Abstract
Purpose:
TP53 mutations are highly prevalent in head and neck squamous cell carcinoma (HNSCC) and associated with increased resistance to conventional treatment primarily consisting of chemotherapy and radiation. Restoration of wildtype p53 function in TP53 mutant cancer cells represents an attractive therapeutic approach and has been explored in recent years. In this study, the efficacy of a putative p53 reactivator called COTI-2, was evaluated in HNSCC cell lines with different TP53 status.
Experimental Design:
Clonogenic survival assays and an orthotopic mouse model of oral cancer were used to examine in vitro and in vivo sensitivity of HNSCC cell lines with either wildtype, null or mutant TP53 to COTI-2 alone, and in combination with cisplatin and/or radiation. Western blotting, cell cycle, live cell imaging, RNA sequencing, RPPA, chromatin immunoprecipitation (ChIP) and apoptosis analyses were performed to dissect molecular mechanisms.
Results:
COTI-2 decreased clonogenic survival of HNSCC cells and potentiated response to cisplatin and/or radiation in vitro and in vivo irrespective of TP53 status. Mechanistically, COTI-2 normalized wildtype p53 target gene expression and restored DNA binding properties to the p53 mutant protein in HNSCC. Additionally, COTI-2 induced DNA damage and replication stress responses leading to apoptosis and/or senescence. Furthermore, COTI-2 lead to activation of AMPK and inhibition of the mTOR pathways in vitro in HNSCC cells.
Conclusions:
COTI-2 inhibits tumor growth in vitro and in vivo in HNSCC likely through p53-dependent and p53-independent mechanisms. Combination of COTI-2 with cisplatin or radiation may be highly relevant in treating patients with HNSCC harboring TP53 mutations.
Keywords: HNSCC cells, high-risk mutant p53, TP53, TAp63, COTI-2, p53 target genes, Cisplatin, Radiation, DNA damage, CDK1, Chk1, replication stress, ChIP assay, AMPK, mTOR, apoptosis, Senescence, Live cell imaging, RNA Seq, RPPA
Introduction
The overall five-year survival rate for patients with advanced head and neck squamous cell carcinoma (HNSCC) remains in the 25–40% range due to resistance to standard therapy, primarily consisting of platinum-based chemoradio-therapy (1). Recent studies have revealed that TP53 is the most common somatically mutated gene in HNSCC, with mutations detected in 65–85% of non-human papillomavirus (HPV) associated HNSCC (2–4). The p53 protein, which is encoded by the TP53 gene, plays a central role in coordinating the response to cell stressors such as DNA damage, hypoxia, and oncogenic stress, largely through sequence-specific transcriptional regulation of multiple important target genes leading to G1 arrest, senescence, and induction of apoptosis. While most mutations in TP53 lead to a decrease in DNA-binding activity to p53 responsive elements, some p53 mutant proteins also have a dominant negative effect as they can bind and inhibit the function of remaining wildtype (wt) p53 protein (5). Moreover, some mutant p53s display oncogenic properties, termed “gain-of-function (GOF),” which are independent of wtp53 functions (5). Accordingly, GOF p53 mutant proteins can enhance cell transformation, increase tumor formation in mice, and confer cellular resistance to chemotherapy and radiation (5, 6).
