Abstract
Background
Despite limitations as a stand‐alone parameter, left ventricular (LV) ejection fraction is the preferred measure of myocardial function and marker for postinfarction risk stratification. LV myocardial uniformity alterations may provide superior prognostic information after acute myocardial infarction, which was the subject of this study.
Methods and Results
Consecutive patients with acute myocardial infarction (n=1082; median age: 63 years; 75% male) undergoing cardiac magnetic resonance at a median of 3 days after infarction were included in this multicenter observational study. Circumferential and radial uniformity ratio estimates were derived from cardiac magnetic resonance feature tracking as markers of mechanical uniformity alterations (values between 0 and 1 with 1 reflecting perfect uniformity). The clinical end point was the 12‐month rate of major adverse cardiac events, consisting of all‐cause death, reinfarction, and new congestive heart failure. Patients with major adverse cardiac events (n=73) had significantly impaired circumferential uniformity ratio estimates (0.76 [interquartile range: 0.67–0.86] versus 0.84 [interquartile range: 0.76–0.89]; P<0.001) and radial uniformity ratio estimates (0.69 [interquartile range: 0.60–0.79] versus 0.76 [interquartile range: 0.67–0.83]; P<0.001) compared with patients without events. Although uniformity estimates did not provide independent prognostic information in the overall cohort, a circumferential uniformity ratio estimate below the median of 0.84 emerged as an independent predictor of outcome in postinfarction patients with LV ejection fraction >35% (n=959), even after adjustment for established risk factors (hazard ratio: 1.99; 95% CI, 1.06–3.74; P=0.033 in multivariable Cox regression analysis). In contrast, LV ejection fraction was not associated with adverse events in this subgroup of patients with acute myocardial infarction.
Conclusions
Cardiac magnetic resonance–derived estimates of mechanical uniformity alterations are novel markers for risk assessment after acute myocardial infarction, and the circumferential uniformity ratio estimate provides independent prognostic information for patients with preserved or only moderately reduced LV ejection fraction.
Keywords: acute myocardial infarction, cardiac magnetic resonance, feature tracking, mechanical uniformity, prognosis
Subject Categories: Myocardial Infarction, Prognosis
Clinical Perspective
What Is New?
In this study, cardiac magnetic resonance myocardial feature tracking–derived estimates of left ventricular uniformity alterations emerged as novel markers for risk assessment after acute myocardial infarction and provided independent prognostic information in postinfarction patients with preserved or only moderately impaired left ventricular ejection fraction >35%.
What Are the Clinical Implications?
Left ventricular mechanical uniformity alterations have great potential to improve postinfarction risk stratification (eg, regarding arrhythmic events or adverse remodeling) beyond left ventricular ejection fraction and might help to improve outcome by enabling a more tailored pharmacological or device therapy.
Introduction
The prognosis of patients with acute myocardial infarction (AMI) has significantly improved in recent years, primarily as a result of advances in interventional and medical treatment options.1 Nevertheless, AMI survivors still face substantial risk of recurrent cardiovascular events, and early risk assessment is recommended to reduce morbidity and mortality following AMI.2, 3 Left ventricular ejection fraction (LVEF) is a powerful predictor of adverse events and the preferred functional marker for routine risk stratification and therapeutic decision‐making.2, 3, 4, 5, 6 However, LVEF is mainly determined by global, systolic function without adequately reflecting other components of cardiac contractility or subtle focal changes. Furthermore, the majority of AMI survivors maintain preserved or only moderately reduced LVEF. Consequently, the greatest numbers of recurrent adverse events occur in these patients despite their lower relative risk compared with the high‐risk but small group of patients with severely impaired LVEF. For these reasons, LVEF has major limitations as a stand‐alone parameter for postinfarction outcome, and increasing efforts have been directed toward improving risk stratification beyond sole calculation of LVEF.6 Cardiac magnetic resonance (CMR) imaging allows detailed visualization of morphological and microvascular alterations after AMI and provides incremental prognostic information over and above established clinical variables and LVEF.4, 7 Moreover, CMR myocardial feature tracking (CMR‐FT)–derived deformation indexes emerged as superior measures of left ventricular (LV) performance and valuable tools for optimized postinfarction risk assessment.8, 9 CMR‐FT techniques have also been successfully applied for quantification of LV mechanical uniformity alterations, which may represent surrogate markers of LV dyssynchrony and potentially also be useful prognostic markers in patients with AMI.10, 11, 12 Postinfarction uniformity alterations have been associated with hemodynamic alterations, adverse LV remodeling, and clinical outcome.13, 14, 15, 16, 17, 18, 19 However, the usefulness of LV mechanical uniformity alterations for the prediction of future cardiovascular events in AMI survivors has not yet been comprehensively evaluated in an adequately sized multicenter trial. The aim of this study was to determine the prognostic value of CMR‐FT–based assessment of LV mechanical uniformity alterations in a large multicenter AMI population including patients with ST‐segment–elevation myocardial infarction (STEMI) and non‐STEMI (NSTEMI).
Methods
Study Population
The data that support the findings of this study are available from the corresponding author on reasonable request. The population of this multicenter CMR study consisted of 1235 patients with AMI participating in 2 randomized trials, AIDA‐STEMI (Abciximab Intracoronary versus intravenously Drug Application in ST‐Elevation Myocardial Infarction) and TATORT‐NSTEMI (Thrombus Aspiration in Thrombus Containing Culprit Lesions in Non‐ST‐Elevation Myocardial Infarction).20, 21, 22 Detailed study protocols and main results have been published previously. In brief, AIDA‐STEMI randomly assigned patients presenting with STEMI in the first 12 hours after symptom onset to intracoronary or intravenous abciximab bolus during primary percutaneous coronary intervention with subsequent 12‐hour intravenous infusion in both groups.20 Consecutive patients at 8 sites in Germany with proven expertise in CMR imaging were enrolled in the CMR substudy (n=795).21 The results did not show a difference regarding clinical outcome or CMR parameters of myocardial damage between the treatment groups.20, 21 TATORT‐NSTEMI randomized 440 patients with NSTEMI at 7 sites in Germany to investigate the effect of aspiration thrombectomy on microvascular damage in CMR imaging.22 Compared with standard percutaneous coronary intervention, additional aspiration thrombectomy did not improve reperfusion injury, infarct size, or clinical outcome. Patients in both studies received reperfusion therapy with primary percutaneous coronary intervention and state‐of‐the‐art postinfarction medical treatment according to guideline recommendations.2, 3
Infarct patients were compared with a control group consisting of 40 consecutive patients undergoing CMR imaging within clinical routine at University Medical Center Göttingen. Patients were eligible as controls provided that cardiac morphology and function did not show any alterations.
