Abstract
Background
Preeclampsia is a leading contributor to maternal and perinatal morbidity and mortality. In mice experiments, manganese (Mn) and selenium (Se) are protective whereas cadmium (Cd) is promotive for preeclampsia. Epidemiologic findings on these chemical elements have been inconsistent. To confirm experimental findings in mice, we examined associations of trace minerals (Mn and Se) and heavy metals (Cd, lead [Pb], and mercury [Hg]) with preeclampsia in a birth cohort.
Methods and Results
A total of 1274 women from the Boston Birth Cohort (enrolled since 1998) had complete data on the exposures and outcome. We measured Mn, Se, Cd, Pb, and Hg from red blood cells collected within 24 to 72 hours after delivery. We ascertained preeclampsia diagnosis from medical records. We used Poisson regression with robust variance models to estimate prevalence ratios (PRs) and 95% CIs. A total of 115 (9.0%) women developed preeclampsia. We observed evidence of a dose–response trend for Mn (P for trend<0.001) and to some extent for Cd (P for trend=0.009) quintiles. After multivariable adjustment, a 1 SD increment in Mn was associated with 32% lower risk of developing preeclampsia (PR=0.68; 95% CI, 0.54–0.86), whereas a 1 SD increment in Cd was associated with 15% higher risk of preeclampsia (PR=1.15; 95% CI, 0.98–1.36). Null associations were observed for Se, Pb, and Hg.
Conclusions
Findings from our cohort, consistent with evidence from mice experiments and human studies, indicate that women with lower blood concentration of Mn or higher Cd are more likely to develop preeclampsia.
Keywords: cadmium, epidemiology, manganese, metal, preeclampsia/pregnancy, trace mineral
Subject Categories: Preeclampsia, Pregnancy, Epidemiology
Clinical Perspective
What Is New?
Higher manganese concentration, measured in maternal red blood cells obtained shortly after delivery, was associated with lower risk of preeclampsia.
Higher cadmium concentration in maternal red blood cells was associated with higher risk of preeclampsia.
No significant associations of selenium, lead, and mercury with preeclampsia were identified.
What Are the Clinical Implications?
The inverse association between manganese and preeclampsia provides new insight into a potentially modifiable way to prevent preeclampsia.
The hazardous effect of cadmium highlights the need to limit exposure to toxic metals for pregnant women in eliminating risk of preeclampsia.
Preeclampsia is a pregnancy‐specific complication chiefly characterized by new‐onset hypertension and proteinuria after 20 weeks of gestation, and accompanied by signs of damage to other organ systems, such as hemolysis, elevated liver enzymes, and low platelet count (HELLP syndrome).1 It is a leading contributor to maternal and perinatal morbidity and mortality, accounting for an estimated 50 000 to 60 000 deaths of mothers and their offspring per year worldwide.2, 3 In addition, the American Heart Association recently added preeclampsia as a risk factor for future cardiovascular disease in women,4 increasing risk at a similar magnitude as diabetes mellitus.5 In the United States, the incidence of preeclampsia has increased by 25% during the past 2 decades.6 Though strategies to prevent preeclampsia have been studied extensively, pharmacologic prevention is at best minimally effective among general pregnant women,7 with the only exception that low‐dose aspirin may be effective in preventing preeclampsia in women at high risk.8
Several lines of evidence suggest that essential trace minerals (eg, manganese [Mn] and selenium [Se]) and toxic heavy metals (cadmium [Cd], lead [Pb], and mercury [Hg]) may have opposing roles in the development of preeclampsia. Mn has antioxidant effects and has been shown in experimental mouse models to reduce risk of preeclampsia.9, 10, 11 Yet epidemiologic studies on Mn and preeclampsia have been limited to small case–control studies with inconsistent results.12, 13, 14, 15, 16, 17 It has also been shown that increasing the antioxidant Se in pregnancy reduced oxidative stress and improved preeclampsia features in rats18 and preeclampsia biomarkers in clinical trials19; however, larger human studies are needed.
In contrast, the heavy metal Cd has been shown to induce oxidative stress,20 endothelial dysfunction,21 and immune abnormality22 in mice. Moreover, a recent literature review of epidemiologic studies considered Cd and Pb as possible risk factors for preeclampsia,23 but the sample sizes of the observational studies reviewed were small. Furthermore, a hazardous association of Hg with preeclampsia was also reported by 1 recent study of 124 adult dental staff24; however, this study was small and needs to be replicated.
Overall, the majority of the epidemiologic studies on the association of trace minerals and heavy metals with preeclampsia have been small and they have not controlled for potentially confounding factors (ie, factors that affect both chemical elements and preeclampsia25). They have also been conducted in settings (eg, places outside the United States12, 13, 14, 15, 17 or among dental staff24) that may not be generalizable to exposure levels for typical pregnancies in the United States. Furthermore, no studies, to our knowledge, have measured concentrations of aforementioned trace minerals and metals in red blood cells (RBCs), a biomarker believed to be more precise and stable in reflecting the concentration of these chemical elements compared with serum, plasma, and whole blood in pregnant women.26, 27, 28, 29
In light of these literature gaps, we examined the associations of trace minerals (Mn and Se) and heavy metals (Cd, Pb, and Hg) with preeclampsia in women from the Boston Birth Cohort, using concentrations measured in RBCs obtained shortly after delivery. We hypothesized that higher Mn and Se concentration in RBCs are associated with lower risk of preeclampsia, whereas higher Cd, Pb, and Hg concentration are associated with higher risk of preeclampsia, after appropriate adjustment.
Methods
The data, analytic methods, and study materials that support the findings of this study will be available from Dr Xiaobin Wang (xwang82@jhu.edu) on reasonable request.
Study Population
The Boston Birth Cohort was initially designed as a molecular epidemiology study on environmental and genetic determinants on low birth weight and preterm birth.30 Pregnant women were recruited at delivery since 1998 at Boston Medical Center on a rolling basis. Multiple‐gestation pregnancies, pregnancies resulting from in vitro fertilization, deliveries induced by maternal trauma, or newborns with major birth defects were not included.
