Abstract
Urban areas become a new habitat for an increasing number of species as they gradually adapt to the expanding, human-associated environment. The barn swallow (Hirundo rustica) has constructed nests on human buildings that provide good protection against bad weather and predators over centuries. In contrast, the Taiwan whistling thrush (Myophonus insularis) is one of new urban-invading species. The interactions between old and new urban avian species can determine the structure of this growing avian community. Here we report the first case of Taiwan whistling thrushes’ predation on other birds in an urban area. Taiwan whistling thrushes were observed to eat all barn swallows’ chicks and eggs on one street within one week and thus dramatically reduced their reproductive success, even severer than did a typhoon. The newly evolving predation behavior of Taiwan whistling thrushes could threaten the survival of barn swallows. If the latter cannot conquer the new challenge, the nesting ground may become an ecological trap to them driving population extirpation. This reported case implies that urbanization could intensify the interactions, such as predation or competition, between old and new urban species, leading to their population decline or growth and thus community dynamics of urban wildlife.
Keywords: Urban bird, Community Evolution, Nest Predation, Ecological Trap
BACKGROUND
While human construction or urbanization has caused habitat loss for most species, some with high tolerance to anthropogenic disturbance have been gradually adapting to urban environments (Bonier et al. 2007). As more and more species colonize urban areas, new interspecific interactions may be generated. The dynamic interactions can change the structure of the growing communities (Svenning et al. 2014). Consequently, some species may thrive while others may be extirpated from the new, expanding urban habitats. Thus, understanding how early urban-dwelling species respond to recently colonizing species in cities is of importance in community evolution and conservation.
The barn swallow (Hirundo rustica) is a typical synanthropic species that intensively uses urban habitats and has built nests almost always in human buildings over centuries (Turner 2004). Ever since human buildings appeared (e.g., barns, farmhouses or arcades), barn swallows have quickly changed nest sites from natural habitats to these artificial structures around the world (Chace and Walsh 2006; Speich et al. 1986; Marzluff 1997). The reasons for the barn swallow’s fast urban adaptation could be (1) a higher number of nest sites with (2) lower access by their predators, (3) better protection from severe weather and/or (4) better thermal environment in urban areas than natural habitats (Speich et al. 1986). The strong selective benefits have led almost all barn swallows to breed in human-created environments, rather than natural habitats.
During hundreds or even thousands of years of nesting on artificial structures, barn swallows have gained high breeding success, reached remarkable population densities and colonized regions that they would not have been able to survive without human buildings (Chace and Walsh 2006; Speich et al. 1986; Marzluff 1997). Interestingly, with the expansion of urban areas or increasing green space in cities, an increasing number of avian species have begun to use urban environments (Hildén 1965; Lancaster and Rees 1979; Evans et al. 2011). Consequently, competition or interference between urban avian species might become increasingly intense. The disturbance from newly colonizing species may have a negative impact on barn swallow populations.
The Taiwan whistling thrush (Myophonus insularis) is an endemic passerine in Taiwan, mainly occurring in a broadleaf forest from lowland to 2,100 m a.s.l. (Collar 2018). This bird is rare in cities probably due to the lack of riparian habitats - its main nesting and foraging grounds. However, in recent years there have been increasing observations of Taiwan whistling thrushes in urban areas (Walther 2015 2018). Similarly, its sister species, the blue whistling thrush (Myophonus caeruleus), has also recently been found in urban areas, especially the city parks in Hong Kong (Zhou and Chu 2012). These observations suggest on-going urban invasions by these whistling thrush species.
The Taiwan whistling thrush mainly feeds on invertebrates, frogs, and lizards (Huang et al. 2013). Although the blue whistling thrush is occasionally observed to prey on other birds (e.g., Yoong 2012), the Taiwan whistling thrush has not been recorded for such behavior before. Here, we report the first case of barn swallow nest predation by the Taiwan whistling thrush in Taipei, the largest city in Taiwan. This record indicates that the Taiwan whistling thrush might treat barn swallow chicks and eggs as a new food resource. We further analyze and discuss the potential impacts of the Taiwan whistling thrush’s predation behavior on barn swallow populations and urban wildlife communities.
