Abstract
Background:
Oxygen and glucose deprivation is a common feature of the solid tumor. Regulatory network underlying the adaptation of cancer cells to the harsh microenvironment remains unclear. We determined the mechanistic role of LIM and senescent cell antigen-like-containing domain protein 1 (LIMS1) in cancer cell survival under oxygen-glucose deprivation conditions.
Methods:
The expression level of LIMS1 was determined by immunohistochemical staining and analysing the mRNA expression profiles from The Cancer Genome Atlas of three human solid tumors. Roles of LIMS1 in cancer cell metabolism and growth were determined by molecular and cell biology methods. A jetPEI nanocarrier was used as the vehicle for anti-LIMS1 siRNAs in mouse models of cancer therapeutics.
Results:
LIMS1 expression was drastically elevated in PDAC. High LIMS1 level was associated with advanced TNM stage and poor prognosis of tumour patients. Increased LIMS1 expression was pivotal for tumour cells to survive in the oxygen-glucose deprivation conditions. Mechanistically, LIMS1 enhanced GLUT1 expression and membrane translocation, which facilitated tumor cell adaptation to the glucose deprivation stress. Furthermore, LIMS1 promoted HIF1A protein translation by activating AKT/mTOR signalling, while HIF1 transactivated LIMS1 transcription, thus forming a positive feedback loop in PDAC cell adaptation to oxygen deprivation stress. Inhibition of LIMS1 with jetPEI nanocarrier-delivered anti-LIMS1 siRNAs significantly increased cell death and suppressed tumour growth.
Conclusions:
LIMS1 promotes pancreatic cancer cell survival under oxygen-glucose deprivation conditions by activating AKT/mTOR signalling and enhancing HIF1A protein translation. LIMS1 is crucial for tumor adaptation to oxygen-glucose deprivation conditions and is a promising therapeutic target for cancer treatment.
Keywords: Oxygen-glucose deprivation, Glucose uptake, LIMS1, HIF1
Introduction
In solid tumors, the poorly formed tumor vasculature leads to diffusion-limited hypoxia, accumulation of waste products and lack of nutrients, including glucose (1). The oxygen shortage causes major metabolic discrepancies between the microenvironments of solid tumor tissues and normal tissues. In normal tissues, aerobic oxidation generates approximately 90% of the cell’s energy, whereas in tumor tissues, over 50% of the cellular ATP is generated by anaerobic glycolysis (Warburg effect) (2). Due to increased glucose consumption and limited supplies, the glucose concentrations in the tumor microenvironment are frequently 3- to 10-fold lower than that in non-tumor tissues (3). Hypoxia-inducible factor 1 (HIF1) is the master regulator of tumor cell adaptation to the oxygen-glucose deprivation microenvironment. HIF1 levels in tumor tissues are elevated in response to hypoxia; activation of some oncogenes, such as Ras and PI3K/Akt; and inactivation of tumor suppressors, such as VHL or PTEN (4). Elevated HIF1 levels, in turn, enhance glucose influx by activating genes involved in glucose uptake (such as GLUT1) (5) and glycolysis (such as PFK1) (6) and inhibit oxidative phosphorylation by transactivating genes such as PDK1 (7). Thus, HIF1 plays crucial roles in tumor glucose metabolism, facilitating cancer cell survival and tumor growth in the oxygen-glucose deprivation http://cn.bing.com/dict/clientsearch?mkt=zh-CN&setLang=zh&form=BDVEHC&ClientVer=BDDTV3.5.0.4311&q=glucose-deprivation microenvironment.
LIMS1 (also known as PINCH1) was originally identified as a marker for senescent erythrocytes and is widely expressed in mammalian cells (8). As an adaptor protein that consists of five LIM domains and tandem nuclear localisation signals (9), LIMS1 binds to integrin-linked kinase (ILK) and parvin to form a ternary protein complex (10), which is essential for the control of cell–extracellular matrix adhesion-mediated cell behaviour (11). Additionally, LIMS1 also binds to Nck2 (12), Ras1 (13), and thymosin β4 (14). In response to transmembrane integrin and growth factor receptors, the LIMS1/ILK/parvin complex regulates cell survival via the PI3K/PKB/Akt1 and Ras/MAPK signalling pathways (13). LIMS1 was also shown to directly inhibit phosphatase 1α (PP1α)—an Akt1-regulating protein—to activate Akt1 phosphorylation (15).
Although LIMS1 was reported to be overexpressed in some types of tumors (16), the roles of LIMS1 in tumors are not fully understood. In the present study, we demonstrated that LIMS1 was highly expressed in tumor tissues, and LIMS1 overexpression was correlated with advanced TNM staging and poor survival of cancer patients. We further identified a novel role of LIMS1 in promoting tumor cell survival in the oxygen-glucose-deprived microenvironment.
Materials and Methods
Cell culture and human sample collection
The human tumor cell lines CFPAC-1, BxPC-3, PANC-1, KYSE40, EC109, A549 and PC9 were obtained from the Type Culture Collection Committee of the Chinese Academy of Sciences (Shanghai, China), and the MIA-PaCa-2 cell line was obtained from the American Type Culture Collection in 2013. The cell lines were authenticated through the short tandem repeat analysis method and mycoplasma contamination was excluded in these cell lines at the beginning of this study. These cells were cultured in DMEM, RPMI-1640 or IMDM basic medium supplemented with 10% foetal bovine serum (FBS) at 37°C in a humidified atmosphere of 95% air and 5% CO2.
A total of 109 sequential PDAC tissues, 124 sequential esophageal carcinoma tissues and 113 sequential lung adenocarcinoma tissues were collected from patients who had received radical surgery at the Tianjin Medical University Cancer Institute and Hospital (China). Retrospective clinicopathological data of these patients, including age, sex, tumor size, regional lymph node status, TNM stage, pathologic type, differentiation and PET/CT scanning data, were also obtained.
The usage of these specimens and the patient information were approved by the Ethics Committee of the Tianjin Medical University Cancer Institute and Hospital. All patients provided written consent for the use of their specimens and disease information for future investigations according to the ethics committee and in accordance with recognized ethical guidelines of Declaration of Helsinki.
IHC and immunofluorescence
IHC was used to detect LIMS1, HIF1, pAKT (S473) and pAKT (T308) in tumor tissues. Briefly, paraffin-embedded sections of tumor tissues were deparaffinised and then heated in a pressure pot for 3 minutes to retrieve the antigens. Then, the sections were incubated with primary antibodies (Supplementary Table 6) overnight at 4°C. Antibody binding was detected using a peroxidase–conjugated secondary antibody at 37°C for 30 minutes. A DAB Substrate Kit was used to perform the chromogenic reaction. The intensity of the staining was evaluated using the following criteria: 0, negative; 1, low; 2, medium; 3, high. The extent of staining was scored as 0, 0% stained; 1, 1% to 25% stained; 2, 26% to 50% stained; 3, 51% to 100% stained. Five random fields (20 × magnification) were evaluated under a light microscope. The final scores were calculated by multiplying the scores of the intensity with those of the extent and dividing the samples into four grades: 0, negative (−); 1 to 2, low staining (+); 3 to 5, medium staining (++); 6 to 9, high staining (+++) (17).
Immunofluorescence staining was performed on the PDAC cell lines. Briefly, PDAC cells seeded on coverslips were cultured with anti-LIMS1 antibody at 4°C overnight. Then, the cells were incubated with fluorescent dye-labelled secondary antibodies at room temperature for 1 hour. The cells were again incubated with anti-fade DAPI solution (1:1000), and images were captured with a confocal fluorescence microscope.
Tumor model
All animal studies were approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital and conducted by skilled experimenters under an approved protocol in accordance with the principles and procedures outlined in the NIH Guide for the Care and Use of Laboratory Animals. Four-week-old female BALB/C nude mice were maintained in a barrier facility on high-efficiency particulate air (HEPA)-filtered racks. Tumor cells were harvested by trypsinisation, washed in PBS and resuspended at 1 × 107 cells/mL in Matrigel. A total of 1 × 106 cells were subcutaneously or orthotopically injected into each mouse to develop tumors as previously described (17,18). Tumor size was measured weekly.
In vivo experiments.
jetPEI-si scramble (40 μg), jetPEI-si LIMS1 (40 μg) or 5% glucose control (200 μL) was intravenously injected into the corresponding mouse group every week from the 14th day. Six weeks later, the tumors were harvested. Each group had 6 mice.
TCGA data analysis
TCGA data of 179 patients with pancreatic carcinoma, 184 patients with esophageal carcinoma and 522 patients with lung adenocarcinoma were downloaded from TCGA website (http://cancergenome.nih.gov/). The normalised RNA levels of LIMS1 and HIF1A, the clinicopathological parameters and the follow-up data were extracted and analysed.