After informed consent was obtained, a face‐to‐face interview using a standardized questionnaire was conducted within 24 to 72 hours after delivery to obtain information including sociodemographic characteristics, smoking status, and alcohol consumption during pregnancy, as well as medical and reproductive history. Electronic medical records were reviewed to obtain clinical information including prenatal care and pregnancy complications. Blood samples were collected from all participants during enrollment.
Of the 1448 participants who had complete information on chemical elements, 174 were excluded from this analysis for the following reasons: 98 had chronic hypertension before pregnancy, 76 had missing covariates included in the statistical model (62 on prepregnancy height and/or weight, 10 on smoking status during pregnancy, 3 on education achievement, and 1 on parity) (Figure S1). After these exclusions, 1274 participants were left for the current analysis. The Institutional Review Board of Boston Medical Center and Johns Hopkins Bloomberg School of Public Health approved this study.
Exposures
The primary exposures for this study were concentrations of Mn, Se, Cd, Pb, and Hg in RBCs. Detailed laboratory methods for measurements had been described previously.29 Briefly, plasma and RBCs were separated by centrifugation and kept frozen at −80°C in vials that were certified to be free of those elements after collection. They were transported on dry ice to the Public Health and Environmental Laboratories in the Department of Health of the State of New Jersey, USA and were measured using inductively coupled plasma mass spectrometry. The number and percentage of samples with Mn, Se, Cd, Pb, and Hg concentrations below the limits of detection were 2 (0.2%), 0 (0%), 115 (9.0%), 0 (0%), and 143 (11.2%), respectively. For samples below limits of detection, the concentrations were re‐assigned as the limits of detection divided by the square root of 2.31
Outcome
In assessing outcome, we extracted physician diagnoses directly from medical records. Preeclampsia was defined based on the criteria at the time of diagnosis as newly diagnosed hypertension (a systolic blood pressure of 140 mm Hg or higher or diastolic blood pressure of 90 mm Hg or higher) and proteinuria (excretion of 300 mg or more in a 24‐hour collection, or 0.3 g/g by urine protein:creatinine ratio, or +1 by dipstick if quantitative methods are unavailable) occurring after 20 weeks of gestation.32 HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count), a subtype of preeclampsia, was also included in the outcome definition. One participant developed eclampsia and was assigned as having the outcome. Those who did not have a medical diagnosis of preeclampsia or HELLP syndrome in their medical records were considered as not having the condition.
Covariates
We extracted age at delivery and parity from electronic medical records. Information on prepregnancy weight and height, self‐reported race, education achievement, smoking status, and alcohol consumption during pregnancy were obtained from a questionnaire administered postpartum. Prepregnancy body mass index was calculated as weight in kilograms divided by height in meters squared. Postpartum plasma folate concentrations were measured using chemiluminescent immunoassay with diagnostic kits (Shenzhen New Industries Biomedical Engineering Co., Ltd, Shenzhen, China) and the interassay coefficient of variation was reported to be <4% by previous studies.33, 34
Statistical Analysis
We described participants’ characteristics using percentages for categorical variables, means and standard deviations (SD) for normally distributed variables, and median and interquartile range (IQR) for nonnormally distributed variables. Pearson χ2, ANOVA, and permutation test were used for comparison of categorical, normally, and nonnormally distributed continuous variables, respectively. We used box and whisker plots to display the median, IQR, and range of each trace mineral and heavy metal, and permutation test to determine differences in their distributions by preeclampsia status. We used Spearman rank‐order correlation coefficients to quantify the interelement correlation for each pairwise combination of chemical elements.
We used Poisson regression with robust variance models to examine the prevalence ratio of preeclampsia in relation to concentration of each chemical element. In all models, chemical element concentrations were treated both as categorical variables by quintiles to explore potential nonlinear effects on preeclampsia and continuous variables scaled to 1 SD increment. P values for overall trend were obtained by coding concentration categories as ordinal variables in the regression models.35, 36
To assess confounding, we began with an unadjusted model (model 1) and then added confounders (model 2) defined as covariates known to be associated with both chemical elements and preeclampsia but not in the causal pathway.25 Confounders included age at delivery (continuous), self‐reported race (black, nonblack), education achievement (below high school, high school, college or above), parity (nulliparous, multiparous), prepregnancy body mass index (continuous), and smoking status during pregnancy (never, former, current smoker). In supplemental analyses, we additionally adjusted for postpartum plasma folate concentration, as a surrogate of maternal nutrition, and also adjusted for other trace minerals and metals to further reduce potential bias caused by confounding.
We considered self‐reported race, smoking status during pregnancy, and parity as potential effect measure modifiers. We tested effect measure modification on the multiplicative scale by including an interaction term between the exposure and each effect measure modifier in our final multivariable adjusted model. P values for interaction were obtained from these models. We also conducted subgroup analyses stratified by each effect measure modifier.
All tests were based on a 2‐sided P<0.05 as evidence of statistical significance. Data management and analyses were performed using Stata 14.2 (Stata Statistical Software: Release 14, 2015. College Station, TX: StataCorp LP.).
Results
Participant Characteristics
Of the 1274 women included in the analysis, 115 (9.0%) developed preeclampsia. Women with preeclampsia were more likely to have higher prepregnancy body mass index and higher educational achievement (high school or above; Table 1). Babies born to preeclamptic mothers were more likely to have shorter gestation and lower birth weight. The distributions of trace minerals and metals according to preeclampsia status, shown in Figure 1, indicated that preeclamptic participants had statistically significantly lower Mn concentration than normal counterparts. The interelement correlation coefficients were small to modest, ranging from −0.03 to 0.34 (Table S1). Participant characteristics by Mn, Se, Cd, Pb, and Hg quintiles are provided in Tables S2 through S6, respectively.
Table 1.