MATERIALS AND METHODS
Behavioral observations: Taiwan whistling thrushes prey on barn swallow chicks and eggs
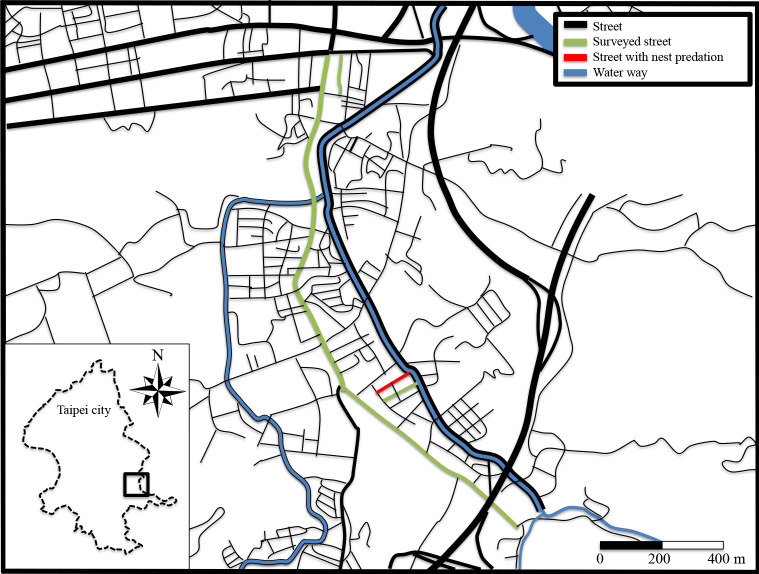
The observations were made by a resident (Mr. Chun-Liang Yu) in Nangang District, Taipei, Taiwan. On June 2nd, 2018, a Taiwan whistling thrush was observed to perform nest predation on one street (Alley 3, Lane 8, Heshun Street), where we found five barn swallow nests containing eggs or chicks and another 11 nests not used. This street was close to a water channel and around 60 meters from a main road, Section 1, Jiuzhuang Street (Fig. 1). This street was about 140 meters in length and about five meters in width; there were verandahs with a width of two meters along the two sides of this street. Barn swallows often built nests facing inward on the road-side beams of the verandahs (Fig. 2). In this observation, the Taiwan whistling thrush searched around the street, jumped up to one barn swallow nest, grabbed up one chick from the nest, threw it to the floor and then swallowed it (Fig. 2). Potentially the same Taiwan whistling thrush was observed again to attack all of the five active nests of this street on June 4th. C.-L. Yu also observed that barn swallows mobbed the invading thrush during this period of time; however, the mobbing behavior did not stop the thrush from preying on swallow chicks (see the supplementary material, Video 1 taken by C.-L. Yu). Unfortunately, on June 14th we were informed that this Taiwan whistling thrush was killed by other residents due to their anger over its nest predation behavior (the potential killing date was between June 8th and 13th). Afterward, there was no nest predation behavior observed until July 16th when another Taiwan whistling thrush was recorded to prey on the only remaining active swallow nest on the same street and ate all four eggs in it (see the supplementary material, Video 2 taken by C.-L. Yu); the second nest predation event occurred about one month after the first predatory Taiwan whistling thrush was killed. Given that the sample size of the second nest predation event was small (N = 1), we conducted downstream analyses (see below) based only on data from the first nest predation event occurring between May 30th and June 7th, 2018.
Fig. 1.
Locations of surveyed streets for studying barn swallow breeding behavior. Barn swallow nest predation by Taiwan whistling thrushes occurred on one of the streets – Alley 3, Lane 8, Heshun Street, Nangang, Taipei (the red line in the map).