RT-PCR
The total RNA of the cells was extracted with TRIzol (Invitrogen) according to the manufacturer’s instructions. Then, the mRNA was reverse transcribed to single-stranded cDNAs using a reverse-transcription PCR (RT-PCR) system (TaKaRa). The primers are listed in Supplementary Table S7. Then, real-time fluorescent quantitative PCR or semi-quantitative PCR was used to analyse the cDNA levels. The products of semi-quantitative PCR were detected by agarose gel electrophoresis, and β-actin was used as a loading control.
Western blotting
Whole-cell extracts were prepared by lysing the cells with RIPA lysis buffer supplemented with a proteinase inhibitor cocktail (Sigma). A membrane and cytosol protein extraction kit was used to extract membrane protein (Pierce). A total of 20 mg protein lysate was separated by SDS-PAGE, and then, the target proteins were detected by Western blotting with the antibodies to LIMS1, HIF1A, pAKT1 (S473), pAKT1 (T308), panAKT1, p-mTOR (s2481), pan-mTOR, p-4EBP1, pan-4EBP1, VEGFA, GLUT1, GLUT3, CA9, ETS1, Na+/K+ -ATPase and β-actin (Supplementary Table S6).
Plasmid construction and stable cell line establishment
The complete coding sequence of the human LIMS1 gene (NM_004987.5) was cloned into pLV-EF1-MCS-IRES-Bsd vectors (Biosettia). Lentiviruses were produced in 293T cells for the stable transfection of the cell lines, per the manufacturer’s instructions, and an empty vector was transfected into cells to be used as a control. A total of 1 × 105 tumor cells in 2 mL medium with 8 μg/mL polybrene were infected with 1 mL lentivirus supernatant. After 48 hours, blasticidin (InvivoGen) was added for selection.
For the cell lines with stable knockdown, shRNA sequences were designed with Biosettia’s shRNA designer (http://biosettia.com/support/shrna-designer). Three recommended sequences for each of the LIMS1 and HIF1A genes were synthesised and cloned into the pLV-hU6-EF1α-puro or pLV-mU6-EF1α-puro vectors (Biosettia). Then, the lentiviruses were produced in 293T cells. Scrambled sequences were transfected into the cells to be used as controls. Of the three stable cell lines, the most efficient one was used for the relevant assays.
CHIP and luciferase analysis.
CHIP assays were performed using a CHIP kit (Millipore), according to the manufacturer’s instructions. Briefly, PANC-1 cells were transiently transfected with or without pcDNA-HIF1A and then immunoprecipitated with anti-HIF1A antibody. The immunoprecipitated products were detected by RT-PCR assays.
Luciferase analysis was performed as described previously with minor changes (19). PANC-1 cells transfected with pcDNA-HIF1A or control vector (pcDNA-vector) were transfected with pGL3-LIMS1-promoter, pGL3-LIMS1-promoter mutation (MUT), or pGL3-empty vectors (pGL3.1 EV). Forty-eight hours later, cells were subjected to dual luciferase analysis. The results are expressed as a fold induction relative to the cells transfected with the control vector (pcDNA3.1) after normalisation to Renilla activity.
Glucose uptake assays in vitro and in vivo
In vitro.
A glucose uptake assay kit was used to detect the uptake of 2-DG in the indicated tumor cells in vitro following the manual instructions (Abcam). In brief, the indicated tumor cells were starved in serum-free culture medium overnight followed by 40 minutes of incubation in Krebs–Ringer–phosphate–HEPES buffer. Subsequently, cells were incubated with 10 mM 2-DG for 20 minutes. Cells were lysed by freezing and thawing procedures. The lysates were neutralised and then diluted with assay buffer. The colourimetric product generation was detected at 412 nm by a microplate reader (Bio-Rad).
In vivo.
The indicated tumor cells were orthotopically transplanted into the pancreases of nude mice to develop tumors (n = 6 for each group). Four weeks later, the mice were intravenously injected with 18FDG (200 μCi in 0.2 mL), anaesthetised and then subjected to PET-CT scanning for 18FDG uptake in vivo. The concentrations of 18FDG uptake in tumor tissues were normalised to %ID/g.
In vitro survival assays under oxygen-glucose deprivation stress
Tumor cells were cultured in the oxygen deprivation conditions (oxygen concentration, 1%) and/or glucose deprivation conditions (glucose concentration, 0.75 mM) as previously described (20). The incubation time varied from 24 hours to 72 hours. The cell morphology was observed by an inverted phase contrast microscope. The cell survival was measured by CCK8 assays and flow cytometry analysis.
Polysome profiling analysis
Polysome RNA preparations and analysis were carried out as previously described.(21) Briefly, cells were seeded in 10-cm dishes, washed with cold PBS containing 100 μg/mL cycloheximide, and then lysed in a hypotonic lysis buffer [5 mM Tris-HCl (pH 7.5), 2.5 mM MgCl2, 1.5 mM KCl, 100 μg/mL cycloheximide, 2 mM DTT, 0.5% Triton X-100, and 0.5% sodium deoxycholate]. A lysate sample was used to isolate the cytoplasmic RNA using TRIzol (Invitrogen). Lysates were loaded onto 10–50% (wt/vol) sucrose density gradients [20 mM HEPES-KOH (pH 7.6), 100 mM KCl, 5 mM MgCl2] and centrifuged at 36,000 rpm for 2 hours at 4°C. Gradients were fractionated, and the optical density at 254 nm was continuously detected by an UV detector and fraction collector (Teledyne ISCO). RNA from each fraction was isolated using TRIzol (Invitrogen). Fractions with mRNA associated with polysomes were pooled (polysomal mRNA) for polysomal mRNA analysis of specific genes.
Statistical analysis
Statistical analyses were performed with the IBM SPSS Statistics Program. Each experiment was performed in triplicate, and the values are presented as the mean ± SD, unless otherwise stated. The variance between the groups was statistically compared. Student’s t test was used to compare the mean values. Kaplan–Meier curves were analysed for relevant variables. The log-rank test was used to analyse the differences in survival times among the patient subgroups. All probability values had a statistical power level of 90%, and a 2-sided level of 5%. P < 0.05 was significant.
Results
LIMS1 is overexpressed in human cancer tissues
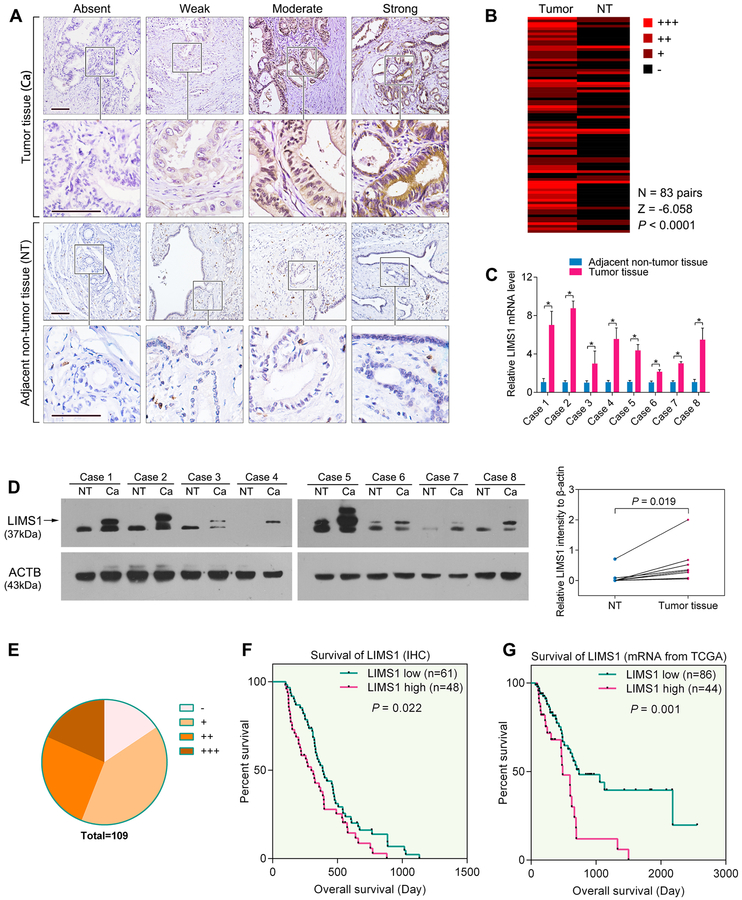
To investigate the expression pattern of LIMS1 in cancers, we performed LIMS1 immunohistochemistry (IHC) staining of PDAC tumor tissues and the paired adjacent non-tumor tissues (pancreatic tissues 2–3 cm around the tumor border). As shown in Fig. 1A, LIMS1 was overexpressed in tumor tissues and rarely expressed in the adjacent non-tumor tissues. When comparing LIMS1 staining in 83 pairs of PDAC tumor tissues and the adjacent non-tumor tissues, we discovered that LIMS1 protein was detected in the majority of PDAC tissues but not in the adjacent non-tumor tissues (Z = −6.058, P < 0.0001, Wilcoxon signed rank test; Fig. 1B).