Characteristics of Mother–Child Pairs in the Boston Birth Cohort By Preeclampsia Status (n=1274)
| Variable, n (%)a | Normal | Preeclampsia | P Value |
|---|---|---|---|
| N | 1159 | 115 | |
| Maternal characteristics | |||
| Age at delivery, years, mean (SD) | 27.99 (6.31) | 29.12 (6.17) | 0.07 |
| Black race | 671 (57.9%) | 68 (59.1%) | 0.80 |
| Education level | 0.02 | ||
| Middle school or below | 318 (27.4%) | 21 (18.3%) | |
| High school | 417 (36.0%) | 56 (48.7%) | |
| College or above | 424 (36.6%) | 38 (33.0%) | |
| Nulliparous | 526 (45.4%) | 60 (52.2%) | 0.16 |
| Married | 362 (31.2%) | 40 (34.8%) | 0.58 |
| Smoking status during pregnancy | 0.09 | ||
| Never smoker | 929 (80.2%) | 99 (86.1%) | |
| Former smoker | 105 (9.1%) | 11 (9.6%) | |
| Current smoker | 125 (10.8%) | 5 (4.3%) | |
| Alcohol consumption during pregnancy | 108 (9.3%) | 7 (6.1%) | 0.18 |
| Prepregnancy BMI (kg/m2), median (IQR) | 24.88 (21.64, 29.18) | 26.58 (23.46, 31.62) | 0.003 |
| Pregestational/gestational diabetes mellitus | 123 (10.6%) | 18 (15.7%) | 0.25 |
| Offspring characteristics | |||
| Boys | 599 (51.7%) | 49 (42.6%) | 0.06 |
| Gestational age (wks), median (IQR) | 39.00 (37.29, 40.14) | 36.29 (32.57, 38.57) | <0.001 |
| Birth weight (kg), median (IQR) | 3.12 (2.67, 3.51) | 2.40 (1.50, 3.17) | <0.001 |
BMI indicates body mass index; IQR, interquartile range; SD, standard deviation.
Unless otherwise indicated.
Figure 1.
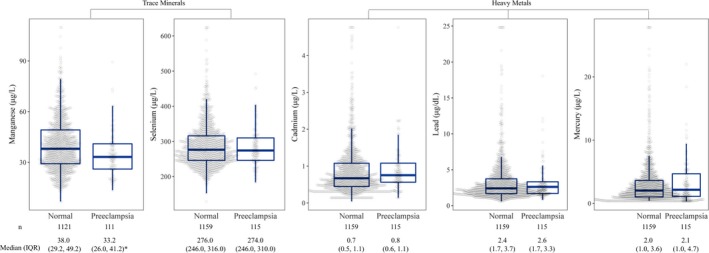
The distributions of trace minerals and heavy metals measured in red blood cells among pregnant women from the Boston Birth Cohort by preeclampsia status. *Statistically significant difference between women diagnosed with preeclampsia versus not (P<0.001) based on permutation test.
Mn and Preeclampsia
In Table 2, we show unadjusted and multivariable‐adjusted associations of trace minerals and metals with preeclampsia risk. We observed evidence of a dose–response trend across Mn quintiles (P for trend<0.001; Figure 2). After multivariable adjustment, the fourth and fifth quintiles of Mn were associated with 0.46 (95% CI, 0.26–0.81) and 0.33 (95% CI, 0.17–0.65) times lower risk of preeclampsia compared with the first quintile, respectively. In linear modeling, each 1 SD (15.52 μg/L) increment of Mn was associated with 0.68 (95% CI, 0.54–0.86) times lower risk of preeclampsia (Table 2).
Table 2.
Prevalence Ratios and 95% CIs for Preeclampsia in Relationship to Concentrations of Trace Minerals and Heavy Metals in RBCs, Before and After Adjustment for Potential Confounders (n=1274)
| n | Cases (%) | Model 1a | Model 2b | |||
|---|---|---|---|---|---|---|
| PR (95% CI) | P Value | PR (95% CI) | P Value | |||
| RBC manganese (μg/L)c | ||||||
| Quintile 1 (6.86–27.00) | 248 | 33 (13.3%) | Ref. | Ref. | ||
| Quintile 2 (27.20–33.80) | 250 | 26 (10.4%) | 0.78 (0.48–1.27) | 0.32 | 0.75 (0.46–1.23) | 0.25 |
| Quintile 3 (34.00–41.20) | 246 | 25 (10.2%) | 0.76 (0.47–1.24) | 0.28 | 0.68 (0.41–1.11) | 0.12 |
| Quintile 4 (41.40–51.80) | 244 | 16 (6.6%) | 0.49 (0.28–0.87) | 0.01 | 0.46 (0.26–0.81) | 0.008 |
| Quintile 5 (52.00–109.80) | 244 | 11 (4.5%) | 0.34 (0.17–0.65) | 0.003 | 0.33 (0.17–0.65) | 0.001 |
| P trend | <0.001 | <0.001 | ||||
| Per 1 SD (15.52) increment | N.A. | 0.70 (0.57–0.86) | 0.001 | 0.68 (0.54–0.86) | 0.001 | |
| RBC selenium (μg/L) | ||||||
| Quintile 1 (129.22–241.12) | 255 | 22 (8.6%) | Ref. | Ref. | ||
| Quintile 2 (242.00–264.00) | 269 | 23 (8.5%) | 0.99 (0.57–1.73) | 0.97 | 0.94 (0.54–1.63) | 0.82 |
| Quintile 3 (266.00–290.00) | 243 | 28 (11.5%) | 1.33 (0.79–2.27) | 0.28 | 1.31 (0.77–2.22) | 0.32 |
| Quintile 4 (291.51–326.00) | 257 | 21 (8.2%) | 0.95 (0.53–1.68) | 0.85 | 0.93 (0.51–1.68) | 0.80 |
| Quintile 5 (328.00–624.00) | 250 | 21 (8.4%) | 0.97 (0.55–1.72) | 0.93 | 0.93 (0.52–1.66) | 0.80 |
| P trend | 0.89 | 0.81 | ||||