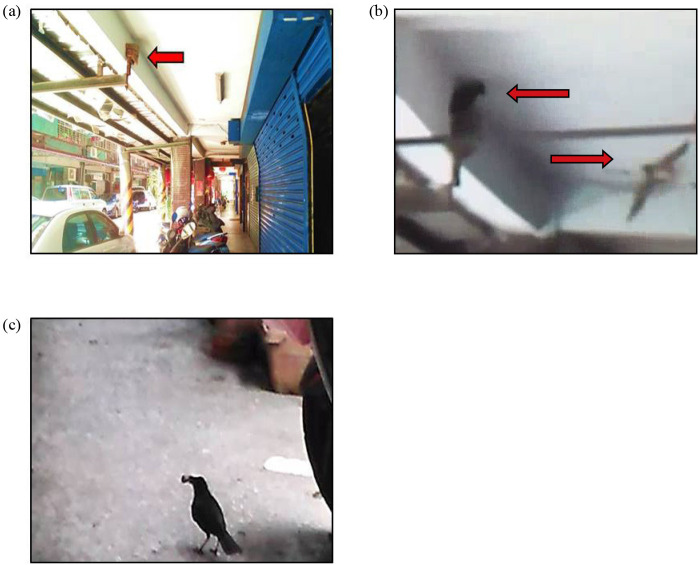
Fig. 2.
(a) One barn swallow nest built on the veranda of a building in Alley 3, Lane 8, Heshun Street, Nangang, Taipei. (b) A Taiwan whistling thrush (pointed by the upper red arrow) was preying on barn swallow chicks from a nest while one barn swallow (pointed by the down red arrow) are mobbing it. (c) The Taiwan whistling thrush was holding a barn swallow chick. It killed the chick by throwing it to the floor and then ate it. The process lasted less than one minute and it continued to attach other chicks after this one. The photograph in (a) was taken by Jhih-Syuan Wang and those in (b) and (c) were taken by Chun-Liang Yu.
Weekly barn swallow breeding survey
The nest predation occurred within the period of time (March 21th -August 29th, 2018) when we conducted weekly surveys to estimate the breeding success rates of barn swallows on several streets with a total length of 2.2 km and 152 nests in Nangang District. Therefore, we were able to compare the barn swallow breeding success rates of the street, where the nest predation occurred, to those of other Nangang streets, where no nest predation by Taiwan whistling thrushes was observed.
In the surveys, we recorded the numbers of barn swallow eggs and chicks in each nest once every week. We used a bluetooth remote control and smartphone (oppo F1f) attached to a rod to take photos or videos for calculating the numbers of eggs and chicks and their survival condition (e.g., numbers of dead chicks and unhatched eggs).
Weekly swallow offspring survival rate estimates and simulation comparisons
We used the weekly survey results from May 30th (before the thrush predation) and June 7th (after the thrush predation) to assess the impact of the thrush predation on barn swallow breeding success. We estimated the weekly survival rate of barn swallow’s offspring between the two survey dates for each nest by dividing the number of eggs plus chicks after predation by that before predation. The weekly survival rate could be larger than one if new eggs were laid during the survey periods. We compared the weekly survival rate from May 30th to June 7th of the predated nests to those of the other surveyed nests that contained eggs and/or chicks on May 30th. In addition, because the eggs were all > one week old and the chicks were all < one week old on May 30th in the five predated nests, we also compared the predated nests’ weekly survival rates to those of other nests that contained eggs or chicks with the same development stages on May 30th to control the effect of eggs’ and checks’ ages.
To test whether the average survival rate of the five predated nests was significantly lower than those of other nests, we generated simulated distributions of barn swallow offspring survival rates in Nangang using a bootstrap resampling approach. To achieve this, we randomly picked five nests from those that contained eggs and/or chicks (1) of any ages (N = 58) or (2) with the matching development stages (i.e., eggs > one week old and chicks < one week old on May 30th; N = 24) on the surveyed streets other than the predated one and calculated an averaged offspring survival rate. We repeated the procedure 1,000 times to generate simulated distributions of average offspring survival rates.
