Abstract
Brain metastases (BM) from pancreatic ductal adenocarcinoma (PDAC) are an infrequent event. We identified n=25 pts from MSKCC with BM and PDAC; median age 58years. Median time to the development of BM was 17 months (range 0 to 79). Median overall survival from the time of BM development was 1.5 months (1 to 31). Six patients had germline testing; with BRCA1 (n=1) or BRCA2 (n=2) alterations detected. Seven patients had molecular profiling with KRAS, TP53 and MYC amplification most frequent.
Background:
The purpose of this study was to assess clinical characteristics of patients with metastatic pancreas ductal adenocarcinoma (PDAC) and brain metastases (BM) as well as the somatic and germline molecular profiles where performed.
Patients and Methods:
Patients with PDAC and BM between January 1990 and January 2016 were identified. Molecular characteristics of somatic and germline testing where performed in the subset of patients that had provided informed consent. Somatic alterations were assessed by either MSK-IMPACT testing (>340 key cancer genes) or Sequenom testing (8 gene panel). Overall survival (OS) was calculated from date of diagnosis to either date of last follow up or death. Survival post brain metastasis was calculated from date of diagnosis of brain metastases by radiology or pathology to either date of last follow up or death.
Results:
From a total of 5,824 patients with PDAC identified from January 2000 to January 2016, twenty-five (0.4%) patients had brain metastases. Median age of PDAC diagnosis was 58 years. Median time to the development of BM from initial PDAC diagnosis was 17 months (0 to 79). Median OS following BM diagnosis was 1.5 months (range 1 to 31). OS for patients that had craniotomy (n=4) was 11 months (1 to 31 months) with two long term survivors; 21 and 31 months respectively. Four patients had leptomeningeal disease. 6/25 pts had germline testing, 3 had BRCA mutations; BRCA1 (n=2) and BRCA2 (n=1). Somatic profiling identified KRAS mutations in 100%; G12D (n=4), G12V (n=2) and Q61K (n=1).
Conclusion:
BM from PDAC is a rare event. We identified a speculitive association of germline BRCA1/2 alterations with BM in PDAC, which requires corroboration. Survival post BM development is poor with prolonged survival in selected patients via a multidisciplinary approach.
Introduction:
Pancreatic ductal adenocarcinoma (PDAC) is the fourth most common cause of cancer related death with an estimated 53,070 cases occurring in the United States in 2016. [1] PDAC is a challenging disease with the majority of patients presenting with either locally advanced or metastatic disease at time of diagnosis. Metastatic disease from PDAC is more commonly evident at sites such as the liver, lymph nodes, peritoneum and lung. In contrast, brain metastases from pancreatic ductal adenocarcinoma are rare with a reported incidence of <0.6%. [2, 3] Given PDAC is underscored by an aggressive tumor biology with an adverse prognostic outlook, a possible hypothesis for the rarity of brain metastases in PDAC is that patients do not survive sufficiently long enough for brain metastases to develop or become clinically apparent. At post mortem, brain metastases from PDAC have been described both in a case report [4] and in a larger series with a rate of 7.9%, [5] suggesting that the actual rate of <0.6% may be potentially underrepresented. Furthermore, since 2011 modest clinical therapeutic progress has been made in PDAC with cytotoxic chemotherapy triplet regimens such as FOLFIRINOX [6] and the doublet regimen of gemcitabine plus nab-paclitaxel [7] improving survival in patients with metastatic disease compared to single agent gemcitabine therapy. We hypothesize that the ability to achieve longer systemic disease control through sequencing of active agents may result in a more frequent incidence of clinically evident brain metastases. We reviewed records of patients with PDAC evaluated at MSKCC over a 16-year period to identify patients with brain metastases to determine associated clinical, pathologic and molecular features and describe the natural history.
Materials and Methods:
Samples:
A retrospective review of patients evaluated at MSKCC with brain metastases from PDAC from January 2000 to January 2016 was undertaken from several prospectively maintained databases following IRB review. Charts were reviewed to determine clinicopathological characteristics including age at diagnosis, stage at presentation, treatment of primary tumor, use of chemotherapy (neoadjuvant, adjuvant or metastatic settings), management of brain metastases (surgery or radiotherapy). In addition, molecular characteristics determined via somatic and germline testing where performed in the subset of patient that had provided informed consent. Somatic alterations were assessed by either MSK-IMPACT testing (>340 key cancer genes) or Sequenom testing (8 gene panel).