Since mutation of TP53 in HNSCC is associated with resistance to standard treatment, there is a major need for novel therapeutic approaches to overcome this resistance. Because wildtype p53 can induce apoptosis and/or senescence when expressed in tumor cells, reactivation of wildtype function in mutant p53 is an attractive therapeutic strategy. A variety of compounds that can restore wildtype (wt) p53 protein function have been identified (7,8). One approach exploits the requirement of WT p53 binding to the metal ion Zn2+ to fold correctly (9, 10). The thiosemicarbazone metal ion chelator NSC319726 (ZMC1), was found to restore wt p53 function in a subset of but not all mutant p53 protein expressing tumor cell lines (10). Unfortunately, ZMC1 demonstrated toxicity in dog models, likely due to its high affinity for Zn2+, precluding further clinical development. COTI-2 is a novel related 3rd generation thiosemicarbazone which has been proposed to modulate p53 protein function (11,12). Upon addition, COTI-2 binds to the misfolded mutant forms of the p53 protein, thereby inducing conformational change that normalizes p53 and restores its activity (11,12). COTI-2 has demonstrated activity against multiple human ovarian and breast cancer cell lines in vitro and in vivo (11,12). However, its efficacy in HNSCC has not been investigated and the specific molecular mechanisms of its action are largely unknown. This exploration will provide important conceptual information as COTI-2 is currently being investigated in a Phase 1 clinical trial in advanced or recurrent gynecologic and head and neck malignancies (13).
In this study, we showed that COTI-2 decreased cell survival of both GOF mutant p53 and wildtype HNSCC cells and synergized with cisplatin (CDDP) and radiation in vitro. Furthermore, COTI-2 potentiated response to cisplatin and radiation in vivo in an orthotopic mouse model of oral cancer. Notably, the reduction in cell survival was associated with DNA damage and replication stress responses leading to apoptosis and/or senescence. Using RNA sequencing coupled with ChIP, COTI-2 lead to normalization of wildtype p53 target gene expression and restoration of DNA binding properties to a GOF p53 mutant protein in HNSCC. Furthermore, pharmacoproteomic profiling revealed that COTI-2 resulted in activation of AMPK and inhibition of the oncogenic mTOR pathways in HNSCC cells independent of p53 status. Our data suggest that combination of COTI-2 with cisplatin or radiation may be novel therapy for treatment of HNSCC harboring TP53 mutations.
Materials and Methods
Tissue culture, reagents and generation of stable cell lines
The HNSCC cell line PCI13 lacking endogenous p53 was obtained from the laboratory of Dr. Jennifer Grandis (University of Pittsburgh, Pittsburgh, PA) in August 2008 and engineered to stably express constructs containing either wildtype p53 or high-risk EA (Evolutionary Action) score mutant p53 (C238F and G245D, R175H, R273H, R282W) as described previously (6). Additional HNSCC cell lines used in this study were obtained from an established cell repository in the laboratory of Dr. Jeffrey N. Myers (University of Texas MD Anderson Cancer Center, Houston, TX) under approved institutional protocols. The cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, L-glutamine, sodium pyruvate, nonessential amino acids, and a vitamin solution, and incubated at 37°C in 5% CO2 and 95% Air. The identity of all cell lines was authenticated using short tandem repeat (STR) testing within 6 months of cell use. COTI-2 drug was supplied by Critical Outcome Technologies Inc (currently known as Cotinga Pharmaceuticals). For in vitro studies, COTI-2 was prepared as a 1.0 mmol/L stock solution in DMSO and stored at −20°C.
Clonogenic survival assay
For the clonogenic survival studies, HNSCC cells were seeded in 6-well plates at predetermined densities, concurrently exposed to different fixed-ratio combinations of COTI-2 (dose range, 0.01–40 nmol/L) and cisplatin (dose range, 0.1–2μmol/L) for 24 hours and clonogenic cell survival was determined as previously described (14). For radiosensitivity assays, cells were treated with different doses of COTI-2, as indicated, then followed by exposure to either 2, 4 or 6 Gray (Gy) radiation and the surviving fraction (SF2) values were determined.
Analysis of combined drug effects
Drug synergy between COTI-2 and cisplatin was assessed by combination-index and conservative isobologram analyses, which were generated according to the median-effect method of Chou and Talalay (15) using CalcuSyn software (Biosoft, Ferguson, MO). See the Supplementary Materials and Methods section for details.