Figure 1. The expression and clinical significance of LIMS1 in PDAC tissues.
A-B, sections of PDAC tissues were used to analyse the expression levels of LIMS1 in tumor tissues and the corresponding adjacent non-tumor (NT) tissues. Representative images are shown for absent, weak, moderate and strong expression of LIMS1 in IHC staining of PDAC tumor and NT tissues (A). The differential expression of LIMS1 in 83 pairs of tumor and NT tissues is shown in a heat-map and was statistically analysed by Wilcoxon signed rank tests (B). C-D, eight pairs of fresh PDAC tumor tissues and NT tissues were subjected to RT-PCR analysis (C) and Western blotting (D) to compare the expression levels of LIMS1. E, the distribution of IHC results in 109 PDAC tissues. F, Kaplan–Meier analysis of overall survival of the 109 PDAC patients according to different LIMS1 levels. G, mRNA profiles and follow-up data of 130 PDAC patients from TCGA analysed for the correlation of the LIMS1 mRNA expression and survival (the minimum follow-up time is 60 days; the cut-off line for the LIMS1 low and LIMS1 high groups is 475.5 RPKM). Paired t tests were used in D; log-rank tests were used in E and G. Tests of significance are two-sided; *P < 0.05. Scale bars, 100 μm.
Subsequently, LIMS1 expression in 8 pairs of fresh PDAC tumor tissues and the adjacent non-tumor tissues was analysed using qPCR (Fig. 1C) and Western blotting. Our data indicated that LIMS1 was abnormally overexpressed in PDAC tumor tissues (P = 0.019, paired t test; Fig. 1D). LIMS1 overexpression was also detected in 5 PDAC cell lines (Suppl. Fig. S1).
To elucidate the clinical significance of LIMS1 in PDAC, we used IHC to determine the expression of LIMS1 in 109 human PDAC specimens (Fig. 1E). As shown in Supplementary Table S1, high expression of LIMS1 was strongly correlated with advanced tumor size (P = 0.021, chi-square tests), T stage (P = 0.036, chi-square tests), regional lymph node involvement (P = 0.019, chi-square tests), pTNM staging (P = 0.017, chi-square tests) and blood vessel infiltration (P = 0.046, chi-square tests). The overall survival times of PDAC patients with low LIMS1 expression were significantly longer than those with high LIMS1 level (P = 0.022, log-rank test; Fig. 1F).
To further investigate the clinical significance of LIMS1 in PDAC progression, we analysed the mRNA sequencing profiles and clinical data of 179 PDAC patients from The Cancer Genome Atlas (TCGA). As shown in Supplementary Table S2, a high mRNA level of LIMS1 was significantly correlated with advanced tumor size (P = 0.044, unpaired t tests), T staging (P = 0.024, unpaired t tests), regional lymph node involvement (P = 0.028, unpaired t tests), differentiation (P = 0.012, unpaired t tests) and pTNM staging (P = 0.005, unpaired t tests). A cohort of 130 PDAC patients with a minimum follow-up time of 60 days was further studied. The overall survival times of PDAC patients with low LIMS1 mRNA levels (≤ 475.5 Reads Per Kilobase per Million mapped reads, RPKM) were significantly longer than those with high LIMS1 mRNA levels (> 475.5 RPKM) (P = 0.001, log-rank test; Fig. 1G). Importantly, a multivariate Cox’s regression analysis revealed that the LIMS1 expression level was an independent prognostic factor for the overall survival (HR, 1.682; 95% CI, 1.097–2.578; P = 0.0170) of patients with PDAC (Suppl Table S3)
The expression and clinical significance of LIMS1 were also examined in two other cancers. Our analysis of esophageal carcinoma and lung adenocarcinoma data revealed that LIMS1 was also frequently overexpressed in esophageal carcinoma (Suppl. Fig. S2A–B) and lung adenocarcinoma tissues (Suppl. Fig. S3A–B). High LIMS1 expression was significantly correlated with poor overall survival of patients (Suppl. Fig. S2C–D, Suppl. Fig. S3C–D), advanced tumor size, T staging, regional lymph node involvement and pTNM staging both in esophageal carcinoma (Supplementary Table S4) and lung adenocarcinoma (Suppl. Table S5).
LIMS1 facilitates cancer cell survival in oxygen-glucose deprivation conditions
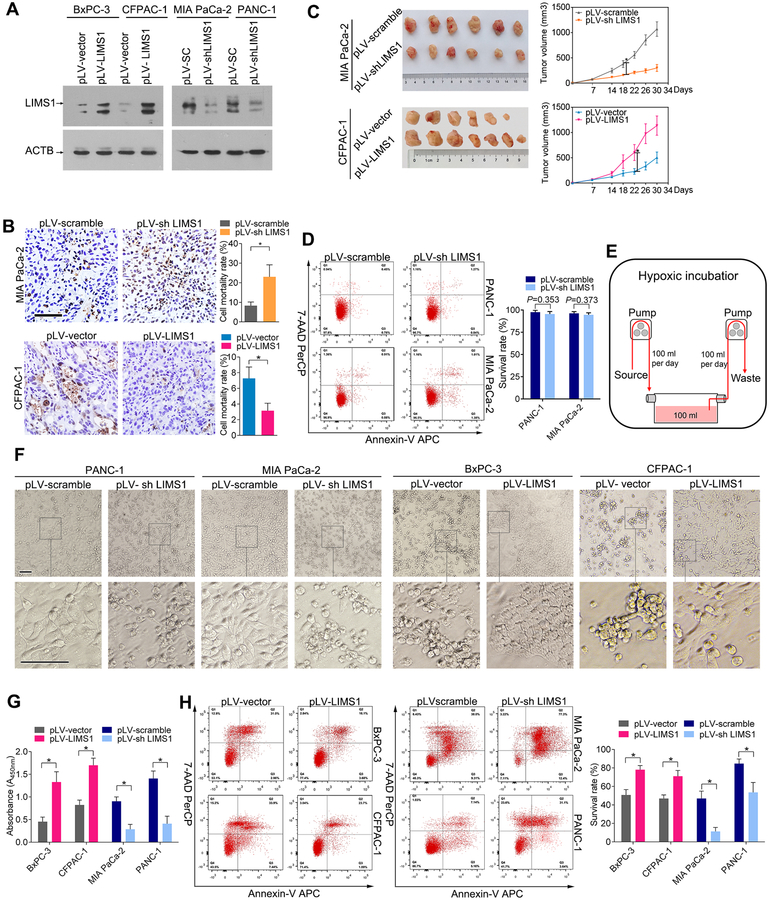
To explore the roles of LIMS1 in tumor progression, we established stable cell lines in which LIMS1 was overexpressed or knocked down (Fig. 2A). We subcutaneously transplanted PDAC cells with LIMS1 overexpression or knockdown into nude mice to generate tumors. The harvested tumors were assessed by a in situ cell death detection kit to determine the cell mortality rate. As shown in Fig. 2B, the cell mortality rate of MIA PaCa-2 tissues with LIMS1 knockdown was strongly elevated (8.33 ± 1.81 vs 23.01 ± 6.14; P < 0.0001, unpaired t test). In contrast, LIMS1 overexpression significantly reduced the mortality rate of CFPAC-1 tumors (7.28 ± 1.44 vs 3.16 ± 0.95; P = 0.0001, unpaired t test). Furthermore, LIMS1 knockdown resulted in reduced tumor growth, whereas LIMS1 overexpression significantly enhanced the tumor growth (Fig. 2C).
Figure 2. LIMS1 facilitates PDAC survival in the oxygen-glucose deprivation microenvironment.
A, the indicated PDAC cells were transfected with lentiviruses to upregulate or knock down LIMS1 expression. Western blotting were performed to verify the stable cell lines. B-C, the indicated tumor cells were subcutaneously transplanted into nude mice to develop tumors (n = 6~7 for each group). The harvested tumors were sliced and then detected by a in situ cell death detection kit to determine the cell mortality rate (B). Tumor size was measured weekly, and the tumor growth curve was generated based on the mean tumor volume (C). D, the indicated cells cultured in whole medium were stained with 7-AAD/Annexin-V and then subjected to flow cytometry analysis to determine the cell viability. E-H, the indicated cells were cultured in the oxygen-glucose deprivation conditions (glucose concentration, 0.75 mM; oxygen concentration, 1%). The incubation time varied from 24 hours to 72 hours. Schematic of the culture conditions (E); morphology of the cells was observed by an inverted phase contrast microscope (F); CCK8 assays (G) and flow cytometry analysis (H) were used to detect the cell viability. Unpaired t tests were used in B, C, D, G and H; data are shown as the mean value ± SD; *P < 0.05; Scale bars, 100 μm.
