Summary
Background
The relation between maternal pre-pregnancy obesity and preterm birth is controversial and inconclusive. We aimed to clarify the association between pre-pregnancy obesity and preterm birth by maternal age and race or ethnicity in a large, multiracial, multiethnic, and diverse population in the USA.
Methods
We did a population-based cohort study using nationwide birth certificate data from the US National Vital Statistics System for 2016 and 2017. We included all mothers who had a live singleton birth and who did not have preexisting hypertension or diabetes. Pre-pregnancy obesity was defined as a pre-pregnancy BMI of at least 30 kg/m2. Preterm birth was defined as gestational age of less than 37 weeks. We used logistic regression models adjusted for maternal age, race or ethnicity, parity, education levels, smoking during pregnancy, previous history of preterm birth, marital status, infant sex, and timing of initiation of prenatal care to estimate the odds ratio (OR) of preterm birth.
Findings
We included 7 141 630 singleton livebirths in our analysis, 527 637 (7·4%) of which were preterm births. 127 611 (7·5%) Hispanic mothers, 244 578 (6·6%) non-Hispanic white mothers, and 102 509 (10·4%) non-Hispanic black mothers had preterm births. In the overall population, maternal pre-pregnancy obesity was significantly associated with an increased risk of preterm birth compared with maternal pre-pregnancy healthy weight (ie, BMI of 18·5–24·9 kg/m2; adjusted OR 1·18 [95% CI 1·18–1·19]). In non-Hispanic white women, maternal obesity was inversely associated with preterm birth among those younger than 20 years (adjusted OR 0·92 [95% CI 0·88–0·97]), but positively associated with preterm birth among those aged 20 years or older (1·04 [1·01–1·06], 1·20 [1·18–1·23], 1·34 [1·31–1·37], 1·40 [1·36–1·43], and 1·39 [1·31–1·46] among those aged 20–24 years, 25–29 years, 30–34 years, 35–39 years, and ≥40 years, respectively). In Hispanic women, maternal obesity was not associated with preterm birth among those younger than 20 years (0·98 [0·93–1·04]), but positively associated with preterm birth among those aged 20 years or older (1·06 [1·03–1·09], 1·21 [1·17–1·24], 1·32 [1·28–1·36], 1·38 [1·33–1·43], and 1·30 [1·22–1·40] among those aged 20–24 years, 25–29 years, 30–34 years, 35–39 years, and ≥40 years, respectively). In non-Hispanic black women, maternal obesity was inversely associated with preterm birth among those younger than 30 years (0·76 [0·71–0·81] in those <20 years, 0·83 [0·80–0·86] in those aged 20–24 years, and 0·98 [0·95–1·01] among those aged 25–29 years), but positively associated with preterm birth among those aged 30 years or older (1·15 [1·11–1·19], 1·26 [1·20–1·32], and 1·29 [1·18–1·42] among those aged 30–34 years, 35–39 years, and ≥40 years, respectively).
Interpretation
Maternal pre-pregnancy obesity is significantly associated with the risk of preterm birth in the general population, but the risk differs according to maternal age and race or ethnicity. Future investigation is warranted to understand the underlying mechanisms.
Funding
US National Institutes of Health.
Introduction
Preterm birth is a public health and clinical concern. It is the leading cause of infant mortality and neonatal morbidity, and is associated with long-term adverse outcomes in children.1 Although the incidence of preterm birth declined between 2007 and 2014, a report from the US National Center for Health Statistics showed that the frequency of preterm birth rose slightly from 2014 to 2017 in the USA.2 In view of the important contribution of preterm birth to infant mortality, identification of risk factors for preterm birth is imperative.
Unlike non-modifiable risk factors—such as race, ethnicity, and genetic predisposition—pre-pregnancy obesity is a potentially modifiable and preventable cause of adverse neonatal outcomes.3 Maternal obesity increases the risk of gestational diabetes, hypertensive disorders of pregnancy, and fetal malformations, all of which can contribute to medically indicated preterm birth.1,4 However, the association between pre-pregnancy obesity and preterm birth is controversial and inconclusive.3,5 Although pre-pregnancy obesity was a risk factor for preterm birth in some studies,6 in others null7 or even inverse8 associations. between pre-pregnancy obesity and preterm birth were noted. Several studies9,10 suggest that the association between pre-pregnancy obesity and preterm birth could differ between white and black populations—ie, a positive association in white populations and an inverse association in black populations. Additionally, the association between pre-pregnancy obesity and preterm birth could differ by maternal age. In the four studies11–14 that have been done in teenage mothers, there was an inverse association between pre-pregnancy obesity and preterm birth.
Although the underlying mechanisms have yet to be elucidated, the effects of maternal age, race, and ethnicity on the association between pre-pregnancy obesity and preterm birth need to be explored further in a large, diverse dataset with substantial available information for potential confounding factors. Addressing this question is important to enable physicians to provide tailored advice and prenatal care to mothers before and during pregnancy. In this study, we used national birth certificate data in the USA to examine the association between pre-pregnancy obesity and preterm birth.
Methods
Study design and data sources
In this US population-based study of mother–child pairs, we used data from the National Vital Statistics System, a major cooperative effort between state and federal agencies that collects nationwide data on births, deaths, marriages, and other events. NVSS uses two uniform documents (a facility worksheet and a maternal worksheet) to collect data for all livebirths in the 50 US states and the District of Columbia. Medical and health information is taken from medical records, and data about maternal characteristics, such as demographics, pre-pregnancy weight and height, and smoking during pregnancy, are taken from the mother using the maternal worksheet, which is usually completed by hospital staff.
