 | −1.6 (−7.0, 3.8) | −2.4 (−7.8, 3.0) | −2.5 (−7.9, 3.0) | |
| Smoking, n (%) | ||||
| Never | Ref | Ref | Ref | |
| Current | −5.5 (−9.3, −1.7)** | −5.4 (−9.2, −1.6)** | −5.3 (−9.7, −0.9)* | |
| Former | −1.1 (−3.6, 1.4) | −1.0 (−3.5, 1.6) | −1.2 (−4.1, 1.6) | |
| Physical activity | ||||
| Low‐intensity activity | Ref | Ref | ||
| Moderate activity | 1.3 (−5.7, 8.3) | 0.3 (−7.6, 8.3) | ||
| Vigorous activity | 0.1 (−5.2, 5.4) | −2.2 (−7.9, 3.5) | ||
| Serum albumin (per 1 g/dL) | 5.3 (2.3, 8.4)*** | |||
Coefficient represents the difference in BP change (in mm Hg) between the category of interest and the reference category;
Low‐intensity activity (activity with <3 metabolic equivalents (MET) h/wk; moderate activity (activity with 3 to <6 MET h/wk); vigorous activity (activity with ≥6 METS h/wk).
Abbreviations: AIC: Akaike's information criterion; BIC: Bayesian information criterion.
P < 0.05;
P < 0.01;
P < 0.001.
Aside from HIV serostatus, the following variables were also predictive of higher SBP in multivariable models: age (each year) (0.3 mm Hg, 95%CI 0.1, 0.5) and serum albumin each 1 g/dL) [5.3 mm Hg (95%CI 2.3, 8.4)] (Table 5, Model 4). In similar models stratified by gender, increased serum albumin remained a predictor of increased SBP and DBP in both genders (Table 6).
Table 5.
Adjusted mixed‐effects regression models for absolute changes in mean diastolic blood pressures, UGANDAC study 2019
|
Model 1 Coeff (95% CI) |
Model 2 Coeff (95% CI) |
Model 3 Coeff (95% CI) |
Model 4 Coeff (95% CI) |
|
|---|---|---|---|---|
| AIC (df) | 6855.1 (16) | 6841.78 (18) | 6663.35 (20) | 4354.0 (17) |
| BIC | 6920.0 | 6914.8 | 6744.5 | 4423.0 |
| β0 coefficient | 75.7 (68.7, 82.7)*** | 77.2 (70.3, 84.2)*** | 78.7 (70.8, 86.7)*** | 66.2 (54.1, 78.4)*** |
| Characteristic | ||||
| Age (y) | −0.1 (−0.1, 0.1) | 0.1 (−0.1, 0.1) | 0.1 (−0.1, 0.1) | 0.1 (−0.1, 0.1) |
| Men | Ref | Ref | Ref | Ref |
| Women | 0.5 (−1.4, 2.4) | −0.7 (−2.7, 1.3) | −0.6 (−2.6, 1.3) | 0.5 (−1.7, 2.7) |
| HIV serostatus and year | ||||
| HIV‐uninfected Year 0 | Ref | Ref | Ref | Ref |
| HIV‐uninfected Year 1 | −4.6 (−6.2, −3.0)*** | −4.7 (−6.3, −3.1)*** | −4.7 (−6.3, −3.1)*** | −5.6 (−7.4, −3.9)*** |
| HIV‐uninfected Year 2 | −3.4 (−5.2, −1.7)*** | −3.6 (−5.3, −1.8)*** | −3.7 (−5.4, −1.9)*** | −4.9 (−7.1, −2.7)*** |
| HIV‐uninfected Year 3 | −0.8 (−2.9, 1.4) | −1.1 (−3.2, 1.1) | −0.9 (−3.1, 1.3) | |
| HIV‐uninfected Year 4 | 0.6 (−4.6, 5.8) | 0.1 (−5.2, 5.3) | 0.3 (−5.0, 5.7) | |
| HIV‐infected Year 0 | −5.6 (−8.0, −3.1)*** | −6.2 (−8.6, −3.8)*** | −6.6 (−9.1, −4.2)*** | −6.4 (−8.8, −3.9)*** |
| HIV‐infected Year 1 | −6.4 (−8.8, −4.0)*** | −7.1 (−9.5, −4.7)*** | −7.4 (−9.8, −5.0)*** | −6.9 (−9.3, −4.5)*** |
| HIV‐infected Year 2 | −4.0 (−6.5, −1.6)** | −4.7 (−7.1, −2.2)*** | −4.7 (−7.1, −2.2)*** | −5.4 (−7.9, −2.9)*** |
| HIV‐infected Year 3 | −2.3 (−5.0, 0.3) | −3.1 (−5.7, −0.4)* | −3.0 (−5.7, −0.4)* | |
| HIV‐infected Year 4 | −0.1 (−3.7, 3.4) | −0.7 (−4.3, 2.8) | −0.8 (−4.4, 2.8) | |
| Smoking, n (%) | ||||
| Never | Ref | Ref | Ref | |
| Current | −4.8 (−7.3, −2.2)*** | −4.6 (−7.2, −2.1)*** | −4.2 (−7.1, −1.3)** | |
| Former | −1.9 (−3.6, −0.2)* | −2.0 (−3.7, −0.3)* | −2.0 (−3.9, −0.1)* | |
| Physical activity | ||||
| Low‐intensity activity | Ref | Ref | ||
| Moderate activity | 0.1 (−4.8, 4.8) | −0.7 (−4.0, 4.6) | ||
| Vigorous activity | −1.5 (−5.2, 2.1) | −2.7 (−6.5, 1.1) | ||
| Serum Albumin (per 1 g/dL) | 2.9 (0.9, 4.9)** | |||
Coefficient represents the difference in BP change (in mm Hg) between the category of interest and the reference category; low‐intensity activity (activity with <3 metabolic equivalents (MET) h/wk; moderate activity (activity with 3 to <6 MET hours/week); vigorous activity (activity with ≥6 METS h/wk).
