Abstract
Background
Heart failure (HF) admissions in adults with congenital heart disease (CHD) are becoming more common. We compared in‐hospital and readmission events among adults with and without CHD admitted for HF.
Methods and Results
We identified all admissions with the primary diagnosis of HF among adults in the California State Inpatient Database between January 1, 2005 and January 1, 2012. International Classification of Disease (ICD) codes identified the type of CHD lesion, comorbidities, and in‐hospital and 30‐day readmissions events. Adjusted odds ratio (AOR, 95% CI) was calculated after adjusting for admission year, age, sex, race, household income, primary payor, and Charlson comorbidity index. Of 203 759 patients admitted for HF, 539 had CHD other than atrial septal defect. Compared with patients admitted for HF without CHD, those with CHD were younger, more often male, and had fewer comorbidities as determined by Charlson comorbidity index. On multivariate analysis, CHD patients admitted for HF had higher odds of length of stay ≥7 days (AOR 2.5 [95% CI 2.0–3.1]), incident arrhythmias (AOR 2.8 [95% CI 1.7–4.5]), and in‐hospital mortality (AOR 1.9 [95% CI 1.1–3.1]). Also, CHD patients had lower odds of readmission for HF (AOR 0.6 [95% CI 0.3–0.9]), but similar odds of other 30‐day readmission events. Complex CHD patients had higher odds of length of stay ≥7 days (AOR 1.9 [95% CI 1.1–3.3]) than patients with noncomplex CHD lesions, but similar odds of all other clinical outcomes.
Conclusions
Among patients admitted with the primary diagnosis of HF in California, adults with CHD have substantially higher odds of longer length of stay, incident arrhythmias, and in‐hospital mortality compared with non‐CHD patients. These results suggest a need for HF risk stratification strategies and management protocols specific for patients with CHD.
Keywords: adult congenital heart disease, arrhythmias, congenital cardiac defect, heart failure, in‐hospital and readmission events
Subject Categories: Congenital Heart Disease, Heart Failure
Clinical Perspective
What Is New?
In this statewide study of all admissions for heart failure (HF) over a 7‐year period, adults with congenital heart disease (CHD)—who are otherwise younger and have fewer comorbidities than non‐CHD patients—nonetheless had significantly higher adjusted risks of adverse clinical outcomes.
Adults with CHD had a 3.5% incidence of new arrhythmias during their admission for HF, representing nearly 3‐fold higher adjusted odds than non‐CHD patients.
Adults admitted for HF with CHD had ≈2‐fold higher adjusted odds of longer length of stay and in‐hospital mortality than those without CHD.
What Are the Clinical Implications?
These data on adverse clinical outcomes for adults with CHD who are admitted for HF highlight the challenges faced by CHD and HF specialists.
Higher odds of incident arrhythmias and mortality during HF hospitalization among CHD patients suggests a need to develop risk prediction tools; these tools might guide clinicians caring for these patients to make appropriate management decisions.
Targeted CHD‐specific prevention and treatment protocols for HF need to be developed to reduce the high burden of adverse clinical outcomes during HF admissions.
Introduction
Congenital heart disease (CHD) is the most common type of birth defect.1 Improved surgical and pediatric CHD care has increased the number of patients surviving to adulthood, including those with complex CHD.2, 3 It is estimated that there are >1.5 million CHD adults currently in the United States.4, 5 Initial surgeries or interventions for CHD often are not curative, and patients frequently develop cardiac complications, including heart failure (HF).6 HF management in the setting of CHD is challenging because of the heterogeneity of the underlying anatomy and surgical repairs, and the paucity of evidence‐based management protocols.6, 7
Prior studies have shown that there has been an increasing burden of inpatient hospitalizations, associated costs, and mortality from HF among adult CHD patients.8, 9, 10, 11 However, studies of in‐hospital clinical outcomes for adults with CHD during a HF hospitalization are lacking. Additionally, it has been shown that, after an index HF admission, 25% of patients get readmitted within 30 days for any cause; 35% get readmitted again for HF.12 But this study did not report readmission rates in CHD patients specifically. Information about clinical outcomes during HF admission and the rate of readmission within 30 days for CHD patients might help physicians treating CHD to develop targeted strategies to improve outcomes in these patients.
