Abstract
Background
Arterial stiffness predicts both cardiovascular events and incident hypertension. However, whether brachial‐ankle pulse wave velocity (baPWV) is predictive of incident hypertension based on the 2017 American College of Cardiology/American Heart Association (ACC/AHA) High Blood Pressure Guidelines has not been established. We performed a large cohort study to investigate whether incident hypertension could be predicted from baPWV measurements as a measure of arterial stiffness, even when applying updated hypertension criteria.
Methods and Results
A total of 10 360 Korean adults who underwent baPWV examination during a health‐screening program between 2010 and 2016 were enrolled. Hypertension was defined according to the 2017 ACC/AHA Guidelines as 130/80 mm Hg. Cox proportional hazard analysis was used to assess the risk of incident hypertension according to baPWV quartiles. The mean age of the study subjects was 40.2 years and 75.6% were men. During the follow‐up period (median 2.17 years), 2000 subjects (19.3%) developed hypertension. The subjects in the highest baPWV quartile group showed an increased risk of hypertension compared with the lowest baPWV quartile group as confirmed by multivariate adjusted hazard ratios of 1.64 (95% CI 1.41–1.89; P<0.001) in men and 12.36 (95% CI 4.41–34.62; P=0.005) in women. The increased risk of developing hypertension was consistent after adjusting for several confounding factors.
Conclusions
Arterial stiffness measured by baPWV is associated with incident hypertension according to the updated 2017 ACC/AHA Guidelines and is a useful independent predictor of incident hypertension among relatively healthy people.
Keywords: arterial stiffness, atherosclerosis, brachial‐ankle pulse wave velocity, hypertension
Subject Categories: Hypertension
Clinical Perspective
What Is New?
Arterial stiffness measured by brachial‐ankle pulse wave velocity is associated with incident hypertension according to the updated 2017 American College of Cardiology/American Heart Association High Blood Pressure Guidelines.
Women were at higher risk than men with respect to developing hypertension according to brachial‐ankle pulse wave velocity.
What Are the Clinical Implications?
In middle‐aged people, brachial‐ankle pulse wave velocity could be additively associated with the risk of incident hypertension.
Introduction
Blood pressure is a strong risk factor of cardiovascular events such as coronary heart disease, stroke, and peripheral artery disease.1, 2, 3 Globally, the prevalence of hypertension is predicted to increase to a total of 1.56 billion people.4 Thus, early detection or prediction of hypertension is important from the perspective that cardiovascular disease is a leading cause of mortality, disability, and global healthcare costs.5
Arterial stiffness is one of the earliest detectable manifestations of adverse structural and functional changes within the vessel wall.6 The relationship between arterial stiffness and hypertension has been reported in several studies.7, 8 Blood pressure and vascular stiffness are interdependent. Increased blood pressure can damage blood vessels and accelerate vascular stiffness, and arterial stiffening increases pressure pulsatility and systolic blood pressure.9 Pulse wave velocity (PWV) is a reliable and noninvasive tool for measuring arterial stiffness. In addition, PWV is a reproducible, accurate, and negative prognostic factor of arterial stiffness.10, 11, 12 Although carotid‐femoral PWV has traditionally been widely used to identify associations with cardiovascular disease such as coronary artery disease, brachial‐ankle pulse wave velocity (baPWV) has also become more widely used in recent years.13, 14, 15
The American College of Cardiology/American Heart Association (ACC/AHA) recently released revised guidelines for hypertension with lower blood pressure thresholds compared with previous guidelines (systolic blood pressure ≥130 versus 140 mm Hg or diastolic blood pressure ≥80 versus 90 mm Hg).16, 17 These changes are expected to increase the prevalence of patients with hypertension, especially in higher‐risk populations compared with the general population.18
Although carotid‐femoral PWV is the gold standard for estimating arterial stiffness,19, 20 baPWV has recently been reported to be correlated with carotid‐femoral PWV.15, 21 In addition, baPWV is easy to measure, requiring no special techniques or training. In this study, we evaluated the relationship between arterial stiffness as measured by baPWV and incident hypertension based on revised guidelines. We assessed whether an increase in PWV could predict the onset of hypertension.
Methods
Study Subjects
The study population consisted of individuals registered in a comprehensive health screening program at Kangbuk Samsung Hospital, Seoul and Suwon, Korea. The purpose of the comprehensive health screening program was to improve health through early detection of chronic diseases and associated risk factors. This study analyzed people who, between 2010 and 2016, underwent baPWV testing as a part of their health‐screening examination (Figure 1). Of the 17 665 individuals for which baPWV and associated data were initially available, we applied the following exclusion criteria: history of hypertension (n=4533); history of malignancy (n=861); history of cardiac surgery or cardiovascular disease (n=636); taking medicine for hypertension, diabetes mellitus, hyperlipidemia, or stroke (n=156); and age <0020 years (n=6). Finally, a total of 10 360 participants were eligible for inclusion in our study (7836 men and 2536 women; mean age, 40.2±7.2 years). The study protocol was approved by the Institutional Review Board of Kangbuk Samsung Hospital. Written informed consent was obtained from all participants. Data supporting the findings of this study are available from the corresponding author upon request.
Figure 1.