Statistics:
Overall survival was calculated from date of diagnosis to either date of last follow up or death. Survival post brain metastasis was calculated from date of diagnosis of brain metastases by radiology or pathology to either date of last follow up or death.
Target capture and sequencing:
Molecular profiling was performed by the MSK-IMPACT assay which detects mutations and copy number alterations using DNA derived from frozen and formalin-fixed, paraffin-embedded (FFPE) tissue [8]. The assay has been designed to sequence all coding exons of >340 cancer-associated genes and provide 98% power to detect mutations with a true variant allele frequency of 10%, novel mutations down to a threshold of 5% variant allele frequency and mutations at recurrent hotspots down to a threshold of 2% variant allele frequency. Prior to the introduction of MSK-IMPACT testing in 2014, a Sequenom mass spectrometry panel which included 8 genes assessing for hotspot mutations in; AKT1, BRAF, EGFR, ERBB2, KRAS, MAP2K1, NRAS and PIK3CA genes was utilized.
Results
Clinical characteristics.
From a total of 5,824 patients with PDAC identified from January 2000 to January 2016, twenty-five (0.4%) patients had brain metastases. Seventeen patients (68%) had locally advanced or metastatic disease at initial diagnosis. Clinical characteristics are outlined in Table 2. Males were more frequently affected (60%) with a median age of pancreas adenocarcinoma diagnosis of 58 years (range 44 to 79 years). The median time to the development of brain metastases from initial diagnosis of pancreas adenocarcinoma was 17 months (range 0 to 79). All patients had evidence of brain metastases by CT or MRI imaging (See Figure 1, Figure 2A and Figure 2B for selected cases). The most common indication for CNS imaging was headache (36%, 9/25). Other neurological symptoms that prompted imaging included; facial/limb weakness (16%), visual disturbance (12%), ataxia (8%) and seizure (8%). One patient had imaging for persistent nausea. Another patient had a diagnosis of brain metastases incidentally discovered as part of work up of a concurrent diagnosis of diffuse large B cell lymphoma and stage IV pancreas adenocarcinoma. Biopsy of the brain lesion confirmed the CNS lesion was of pancreatic origin.
Table 2.
Clinical charachteristics of 24 patients with brain metastases from PDAC.
| Characteristics | N=25 (%) |
|---|---|
| Gender | |
| Male | 15 (60) |
| Female | 10 (40) |
| Race/Ethnicity | |
| White | 20 (80) |
| Asian | 3 (12) |
| Black | 2 (8) |
| Smoking status | |
| Never | 12 (48) |
| Prior/current smoking history | 13 (52) |
| Location of primary pancreas tumor | |
| Head | 10 (40) |
| Body | 8 (32) |
| Tail | 7 (28) |
| Stage at diagnosis | |
| I | 0 (0) |
| IIA | 4 (16) |
| IIB | 4 (16) |
| III | 1 (4) |
| IV | 16 (64) |
Figure 1.