To understand these clinical outcomes in CHD patients, we used the California inpatient database to identify all admissions for HF over a 7‐year period. We compared the outcomes during admissions and the 30‐day readmission rates for adults with and without CHD.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request. The data source for this study is the Health Care Utilization Project California State Inpatient Database. This database includes all in‐hospital patients, regardless of payor, from 98.3% of all California hospitals, providing a unique view of statewide inpatient care, and includes over 3.5 million discharges per year.13
We retrospectively examined all discharges in the California State Inpatient database between January 1, 2005, and January 1, 2012, with an International Classification of Diseases, Ninth Revision (ICD‐9‐CM) diagnosis code for HF (Table 1). State Inpatient Database includes a variable listing the Agency for Healthcare Research and Quality's single‐level Clinical Classification System (CCS) code for the primary diagnosis.14 The CCS system provides a way to classify diagnoses and procedures into a limited number of categories by aggregating individual ICD‐9‐CM codes into broad diagnosis and procedure groups to facilitate statistical analysis and reporting. If the single‐level CCS code of 108 was present, then it was considered that the primary admitting diagnosis for the patient was HF. Individual patients were the unit of analyses.
Table 1.
ICD‐9 Diagnostic Codes
| Diagnosis | ICD‐9 Codes |
|---|---|
| HF | 402.01, 402.11, 402.91, 404.91, 404.93, 404.01, 404.03, 428.x |
| Anemia | 648.2.x, 280.x–285.x |
| Hypertension | 437.2, 401.x–405.x |
| Hyperlipidemia | 272.0, 272.2, 272.4 |
| Diabetes mellitus | 791.5, V458.5, V539.1, V654.6, 249.x, 250.x |
| Coronary artery disease | 360.1, 360.2, 360.3, 360.4, 360.5, 360.6, 360.7, 360.9, 361.0, 361.1, 361.2, 361.3, 361.4, 361.5, 361.6, 361.7, 361.8, 361.9, 411.0, 411, 411.1, 411.8, 411.89, 412, 412.0, V458.1, V458.2, 429.7, 401.x, 413.x, 414.x |
| Atrial arrhythmia | 427.31, 427.32, 427.0 |
| VT/VF or SCA | 427.1, 427.4, 427.41, 427.42, 427.5, V125.3 |
| Lung disease | 491.8, 491.9, 492.0, 492.8, 494, 494.0, 494.1, 496, 491.2, 493.x |
| Chronic renal failure | V420, V451, V451.1, V451.2, V560, V561, V562, V563.1, V563.2, V568, V56, 585.x |
| Cerebrovascular disease | 430, 431, 432.x–435.x, 438.x |
| Depression | 296.2, 296.3, 298.0, 300.4, 309.0, 309.1, 311.0 |
HF indicates heart failure; VT/VF or SCA, ventricular tachycardia, ventricular fibrillation or sudden cardiac arrest; ICD‐9, International Classification of Diseases, Ninth Revision.
x designates all diagnosis codes under the listed category, e.g., 280.x includes 280.0, 280.1, 280.8, and 280.9.
Patients were designated as having CHD if their discharge abstract included any of the ICD‐9 codes for CHD (Table 2). They were then categorized, on the basis of their anatomic subgroups, as complex and noncomplex CHD using the definitions previously described.9, 15, 16 Complex CHD included “any CHD with pulmonary arterial hypertension,” univentricular heart defects (including hypoplastic left heart syndrome), transposition of the great arteries, tetralogy of Fallot, truncus arteriosus, and endocardial cushion defects. All other CHD defects were classified as noncomplex. Patients with both a complex and a noncomplex CHD diagnosis were assigned to the complex CHD group. For patients with codes for more than 1 CHD diagnosis, we used the hierarchical algorithm proposed by Broberg et al17 to designate 1 condition per patient as their principal CHD diagnosis. As described by Broberg et al17 and like Burchill et al,9 we excluded patients with atrial septal defect since their ICD codes have lower specificity for CHD and are often used on patients who only have a patent foramen ovale.
Table 2.
Types of CHD Lesions and Their ICD‐9 Codes
| ICD 9 Codes | |
|---|---|
| Complex lesions | |
| CHD and pulmonary hypertension | Any code below AND 416.0, 416.8, 416.9 |
| Univentricular heart/hypoplastic left heart syndrome | 745.3, 746.7 |
| Transposition complex | 745.10, 745.11, 745.12, 745.19 |
| Tetralogy of Fallot | 745.2 |
| Truncus arteriosus | 745.0 |
| Endocardial cushion defect | 745.6 |
| Noncomplex lesions | |
| Aortic coarctation | 747.10 |
| Ebstein's anomaly | 746.2 |
| Anomalies of the pulmonary valve | 746.0, 746.02, 746.09 |
| Anomalies of veins | 747.4, 747.41, 747.42, 747.49 |
| Ventricular septal defect | 745.4 |
| Patent ductus arteriosus | 747.0 |
| Congenital aortic stenosis/insufficiency | 746.3, 746.4 |
| Congenital mitral stenosis/insufficiency | 746.5, 746.6 |
| Anomalies of the pulmonary artery | 747.3 |
| Congenital tricuspid valve disease | 746.1 |
| Unspecified defect of septal closure | 745.9 |
| Other specified cardiac anomalies | 746.80, 746.81, 746.82, 746.83, 746.84, 746.85, 746.86, 746.87, 746.89 |
CHD indicates congenital heart disease; HF, heart failure; ICD‐9, International Classification of Diseases, Ninth Revision.