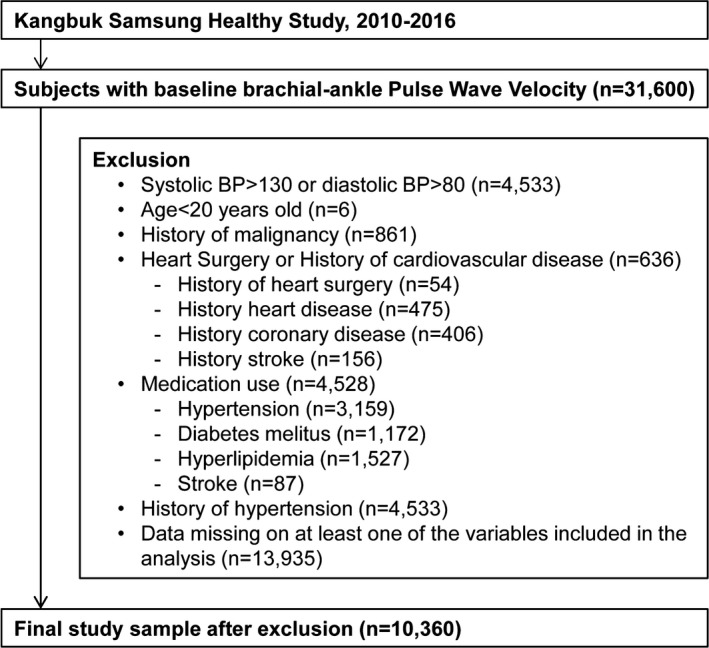
Flowchart summarizing the study population. BP indicates blood pressure.
Data Collection
All examinations were conducted at Kangbuk Samsung Health Screening Center clinics in Seoul and Suwon according to a standardized protocol. Blood was drawn from participants after fasting for at least 10 hours and analyzed at the Laboratory Medicine Department at the Kangbuk Samsung Hospital accredited by the Korean Association of Quality Assurance for Clinical Laboratories. Anthropometric measurements including height, weight, systolic blood pressure, and diastolic blood pressure were performed by well‐trained examiners. Blood pressure was measured using an automated oscillometric device (53 000, Welch Allyn, New York) by trained nurses while participants were in a sitting position with their arm supported at heart level after a 5‐minute rest. We recorded 3 consecutive blood pressure readings and used the average of the second and third readings for our analysis. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
Information about alcohol consumption (type of alcohol and glasses/day), smoking (never smoker, former smoker, or current smoker), frequency of vigorous exercise per week, and level of education were obtained from self‐questionnaires. The baPWV measurement was recorded relative to conventional blood pressure at the same baseline clinic visit.
BaPWV measurements recorded in the supine position were obtained using a volume‐plethysmography device (VP‐1000, OMRON, Kyoto, Japan), which measures both brachial and posterior tibial artery pressure waveforms using an oscillometric method with cuffs placed around both arms and ankles of the participant. baPWV was calculated automatically by time‐phase analysis, and the distance between the upper arm and ankle was estimated based on height. baPWV was obtained from right and left measurements, and the higher of the 2 readings were used for analysis.22
Prior history of surgery, drug, cerebrovascular disease, hypertension, hyperlipidemia, and diabetes mellitus was obtained by questionnaires. Hypertension was defined by systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥80 mm Hg, or current use of anti‐hypertension medication.16
Statistical Analysis
Comparisons of baseline characteristics and cardiovascular risk factors of the participants according to hypertension was performed using the following analysis method. Categorical variables are expressed as the number (%) and compared using the Pearson Chi‐squared test. Continuous variables were expressed as mean (standard deviation) or median (interquartile range) if not normally distributed. For continuous variables, Student t‐test or Mann–Whitney test were used to compare 2 groups, and ANOVA or Kruskal–Wallis tests were used for comparison of multiple groups as appropriate.
The final number of study participants without hypertension was categorized into 4 groups by baPWV. Cox proportional hazard analysis model was used to estimate adjusted hazard ratios (aHRs) and 95% CI for incident hypertension, adjusted for confounding factors identified from baseline characteristics. The predictive accuracy of each Cox model was determined by Harrell c‐index.23
For analyses separated by sex and baPWV quartiles, we estimated aHRs for incident hypertension comparing the 3 highest quartiles of baPWV to the lowest quartile (reference group). In our analyses, we used 4 models to adjust for confounding factors: model 1 was adjusted for age, medical center, year of screening examination, smoking status, alcohol intake, vigorous exercise frequency and education level; model 2 was further adjusted for BMI; model 3 was further adjusted for systolic blood pressure; and model 4 was further adjusted for diastolic blood pressure.
We used Kaplan–Meier estimates to evaluate event rates over time according to baPWV quartiles, and used the log‐rank test for analysis. The interaction effect between variables was also measured. All P values were 2‐tailed, and P<0.05 was considered statically significant. Finally, we used receiver operating characteristic analysis to determine the baPWV cut point for incident hypertension. Statistical analyses were performed using Stata version 15.0 (Stata Corp. 2017, College Station, TX).
Results
We followed 10 360 non‐hypertensive participants over a median period of 2.17 years (interquartile range, 1.74–3.63 years). The mean participant age was 40.2±7.2 years, and 7836 (75.6%) were men. At the end of the follow‐up period, 2000 (19.3%) participants developed hypertension according to the revised 2017 ACC/AHA guidelines.
Table 1 shows the baseline characteristics of the study population including metabolic markers divided into those who develop hypertension compared with those who do not. Participants who developed hypertension were older (42±6.8 versus 39.7±7.2 years) and more obese (BMI, 24.7±2.7 versus 23.1±2.8 kg/m2; waist circumference, 86.1±7.5 versus 81.4±8.4 cm). With the exception of high‐density lipoprotein cholesterol levels, all metabolic values were significantly higher in the hypertension group compared with the normotensive group. Compared with the normotensive group, the proportion of current smokers in the hypertension group was greater, while the level of education and the frequency of vigorous exercise was lower. Among participants who developed hypertension, men tended to be older than women (41.2±7.2 versus 37.1±6.2). In addition, systolic and diastolic BP were higher in men than in women (108.9±7.8 versus 98.9±8.6 mm Hg; 69.6±5.7 versus 63.5±6.6 mm Hg, respectively).
Table 1.