AIDA‐STEMI (NCT00712101) and TATORT‐NSTEMI (NCT01612312) were registered with ClinicalTrials.gov and approved by the ethics committees of the participating sites. This CMR‐FT study was supported by a grant from the German Center for Cardiovascular Research and conducted according to the Declaration of Helsinki. Patients gave written informed consent for study participation.
CMR Imaging Protocol
All patients underwent CMR imaging on clinical 1.5‐ or 3.0‐T scanners within 10 days after infarction. The standardized protocol has been published previously and included ECG‐gated balanced steady‐state free precession sequences to assess LV function and T1‐weighted late gadolinium enhancement images to determine myocardial and microvascular damage.4, 21, 22 All sequences were acquired in 2‐ and 4‐chamber long‐axis views and continuous stacks of short‐axis slices covering the whole left ventricle. The same CMR protocol was used in all AMI patients and in the control group.
CMR Analysis
Infarct characteristics and LVEF were analyzed at a core laboratory by blinded investigators using certified evaluation software (cmr42; Circle Cardiovascular Imaging).4, 21 All parameters were determined in sequential short‐axis planes. Established threshold techniques were applied to assess infarct size and microvascular obstruction as a percentage of LV mass. Furthermore, infarct transmurality and the number of involved LV segments (according to the 17‐segments model) were assessed visually.
CMR‐FT was performed in an experienced core laboratory at the University Medical Center Göttingen using dedicated software (2D CPA MR, Cardiac Performance Analysis v1.1.2; TomTec Imaging Systems). Circumferential and radial strain were derived from balanced steady‐state free precession sequences at basal, midventricular, and apical locations, as described previously.9, 23 In brief, LV endocardial borders were manually traced followed by the application of an automatic border‐tracking algorithm. Accurate tracking was ensured by visual review and manual adjustments, if necessary. Final values were based on the average of 3 independent analyses. Scans that did not allow for reliable tracking were excluded. Uniformity was evaluated based on the assumption that perfectly uniform contraction results in equal strain across the myocardium at a given point in time, whereas opposing walls exhibit opposing strains in nonuniform hearts. Consequently, circumferential and radial strain of 48 evenly distributed locations were plotted against spatial positions for each time frame within the respective apical, midventricular, and basal slices. Corresponding plots were subjected to Fourier analysis. Circumferential uniformity ratio estimate (CURE) and radial uniformity ratio estimate (RURE) were calculated per slice with subsequent averaging across basal, midventricular, and apical slices and then expressed as global myocardial values, as described previously.10, 12, 24 Resulting values for CURE and RURE range between 0 (corresponding to complete nonuniformity) and 1 (corresponding to perfect uniformity; Figure 1). The CMR‐FT core laboratory in Göttingen has repeatedly proven excellent reproducibility and low inter‐ and intraobserver variability for strain assessments and synchrony analyses.9, 10, 23
Figure 1.
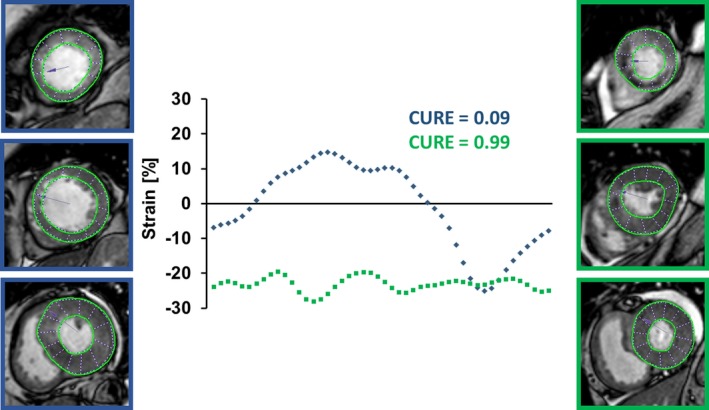
Derivation of uniformity ratio estimates from cardiac magnetic resonance myocardial feature tracking. Global circumferential strain values (GCS) are plotted at 48 evenly distributed locations against their spatial positions during the cardiac cycle. Assuming that equal strain across the myocardium at any given time results in perfect uniformity, CURE/RURE=1. Spatially divergent strain values result in oscillations within the plots representing myocardial uniformity alterations with a peak at CURE/RURE=0. The blue dotted line represents CURE at end‐systole of a patient with extensive uniformity alterations and MACE during follow‐up. The green dotted line represents CURE at end‐systole of a patient with uniform contraction and no MACE during follow‐up. CURE indicates circumferential uniformity ratio estimate; MACE, major adverse cardiac events; RURE, radial uniformity ratio estimate.
Clinical End Points
The clinical end point of this study was the 12‐month rate of major adverse cardiac events (MACE), consisting of all‐cause death, reinfarction, and new congestive heart failure. Each patient contributed only once to the composite end point to avoid double counting in case of multiple events per patient (death, reinfarction, and new congestive heart failure). A fully blinded clinical end points committee adjudicated all events based on data provided by the study sites. More detailed end point definitions have been reported previously.20, 21, 22
Statistical Analysis
Categorical variables are presented as frequencies and percentages. Continuous variables were nonnormally distributed in a Shapiro–Wilk test and are provided as median with interquartile range (IQR). Comparisons were performed with the χ2 test for categorical data and the nonparametric Mann–Whitney U test for continuous variables. Baseline characteristics and CMR findings are described according to the occurrence of MACE. Furthermore, CURE and RURE were compared with the healthy control group and between patients with STEMI and NSTEMI. Correlations between LVEF and infarct size with uniformity ratio estimates were analyzed with the Spearman method. CURE and RURE were additionally assessed according to infarct transmurality (Mann–Whitney U test) and the number of involved segments (Kruskal–Wallis test). Patients were stratified according to median uniformity estimates to assess the composite 12‐month MACE end point with the Kaplan–Meier method and log‐rank testing. Analyses were performed for the overall AMI cohort and separately for patients with STEMI and NSTEMI. Predictors of MACE were identified in univariate and stepwise multivariable Cox regression analyses. Hazard ratios with corresponding 95% CIs are provided. All baseline characteristics and CMR findings were considered for univariate analysis. Only significant predictors in univariate analysis (P<0.05) were included in the multivariable model, which comprised a stepwise approach with P‐value thresholds to keep or remove the variables of 0.05 and 0.1, respectively. Uniformity ratio estimates were entered dichotomized according to median values into the regression models. Furthermore, CURE and RURE were also explored as continuous variables. In case CURE and RURE did not reach statistical significance in univariate testing, we also analyzed a selected multivariable model in which the main variables were retained regardless of statistical significance. The clinical end point was assessed in the overall study cohort and in the subgroup of patients with LVEF >35%, using identical approaches. The cutoff at 35% is of clinical relevance because current heart failure guidelines recommend intensified medical treatment (eg, addition of a mineralocorticoid receptor antagonist or switch to angiotensin receptor neprilysin inhibitor) and evaluation regarding prophylactic cardioverter‐defibrillator implantation or cardiac resynchronization therapy in symptomatic patients with ejection fraction ≤35%.25 Prognostic markers in the predominant group of postinfarction patients with preserved LVEF >35% might help identify additional patients who could benefit from these treatment approaches. All analyses were performed with SPSS v23.0 (IBM Corp). A 2‐tailed P<0.05 was considered statistically significant.