Clinical characteristics assessed at admission included age, sex, race, and comorbidities. Medical comorbidities were identified from the ICD‐9 diagnosis codes previously used in the literature (Table 1)18, 19 and were considered to be present only if the codes were recorded to be present on admission. A Charlson comorbidity index was calculated for each patient.20
Study Outcomes
The primary study outcome was any adverse event, and included any in‐hospital adverse event during the index admission or readmission within 30 days. An adverse event was considered in‐hospital if the relevant diagnosis was not present on admission. In‐hospital events evaluated included length of stay (LOS) ≥7 days, incident arrhythmias (atrial arrhythmia or in‐hospital ventricular tachycardia/sudden cardiac arrest), and all‐cause in‐hospital mortality. We used ICD‐9 diagnosis codes to identify these events (Table 1). The database included a record linkage number that can be used to identify sequential visits for a patient within California, even if those visits occur at a different facility or setting (inpatient, emergency department, or ambulatory surgery) than the index admission. We used this record linkage number to determine whether a patient had an adverse event of any readmission and if the readmission was for HF or arrhythmia within 30 days after their HF hospitalization. Only the first readmission was considered. We determined the primary reason for readmission (HF versus arrhythmia), and all‐cause mortality during the readmission. We assigned patients with the Agency for Healthcare Research and Quality single‐level CCS codes of 108 as having a primary readmission diagnosis of HF and those with CCS codes of 106 or 107 as having a primary readmission diagnosis of arrhythmia.
Statistical Analysis
Data were analyzed from April 4, 2017 through November 1, 2018. Continuous variables are presented as mean (±SD) or median (interquartile range) as appropriate, while categorical variables are presented as percentages. A Student t test or Kruskal–Wallis rank test as appropriate was used for comparisons of continuous variables and Pearson χ2 test for categorical variables. We calculated odds ratios for in‐hospital events comparing patients with CHD to the reference group without CHD, adjusting for the following covariates: admission year, age, sex, race, household income, primary payor, and Charlson comorbidity index. A similar analysis was also performed to compare complex and noncomplex CHD subgroups. Because the data use agreement for the database requires it, results for patient groups with fewer than 10 patients are not reported, although the exact number is known to the investigators and used in the analyses. Statistical analyses were performed using STATA/SE software (version 14; StataCorp).
This study used previously collected deidentified data and was, therefore, exempted from institutional review board approval.
Results
Study Population
From a total of 27 907 535 inpatient hospital discharges for adults in California between January 1, 2005 and January 1, 2012, we identified 203 759 patients who were admitted with the primary diagnosis of HF (Figure 1). After excluding atrial septal defect, 539 of these patients had CHD. The proportion of HF hospitalizations with CHD increased from 0.23% to 0.33% over the study period (P=0.01 for trend).
Figure 1.
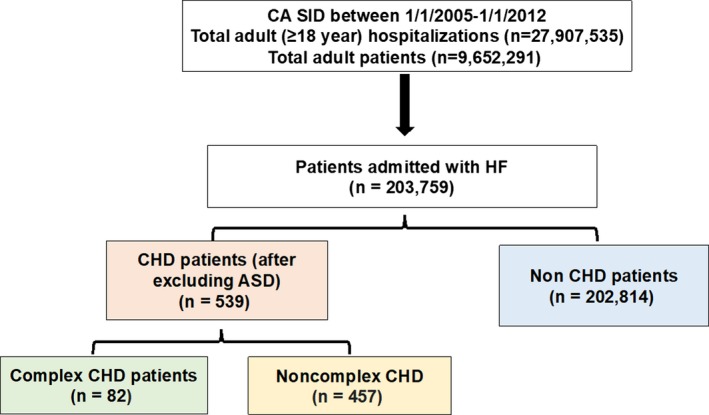
Study population. ASD indicates atrial septal defect; CA, California; CHD, congenital heart disease; HF, heart failure; SID, state inpatient database.