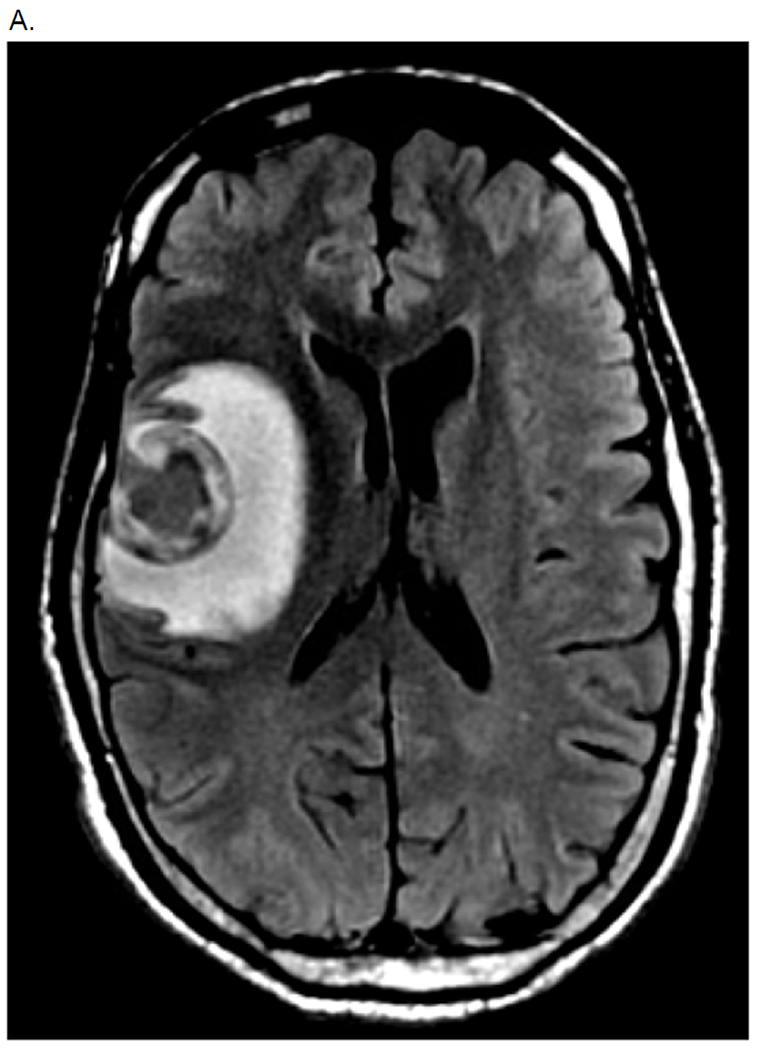
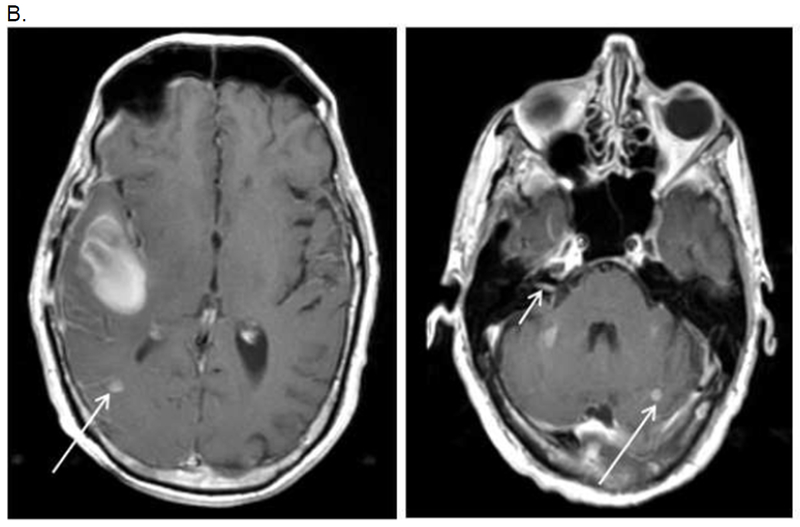
A. MRI Brain (Patient 3 in Table 3 - KRAS mutant pancreas cancer)
Hemorrhagic 3.1cm mass in R frontal love with surrounding edema and mild L midline shift.
B. MRI brain one month post craniotomy with evidence of two new subcentimeter brain metastases (arrow) and leptomeningeal enhancement (arrow). Cytology positve from lumbar puncture.
Figure 2.
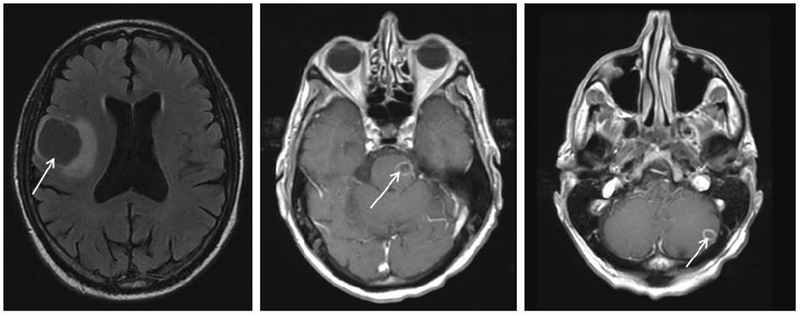
Metastatic KRAS mutant pancreas cancer. (Patient 1 in table 3)
MRI brain with evidence of right frontal lobe, midbrain and cerebellar metastases (arrows).