Since 2016, the 2003 revised standard birth certificate for livebirths, in which more information about socio-demographic and health information were added, has been fully implemented nationwide. In this study, we used birth data for Jan 1, 2016, to Dec 31, 2017, from the NVSS 2016–17, because data for pre-pregnancy BMI were not recorded on birth certificates before the 2003 version. We included all mothers who had a live singleton birth and available data for pre-pregnancy BMI and gestational age at birth. Mothers with pre-existing hypertension or diabetes were excluded, because these diseases are strong risk factors for adverse birth outcomes, including preterm birth. This study followed the reporting guidelines in the Strengthening the Reporting of Observational Studies in Epidemiology statement. Because these records are publicly available and the data are de-identified, this study was exempt from institutional review board approval.
Procedures
Maternal pre-pregnancy weight and height were used by the NVSS to calculate pre-pregnancy BMI. Maternal pre-pregnancy BMIs were classified as underweight (ie, BMI <18·5 kg/m2), healthy (18·5–24·9 kg/m2), overweight (25·0–29·9 kg/m2), or obese (≥30·0 kg/m2) in line with the US National Heart, Lung, and Blood Institute’s categories. Obesity was further subdivided into class 1 (ie, BMI 30·0–34·9 kg/m2), class 2 (35·0–39·9 kg/m2), or class 3 (≥40·0 kg/m2) obesity.
Gestational age was calculated on the basis of the obstetric estimate of gestation at delivery, which was defined as the best obstetric estimate of the infant’s gestation in completed weeks based on the birth attendant’s final estimate of gestation.15 Preterm birth, which was defined as delivery occurring before 37 weeks of gestation, was the main outcome of interest.16 We further subdivided this outcome into three groups: extremely preterm birth (ie, <28 weeks’ gestation), very preterm birth (28–31 weeks’ gestation), and moderately preterm birth (32–36 weeks’ gestation).16
Statistical analysis
The rates of preterm birth according to population characteristics were calculated. Logistic regressions were used to estimate the odds ratios (ORs) of preterm birth and 95% CI. Maternal age (<20 years, 20·0–24·9 years vs 25·0–29·9 years vs 30·0–34·9 years vs 35·0–39·9 years vs ≥40·0 years), race or ethnicity (Hispanic vs non-Hispanic black vs non-Hispanic white vs other [which included Non-Hispanic Native American or Alaskans, non-Hispanic Asians, non-Hispanic Native Hawaiians or other Pacific Islanders, non-Hispanic people of more than one race, people of unknown racial or ethnic origin, or not stated]), parity (one child vs two children vs three children vs four or more children), maternal education level (less than high school vs high school vs more than high school), smoking status during pregnancy (yes vs no), previous history of preterm birth (yes vs no vs nulliparous), marital status (yes vs no), infant sex (boy vs girl), and timing of initiation of prenatal care (during months 1–3 vs months 4–6 vs month 7 or later vs no prenatal care) were covariates that we adjusted for in our analyses. We also calculated ORs and 95% CIs for the subgroups of preterm birth that we defined.
We did stratified analyses according to maternal age and race or ethnicity to explore potential disparities in the association between pre-pregnancy obesity and preterm birth. To further clarify the association, we did analyses to detect the joint effect in each age group by race or ethnicity subgroup. Additionally, we did a series of sensitivity analyses by excluding women who had caesarean sections, in whom labour was induced, with pregnancy-induced hypertension or pre-eclampsia, or with gest ational diabetes, and women who had infants who were small for gestational age, to assess the effects of these complicated pregnancy conditions on the association between pre-pregnancy obesity and preterm birth (appendix p 1). To examine the effect of missing covariate data in our analysis, we did a sensitivity analysis in which women with incomplete covariate data were omitted. Two-sided p values less than 0·05 were considered significant. All analyses were done in SAS (version 9.4).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
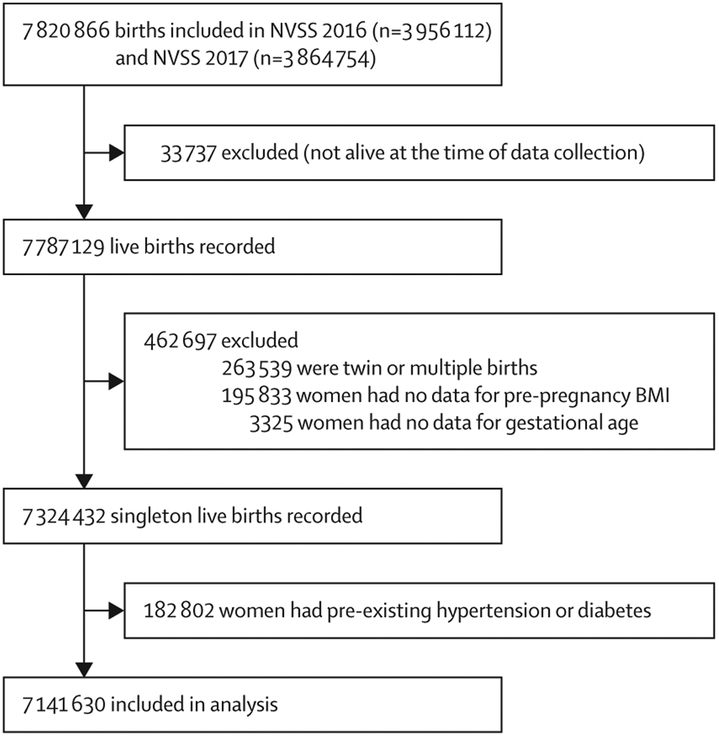
Our final sample consisted of 7 141 630 live singleton births (figure 1), including 527 637 (7·4%) preterm births. The proportion of births that were preterm was higher among women younger than 20 years or older than 40 years than among other age groups, non-Hispanic black women than among other races or ethnicities, unmarried women than among married women, women with less education than among those with more education, women who smoked during pregnancy than among those who did not, women who did not have prenatal care than among those who did, and women with BMIs lower than 18·5 kg/m2 or higher than 40 kg/m2 than among those with other BMIs (table 1). 1 825 112 (25·6%) women had pre-pregnancy obesity. The prevalence of each BMI category by population characteristics is depicted in the appendix (pp 2–4).