Baseline Characteristics of the Study Cohort Stratified by Incident Hypertension
| Characteristics | No incident Hypertension (n=8360) | Hypertension (n=2000) | P Value |
|---|---|---|---|
| Age, y | 39.7±7.2 | 42±6.8 | <0.001 |
| Sex | |||
| Men | 5941 (71.0) | 1895 (94.7) | <0.001 |
| Women | 2419 (28.9) | 105 (5.2) | |
| Glucose, mg/dL | 92.0 (11.1) | 95.1 (14.7) | <0.001 |
| HbA1c, % | 5.55 (0.35) | 5.64 (0.49) | <0.001 |
| AST (SGOT), IU/L | 22.0 (11.9) | 24.8 (12.6) | <0.001 |
| ALT (SGPT), U/L | 23.0 (17.9) | 29.1 (24.5) | <0.001 |
| GGT, IU/L | 30.5 (30.6) | 45.9 (44.0) | <0.001 |
| Triglyceride, mg/dL | 110.9 (69.9) | 139.5 (79.6) | <0.001 |
| HDL‐C, mg/dL | 56.7 (14.6) | 51.9 (12.4) | <0.001 |
| LDL‐C, mg/dL | 120.8 (30.4) | 129.5 (29.5) | <0.001 |
| Uric acid, mg/dL | 5.4 (1.3) | 6.0 (1.2) | <0.001 |
| hsCRP, mg/L | 0.09 (0.28) | 0.11 (0.29) | <0.001 |
| Insulin, IU/mL | 5.31 (3.06) | 5.94 (3.61) | <0.001 |
| HOMA‐IR | 1.23 (0.79) | 1.42 (0.99) | <0.001 |
| BMI, kg/m2 | 23.1 (2.8) | 24.7 (2.7) | <0.001 |
| Waist, cm | 81.4 (8.4) | 86.1 (7.5) | <0.001 |
| Systolic BP, mm Hg | 105.1 (9.0) | 112.3 (6.9) | <0.001 |
| Diastolic BP, mm Hg | 67.0 (6.3) | 72.7 (4.8) | <0.001 |
| Education level | |||
| ≥College graduate | 7246 (86.6) | 1717 (85.8) | 0.02 |
| ≤High school | 774 (9.2) | 174 (8.7) | |
| Unknown | 340 (4.0) | 109 (5.4) | |
| Smoking status | |||
| Never/former smoker | 5858 (70.1) | 1188 (59.4) | <0.001 |
| Current smoker | 2338 (28.0) | 787 (39.3) | |
| Unknown | 164 (2.0) | 25 (1.2) | |
| Alcohol, g/day | 3.0 (7.0–18.0) | 6.0 (14.0–34.0) | <0.001 |
| Vigorous exercise frequency | |||
| <1 time/wk | 5163 (61.7) | 1108 (55.4) | <0.001 |
| ≥1 time/wk | 3117 (37.2) | 869 (43.4) | |
| Unknown | 80 (0.9) | 23 (1.1) | |
Data are presented as n (%) or mean±SD or median (interquartile range). ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; GGT, gamma glutamyl transferase; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; SGOT, serum glutamic‐oxaloacetic transaminase; SGPT, serum glutamate‐pyruvate transaminase.
Tables 2 and 3 show the baseline characteristics of male and female patients in the hypertension population divided into baPWV quartiles. In general, the mean values of the systolic and diastolic blood pressures were highest among patients in the top baPWV quartile. This trend generally held true for other metabolic measurements in the top baPWV quartile as well.
Table 2.
Baseline Characteristics of the Male Study Cohort Stratified by baPWV Quartile
| Characteristics | PWV Quartiles, cm/s | P Value | |||
|---|---|---|---|---|---|
| Q1 (865‐1218) (n=1945) | Q2 (1219‐1301) (n=1962) | Q3 (1302‐1388) (n=1956) | Q4 (1389‐2468) (n=1973) | ||
| Age, y | 39.7 (6.4) | 40.6 (6.5) | 41.3 (6.7) | 43.0 (8.4) | <0.001 |
| Glucose, mg/dL | 92.2 (10.0) | 93.1 (11.3) | 94.8 (14.8) | 95.2 (13.9) | <0.001 |
| HbA1c, % | 5.55 (0.32) | 5.57 (0.35) | 5.61 (0.46) | 5.63 (0.47) | <0.001 |
| AST (SGOT), IU/L | 23.4 (15.5) | 23.1 (9.6) | 24.2 (13.7) | 24.6 (10.7) | <0.001 |
| ALT (SGPT), IU/L | 26.2 (23.7) | 26.0 (16.6) | 27.5 (19.2) | 28.7 (19.4) | <0.001 |
| GGT, IU/L | 34.5 (35.5) | 37.0 (31.5) | 40.4 (36.7) | 43.4 (40.8) | <0.001 |
| Triglyceride, mg/dL | 120.0 (69.1) | 124.0 (70.2) | 132.3 (81.1) | 139.1 (83.5) | <0.001 |
| HDL‐C, mg/dL | 52.6 (12.2) | 52.9 (12.8) | 52.2 (12.8) | 52.3 (12.5) | 0.321 |
| LDL‐C, mg/dL | 124.9 (29.6) | 125.9 (28.9) | 128.3 (29.1) | 131.0 (30.4) | <0.001 |
| Uric acid, mg/dL | 6.00 (1.15) | 6.01 (1.17) | 5.99 (1.18) | 6.03 (1.17) | 0.720 |
| hsCRP, mg/L | 0.09 (0.19) | 0.10 (0.24) | 0.11 (0.33) | 0.12 (0.36) | 0.006 |
| Insulin, IU/mL | 5.40 (3.24) | 5.41 (3.04) | 5.58 (3.29) | 5.72 (3.31) | 0.005 |
| HOMA‐IR | 1.25 (0.82) | 1.26 (0.78) | 1.33 (0.93) | 1.37 (0.91) | <0.001 |
| BMI, kg/m2 | 24.45 (2.79) | 24.16 (2.53) | 24.03 (2.58) | 23.92 (2.58) | <0.001 |
| Waist, cm | 85.3 (7.5) | 84.8 (6.8) | 84.6 (7.1) | 84.6 (7.1) | 0.008 |
| Systolic BP, mm Hg | 106.5 (7.9) | 108.2 (7.6) | 109.6 (7.5) | 111.4 (7.5) | <0.001 |
| Diastolic BP, mm Hg | 67.8 (5.9) | 69.3 (5.6) | 70.2 (5.4) | 71.2 (5.3) | <0.001 |