Brain metastases were evident at time of initial diagnosis of metastatic disease in three patients. All patients developed extracranial disease at some point during their disease trajectory. One patient developed a solitary brain metastases seventeen months post initial diagnosis of stage III pancreas adenocarcinoma but developed peritoneal disease twelve months post craniotomy. The majority (68%, n=17) had evidence of liver metastases at time of metastatic presentation followed by lung metastases (52%, n=13) and peritoneal disease (20%, n=5).
Treatment.
First line systemic chemotherapy regimens are listed in Table 3. Mean number of systemic treatments was 2 (range 0 to 8). Six patients received prior adjuvant therapy, four received single agent gemcitabine and one patient each received adjuvant GTX and FOLFIRINOX. The treatment modalities employed in 24 patients with available follow up of their brain metastases are outlined in Table 3. Four (16.6%) patients underwent craniotomy and fifteen (62.5%) patients receiving radiotherapy. Eight (33.3%) patients received best supportive care alone. Of the patients who received radiotherapy, the majority (n=13) received whole brain radiotherapy (WBRT), with two patients receiving stereotactic radiosurgery (SRS). Median overall survival for all patients following diagnosis of brain metastases was 1.5 months (range 1 to 31 months). Median overall survival patients (n=4) post craniotomy was 11 months (1 to 31 months). Location of brain metastases varied with >50% having evidence of frontal lobe involvement. Four patients had evidence of leptomeningeal disease and five (21%) had cerebellar metastases, Table 3.
Table 3.
Location, treatment, survival and molecular charachteristics of 24 patients with brain metastases from PDAC
| Pt | Molecular | Site of BM | Surgery Y/N | RT | LMD | BM Survival (days) | 1ST Line chemo. | OS advanced disease (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | KRAS G12D | Cerebellum, Right frontal | N | WBRT | N | 2 | Cisplatin + gemcitabine | 82 |
| 2 | KRAS Q61K, MYC Amp, FGFR1 Amp | Right occipital, Right frontal | N | WBRT | N | 77 | FOLFIRINOX | 3 |
| 3 | KRAS G12D, TP53 R273H | Right frontal | Y | WBRT | Y | 52 | Gemcitabine plus nab-paclitaxel | 10 |
| 4 | KRAS G12V BRCA2 Germline | Right parietal, Right temporoocciptal | N | WBRT | Y | 233 | GemOX | 24 |
| 5 | No testing | Left frontal | Y | N | N | 975 | GTX | 31 |
| 6 | No testing | Multiple cerebral hemisphere, cerebellum | N | N | N | 28 | Supportive care | <1 |
| 7 | No testing | Multiple cerebral | N | N | N | 12 | Supportive care | <1 |
| 8 | No testing | Right frontal then parietal, cerebellum | N | SRS then WBRT (new lesions) | N | 165 | Gemcitabine | 30 |
| 9 | No testing | Right frontal, Left parietal, Left frontal | N | N | N | 20 | Gemcitabine plus irinotecan | 7 |
| 10 | KRAS G12D | Precentral gyrus, cerebellum | N | WBRT | N | 50 | Gemcitabine | 9 |
| 11 | No testing | Left frontal, right temporal, left occipital | N | N | N | 11 | GemOX | 12 |
| 12 | No testing | Multiple cerebral hemisphere | N | N | N | 21 | Gemcitabine plus erlotinib | 22 |
| 13 | No testing | Left occipital | N | SRS | N | 56 | GemOX | 18 |
| 14 | No testing | Left frontal, Left temporal | N | WBRT | N | 27 | Supportive care | <1 |
| 15 | No testing | Left frontal, left parietal, cerebellum | N | WBRT | N | 193 | Unknown | 6 |
| 16 | No testing | Left frontal, Right occipital | Y | WBRT | N | 656 | Cisplatin plus gemcitabine | 39 |
| 17 | No testing | Left frontal | N | SRS | N | 184 | GemOX | 16 |
| 18 | No testing | Left parietal | N | N | N | 41 | FOLFIRINOX | 43 |
| 19 | No testing | Multiple cerebral hemisphere | N | WBRT | Y | 23 | GemOX | 18 |
| 20 | No testing | Right frontal, superior vermis | Y | WBRT | N | 26 | GTX | 29 |
| 21 | No testing | Left hemisphere, cerebellum | N | N | N | 7 | FOLFIRINOX | 10 |
| 22 | BRCA1 Germline | Left frontal, right occipital | N | N | N | 23 | Cisplatin plus gemcitabine +/−PARP inhibitor | 22 |
| 23 | KRAS G12V, TP53 1251F | Left Frontal, left precentral gyrus | N | WBRT | N | 76 | FOLFIRINOX | 57 |
| 24 | BRCA1 Germline | Right Temporal lobe, Cerebellum | N | WBRT | Y | 118 | Cisplatin plus gemcitabine +/−PARP inhibitor | 14 |
Molecular Testing.