Results
Of the 1235 patients with AMI participating in AIDA‐STEMI and TATORT‐NSTEMI, 1082 patients had complete CMR protocols with sufficient quality to assess LV mechanical uniformity alterations (STEMI: n=762; NSTEMI: n=320; Figure 2). CMR was performed in a median of 3 days (IQR: 2–4 days) after infarction. Follow‐up data 12 months after the index event were available for 1080 patients (99.8%) and showed 73 MACE (death: n=32; reinfarction: n=21; congestive heart failure: n=20).
Figure 2.
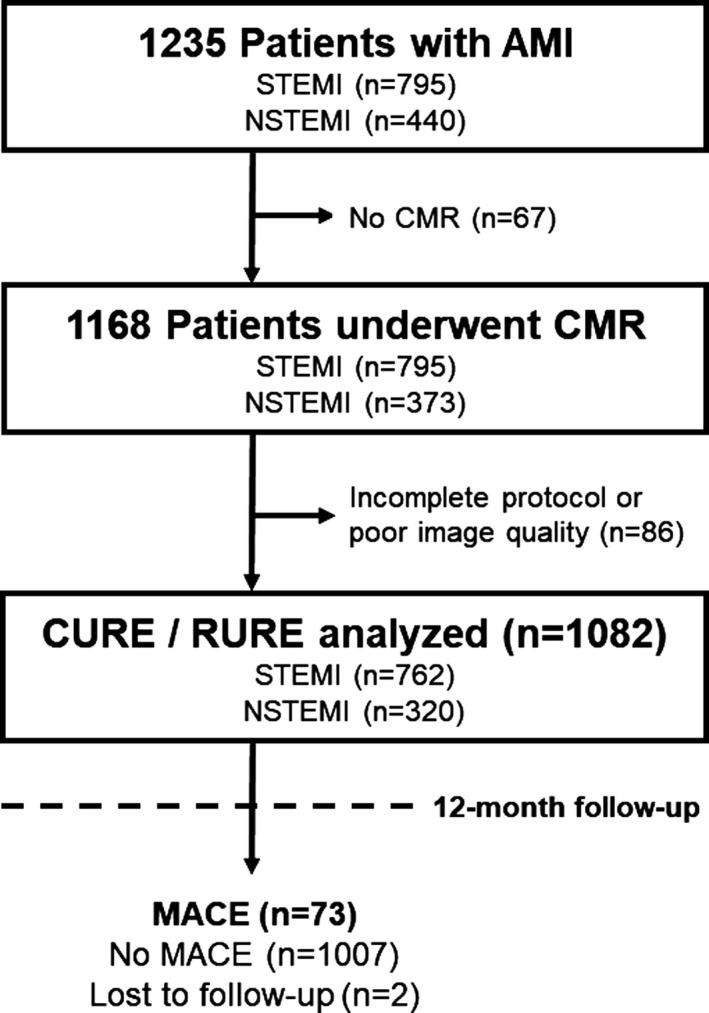
Study flowchart. AMI indicates acute myocardial infarction; CMR, cardiac magnetic resonance; CURE, circumferential uniformity ratio estimate; MACE, major adverse cardiac events; NSTEMI, non–ST‐segment–elevation myocardial infarction; RURE, radial uniformity ratio estimate; STEMI, ST‐segment–elevation myocardial infarction.
Patient Characteristics
Baseline clinical and angiographic characteristics and their association with MACE are illustrated in Table 1. The patient population was predominantly male (75%) with a median age of 63 years (IQR: 53–72 years). Patients with MACE at 12‐month follow‐up were significantly older (P<0.001), less often male (P=0.030) or smokers (P=0.015), and had a higher prevalence of hypertension (P=0.006) and diabetes mellitus (P=0.006). Furthermore, significant differences existed regarding Killip class on admission (P<0.001) and the number of diseased coronary vessels (P=0.012).
Table 1.
Baseline Characteristics
| Variable | All Patients (n=1082) | MACE (n=73) | No MACE (n=1007) | P Value |
|---|---|---|---|---|
| Age, y | 63 (53–72) | 72 (61–77) | 63 (52–72) | <0.001 |
| Male sex | 811/1082 (75.0) | 47/73 (64.4) | 763/1007 (75.8) | 0.030 |
| Cardiovascular risk factors | ||||
| Current smoking | 432/1002 (43.1) | 19/66 (28.8) | 412/934 (44.1) | 0.015 |
| Hypertension | 767/1080 (71.0) | 62/73 (84.9) | 703/1005 (70.0) | 0.006 |
| Hyperlipoproteinemia | 410/1074 (38.2) | 25/73 (34.2) | 384/999 (38.4) | 0.477 |
| Diabetes mellitus | 246/1080 (22.8) | 26/73 (35.6) | 219/1005 (21.8) | 0.006 |
| Body mass index, kg/m2 | 27.4 (25.0–30.4) | 27.0 (25.2–31.0) | 27.4 (24.9–30.3) | 0.899 |
| Previous myocardial infarction | 75/1080 (6.9) | 5/73 (6.8) | 69/1005 (6.9) | 0.996 |
| Previous PCI | 90/1081 (8.3) | 5/73 (6.8) | 84/1006 (8.3) | 0.653 |
| Previous CABG | 20/1081 (1.9) | 2/73 (2.7) | 18/1006 (1.8) | 0.561 |
| ST‐segment elevation | 762/1082 (70.4) | 51/73 (69.9) | 711/1007 (70.6) | 0.893 |
| Time from symptom onset to PCI hospital admission, min* | 180 (109–317) | 191 (116–363) | 180 (109–310) | 0.397 |
| Door‐to‐balloon time, min* | 30 (22–42) | 28 (24–40) | 30 (22–42) | 0.497 |
| Killip class on admission | <0.001 | |||
| 1 | 964/1082 (89.1) | 49/73 (67.1) | 913/1007 (90.7) | |
| 2 | 80/1082 (7.4) | 15/73 (20.5) | 65/1007 (6.5) | |
| 3 | 21/1082 (1.9) | 4/73 (5.5) | 17/1007 (1.7) | |
| 4 | 17/1082 (1.6) | 5/73 (6.8) | 12/1007 (1.2) | |
| Number of diseased vessels | 0.012 | |||
| 1 | 541/1082 (50.0) | 26/73 (35.6) | 514/1007 (51.0) | |
| 2 | 327/1082 (30.2) | 24/73 (32.9) | 303/1007 (30.1) | |
| 3 | 214/1082 (19.8) | 23/73 (32.5) | 190/1007 (18.9) | |
| Infarct‐related artery | 0.109 | |||
| Left anterior descending | 443/1082 (40.9) | 39/73 (53.4) | 404/1007 (40.1) | |
| Left circumflex | 218/1082 (20.1) | 13/73 (17.8) | 203/1007 (20.2) | |
| Left main | 6/1082 (0.6) | 1/73 (1.4) | 5/1007 (0.5) | |
| Right coronary artery | 408/1082 (37.7) | 19/73 (26.0) | 389/1007 (38.6) | |
| Bypass graft | 7/1082 (0.6) | 1/73 (1.4) | 6/1007 (0.6) | |
| TIMI flow grade before PCI | 0.617 | |||
| 0 | 550/1082 (50.8) | 42/73 (57.5) | 507/1007 (50.3) | |
| 1 | 121/1082 (11.2) | 5673 (8.2) | 115/1007 (11.4) | |
| 2 | 216/1082 (20.0) | 12/73 (16.4) | 203/1007 (20.2) | |
| 3 | 195/1082 (18.0) | 13/73 (17.8) | 182/1007 (18.1) | |
| TIMI flow grade after PCI | 0.650 | |||
| 0 | 20/1082 (1.8) | 1/73 (1.4) | 19/1007 (1.9) | |
| 1 | 21/1082 (1.9) | 2/73 (2.7) | 19/1007 (1.9) | |
| 2 | 82/1082 (7.6) | 8/73 (11.0) | 74/1007 (7.3) | |
| 3 | 959/1082 (88.6) | 62/73 (84.9) | 895/1007 (88.9) | |
| Concomitant medications | ||||
| Aspirin | 1080/1082 (99.8) | 73/73 (100) | 1005/1007 (99.8) | 0.703 |
| Clopidogrel/prasugrel/ticagrelor | 1082/1082 (100) | 73/73 (100) | 1007/1007 (100) | ··· |
| β‐Blocker | 1032/1080 (95.6) | 71/73 (97.3) | 959/1005 (95.4) | 0.462 |
| ACEI/AT‐1 antagonist | 991/1080 (91.8) | 69/73 (94.5) | 921/1005 (91.6) | 0.386 |
| Aldosterone antagonist | 140/1080 (13.0) | 22/73 (30.1) | 118/1005 (11.7) | <0.001 |
| Statin | 1032/1080 (95.6) | 70/73 (95.9) | 960/1005 (95.5) | 0.883 |