Baseline Demographics
Patients with CHD had fewer medical comorbidities upon admission than non‐CHD patients, including the comorbidities of anemia, hypertension, hyperlipidemia, diabetes mellitus, coronary artery disease, lung disease, chronic renal failure, and cerebrovascular disease (Table 3). Charlson Comorbidity Index was thus lower among patients with CHD than non‐CHD patients. Only history of ventricular arrhythmias was more common in CHD patients than non‐CHD patients.
Table 3.
Baseline Characteristics of Patients Admitted for HF
| CHD (n=539) | No CHD (n=202 814) | P Valuea | |
|---|---|---|---|
| Age (y), mean±SD | 54.9±14.7 | 72.6±14.7 | 0.0001 |
| 18–39 y, n (%) | 117 (21.7) | 5102 (2.5) | <0.0001 |
| 40–64 y, n (%) | 252 (46.8) | 51 369 (25.3) | |
| 65+ y, n (%) | 170 (31.5) | 146 343 (72.2) | |
| Males | 310 (57.5) | 101 996 (50.3) | 0.001 |
| Race/ethnicityb | |||
| White | 306 (60.1) | 11 661 (62.4) | 0.09 |
| Black | 42 (8.3) | 20 146 (10.3) | |
| Hispanic | 113 (22.2) | 34 095 (18.0) | |
| Asian or Pacific Islander | 37 (7.3) | 14 508 (7.4) | |
| Other | 11 (2.2) | 3631 (1.9) | |
| Comorbidities present on admission | |||
| Anemia | 104 (19.3) | 57 085 (28.2) | <0.001 |
| Hypertension | 232 (43.0) | 143 832 (70.9) | <0.001 |
| Hyperlipidemia | 126 (23.4) | 71 162 (35.1) | <0.001 |
| Diabetes mellitus | 95 (17.6) | 76 814 (37.9) | <0.001 |
| CAD | 136 (25.2) | 88 929 (43.9) | <0.001 |
| Atrial arrhythmias | 176 (32.7) | 65 855 (32.5) | 0.9 |
| Ventricular arrhythmias | 43 (7.9) | 7604 (3.8) | <0.001 |
| Lung disease | 96 (17.8) | 55 454 (27.3) | <0.001 |
| Chronic renal failure | 59 (10.9) | 44 079 (21.7) | <0.001 |
| Cerebrovascular disease | ≤10 (1.3)c | 6879 (3.4) | 0.007 |
| Depression | 34 (6.3) | 13 750 (6.8) | 0.62 |
| Charlson comorbidity index | 2.1±0.9 | 2.5±1.2 | 0.0001 |
Values are mean±SD or number (percent of total). CAD indicates coronary artery disease; CHD, congenital heart disease; HF, heart failure.
Calculated using Pearson χ2 test.
Because of missing data, totals may not equal column heads.
Health Care Cost and Utilization Project policy prohibits reporting cell frequencies of <10.
Clinical Events
Patients with CHD had higher incidence of any adverse event than non‐CHD patients (40.5% versus 29.0%, P<0.001). The rates of any in‐hospital event, LOS ≥7 days, and incident arrhythmia were higher in CHD patients than non‐CHD patients (Figure 2A). Median LOS was longer for CHD patients than non‐CHD patients (4 [interquartile range: 2.7] versus 3 [interquartile range: 2.6] days, P=0.0001). Fifteen percent of patients with HF hospitalizations (30 317 of 203 353) had any 30‐day readmission; 88 (16.3%) had CHD, and 30 229 (14.9%) did not. We found no significant difference in the rates of readmission events between CHD patients and non‐CHD patients (Figure 2B).
Figure 2.