Seven patients had somatic mutational profiling, four had MSK-IMPACT and three had Sequenom testing (8 gene panel), Figure 3. KRAS mutations were detected in all seven cases. Six of seven KRAS mutations were located at codon 12, four of which were KRAS G12D and two were KRAS G12V mutations. One patient had a KRAS Q61K mutation. Sample origins for molecular testing for MSK-IMPACT included; skin (n=1), lung (n=1), pancreas (n=1) and brain (n=1). For patients that had the limited eight-gene panel, one sample each was from brain, pleura and pancreas.
Figure 3:
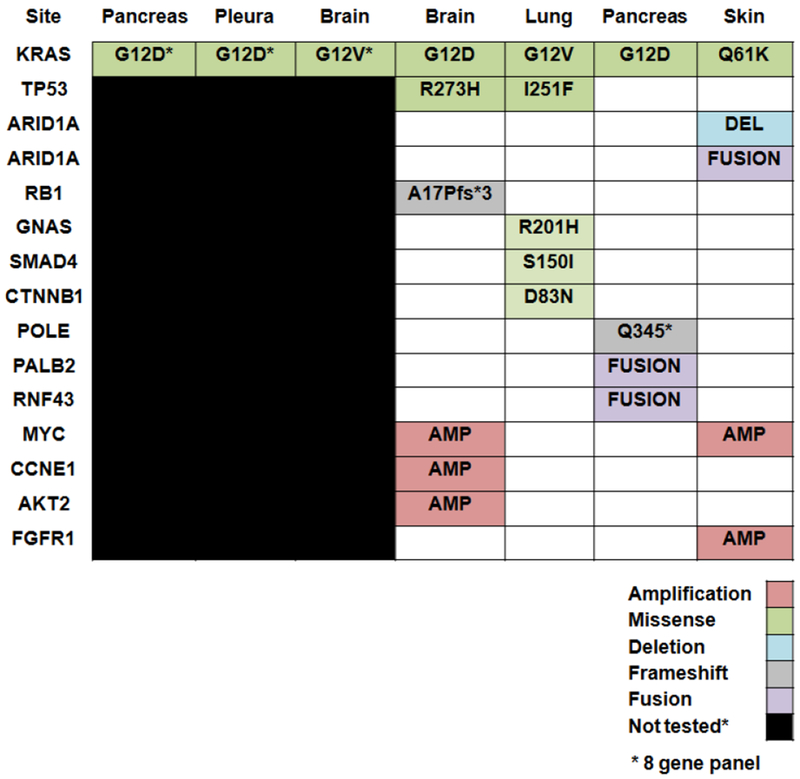
Molecular profiling of seven patients with brain metastases from PDAC
Two patients with brain metastases from PDAC were known BRCA1 and BRCA2 carriers. Four other patients had germline BRCA testing of which one had a BRCA2 V2111 germline alteration. The germline mutation status of the other 19 patients was unknown.
Discussion.
This is the largest reported series to date of brain metastases in patients with PDAC. Previously reported cases include case reports, and a single institution experience [9], are summarized in Table 1. Consistent with prior data reported, males were more frequently affected in our dataset and represented 60% of patients. Median age at time of pancreas adenocarcinoma diagnosis within our patient cohort was 58 years (range 44 to 79 years) similar to a median age of 61.5 years reported previously [9]. The median time to development of brain metastases was 17 months for the 24 patients within our dataset, one year shorter than the 29 months reported previously [9]. Notably, our series included a higher number of patients with locally advanced or de novo metastatic disease at presentation; 71% (17/24pts) compared to 11% (1/9) [9] and no patients had ampullary carcinoma.
Table 1.