A typhoon (named Maria) hit Taiwan on July 10th and thus provided a chance to examine whether the impact of the Taiwan whistling thrush’s predation was more serious than the typhoon on barn swallows’ breeding success. Therefore, we also compared the average offspring survival rate of the predated nests to those of other nests between the survey dates right before (July 4th) and right after (July 11th) Typhoon Maria hit Taiwan. We generated simulated distributions of average offspring survival rates after the typhoon hit using the same bootstrap resampling approach as above. However, we based the bootstrap resampling only on nests contained eggs and/or chicks of all ages (N = 44), but not on those containing offspring with the matching development stages because all of latter had 100% survival rates from July 4th to July 11th. In addition, we generated another distribution by excluding nests having potentially fledging individuals (i.e., chicks > two weeks old on July 4th) from the dataset (N = 26) because there were a good number of fledglings that were more likely to leave nests between July 4th and 11th than be killed by typhoon Maria.
Occurrence records of Taiwan whistling thrushes in the Taipei city
To assess the Taiwan whistling thrush’s recent occurrence changes in urban areas, we downloaded the observation records of this bird from the Global Biodiversity Information Facility database (GBIF.org, 15th December 2018). We examined the records in the central (latitudinal range: 25.024042 -25.081733; longitudinal range: 121.506580 -121.624210) and non-central areas of the Taipei city from 1996 to 2016. The central area had a higher building density than the non-central area.
RESULTS
The nest predation dramatically reduced the survival rates of the barn swallow eggs and chicks; this negative effect was even stronger than that of a typhoon. The offspring survival rates of the nests predated by the Taiwan whistling thrush were all zero (Table 1). The average offspring survival rate of the predated nests was lower and located outside the simulated distributions of those of other nests containing eggs and/or chicks (1) of all ages or (2) with the same development stages (Fig. 3a and 3b). Furthermore, the predated nests’ average offspring survival rate was also lower than 97.5% of the simulated values based on those impacted by Typhoon Maria (Fig. 4a) and located completely outside the simulated distribution excluding potential fledglings (Fig. 4b).
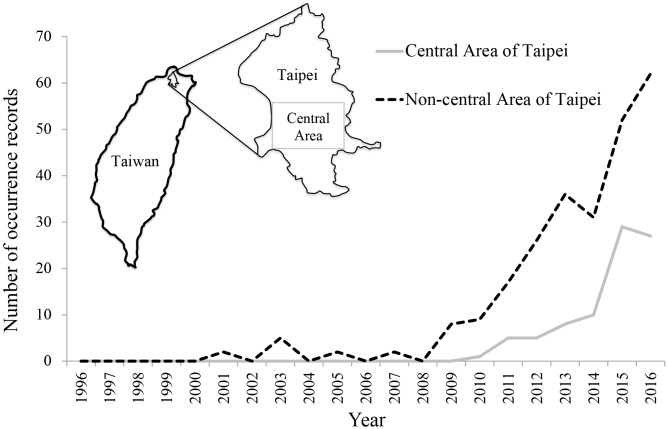
Taiwan whistling thrushes’ occurrence records in the Taipei city dramatically increased in the past decade (Fig. 5). The trend of increasing occurrence records in the central area of Taipei began more lately than that of the non-central area (Fig. 5).
Table 1.
Changes in eggs’ and chicks’ numbers in nests predated by a Taiwan whistling thrush. We calculated the numbers of eggs and chicks before and after the nest predation (on May 30th and June 7th, respectively)
| Before predation | After predation | |||
| Nest site | Egg(s) | Chick(s) | Egg(s) | Chick(s) |
| A | 2 | 3 | 0 | 0 |
| B | 0 | 4 | 0 | 0 |
| C | 5 | 0 | 0 | 0 |
| D | 5 | 0 | 0 | 0 |
| E | 5 | 0 | 0 | 0 |
Fig. 3.