Western blot analysis
Cells grown on 10-cm plates were treated with physiologically relevant-doses of COTI-2 (1.0 μmol/L), CDDP (1.5 μmol/L) either alone or in combination for 16 or 48 hours. For radiosensitization studies, cells were radiated with 4 Gray. Whole cell lysates were prepared and Western blot analyses were conducted with indicated antibodies as described previously (14). Densitometric quantifications were performed with ImageJ (v1.50i). Antibodies used for Western blotting are described in Supplementary Materials and Methods section.
Cell cycle analysis and Annexin V-FITC/PI staining
Cells were seeded in 60-mm dishes, treated the next day with COTI-2 (1 μmol/L), CDDP (1.5 μmol/L) either alone or in combination and then harvested at 12, 24, 48, or 72 hours. The cell cycle analysis was performed as previously described (14). Annexin V-FITC/PI staining was used to detect apoptotic cell death using the BD Bioscience apoptotic detection kit according to the manufacturer’s instructions.
Live cell imaging and EdU labeling
HNSCC cell lines (PCI13-pBabe, PCI13-G245D) were stably transfected with histone H2B-RFP lentiviral vector (Addgene) and sorted by flow cytometry to enrich for highly expressing cells. Cells were treated with drugs as indicated and live video imaging, EdU labeling, and DNA content measured by laser scanning cytometry analyses, were all carried out as described previously (16).
RNA-Seq profiling
HNSCC cell lines (PCI13) stably expressing either wildtype p53 or high-risk mutp53 (G245D) were treated with COTI-2 (1.0 μmol/L) and subjected to RNA sequencing analysis as described in the Supplementary Materials and Methods section.
Quantitative reverse transcription PCR (qRT-PCR) Analyses
PCI13 cells expressing pBabe (null TP53), wildtype TP53, or TP53 mutant (G245D) constructs were treated with COTI-2 (1.0 mmol/L) for 24 or 48 hours before isolation of total RNA using RNeasy mini kit (QIAGEN). Detailed description of the qRT-PCR procedures is included in the Supplementary Materials and Methods.
Chromatin Immunoprecipitation (ChIP)
PCI13 cells expressing pBabe, wildtype TP53 or high-risk mutant TP53 (G245D) were treated with DMSO (controls) or COTI-2 as indicated for 24 hours and cross-linked using paraformaldehyde. The DNA lysates were then immunoprecipitated with anti-p53 (DO-1X) antibody and the recovered ChIP DNA was subjected to PCR analysis. The experimental procedures were described in details in the Supplementary Materials and Methods.
3D cell culture and in vivo TUNEL assay
A “3D cell culture” was established as described in previous publication (17,18). Briefly, a total of 3000 cells (PCI13-G245D) embedded in 150 μl of collagen gel (n = 3/group) were then layered on top of the solidified collagen gel. The “3D tumors” were treated with a calculated corresponding maximum physiological (given to mice) dose of COTI-2 (2 μmol/L) for 1 day or 5 days and effect of the drug on apoptosis was determined using the in vivo TUNEL assay as described in Supplementary Materials and Methods.
In vivo orthotopic mouse model of oral tongue cancer
All animal experimentation was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas MD Anderson Cancer Center. Our orthotopic nude mouse tongue model has been previously described (14,16,17). PCI13-cells (30 × 103) containing either wildtype p53 or high risk mutant p53 (G245D), and HN31 were injected into the tongues of male athymic nude mice since oral cancer is mostly prevalent and represented in male patients. Mice were randomized into different groups 8–10 days after injection. Treatment with COTI-2 (75 mg/kg), cisplatin, or in combination was initiated when tumors were about 1–3 mm3 in size. Treatment with COTI-2, radiation, or in combination was initiated when tumors reached about 10–20 mm3 in size. Details of the treatment protocol and tumor growth inhibition measurement are described in the Supplementary Materials and Methods.
Statistical analysis
The Student’s t and one-way ANOVA tests were utilized to analyze in vitro data. For mouse studies, a two-way ANOVA test was used to compare tumor volumes between control and treatment groups. Survival following drug treatment and radiation was analyzed by the Kaplan–Meier method and compared with log-rank test. All data were expressed as mean ± standard error, and P values of 0.05 or less were considered to indicate statistical significance.