Clinical characteristics, treatment and outcomes of patients with brain metastases from pancreas cancer previously reported in the literature
| Author Year | BM at diagnosis | Pt. | Primary | Location BM | Treatment BM | OS from BM |
|---|---|---|---|---|---|---|
| Kuratsu [21] | N N |
56yo M 58yo M |
Head Not reported (NR) | Thalamus Cerebellum |
Ommaya + RT Resection |
9 months 2 weeks |
| Chiang [17] | N | 54yo M | Uncinate process (KRAS G12V) | Left Frontal | Resection + RT | 20 months (ongoing) |
| Caricato [22] | N | 67yo M | Head | Cerebellum | Resection | 12 months (ongoing) |
| Park [2] | N Y N Y |
48yo, M 51yo, M 52yo, M 62yo, M |
NR NR NR NR |
Multiple Left frontal Left parietal Left frontal, L basal ganglia |
RT Supportive care Radiation Supportive care |
Median Survival 2.9 months (1.5-3.8) |
| El Kamar [23] | N | 56yo, M | NR | Multiple cerebral and pons | Supportive care | 3 days |
| Lemke [20] | N | 48yo, F 66yo, M |
Tail Tail |
Cerebellum Right cerebrum |
Resection + RT Resection + RT |
>10yrs >6 yrs |
| Matsumura[24] | N | 64yo M | Tail | Cerebrum | Resection + RT | >10 months |
| Marepaily[25] | N | 36yo, F | Tail | Cerebellum (adenosquamous) | Resection | <1 month |
| Matsumoto[26] | Y | 68yo, M | Head | Right Cerebrum | Resection | 3 months |
| Rajappa [27] | N | 67 yo, M | Tail | Right Occipital | Resection + SRS | 36 months |
| Zaanan [28] | N | 57yo, M | Head | Multiple cerebrum | Supportive care | 3 days |
| Rao [29] | Y | 57yo, M | Mass neck, body, tail | Multiple cerebral and cerebellar | Radiation | <3 months |
| Kumar [9] | N | Median 61.5 yrs (N=8) | Head n=6 Ampullary n=1 Tail n=1 |
Cerebral Cerebellar n=1 Vermis n=1 Choroid n=1 |
Reported (n=4) Resection + RT (n=1) Resection (n=1) RT (n=2) |
One pt >9 yrs post resection |
*NR= not reported
RT= radiotherapy
SRS= stereotactic radiosurgery
We sought to identify somatic genetic alterations in PDAC which may predispose to the development of brain metastases. In a prior series, TP53 mutations were detected in three patients with brain metastases and all were SMAD4 wild-type by immunohistochemistry. [9] In a cohort of ten patients with ERBB2 amplified PDAC cases, one of eight patients with metastatic disease developed brain metastases [10]. In our dataset, we identified KRAS mutations in all patients, predominantly located at codon 12 in 86% (n=6) cases. TP53 was altered in two of four patients that had broader profiling by MSK-IMPACT in our dataset. We did not identify any ERBB2 mutations in either the eight-gene panel or ERBB2 amplifications/mutations by broader MSK-IMPACT testing. Two patients in this dataset had molecular testing performed on their brain metastases, Figure 1. Both harbored codon 12 KRAS mutations. Concurrent TP53 and RB1 loss in one patient sample coexisting with KRAS by MSK-IMPACT which would indicate a possible small cell phenotype [11]. Additionally, the presence of a GNAS R201 mutation concurrent with a KRAS G12V mutation in one patient infers the likelihood of this tumor arising from a precursor intraductal pancreatic neoplasm (IPMN) [12]. This patient developed brain metastases seventy months post diagnosis of a stage IIA tumor and 54 months from diagnosis with metastatic disease consistent with a more favorable biological profile [13].Similarly, a case report of oncocytic carcinoma derived from an IPMN and metachronous brain metastases harbored a KRAS G12V mutation with the presence of GNAS not commented. [14] MYC amplification was noted in two of four samples by MSK-IMPACT (Figure 3) and has been reported as an inducer for metastases in in-vivo models of lung cancer [15].