Legend:
Abbreviations: AIC: Akaike's information criterion; BIC: Bayesian information criterion.
P < 0.05;
P < 0.01;
P < 0.001.
Table 6.
Adjusted mixed‐effects regression models for between‐group difference in absolute changes in mean blood pressures between HIV‐infected and HIV‐uninfected participants stratified by gender, UGANDAC study 2019
| Characteristic | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean systolic blood pressure (mm Hg) | Mean diastolic blood pressure (mm Hg) | Mean systolic blood pressure (mm Hg) | Mean diastolic blood pressure (mm Hg) | |||||
|
Model 1 Coeff (95% CI) |
Model 2 Coeff (95% CI) |
Model 1 Coeff (95% CI) |
Model 2 Coeff (95% CI) |
Model 1 Coeff (95% CI) |
Model 2 Coeff (95% CI) |
Model 1 Coeff (95% CI) |
Model 2 Coeff (95% CI) |
|
| Age (y) | 0.2 (−0.1, 0.5) | 0.4 (0.04, 0.7)* | 0.1 (−0.2, 0.2) | 0.1 (−0.2, 0.3) | 0.2 (−0.1, 0.5) | 0.2 (−0.1, 0.5) | −0.1 (−0.2, 0.2) | −0.1 (−0.2, 0.2) |
| HIV‐infected | −8.8 (−14.3, −3.2)** | −8.4 (−14.0, −2.8)** | −7.0 (−10.5, −3.6)*** | −6.6 (−10.0, −3.1)*** | −7.4 (−12.2, −2.6)** | −7.4 (−12.2, −2.6)** | −6.1 (−9.6, −2.7)*** | −5.9 (−9.4, −2.4)*** |
| HIV‐uninfected | Ref | Ref | Ref | Ref | Ref | Ref | Ref | |
| HIV serostatus and year | ||||||||
| HIV‐uninfected year 0 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | |
| HIV‐uninfected year 1 | −7.1 (−10.3, −3.8)*** | −6.7 (−10.7, −2.7)*** | −6.1 (−8.4, −3.8)*** | −6.0 (−8.7, −3.3)*** | −4.9 (−6.0, −1.7)** | −6.1 (−9.8, −2.5)*** | −3.7 (−5.9, −1.4)*** | −5.2 (−7.7, −1.0)*** |
| HIV‐uninfected year 2 | −9.1 (−11.0, −5.2)*** | −8.5 (−14.1, −2.9)** | −5.6 (−8.2, −3.0)*** | −5.8 (−9.6, −2.1)** | −3.5 (−7.0, 0.1)* | −3.6 (−6.0, 0.7) | −2.2 (−4.6, 0.2) | −4.2 (−5.0, −1.4)** |
| HIV‐uninfected year 3 | −7.3 (−12.6, −2.1)** | −2.0 (−5.5, 1.4) | −2.7 (−7.0, 1.6) | 0.2 (−2.8, 3.1) | ||||
| HIV‐uninfected year 4 | −1.5 (−15.2, 12.2) | 2.0 (−7.4, 11.3) | 2.9 (−6.5, 12.3) | −0.1 (−6.5, 6.4) | ||||
| HIV‐infected year 0 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | |
| HIV‐infected year 1 | −1.4 (−4.5, 1.7) | −1.2 (−4.4, 1.9) | −2.0 (−4.2, 0.2) | −2.2 (−4.3, 0.1) | 0.5 (−2.7, 3.8) | 1.1 (−2.3, 4.5) | 0.5 (−1.8, 2.8) | 1.0 (−1.2, 3.2) |