| Per 1 SD (61.55) increment | N.A. | 0.95 (0.79–1.13) | 0.54 | 0.93 (0.78–1.12) | 0.46 | |
| RBC cadmium (μg/L) | ||||||
| Quintile 1 (0.04–0.39) | 256 | 17 (6.6%) | Ref. | Ref. | ||
| Quintile 2 (0.40–0.59) | 256 | 18 (7.0%) | 1.06 (0.56–2.01) | 0.86 | 1.09 (0.58–2.06) | 0.77 |
| Quintile 3 (0.59–0.80) | 253 | 24 (9.5%) | 1.43 (0.79–2.59) | 0.24 | 1.48 (0.81–2.71) | 0.21 |
| Quintile 4 (0.80–1.18) | 255 | 31 (12.2%) | 1.83 (1.04–3.22) | 0.04 | 1.97 (1.08–3.59) | 0.03 |
| Quintile 5 (1.19–4.76) | 254 | 25 (9.8%) | 1.48 (0.82–2.68) | 0.19 | 1.86 (0.98–3.50) | 0.06 |
| P trend | 0.04 | 0.009 | ||||
| Per 1 SD (0.69) increment | N.A. | 1.03 (0.90–1.19) | 0.65 | 1.15 (0.98–1.36) | 0.09 | |
| RBC lead (μg/dL) | ||||||
| Quintile 1 (0.58–1.56) | 255 | 22 (8.6%) | Ref. | Ref. | ||
| Quintile 2 (1.57–2.10) | 258 | 22 (8.5%) | 0.99 (0.56–1.74) | 0.97 | 1.05 (0.59–1.85) | 0.87 |
| Quintile 3 (2.12–2.80) | 254 | 26 (10.2%) | 1.19 (0.69–2.04) | 0.53 | 1.19 (0.68–2.10) | 0.54 |
| Quintile 4 (2.81–4.28) | 253 | 23 (9.1%) | 1.05 (0.60–1.84) | 0.85 | 1.03 (0.57–1.88) | 0.91 |
| Quintile 5 (4.30–24.80) | 254 | 22 (8.7%) | 1.00 (0.57–1.77) | 0.99 | 0.90 (0.48–1.68) | 0.74 |
| P trend | 0.99 | 0.75 | ||||
| Per 1 SD (3.07) increment | N.A. | 0.95 (0.80–1.13) | 0.57 | 0.91 (0.74–1.11) | 0.36 | |
| RBC mercury (μg/L) | ||||||
| Quintile 1 (0.30–0.89) | 255 | 23 (9.0%) | Ref. | Ref. | ||
| Quintile 2 (0.90–1.60) | 256 | 23 (9.0%) | 1.00 (0.57–1.73) | 0.99 | 0.94 (0.54–1.61) | 0.82 |
| Quintile 3 (1.59–2.58) | 254 | 17 (6.7%) | 0.74 (0.41–1.35) | 0.33 | 0.69 (0.38–1.26) | 0.23 |
| Quintile 4 (2.60–4.28) | 257 | 21 (8.2%) | 0.91 (0.51–1.59) | 0.73 | 0.84 (0.47–1.49) | 0.55 |
| Quintile 5 (4.30–27.80) | 252 | 31 (12.3%) | 1.36 (0.82–2.27) | 0.23 | 1.21 (0.72–2.05) | 0.47 |
| P trend | 0.34 | 0.58 | ||||
| Per 1 SD (3.60) increment | N.A. | 1.06 (0.92–1.22) | 0.45 | 1.03 (0.88–1.20) | 0.71 | |
N.A. indicates not applicable; PR, prevalence ratio; RBC, red blood cell; Ref, reference group.
Model 1 was an unadjusted model.
Model 2 was adjusted for age at delivery (continuous), self‐reported race (black, nonblack), education (below high school, high school, college or above), parity (nulliparous, multiparous), prepregnancy body mass index (continuous), and smoking status during pregnancy (never, former, current).
There are 42 missing values for manganese (n=1232).
Figure 2.
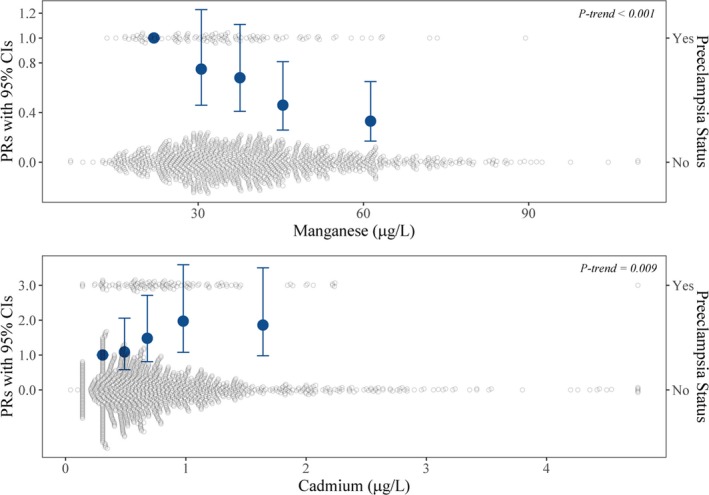
Prevalence ratios (PRs) and 95% CIs of preeclampsia in relationship to quintiles of manganese and cadmium measured in red blood cells. Quintile points were set at the median value of each quintile. Models adjusted for age at delivery, self‐reported race, education, parity, prepregnancy body mass index, and smoking status during pregnancy.
Cd and Preeclampsia
Higher Cd levels were associated with greater preeclampsia risk, in somewhat of a dose‐dependent manner (P for trend=0.009) (Figure 2). Compared with the first quintile, the fourth and fifth quintiles were associated with 1.97 (95% CI, 1.08–3.59) and 1.86 (95% CI, 0.98–3.50) times greater risk after adjustment, respectively. In linear modeling, each 1 SD (0.69 μg/L) increment of Cd was associated with 1.15 (95% CI, 0.98–1.36) times higher risk of preeclampsia (Table 2). Estimates for both Mn and Cd were not materially changed after additional adjustment for maternal plasma folate concentration (Table S7) or upon additional adjustment for other trace minerals or metals measured in RBCs (Tables S8 and S9).
Effect Measure Modification
In subgroup analyses, associations of (1‐SD increment in) Mn and Cd with preeclampsia were consistent across strata of self‐reported race, smoking status during pregnancy, and parity (Figure 3). Furthermore, all P values for interaction on the multiplicative scale were not statistically significant, suggesting no evidence of effect measure modification.
Figure 3.