A novel aspect to our cohort was the finding of germline BRCA1 or BRCA2 in three of six patients that had germline testing. Germline BRCA1 alterations have been associated with an increased propensity for brain metastases in breast cancer [16] and ovarian cancer [17]. Germline BRCA1 or BRCA2 mutations have not been associated with brain metastases in PDAC to date. We anticipate that ongoing prospective studies in patients with BRCA1/2 associated PDAC will contribute more information in this regard. Very specifically, data from our group and others have demonstrated the potential value of platinum based therapies and Poly ADP ribose polymerase (PARP) inhibitors in patients with germline BRCA mutations[18–20]. As these patients may live longer compared to patients without germline BRCA mutations, speculatively they may be at higher risk of developing rarer sites of metastases, including brain metastases.
Survival is poor for the majority of patients upon development of brain metastases with rare exceptions in select patients who undergo resection; with long term survival reported in three patients who had oligo-metastatic disease.[9, 21] Median survival post brain metastasis was 1.5 months in our dataset. For the selected patients who had resection within our described cohort a longer median survival time post diagnosis of brain metastases was noted at 11 months (range 1 to 31 months). Two patients survived >1-year post brain metastases development for 21 and 31 months respectively. Both had surgical resection and received subsequent systemic chemotherapy. One patient had a solitary brain metastasis resected followed by GTX chemotherapy and two subsequent lines of therapy upon systemic progression. The other longterm survivor had resection of a dominant mass followed by WBRT followed by two lines of chemotherapy. Both patients died of extracranial disease. Less common sites of CNS related disease from PDAC reported in the literature include the presence leptomeningeal disease and cerebellar metastases. Leptomeningeal disease from PDAC has been described at initial presentation of PDAC [22–24], both in the presence [25] and absence [26] of parenchymal brain metastases. Four patients had evidence of leptomeningeal disease all occurred late in the disease trajectory with parenchymal brain metastases. Additionally, cerebellar metastases has also been reported [9, 27], and was evident in five patients in our dataset.
Conclusion
This is the largest reported clinical dataset of brain metastases in pancreas ductal adenocarcinoma. Brain metastases from pancreas ductal adenocarcinoma are rare and not exclusively seen in patients with prolonged survival. Indeed, the minority (32%, n=8) of patients within our dataset had evidence of brain metastases after 2011 which is following the introduction of both FOLFIRINOX and gemcitabine plus nab-paclitaxel for metastatic PDAC, suggesting no definitive increase in incidence since these regimens have been introduced. We provide insights into the somatic and germline alterations identified in patients that had molecular testing. Acknowledging small numbers that had germline testing in this dataset, a putative association of brain metastases with germline BRCA1/2 alterations was noted. Survival is poor upon metastatic brain disease development, however in select patients long term survival can be achieved utilizing a multidisciplinary approach to treatment, highlighted by two long term survivors. With advances in germline and somatic profiling our findings of a possible association of germline BRCA1/2 alterations with as well as other molecular patterns may be uncovered to provide insight into the molecular features of this uncommon clinical entity.
Clinical Practice Points.
Brain metastases from PDAC are a rare event, 0.4% frequency in our series. Brain metastases confer a poor prognostic implication with a median survival following diagnosis of BM of 1.5 months (range 1 to 31).
In select patients long term survival can be achieved when incorporating a multidisciplinary approach, for example the median survival for the four patients that were able to undergo craniotomy post BM development was 11 months (range 1 to 31), with two patients surviving >1-year; at 21 and 31 months respectively.
The molecular profile of patients who develop brain metastases from PDAC is poorly understood, our results a speculative association of BM in PDAC with germline BRCA1/2 alterations which occurred in three of six patients that had germline testing.
Somatic profiling of six patients identified KRAS mutations in all patients; G12D (n=4), G12V (n=2), Q61K (1) with TP53 mutations and MYC amplification most commonly in two of four patients that had broader profiling by MSK-IMPACT.
Median time to development of BM was 17 months (0 to 79) with three patients harboring de novo BM involvement, which indicate that development of BM is not confined to patients with PDAC that achieve long term systemic disease control.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no potential conflict of interest.
References
- 1. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/.