LIMS1 was previously shown to contribute to apoptosis resistance in cancer cells(22,23). However, surprisingly, under normal cell culture conditions, LIMS1 expression led to negligible effect on cell viability in cancer cells (Fig. 2D & Supplementary Fig. S4). Oxygen-glucose deprivation is a common characteristic of solid tumors(24). Therefore, we examined the effects of LIMS1 on PDAC cell survival under oxygen-glucose deprivation. We used a continuous-flow culture system to maintain cancer cells in the proliferative phase in a reduced but steady glucose concentration (0.75 mM) in a hypoxic incubator (1% oxygen) to mimic oxygen-glucose deprivation conditions (20) (Fig. 2E). As shown in Fig. 2F–2H, LIMS1 overexpression strongly enhanced the survival of BxPC-3 and CFPAC-1 cells in oxygen-glucose deprivation environments (unpaired t tests). In contrast, LIMS1 knockdown significantly reduced the survival of MIA PaCa-2 and PANC-1 cells (unpaired t tests). Similar results were observed in esophageal cancer cells (Supplementary Fig. S5) and lung adenocarcinoma cells (Supplementary Fig. S6), indicating a critical role of LIMS1 in tumor cell adaptation to oxygen-glucose deprivation.
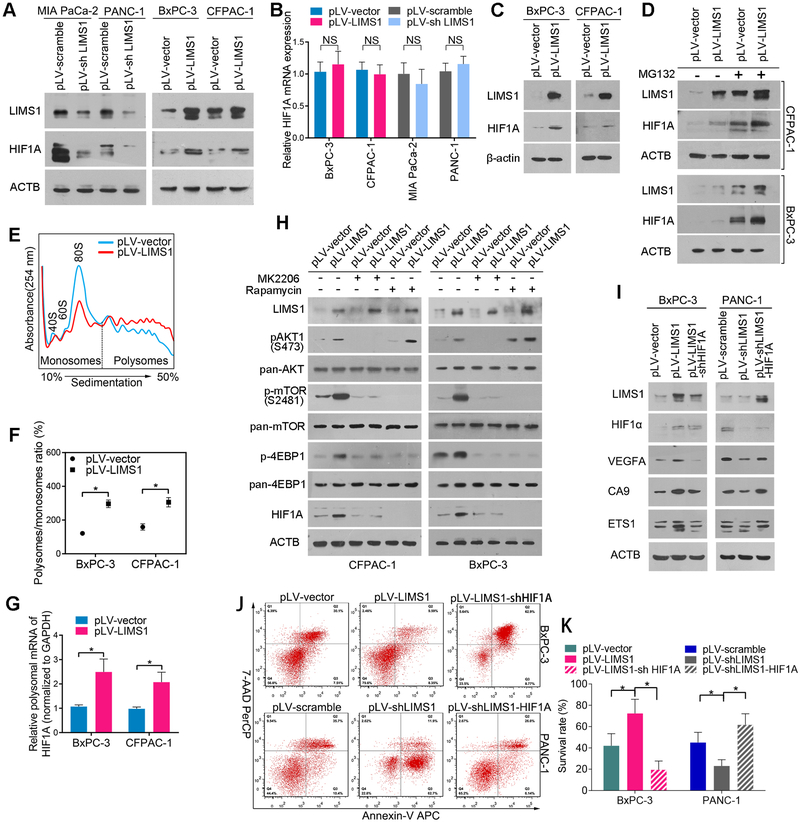
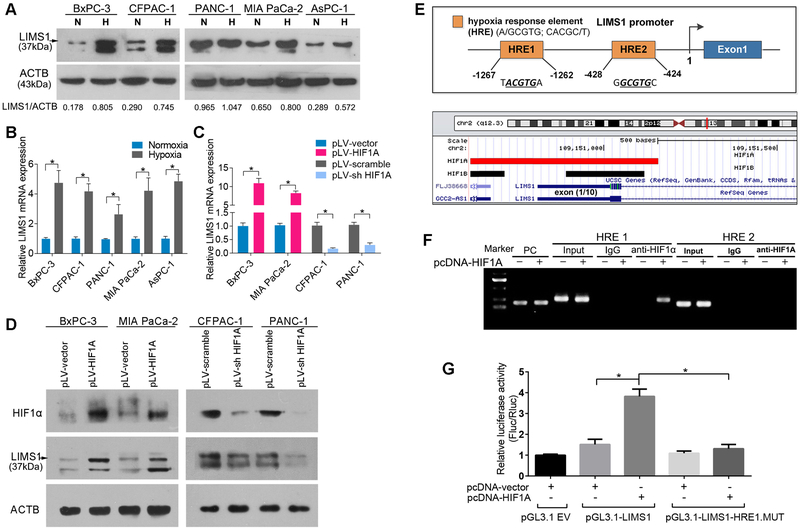
LIMS1 facilitates the response to hypoxic stress by upregulating HIF1A translation
HIF1 is a master regulator of tumor cell adaptation to oxygen-glucose deprivation. We found that LIMS1 knockdown substantially inhibited the HIF1A protein level, whereas LIMS1 overexpression significantly elevated the HIF1A protein level (Fig. 3A). Upregulation of HIF1A protein in tumor tissues could be achieved by promoting transcription, inhibiting proteasomal degradation or enhancing mRNA translation (25). The upregulation or knockdown of LIMS1 did not change the mRNA level of HIF1A (Fig. 3B; unpaired t tests), indicating that LIMS1 did not enhance HIF1A levels by facilitating the transcriptional activity. To determine the effects of LIMS1 on HIF1A proteasomal degradation, we detected the HIF1A protein level in the indicated cells cultured in hypoxic conditions. As shown in Fig. 3C, under hypoxic conditions, overexpression of LIMS1 still significantly enhanced the protein level of HIF1A. The proteasome inhibitor MG132 was used to prevent HIF1A degradation and LIMS1 still increased HIF1A expression (Fig. 3D). Furthermore, the results of pulse chase experiments demonstrated that LIMS1 expression did not alter the half-life of HIF1A (Supplementary Fig. S7). These results indicated that the regulation of HIF1A by LIMS1 was not through inhibiting proteasomal degradation.
Figure 3. LIMS1 facilitates the response to hypoxic stress by upregulating HIF1A translation.
A-B, the indicated PDAC cells with LIMS1 upregulation or downregulation were subjected to Western blotting (A) and RT-PCR (B) to detect the expression level of HIF1A. C, the indicated PDAC cells were incubated in hypoxic conditions (1% O2) for 48 hours and then subjected to Western blotting to detect the level of HIF1A. D, the indicated PDAC cells were incubated with or without 10 nM MG132 for 24 hours and then subjected to Western blotting to detect the level of HIF1A. E-G, polysome profiles from the indicated cells. Absorbance at 254 nm is shown as a function of sedimentation (E). The area under the curve for polysomes and the 80S peak were calculated, and the ratio is shown (F). The fractions of polysomes were mixed together, and the RNA of the mixture was isolated and subjected to real-time PCR assays to determine the polysomal mRNA level of HIF1A (G). H, the indicated PDAC cells were incubated with or without 10 nM MK2206/0.1 nM rapamycin and then subjected to Western blotting to detect the expression level of p-AKT1, p-mTOR (S2481), p-4EBP1 and HIF1A. The incubation times are as follows: MK2206, 24 hours; rapamycin, 30 minutes for detection of p-4EBP1 and 24 hours for detection of HIF1A. I, the indicated cells were subjected to Western blotting to detect the expression levels of three markers of HIF1 signalling: VEGFA, CA9 and ETS1. J-K, the indicated tumor cells were incubated in hypoxic conditions (oxygen concentration, 0.5%) for 48 hours and then subjected to flow cytometry analysis to detect cell viability. Unpaired t tests were used in B; data are shown as the mean value ± SD; *P < 0.05.
To determine whether LIMS1 regulates HIF1A through mRNA translation, we next examined the impact of LIMS1 expression on overall mRNA translation by studying polysome formation. The number of ribosomes engaged in polysomes is directly proportional to the translation initiation speed (26). Overexpression of LIMS1 led to an increase in the polysome/monosome ratio compared with the control treatment (Fig. 3E–3F). The polysomal mRNA levels of HIF1A were significantly elevated in LIMS1-overexpresing cells (Fig. 3G), suggesting that the translational activity of HIF1A was enhanced by LIMS1.