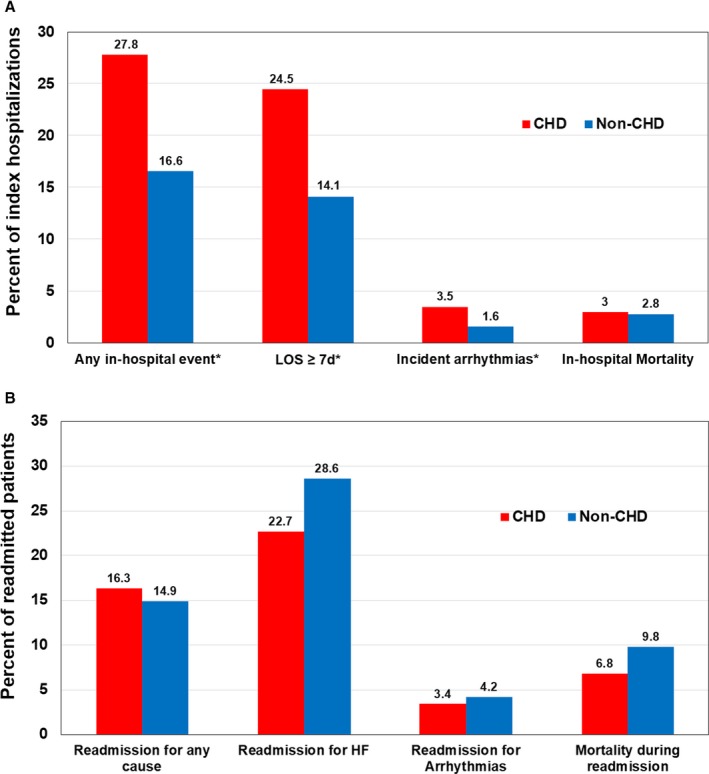
Rates of in‐hospital and 30‐day readmission events in CHD and non‐CHD patients admitted for HF. A, In‐hospital events (*P<0.01). B, 30‐day readmission events. CHD indicates congenital heart disease; HF, heart failure; LOS, length of stay.
After adjusting for covariates, CHD patients had higher odds of an adverse event than non‐CHD patients (adjusted odds ratio 2.1 (95% CI: 1.7–2.5), P<0.001). Of the individual adverse events, CHD patients had higher adjusted odds of any in‐hospital event, LOS ≥7 days, incident arrhythmias, in‐hospital mortality, and a lower adjusted odds of 30‐day readmission for HF (Figure 3A). Among patients admitted for HF with CHD, those with complex lesions had significantly higher odds of LOS ≥7 days than patients with noncomplex lesions but no difference in the odds of other outcomes (Figure 3B).
Figure 3.
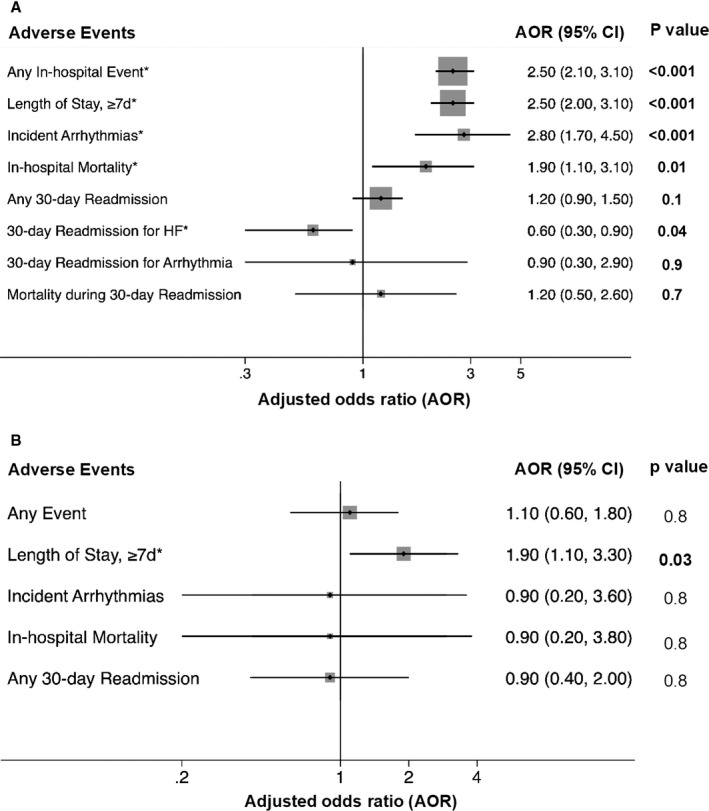
Multivariate analysis for adverse clinical outcomes in patients admitted for HF, comparing (A) CHD and non‐CHD patients; and (B) complex and noncomplex CHD patients. *Adjusted for admission year, age, sex, race, household income, primary payor, and Charlson comorbidity index. AOR indicates adjusted odds ratio; CHD, congenital heart disease; HF, heart failure.
Discussion
It has been reported that adults with CHD have higher resource use during their HF hospitalizations than HF patients who do not have CHD.9 Yet it has not been known whether the clinical outcomes for CHD patients with HF differ from the outcomes for non‐CHD patients with HF. In this study of ≈200 000 patients admitted for HF in California during a 7‐year period, we found that adults with CHD had significantly more adverse in‐hospital clinical events than those without CHD, including 2.5‐, 2.8‐, and 1.9‐fold higher adjusted odds of longer LOS, incident arrhythmias, and in‐hospital mortality, respectively. On the other hand, CHD patients had 40% lower adjusted odds of 30‐day readmission for HF after their HF admission.