- 2.Park KS, et al. , Nervous system involvement by pancreatic cancer. J Neurooncol, 2003. 63(3): p. 313–6. [DOI] [PubMed] [Google Scholar]
- 3.Go PH, et al. , Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer, 2011. 117(16): p. 3630–40. [DOI] [PubMed] [Google Scholar]
- 4.Yamada K, et al. , Brain metastases from asymptomatic adenocarcinoma of the pancreas: an autopsy case report. Surg Neurol, 2002. 58(5): p. 332–6; discussion 336–7. [DOI] [PubMed] [Google Scholar]
- 5.Lee YT and Tatter D, Carcinoma of the pancreas and periampullary structures. Pattern of metastasis at autopsy. Arch Pathol Lab Med, 1984. 108(7): p. 584–7. [PubMed] [Google Scholar]
- 6.Conroy T, et al. , FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med, 2011. 364(19): p. 1817–25. [DOI] [PubMed] [Google Scholar]
- 7.Von Hoff DD, et al. , Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med, 2013. 369(18): p. 1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng DT, et al. , Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn, 2015. 17(3): p. 251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar A, et al. , CNS involvement in pancreatic adenocarcinoma: a report of eight cases from the Johns Hopkins Hospital and review of literature. J Gastrointest Cancer, 2015. 46(1): p. 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou A, et al. , Clinical and molecular characterization of HER2 amplified-pancreatic cancer. Genome Med, 2013. 5(8): p. 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rekhtman N, et al. , Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-like and Non-Small Cell Carcinoma-like Subsets. Clin Cancer Res, 2016. 22(14): p. 3618–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furukawa T, et al. , Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep, 2011. 1: p. 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaguchi K, et al. , Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas, 2011. 40(4): p. 571–80. [DOI] [PubMed] [Google Scholar]
- 14.Chiang KC, et al. , Oncocytic-type intraductal papillary mucinous neoplasm (IPMN)-derived invasive oncocytic pancreatic carcinoma with brain metastasis - a case report. World J Surg Oncol, 2012. 10: p. 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rapp UR, et al. , MYC is a metastasis gene for non-small-cell lung cancer. PLoS One, 2009. 4(6): p. e6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albiges L, et al. , Spectrum of breast cancer metastasis in BRCA1 mutation carriers: highly increased incidence of brain metastases. Ann Oncol, 2005. 16(11): p. 1846–7. [DOI] [PubMed] [Google Scholar]
- 17.Sekine M, et al. , Increased incidence of brain metastases in BRCA1-related ovarian cancers. J Obstet Gynaecol Res, 2013. 39(1): p. 292–6. [DOI] [PubMed] [Google Scholar]
- 18.O’Reilly EM, et al. , Phase 1 trial evaluating cisplatin, gemcitabine, and veliparib in 2 patient cohorts: Germline BRCA mutation carriers and wild-type BRCA pancreatic ductal adenocarcinoma. Cancer, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowery MA, et al. , Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur J Cancer, 2018. 89: p. 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman B, et al. , Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol, 2015. 33(3): p. 244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemke J, et al. , Long-term survival following resection of brain metastases from pancreatic cancer. Anticancer Res, 2011. 31(12): p. 4599–603. [PubMed] [Google Scholar]
- 22.Trinh VT, Medina-Flores R, and Chohan MO, Leptomeningeal carcinomatosis as primary manifestation of pancreatic cancer. J Clin Neurosci, 2016. 30: p. 124–7. [DOI] [PubMed] [Google Scholar]
- 23.Yoo IK, et al. , Rare case of pancreatic cancer with leptomeningeal carcinomatosis. World J Gastroenterol, 2015. 21(3): p. 1020–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minchom A, et al. , An unusual case of pancreatic cancer with leptomeningeal infiltration. J Gastrointest Cancer, 2010. 41(2): p. 107–9. [DOI] [PubMed] [Google Scholar]
- 25.Rao R, et al. , An extremely rare case of pancreatic cancer presenting with leptomeningeal carcinomatosis and synchronous intraparenchymal brain metastasis. Gastrointest Cancer Res, 2013. 6(3): p. 90–2. [PMC free article] [PubMed] [Google Scholar]
- 26.Hong CS, Kurt H, and Elder JB, Asynchronous leptomeningeal carcinomatosis from pancreatic cancer: a case report and review of the literature. Clin J Gastroenterol, 2014. 7(5): p. 434–40. [DOI] [PubMed] [Google Scholar]
- 27.Caricato M, et al. , Cerebellar metastasis from pancreatic adenocarcinoma. A case report. Pancreatology, 2006. 6(4): p. 306–8. [DOI] [PubMed] [Google Scholar]