| HIV‐infected year 2 | 0.1 (−3.6, 3.8) | −2.2 (−6.1, 1.8) | 0.9 (−1.5, 3.4) | −0.2 (−2.8, 2.5) | 0.4 (−3.0, 3.8) | 0.1 (−2.0, 4.1) | 2.8 (0.4, 5.2)* | 2.1 (−0.5, 4.7) |
| HIV‐infected year 3 | 3.4 (−1.4, 8.2) | 2.9 (−0.2, 6.1) | 2.0 (−2.2, 6.1) | 4.1 (1.3, 7.0)** | ||||
| HIV‐infected year 4 | 4.7 (−2.8, 12.1) | 3.9 (−1.0, 8.8) | 7.9 (1.2, 14.5)* | 8.2 (3.6, 12.7)*** | ||||
| Smoking, n (%) | ||||||||
| Never | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Current | −2.1 (−9.7, 5.4) | 3.3 (−6.6, 13.2) | −6.0 (−11.2, −0.8)* | −5.3 (−11.7, 1.2) | −8.1 (−12.7, −3.5)*** | −8.3 (−13.3, −3.3)*** | −5.02 (−8.2, −1.8)** | −4.12 (−7.7, −0.5)* |
| Former | 2.0 (−1.4, 5.4) | 0.9 (−3.2, 4.9) | −0.9 (−3.2, 1.4) | −1.5 (−4.1, 1.1) | −3.7 (−7.5, −0.1)* | −3.5 (−7.5, 0.5) | −3.37 (−5.9, −0.8) | −2.72 (−5.5, 0.1) |
| Physical activity | ||||||||
| Low‐intensity activity | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Moderate activity | −3.0 (−13.2, 7.2) | −2.0 (−13.7, 9.6) | −2.3 (−9.4, 4.8) | −3.8 (−11.6, 4.0) | 6.5 (−2.4, 15.5) | 3.9 (−6.8, 14.6) | 3.5 (−2.9, 9.9) | 3.7 (−3.5, 10.9) |
| Vigorous activity | −4.4 (−11.9, 3.1) | −4.6 (−12.3, 3.0) | −5.1 (−10.3, 0.1)* | −4.7 (−9.9, 0.4) | 5.0 (−1.9, 11.9) | 1.5 (−6.7, 9.7) | 2.4 (−2.6, 7.3) | 0.7 (−4.7, 6.3) |
| Serum albumin (per 1 g/dL) | 4.4 (0.1, 8.8)* | 3.5 (0.6, 6.4)* | 6.1 (1.9, 10.3)** | 2.7 (−0.1, 5.5) | ||||
The coefficient represents the difference in BP change per year between the category of interest and the reference category. Physical activity: low‐intensity activity (activity with <3 metabolic equivalents [MET] h/wk; moderate activity (activity with 3 to <6 MET h/wk); vigorous activity (activity with ≥6 METS h/wk).
*P < 0.05; **P < 0.01; ***P < 0.001
3.2. Longitudinal changes in blood pressure
During the first year of follow‐up, HIV‐uninfected experienced a 7 mm Hg decrease in SBP (from 121 to 114 mm Hg) compared to no SBP change in HIV‐infected persons (P < 0.0001), after controlling for age, gender, smoking, and physical activity. Though not statistically significant, SBP rose in the period between year 2 and year 4 in both HIV‐infected and HIV‐uninfected beyond the baseline (year 1) levels of HIV‐infected but not that of the HIV‐uninfected. A similar trend was observed for diastolic blood pressure (Figure 2).
Figure 2.