Figure 1: Participant flow chart.
NVSS=US National Vital Statistics System.
Table 1:
Prevalence of preterm birth in the study population
| N | Preterm birth | Moderately preterm birth |
Very preterm birth |
Extremely preterm birth |
|
|---|---|---|---|---|---|
| Overall | 7141 630 | 527 637 (7·4%) | 458 916 (6·4%) | 43 690 (0·6%) | 25 031 (0·4%) |
| Age, years | |||||
| <20 | 384 699 | 33 473 (8·7%) | 28 312 (7·4%) | 3064 (0·8%) | 2097 (0·5%) |
| 20–24 | 1465 686 | 111 116 (7·6%) | 96 143 (6·6%) | 9379 (0·6%) | 5594 (0·4%) |
| 25–29 | 2 099 891 | 144 982 (6·9%) | 126 58 (6·0%) | 11 619 (0·6%) | 6805 (0·3%) |
| 30–34 | 2 003 581 | 137 853 (6·9%) | 120 628 (6·0%) | 11 104 (0·6%) | 6081 (0·3%) |
| 35–39 | 977 659 | 78 532 (8·0%) | 68 490 (7·0%) | 6510 (0·7%) | 3532 (0·4%) |
| ≥40 | 210 114 | 21 681 (10·3%) | 18 785 (8·9%) | 1974 (0·9%) | 922 (0·4%) |
| Race or ethnicity | |||||
| Hispanic | 1692 126 | 127 611 (7·5%) | 111 603 (6·6%) | 10 111 (0·6%) | 5897 (0·3%) |
| Non-Hispanic white | 3 723 040 | 244 578 (6·6%) | 217 784 (5·8%) | 18 028 (0·5%) | 8766 (0·2%) |
| Non-Hispanic black | 981 654 | 102 509 (10·4%) | 82 914 (8·4%) | 11 361 (1·2%) | 8234 (0·8%) |
| Other | 744 810 | 52 939 (10·0%) | 46 615 (6·3%) | 4190 (0·6%) | 2134 (0·3%) |
| Education level | |||||
| Less than high school | 954 384 | 85 314 (8·9%) | 73 800 (7·7%) | 7283 (0·8%) | 4231 (0·4%) |
| High school | 1791 583 | 148 661 (8·3%) | 128 132 (7·2%) | 12 759 (0·7%) | 7770 (0·4%) |
| More than high school | 4315 516 | 287 435 (6·7%) | 251 641 (5·8%) | 23 079 (0·5%) | 12 715 (0·3%) |
| Missing | 80 147 | 6227 (7·8%) | 5343 (6·7%) | 569 (0·7%) | 315 (0·4%) |
| Marital status | |||||
| Married | 4036 400 | 260 640 (6·5%) | 230 882 (5·7%) | 19 619 (0·5%) | 10 139 (0·3%) |
| Unmarried | 2 669 954 | 238 796 (8·9%) | 203 117 (7·6%) | 21 892 (0·8%) | 13 787 (0·5%) |
| Missing | 435 276 | 28 201 (6·5%) | 24 917 (5·7%) | 2179 (0·5%) | 1105 (0·3%) |
| Smoked during pregnancy | |||||
| Yes | 497 527 | 54 508 (11·0%) | 47 221 (9·5%) | 4749 (1·0%) | 2538 (0·5%) |
| No | 6616 141 | 470 339 (7·1%) | 409 329 (6·2%) | 38 684 (0·6%) | 22 326 (0·3%) |
| Missing | 27 962 | 2790 (10·0%) | 2366 (8·5%) | 257 (0·9%) | 167 (0·6%) |
| Parity | |||||
| One child | 2757 355 | 206 868 (7·5%) | 175 343 (6·4%) | 19 262 (0·7%) | 12 263 (0·4%) |
| Two children | 2 292 577 | 146 464 (6·4%) | 129 516 (5·6%) | 11 028 (0·5%) | 5920 (0·3%) |
| Three children | 1205 538 | 89 193 (7·4%) | 78 992 (6·6%) | 6758 (0·6%) | 3443 (0·3%) |
| Four or more children | 868 564 | 83 264 (9·6%) | 73 511 (8·5%) | 6457 (0·7%) | 3296 (0·4%) |
| Missing | 17 596 | 1848 (10·5%) | 1554(8·8%) | 185 (1·1%) | 109 (0·6%) |
| Timing of initiation of prenatal care | |||||
| 1st–3rd month | 5399 926 | 384 450 (7·1%) | 335 914 (6·2%) | 30 950 (0·6%) | 17 586 (0·3%) |
| 4th–6th month | 1162 737 | 81 491 (7·0%) | 71 020 (6·1%) | 6813 (0·6%) | 3658 (0·3%) |
| 7th to final month | 319 100 | 18 937 (5·9%) | 18 233 (5·7%) | 693 (0·2%) | 11 (<0·1%) |
| No prenatal care | 103 625 | 22 314 (21·5%) | 17 523 (16·9%) | 2711 (2·6%) | 2080 (2·0%) |
| Missing | 156 242 | 20 445 (13·1%) | 16 226 (10·4%) | 2523 (1·6%) | 1696 (1·1%) |
| BMI, kg/m2 | |||||
| <18.5 | 252 685 | 23 804 (9·4%) | 20 820 (8·2%) | 2016 (0·8%) | 968 (0·4%) |
| 18.5–24.9 | 3189 165 | 217 098 (6·8%) | 190 998 (6·0%) | 17152(0·5%) | 8948 (0·3%) |
| 25.0–29.9 | 1874 668 | 133 593 (7·1%) | 116 399 (6·2%) | 10 915 (0·6%) | 6279 (0·3%) |
| 30.0–34.9 | 1017 698 | 80 838 (7·9%) | 69 283 (6·8%) | 7052 (0·7%) | 4503 (0·4%) |
| 35.0–39.9 | 482 914 | 41 462 (8·6%) | 35189 (7·3%) | 3783 (0·8%) | 2490 (0·5%) |
| ≥40.0 | 324 500 | 30 842 (9·5%) | 26 227 (8·1%) | 2772 (0·9%) | 1843 (0·6%) |
| Previous history of preterm birth | |||||
| Yes | 212 163 | 52 666 (24·8%) | 45 011 (21·2%) | 5065 (2·4%) | 2590 (1·2%) |
| No | 4169 248 | 267 745 (6·4%) | 238 258 (5·7%) | 19 325 (0·5%) | 10 162 (0·2%) |
| Nulliparous | 2755 067 | 206 601 (7·5%) | 175 121 (6·4%) | 19 241 (0·7%) | 12 239 (0·4%) |
| Missing | 5152 | 625 (12·1%) | 526 (10·2%) | 59 (1·1%) | 40 (0·8%) |
Preterm birth was defined as delivery occurring before 37 weeks of gestation. Moderately preterm births were those at 32–37 weeks’ gestation. Very preterm births were those at 28–31 weeks’ gestation. Extremely preterm births were those before 28 weeks’ gestation.