Results
COTI-2 shows single agent activity and synergy with cisplatin and radiation in vitro in HNSCC cells expressing GOF mutant p53.
To determine the relative degree of sensitivity of HNSCC cells to COTI-2 as a single agent, isogenic HNSCC PCI13 cells either lacking p53 expression (pBabe null), or expressing wildtype p53, or p53 mutations (C238F, G245D, R175H, R273H, R282W) were treated with COTI-2 for 24 hour and then assessed for clonogenic survival. As shown in Fig. 1A and B, the PCI13 isogenic cells displayed exquisite sensitivity to COTI-2 as a single agent (IC50; 1.4–13.2 nmol/L). Representative images of clonogenic survival assays with corresponding IC50 for COTI-2 as a single agent are shown in Supplementary Figure S1A–C. The results suggest that COTI-2 appears to target HNSCC regardless of TP53 status. Additionally, MTT viability assays demonstrated that various HNSCC cell lines with naturally occurring p53 mutations and HPV positivity (wtp53) were sensitive to COTI-2 as monotherapy (Supplementary Fig. S1D). HNSCC cells expressing mutant p53 but not wildtype p53, are relatively resistant to cisplatin and radiation in vitro and in vivo (3,14,19). We next examined whether COTI-2 was synergistic with CDDP treatment in isogenic PCI13 cell lines, using the combination index (CI) method of Chou and Talalay (15). HNSCC cells expressing mutant p53 were resistant to cisplatin, and addition of COTI-2 significantly decreased their survival in vitro, compared to each drug alone (Fig. 1C, E and G). Conservative isobologram plots of effective doses (ED50; 50% inhibition), ED75 (75% inhibition), and ED90 (90% inhibition) show the combination index (CI) at each inhibitory concentration is less than 1.0, indicating strong synergism in all HNSCC cells tested (Fig. 1D, F and H). Strong synergy between COTI-2 and cisplatin was also demonstrated in additional HNSCC cell lines with naturally occurring mutant p53 (UMSCC10A) and HPV+ (UMSCC47) (Supplementary Fig. S2A–D). To evaluate if COTI-2 radiosensitizes HNSCC cell lines, PCI13-Wtp53 and PCI13-G245D cells were treated with radiation and COTI-2 and subjected to clonogenic survival assays as indicated. The degree of radiosensitization was quantified from the survival curves by comparing surviving fractions at the radiation dose of 2 Gy (SF2). The SF2 is particularly relevant since 2 Gy is the typical dose given on a daily basis in clinical radiotherapy for HNSCC. Compared to control and each treatment alone, COTI-2 markedly decreased the SF2 fractions when combined with radiation in vitro in HNSCC cells in a dose dependent manner. (Supplementary Fig. S3). Taken together, these data clearly demonstrated that COTI-2 synergized with cisplatin and/or radiation and inhibited in vitro tumor cell growth in HNSCC independent of the TP53 status.
Figure 1. COTI-2 shows single agent activity and strong synergy with cisplatin and radiation in vitro in HNSCC cells expressing GOF mutant p53.
A and B, clonogenic survival curves for isogenic HNSCC PCI13 cells lacking p53 (pBabe null), wtp53, or various GOF mutp53 (C238F, G245D, R175H, R273H, R282W) treated with a range of COTI-2 concentrations (0.01–40 nmol/L) for 24 hours to determine the IC50. C, E and G, representative images of clonogenic survival assays for combined treatment with COTI-2 and cisplatin (CDDP). D, F and H, assessment of the degree of synergy between COTI-2 and CDDP in PCI13-pBabe (p53 null), PCI13-G245D and PCI13-R273H cells, using the Chou and Talalay method. CDDP and COTI-2 were used at constant ratios (50:1 respectively). The CI values for combination of effective drug doses (ED) that result in growth inhibition of 50% (ED50; fraction affected; fa = 0.5), 75% (ED75; fa = 0.75), and 90% (ED90; fa = 0.90) were generated from the conservative isobolograms. The ED50 (red X), ED75 (green crosses) and ED90 (blue circles) graphed against fractional concentrations of CDDP and COTI-2 on the y and x axis, respectively are indicated. The diagonal line is the line of additivity. The conservative isobologram plots show CI values less than 1.0 falling below diagonal line, demonstrating that COTI-2 and CDDP acts synergistically to inhibit in vitro growth of HNSCC cells. All treatments were performed in triplicate and each experiment was repeated at least three times.