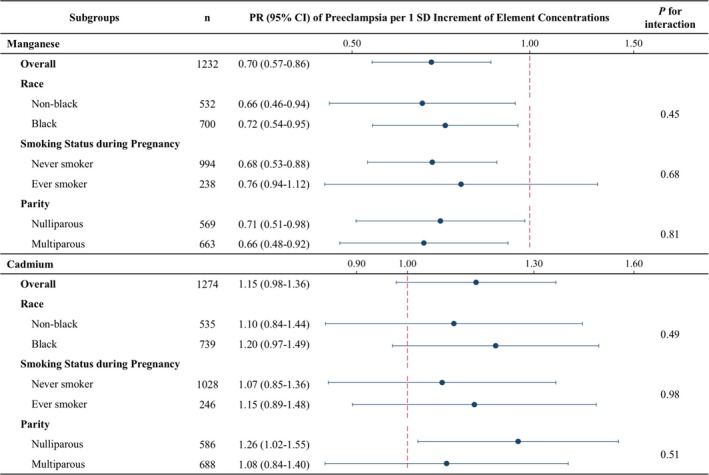
Subgroup analysis of the association of manganese and cadmium in red blood cells with preeclampsia, by potential effect measure modifiers. Model adjusted for age at delivery, self‐reported race (when not testing race), education, parity (when not testing parity), prepregnancy body mass index, and smoking status during pregnancy (when not testing smoking status). PR indicates prevalence ratio; SD, standard deviation.
Other Trace Minerals, Metals, and Preeclampsia
We did not observe significant associations (linear or nonlinear) of Se, Pb, and Hg with preeclampsia (Table 2). The fifth quintile of Se, Pb, and Hg was associated with a prevalence ratio of 0.93 (95% CI: 0.52–1.66), 0.90 (95% CI: 0.48–1.68), and 1.12 (95% CI: 0.72–2.05) as compared with the first quintile, respectively. There was also no evidence that race, smoking status, or parity modified these associations.
Discussion
In this multiethnic, predominantly urban and low‐income cohort of pregnant women with high burden of preeclampsia, higher Mn levels (measured in RBCs) were associated, in a dose–response fashion, with lower preeclampsia risk. Independently, Cd had a positive association with preeclampsia. These associations persisted after controlling for potential confounders and were consistent across levels of race, smoking status during pregnancy, and parity. The other chemical elements measured in our study (Se, Pb, and Hg) were not associated with preeclampsia.
Our findings contribute to an emerging literature base on the association of the essential trace mineral Mn with preeclampsia. Consistent with our findings on Mn, 3 small case–control studies conducted in Saudi Arabia (n=120, 40 cases),14 Bangladesh (n=108, 50 cases),15 and South Africa (n=66, 43 cases)12 all reported significantly lower Mn concentration in serum among women with preeclampsia compared with healthy controls. Furthermore, in a prospective cohort study of 620 pregnant women in Iran, Goodarzi Khoigani et al16 reported that Mn intake in the third trimester, estimated using 48‐hour dietary recalls, was significantly lower among women who developed preeclampsia compared with those who did not. In contrast, in a case–control study of 396 participants (31 preeclampsia cases) in Iran, Vigeh et al17 reported that Mn concentration in umbilical cord blood, but not maternal blood, was associated with higher odds of preeclampsia after adjustment. However, their study was limited by having few cases of preeclampsia and Mn concentration measured in whole blood, which, unlike in RBCs, is subject to the extent of plasma volume expansion in pregnant women with and without preeclampsia.26, 27
Our findings on Cd are also largely consistent with previous studies from around the world. A nested case–control study of 172 participants (86 cases) in the United States reported that each 1 ng/g increment in placenta Cd was associated with an odds ratio of 1.50 (95% CI: 1.10–2.20) after adjustment.37 Similarly, another case–control study of 102 participants (51 cases) in China reported that the odds ratio comparing the third tertile with the first tertile of blood Cd in the third trimester was 7.83 (95% CI: 1.64–37.26) after adjustment.38 In DR Congo, Elongi Moyene et al13 also found significantly higher urinary Cd concentration among 88 women with preeclampsia compared with 88 controls that were matched on age, parity, and duration of pregnancy. However, Maduray et al12 did not detect such association using either serum or hair Cd in a case–control study conducted in 43 preeclamptic and 23 normotensive pregnant women in South Africa. The null finding may have been because of the small sample size, lack of adjustment for potential confounders, and/or the aforementioned effect of plasma volume expansion on metal concentrations measured in serum.
In our study, we did not find evidence that Pb was associated with preeclampsia. However, Pb has been linked to increased risk of preeclampsia for more than a century, and a recently published systematic review and meta‐analysis reported that an increment of 1 μg/dL blood Pb was associated with a 1.6% higher likelihood of preeclampsia.39 Thus, considering the preponderance of evidence linking Pb and preeclampsia, null findings from our single study should be interpreted cautiously. One reason for the lack of association in our study could be that Pb concentration in our sample was lower than the majority of the studies reviewed. This could be partially because of the substantial decline in blood Pb concentration of the entire US population over the past 40 years,40, 41 whereas that half of the reviewed studies were conducted before year 2000 in low‐ and middle‐income countries.
Our findings also do not support associations of Hg, measured in RBCs, with risk of preeclampsia. A single prospective cohort study of 64 pregnant dental staff and 60 pregnant administrators conducted by El‐Badry et al in Egypt reported that the dental staff had significantly higher urinary Hg concentration and higher odds of preeclampsia compared with the administrators.24 However, this study was small (n=124) and concentrations of Hg in the dental staff may be higher than what pregnant women are typically exposed to.
As for Se, 1 randomized clinical trial conducted in 166 pregnant Iranian women suggested that Se supplementation may be associated with a lower incidence of preeclampsia; however, they only had 3 preeclampsia events in the control arm and 0 in the active treatment arm.42 In another randomized trial of 230 pregnant women in the United Kingdom, Se supplementation improved a preeclampsia biomarker (serum soluble vascular endothelial growth factor receptor 1), implying that Se may prevent preeclampsia.19 However, these trials have not been definitive, and thus the null association observed in our study cannot be ruled out. Other explanations for the incongruent findings from ours and prior studies include the different time points studied, differences in the population studied, and differences in the covariate adjustment.