Data presented as n/N (%) or median (interquartile range). P values were calculated for the comparison between patients with and without MACE. AT‐1 antagonist indicates angiotensin II type I receptor antagonist; ACEI, angiotensin‐converting enzyme inhibitor; CABG, coronary artery bypass grafting; MACE, major adverse cardiac events; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction. Two patients were lost to follow‐up regarding MACE.
Assessed only in patients with ST‐segment–elevation myocardial infarction (n=795).
CMR Infarct Characteristics and Uniformity Alterations
Structural and functional CMR imaging parameters are provided in Table 2. The median infarct size was 13.3% of LV mass (IQR: 5.4–21.7%) with a microvascular obstruction zone of 0.4% of LV mass (IQR: 0–2.0%) and LVEF of 50.5% (IQR: 43.5–57.6%). Uniformity ratio estimates in the overall study population were as follows: CURE of 0.84 (IQR: 0.75–0.89) and RURE of 0.75 (IQR: 0.67–0.83). In comparison, a healthy control group (n=40; 50% male; median age: 64 years [IQR: 46–76 years]; median LVEF: 69% [IQR: 65–72%]) showed significantly higher values for CURE (0.92 [IQR: 0.89–0.94]; P<0.001) and RURE (0.79 [IQR: 0.74–0.85]; P=0.020). Uniformity ratio estimates correlated significantly with LVEF and infarct size (Figure S1). Furthermore, transmural infarction and an increasing number of involved segments were associated with more pronounced LV uniformity alterations (Figures S2 and S3). Although CURE was similarly reduced in STEMI and NSTEMI (0.83 [IQR: 0.75–0.89] versus 0.84 [IQR: 0.76–0.89]; P=0.544), RURE was significantly lower in STEMI patients (0.74 [IQR: 0.66–0.82] versus 0.78 [IQR: 0.68–0.84]; P=0.001). Patients with MACE had significantly larger infarcts (P=0.001), more microvascular obstruction (P=0.029), lower LVEF (P<0.001), and lower uniformity ratio estimates (P<0.001 for CURE and RURE; Table 2).
Table 2.
CMR Imaging Results
| Variable | All Patients (n=1082) | MACE (n=73) | No MACE (n=1007) | P Value |
|---|---|---|---|---|
| Infarct size (% LV)* | 13.3 (5.4–21.7) | 20.4 (9.3–28.9) | 13.1 (5.3–21.3) | 0.001 |
| Microvascular obstruction (% LV)* | 0.4 (0–2.0) | 1.1 (0–3.2) | 0.3 (0–1.9) | 0.029 |
| LVEF (%) | 50.5 (43.5–57.6) | 40.0 (33.0–51.9) | 50.9 (44.3–57.6) | <0.001 |
| LV end‐diastolic volume, mL | 143 (116–171) | 145 (122–170) | 143 (116–171) | 0.820 |
| LV end‐systolic volume, mL | 70 (53–91) | 86 (61–110) | 69 (53–89) | 0.001 |
| CURE | 0.84 (0.75–0.89) | 0.76 (0.67–0.86) | 0.84 (0.76–0.89) | <0.001 |
| RURE | 0.75 (0.67–0.83) | 0.69 (0.60–0.79) | 0.76 (0.67–0.83) | <0.001 |
Data presented as median (interquartile range). P values were calculated for comparison of patients with and without MACE. CMR indicates cardiac magnetic resonance; CURE, circumferential uniformity ratio estimate; LV, left ventricular; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events; % LV, percentage of left ventricular mass; RURE, radial uniformity ratio estimate. Two patients were lost to follow‐up regarding MACE.
Late gadolinium enhancement imaging was available for 1055 patients (MACE, n=68; no MACE, n=985).
Prognostic Value of LV Uniformity Alterations
Kaplan–Meier plots showing the risk of MACE according to median CURE and RURE in the overall study cohort and in patients with STEMI and NSTEMI are illustrated in Figure 3A and 3B. Uniformity ratio estimates below median were associated with significantly higher 12‐month event rates in the overall AMI population and in the subgroup of patients with STEMI. NSTEMI patients with more pronounced mechanical uniformity alterations had numerically more MACE with a strong trend toward significance in log‐rank testing (P=0.050 for CURE and P=0.067 for RURE). In the overall AMI cohort, CURE and RURE below median were significantly associated with MACE in univariate Cox regression analysis but did not add to the profound prognostic implications of age (P=0.002), Killip class (P=0.024), and particularly LVEF (P<0.001) in stepwise multivariable testing (Table 3). However, considering only patients with LVEF >35% (n=959), CURE below median was a significant predictor of MACE (P=0.033) in addition to age (P=0.006) and the number of diseased coronary vessels (P=0.016; Table 4). In contrast, LVEF was no longer independently associated with adverse events in this subgroup of AMI patients with preserved or only moderately reduced LV function. The results were consistent in selected multivariable models including the main variables regardless of statistical significance and when using uniformity ratio estimates as continuous variables (Tables S1–S3). Kaplan–Meier curves according to median uniformity ratio estimates illustrate the prognostic implications of CURE (Figure 4A), whereas RURE was not predictive for MACE in this subgroup of patients (Figure 4B).