| Education level | |||||
| ≥College graduate | 1798 (92.4) | 1800 (91.7) | 1716 (87.7) | 1667 (89.0) | <0.001 |
| ≤High school | 90 (4.6) | 107 (5.4) | 141 (7.2) | 168 (8.5) | |
| Unknown | 57 (2.9) | 55 (2.8) | 99 (5.0) | 138 (6.9) | |
| Smoking status | |||||
| Never/former smoker | 1184 (60.9) | 1152 (58.7) | 1153 (58.9) | 1169 (59.2) | 0.059 |
| Current smoker | 743 (38.2) | 785 (40.0) | 769 (39.3) | 764 (38.7) | |
| Unknown | 17 (0.8) | 25 (1.2) | 34 (1.7) | 40 (2.0) | |
| Alcohol, g/day | 4.0 (11.0–23.0) | 5.0 (11.0–27.0) | 5.0 (11.0–27.0) | 5.0 (11.0–27.0) | 0.026 |
| Vigorous exercise frequency | |||||
| <1 time/wk | 1024 (52.6) | 1086 (55.3) | 1105 (56.4) | 1155 (58.5) | 0.001 |
| ≥1 time/wk | 910 (46.7) | 850 (43.3) | 835 (42.6) | 795 (40.2) | |
| Unknown | 11 (0.5) | 26 (1.3) | 16 (0.8) | 23 (1.1) | |
Data are presented as n (%) or mean±SD or median (interquartile range). ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; baPWV, brachial‐ankle pulse wave velocity; BMI, body mass index; BP, blood pressure; GGT, gamma glutamyl transferase; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; SGOT, serum glutamic‐oxaloacetic transaminase; SGPT, serum glutamate‐pyruvate transaminase.
Table 3.
Baseline Characteristics of the Female Study Cohort Stratified by baPWV Quartile
| Characteristics | PWV Quartiles, cm/s | P Value | |||
|---|---|---|---|---|---|
| Q1 (781‐1104) (n=625) | Q2 (1105‐1188) (n=626) | Q3 (1189‐1272) (n=639) | Q4 (1273‐1961) (n=634) | ||
| Age, y | 35.0 (4.9) | 36.4 (5.4) | 37.3 (5.9) | 39.9 (7.3) | <0.001 |
| Glucose, mg/dL | 87.8 (7.4) | 88.5 (7.2) | 88.5 (7.6) | 89.8 (8.8) | 0.0001 |
| HbA1c, % | 5.46 (0.25) | 5.49 (0.26) | 5.48 (0.26) | 5.54 (0.30) | <0.001 |
| AST (SGOT), IU/L | 17.9 (5.9) | 18.4 (6.4) | 19.0 (14.3) | 19.0 (8.6) | 0.100 |
| ALT (SGPT), IU/L | 14.0 (9.5) | 14.9 (10.4) | 16.0 (24.2) | 15.5 (9.7) | 0.113 |
| GGT, IU/L | 15.1 (12.8) | 16.0 (12.3) | 17.2 (22.7) | 18.6 (17.2) | 0.001 |
| Triglyceride, mg/dL | 73.0 (32.9) | 75.8 (42.5) | 75.6 (34.0) | 86.7 (44.5) | <0.001 |
| HDL‐C, mg/dL | 66.7 (14.6) | 66.2 (13.7) | 66.9 (14.8) | 64.8 (15.0) | 0.045 |
| LDL‐C, mg/dL | 101.7 (24.5) | 105.5 (26.8) | 105.8 (25.9) | 113.7 (29.9) | <0.001 |
| Uric acid, mg/dL | 4.16 (0.89) | 4.18 (0.83) | 4.25 (0.85) | 4.22 (0.85) | 0.322 |
| hsCRP, mg/L | 0.07 (0.21) | 0.07 (0.18) | 0.07 (0.21) | 0.09 (0.36) | 0.243 |
| Insulin, IU/mL | 5.03 (2.89) | 5.09 (2.99) | 5.04 (2.96) | 5.30 (3.25) | 0.344 |
| HOMA‐IR | 1.11 (0.68) | 1.13 (0.73) | 1.12 (0.73) | 1.20 (0.79) | 0.145 |
| BMI, kg/m2 | 21.47 (2.93) | 21.32 (2.72) | 21.31 (2.95) | 21.68 (2.89) | 0.07 |
| Waist, cm | 74.09 (7.54) | 74.20 (7.70) | 74.56 (7.84) | 75.36 (7.56) | 0.014 |
| Systolic BP, mm Hg | 95.6 (7.9) | 97.3 (8.0) | 99.1 (7.9) | 103.8 (8.5) | <0.001 |
| Diastolic BP, mm Hg | 60.8 (6.1) | 62.4 (6.3) | 63.9 (6.1) | 66.8 (6.3) | <0.001 |
| Education level | |||||
| ≥College graduate | 509 (81.4) | 494 (78.9) | 515 (80.5) | 464 (73.1) | 0.003 |
| ≤High school | 98 (15.6) | 105 (16.7) | 95 (14.8) | 144 (22.7) | |
| Unknown | 18 (2.8) | 27 (4.3) | 29 (4.5) | 26 (4.1) | |
| Smoking status | |||||
| Never/former smoker | 605 (96.8) | 602 (96.1) | 597 (93.4) | 583 (91.9) | 0.001 |
| Current smoker | 13 (2.0) | 12 (1.9) | 17 (2.6) | 22 (3.4) | |
| Unknown | 7 (1.1) | 12 (1.9) | 25 (3.9) | 29 (4.5) | |
| Alcohol, g/day | 1.0 (3.0–7.0) | 0.0 (3.0–6.0) | 0.0 (3.0–6.0) | 0.0 (3.0–7.0) | 0.422 |
| Vigorous exercise frequency | |||||
| <1 time/wk | 473 (75.6) | 481 (76.8) | 483 (75.5) | 464 (73.1) | 0.757 |
| ≥1 time/wk | 147 (23.5) | 137 (21.8) | 150 (23.4) | 162 (25.5) | |
| Unknown | 5 (0.8) | 8 (1.2) | 6 (0.9) | 8 (1.2) | |
Data are presented as n (%) or mean±SD or median (interquartile range). ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; baPWV, brachial‐ankle pulse wave velocity; BMI, body mass index; BP, blood pressure; GGT, gamma glutamyl transferase; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; SGOT, serum glutamic‐oxaloacetic transaminase; SGPT, serum glutamate‐pyruvate transaminase.