There are several possible biologic mechanisms underlying the opposing associations of Mn and Cd with preeclampsia, some of which may operate through a shared pathway. According to in vivo and animal models, Mn and Cd could modulate reactive oxygen species and oxidative stress, factors on the pathway to preeclampsia.43, 44 Mn is an essential component of manganese superoxide dismutase, which is located within mitochondria and serves as the first line of defense against superoxide and free radical.9, 10 In contrast, Cd may interfere with enzymes of the cellular antioxidant system and induce oxidative stress.20 Therefore, low concentration of Mn, as well as high concentration of Cd, could cause accumulation of reactive oxygen species and oxidative stress, which may eventually lead to preeclampsia. Other potential mechanisms include endothelial dysfunction10, 21 and immune abnormality.22 Additionally, Cd concentrates in the kidney and has been shown to induce proteinuria and renal dysfunction from studies both in animal and general population.45, 46
A major strength of our study is the measurement of trace minerals and heavy metals in RBCs, which has been purported to be a more precise and stable biomarker than other hematological indices, including whole blood, serum, and plasma, in pregnant women.26, 27, 28, 29 Therefore, using RBCs may reduce measurement error. Furthermore, because of the long biological half‐lives of those chemical elements in the human body, RBCs are believed to reflect their long‐term body burden. Second, the extensive covariate information afforded by the Boston Birth Cohort enabled us to control for a variety of confounders and stratify by potential modifiers in the analysis. Finally, the participant diversity of our cohort improves the external validity of the conclusion.
There are also limitations of the current study that merit mention. First, we only had blood available at delivery, which precluded us from firmly establishing a temporal association between concentrations of trace minerals and metals with preeclampsia. No study has been done to establish the exact exposure period reflected by RBCs by comparing with other widely accepted biomarkers for those chemical elements. Therefore, while we assume that chemical elements measured in our study reflect pregnancy levels before development of preeclampsia, we cannot rule out the possibility that the concentrations were influenced by preeclampsia. Second, misclassification of preeclampsia could exist, since the diagnosis was extracted directly from electronic medical records. It is reasonable to consider the misclassification as independent and nondifferential, which would bias the observed association towards the null. Third, because of the lack of information on the onset time of preeclampsia in our cohort, we were unable to distinguish between early‐ and late‐onset preeclampsia, which may differ with respect to pathogenesis and clinical managements.47 Therefore, we cannot specifically discern whether trace minerals and metals examined in our analysis have different associations with early‐ versus late‐onset preeclampsia. Finally, because our study is observational, we cannot rule out the possibility of residual or unmeasured confounding of the reported associations.
Conclusions
Our finding on Mn, an essential trace mineral, provides new insight into a potentially modifiable way to prevent preeclampsia, while the observation of a potentially hazardous effect of Cd on preeclampsia reinforces the recommendations by the American College of Obstetricians and Gynecologists that healthcare professionals provide useful information and necessary interventions for pregnant women to limit exposure to toxic metals.48 Nevertheless, the associations observed in our study need to be replicated by longitudinal studies with measurements of trace minerals and metals in both RBCs and other biomarkers, as well as their original sources (eg, diet). If replicated, randomized clinical trials are needed to determine whether the associations are causal. Moreover, further research with information on characteristic features of preeclampsia (such as severity of blood pressure and proteinuria) and clinical subtypes of preeclampsia (eg, early‐onset versus late‐onset) is needed to elucidate the potential heterogeneity of mechanisms that are responsible for the effect of trace minerals and metals, which is in line with recent research recommendations by the National Institutes of Health.7
Sources of Funding
This study is supported in part by the National Institute of Health grants (R01HD086013, 2R01HD041702, R01HD098232). Dr Mueller is supported by the National Heart, Lung, and Blood Institute grant (K01HL141589). The Boston Birth Cohort (the parent study) is supported in part by the Health Resources and Services Administration of the US Department of Health and Human Services under grant numbers R40MC27443 and UJ2MC31074.
Disclosures
None.
Supporting information
Table S1. Spearman Rank‐Order Correlation Coefficients Among Trace Minerals and Heavy Metals (n=1274)
Table S2. Characteristics of Participants in the Boston Birth Cohort by RBC Manganese Level (n=1232)
Table S3. Characteristics of Participants in the Boston Birth Cohort by RBC Selenium Level (n=1274)
Table S4. Characteristics of Participants in the Boston Birth Cohort by RBC Cadmium Level (n=1274)
Table S5. Characteristics of Participants in the Boston Birth Cohort by RBC Lead Level (n=1274)
Table S6. Characteristics of Participants in the Boston Birth Cohort by RBC Mercury Level (n=1274)
Table S7. Prevalence Ratios and 95% CIs for Preeclampsia in Relationship to Manganese and Cadmium in RBCs, Before and After Adjustment for Folate
Table S8. Prevalence Ratios and 95% CIs for Preeclampsia in Relationship to Manganese in RBCs, Before and After Adjustment for Other Trace Minerals and Heavy Metals (n=1232)
Table S9. Prevalence Ratios and 95% CIs for Preeclampsia in Relationship to Cadmium in RBCs, Before and After Adjustment for Other Trace Minerals and Heavy Metals (n=1274)
Figure S1. Flow chart of sample selection for the current analysis.
Acknowledgments
The authors thank all of the study participants in the Boston Birth Cohort, the Boston University Medical Center Labor and Delivery Nursing Staff, the Boston Birth Cohort field team, and the Public Health and Environmental Laboratories in the Department of Health of the State of New Jersey for their invaluable contributions to this research.
(J Am Heart Assoc. 2019;8:e012436 DOI: 10.1161/JAHA.119.012436.)