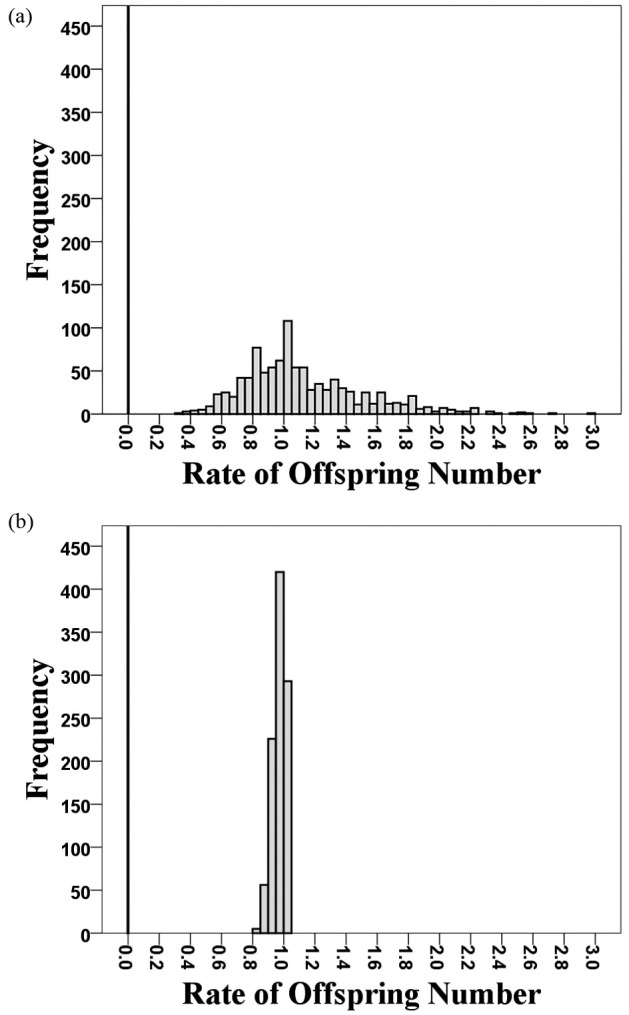
Simulated distributions of weekly offspring survival rates of barn swallows in Nangang based on 1,000 times of bootstrap resampling. The average offspring (i.e., eggs and chicks) survival rates were estimated between May 30th (before a thrush’s predation) and June 7th (after a thrush’s predation). (a) The first distribution was generated from nests that contained eggs and/or chicks of all ages on May 30th. (b) The second distribution was based on nests that contained eggs of > one week old and/or chicks < one week old on May 30th. The black line indicates the average offspring survival rate of five nests predated by a Taiwan whistling thrush.
Fig. 4.
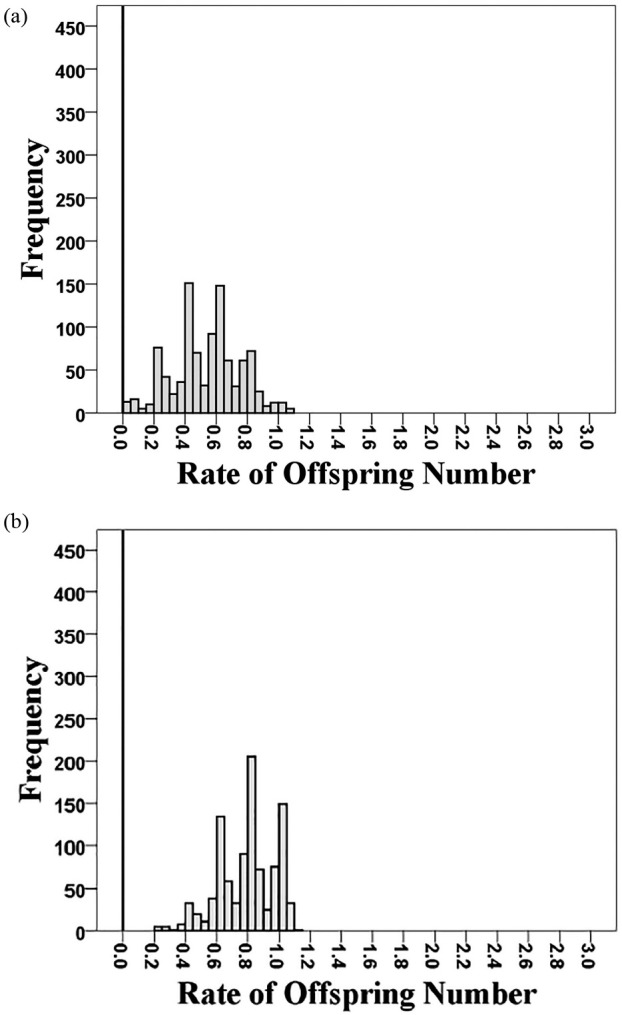
Simulated distributions of weekly offspring survival rates of barn swallows through Typhoon Maria in Nangang based on 1,000 times of bootstrap resampling. The average offspring survival rates were estimated between July 4th (before Typhoon Maria hit Taiwan) and July 11th (after Typhoon Maria hit Taiwan). (a) The first distribution was generated from nests that contained eggs and/or chicks of all ages on July 4th. (b) The second distribution was generated from the same nests as above but excluding ones containing chicks > two weeks on July 4th that were potential fledglings leaving nests before July 11th. The black line indicates the average offspring survival rate of five nests predated by a Taiwan whistling thrush between May 30th and June 7th.
Fig. 5.
Occurrence records of Taiwan whistling thrushes in the Taipei city. The occurrence data are downloaded from the Global Biodiversity Information Facility database (GBIF.org, 15th December 2018, https://doi.org/10.15468/dl.svzckk). The records for the central and non-central areas of the Taipei city from 1996 to 2016 are shown.
DISCUSSION