LIMS1 was previously shown to activate the PI3K/PKB/Akt1 signalling pathway(13), which plays essential roles in HIF1A translation(27). In Supplementary Figure S8, we confirmed that LIMS1 was crucial in maintaining AKT signalling activation in PDAC cells and tissues. Consequently, we explored whether LIMS1 promotes HIF1A translation by activating AKT signalling. In Fig. 3H, the data showed that LIMS1 overexpression enhanced the phosphorylation of AKT1, mTOR and 4EBP1. The AKT1 inhibitor MK2206 completely abrogated the LIMS1-induced phosphorylation AKT1, mTOR and 4EBP1, as well as LIMS1-induced HIF1A expression. Moreover, the mTOR inhibitor rapamycin also completely inhibited the LIMS1-induced phosphorylation mTOR and 4EBP1, as well as LIMS1-induced HIF1A expression. In vivo, MK2206 and rapamycin showed similar growth-inhibiting effects as well as LIMS1 knockdown (Suppl. Fig. S9). These results suggested that LIMS1 enhanced HIF1A translation by activating AKT1/mTOR/4EBP1 signalling.
When HIF1A was knocked down, the LIMS1-induced HIF1A signalling and survival were completely inhibited (Fig. 3I–3K), suggesting that LIMS1 played crucial roles in the response to hypoxic stress by activating HIF1A translation in tumor cells.
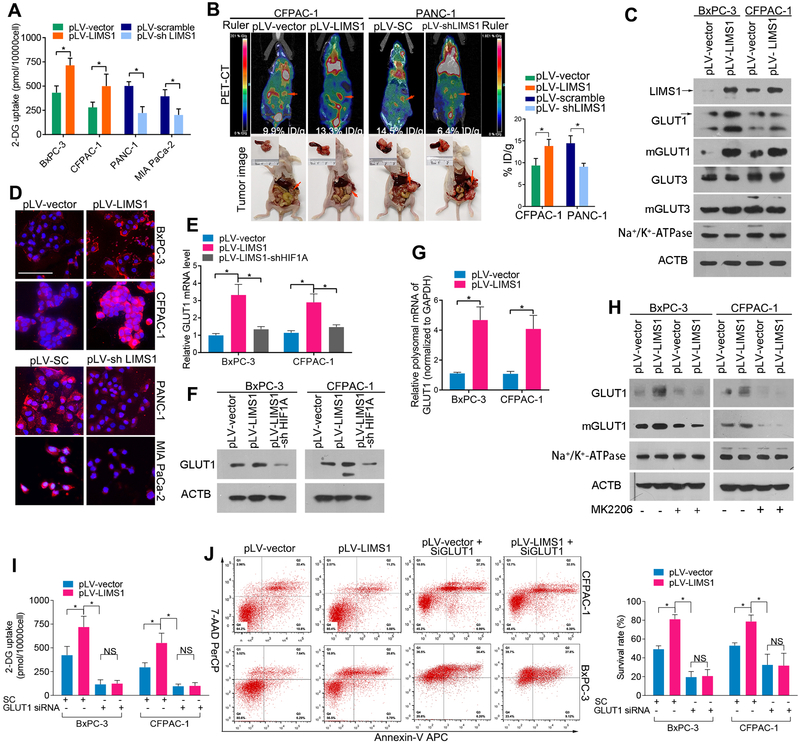
LIMS1 promotes glucose uptake by enhancing GLUT1 expression and membrane translocation
Since LIMS1 facilitates the survival of tumor cells in oxygen-glucose deprivation conditions, we examined the effects of LIMS1 on PDAC glucose uptake using 2-deoxyglucose (2-DG) in vitro. As shown in Fig. 4A, LIMS1 overexpression significantly increased the uptake of 2-DG in PDAC (unpaired t test). Conversely, LIMS1 knockdown substantially reduced the uptake of 2-DG in PDAC cells. This was well supported by the evidence that tumor cells with high LIMS1 expression had an elevated extracellular acidification rate in vitro (Suppl. Fig. S10). The role of LIMS1 in glucose uptake was also assessed in esophageal carcinoma cells (Suppl. Fig. S5B) and lung adenocarcinoma cells (Suppl. Fig. S6B). To determine whether LIMS1 regulates glucose uptake in vivo, we measured the uptake of 18fluorine-fluorodeoxyglucose (18FDG) by orthotopically implanted PDAC in nude mice by PET/CT scanning. Our data indicated that LIMS1 significantly enhanced the uptake of 18FDG (Fig. 4B; unpaired t tests) in orthotopic PDAC xenograft models. To determine whether LIMS1 correlates with glucose uptake in human cancer patients, we used PET/CT scanning to compare the standardised 18FDG uptake between LIMS1 high and LIMS1 low patients. As shown in Suppl. Fig. S11, PDAC, esophageal carcinoma and lung adenocarcinoma patients with high LIMS1 expression levels also had substantially higher standardised 18FDG uptake values. GLUT1 and GLUT3 are the two major glucose transporters that are overexpressed in cancer (28,29). Our data showed that LIMS1 enhanced the expression and membrane transport of GLUT1 but not GLUT3 in tumor cells (Fig. 4C–4D). Next, we investigated the mechanism underlying LIMS1 activation of GLUT1 expression to facilitate glucose uptake. We found that LIMS1 overexpression increased the mRNA and protein levels of GLUT1, and LIMS1-mediated upregulation of GLUT1 was inhibited by HIF1A knockdown, suggesting that LIMS1 increases GLUT1 transcription by activating HIF1 signalling (Fig. 4E–4F). Intriguingly, LIMS1 also increased the GLUT1 mRNA levels in polysomes (Fig. 4G), suggesting that GLUT1 translation was promoted by LIMS1. Both LIMS1-mediated GLUT1 transcription and translation were directly or indirectly mediated by AKT signalling, which was further confirmed by blocking assays. As shown in Fig. 4H, LIMS1-mediated GLUT1 upregulation was completely abrogated by the AKT inhibitor MK2206 (Fig. 4H). Additionally, when AKT signalling was inhibited by MK2206, the LIMS1-induced GLUT1 overexpression was inhibited(Fig. 4H). When GLUT1 expression was knocked down, the LIMS1-induced 2-DG uptake (Fig. 4I) and elevated tumor survival rate (Fig. 4J) were completely abrogated.
Figure 4. LIMS1 promotes glucose uptake by enhancing GLUT1 expression and membrane translocation.
A, a glucose uptake assay kit was used to detect the uptake of 2-deoxyglucose (2-DG) (structurally similar to glucose) in the indicated tumor cells in vitro. B, the indicated tumor cells were orthotopically transplanted into the nude mouse pancreases to develop tumors (n = 6 for each group). Four weeks later, the mice were intravenously injected with 18FDG, anaesthetised and then subjected to PET-CT scanning for 18FDG uptake in vivo. The representative images of PET-CT scans of each groups were shown (Left top). Then the abdomen of mice were opened to harvest the tumors. Representative images of tumors in situ were shown (Left bottom). The concentrations of 18FDG in tumor tissues were normalised to %ID/g (Right bottom). C, the indicated tumor cells were subjected to Western blotting to detect the expression levels of GLUT1, GLUT3, mGLUT1 and mGLUT3; Na+/K+-ATPase was used as a loading control for membrane protein; β-actin was used as a loading control for whole protein. D, immunofluorescence images showing the expression and localisation of GLUT1 in the indicated tumor cells. E-F, the indicated tumor cells were subjected to real-time PCR (E) and Western blotting (F) to detect GLUT1 levels. G, the polysomal mRNA level of GLUT1 in the indicated tumor cells was determined as in Fig. 3G. H, the indicated tumor cells were incubated with/without 10 nM MK2206 for 24 hours and then subjected to Western blotting to detect the levels of GLUT1 and mGLUT1.
I-J, the indicated tumor cells were transiently transfected with anti-GLUT1 silencing RNA (GLUT1 siRNA) or scramble control RNA (SC) and then subjected to glucose uptake assays (I) or incubated in glucose-deprivation conditions (glucose concentration, 0.75 M) for 48 hours and then subjected to flow-cytometry analysis to detect cell viability (J). Unpaired t tests were used in A, B, E, G, I and J; data are shown as the mean value ± SD; *P < 0.05; Scale bars, 100 μm.
These results indicated that LIMS1 played a crucial role in response to glucose deprivation stress by enhancing GLUT1 expression and membrane translocation.