The risk of incident hypertension increased progressively across baPWV quartiles in both men and women (Table 4). In the fully adjusted multivariate models, including adjustments for age, medical center, year of screening examination, smoking status, alcohol intake, exercise, education level, body mass index, systolic blood pressure and diastolic blood pressure, the aHRs (95% CI) for incident hypertension comparing the highest with the lowest quartiles (reference group) of baPWV were 1.64 (1.41–1.89) in men and 12.36 (4.41–34.62) in women, the difference of which was statistically significant. In this result, the aHRs in women have wide CIs, driven in large part by the chosen reference group having only 4 events. Because statistically operating at such small numbers could cause questions about the accuracy of large‐sample approximations that provide the CIs used and P value, so we further analyzed baPWV in women by dividing them into 2 groups (Figure S1, Tables S1 and S2). As a result, the risk of incident hypertension was 76% higher in the group with higher baPWV than in the group with lower baPWV, which was statistically significant.
Table 4.
Risk of Incident Hypertension According to baPWV in Men and Women
| Person‐Years | Events (No.) | Incident Rate (10 000 person‐years) | Age‐Adjusted HR (95% CI) | Multivariable‐Adjusted HRa (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| Men | ||||||||
| Q1 | 5360.3 | 284 | 529.8 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 5763.7 | 409 | 709.6 | 1.27 (1.09–1.48) | 1.38 (1.18–1.60) | 1.41 (1.21–1.65) | 1.32 (1.13–1.54) | 1.23 (1.05–1.43) |
| Q3 | 5851.4 | 527 | 900.6 | 1.61 (1.39–1.86) | 1.72 (1.49–1.99) | 1.79 (1.55–2.07) | 1.59 (1.37–1.84) | 1.45 (1.25–1.68) |
| Q4 | 5835.8 | 675 | 1156.6 | 2.06 (1.79–2.37) | 2.16 (1.88–2.49) | 2.28 (1.98–2.62) | 1.8 (1.55–2.08) | 1.64 (1.41–1.89) |
| Women | ||||||||
| Q1 | 1503.8 | 4 | 26.5 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 1532.2 | 17 | 110.9 | 3.81 (1.28–11.34) | 3.68 (1.23–10.97) | 3.81 (1.28–11.34) | 3.68 (1.23–10.97) | 4.2 (1.41–12.55) |
| Q3 | 1542.5 | 22 | 142.6 | 4.74 (1.63–13.80) | 4.98 (1.71–14.56) | 4.74 (1.63–13.8) | 4.98 (1.71–14.56) | 5.12 (1.75–14.98) |
| Q4 | 1525.8 | 62 | 406.3 | 11.09 (3.96–31.05) | 11.11 (3.97–31.11) | 11.09 (3.96–31.05) | 11.11 (3.97–31.11) | 12.36 (4.41–34.62) |
baPWV indicates brachial‐ankle pulse wave velocity; HR, hazard ratio.
Model 1: adjustment for age, medical center, year of screening examination, smoking status, alcohol intake, exercise and educationa level; Model 2: Model 1 and adjustment for BMI; Model 3: Model 2 and adjustment for systolic blood pressure; Model 4: Model 3 and adjustment for diastolic blood pressure.
We used Harrell C‐index to check the predictive power of the model with PWV in the incident hypertension risk, which was 0.823 (95% CI 0.812–0.836) for men and 0.894 (95% CI 0.855–0.934) for women.
The Kaplan–Meier curves for risk of incident hypertension according to baPWV quartiles are shown in Figure 2. The rate was higher for groups with higher baPWV values in both men and women (P<0.001) (Figure 2). The results were similar when changing the cutoffs of blood pressure to define hypertension (systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg; Figure S2, Table S3 through S6). The interaction between baseline blood pressure, baPWV, and incident hypertension was illustrated by 2‐dimensional plot by quartiles of baPWV and quartiles of baseline systolic blood pressure (Figure 3, Table S7).
Figure 2.
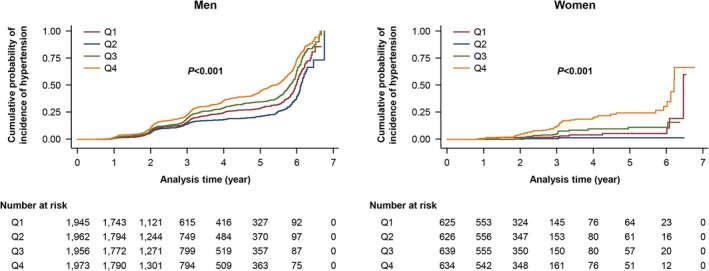
Kaplan–Meier Curves for incident hypertension according to baPWV in men and women. baPWV indicates brachial‐ankle pulse wave velocity.
Figure 3.
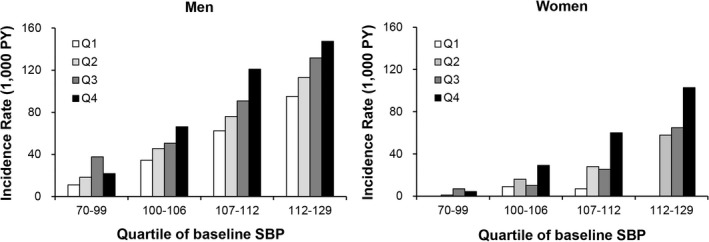
Incident hypertension rate by quartiles of baPWV and quartiles of baseline systolic blood pressure. baPWV indicates brachial‐ankle pulse wave velocity; SBP, systolic blood pressure; PY, person‐years.