Figure 3.
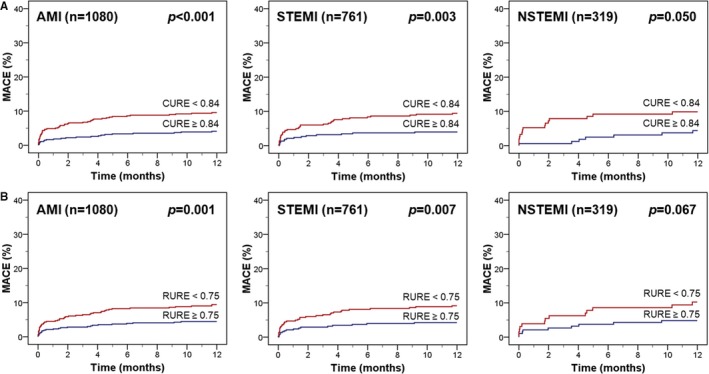
Kaplan–Meier plots according to median uniformity ratio estimates. MACE is illustrated according to CURE (A) and RURE (B). The cutoffs for CURE and RURE are median values in the overall study population. AMI indicates acute myocardial infarction; CURE, circumferential uniformity ratio estimate; MACE, major adverse cardiac events; NSTEMI, non–ST‐segment–elevation myocardial infarction; RURE, radial uniformity ratio estimate; STEMI, ST‐segment–elevation myocardial infarction.
Table 3.
Predictors of MACE in Univariate and Multivariable Cox Regression Analysis
| Variable | Univariate | Stepwise Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, y | 1.05 (1.03–1.07) | <0.001 | 1.04 (1.02–1.07) | 0.002 |
| Male sex | 0.59 (0.37–0.96) | 0.032 | ··· | ··· |
| Current smoking | 0.53 (0.31–0.90) | 0.018 | ··· | ··· |
| Diabetes mellitus | 1.93 (1.20–3.12) | 0.007 | ··· | ··· |
| Hypertension | 2.36 (1.24–4.48) | 0.009 | ··· | ··· |
| Killip class on admission | 2.04 (1.61–2.58) | <0.001 | 1.47 (1.05–2.04) | 0.024 |
| Number of diseased vessels | 1.51 (1.15–2.00) | 0.004 | ··· | ··· |
| LVEF (%) | 0.94 (0.92–0.96) | <0.001 | 0.94 (0.92–0.97) | <0.001 |
| Infarct size (% LV) | 1.03 (1.01–1.05) | <0.001 | ··· | ··· |
| Microvascular obstruction (% LV) | 1.09 (1.03–1.15) | 0.003 | ··· | ··· |
| CURE <0.84* | 2.42 (1.47–3.98) | 0.001 | ··· | ··· |
| RURE <0.75* | 2.17 (1.34–3.53) | 0.002 | ··· | ··· |
CURE indicates circumferential uniformity ratio estimate; HR, hazard ratio; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events; % LV indicates percentage of left ventricular mass; RURE, radial uniformity ratio estimate.
Cutoffs for CURE and RURE are median values in the study population.
Table 4.
Predictors of MACE in Patients With Ejection Fraction >35%
| Variable | Univariate | Stepwise Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, y | 1.05 (1.02–1.08) | <0.001 | 1.04 (1.01–1.07) | 0.006 |
| Male sex | 0.53 (0.30–0.96) | 0.035 | ··· | ··· |
| Current smoking | 0.46 (0.23–0.92) | 0.027 | ··· | ··· |
| Diabetes mellitus | 2.75 (1.55–4.86) | 0.001 | ··· | ··· |
| Hypertension | 2.14 (1.00–4.58) | 0.049 | ··· | ··· |
| Killip class on admission | 1.87 (1.33–2.63) | <0.001 | ··· | ··· |
| Number of diseased vessels | 1.59 (1.13–2.24) | 0.009 | 1.61 (1.09–2.38) | 0.016 |
| LVEF (%) | 0.96 (0.92–0.99) | 0.013 | ··· | ··· |
| Infarct size (% LV) | 1.03 (1.00–1.05) | 0.029 | ··· | ··· |
| CURE <0.84* | 2.57 (1.41–4.68) | 0.002 | 1.99 (1.06–3.74) | 0.033 |
CURE indicates circumferential uniformity ratio estimate; HR, hazard ratio; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events; % LV indicates percentage of left ventricular mass.
The cutoff for CURE is the median value in the study population.
Figure 4.
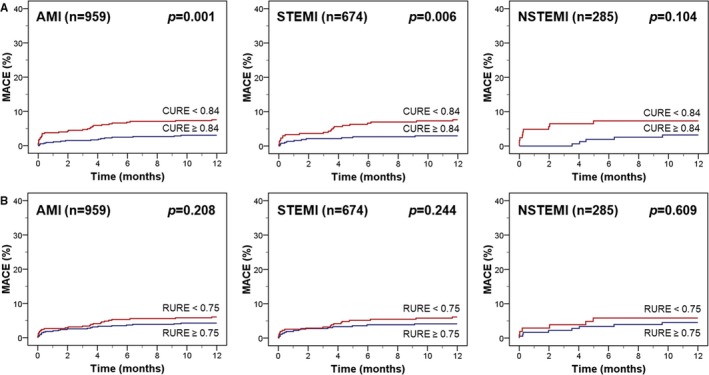
Kaplan–Meier plots according to median uniformity ratio estimates in patients with an ejection fraction >35%. MACE is illustrated in patients with an ejection fraction >35% according to CURE (A) and RURE (B). The cutoffs for CURE and RURE are median values in the overall study population. AMI indicates acute myocardial infarction; CURE, circumferential uniformity ratio estimate; MACE, major adverse cardiac events; NSTEMI, non–ST‐segment–elevation myocardial infarction; RURE, radial uniformity ratio estimate; STEMI, ST‐segment–elevation myocardial infarction.
Discussion
This study is the first to comprehensively assess the prognostic value of LV mechanical uniformity alterations determined by CMR‐FT in a large multicenter population of patients with AMI. The results indicate a significantly higher 12‐month MACE rate in case of ventricular uniformity alterations, although the prognostic implications of LVEF remained superior in the overall study population. In patients with LVEF >35%, however, CURE emerged as an independent predictor of postinfarction adverse events. Consequently, CMR‐FT–derived LV mechanical uniformity alterations enable risk assessment after AMI and expand and complement the prognostic significance of LVEF, the preferred functional marker in clinical routine.