COTI-2 increases the levels of DNA damage response and replication stress markers with subsequent induction of apoptosis and cellular senescence in HNSCC cells.
Chemotherapy and radiation elicit DNA damage responses and can confer anti-cancer synergy in tumor cells. Therefore, phosphorylation levels of the DNA damage and replicative stress indicators, γH2AX (S139), CHK1 (S345) and CDK1 (Y15) were examined by western blotting. Increased phosphorylation of γH2AX, CHK1, and increased activity of CDK1 (manifested by decreased level of phosphorylation at tyrosine 15) were observed following COTI-2 treatment and in combination with cisplatin and/or radiation (Fig. 2A–B). Modification of these proteins occurred in a time dependent manner, indicating induction of DNA damage and replication stress responses during cell cycle in these cells. Blots were quantified and results were shown in Supplementary Figure S4A–B. The p21 protein levels were induced in all cells tested in a time-dependent manner (Fig. 2A–B, and Supplementary Fig. S4A–B), suggesting that COTI-2 activates p53 dependent transcription similar to that regulated by wildtype p53. To determine whether COTI-2 inhibits growth of HNSCC cells via apoptosis, the apoptotic marker, PARP cleavage was examined at 16 and 48 hours of treatment by Western blotting. Cleavage of PARP, caspase-3 and to less extent caspase-9 was observed only in cell lines with mutant (G245D) or null (PCI13-pBabe) p53 when exposed to COTI-2 alone or in combination with cisplatin and radiation (Fig. 2A–B, and Supplementary Fig. S5A–C). Induction of apoptosis in these cells following treatment with cisplatin and COTI-2 was further confirmed with AnnexinV/PI positive staining (Fig. 2C–D). Treatment of the wildtype p53 cells with COTI-2 showed very low levels of AnnexinV/PI positive staining (Supplementary Fig. S6A–B). The p53 can cause growth arrest through senescence and can be assessed by expression of the acidic senescence-associated β-galactosidase (SA-β-gal) activity. The isogenic PCI13-WTp53 and naturally occurring wildtype p53 (HN30) cell lines were treated as indicated, and tested for SA-β-gal positivity. A substantial fraction of cells showed increased levels of SA-β-gal activity following COTI-2 treatment (Supplementary Fig. S6C). Cells treated with cisplatin served as positive controls. Quantification of senescence staining is shown in Supplementary Figure S6D–E. These results indicate that HNSCC cells expressing wildtype p53 are sensitive to COTI-2 treatment through induction of cellular senescence rather than apoptosis at the concentrations of drug evaluated.
Figure 2. COTI-2 increases the levels of DNA damage response and replication stress markers with subsequent induction of apoptosis in defective TP53 HNSCC cells.