No significant interactions were observed between baPWV and any of the subgroups with respect to the incidence of hypertension, as shown in the hazard‐ratio plots in Figure 4. In addition, we used the Youden Index on the receiver operating characteristic curve to determine the baPWV cut point for predicting incident hypertension.24 As a result, the baPWV cut point for predicting incident hypertension was 1319 cm/s for male (sensitivity: 58.5%, specificity: 59.7%) and 1246 cm/s (sensitivity: 69.5%, specificity: 69.5%) for female. The Harrell C‐index was not significantly increased when baPWV is added to a Model 4 in Table 4.
Figure 4.
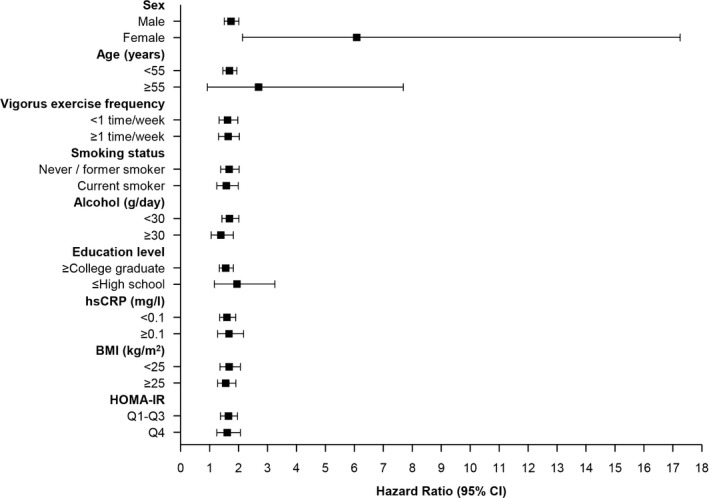
Subgroup analyses of incident hypertension according to baPWV. baPWV indicates brachial‐ankle pulse wave velocity; BMI, bone mass index, HOMA‐IR, homeostasis model assessment of insulin resistance; hsCRP, high‐sensitity C‐reactive protein.
Discussion
The American College of Cardiology/American Heart Association (ACC/AHA) recently released revised guidelines16 for hypertension. These guidelines consist of lower blood pressure thresholds (systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥80 mm Hg) than previous guidelines (systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥80 mm Hg).25 Adopting the 2017 ACC/AHA high blood pressure guidelines is expected to markedly increase the number of people labeled as having hypertension and treated with medication in both the US and China. Indeed, according to these criteria, more than half of those aged 45 to 75 years in both countries can be considered as hypertensive.26 Accordingly, the usefulness of previously studied tools may also change as patients with perhaps greater risk than the general population become newly included in high blood pressure groups.
In the present study, we evaluate the relationship between blood pressure and baPWV reflective of arterial stiffness among a large cohort of young and middle‐aged asymptomatic adults. There were 3 main findings of our study. First, baPWV as a measure of arterial stiffness was independently associated with development of hypertension even under the new 2017 ACC/AHA guidelines. Second, we found that women were at higher risk than men with respect to developing hypertension according to baPWV.
We evaluated the association between high blood pressure and baPWV as a representative marker of arterial stiffness.27 Several studies have utilized PWV as a predictor of cardiovascular disease,28, 29, 30 and carotid‐femoral PWV has traditionally been used as a standard method to evaluate vascular damage and cardiovascular disease. However, several recent studies have also demonstrated the effectiveness and validity of baPWV for vascular damage and cardiovascular disease as well.15, 21, 31, 32 Importantly, measuring baPWV does not require any specialized techniques, and instead is automatically measured by wrapping cuffs on the brachium and ankle. Because of this simple measurement method, baPWV can be used in health examination programs, resulting in higher utilization as a predictive tool.
Arterial stiffness is one of the earliest detectable manifestations of adverse structural and functional changes within the vessel wall.6 There are several mechanisms of arterial stiffness, including vascular growth factor expression, calcification, inflammation, and environmental factors.26, 33, 34, 35 Arterial stiffness increases as a complication of hypertension, whereas more recent studies have shown that arterial stiffness contributes to hypertension. Thus, early diagnosis of arterial stiffness may provide insight into future disease, including hypertension.
Several earlier studies have shown that arterial stiffness is associated with hypertension.8, 36, 37, 38 However there are limitations, in that the studies were relatively small in number or did not adjust for socioeconomic status. In addition, their study did not utilize the new 2017 ACC/AHA criteria.
The results of our study are consistent with those from previous studies on the association between arterial stiffness and hypertension. The increased risk of incident hypertension in those individuals in the greatest baPWV quartile was the same for both men and women, and this result was maintained even after multivariable adjustment. Some of the strengths of this study relative to prior studies were the relatively large number participants (n=10 360), use of new hypertension guidelines, and adjusting for socioeconomic status.