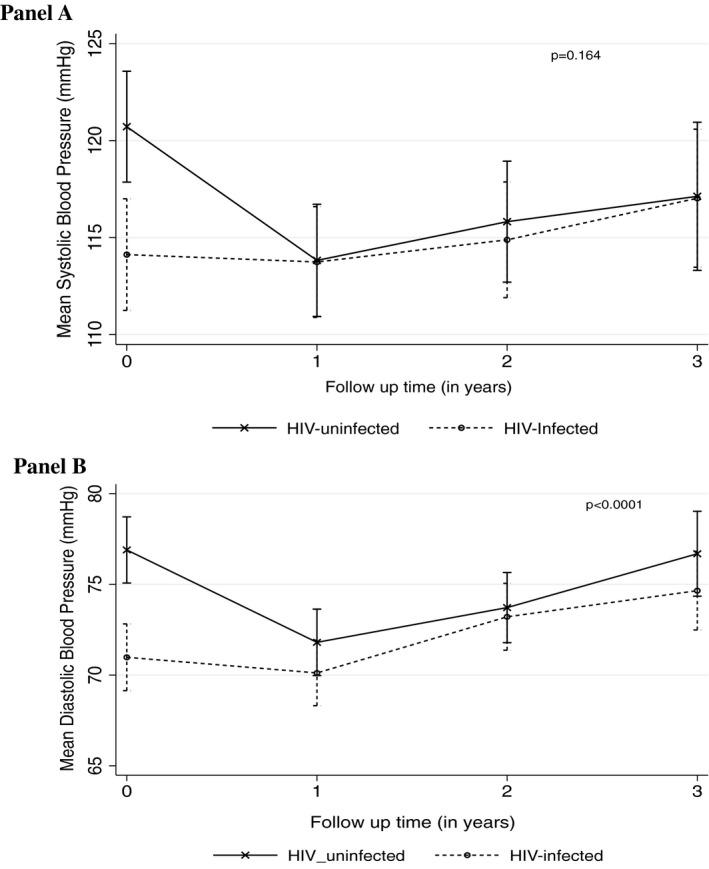
Adjusted changes in mean blood pressures (panel A–trends of mean systolic blood pressure by HIV serostatus, panel B—trends of mean diastolic blood pressure by HIV serostatus, UGANDAC study 2019)
Overall HIV‐uninfected participants had higher BPs than HIV‐uninfected counterparts. On average, for each additional year of follow‐up, among HIV‐uninfected both SBP and DBP decreased by 1.7 mm Hg/y (95%CI 0.7, 2.7) and 0.6 mm Hg/y (95%CI 0.1, 1.3), respectively, whereas among HIV‐infected SBP increased by 1.1 (95%CI 0.1, 2.0) and 1.4 mm Hg (95%CI 0.8, 2.0) for DBP. We found a significant difference in the overall rate of change over time between groups for DBP (P < 0.0001) but not SBP (P = 0.1638; Figure 2).
3.3. Mediation analyses
In models exploring the potential mediating pathways, we found that BMI mediated the largest proportion of the association between HIV and BP (Figure 3). The mediated effect of BMI (per kg/m2) on the association between HIV and blood pressure was 72% (95% CI 57%, 97%) for SBP and 69% (95% CI 56%, 88%) for DBP (Figure 3, Table 7). In contrast, we found little evidence of a mediating effect of biomarkers of inflammation and immune activation on differences in BP between people with and without HIV in rural Uganda (Figure 3). Of the four biomarkers tested, only sCD14 was a significant mediator for SBP (9%, 95% CI 5%, 21%) and DBP (3%, 95%CI 2%, 5%). In the sensitivity analyses, only the putative mediation by BMI was robust.
Figure 3.
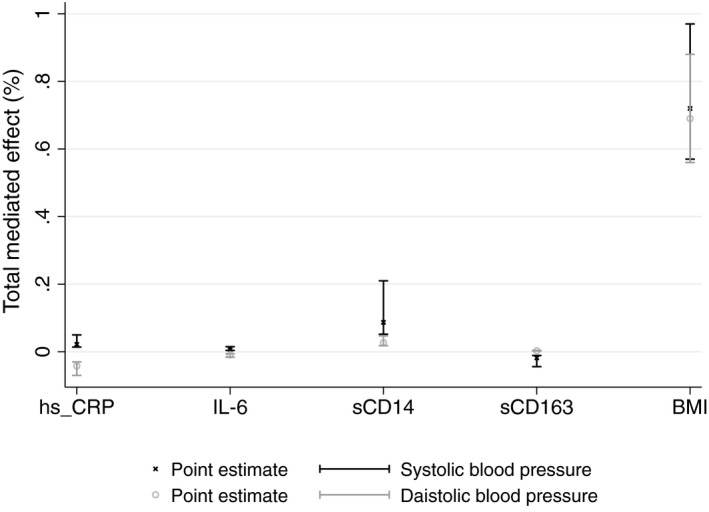
Mediated effects by markers on association of HIV with blood pressure, UGANDAC study 2019
Table 7.