Our results suggest that the nest predation by Taiwan whistling thrushes could have a remarkable impact on barn swallow’s breeding success. If the omnivorous Taiwan whistling thrush becomes widely adapted to urban environment and treats other urban species as new food resources, the urban avian community could be reconstructed (Chace and Walsh 2006, Moya-Larano 2011, Shochat et al. 2010). The nest predation pressure could reduce the survival rate of barn swallows’ offspring to an extremely low point. If so, the urban environment will no longer be an ideal reproduction habitat for barn swallows.
Human buildings usually provide barn swallows with effective protection from the attack of most predators. For example, raptors seldom fly into human buildings for hunting thanks to narrow space inside or between buildings and strong disturbance from human activities. However, the Taiwan whistling thrush is a medium-sized bird (with a body length of 28 -30 cm; Collar 2018) and appears to be tolerant to human disturbance (pers. obs.). According to several observations and studies of the Taiwan whistling thrush (Fang and Wang 2002; Walther 2015 2018), we believe that some populations have already adapted to urban environment and even use human buildings as nest sites (e.g., bridges, city parks or apartments). The analysis based on GBIF occurrence data (Fig. 5) implies that Taiwan whistling thrushes might have increased their use of urban areas recently. Even though growing data deposition in the GBIF dataset could also cause the increasing records, the more recent trend of increasing records in the central area than the non-central area suggests that Taiwan whistling thrushes are possibly expanding their ranges towards central Taipei. Although the bird-hunting behavior has only been reported in its sister species, the blue whistling thrush (Yoong 2012), the Taiwan whistling thrush is highly likely to develop such behavior, as evidenced by our observations. Such emerging predatory behavior would make the Taiwan whistling thrush a new, devastating predator to barn swallows in cities. Our analytic results suggest that predation by Taiwan whistling thrushes would severely reduce barn swallows’ reproductive success if the behavior spreads through Taiwan whistling thrush populations.