HIF1 transactivates LIMS1 transcription
We next explored the mechanism underlying LIMS1 overexpression in cancer cells. We found that in hypoxic conditions, LIMS1 expression was significantly elevated (Fig. 5A–5B). To determine whether HIF1 transcriptionally regulates LIMS1 expression in cancer cells, we established stable cell lines in which HIF1A was overexpressed or knocked down (Fig. 5D). HIF1A knockdown significantly inhibited the mRNA and protein levels of LIMS1. Moreover, when HIF1A was overexpressed, LIMS1 expression was substantially increased (Fig. 5C–5D; unpaired t tests).
Figure 5. HIF1 transactivates LIMS1 transcription.
A-B, the indicated PDAC tumor cells were incubated in normoxic conditions (N) or hypoxic conditions (H; 1% O2) for 16 hours and subjected to Western blotting (A) and RT-PCR assays (B). C-D, the indicated PDAC tumor cells with HIF1A upregulated or knocked down were subjected to RT-PCR assays (C) and Western blotting (D). E, top: a HIF1 CHIP sequencing data profile (GEO-GSE59937) was downloaded and analysed in UCSC Genome Browser (http://genome.ucsc.edu/). Red rectangle, HIF1A binding site; black rectangle, HIF1β binding site; blue rectangle, the first exon of LIMS1. Bottom: schematic of the human LIMS1 promoter, two hypoxia responsive elements (HREs) were identified (UCSC gene ID: uc002teg.4.). F, CHIP analysis of PANC-1 cells transfected with or without HIF1A-overexpressing plasmids (pcDNA-HIF1A). Chromatin was immunoprecipitated with anti-HIF1A antibody and then subjected to PCR analysis. G, luciferase analysis of Panc-1 cells. Panc-1 cells transfected with pcDNA-HIF1A or control vector (pcDNA-vector) were transfected with pGL3-LIMS1-promoter, pGL3-LIMS1-promoter mutation (MUT), or pGL3-empty vectors (pGL3.1 EV). Forty-eight hours later, cells were subjected to dual luciferase analysis. The results are expressed as a fold induction relative to the cells transfected with the control vector (pcDNA3.1) after normalisation to Renilla activity. Unpaired t tests were used in B, C and G; data are shown as the mean value ± SD; *P < 0.05.
We analysed the promoter region of the human LIMS1 gene and identified two hypoxia response elements (HREs) (Fig. 5E, top). Then, we analysed a chromatin immunoprecipitation (CHIP) sequencing profile deposited in the GEO database (GEO-GSE59937; Fig. 5E, bottom) and found that both HIF1A and HIF1β can bind to the promoter region of LIMS1.
CHIP assays were performed in PANC-1 cells with or without HIF1A overexpression. In DNA fractions pulled down by an anti-HIF1A antibody, only the HRE of the LIMS1 promoter located at −1267 to −1262 was detected when HIF1A was upregulated (Fig. 5F), suggesting that HIF1A can bind to the promoter of LIMS1.
To determine whether HIF1A binding activates LIMS1 transcription, we constructed a full-length LIMS1 luciferase promoter vector (containing HRE1) and co-transfected this reporter with or without the HIF1A overexpression vector (pcDNA-HIF1A) into PANC-1 cells. Luciferase analysis showed that HIF1A overexpression substantially increased the transcriptional activity of the LIMS1 promoter. To determine whether HRE1 is necessary for HIF1A to transactivate the LIMS1 promoter, we mutated this HIF1A binding site from ACGTG to ACATG. As shown in Fig. 5G, the mutation of HRE1 almost abrogated the HIF1A-induced transactivation of the LIMS1 promoter.
Taken together, our data indicated that LIMS1 and HIF1 mutually upregulated the expression of each other, thus forming a positive feedback signalling loop.
The LIMS1-HIF1 positive feedback loop is pivotal to oxygen-glucose deprivation stress resistance
Given that LIMS1 and HIF1A mutually regulate the expression of each other, forming a positive feedback loop, we explored their relationship in vivo. First, the data from TCGA were analysed. As shown in Suppl. Fig. S12A, the correlation between the mRNA levels of LIMS1 and HIF1A reached 0.702 (P < 0.0001, Spearman correlation analysis). The LIMS1 mRNA level in HIF1A high patients was significantly higher than that in HIF1A low patients (Suppl. Fig. S12B; 564.80 ± 228.10 vs 290.06 ± 158.28; P < 0.0001, unpaired t test), suggesting that HIF1 increased LIMS1 expression in PDAC. Next, we examined the correlation between the protein levels of LIMS1 and HIF1A by immunohistochemical staining in a cohort of 114 PDAC specimens. As shown in Suppl. Fig. S12C, LIMS1 expression co-localised with HIF1A in consecutive sections of the PDAC tissues. LIMS1 overexpression lead to elevated HIF1A expression both in normoxia area and hypoxia area of tumor tissues (Suppl. Fig. S13). The LIMS1 expression level in PDAC tissues was significantly associated with the HIF1A expression level (r = 0.505, P < 0.0001, Spearman correlation analysis), i.e., tumor tissues with a high LIMS1 level usually had high HIF1A expression (Suppl. Fig. S12D–E). Then, in transplanted tumor tissues from mice, HIF1A overexpression significantly increased LIMS1 level and vice versa (Suppl. Fig. S12F).
We then explored the roles of the HIF1-LIMS1 loop under glucose-oxygen deprivation conditions, a characteristic stressor in solid tumors. The ectopic expression of HIF1A significantly promoted PDAC cell survival, and HIF1A knockdown strongly inhibited survival under oxygen-glucose deprivation stress. The pro-survival effects of HIF1A were abrogated by LIMS1 knockdown. LIMS1 overexpression in HIF1A knockdown cells rescued the survival under oxygen-glucose deprivation (Suppl. Fig. S12G; unpaired t test). These data indicated that LIMS1 is crucial for HIF1A-mediated resistance to oxygen-glucose deprivation. We further examined the HIF1-LIMS1 loop in oxygen-glucose deprivation resistance in vivo. The results of in vivo experiments were consistent with those of in vitro assays. LIMS1 knockdown abrogated the HIF1-induced increase in cell survival and tumor growth (Suppl. Fig. S12H & I; unpaired t test), whereas LIMS1 overexpression relieved the inhibitory effect of HIF1A knockdown on cell survival and tumor growth (Suppl. Fig. S12H & J; unpaired t test). Vice versa, HIF1A had important roles in LIMS1-induced tumor growth in mice models (Suppl. Fig. S14).
Taken together, these results suggested that the HIF1-LIMS1 positive feedback loop was pivotal for cancer cells to survive in the oxygen-glucose deprivation microenvironment.
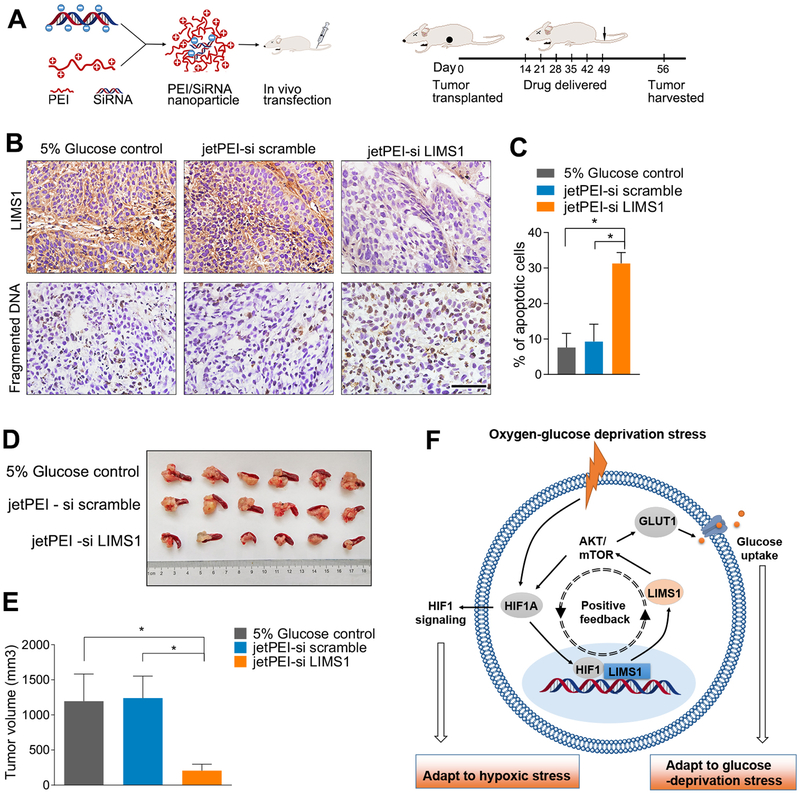
LIMS1-targeted therapy strikingly inhibits tumor growth in vivo
To investigate the anti-tumor effects of LIMS1-targeted therapy in vivo, we used a jetPEI nanocarrier (30,31) as the vehicle for anti-LIMS1 siRNAs to inhibit LIMS1 expression in mouse models (Fig. 6A). Our data showed that the jetPEI-delivered anti-LIMS1 siRNA reagent efficiently inhibited LIMS1 expression (Fig. 6B). The inhibition of LIMS1 blocked AKT/mTOR signal pathway and resulted in decreased HIF1A and GLUT1 expression in tumor tissues (Supplementary Figure S15), leading to an remarkable increase of cell mortality (Fig. 6C; unpaired t test) and significant tumor inhibitory effect (Fig. 6D–6E; unpaired t test). These results suggested that LIMS1 could be a promising therapeutic target for cancer therapy.