We found CHD patients to be younger than non‐CHD patients during their HF admission, similar to the prior study by Burchill et al.9 This may be because of the direct time‐dependent impact of the underlying structural and functional abnormalities on cardiac function, resulting in HF at a younger age. The rates of some comorbidities (ie, diabetes mellitus, coronary artery disease, lung, and renal disease) in our adult CHD HF patients was similar to what was previously reported by Burchill et al,9 but our CHD HF patients had higher rates of hypertension (43% versus 28%). This is likely because of the differences in the ICD‐9 codes used to define hypertension. Overall, in both our study and the Burchill et al study, CHD patients admitted for HF had lower comorbidity indices than non‐CHD patients.9
While the median LOS for CHD adults in our study was 4 days, it was much higher (7 and 7.2 days) in the 2 prior studies (Burchill et al and Chan et al, respectively)9, 21 that reported LOS during a HF hospitalization among CHD patients. This could be related to differences in the study population. Burchill et al included in their study any patient with a HF diagnosis, not just those for whom HF was the primary diagnosis. As a result, their study population included many patients admitted for procedures, and those patients have longer average LOS. Chan et al obtained data for adult CHD patients from pediatric hospitals. While these patients had longer median LOS among high resource use pediatric centers, the median LOS for lower resource use centers was similar to our study of nearly all hospitals statewide. Additionally, similar to Burchill et al, we also found that CHD patients had a longer LOS than non‐CHD patients during their HF hospitalization.9 This might be because of the differences in HF management between the 2 study groups. The management of HF in CHD patients is usually directed towards identifying and correcting any underlying mechanical or hemodynamic abnormalities.22, 23 However, conventional HF medications have been less effective and may even be harmful, such as the use of beta blockers in CHD patients with prevalent pre‐existing sinus node dysfunction, heart block, baffle stenosis, nondistensible atria, or restrictive ventricular physiology.6
For some CHD patients, arrhythmias are intrinsic to the structural malformation itself, while for most others, arrhythmias represent an acquired condition related to the unique myocardial substrate created by surgical scars in conjunction with cyanosis and abnormal pressure/volume loads of long duration.24 We observed ≈33% and 8% prevalence of atrial and ventricular arrhythmia, respectively, at the time of admission for HF in adults with CHD. This estimate was higher than the 25% to 32% prevalence of all arrhythmia noted in a national sample of adult CHD admissions for any cause.25, 26 Our finding also supports the notion that arrhythmias have serious implications in CHD patients, whether it is a cause of or result from HF.6, 27 There are little data on the incidence of new arrhythmias during admissions for HF among CHD patients. In our study, 3.5% of adult CHD patients had incident arrhythmias during their HF hospitalization, representing a 2.8‐fold higher adjusted odds for this adverse outcome than for non‐CHD patients.
The overall in‐hospital mortality rate of 3% in our study is lower than the 6.5% noted by Burchill et al and 7.3% noted by Chan et al.9, 21 The increased mortality noted in those studies may be related to more complicated admissions, as suggested by their longer LOS. Similar to Burchill et al, we found the in‐hospital mortality rates to be similar for CHD and non‐CHD HF patients, but we found that the adjusted odds of inpatient mortality was nearly 2‐fold higher in CHD patients. Given the limited sample size of the CHD patients in our data set and the absence of clinical details, we were not able to explore the predictors of high mortality among them. However, future studies could address the hypotheses that heterogeneity of the underlying anatomy, surgical repairs, and the physiologic severity of the CHD lesions as well as the chronic nature of cardiac remodeling may cause progressive modulation of the arrhythmia and hemodynamic substrate, leading to higher inpatient mortality.
We found that only 15% of all patients with a HF admission had a 30‐day readmission. This is lower than the 25% rate of 30‐day readmission noted in a prior study of Medicare beneficiaries.12 In that study, 35% of patients who were readmitted were rehospitalized with a primary diagnosis of HF, while we found these readmission rates to be 23% and 29% in our CHD and non‐CHD patients, respectively. CHD patients in our study also had 40% lower adjusted odds of 30‐day readmission for HF after their HF admission than non‐CHD patients. As mentioned earlier, the management of HF during admission is usually targeted toward correcting the underlying structural or hemodynamic abnormalities. This might reduce the risk for recurrent HF hospitalization, at least in the short term. This could explain why we observed significantly lower 30‐day readmission for HF in CHD patients despite no difference in the odds of 30‐day readmission between the CHD and non‐CHD groups. In general, readmission is known to be a major healthcare burden for CHD patients after a hospitalization, and the type of CHD lesion is shown to be the primary risk factor for the readmission.28, 29 Because of the small sample size of CHD patients who had a readmission after their HF admission, we were unable to explore potential differences in readmission events specific to each lesion.