Study Limitations
The results of this study should be interpreted in the context of several potential limitations. First, the diagnosis of hypertension were based on measurements taken at a single visit, which may be unreliable and lead to misclassification of blood pressure status. Well‐trained examiners measured blood pressure 3 times using the standard method of measurement, but we cannot completely rule out white coat or masked hypertension that may occur in some patients. The 24‐hour ambulatory BP measurement can be used as a good tool to rule out white coat or masked hypertension. Unfortunately, we didn't measure 24‐hour ambulatory BP to confirm hypertension, so we couldn't completely rule out the possibility of white coat or masked hypertension. Second, although we adjusted for age, BMI, smoking status, blood pressure, and several other possible risk factors, we could not exclude all possible residual confounding factors. To address this limitation, we performed multivariate analysis to account for confounding factors. Third, there is a notable dispersion at the end of the follow‐up period across baPWV quartiles. When referring to Kaplan Meier plots in Figure 2, the difference in the number at risk between Q1 and Q4 for men at year 3 is (794–615)/615=29%. However, the absolute difference in cumulative incidence of hypertension between Q1 and Q4 is ≈ 10%. This result can be interpreted as likely to have visited patients in Q4 more frequently, which suggests the possibility of ascertainment bias. The Occupational Safety and Health Act in Korea require employers to conduct annual or biennial health examinations of all employees, and employees are required to participate. About 80 percent of the population who participated in this study were conducted under the Occupational Safety and Health Act in Korea, so there is little room for individual will to intervene. However, since the remaining 20% were the people who underwent medical checkups on their own, it is likely that these people underwent medical checkups more frequently. For this reason, there is a possibility that ascertainment bias has occurred. Fourth, our study consisted of relatively healthy young middle‐aged subjects. Therefore, it may be unreasonable to generalize our results to the entire population. Accordingly, our findings may differ for different age groups or patients from other races/ethnicities beyond Korea. Fifth, we did not measure the heart rate when collecting baPWV results. Heart rate is known as an important confounding factor in interpreting PWV results.39 Therefore, in this study, it was also necessary to analyze the results by adding heart rate as a covariate to the model. Finally, there is a possibility that the blood pressure measurements may not be an accurate reflection of the true blood pressure and this may influence findings.40 However, several aspects of this large cohort study add significant strength to our findings. The participants in this study were generally young and healthy, and thus may be less biased to use of medications or co‐morbidities that affect elderly individuals. In addition, the data were collected by well‐trained expert staff according to standardized and uniform conditions.
Conclusions
We found that baPWV, as a measurement of arterial stiffness, was strongly and independently correlated with hypertension among relatively healthy people. In addition, baPWV was predictive of incident hypertension in patients with known risk factors.
Disclosures
None.
Supporting information
Table S1. Baseline Characteristics of the Female Study Cohort Stratified by Dichotomizing baPWV
Table S2. Risk of Incident Hypertension According to baPWV in Men and Women
Table S3. Baseline Characteristics of the Cohort Stratified by Incident Hypertension
Table S4. Baseline Characteristics of the Cohort Stratified by baPWV Quartile in Men
Table S5. Baseline Characteristics of the Cohort Stratified by baPWV Quartile in Women
Table S6. Risk of Incident Hypertension According to baPWV in Men and Women
Table S7. The Incidence Rate of Hypertension According to baPWV Quartile and SBP Quartile in Men and Women
Figure S1. Incident hypertension rate by quartiles of baseline systolic blood pressure and dichotomization of baPWV in women.
Figure S2. Kaplan–Meier Curves for incident hypertension according to baPWV in men and women.
Acknowledgments
The authors would like to acknowledge the efforts of the health screening group at Kangbuk Samsung Hospital, Korea. Dr Ki‐Chul Sung developed the hypothesis, wrote the introduction, methods and results and contributed to discussion. Dr Seung Jae Lee wrote the methods and the results, contributed to discussion and edited the whole paper. Mi Yeon Lee analyzed the data. Dr Bum Soo Kim, Dr Jin Ho Kang, Dr Dae Chul Seo and Prof Alberto Avolio contributed to discussion. Dr Ki Chul Sung is the guarantor for the article. All authors have read the manuscript and agree with its findings.
(J Am Heart Assoc. 2019;8:e013019 DOI: 10.1161/JAHA.119.013019.)
References
- 1. Lawes CM, Bennett DA, Parag V, Woodward M, Whitlock G, Lam TH, Suh I, Rodgers A; Asia Pacific Cohort Studies Collaboration . Blood pressure indices and cardiovascular disease in the Asia Pacific Region: a pooled analysis. Hypertension. 2003;42:69–75. [DOI] [PubMed] [Google Scholar]
- 2. Rapsomaniki E, Timmis A, George J, Pujades‐Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shen L, Ma H, Xiang MX, Wang JA. Meta‐analysis of cohort studies of baseline prehypertension and risk of coronary heart disease. Am J Cardiol. 2013;112:266–271. [DOI] [PubMed] [Google Scholar]
- 4. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 5. GBD 2016 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1345–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cavalcante JL, Lima JA, Redheuil A, Al‐Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522. [DOI] [PubMed] [Google Scholar]
- 7. Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross‐sectional correlates of increased aortic stiffness in the community: the Framingham heart study. Circulation. 2007;115:2628–2636. [DOI] [PubMed] [Google Scholar]
- 8. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aatola H, Hutri‐Kahonen N, Juonala M, Viikari JS, Hulkkonen J, Laitinen T, Taittonen L, Lehtimaki T, Raitakari OT, Kahonen M. Lifetime risk factors and arterial pulse wave velocity in adulthood: the cardiovascular risk in young Finns study. Hypertension. 2010;55:806–811. [DOI] [PubMed] [Google Scholar]
- 10. Kelly R, Hayward C, Avolio A, O'Rourke M. Noninvasive determination of age‐related changes in the human arterial pulse. Circulation. 1989;80:1652–1659. [DOI] [PubMed] [Google Scholar]