Total, direct, and mediated effects of BMI, biomarkers on the association between HIV and BP, UGANDAC study 2019
| Mediators |
Total effect Coeff (95% CI) |
Natural direct effect Coeff (95% CI) |
Total effect mediated Coeff (95% CI) |
|---|---|---|---|
| Systolic blood pressure | |||
| Body mass index (BMI) (per kg/m2) | −20.2 (−25.5, −14.9) | −5.7 (−8.8, −2.7) | 0.7 (0.6, 1.0) |
| Chronic inflammation | |||
| hs C‐reactive protein (per μg/mL) | −6.2 (−9.7, −2.6) | −6.1 (−9.6, −2.2) | 0.1 (0.1, 0.1) |
| Interleukin‐6 (per pg/mL) | −5.7 (−9.2, −2.3) | −5.7 (−9.2, −2.3) | 0.1 (0.1, 0.1) |
| Immune activation | |||
| sCD14 (per pg/mL) | −5.6 (−9.3, 2.3) | −5.1 (−8.9, −1.5) | 0.1 (0.1, 0.2) |
| sCD163 (per pg/mL) | −5.7 (−9.3, −2.3) | −5.8 (−9.5, −2.3) | −0.1 (−0.1, −0.1) |
| Multipulative effects | |||
| hs C‐reactive protein, interleukin‐6 | −4.9 (−8.4, −1.3) | −5.6 (−9.1, −2.0) | −0.1 (−0.5, −0 0.1) |
| sCD14, sCD163 | 0.2 (−11.3, 12.4) | −5.4 (−9.1, −2.0) | 0.4 (−18.5, 21.8) |
| Diastolic blood pressure | |||
| Body mass index (BMI) (per kg/m2) | −15.2 (−18.6, −11.9) | −4.8 (−6.8, −2.8) | 0.7 (0.6, 0.9) |
| Chronic inflammation | |||
| hs C‐reactive protein (per μg/mL) | −5.6 (−7.9, −3.1) | −5.9 (−8.1, −3.3) | −0.1 (−0.1, −0.1) |
| Interleukin‐6 (per pg/mL) | −5.4 (−7.7, −3.1) | −5.4 (−7.7, −3.1) | −0.1 (−0.2, −0.1) |
| Immune activation | |||
| sCD14 (per pg/mL) | −5.3 (−7.8, −3.0) | −5.2 (−7.7, −2.7) | 0.1 (0.1, 0.1) |
| sCD163 (per pg/mL) | −5.4 (−7.9, −3.1) | −5.4 (−7.9, −3.1) | 0.1 (0.1, 0.1) |
| Multipulative effects | |||
| hs C‐reactive protein, interleukin‐6 | −4.9 (−7.3, −2.4) | −5.4 (−7.7, −2.9) | −0.1 (−0.19, −0.1) |
| sCD14, sCD163 | −5.6 (−13.3, 2.5) | −5.4 (−7.9, −3.1) | 0.1 (−0.3, 0.4) |
Chronic inflammation (high‐sensitivity C‐reactive protein and interleukin‐6) (sCD14 and sCD163).
4. DISCUSSION
In this longitudinal cohort of HIV‐infected and population‐based controls, we found evidence that people living with HIV taking ART in rural Uganda consistently have lower blood pressures compared to HIV‐uninfected counterparts over 4 years of follow‐up. Both groups experienced a decline in BP in the first year of follow‐up, presumably due to referral into hypertension care, and then experiences increase over time during the next 2‐4 years of observation. The rise in BP among people with HIV appeared to be largely mediated by increased BMI, but we found little evidence that biomarkers of inflammation or immune activation, despite remaining elevated among people with HIV on ART, result in significant differences in BP by HIV serostatus in Uganda.
Contrary to results from high‐income countries, reports from sub‐Saharan Africa tend to demonstrate higher BP in HIV‐uninfected compared with HIV‐infected individuals.7, 19, 25, 26 This difference could be because people with HIV in high‐income countries tend to be older with prevalent cardiovascular comorbidities27 or because of a disparity in access to care that may provide people with HIV with better access to care than uninfected people in SSA (whereas this disparity perhaps is not replicated, or is even reversed, in high‐income countries).26, 28 Alternatively, the phenomenon of cardiovascular autonomic dysfunction in HIV infection might result in lower blood pressure among HIV‐infected individuals.29, 30 Our study was age‐matched by HIV serostatus, so the differences we detected are unlikely to be due to increased BP risk among older HIV‐uninfected individuals. It is beyond the scope of this study to determine whether the BP difference by HIV serostatus detected in our study by HIV serostatus was due to HIV‐related pathophysiology or due to improved clinical care. To test this hypothesis, we and other groups intend to assess the impact of healthcare access for HIV‐infected persons in larger population‐based cohorts.