In the overall population, mothers who were overweight (adjusted OR 1·02 [95% CI 1·01–1·03]) or obese (1·18 [1·18–1·19]) had a significantly increased risk of preterm birth compared with healthy weight mothers (table 2). The adjusted ORs of preterm birth increased with increasing obesity severity (table 2; crude ORs are in the appendix [p 6])). The associations between pre-pregnancy BMI and moderately, very, and extremely preterm births are shown in the appendix (p 5). Compared with healthy weight, underweight was also positively and significantly associated with preterm birth overall (adjusted OR 1·33 [95% CI 1·31–1·35]) and consistently across maternal age groups and race or ethnicity groups (table 2).
Table 2:
Odds ratios (95% CI) for the associations between pre-pregnancy BMI and preterm birth in the US National Vital Statistics System 2016–17
| Maternal pre-pregnancy BMI | Obesity category | ||||||
|---|---|---|---|---|---|---|---|
| Underweight (BMI <18.5 kg/m2) |
Healthy weight (BMI 18.5–24.9 kg/m2) |
Overweight (BMI 25.0–29.9 kg/m2) |
Obese (BMI ≥30.0 kg/m2) |
Class 1 | Class 2 | Class 3 | |
| Overall | 1·33 (1·31–1·35) | Ref | 1·02 (1·01–1·03) | 1·18 (1·18–1·19) | 1·12 (1·11–1·13) | 1·21 (1·20–1·23) | 1·34 (1·32–1·35) |
| Age group, years* | |||||||
| <20 | 1·30 (1·25–1·36) | Ref | 0·85 (0·83–0·88) | 0·89 (0·86–0·92) | 0·88 (0·85–0·92) | 0·86 (0·82–0·92) | 0·96 (0·89–1·04) |
| 20–24 | 1·39 (1·35–1·42) | Ref | 0·93 (0·91–0·94) | 0·98 (0·97–1·00) | 0·95 (0·93–0·96) | 0·99 (0·97–1·02) | 1·07 (1·04–1·11) |
| 25–29 | 1·30 (1·27–1·34) | Ref | 1·00 (0·99–1·02) | 1·16 (1·15–1·18) | 1·10 (1·08–1·12) | 1·19 (1·17–1·22) | 1·31 (1·28–1·34) |
| 30–34 | 1·25 (1·21–1·30) | Ref | 1·09 (1·08–1·11) | 1·32 (1·30–1·34) | 1·24 (1·22–1·26) | 1·35 (1·32–1·38) | 1·51 (1·48–1·55) |
| 35–39 | 1·23 (1·17–1·29) | Ref | 1·13 (1·11–1·15) | 1·39 (1·36–1·41) | 1·29 (1·26–1·32) | 1·46 (1·42–1·51) | 1·58 (1·53–1·63) |
| ≥40 | 1·03 (0·92–1·14) | Ref | 1·13 (1·09–1·17) | 1·36 (1·31–1·41) | 1·28 (1·23–1·34) | 1·43 (1·35–1·51) | 1·55 (1·45–1·66) |
| Race or ethnicity† | |||||||
| Hispanic | 1·33 (1·28–1·37) | Ref | 1·03 (1·02–1·04) | 1·20 (1·18–1·22) | 1·14 (1·12–1·16) | 1·24 (1·22–1·27) | 1·38 (1·34–1·41) |
| Non-Hispanic white | 1·41 (1·39–1·44) | Ref | 1·03 (1·02–1·04) | 1·23 (1·22–1·25) | 1·15 (1·13–1·16) | 1·26 (1·24–1·28) | 1·45 (1·43–1·48) |
| Non-Hispanic black | 1·32 (1·28–1·37) | Ref | 0·91 (0·89–0·92) | 0·99 (0·97–1·00) | 0·95 (0·93–0·97) | 1·02 (0·99–1·04) | 1·05 (1·02–1·08) |
| Other | 1·07 (1·03–1·11) | Ref | 1·13 (1·10–1·15) | 1·26 (1·23–1·29) | 1·25 (1·21–1·28) | 1·26 (1·20–1·31) | 1·32 (1·25–1·39) |
Maternal age, race or ethnicity, parity, education levels, smoking during pregnancy, previous history of preterm birth, marital status, infant sex, and timing of initiation of prenatal care were adjusted for in models. Ref=reference.
Maternal age was not included in this model.
Race or ethnicity was not included in this model.