Role of CMR for Postinfarction Risk Assessment
According to current guidelines, it is recommended that myocardial function be determined as a key prognostic factor in all patients with AMI before hospital discharge.2, 3 Routine echocardiography with calculation of LVEF is usually the preferred modality given its broad and easy availability. Nevertheless, CMR imaging allows for more accurate assessment of LVEF and provides additional insights into postinfarction myocardial and microvascular damage. Numerous trials have repeatedly shown incremental prognostic information about infarct size and microvascular obstruction beyond established risk factors and thus emphasize the benefits of visualizing structural changes after AMI.4, 7 Furthermore, extended CMR protocols with T1 mapping techniques and T2* imaging enable even more detailed tissue characterization with additional value for prognostication in AMI survivors.26, 27, 28 Most recently, CMR studies also investigated approaches to overcome the drawbacks of sole LVEF calculation for analysis of myocardial function and identified CMR‐FT as a promising tool. CMR‐FT–derived multidirectional myocardial strain emerged as a superior measure of LV performance and a valuable marker for adverse events following AMI over and above LVEF.9 The current CMR‐FT trial focused on LV mechanical uniformity alterations, an important aspect of ventricular performance that is not sufficiently reflected in LVEF and rather represents postinfarction dyssynchrony. LV mechanical uniformity alterations were associated with adverse outcomes in asymptomatic individuals participating in MESA (Multiethnic Study of Atherosclerosis) and in patients with coronary artery disease.29, 30 Previous studies in AMI cohorts mainly targeted the prediction of postinfarction LV remodeling, whereas clinical outcome data are sparse and mostly derived from small populations.13, 14, 15, 16, 17, 18, 19 Moreover, these investigations used different imaging modalities to assess uniformity (eg, speckle‐tracking echocardiography, single‐photon emission computed tomography, or CMR tagging) with known limitations (eg, image quality and observer dependency, radiation exposure, or time‐consuming acquisition of additional CMR sequences). In contrast, CMR‐FT–derived uniformity ratio estimates are based on high‐quality balanced steady‐state free precession images, which are part of standard CMR protocols. Using this innovative technique, our study proved the association between LV mechanical uniformity alterations and clinical outcome in AMI survivors with independent prognostic implications in patients with LVEF >35%. The results were driven by significantly higher event rates in STEMI patients with LV uniformity alterations. In contrast, the NSTEMI cohort showed a trend without reaching statistical significance, which might be due to lesser myocardial damage or the lower sample size. Regarding the investigated uniformity estimates, CURE turned out to be more suitable for postinfarction risk assessment compared with RURE. This finding is in line with previous studies that identified uniformity measures based on circumferential strain as the most robust and reproducible approach.10 Furthermore, the extent of myocardial injury might also play a role for the superiority of CURE in the overall population with AMI. CURE is already sensitive to subendocardial fiber damage, which can be found in all patients with STEMI and NSTEMI. In contrast, RURE responds after more pronounced transmural infarction, as usually seen in STEMI patients.
Clinical Implications and Future Directions
LVEF is currently the only imaging parameter with direct implications for the management of postinfarction patients (eg, in terms of medical treatment or prophylactic cardioverter‐defibrillator implantation). Other functional or morphological CMR parameters have not yet found their roles in clinical practice despite proven prognostic relevance in multiple studies and even superiority to sole LVEF‐based risk assessment. A few factors may account for this imbalance. First, some clinicians still consider CMR to be a complex and time‐consuming examination restricted to some highly specialized centers. However, contrary to this assumption, local expertise and availability have significantly increased in recent years and a postinfarction CMR protocol can be acquired in roughly 30 minutes, which only marginally exceeds the duration of a comprehensive transthoracic echocardiography. Second, the variety of different CMR parameters for risk stratification impedes clinical use and may be confusing for physicians without advanced CMR knowledge. Risk‐scoring models that incorporate several prognostic markers into a simple score have been introduced recently to overcome this drawback.7 The third and probably most important reason for the slow implementation of CMR‐based risk assessment in clinical routine is the lack of studies investigating CMR‐guided management approaches in patients with AMI. Despite the proven prognostic value of morphological and functional alterations in CMR imaging, any benefit of considering these findings for treatment decisions remains speculative in the absence of randomized trials. However, the scientific basis to assume improved outcome and to initiate such studies is solid. For instance, current decision‐making on postinfarction primary prophylactic cardioverter‐defibrillator implantation, which relies almost exclusively on LVEF, is suboptimal. Only a small portion of patients with implanted devices require interventions after AMI, and patients with preserved ventricular function are not considered for device implantation although arrhythmic events are not uncommon in this population.31 Consequently, additional factors representing pathology beyond LVEF, such as LV mechanical uniformity alterations as surrogate markers of LV dyssynchrony, have great potential to improve postinfarction arrhythmic risk stratification. Furthermore, LV uniformity alterations might help prevent adverse remodeling after AMI by enabling tailored pharmacological therapy (eg, aldosterone antagonists in patients with preserved LVEF but nonuniform contraction). These and other management approaches deserve further exploration in future studies.
Limitations
This multicenter CMR study assessed LV mechanical uniformity alterations caused by regional contraction abnormalities related to ischemic scars and not electrical dyssynchrony because of time delays in contraction. The population was recruited at several sites in Germany using different CMR vendors. However, the scanning protocol was identical at all centers, and data analysis was performed centrally in a core laboratory. In the absence of specific recommendations regarding the optimal time of CMR imaging after AMI, scans were performed within several days after the acute event. It cannot be excluded that CMR‐FT parameters may change over time due to ongoing remodeling processes, similar to the discussed time dependency of myocardial edema.32, 33 Consequently, later assessment of LV mechanical uniformity alterations might have resulted in even better prediction of future cardiovascular events. Furthermore, the results of this study are restricted to stable AMI patients without contraindications to undergo CMR imaging. CMR‐FT–based assessment of LV uniformity alterations was not compared with other techniques (eg, CMR tagging or displacement encoding with stimulated echoes), and reproducibility of CMR‐FT analyses in our core laboratory has been reported in several previous publications and was not repeated in this study.9, 10, 23
Conclusions
This large multicenter study suggests that CMR‐FT–based assessment of LV mechanical uniformity alterations is a novel marker for risk assessment after AMI and that CURE provides independent prognostic information in postinfarction patients with preserved or only moderately reduced LVEF.
Sources of Funding
The study was supported by a DZHK (German Center for Cardiovascular Research) research grant. Lamata holds a Wellcome Trust Senior Research Fellowship (g.a. 209450/Z/17/Z).