Body mass index mediated 70% of the association between HIV and SBP. This is similar to, though larger in magnitude, a previous cross‐sectional finding in Uganda in which BMI mediated over 25% of the association between HIV and SBP.19 BMI appears to be a major contributor to the BP increase among people with HIV partly due to better access to care and healthier lifestyles (more exercise, cessation of smoking, and less salty/fatty diets).14 Though HIV infection is associated with weight loss via multifactorial mechanisms including socioeconomic status, access to care, cultural practices, psychological factors, HIV virus, and ART,31 stabilization on ART32 leads to weight gain termed “trend toward normal weight.” Though beneficial to health recovery and return to pre‐HIV workforce productivity,33 this represents a trend toward overweight and obesity18 and later blood pressure increase.19
In contrast, we found relatively little evidence that chronic inflammation or immune activation is important contributor to BP increase. Of the markers of immune activation, we found soluble CD (cluster of differentiation) 14 mediated about a tenth of the effect of HIV on blood pressure, while the others had negligible mediated effects. These findings are similar to prior work that showed increased sCD14 levels predicted elevated blood pressure34, 35 as well as subclinical atherosclerosis in infected and uninfected individuals.36 In contrast, our previous retrospective cohort of participants with advanced HIV disease at ART initiation lower sCD14 was associated with elevated blood pressure.37 The relationship between sCD14 and blood pressure may be important for two reasons. First, this macrophage activation pathway may provide targets for interventions to prevent hypertension. Second, elevated blood pressure may be a modifiable mediator in the relationship between higher sCD14 levels and cardiovascular events. Long‐term data with outcomes remain a missing link in this field and will be needed to corroborate the relationship between sCD14, hypertension, and ultimate impacts on morbidity and mortality.
Notably, we found that higher serum albumin predicts increases in BP among both HIV‐infected and HIV‐uninfected adults. It is known that albumin triggers the tubular rennin‐angiotensin system (RAAS) and thus can theoretically lead to elevation of blood pressure. The mechanism of action is believed to be through production of intracellular reactive oxygen species by a protein kinase C‐induced activation of nicotinamide adenine dinucleotide phosphate (NADPH), which in turn activates nuclear factor‐[kappa]B (NF‐[kappa]B) and protein‐1 (AP‐1).38 Moreover, heightened angiotensin II may further activate NF‐[kappa]B and, thus, induce a spiral of renal injury and higher BP.39 Future work should explore the predictive value of albumin to detect hypertension risk and/or whether these pathways might over therapeutic targets for elevated BP.
We cannot explain the inverse relationship we found between diastolic blood pressure and smoking observed in our study. Although smoking data were collected and analyzed as a categorical explanatory covariate (never/former/current smoker), the nicotine/cotinine concentrations might be more sensitive measures for lifetime smoking, to be explored in future studies.
Our study has some distinct strengths: We used data from a longitudinal cohort study including rich clinical and biomarker data, which enabled us to take advantage of the longitudinal design to evaluate the relationship between HIV and blood pressure changes over 4 years. On the other hand, some limitations must be acknowledged, the unmeasured confounding and measurement error of our proposed mediators could potentially offer biased estimates for direct and indirect mediated effects. We minimized it by using instruments capable of more precise measurements and obtaining repeated measurements from an individual to better estimate the true values.
In conclusion, older people living with HIV on ART have lower blood pressure then age‐ and sex‐matched HIV‐uninfected counterparts over four years of follow‐up. Despite the higher levels of inflammation in HIV‐infected people, we found little mediated effects of inflammatory markers on the association between HIV and BP. In contrast, BMI significantly mediated the effects of HIV on blood pressure. Thus, interventions focused on body weight may be strategic targets for blood pressure control in Uganda and in the clinical prediction of hypertension in HIV‐infected patients on ART.
AUTHOR CONTRIBUTIONS
SO, ACT, RT, BK, and MJS designed the research study; SO, RNS, JK, RT, and MJS collected the data and performed laboratory tests; SO, ACT, and MJS analyzed the data, and SO, RNS, JK, ACT, RT, BK, and MJS wrote the manuscript. All authors read and approved the final manuscript.
DISCLOSURE OF PREVIOUS PRESENTATIONS
None.
ACKNOWLEDGMENTS
The authors would like to thank the staff at the Immune Suppression Syndrome (ISS) clinic and the Uganda Non‐Communicable Diseases and Aging Cohort study staff: Alan Babweteera, Zulaika Namboga, Sheila Abaasabyoona, and Doreen Kyomuhendo.
Okello S, Kim J‐H, Sentongo RN, et al. Blood pressure trajectories and the mediated effects of body mass index and HIV‐related inflammation in a mixed cohort of people with and without HIV in rural Uganda. J Clin Hypertens. 2019;21:1230–1241. 10.1111/jch.13621
Funding information
This study was supported by funding from the National Institutes of Health (R21HL124712, K23MH099916, K43TW010715, R01MH113494, P30AI060354, R24AG044325, P30AG024409, and U01AI069911) and the Massachusetts General Hospital Executive Committee on Research). The funders had no role in study design, conduct, data analysis, or production of manuscript.