- 11. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group . 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 12. Asmar RG, Topouchian JA, Benetos A, Sayegh FA, Mourad JJ, Safar ME. Non‐invasive evaluation of arterial abnormalities in hypertensive patients. J Hypertens Suppl. 1997;15:S99–S107. [DOI] [PubMed] [Google Scholar]
- 13. Vishnu A, Choo J, Wilcox B, Hisamatsu T, Barinas‐Mitchell EJ, Fujiyoshi A, Mackey RH, Kadota A, Ahuja V, Kadowaki T, Edmundowicz D, Miura K, Rodriguez BL, Kuller LH, Shin C, Masaki K, Ueshima H, Sekikawa A; ERA JUMP Study Group . Brachial‐ankle pulse wave velocity is associated with coronary calcification among 1131 healthy middle‐aged men. Int J Cardiol. 2015;189:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seo WW, Chang HJ, Cho I, Yoon YY, Suh JW, Kim KI, Cho YS, Youn TJ, Chae IH, Choi DJ, Kim CH, Chun EJ, Choi SI. The value of brachial‐ankle pulse wave velocity as a predictor of coronary artery disease in high‐risk patients. Korean Circ J. 2010;40:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y. Validity, reproducibility, and clinical significance of noninvasive brachial‐ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–364. [DOI] [PubMed] [Google Scholar]
- 16. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 17. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee . The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 18. Vaduganathan M, Pareek M, Qamar A, Pandey A, Olsen MH, Bhatt DL. Baseline blood pressure, the 2017 ACC/AHA high blood pressure guidelines, and long‐term cardiovascular risk in sprint. Am J Med. 2018;131:956–960. [DOI] [PubMed] [Google Scholar]
- 19. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker‐Boudier H; European Network for Non‐invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 20. Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension . Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, Tomiyama H, Yamashina A, Yasuda H, Sawayama T, Ozawa T. Comparison between carotid‐femoral and brachial‐ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27:2022–2027. [DOI] [PubMed] [Google Scholar]
- 22. Lee JY, Ryu S, Lee SH, Kim BJ, Kim BS, Kang JH, Cheong ES, Kim JY, Park JB, Sung KC. Association between brachial‐ankle pulse wave velocity and progression of coronary artery calcium: a prospective cohort study. Cardiovasc Diabetol. 2015;14:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 24. Fluss R, Faraggi D, Reiser B. Estimation of the Youden index and its associated cutoff point. Biom J. 2005;47:458–472. [DOI] [PubMed] [Google Scholar]
- 25. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 26. Zachariah JP, Xanthakis V, Larson MG, Vita JA, Sullivan LM, Smith HM, Safa R, Peng X, Hamburg N, Levy D, Sawyer DB, Mitchell GF, Vasan RS. Circulating vascular growth factors and central hemodynamic load in the community. Hypertension. 2012;59:773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. [DOI] [PubMed] [Google Scholar]
- 28. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham heart study. Circulation. 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 30. Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end‐stage renal disease. Circulation. 1999;99:2434–2439. [DOI] [PubMed] [Google Scholar]
- 31. Yu WC, Chuang SY, Lin YP, Chen CH. Brachial‐ankle vs carotid‐femoral pulse wave velocity as a determinant of cardiovascular structure and function. J Hum Hypertens. 2008;22:24–31. [DOI] [PubMed] [Google Scholar]
- 32. Yamashina A, Tomiyama H, Arai T, Hirose K, Koji Y, Hirayama Y, Yamamoto Y, Hori S. Brachial‐ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res. 2003;26:615–622. [DOI] [PubMed] [Google Scholar]
- 33. Smith ER, Tomlinson LA, Ford ML, McMahon LP, Rajkumar C, Holt SG. Elastin degradation is associated with progressive aortic stiffening and all‐cause mortality in predialysis chronic kidney disease. Hypertension. 2012;59:973–978. [DOI] [PubMed] [Google Scholar]
- 34. van Bussel BC, Schouten F, Henry RM, Schalkwijk CG, de Boer MR, Ferreira I, Smulders YM, Twisk JW, Stehouwer CD. Endothelial dysfunction and low‐grade inflammation are associated with greater arterial stiffness over a 6‐year period. Hypertension. 2011;58:588–595. [DOI] [PubMed] [Google Scholar]
- 35. Mitchell GF. Arterial stiffness and hypertension. Hypertension. 2014;64:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore longitudinal study of aging. J Am Coll Cardiol. 2008;51:1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takase H, Dohi Y, Toriyama T, Okado T, Tanaka S, Sonoda H, Sato K, Kimura G. Brachial‐ankle pulse wave velocity predicts increase in blood pressure and onset of hypertension. Am J Hypertens. 2011;24:667–673. [DOI] [PubMed] [Google Scholar]
- 38. Koivistoinen T, Lyytikainen LP, Aatola H, Luukkaala T, Juonala M, Viikari J, Lehtimaki T, Raitakari OT, Kahonen M, Hutri‐Kahonen N. Pulse wave velocity predicts the progression of blood pressure and development of hypertension in young adults. Hypertension. 2018;71:451–456. [DOI] [PubMed] [Google Scholar]
- 39. Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002;39:1083–1087. [DOI] [PubMed] [Google Scholar]
- 40. Picone DS, Schultz MG, Otahal P, Aakhus S, Al‐Jumaily AM, Black JA, Bos WJ, Chambers JB, Chen CH, Cheng HM, Cremer A, Davies JE, Dwyer N, Gould BA, Hughes AD, Lacy PS, Laugesen E, Liang F, Melamed R, Muecke S, Ohte N, Okada S, Omboni S, Ott C, Peng X, Pereira T, Pucci G, Rajani R, Roberts‐Thomson P, Rossen NB, Sueta D, Sinha MD, Schmieder RE, Smulyan H, Srikanth VK, Stewart R, Stouffer GA, Takazawa K, Wang J, Westerhof BE, Weber F, Weber T, Williams B, Yamada H, Yamamoto E, Sharman JE. Accuracy of cuff‐measured blood pressure: systematic reviews and meta‐analyses. J Am Coll Cardiol. 2017;70:572–586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of the Female Study Cohort Stratified by Dichotomizing baPWV
Table S2. Risk of Incident Hypertension According to baPWV in Men and Women
Table S3. Baseline Characteristics of the Cohort Stratified by Incident Hypertension
Table S4. Baseline Characteristics of the Cohort Stratified by baPWV Quartile in Men
Table S5. Baseline Characteristics of the Cohort Stratified by baPWV Quartile in Women
Table S6. Risk of Incident Hypertension According to baPWV in Men and Women
Table S7. The Incidence Rate of Hypertension According to baPWV Quartile and SBP Quartile in Men and Women
Figure S1. Incident hypertension rate by quartiles of baseline systolic blood pressure and dichotomization of baPWV in women.
Figure S2. Kaplan–Meier Curves for incident hypertension according to baPWV in men and women.