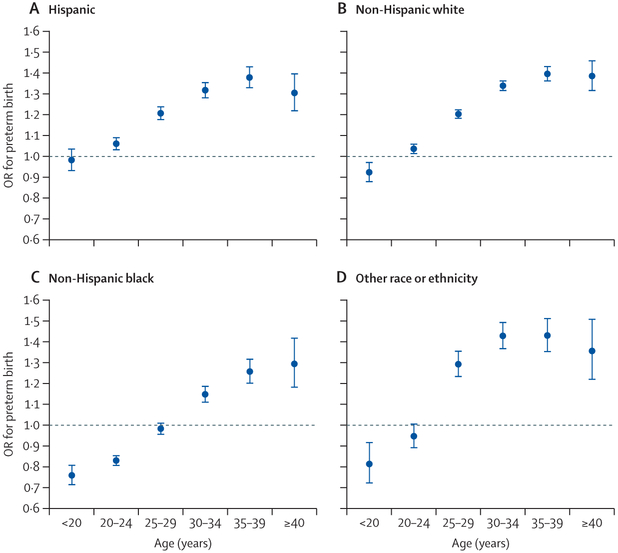
The association between pre-pregnancy obesity and preterm birth differed significantly by maternal age and race or ethnicity (pinteraction<0·0001). Stratified analysis showed that the association between pre-pregnancy obesity and preterm birth was inverse among women younger than 25 years and among non-Hispanic black women (table 2). Therefore, we did further analyses by age × race or ethnicity and noted a crossover effect of age in the association between pre-pregnancy obesity and preterm birth (figure 2). In non-Hispanic white women, maternal obesity was inversely associated with preterm birth among those younger than 20 years (adjusted OR 0·92 [95% CI 0·88–0·97]), but positively associated with preterm birth among those aged 20 years or older (1·04 [1·01–1·06], 1·20 [1·18–1·23], 1·34 [1·31–1·37], 1·40 [1·36–1·43], and 1·39 [1·31–1·46] among those aged 20–24 years, 25–29 years, 30–34 years, 35–39 years, and ≥40 years, respectively). In Hispanic women, maternal obesity was not associated with preterm birth among those younger than 20 years (0·98 [0·93–1·04]), but positively associated with preterm birth among those aged 20 years or older (1·06 [1·03–1·09], 1·21 [1·17–1·24], 1·32 [1·28–1·36], 1·38 [1·33–1·43], and 1·30 [1·22–1·40] among those aged 20–24 years, 25–29 years, 30–34 years, 35–39 years, and ≥40 years, respectively). In non-Hispanic black women, maternal obesity was inversely associated with preterm birth among those younger than 30 years (0·76 [0·71–0·81] in those <20 years, 0·83 [0·80–0·86] in those aged 20–24 years, and 0·98 [0·95–1·01] among those aged 25–29 years), but positively associated with preterm birth among those aged 30 years or older (1·15 [1·11–1·19], 1·26 [1·20–1·32], and 1·29 [1·18–1·42] among those aged 30–34 years, 35–39 years, and ≥40 years, respectively). In all racial and ethnic groups the OR for preterm birth increased with increasing age (at least up to age 40 years; figure 2).
Figure 2: Association between pre-pregnancy obesity and risk of preterm birth in Hispanic (A), non-Hispanic white (B), non-Hispanic black (C), and other racial or ethnic (D) populations, by maternal age group.
Data are from the US National Vital Statistics System for 2016 and 2017. Parity, education level, smoking during pregnancy, previous history of preterm birth, marital status, infant sex, and timing of initiation of prenatal care were adjusted for in models. Women who had a healthy pre-pregnancy BMI (ie, 18·5–24·9 kg/m2) were the reference group. Error bars represent 95% CIs. OR=odds ratio.
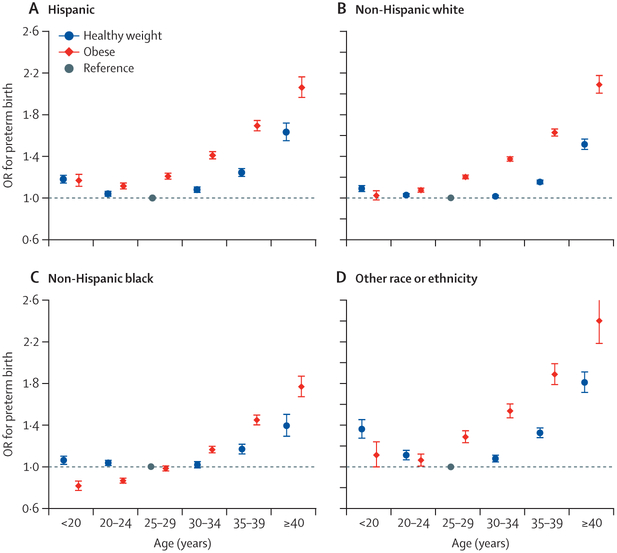
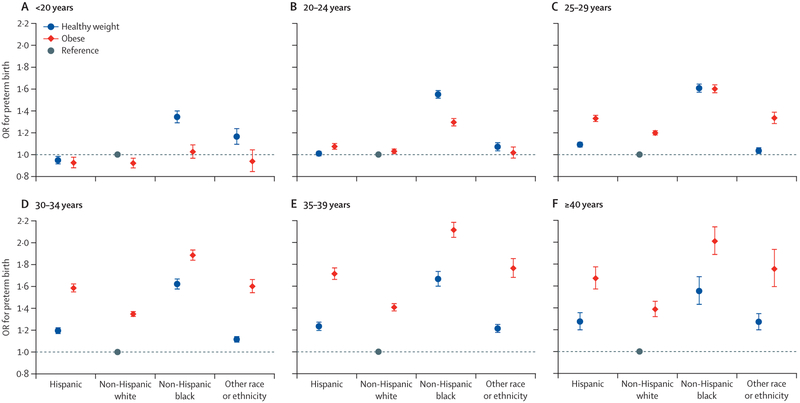
To further explore the relations between maternal obesity and preterm birth, we examined joint effects of maternal age or race or ethnicity with pre-pregnancy obesity on the risk of preterm birth. Among Hispanic women, non-Hispanic white women, and women in the other race or ethnicity category, those younger or older than 25–29 years, irrespective of obesity, had increased odds of preterm delivery compared with healthy weight women aged 25–29 years did (figure 3). Among non-Hispanic black women, obese women younger than 25 years had a lower risk of preterm delivery than healthy weight black women aged 25–29 years (figure 3). Among women within the same BMI groups (healthy weight vs obese), non-Hispanic black women in all age groups had the highest risk of preterm delivery (figure 4). Similar results were noted in the association between overweight and preterm birth (appendix pp 10–12).