Our findings highlight that during admission for HF, CHD patients—who are younger with fewer comorbidities than non‐CHD patients admitted with HF—nonetheless had significantly higher adjusted risks of adverse clinical outcomes than non‐CHD patients. Further studies to identify predictors of incident arrhythmias and in‐hospital mortality during HF admission in CHD patients, such as those specific to CHD lesions, may assist in the development of lesion‐specific risk prediction models and targeted prevention and treatment protocols.
Limitations
This study has several limitations, primarily intrinsic to the use of the hospital discharge abstract database.30 First, ICD‐9 codes have imperfect sensitivity and specificity, and CHD may have been incorrectly coded. Because of this, we excluded patients with atrial septal defect, since it is known that coding for atrial septal defect versus patent foramen ovale is frequently incorrect.17 Second, clinical detail is often missing from discharge abstracts; thus inherent patient differences, variations in clinical presentation, information regarding medication use, and similar characteristics during the hospitalization could not be studied. Finally, the inpatient nature of this database did not allow us to capture out‐of‐hospital events or mortality, or intensity and quality of care before and after hospitalization with HF. Similarly, only patients who were readmitted to a California hospital were captured; therefore, we had no information about deaths among patients who were admitted or died out of state.
Conclusions
In this study of nearly all patients with HF hospitalizations in California hospitals during a 7‐year period, CHD patients admitted for HF are younger and have fewer comorbidities than patients without CHD. Despite this, patients with CHD have higher adjusted odds of longer LOS, incident arrhythmias, and in‐hospital mortality than non‐CHD patients. The higher risks for adverse clinical outcomes during their admissions for HF among CHD patients suggest a need for CHD‐specific risk prediction tools and HF treatment recommendations to improve outcomes in these patients.
Sources of Funding
This work was supported in part from the American Heart Association/Childrens’ Heart Foundation Mentored Clinical & Population Research Award 17MCPRP33240000 (Agarwal). Dr Tseng received support from NIH/NHLBI 5 R01 HL126555 during the conduct of the study. There are no relationships with industry.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e012595 DOI: 10.1161/JAHA.119.012595.)
References
- 1. Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J. 2004;147:425–439. [DOI] [PubMed] [Google Scholar]
- 2. Khairy P, Ionescu‐Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Marelli AJ. Changing mortality in congenital heart disease. J Am Coll Cardiol. 2010;56:1149–1157. [DOI] [PubMed] [Google Scholar]
- 3. Marelli AJ, Mackie AS, Ionescu‐Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. [DOI] [PubMed] [Google Scholar]
- 4. Gilboa SM, Devine OJ, Kucik JE, Oster ME, Riehle‐Colarusso T, Nembhard WN, Xu P, Correa A, Jenkins K, Marelli AJ. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krasuski RA, Bashore TM. Congenital heart disease epidemiology in the United States: blindly feeling for the charging elephant. Circulation. 2016;134:110–113. [DOI] [PubMed] [Google Scholar]
- 6. Stout KK, Broberg CS, Book WM, Cecchin F, Chen JM, Dimopoulos K, Everitt MD, Gatzoulis M, Harris L, Hsu DT, Kuvin JT, Law Y, Martin CM, Murphy AM, Ross HJ, Singh G, Spray TL. Chronic heart failure in congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2016;133:770–801. [DOI] [PubMed] [Google Scholar]
- 7. Piran S, Veldtman G, Siu S, Webb GD, Liu PP. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation. 2002;105:1189–1194. [DOI] [PubMed] [Google Scholar]
- 8. Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the US. J Am Coll Cardiol. 2009;54:460–467. [DOI] [PubMed] [Google Scholar]
- 9. Burchill LJ, Gao L, Kovacs AH, Opotowsky AR, Maxwell BG, Minnier J, Khan AM, Broberg CS. Hospitalization trends and health resource use for adult congenital heart disease—related heart failure. J Am Heart Assoc. 2018;7:e008775 DOI: 10.1161/JAHA.118.008775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verheugt CL, Uiterwaal CS, van der Velde ET, Meijboom FJ, Pieper PG, Sieswerda GT, Plokker HW, Grobbee DE, Mulder BJ. The emerging burden of hospital admissions of adults with congenital heart disease. Heart. 2010;96:872–878. [DOI] [PubMed] [Google Scholar]