References
- 1. Task Force on Hypertension in Pregnancy . Hypertension in Pregnancy, Washington, DC: American College of Obstetricians and Gynecologists: 2013. [DOI] [PubMed] [Google Scholar]
- 2. Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. Br J Obstet Gynaecol. 1992;99:547–553. [DOI] [PubMed] [Google Scholar]
- 3. Van Lerberghe W. The World Health Report 2005: make every mother and child count. Geneva, Switzerland: World Health Organization: 2005. [Google Scholar]
- 4. Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd‐Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D'Armiento J, Kris‐Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC Jr, Sopko G, Chandra‐Strobos N, Urbina EM, Vaccarino V, Wenger NK. Effectiveness‐based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the american heart association. Circulation. 2011;123:1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation. 2010;122:579–584. [DOI] [PubMed] [Google Scholar]
- 6. Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21:521–526. [DOI] [PubMed] [Google Scholar]
- 7. Maric‐Bilkan C, Abrahams VM, Arteaga SS, Bourjeily G, Conrad KP, Catov JM, Costantine MM, Cox B, Garovic V, George EM, Gernand AD, Jeyabalan A, Karumanchi SA, Laposky AD, Miodovnik M, Mitchell M, Pemberton VL, Reddy UM, Santillan MK, Tsigas E, Thornburg KLR, Ward K, Myatt L, Roberts JM. Research recommendations from the National Institutes of Health workshop on predicting, preventing, and treating preeclampsia. Hypertension. 2019;73:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rolnik DL, Wright D, Poon LC, O'Gorman N, Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D, Singh M, Molina FS, Persico N, Jani JC, Plasencia W, Papaioannou G, Tenenbaum‐Gavish K, Meiri H, Gizurarson S, Maclagan K, Nicolaides KH. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–622. [DOI] [PubMed] [Google Scholar]
- 9. Miriyala S, Spasojevic I, Tovmasyan A, Salvemini D, Vujaskovic Z, St Clair D, Batinic‐Haberle I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim Biophys Acta. 2012;1822:794–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li L, Yang X. The essential element manganese, oxidative stress, and metabolic diseases: links and interactions. Oxid Med Cell Longev. 2018;2018:7580707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Banerjee S, Randeva H, Chambers AE. Mouse models for preeclampsia: disruption of redox‐regulated signaling. Reprod Biol Endocrinol. 2009;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maduray K, Moodley J, Soobramoney C, Moodley R, Naicker T. Elemental analysis of serum and hair from pre‐eclamptic South African women. J Trace Elem Med Biol. 2017;43:180–186. [DOI] [PubMed] [Google Scholar]
- 13. Elongi Moyene JP, Scheers H, Tandu‐Umba B, Haufroid V, Buassa‐Bu‐Tsumbu B, Verdonck F, Spitz B, Nemery B. Preeclampsia and toxic metals: a case‐control study in Kinshasa, DR Congo. Environ Health. 2016;15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al‐Jameil N, Tabassum H, Al‐Mayouf H, Aljohar HI, Alenzi ND, Hijazy SM, Khan FA. Analysis of serum trace elements‐copper, manganese and zinc in preeclamptic pregnant women by inductively coupled plasma optical emission spectrometry: a prospective case controlled study in Riyadh, Saudi Arabia. Int J Clin Exp Pathol. 2014;7:1900–1910. [PMC free article] [PubMed] [Google Scholar]
- 15. Sarwar MS, Ahmed S, Ullah MS, Kabir H, Rahman GK, Hasnat A, Islam MS. Comparative study of serum zinc, copper, manganese, and iron in preeclamptic pregnant women. Biol Trace Elem Res. 2013;154:14–20. [DOI] [PubMed] [Google Scholar]
- 16. Goodarzi Khoigani M, Paknahad Z, Mardanian F. The relationship between nutrients intake and preeclampsia in pregnant women. J Res Med Sci. 2012;17:S217. [Google Scholar]
- 17. Vigeh M, Yokoyama K, Ramezanzadeh F, Dahaghin M, Sakai T, Morita Y, Kitamura F, Sato H, Kobayashi Y. Lead and other trace metals in preeclampsia: a case‐control study in Tehran, Iran. Environ Res. 2006;100:268–275. [DOI] [PubMed] [Google Scholar]
- 18. Vanderlelie J, Venardos K, Perkins A. Selenium deficiency as a model of experimental pre‐eclampsia in rats. Reproduction. 2004;128:635–641. [DOI] [PubMed] [Google Scholar]
- 19. Rayman MP, Searle E, Kelly L, Johnsen S, Bodman‐Smith K, Bath SC, Mao J, Redman CW. Effect of selenium on markers of risk of pre‐eclampsia in UK pregnant women: a randomised, controlled pilot trial. Br J Nutr. 2014;112:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nair AR, Degheselle O, Smeets K, Van Kerkhove E, Cuypers A. Cadmium‐induced pathologies: where is the oxidative balance lost (or not)? Int J Mol Sci. 2013;14:6116–6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kolluru GK, Tamilarasan KP, Geetha Priya S, Durgha NP, Chatterjee S. Cadmium induced endothelial dysfunction: consequence of defective migratory pattern of endothelial cells in association with poor nitric oxide availability under cadmium challenge. Cell Biol Int. 2006;30:427–438. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Q, Huang YP, Zhang KK, Huang YJ, Yan Y, Wang F, Wu J, Wang X, Xu ZY, Chen YT, Cheng X, Li Y, Jiao JY, Ye DY. Cadmium‐induced immune abnormality is a key pathogenic event in human and rat models of preeclampsia. Environ Pollut. 2016;218:770–782. [DOI] [PubMed] [Google Scholar]
- 23. Kahn LG, Trasande L. Environmental toxicant exposure and hypertensive disorders of pregnancy: recent findings. Curr Hypertens Rep. 2018;20:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. El‐Badry A, Rezk M, El‐Sayed H. Mercury‐induced oxidative stress may adversely affect pregnancy outcome among dental staff: a cohort study. Int J Occup Environ Med. 2018;9:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. VanderWeele TJ, Shpitser I. On the definition of a confounder. Ann Stat. 2013;41:196–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension. 2006;47:203–208. [DOI] [PubMed] [Google Scholar]
- 27. Faupel‐Badger JM, Hsieh CC, Troisi R, Lagiou P, Potischman N. Plasma volume expansion in pregnancy: implications for biomarkers in population studies. Cancer Epidemiol Biomarkers Prev. 2007;16:1720–1723. [DOI] [PubMed] [Google Scholar]