Figure 6. jetPEI nanocarrier-delivered anti-LIMS1 siRNAs significantly inhibit tumor growth in PDAC mouse models.
A, jetPEI nanocarrier was used as the vehicle of anti-LIMS1 siRNAs to inhibit LIMS1 expression in vivo. Schematic of the nanoparticle (left); the time line of the drug administration (right). B-D, CFPAC-1 cells were orthotopically transplanted into the nude mice to develop tumors (n = 6 for each group). jetPEI-si scramble or jetPEI anti-LIMS1 siRNAs nanoparticles were intravenously injected into mice. The 5% glucose group was used as a control group. When the mice were sacrificed, the tumors were sliced and then detected by a in situ cell death detection kit to determine the cell mortality rate (B-C). The tumor volumes were analysed (D-E). F, schematic of the roles of LIMS1 in mediating tumor cell survival in oxygen-glucose deprivation conditions. The hypoxia-glucose deprivation microenvironment is a general feature of solid tumors. In hypoxic conditions, HIF1 directly activates the transcription of LIMS1. However, by activating AKT-mTOR signalling, LIMS1 increases the translation of HIF1A in response to hypoxic stress. LIMS1 and HIF1 form a positive feedback loop, which is essential for the abnormally elevated levels of LIMS1 and HIF1 in tumors. More importantly, by activating AKT signalling and HIF1A translation, LIMS1 enhances GLUT1 production and membrane translocation, promotes glucose uptake and facilitates tumor cell survival in the glucose deprivation environment. Unpaired t tests were used in B and C; data are shown as the mean value ± SD; *P < 0.05; Scale bars, 100 μm.
Discussion
In solid tumors, the poorly formed tumor vasculature and the elevated glucose consumption result in a characteristic oxygen-glucose deprivation microenvironment. To survive, cancer cells have evolved complicated regulatory network to adapt to the harsh conditions.
In this study, we identified LIMS1 as a critical regulator of tumor cell resistance to oxygen-glucose deprivation. LIMS1 can form a ternary protein complex with integrin-linked kinase and α-parvin in focal complexes and focal adhesions (10). LIMS1 is required for the maintenance of ILK protein expression level and is indispensable for some integrin-dependent functions (11,32). Although LIMS1 has been implicated in cell spreading (11) and survival (22), the roles of LIMS1 in cancer progression are poorly understood. Here, we demonstrated that LIMS1 expression was significantly elevated in multiple cancer tissues, including pancreatic adenocarcinoma, lung adenocarcinoma and esophageal carcinoma. High levels of LIMS1 correlated with advanced tumor size, tumor staging, nodal involvement and poor survival of tumor patients. Although knockdown of LIMS1 had negligible effects on cancer cell apoptosis under normal culture conditions, LIMS1 was critical for cancer cell survival under oxygen-glucose deprivation.
Mechanistically, LIMS1 enhances glucose uptake by increasing the expression levels of GLUT1 (33), possibly through ILK-mediated phosphorylation and activation of PKB/AKT (34). LIMS1 was shown to be crucial for the stability and localisation of ILK (10) and is important for ILK-induced phosphorylation of AKT (15,32). Our results showed that when AKT signalling was inhibited, the LIMS1-induced glucose uptake was completely abrogated, indicating that LIMS1-induced glucose uptake is mediated by AKT/GLUT1 signalling.
In the oxygen-glucose deprivation microenvironment, HIF1 signalling is another master regulator that plays crucial roles in tumor angiogenesis, tumor metastasis and survival.
There are complicated correlations between HIF1 signalling and the AKT pathway. PKB/AKT was shown to be activated by hypoxia in various tumor types (35). Several studies demonstrated that hypoxia-induced resistance to apoptosis was largely mediated by the AKT pathway (36,37). Nevertheless, how AKT signalling is activated by hypoxia is currently unclear. In this study, we reported a new link between these two pathways: the HIF1-LIMS1-AKT signalling-HIF1 axis transcriptionally initiated LIMS1 expression to activate AKT signalling. Additionally, the hypoxia-induced AKT pathway activation is cell-specific (38). This might be due to the differential expression of LIMS1 in different tumor cells. AKT signalling is considered the major regulator of HIF1α synthesis in cancer cells (39). AKT initiates the mTOR pathway, which regulates HIF1A protein biosynthesis by regulating cap-dependent translation and ribosome biogenesis (40). This was further verified by the evidence that LIMS1 did not have a direct interaction with the basal translational apparatus and did not affect HIF1A translation through the Cap-independent translation (Supplementary Figure S16). Our data showed that LIMS1 overexpression significantly increased the protein level of HIF1A in tumor cells. When LIMS1 was knocked down, HIF1A expression was substantially reduced, suggesting that LIMS1 plays crucial roles in HIF1A expression. Instead of promoting the transcription and stabilisation of HIF1α, LIMS1 facilitates the translation of HIF1A, which is mediated by the AKT/mTOR pathway. Thus, LIMS1 and HIF1 form a positive feedback loop, which is a crucial link between HIF1 and AKT signalling.
As a transcription factor, HIF1 can initiate the transcription of GLUT1 and facilitate glucose uptake in the oxygen-glucose deprivation microenvironment (24). In this study, we found that LIMS1 promoted GLUT1 transcription via HIF1A and translation by activating AKT signalling. LIMS1/AKT signalling was important in maintaining the HIF1A translational expression in tumor cells. LIMS1 overexpression significantly rescued the inhibitory effect of HIF1A knockdown on GLUT1 expression. These results suggest that LIMS1/AKT signalling strongly contributes to GLUT1 expression and glucose uptake in tumor cells. Hence, LIMS1 is probably a key molecule in maintaining glucose homeostasis of cancer cells in the oxygen-glucose deprivation microenvironment.
The important roles of LIMS1 in glucose uptake of tumor cells were verified in vivo. When we inhibited LIMS1 expression, the tumor cell viability and growth speed decreased significantly, suggesting that LIMS1 could be a promising therapeutic target for cancer treatment. However, as LIMS1 is not ubiquitously overexpressed in all tumor tissues, anti-LIMS1 therapy might only be efficacious in tumor patients with LIMS1 overexpression. As the SUVmax in PET-CT scanning of tumor patients was positively correlated with the expression level of LIMS1 in tumor tissues, PET-CT scanning is a potential non-invasive approach to identify the potential beneficiaries of anti-LIMS1 therapy. Further investigations are needed to verify this hypothesis.
Several limitations to the present study are noted. As no anti-LIMS1 inhibitors are currently available, in the translational experiments, we used the anti-LIMS1 siRNA to inhibit LIMS1 expression in vivo. Although the anti-LIMS1 siRNA significantly inhibited LIMS1 expression and tumor growth, siRNAs were not yet an ideal agent for clinical usage. Specific inhibitors for LIMS1 are demanded to verify these results before launch a clinical study. Second, as a single agent LIMS1 might be not strong enough to suppress tumor in clinical application, considering pancreatic cancer is such a tenacious disease. The combination of anti- LIMS1 agents and chemotherapy drugs might be more effective for curing pancreatic cancer. Further investigations are needed to confirm this.
Supplementary Material
Translational relevance.
Pancreatic ductal adenocarcinoma (PDAC) is a deadly disease. The mechanisms underlying these aggressive behaviors are poorly understood. In the present study, we demonstrated that LIMS1 was highly expressed in tumor tissues, and LIMS1 overexpression was correlated with advanced TNM staging and poor survival of cancer patients. We further identified a novel mechanistic role of LIMS1 in promoting tumor cell survival in the oxygen-glucose-deprived microenvironment by activating AKT/mTOR signaling and enhancing HIF1A protein translation. Our findings suggested that LIMS1 signaling promoted PDAC cell survival under oxygen-glucose deprivation conditions. Targeting LIMS1 signaling may be an effective therapeutic strategy for PDAC. Therefore, our study may have a significant effect on clinical management of PDAC.