Disclosures
None.
Supporting information
Table S1. Predictors of Major Adverse Cardiac Events in Patients With an Ejection Fraction >35% in a Selected Model Including RURE
Table S2. Predictors of Major Adverse Cardiac Events in Univariate and Multivariable Cox Regression Analysis Including Uniformity Ratio Estimates as Continuous Variables
Table S3. Predictors of Major Adverse Cardiac Events in Patients With an Ejection Fraction >35% Including Uniformity Ratio Estimates as Continuous Variables
Figure S1. Correlation between circumferential and radial uniformity ratio estimates with left ventricular ejection fraction and infarct size.
Figure S2. Association of circumferential and radial uniformity ratio estimates with scar transmurality.
Figure S3. Association of circumferential and radial uniformity ratio estimates with the number of infarcted left ventricular segments.
(J Am Heart Assoc. 2019;8:e011576 DOI: 10.1161/JAHA.118.011576.)
Contributor Information
Ingo Eitel, Email: ingo.eitel@uskh.de.
Andreas Schuster, Email: andreas_schuster@gmx.net.
References
- 1. Jernberg T, Johanson P, Held C, Svennblad B, Lindback J, Wallentin L. Association between adoption of evidence‐based treatment and survival for patients with ST‐elevation myocardial infarction. JAMA. 2011;305:1677–1684. [DOI] [PubMed] [Google Scholar]
- 2. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimsky P. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 3. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 4. Eitel I, de Waha S, Wohrle J, Fuernau G, Lurz P, Pauschinger M, Desch S, Schuler G, Thiele H. Comprehensive prognosis assessment by CMR imaging after ST‐segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:2017–2026. [DOI] [PubMed] [Google Scholar]
- 5. Rouleau JL, Talajic M, Sussex B, Potvin L, Warnica W, Davies RF, Gardner M, Stewart D, Plante S, Dupuis R, Lauzon C, Ferguson J, Mikes E, Balnozan V, Savard P. Myocardial infarction patients in the 1990s‐their risk factors, stratification and survival in Canada: the Canadian Assessment of Myocardial Infarction (CAMI) Study. J Am Coll Cardiol. 1996;27:1119–1127. [DOI] [PubMed] [Google Scholar]
- 6. Dagres N, Hindricks G. Risk stratification after myocardial infarction: is left ventricular ejection fraction enough to prevent sudden cardiac death? Eur Heart J. 2013;34:1964–1971. [DOI] [PubMed] [Google Scholar]
- 7. Stiermaier T, Jobs A, de Waha S, Fuernau G, Poss J, Desch S, Thiele H, Eitel I. Optimized prognosis assessment in ST‐segment‐elevation myocardial infarction using a cardiac magnetic resonance imaging risk score. Circ Cardiovasc Imaging. 2017;10:e006774. [DOI] [PubMed] [Google Scholar]
- 8. Schuster A, Hor KN, Kowallick JT, Beerbaum P, Kutty S. Cardiovascular magnetic resonance myocardial feature tracking: concepts and clinical applications. Circ Cardiovasc Imaging. 2016;9:e004077. [DOI] [PubMed] [Google Scholar]
- 9. Eitel I, Stiermaier T, Lange T, Rommel KP, Koschalka A, Kowallick JT, Lotz J, Kutty S, Gutberlet M, Hasenfuss G, Thiele H, Schuster A. Cardiac magnetic resonance myocardial feature tracking for optimized prediction of cardiovascular events following myocardial infarction. JACC Cardiovasc Imaging. 2018;11:1433–1444. [DOI] [PubMed] [Google Scholar]
- 10. Kowallick JT, Morton G, Lamata P, Jogiya R, Kutty S, Hasenfuss G, Lotz J, Chiribiri A, Nagel E, Schuster A. Quantitative assessment of left ventricular mechanical dyssynchrony using cine cardiovascular magnetic resonance imaging: inter‐study reproducibility. JRSM Cardiovasc Dis. 2017;6:2048004017710142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Onishi T, Saha SK, Ludwig DR, Onishi T, Marek JJ, Cavalcante JL, Schelbert EB, Schwartzman D, Gorcsan J. Feature tracking measurement of dyssynchrony from cardiovascular magnetic resonance cine acquisitions: comparison with echocardiographic speckle tracking. J Cardiovasc Magn Reson. 2013;15:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor RJ, Umar F, Moody WE, Meyyappan C, Stegemann B, Townend JN, Hor KN, Miszalski‐Jamka T, Mazur W, Steeds RP, Leyva F. Feature‐tracking cardiovascular magnetic resonance as a novel technique for the assessment of mechanical dyssynchrony. Int J Cardiol. 2014;175:120–125. [DOI] [PubMed] [Google Scholar]
- 13. Mollema SA, Liem SS, Suffoletto MS, Bleeker GB, van der Hoeven BL, van de Veire NR, Boersma E, Holman ER, van der Wall EE, Schalij MJ, Gorcsan J, Bax JJ. Left ventricular dyssynchrony acutely after myocardial infarction predicts left ventricular remodeling. J Am Coll Cardiol. 2007;50:1532–1540. [DOI] [PubMed] [Google Scholar]
- 14. Nucifora G, Bertini M, Ajmone Marsan N, Scholte AJ, Siebelink HM, Holman ER, Schalij MJ, van der Wall EE, Bax JJ, Delgado V. Temporal evolution of left ventricular dyssynchrony after myocardial infarction: relation with changes in left ventricular systolic function. Eur Heart J Cardiovasc Imaging. 2012;13:1041–1046. [DOI] [PubMed] [Google Scholar]
- 15. Shin SH, Hung CL, Uno H, Hassanein AH, Verma A, Bourgoun M, Kober L, Ghali JK, Velazquez EJ, Califf RM, Pfeffer MA, Solomon SD. Mechanical dyssynchrony after myocardial infarction in patients with left ventricular dysfunction, heart failure, or both. Circulation. 2010;121:1096–1103. [DOI] [PubMed] [Google Scholar]
- 16. Ng AC, da Tran T, Allman C, Vidaic J, Leung DY. Prognostic implications of left ventricular dyssynchrony early after non‐ST elevation myocardial infarction without congestive heart failure. Eur Heart J. 2010;31:298–308. [DOI] [PubMed] [Google Scholar]
- 17. Antoni ML, Boden H, Hoogslag GE, Ewe SH, Auger D, Holman ER, van der Wall EE, Schalij MJ, Bax JJ, Delgado V. Prevalence of dyssynchrony and relation with long‐term outcome in patients after acute myocardial infarction. Am J Cardiol. 2011;108:1689–1696. [DOI] [PubMed] [Google Scholar]