- 28. Chandra S, Tripathi AK, Mishra S, Amzarul M, Vaish AK. Physiological changes in hematological parameters during pregnancy. Indian J Hematol Blood Transfus. 2012;28:144–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Z, Myers R, Wei T, Bind E, Kassim P, Wang G, Ji Y, Hong X, Caruso D, Bartell T, Gong Y, Strickland P, Navas‐Acien A, Guallar E, Wang X. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J Expo Sci Environ Epidemiol. 2014;24:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287:195–202. [DOI] [PubMed] [Google Scholar]
- 31. Croghan C, Egeghy PP. Methods of dealing with values below the limit of detection using SAS. Southern SAS User Group. 2003;22:24. [Google Scholar]
- 32. Schroeder BM; American College of O and Gynecologists . ACOG practice bulletin on diagnosing and managing preeclampsia and eclampsia. American College of Obstetricians and Gynecologists. Am Fam Physician. 2002;66:330–331. [PubMed] [Google Scholar]
- 33. Wang GY, Hu FB, Mistry KB, Zhang CL, Ren FZ, Huo Y, Paige D, Bartell T, Hong XM, Caruso D, Ji ZC, Chen Z, Ji YL, Pearson C, Ji HK, Zuckerman B, Cheng TL, Wang XB. Association between maternal prepregnancy body mass index and plasma folate concentrations with child metabolic health. JAMA Pediatrics. 2016;170:e160845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huo Y, Li JP, Qin XH, Huang YN, Wang XB, Gottesman RF, Tang GF, Wang BY, Chen DF, He ML, Fu J, Cai YF, Shi XL, Zhang Y, Cui YM, Sun NL, Li XY, Cheng XS, Wang JA, Yang XC, Yang TL, Xiao CS, Zhao G, Dong Q, Zhu DL, Wang X, Ge JB, Zhao LY, Hu DY, Liu LS, Hou FF, Investigators C. Efficacy of Folic Acid Therapy in Primary Prevention of Stroke Among Adults With Hypertension in China The CSPPT Randomized Clinical Trial. JAMA. 2015;313:1325–1335. [DOI] [PubMed] [Google Scholar]
- 35. Maclure M, Greenland S. Tests for trend and dose response: misinterpretations and alternatives. Am J Epidemiol. 1992;135:96–104. [DOI] [PubMed] [Google Scholar]
- 36. Mantel N. Chi‐square tests with one degree of freedom; extensions of the mantel‐haenszel procedure. J Am Stat Assoc. 1963;58:690–700. [Google Scholar]
- 37. Laine JE, Ray P, Bodnar W, Cable PH, Boggess K, Offenbacher S, Fry RC. Placental cadmium levels are associated with increased preeclampsia risk. PLoS One. 2015;10:e0139341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang F, Fan F, Wang L, Ye W, Zhang Q, Xie S. Maternal cadmium levels during pregnancy and the relationship with preeclampsia and fetal biometric parameters. Biol Trace Elem Res. 2018;186:322–329. [DOI] [PubMed] [Google Scholar]
- 39. Poropat AE, Laidlaw MAS, Lanphear B, Ball A, Mielke HW. Blood lead and preeclampsia: a meta‐analysis and review of implications. Environ Res. 2018;160:12–19. [DOI] [PubMed] [Google Scholar]
- 40. Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, Matte TD. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). JAMA. 1994;272:284–291. [PubMed] [Google Scholar]
- 41. Tsoi MF, Cheung CL, Cheung TT, Cheung BM. Continual decrease in blood lead level in Americans: United Sstates National Health Nutrition and Examination Survey 1999–2014. Am J Med. 2016;129:1213–1218. [DOI] [PubMed] [Google Scholar]
- 42. Tara F, Maamouri G, Rayman MP, Ghayour‐Mobarhan M, Sahebkar A, Yazarlu O, Ouladan S, Tavallaie S, Azimi‐Nezhad M, Shakeri MT, Boskabadi H, Oladi M, Sangani MT, Razavi BS, Ferns G. Selenium supplementation and the incidence of preeclampsia in pregnant Iranian women: a randomized, double‐blind, placebo‐controlled pilot trial. Taiwan J Obstet Gynecol. 2010;49:181–187. [DOI] [PubMed] [Google Scholar]
- 43. Matsubara K, Higaki T, Matsubara Y, Nawa A. Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. Int J Mol Sci. 2015;16:4600–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chappell LC, Seed PT, Briley AL, Kelly FJ, Lee R, Hunt BJ, Parmar K, Bewley SJ, Shennan AH, Steer PJ, Poston L. Effect of antioxidants on the occurrence of pre‐eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354:810–816. [DOI] [PubMed] [Google Scholar]
- 45. Jacobo‐Estrada T, Santoyo‐Sanchez M, Thevenod F, Barbier O. Cadmium handling, toxicity and molecular targets involved during pregnancy: lessons from experimental models. Int J Mol Sci. 2017;18:1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Houston MC. The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease, and myocardial infarction. Altern Ther Health Med. 2007;13:S128–S133. [PubMed] [Google Scholar]
- 47. Raymond D, Peterson E. A critical review of early‐onset and late‐onset preeclampsia. Obstet Gynecol Surv. 2011;66:497–506. [DOI] [PubMed] [Google Scholar]
- 48. American College of Obstetricians and Gynecologists . Exposure to toxic environmental agents. Committee opinion No.575. J Obstet Gynecol. 2013;122:931–935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Spearman Rank‐Order Correlation Coefficients Among Trace Minerals and Heavy Metals (n=1274)
Table S2. Characteristics of Participants in the Boston Birth Cohort by RBC Manganese Level (n=1232)
Table S3. Characteristics of Participants in the Boston Birth Cohort by RBC Selenium Level (n=1274)
Table S4. Characteristics of Participants in the Boston Birth Cohort by RBC Cadmium Level (n=1274)
Table S5. Characteristics of Participants in the Boston Birth Cohort by RBC Lead Level (n=1274)
Table S6. Characteristics of Participants in the Boston Birth Cohort by RBC Mercury Level (n=1274)
Table S7. Prevalence Ratios and 95% CIs for Preeclampsia in Relationship to Manganese and Cadmium in RBCs, Before and After Adjustment for Folate
Table S8. Prevalence Ratios and 95% CIs for Preeclampsia in Relationship to Manganese in RBCs, Before and After Adjustment for Other Trace Minerals and Heavy Metals (n=1232)
Table S9. Prevalence Ratios and 95% CIs for Preeclampsia in Relationship to Cadmium in RBCs, Before and After Adjustment for Other Trace Minerals and Heavy Metals (n=1274)
Figure S1. Flow chart of sample selection for the current analysis.