Acknowledgments
Financial support: This work was supported in part by the National Natural Science Foundation of China (grants 81602017, 81772633, 81720108028, 81525021, 81502067, 81302082, 81272685, 31301151, 81172355, 31471340, 31470957, 81472264 and 81401957), the Natural Science Foundation of Tianjin (grants 16JCQNJC09900, 13YCYBYC37400 and 11JCZDJC18400); by the NIH grants R01CA175741 (Shengyu Yang); and R01CA173322 and CA220236 (Keping Xie).
Footnotes
Competing Interests: No potential conflicts of interest were disclosed.
References
- 1.Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clinical and Experimental Metastasis 2003;20:237–50 [DOI] [PubMed] [Google Scholar]
- 2.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nature Reviews Cancer 2008;8:705–13 [DOI] [PubMed] [Google Scholar]
- 3.Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, et al. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature 2014;508:108–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nature reviews Drug discovery 2003;2:803–11 [DOI] [PubMed] [Google Scholar]
- 5.Fulda S, Debatin KM. HIF-1-regulated glucose metabolism: a key to apoptosis resistance? Cell Cycle 2007;6:790–2 [DOI] [PubMed] [Google Scholar]
- 6.Cairns RA, Papandreou I, Sutphin PD, Denko NC. Metabolic targeting of hypoxia and HIF1 in solid tumors can enhance cytotoxic chemotherapy. Proceedings of the National Academy of Sciences of the United States of America 2007;104:9445–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prigione A, Rohwer N, Hoffmann S, Mlody B, Drews K, Bukowiecki R, et al. HIF1α modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1–3 and PKM2. Stem Cells 2014;32:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rearden A A new LIM protein containing an autoepitope homologous to” senescent cell antigen”. Biochemical and biophysical research communications 1994;201:1124–31 [DOI] [PubMed] [Google Scholar]
- 9.Hobert O, Moerman DG, Clark KA, Beckerle MC, Ruvkun G. A conserved LIM protein that affects muscular adherens junction integrity and mechanosensory function in Caenorhabditis elegans. The Journal of cell biology 1999;144:45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legate KR, Montañez E, Kudlacek O, Füssler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nature reviews Molecular cell biology 2006;7:20–31 [DOI] [PubMed] [Google Scholar]
- 11.Ito S, Takahara Y, Hyodo T, Hasegawa H, Asano E, Hamaguchi M, et al. The roles of two distinct regions of PINCH-1 in the regulation of cell attachment and spreading. Molecular biology of the cell 2010;21:4120–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu Y, Li F, Wu C. Nck-2, a novel Src homology2/3-containing adaptor protein that interacts with the LIM-only protein PINCH and components of growth factor receptor kinase-signaling pathways. Molecular biology of the cell 1998;9:3367–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dougherty GW, Jose C, Gimona M, Cutler ML. The Rsu-1-PINCH1-ILK complex is regulated by Ras activation in tumor cells. European journal of cell biology 2008;87:721–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bock-Marquette I, Saxena A, White MD, DiMaio JM, Srivastava D. Thymosin β4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 2004;432:466–72 [DOI] [PubMed] [Google Scholar]
- 15.Eke I, Koch U, Hehlgans S, Sandfort V, Stanchi F, Zips D, et al. PINCH1 regulates Akt1 activation and enhances radioresistance by inhibiting PP1α. The Journal of clinical investigation 2010;120:2516–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmqvist A, Gao J, Holmlund B, Adell G, Carstensen J, Langford D, et al. PINCH is an independent prognostic factor in rectal cancer patients without preoperative radiotherapy--a study in a Swedish rectal cancer trial of preoperative radiotherapy. BMC cancer 2012;12:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang M, Wang X, Wang H, Dong J, Lan C, Hao J, et al. Arsenic trioxide plus PX-478 achieves effective treatment in pancreatic ductal adenocarcinoma. Cancer letters 2016;378:87–96 [DOI] [PubMed] [Google Scholar]
- 18.Huang C, Li N, Li Z, Chang A, Chen Y, Zhao T, et al. Tumour-derived Interleukin 35 promotes pancreatic ductal adenocarcinoma cell extravasation and metastasis by inducing ICAM1 expression. Nature communications 2017;8:14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao T, Ren H, Li J, Chen J, Zhang H, Xin W, et al. LASP1 is a HIF1alpha target gene critical for metastasis of pancreatic cancer. Cancer Res 2015;75:111–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, et al. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature 2014;508:108–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsson O, Morita M, Topisirovic I, Alain T, Blouin MJ, Pollak M, et al. Distinct perturbation of the translatome by the antidiabetic drug metformin. Proceedings of the National Academy of Sciences of the United States of America 2012;109:8977–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen K, Tu Y, Zhang Y, Blair HC, Zhang L, Wu C. PINCH-1 regulates the ERK-Bim pathway and contributes to apoptosis resistance in cancer cells. Journal of Biological Chemistry 2008;283:2508–17 [DOI] [PubMed] [Google Scholar]
- 23.Montanez E, Karaköse E, Tischner D, Villunger A, Fässler R. PINCH-1 promotes Bcl-2-dependent survival signalling and inhibits JNK-mediated apoptosis in the primitive endoderm. J Cell Sci 2012;125:5233–40 [DOI] [PubMed] [Google Scholar]
- 24.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nature Reviews Cancer 2008;8:705–13 [DOI] [PubMed] [Google Scholar]
- 25.Zhou J, Schmid T, Frank R, Brune B. PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1alpha from pVHL-independent degradation. The Journal of biological chemistry 2004;279:13506–13 [DOI] [PubMed] [Google Scholar]
- 26.Larsson O, Morita M, Topisirovic I, Alain T, Blouin MJ, Pollak M, et al. Distinct perturbation of the translatome by the antidiabetic drug metformin. Proceedings of the National Academy of Sciences of the United States of America 2012;109:8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semenza GL. Targeting HIF-1 for cancer therapy. Nature Reviews Cancer 2003;3:721. [DOI] [PubMed] [Google Scholar]
- 28.Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. Journal of cellular physiology 2005;202:654–62 [DOI] [PubMed] [Google Scholar]
- 29.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nature Reviews Cancer 2004;4:891–9 [DOI] [PubMed] [Google Scholar]
- 30.Bolcato-Bellemin A-L, Bonnet M-E, Creusat G, Erbacher P, Behr J-P. Sticky overhangs enhance siRNA-mediated gene silencing. Proceedings of the National Academy of Sciences 2007;104:16050–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poeck H, Besch R, Maihoefer C, Renn M, Tormo D, Morskaya SS, et al. 5′-Triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. Nature medicine 2008;14:1256–63 [DOI] [PubMed] [Google Scholar]
- 32.Fukuda T, Chen K, Shi X, Wu C. PINCH-1 is an obligate partner of integrin-linked kinase (ILK) functioning in cell shape modulation, motility, and survival. Journal of Biological Chemistry 2003;278:51324–33 [DOI] [PubMed] [Google Scholar]
- 33.Kim D, Chung J. Akt: versatile mediator of cell survival and beyond. Journal of biochemistry and molecular biology 2002;35:106–15 [DOI] [PubMed] [Google Scholar]
- 34.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proceedings of the National Academy of Sciences 1998;95:11211–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stegeman H, Span PN, Peeters WJ, Verheijen MM, Grénman R, Meijer TW, et al. Interaction between hypoxia, AKT and HIF-1 signaling in HNSCC and NSCLC: implications for future treatment strategies. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S-M, Lee C-T, Kim YW, Han SK, Shim Y-S, Yoo C-G. Hypoxia confers protection against apoptosis via PI3K/Akt and ERK pathways in lung cancer cells. Cancer letters 2006;242:231–8 [DOI] [PubMed] [Google Scholar]
- 37.Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E, et al. Hypoxia inhibits paclitaxel-induced apoptosis through adenosine-mediated phosphorylation of bad in glioblastoma cells. Molecular pharmacology 2007;72:162–72 [DOI] [PubMed] [Google Scholar]
- 38.Alvarez-Tejado M, Alfranca A, Aragonés J, Vara A, Landázuri MO, del Peso L. Lack of evidence for the involvement of the phosphoinositide 3-kinase/Akt pathway in the activation of hypoxia-inducible factors by low oxygen tension. Journal of Biological Chemistry 2002;277:13508–17 [DOI] [PubMed] [Google Scholar]
- 39.Semenza GL. Targeting HIF-1 for cancer therapy. Nature reviews cancer 2003;3:721–32 [DOI] [PubMed] [Google Scholar]
- 40.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, et al. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Molecular and cellular biology 2002;22:7004–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.