Taiwan whistling thrushes can quickly eat all barn swallow chicks and eggs in one location, and the defensive behaviors, such as mobbing, from parental swallows, apparently cannot stop their predation behavior. Our results suggest that the threat from Taiwan whistling thrushes could be even stronger than that from natural disasters, such as typhoons, to barn swallows. The thrush’s predation can cause entire swallow clutches to fail while typhoons may not have strong impacts on the survival rates of swallow eggs and chicks thanks to the shielding effect of human buildings. If the swallow-hunting behavior is established in Taiwan whistling thrushes’ urban populations, we predict that (1) some nest sites will become an “ecological trap” to barn swallows to reduce their local population density in the short term (Best 1986, Donovan and Thompson 2001) and (2) the selection pressure will eventually force barn swallows to avoid nesting grounds where Taiwan whistling thrushes occur in the long term. As adult barn swallows tend to return to the same sites or nearby regions for breeding (Shields 1984), swallows that nest in regions where Taiwan whistling thrushes prey on their offspring might suffer low reproductive success year after year. If so, these particular regions will become an ecological trap, in which the barn swallow cannot sustain their populations (Donovan and Thompson 2001). Consequently, the barn swallow populations might be extirpated from these urban regions. However, barn swallow nest predation might also be reduced via human interference, as happened in this instance. That is because people in Taiwan are often fond of barn swallows and thus could act as protectors against predators such as Taiwan whistling thrushes although killing wild birds can be illegal in Taiwan. Such protective behavior could be another factor leading to the widespread use of human buildings by barn swallows especially in swallow-friendly areas.
The new swallow-hunting behavior of the recently urban-invading Taiwan whistling thrush reveals on-going shifts in urban avian community structure. As more and more species colonize urban areas, new immigrants may dramatically influence or even threaten earlier urban species. The interspecific interactions such as predation or competition could reduce the reproductive performance or survival rates of prey or inferior species. Consequently, the originally common urban species may decline from population peaks and the urban wildlife community structure may reshape quickly (Puga-Caballero et al. 2014; Rebele 1994). Therefore, the urban habitat, where recently invading species prey on or compete with original urban species, provides a natural laboratory for examining the process of community assembly and thus warrants further studies.
Supplementary Material
Acknowledgments
We are grateful to Chun-Liang Yu for providing the photos, videos and observation records of Taiwan whistling thrushes’ nest predation behavior. We thank Chen-Tau Lin’s assistant in the swallow breeding survey and Mao-Ning Tuanmu’s help in data analysis. This research was supported by the summer internship of Biodiversity Research Center, Academia Sinica.
Footnotes
Authors’ contributions: JSW and CHM conceived the research ideas. JSW collected and analyzed data. JSW and CHM wrote the manuscript.
Competing interests: JSW and CHM declare that they have no conflict of interest.
Availability of data and materials: The raw data will be deposited to a public dataset upon acceptance.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Best LB. 1986. Conservation tillage: ecological traps for nesting birds? Wildl Soc Bull 14:308–317.
- Bonier F, Martin PR,Wingfield JC. 2007. Urban birds have broader environmental tolerance. Biol Lett 3(6):670–673. doi:10.1098/rsbl.2007.0349. [DOI] [PMC free article] [PubMed]
- Collar N. 2018. Taiwan whistling-thrush (Myophonus insularis). In: del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E (eds) Handbook of the Birds of the World Alive. Available via Lynx Edicions, Barcelona. https://www.hbw.com/node/58398. Accessed 2 Aug. 2018.
- Chace JF, Walsh JJ. 2006. Urban effects on native avifauna: a review. Landsc Urban Plan 74(1):46–69. doi:10.1016/j.landurbplan.2004.08.007.
- Donovan TM, Thompson FR. 2001. Modeling the ecological trap hypothesis: a habitat and demographic analysis for migrant songbirds. Ecol Appl 11(3):871-882. doi:10.2307/3061122.
- Evans KL, Chamberlain DE, Hatchwell BJ, Gregory RD, Gaston KJ. 2011. What makes an urban bird? Glob Change Biol 17(1):32– 44. doi:10.1111/j.1365-2486.2010.02247.x.