PCI13-pBabe (null TP53) and PCI13-G245D (mutant p53) cells were treated with either COTI-2, cisplatin, radiation (XRT) alone or in combination for 16 and 48 hours and subjected to immunoblot analysis using antibodies as indicated. A and B, protein expression levels of p53, p21, phospho-γH2AX (S139), phospho-Chk1 (S345), and total Chk1. The mitotic entry marker histone H3 S10 phosphorylation, CDK1 (CDC2) tyr15 phosphorylation, and total protein levels were also analyzed. The presence of PARP-1 cleavage as marker of apoptosis is shown. β-actin served as loading control .C and D, AnnexinV/PI positive staining confirming induction of apoptosis in PCI13-pBabe and PCI13-G245D HNSCC cells following treatment with COTI-2 and cisplatin. E and F, unsynchronized PCI13-pBabe and PCI13-G245D cells were treated with COTI-2, CDDP alone or in combination for various time points as indicated and subjected to cell cycle analysis. NT (no treatment) control. Percentage of cell cycle distribution determined by flow cytometry is presented as bar graphs. An increase in S-phase and sub G1 fractions indicative of replicative stress and cumulative apoptosis respectively were observed over time. Data shown are representative of two independent experiments.
To better understand the longer term effects of drug treatment, PCI13-pBabe and G245D cells were treated with cisplatin and COTI-2, and examined at 12–72 hours for cell cycle kinetics. Treatment with COTI-2 arrested the cells at G1 and G2/M cell cycle phases in a time dependent manner (12–48 hours), however, at 72 hour, cell number in these phases gradually declined because proportion of cells went into apoptosis as revealed by increased subG1 fractions (Fig. 2E–F). Moreover, cisplatin arrested the cells at the G2/M phase within 48 hour and upon addition of COTI-2, increased fraction of cells was observed in the S-phase, suggesting slow rate of DNA synthesis associated with induction of replication stress (Fig. 2E–F). While cells with no p53 (pBabe) showed increased subG1 fraction only at 72 hour (Fig.2E), a significant increase in the subG1 fraction of cells indicative of apoptosis was seen in PCI13-G245D cells after 48 hours of combination treatment and remained elevated at 72 hours (Fig. 2E). Interestingly, cells with wildtype p53 were arrested at the G1 cell cycle phase upon treatment with COTI-2 or in combination with cisplatin (Supplementary Fig. S7). Furthermore, these cells showed insignificant subG1 fraction of cells, consistent with the low levels of the PARP-1 cleavage and AnnexinV/PI staining observed previously with the drug treatment (Supplementary Fig. S6A–B).
Multiple cell cycle perturbations leading to significant apoptosis in HNSCC cells treated with combination of COTI-2 and cisplatin.
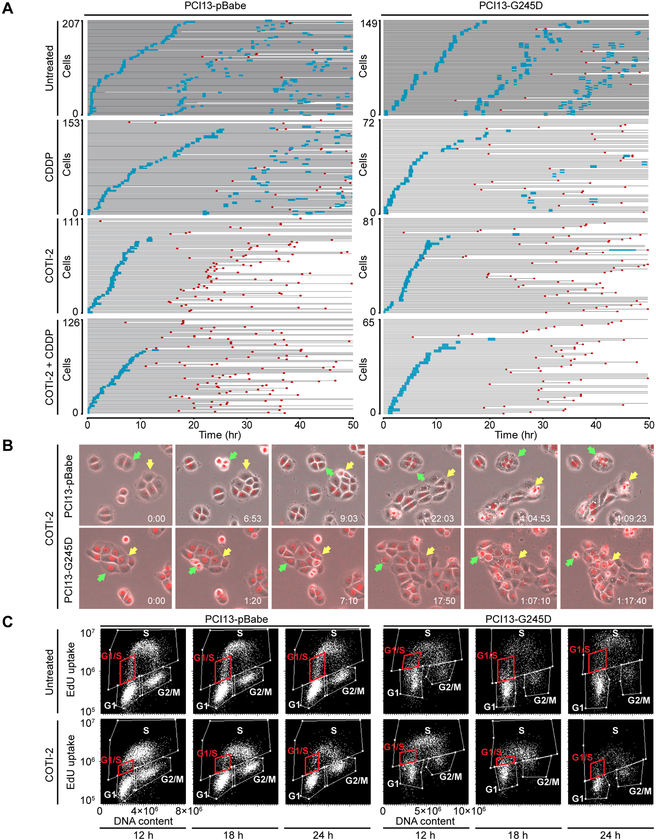
To better understand the dynamics of cell cycle perturbations and cell death response to COTI-2 treatment alone and in combination with cisplatin, live cell imaging studies were carried out on histone H2B-RFP-tagged HNSCC cells. The results of these studies are depicted in Figure 3A as event charts for the cell lines PCI13-pBabe (p53 null) and PCI13-G245D (mutp53), where each graphed line represents the history of a cell over time and the color of the line represents whether the cell was in interphase (gray) or in mitosis (blue). A red asterisk marks the time of apoptosis. Over the 50 hour time period of these studies, both untreated cell lines were able to