Figure 3: Joint association of maternal age and pre-pregnancy obesity status with risk of preterm birth in Hispanic (A), non-Hispanic white (B), non-Hispanic black (C), and other racial or ethnic (D) populations.
Data are from the US National Vital Statistics System for 2016 and 2017. Parity, education level, smoking during pregnancy, previous history of preterm birth, marital status, infant sex, and timing of initiation of prenatal care were adjusted for in models. Healthy weight refers to a BMI of 18·5–24·9 kg/m2. Obese refers to a BMI ≥30 kg/m2. Women aged 25–29 years who were a healthy weight before pregnancy were the reference group. Error bars represent 95% CIs. OR=odds ratio.
Figure 4: Joint association of maternal race or ethnicity and pre-pregnancy obesity status with risk of preterm birth among women aged younger than 20 years (A), 20–24 years (B),25–29 years (C), 30–34 years (D), 35–39 years (E), and 40 years or older (F).
Data are from the US National Vital Statistics System for 2016 and 2017. Parity, education level, smoking during pregnancy, previous history of preterm birth, marital status, infant sex, and timing of initiation of prenatal care were adjusted for in models. Healthy weight refers to a BMI of 18·5–24·9 kg/m2. Obese refers to a BMI ≥30 kg/m2. The reference group were non-Hispanic white women who had a healthy BMI before pregnancy. Error bars represent 95% CIs. OR=odds ratio.
Sensitivity analyses, in which we excluded women with complicated pregnancy conditions, yielded similar results as our main analyses (appendix pp 7, 13). The frequency of missing data was low for almost all covariates, but data for marital status was missing for 435 276 (6·1%) women (table 1). In a sensitivity analysis that omitted participants with incomplete covariate data, the results were almost the same as those of the main analysis (appendix pp 8, 14). Finally, in a sensitivity analysis in which we included gestational weight gain per week in models, our findings did not change significantly (appendix pp 9, 15).
Discussion
In this population-based study of more than 7 million multiracial, multiethnic mother–child pairs, we noted a significantly increased risk of preterm birth among underweight women and obese women compared with healthy weight women. We showed a crossover effect for the association (ie, a change from an inverse association to a positive association) between pre-pregnancy obesity and preterm birth by maternal age. The age at which the effect crosses over differs across racial and ethnic groups. Consistent with the findings of previous studies, we found that underweight women, irrespective of age and race or ethnicity, had increased odds of preterm birth compared with healthy weight women.6,17,18
Findings in previous studies for the association between pre-pregnancy obesity and preterm birth were inconsistent and inconclusive. In a study19 of women aged 20–30 years who were predominantly black, Hendler and colleagues showed an inverse association between pre-pregnancy obesity and preterm birth. However, in another study20 of 11 726 people—5181 (44%) of whom were Hispanic and 4038 (34%) of whom were non-Hispanic white—with a mean age of 26·7 years (SD 6·3), a positive association between pre-pregnancy obesity and preterm birth was reported. Four studies11–14 have examined the association between pre-pregnancy obesity and preterm birth in teenage mothers, and an inverse association has consistently been reported. Our results, which are based on a large and diverse US population, provide for the first time a comprehensive review of the association between maternal obesity and preterm birth for women with a broad range of ages, and suggest that the differing age and racial or ethnic makeup of previous study populations could have contributed to the inconsistent findings.21
The effect of age on the association between pre-pregnancy obesity and preterm birth suggests that the underlying mechanisms causing pre-term birth might differ by age. In women aged 20 years or older, maternal obesity could increase the risk of gestational diabetes and pre-eclampsia, which might subsequently increase the risk of preterm delivery.6 Additionally, obesity can enhance the inflammatory status induced by pregnancy.22 Inflammation, which is related to both advanced maternal age and obesity, has been proposed as an important risk factor in preterm birth.23 The biological mechanisms of the inverse association between pre-pregnancy obesity and preterm birth in teenagers and younger women is unknown. A potential explanation is that teenagers, who are still growing and developing, might compete with the fetus for nutrients, which could subsequently affect physiological and metabolic systems involved with parturition.24–27 A previous study25 showed that 144 (45%)of 318 pregnant adolescents continued to grow as indicated by knee height during pregnancy and post partum. Children who are obese have advanced bone maturation and usually lose their growth advantage during puberty.28 This finding could suggest that pregnant teenagers who are obese might not need to compete (or compete to a lesser extent) for nutrients with their babies for their own growth. This notion was supported by animal studies. A previous study27 among adolescent sheep showed that adiposity, which could indicate more nutrition reserves, at conception is important for still-growing adolescents. Another study29 showed that sheep that were thin at conception had lower concentrations of peripheral nutrients and metabolic hormones than sheep who were fat at conception.29 Nonetheless, further investigation is warranted to elucidate the underlying mechanisms for the intriguing inverse association between pre-pregnancy obesity and preterm birth among young women. Additionally, higher adiposity implies higher levels of circulating oestrogen, which has an important role during pregnancy, including triggering fetal development.30 Another study31 suggested that healthy weight teenagers reported higher levels of psychosocial stress, a risk factor of preterm birth, than their obese peers did. More research is needed to elucidate the underlying mechanisms.