- 18. Chang SA, Chang HJ, Choi SI, Chun EJ, Yoon YE, Kim HK, Kim YJ, Choi DJ, Sohn DW, Helm RH, Lardo AC. Usefulness of left ventricular dyssynchrony after acute myocardial infarction, assessed by a tagging magnetic resonance image derived metric, as a determinant of ventricular remodeling. Am J Cardiol. 2009;104:19–23. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Yip GW, Chan AK, Wang M, Lam WW, Fung JW, Chan JY, Sanderson JE, Yu CM. Left ventricular systolic dyssynchrony is a predictor of cardiac remodeling after myocardial infarction. Am Heart J. 2008;156:1124–1132. [DOI] [PubMed] [Google Scholar]
- 20. Thiele H, Wohrle J, Hambrecht R, Rittger H, Birkemeyer R, Lauer B, Neuhaus P, Brosteanu O, Sick P, Wiemer M, Kerber S, Kleinertz K, Eitel I, Desch S, Schuler G. Intracoronary versus intravenous bolus abciximab during primary percutaneous coronary intervention in patients with acute ST‐elevation myocardial infarction: a randomised trial. Lancet. 2012;379:923–931. [DOI] [PubMed] [Google Scholar]
- 21. Eitel I, Wohrle J, Suenkel H, Meissner J, Kerber S, Lauer B, Pauschinger M, Birkemeyer R, Axthelm C, Zimmermann R, Neuhaus P, Brosteanu O, de Waha S, Desch S, Gutberlet M, Schuler G, Thiele H. Intracoronary compared with intravenous bolus abciximab application during primary percutaneous coronary intervention in ST‐segment elevation myocardial infarction: cardiac magnetic resonance substudy of the AIDA STEMI trial. J Am Coll Cardiol. 2013;61:1447–1454. [DOI] [PubMed] [Google Scholar]
- 22. Thiele H, de Waha S, Zeymer U, Desch S, Scheller B, Lauer B, Geisler T, Gawaz M, Gunkel O, Bruch L, Klein N, Pfeiffer D, Schuler G, Eitel I. Effect of aspiration thrombectomy on microvascular obstruction in NSTEMI patients: the TATORT‐NSTEMI trial. J Am Coll Cardiol. 2014;64:1117–1124. [DOI] [PubMed] [Google Scholar]
- 23. Schuster A, Stahnke VC, Unterberg‐Buchwald C, Kowallick JT, Lamata P, Steinmetz M, Kutty S, Fasshauer M, Staab W, Sohns JM, Bigalke B, Ritter C, Hasenfuss G, Beerbaum P, Lotz J. Cardiovascular magnetic resonance feature‐tracking assessment of myocardial mechanics: intervendor agreement and considerations regarding reproducibility. Clin Radiol. 2015;70:989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leclercq C, Faris O, Tunin R, Johnson J, Kato R, Evans F, Spinelli J, Halperin H, McVeigh E, Kass DA. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle‐branch block. Circulation. 2002;106:1760–1763. [DOI] [PubMed] [Google Scholar]
- 25. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 26. Carrick D, Haig C, Rauhalammi S, Ahmed N, Mordi I, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S, Lindsay M, Mahrous A, Ford I, Tzemos N, Sattar N, Welsh P, Radjenovic A, Oldroyd KG, Berry C. Prognostic significance of infarct core pathology revealed by quantitative non‐contrast in comparison with contrast cardiac magnetic resonance imaging in reperfused ST‐elevation myocardial infarction survivors. Eur Heart J. 2016;37:1044–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reinstadler SJ, Stiermaier T, Liebetrau J, Fuernau G, Eitel C, de Waha S, Desch S, Reil JC, Poss J, Metzler B, Lucke C, Gutberlet M, Schuler G, Thiele H, Eitel I. Prognostic significance of remote myocardium alterations assessed by quantitative noncontrast T1 mapping in ST‐segment elevation myocardial infarction. JACC Cardiovasc Imaging. 2018;11:411–419. [DOI] [PubMed] [Google Scholar]
- 28. Reinstadler SJ, Stiermaier T, Reindl M, Feistritzer HJ, Fuernau G, Eitel C, Desch S, Klug G, Thiele H, Metzler B, Eitel I. Intramyocardial haemorrhage and prognosis after ST‐elevation myocardial infarction. Eur Heart J Cardiovasc Imaging. 2018;20:138–146. [DOI] [PubMed] [Google Scholar]
- 29. Fudim M, Fathallah M, Shaw LK, Liu PR, James O, Samad Z, Piccini JP, Hess PL, Borges‐Neto S. The prognostic value of diastolic and systolic mechanical left ventricular dyssynchrony among patients with coronary heart disease. JACC Cardiovasc Imaging. 2019;12(7 Pt 1):1215–1226. [DOI] [PubMed] [Google Scholar]
- 30. Sharma RK, Volpe G, Rosen BD, Ambale‐Venkatesh B, Donekal S, Fernandes V, Wu CO, Carr J, Bluemke DA, Lima JA. Prognostic implications of left ventricular dyssynchrony for major adverse cardiovascular events in asymptomatic women and men: the Multi‐Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2014;3:e000975 DOI: 10.1161/JAHA.114.000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buxton AE, Ellison KE, Lorvidhaya P, Ziv O. Left ventricular ejection fraction for sudden death risk stratification and guiding implantable cardioverter‐defibrillators implantation. J Cardiovasc Pharmacol. 2010;55:450–455. [PubMed] [Google Scholar]
- 32. Fernandez‐Jimenez R, Sanchez‐Gonzalez J, Aguero J, Garcia‐Prieto J, Lopez‐Martin GJ, Garcia‐Ruiz JM, Molina‐Iracheta A, Rossello X, Fernandez‐Friera L, Pizarro G, Garcia‐Alvarez A, Dall'Armellina E, Macaya C, Choudhury RP, Fuster V, Ibanez B. Myocardial edema after ischemia/reperfusion is not stable and follows a bimodal pattern: imaging and histological tissue characterization. J Am Coll Cardiol. 2015;65:315–323. [DOI] [PubMed] [Google Scholar]
- 33. Stiermaier T, Thiele H, Eitel I. Early myocardial edema after acute myocardial infarction is stable and not bimodal in humans—evidence from a large CMR multicenter study. Int J Cardiol. 2017;246:87–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Predictors of Major Adverse Cardiac Events in Patients With an Ejection Fraction >35% in a Selected Model Including RURE
Table S2. Predictors of Major Adverse Cardiac Events in Univariate and Multivariable Cox Regression Analysis Including Uniformity Ratio Estimates as Continuous Variables
Table S3. Predictors of Major Adverse Cardiac Events in Patients With an Ejection Fraction >35% Including Uniformity Ratio Estimates as Continuous Variables
Figure S1. Correlation between circumferential and radial uniformity ratio estimates with left ventricular ejection fraction and infarct size.
Figure S2. Association of circumferential and radial uniformity ratio estimates with scar transmurality.
Figure S3. Association of circumferential and radial uniformity ratio estimates with the number of infarcted left ventricular segments.