REFERENCES
- 1. Gazzaruso C, Bruno R, Garzaniti A, et al. Hypertension among HIV patients: prevalence and relationships to insulin resistance and metabolic syndrome. J Hypertens. 2003;21(7):1377‐1382. [DOI] [PubMed] [Google Scholar]
- 2. Baekken M, Os I, Sandvik L, Oektedalen O. Hypertension in an urban HIV‐positive population compared with the general population: influence of combination antiretroviral therapy. J Hypertens. 2008;26(11):2126‐2133. [DOI] [PubMed] [Google Scholar]
- 3. Bergersen B, Sandvik L, Dunlop O, Birkeland K, Bruun J. Prevalence of hypertension in HIV‐positive patients on highly active retroviral therapy (HAART) compared with HAART‐naive and HIV‐negative controls: results from a Norwegian study of 721 patients. Eur J Clin Microbiol Infect Dis. 2003;22(12):731‐736. [DOI] [PubMed] [Google Scholar]
- 4. Khalsa A, Karim R, Mack WJ, et al. Correlates of prevalent hypertension in a large cohort of HIV‐infected women: Women's Interagency HIV Study. AIDS. 2007;21(18):2539‐2541. [DOI] [PubMed] [Google Scholar]
- 5. Jerico C, Knobel H, Montero M, et al. Hypertension in HIV‐infected patients: prevalence and related factors. Am J Hypertens. 2005;18(11):1396‐1401. [DOI] [PubMed] [Google Scholar]
- 6. Kwarisiima D, Balzer L, Heller D, et al. Population‐based assessment of hypertension epidemiology and risk factors among HIV‐positive and general populations in Rural Uganda. PLoS ONE. 2016;11(5):e0156309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schutte AE, Schutte R, Huisman HW, et al. Are behavioural risk factors to be blamed for the conversion from optimal blood pressure to hypertensive status in Black South Africans? A 5‐year prospective study. Int J Epidemiol. 2012;41(4):1114‐1123. [DOI] [PubMed] [Google Scholar]
- 8. Ford N, Shubber Z, Meintjes G, et al. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta‐analysis. Lancet HIV. 2015;2(10):e438‐e444. [DOI] [PubMed] [Google Scholar]
- 9. Ali MK, Magee MJ, Dave JA, et al. HIV and metabolic, body, and bone disorders: what we know from low‐and middle‐income countries. J Acquir Immune Defic Syndr. 2014;67:S27‐S39. [DOI] [PubMed] [Google Scholar]
- 10. Gari S, Doig‐Acuña C, Smail T, Malungo JR, Martin‐Hilber A, Merten S. Access to HIV/AIDS care: a systematic review of socio‐cultural determinants in low and high income countries. BMC Health Serv Res. 2013;13(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eric N, Janet L, Steven KG. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS. 2016;30(10):1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harrison DG. The mosaic theory revisited: common molecular mechanisms coordinating diverse organ and cellular events in hypertension. J Am Soc Hypertens. 2013;7(1):68‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lo J, Plutzky J. The biology of atherosclerosis: general paradigms and distinct pathogenic mechanisms among HIV‐infected patients. J Infect Dis. 2012;205(Suppl 3);S368‐S374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feinstein MJ, Kim J‐H, Bibangambah P, et al. Ideal cardiovascular health and carotid atherosclerosis in a mixed cohort of HIV‐infected and uninfected Ugandans. AIDS Res Hum Retroviruses. 2017;33(1):49‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haas AD, Keiser O, Balestre E, et al. Monitoring and switching of first‐line antiretroviral therapy in adult treatment cohorts in sub‐Saharan Africa: collaborative analysis. Lancet HIV. 2015;2(7):e271‐e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sobngwi E, Mbanya JC, Unwin NC, Aspray TJ, Alberti KG. Development and validation of a questionnaire for the assessment of physical activity in epidemiological studies in Sub‐Saharan Africa. Int J Epidemiol. 2001;30(6):1361‐1368. [DOI] [PubMed] [Google Scholar]
- 17. Geldsetzer P, Feigl AB, Tanser F, Gareta D, Pillay D, Bärnighausen T. Population‐level decline in BMI and systolic blood pressure following mass HIV treatment: evidence from rural KwaZulu‐Natal. Obesity. 2017;25(1):200‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feigl AB, Bloom DE, Danaei G, et al. The effect of HIV and the modifying effect of anti‐retroviral therapy (ART) on body mass index (BMI) and blood pressure levels in rural South Africa. PLoS ONE. 2016;11(8):e0158264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okello S, Ueda P, Kanyesigye M, et al. Association between HIV and blood pressure in adults and role of body weight as a mediator: Cross‐sectional study in Uganda. J Clin Hypertens. 2017;19(11):1181‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nduka CU, Uthman OA, Kimani PK, Malu AO, Stranges S. Impact of body fat changes in mediating the effects of antiretroviral therapy on blood pressure in HIV‐infected persons in a sub‐Saharan African setting. Infect Dis Poverty. 2016;5(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Banack HR, Kaufman JS. From bad to worse: collider stratification amplifies confounding bias in the “obesity paradox”. Eur J Epidemiol. 2015;30(10):1111‐1114. [DOI] [PubMed] [Google Scholar]