By contrast with the findings of previous studies that showed that pre-pregnancy obesity was a protective factor against preterm birth in non-Hispanic black populations,20 our results suggest that the pattern of the association in non-Hispanic black populations was similar to that in other racial or ethnic groups. However, the age at which the association between pre-pregnancy obesity and preterm birth changes from inverse to positive is older in non-Hispanic black women than in other racial or ethnic groups. Although differences in muscle and fat distribution, nutritional status, and genetics have been put forward as possible explanations for this disparity,32,33 the exact underlying mechanisms remain unknown.
It is worth noting that our findings do not advocate weight gain as a preventive measure against preterm birth among non-underweight young women. In fact, younger women, whether obese or not, have a higher risk of preterm birth than women aged 25–29 years do in Hispanic and in non-Hispanic white populations. Additionally, the adverse effects that maternal obesity has on other perinatal and neonatal outcomes should not be overlooked.3 However, understanding the disparities in the effects of obesity on preterm birth among women at different ages and in different racial or ethnic groups could help to identify novel pathways in the pathogenesis of preterm birth. Our study also highlights that specific tailored recommendations for pregnancy according to maternal age and race or ethnicity are urgently need to accurately stratify the risk of preterm delivery, although more work is needed.
The main strength of our study is the large sample size, which provided sufficient statistical power to examine the association between maternal obesity and preterm birth in sub populations. Our study also has several limitations. First, pre-pregnancy weights and heights were self-reported. However, self-reported height and weight was previously shown to give an accurate representation of true BMI among US women of reproductive age.34 Second, the NVSS birth data did not specify the reason for each case of preterm birth, which precludes us from doing analyses for each subtype of preterm birth. Third, women with preexisting diabetes or hypertension were excluded from the analysis, and thus our findings cannot be generalised to these populations. Fourth, gestational weight gain was not included in our main analysis. However, in a sensitivity analysis, it did not seem to substantially affect results. Although gestational weight gain might be associated with preterm birth, the magnitude of the association is probably much lower than that of the association between pre-pregnancy obesity and preterm birth.35,36 Furthermore, gestational weight gain acts more like a mediator than a confounder in the association between pre-pregnancy obesity and preterm birth.37 Fifth, we cannot identify whether the association between pre-pregnancy obesity and preterm birth was modified by pregnancy health-care access because of a paucity of such data (other than data for the timing of initiation of prenatal care). Finally, although many potential confounders were adjusted for, we cannot rule out the possibility of residual confounding by unknown factors.
In conclusion, the association between maternal pre-pregnancy obesity and risk of preterm birth in the general population differs according to maternal age and race or ethnicity. Although age and racial or ethnic disparities in pregnancy outcomes are increasingly recognised, no tailored recommendations are available. Physicians who provide preconceptional or prenatal care pertaining to preterm birth should take maternal age and race or ethnicity into account. Further investigations are warranted to explore the underlying mechanisms behind the differing associations between maternal pre-pregnancy obesity and risk of preterm birth. Additionally, risk prediction models for preterm birth should consider more refined categories of maternal age and race or ethnicity, especially in diverse populations.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed with the terms “preterm birth”, “preterm delivery”, “premature”, “maternal obesity”, “pre-pregnancy obesity”, “maternal body mass index”, and “maternal BMI”for original articles and reviews published up to Feb 20, 2019. Findings on the association between pre-pregnancy obesity and preterm birth are inconsistent. For example, several studies suggest that the association could differ between white and black populations. In four studies done in teenage mothers, pre-pregnancy obesity and preterm birth were inversely associated. However, we identified no large studies in which age, racial, or ethnic disparities in the association between pre-pregnancy BMI and preterm birth were comprehensively investigated.
Added value of this study
Our study provides a comprehensive overview of the association between maternal obesity and preterm birth by using US nationwide birth certificate data. In the overall population, pre-pregnancy obesity was associated with an increased risk of preterm birth. However, we showed a crossover effect of the association between pre-pregnancy obesity and preterm birth by age. Furthermore, the age at which the effect of the association changes from inverse to positive differed between racial or ethnic groups (age 20 years in Hispanic and in non-Hispanic white populations, age 30 years in non-Hispanic black populations).
Implications of all the available evidence
By clarifying the age and racial or ethnic disparities in the association between pre-pregnancy obesity and preterm birth, our study has important clinical and public health implications. Although encouraging pregnancy at the best reproductive age is still the priority, our results suggest that future clinical guidelines for maternal care before and during pregnancy should include age-specific and race-specific or ethnicity-specific recommendations
Acknowledgments
This work was partly supported by a research grant from the US National Institutes of Health (R21 HD091458).