- Fang JR, Wang Y. 2002. Environmental characteristics of bridges selected by nesting Formosan whistling thrush Myiophoneus insularis. BioFormosa 37:17–23.
- GBIF.org. 2018. GBIF Occurrence (15 December 2018) download https://doi.org/10.15468/dl.svzckk.
- Hildén O. 1965. Board habitat selection in birds: a review. Ann Zool Fenn 2(1):53–75.
- Huang S-Y, Hsu S-H, Wang Y. 2013. Effects of rainfall, breeding month, and brood size on food provision in the Formosan whistling trush Myiophoneus insularis. TW J of Biodivers 15(1):21–31.
- Lancaster RK, Rees WE. 1979. Bird communities and the structure of urban habitats. Can J Zool 57(12):2358–2368. doi:10.1139/z79-307.
- Marzluff JM. 1997. Effects of urbanization and recreation on songbirds. In: Block WM, Finch DM (eds) Songbird Ecology in Southwestern Ponderosa Pine Forests: A Literature Review. Gen. Tech. Re RM-GTR-292. US Department of Agriculture, Forest Service, Rocky Mountain Fores stand Range Experiment Station, Fort Collins, CO, pp. 89–102.
- Moya-Larano J. 2011. Genetic variation, predator-prey interactions and food web structure. Phil Trans R Soc B 366(1569):1425– 1437. doi:10.1098/rstb.2010.0241. [DOI] [PMC free article] [PubMed]
- Rebele F. 1994. Urban ecology and special features of urban ecosystems. Glob Ecol Biogeogr 4(6):173–187. doi:10.2307/2997649.
- Puga-Caballero A, MacGregor-Fors I, Ortega-Álvarez R. 2014. Birds at the urban fringe: avian community shifts in different peri-urban ecotones of a megacity. Ecol Res 29(4):619–628. doi:10.1007/s11284-014-1145-2.
- Shields WM. 1984. Factors affecting nest and site fidelity in Adirondack barn swallows (Hirundo rustica). The Auk 101:780– 789. doi:10.1007/s11284-014-1145-2.
- Shochat E, Lerman S, Fernández-Juricic E. 2010. Birds in urban ecosystems: population dynamics, community structure, biodiversity, and conservation. In: Aitkenhead-Peterson J, Volder A (eds.) Urban Ecosystem Ecology, Agronomy Monograph 55. ASA-CSSA-SSSA, Madison, pp. 75–86.
- Speich SM. Jones HL, Benedict EM. 1986. Review of the natural nesting of the Barn Swallow in North America. Am Midl Nat 115(2):248–254. doi:10.2307/2425861.
- Svenning J-C, Gravel D, Holt RD, Schurr FM, Thuiller W, Münkemüller T, Schiffers KH, Dullinger S, Edwards TC, Jr Hickler T, Higgins SI, Nabel JEMS, Pagel J, Normand S. 2014. The influence of interspecific interactions on species range expansion rates. Ecography 37(12):1198–1209. doi:10.1111/j.1600-0587.2013.00574.x. [DOI] [PMC free article] [PubMed]
- Turner A. 2004. The Barn Swallow. T. & A.D. Poyser, London.
- Walther BA. 2015. Two unusual nest-sites of the Taiwan whistling thrush Myophonus insularis: part of a trend towards urbanisation? BirdingASIA 24:122–125.
- Walther BA. 2018. Urban birds in the shadow of Taipei 101. Feather 31:39–46.
- Yoong KS. 2012. Meat-eating chats: more observations on blue whistling thrushes Myophonus caeruleus in Peninsular Malaysia. BirdingASIA 17:60–64.
- Zhou D, Chu LM. 2012. How would size, age, human disturbance, and vegetation structure affect bird communities of urban parks in different seasons. J Ornith 153(4):1101–1112. doi:10.1007/s10336-012-0839-x.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.