complete nearly three complete cell cycles (i.e., three waves of mitoses). While most cells treated with cisplatin were able to reach the first mitosis, albeit increasingly delayed with duration of treatment, subsequent cell cycles were slowed as demonstrated by longer time intervals from the first to the second mitosis and fewer transitions through subsequent cell cycles. On the other hand, only two-thirds of COTI-2 treated cells were able to reach the first mitosis with similar kinetics as untreated cells (Supplementary Fig. S8A), suggesting a block in the early phase of the cell cycle. Moreover, they were not able to attain a second mitosis and a high fraction of cells underwent apoptosis at variable times after the first mitosis. Interestingly, COTI-2 treated cells showed increasing cytoplasmic vacuolization after 12 hours that persisted until apoptosis (Fig. 3B, and Supplementary Fig. S9, and Supplementary Videos S1–S4). Combined treatment with cisplatin and COTI-2 resulted in fewer cells reaching the first mitosis and slightly earlier onset and extent of apoptosis. Cells expressing wildtype p53 showed very little apoptosis upon treatment with COTI-2 alone which increased with addition of cisplatin (Supplementary Figure S10), but seemed much lower than that observed in cells with pBabe or G245D respectively. Since COTI-2 treatment was found to block cells from reaching a second mitosis, it was of interest to determine where and how COTI-2 treatment delayed cell cycle progression. PCI13-pBabe and PCI13-G245D cells were therefore treated with COTI-2, pulsed with EdU for one hour prior to cell harvest after 12, 18, and 24 hours of treatment, and analyzed for cell cycle position and EdU uptake levels (i.e., rate of DNA synthesis). As shown in Figure 3C and Supplementary Figure S8B, by 12 hours of COTI-2 treatment, there was an increase in the fraction of cells in S phase but with decreased EdU uptake per cell, suggesting a generalized slowing of DNA synthesis. By 18 hours of COTI-2 treatment, fewer cells were transitioning from G1 to S-phase, and DNA synthesis was increasingly slowed, resulting in an increased fraction of cells in late S-phase. By 24 hours of COTI-2 treatment, the rate of DNA synthesis was even lower but the relative fraction of cells in S-phase dropped, suggesting that cells were dying in late S/G2 phase and were not able to reach the second mitosis.
Figure 3. Cell cycle perturbation and subsequent apoptosis in HNSCC cells treated with combination of COTI-2 and cisplatin.
Histone-H2B-RFP expressing PCI13-pBabe and PC13-G245D cells were treated with the drugs as indicated and followed by live cell imaging. A, live cell imaging results depicted as event charts, where each line represents one cell. The cell’s time in interphase is labeled gray, its time in mitosis is labeled blue, and the time of apoptosis is marked by a red symbol. B, Representative still image frames from the live cell imaging movies illustrating mitotic and interphase events leading to increased cytoplasmic vacuolization and apoptosis in PCI13-G245D cell lines treated with COTI-2 compared to untreated control. C, scatter plots showing cell cycle position and EdU uptake levels (i.e., rate of DNA synthesis) in PCI13-pBabe and PCI13-G245D cells harvested and analyzed after 12, 18, or 24 hour time points following treatment with COTI-2 as indicated. Decreased EdU uptake and eventual disappearance of S-phase cells at later time point is shown.