Footnotes
Declaration of interests
We declare no competing interests.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008; 371: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2017. Natl Vital Stat Rep 2018; 67: 1–50. [PubMed] [Google Scholar]
- 3.Liu P, Xu L, Wang Y, et al. Association between perinatal outcomes and maternal pre-pregnancy body mass index. Obes Rev 2016; 17: 1091–102. [DOI] [PubMed] [Google Scholar]
- 4.Poston L, Caleyachetty R, Cnattingius S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol 2016; 4: 1025–36. [DOI] [PubMed] [Google Scholar]
- 5.McDonald SD, Han Z, Mulla S, Beyene J, Grp KS. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ 2010; 341: c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cnattingius S, Villamor E, Johansson S, et al. Maternal obesity and risk of preterm delivery. JAMA 2013; 309: 2362–70. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Du J, Wang G, Chen Z, Wang W, Xi Q. Effect of pre-pregnancy body mass index on adverse pregnancy outcome in north of China. Arch Gynecol Obstet 2011; 283: 65–70. [DOI] [PubMed] [Google Scholar]
- 8.Kumari AS. Pregnancy outcome in women with morbid obesity. Int J Gynaecol Obstet 2001; 73: 101–07. [DOI] [PubMed] [Google Scholar]
- 9.de Jongh BE, Paul DA, Hoffman M, Locke R. Effects of pre-pregnancy obesity, race/ethnicity and prematurity. Matern Child Health J 2014; 18: 511–17. [DOI] [PubMed] [Google Scholar]
- 10.Torloni MR, Fortunato SJ, Betran AP, et al. Ethnic disparity in spontaneous preterm birth and maternal pre-pregnancy body mass index. Arch Gynecol Obstet 2012; 285: 959–66. [DOI] [PubMed] [Google Scholar]
- 11.Baker AM, Haeri S. Estimating risk factors for spontaneous preterm delivery in teen pregnancies. Arch Gynecol Obstet 2014; 289: 1203–06. [DOI] [PubMed] [Google Scholar]
- 12.Salihu HM, Luke S, Alio AP, Deutsch A, Marty PJ. The impact of obesity on spontaneous and medically indicated preterm birth among adolescent mothers. Arch Gynecol Obstet 2010; 282: 127–34. [DOI] [PubMed] [Google Scholar]
- 13.Haeri S, Guichard I, Baker AM, Saddlemire S, Boggess KA. The effect of teenage maternal obesity on perinatal outcomes. Obstet Gynecol 2009; 113: 300–04. [DOI] [PubMed] [Google Scholar]
- 14.Hope AC. Pre-pregnancy BMI and preterm birth among Hispanic teens Masters thesis, University of Massachusetts, 2015. [Google Scholar]
- 15.US National Center for Health Statistics. Guide to completing the facility worksheets for the certificate of live birth and report of fetal death (2003 revision). https://www.cdc.gov/nchs/data/dvs/GuidetoCompleteFacilityWks.pdf (accessed Sept 20, 2018).
- 16.Tucker J, McGuire W. Epidemiology of preterm birth. BMJ 2004; 329: 675–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han Z, Mulla S, Beyene J, Liao G, McDonald SD. Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. Int J Epidemiol 2011; 40: 65–101. [DOI] [PubMed] [Google Scholar]
- 18.Santos S, Voerman E, Amiano P, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American, and Australian cohorts. BJOG 2019; 126: 984–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendler I, Goldenberg RL, Mercer BM, et al. The preterm prediction study: association between maternal body mass index and spontaneous and indicated preterm birth. Am J Obstet Gynecol 2005; 192: 882–86. [DOI] [PubMed] [Google Scholar]
- 20.Lynch AM, Hart JE, Agwu OC, Fisher BM, West NA, Gibbs RS. Association of extremes of prepregnancy BMI with the clinical presentations of preterm birth. Am J Obstet Gynecol 2014; 210: 428.e1–9. [DOI] [PubMed] [Google Scholar]
- 21.Salihu HM, Luke S, Alio AP, et al. The superobese mother and ethnic disparities in preterm birth. J Natl Med Assoc 2009; 101: 1125–31. [DOI] [PubMed] [Google Scholar]
- 22.Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab 2007; 92: 969–75. [DOI] [PubMed] [Google Scholar]
- 23.Cappelletti M, Della Bella S, Ferrazzi E, Mavilio D, Divanovic S. Inflammation and preterm birth. J Leukoc Biol 2016; 99: 67–78. [DOI] [PubMed] [Google Scholar]
- 24.King JC. The risk of maternal nutritional depletion and poor outcomes increases in early or closely spaced pregnancies. J Nutr 2003; 133: 1732S–36S. [DOI] [PubMed] [Google Scholar]
- 25.Scholl TO, Hediger ML, Schall JI, Khoo CS, Fischer RL. Maternal growth during pregnancy and the competition for nutrients. Am J Clin Nutr 1994; 60: 183–88. [DOI] [PubMed] [Google Scholar]
- 26.Bloomfield FH. How is maternal nutrition related to preterm birth? Annu Rev Nutr 2011; 31: 235–61. [DOI] [PubMed] [Google Scholar]
- 27.Wallace JM. Competition for nutrients in pregnant adolescents: consequences for maternal, conceptus and offspring endocrine systems. J Endocrinol 2019; 242: T1–19. [DOI] [PubMed] [Google Scholar]
- 28.De Leonibus C, Marcovecchio ML, Chiavaroli V, de Giorgis T, Chiarelli F, Mohn A. Timing of puberty and physical growth in obese children: a longitudinal study in boys and girls. Pediatr Obes 2014; 9: 292–99. [DOI] [PubMed] [Google Scholar]
- 29.Wallace JM, Milne JS, Adam CL, Aitken RP. Impact of donor and recipient adiposity on placental and fetal growth in adolescent sheep. Reproduction 2017; 153: 381–94. [DOI] [PubMed] [Google Scholar]
- 30.Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol 2001; 45: S116–24. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro GD, Fraser WD, Frasch MG, Seguin JR. Psychosocial stress in pregnancy and preterm birth: associations and mechanisms. J Perinat Med 2013; 41: 631–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman M, Temple JR, Breitkopf CR, Berenson AB. Racial differences in body fat distribution among reproductive-aged women. Metabolism 2009; 58: 1329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burris HH, Thomas A, Zera CA, McElrath TF. Prenatal vitamin use and vitamin D status during pregnancy, differences by race and overweight status. J Perinatol 2015; 35: 241–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunner Huber LR. Validity of self-reported height and weight in women of reproductive age. Matern Child Health J 2007; 11: 137–44. [DOI] [PubMed] [Google Scholar]
- 35.Voerman E, Santos S, Inskip H, et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA 2019; 321: 1702–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nohr EA, Vaeth M, Baker JL, Sorensen T, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr 2008; 87: 1750–59. [DOI] [PubMed] [Google Scholar]
- 37.O’Dwyer V, O’Toole F, Darcy S, Farah N, Kennelly MM, Turner MJ. Maternal obesity and gestational weight gain. J Obstet Gynaecol 2013; 33: 671–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.