- 22. Hafeman DM. Confounding of indirect effects: a sensitivity analysis exploring the range of bias due to a cause common to both the mediator and the outcome. Am J Epidemiol. 2011;174(6):710‐717. [DOI] [PubMed] [Google Scholar]
- 23. Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods. 2010;15(4):309. [DOI] [PubMed] [Google Scholar]
- 24. Siedner MJ, Zanni M, Tracy RP, et al. Increased Systemic inflammation and gut permeability among women with treated HIV infection in Rural Uganda. J Infect Dis. 2018;40:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dillon DG, Gurdasani D, Riha J, et al. Association of HIV and ART with cardiometabolic traits in sub‐Saharan Africa: a systematic review and meta‐analysis. Int J Epidemiol. 2013;42(6):1754‐1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scholten F, Mugisha J, Seeley J, et al. Health and functional status among older people with HIV/AIDS in Uganda. BMC Public Health. 2011;11(1):886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guaraldi G, Orlando G, Zona S, et al. Premature age‐related comorbidities among HIV‐infected persons compared with the general population. Clin Infect Dis. 2011;53(11):1120‐1126. [DOI] [PubMed] [Google Scholar]
- 28. Nyirenda M, Chatterji S, Falkingham J, et al. An investigation of factors associated with the health and well‐being of HIV‐infected or HIV‐affected older people in rural South Africa. BMC Public Health. 2012;12(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nzuobontane D, Ngu BK, Christopher K. Cardiovascular autonomic dysfunction in Africans infected with human immunodeficiency virus. J R Soc Med. 2002;95(9):445‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mattana J, Siegal FP, Sankaran RT, Singhal PC. Absence of age‐related increase in systolic blood pressure in ambulatory patients with HIV infection. Am J Med Sci. 1999;317(4):232‐237. [DOI] [PubMed] [Google Scholar]
- 31. Mangili A, Murman DH, Zampini AM, Wanke CA, Mayer KH. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort. Clin Infect Dis. 2006;42(6):836‐842. [DOI] [PubMed] [Google Scholar]
- 32. Brown TT, Chu H, Wang Z, et al. Longitudinal increases in waist circumference are associated with HIV‐serostatus, independent of antiretroviral therapy. AIDS. 2007;21(13):1731‐1738. [DOI] [PubMed] [Google Scholar]
- 33. Bor J, Tanser F, Newell M‐L, Bärnighausen T. In a study of a population cohort in South Africa, HIV patients on antiretrovirals had nearly full recovery of employment. Health Aff. 2012;31(7):1459‐1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manner IW, Baekken M, Kvale D, et al. Markers of microbial translocation predict hypertension in HIV‐infected individuals. HIV Med. 2013;14(6):354‐361. [DOI] [PubMed] [Google Scholar]
- 35. Tenorio AR, Zheng YU, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T‐cell activation predict non–AIDS‐defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McKibben RA, Margolick JB, Grinspoon S, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2014;211(8):1219‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Okello S, Asiimwe SB, Kanyesigye M, et al. D‐dimer levels and traditional risk factors are associated with incident hypertension among HIV‐infected individuals initiating antiretroviral therapy in Uganda. J Acquir Immune Defic Syndr. 2016;73(4):396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cao W, Zhou QG, Nie J, et al. Albumin overload activates intrarenal renin–angiotensin system through protein kinase C and NADPH oxidase‐dependent pathway. J Hypertens. 2011;29(7):1411‐1421. [DOI] [PubMed] [Google Scholar]
- 39. Tikellis C, Bernardi S, Burns WC. Angiotensin‐converting enzyme 2 is a key modulator of the renin–angiotensin system in cardiovascular and renal disease. Curr Opin Nephrol Hypertens. 2011;20(1):62‐68. [DOI] [PubMed] [Google Scholar]