- 11. Yu C, Moore BM, Kotchetkova I, Cordina RL, Celermajer DS. Causes of death in a contemporary adult congenital heart disease cohort. Heart. 2018;104:1678–1682. [DOI] [PubMed] [Google Scholar]
- 12. Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto‐Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. HCUP central distributor SID California file composition. Healthcare cost and utilization project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; August 2006. Available at: https://www.hcup-us.ahrq.gov/db/state/siddist/siddist_filecompca.jsp. Accessed 11/06/2018. [Google Scholar]
- 14. HCUP CCS . Healthcare cost and utilization project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; March 2017. Available at: https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Updated 2017. Accessed 11/03/2018. [Google Scholar]
- 15. Hayward RM, Foster E, Tseng ZH. Maternal and fetal outcomes of admission for delivery in women with congenital heart disease. JAMA Cardiol. 2017;2:664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mackie AS, Pilote L, Ionescu‐Ittu R, Rahme E, Marelli AJ. Health care resource utilization in adults with congenital heart disease. Am J Cardiol. 2007;99:839–843. [DOI] [PubMed] [Google Scholar]
- 17. Broberg C, McLarry J, Mitchell J, Winter C, Doberne J, Woods P, Burchill L, Weiss J. Accuracy of administrative data for detection and categorization of adult congenital heart disease patients from an electronic medical record. Pediatr Cardiol. 2015;36:719–725. [DOI] [PubMed] [Google Scholar]
- 18. Shah RU, Freeman JV, Shilane D, Wang PJ, Go AS, Hlatky MA. Procedural complications, rehospitalizations, and repeat procedures after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2012;59:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bansil P, Kuklina EV, Meikle SF, Posner SF, Kourtis AP, Ellington SR, Jamieson DJ. Maternal and fetal outcomes among women with depression. J Womens Health (Larchmt). 2010;19:329–334. [DOI] [PubMed] [Google Scholar]
- 20. Austin SR, Wong YN, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson comorbidity index and Elixhauser score work. Med Care. 2015;53:e65–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan J, Collins RT II, Hall M, John A. Resource utilization among adult congenital heart failure admissions in pediatric hospitals. Am J Cardiol. 2019;123:839–846. [DOI] [PubMed] [Google Scholar]
- 22. Sabanayagam A, Cavus O, Williams J, Bradley E. Management of heart failure in adult congenital heart disease. Heart Fail Clin. 2018;14:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Opina AD, Franklin WJ. Management of heart failure in adult congenital heart disease. Prog Cardiovasc Dis. 2018;61:308–313. [DOI] [PubMed] [Google Scholar]
- 24. Masarone D, Limongelli G, Rubino M, Valente F, Vastarella R, Ammendola E, Gravino R, Verrengia M, Salerno G, Pacileo G. Management of arrhythmias in heart failure. J Cardiovasc Dev Dis. 2017;4:3 DOI: 10.3390/jcdd4010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loomba RS, Buelow MW, Aggarwal S, Arora RR, Kovach J, Ginde S. Arrhythmias in adults with congenital heart disease: what are risk factors for specific arrhythmias? Pacing Clin Electrophysiol. 2017;40:353–361. [DOI] [PubMed] [Google Scholar]
- 26. O'Leary JM, Siddiqi OK, de Ferranti S, Landzberg MJ, Opotowsky AR. The changing demographics of congenital heart disease hospitalizations in the United States, 1998 through 2010. JAMA. 2013;309:984–986. [DOI] [PubMed] [Google Scholar]
- 27. Bouchardy J, Therrien J, Pilote L, Ionescu‐Ittu R, Martucci G, Bottega N, Marelli AJ. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679–1686. [DOI] [PubMed] [Google Scholar]
- 28. Cedars AM, Burns S, Novak EL, Amin AP. Predictors of rehospitalization among adults with congenital heart disease are lesion specific. Circ Cardiovasc Qual Outcomes. 2016;9:566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cedars AM, Burns S, Novak EL, Amin AP. Rehospitalization is a major determinant of inpatient care costs in adult congenital heart disease. J Am Coll Cardiol. 2016;67:1254–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Humphries KH, Rankin JM, Carere RG, Buller CE, Kiely FM, Spinelli JJ. Co‐morbidity data in outcomes research are clinical data derived from administrative databases a reliable alternative to chart review? J Clin Epidemiol. 2000;53:343–349. [DOI] [PubMed] [Google Scholar]