COTI-2 restores the DNA binding properties and transcriptional activity of a subset of p53 mutant proteins in HNSCC cells.
To further assess the cellular phenotype associated with the activity of COTI-2 monotherapy in HNSCC cells expressing wildtype or mutp53, cells were treated for 48 hours with the drug and differentially expressed genes (DEGs) were examined by RNA sequencing (RNA-seq). A linear regression model (false discovery rate (FDR) cutoff = 0.05) and filtering criteria (i.e. DEG with an adjusted P-value of < 0.01, genes with 50 average reads among the comparison groups, exclusion of noncoding RNAs) were used. Clustering via principal component analysis (PCA) revealed that DEGs among treatment groups are different between cell types. Approximately, 1772 and 2764 genes (up and down-regulated) out of the total 26119 DEGs that are significantly different between COTI-2 and untreated groups with p<0.01 were identified in PCI13-wtp53 and PCI13-G245D cells respectively (Supplementary Table S1). Because our in vitro western blotting data suggested that COTI-2 induced p21 (CDKN1A), a known p53 target gene in HNSCC cells tested, (Fig. 2), we focused our analysis on comparing the p53 induced gene expression levels in PCI13 cells expressing wildtype and mutant TP53 following COTI-2 treatment within 48 hours as an indication of normalization of p53 transcriptional activity. A list of high-confidence p53 target genes has been described in the literature (20). Results demonstrated that COTI-2 treatment produced a p53 target expression signature remarkably different from untreated controls (Fig. 4A–B, and Supplementary Table S2–4), suggesting that this agent restored normal DNA binding activity to the p53 G245D mutant similar to wildtype p53 and resulted in a transcriptionally active protein. The results further suggest that COTI-2 inhibits cell growth through a p53–dependent mechanism. The cluster heatmap represents significant differentially expressed p53 target genes (82 and 94 genes for wtp53 and mutp53 respectively) as indicated with the color scale (Fig. 4A–B). mRNA expression levels of selected p53 target genes presented in the heatmap, including p21, PUMA, NOXA, MDM2, EphA2, PLK2, and ATF3 in PCI13-Wtp53 and PCI13-G245D cells treated with COTI-2 for 24 hours were validated with qRT-PCR analysis (Fig. 4C–D). In HNSCC cells with defective p53, basal mRNA and protein expression levels of the key pro-survival gene MDM2 were increased compared to wildtype p53 cells (Supplementary Fig. S11A–D). Increased expression of TAp63 was also observed in the PCI13-G245D cells following COTI-2 treatment (Fig 4A–B), suggesting that the drug can enhance tumor suppression through induction of this TP53 family related gene. We further confirmed the ability of COTI-2 to restore DNA binding properties to the p53 G245D mutant protein by chromatin immunoprecipitation (ChIP) coupled with qRT-PCR analyses. Results in PCI13-G245D cells treated with COTI-2 for 24 hours revealed significant restoration of site-specific DNA binding of p53 G245D mutant to the promoters of p21, PUMA, NOXA, MDM2, PLK3 and ATF3 (Fig. 4F). While wtp53 showed significant binding activity to MDM2 and ATF3 promoters upon COTI-2 treatment, this binding activity was not significant towards p21, PUMA, NOXA and PLK3 promoters in these cells (Fig. 4E), consistent with published data (9). No p53 DNA binding activity to these promoters was detected in the PCI13-pBabe (p53 null) cells following COTI-2 treatment (Supplementary Figure S12). As TAp63α can also induce many of the prototypical p53 inducible target genes (PIGs) that can mediate cell cycle arrest and induction of senescence and apoptosis (21), it is conceivable that activation of this isoform could mediate COTI-2 anti-tumor effects observed in in the p53 null HNSCC cells. qRT-PCR analysis revealed that treatment with COTI-2 for 48 hours increased expression of the p53 target genes and TAp63α isoform following COTI-2 treatment in HNSCC cells lacking TP53 (PCI13-pBabe) (Supplementary Fig. 13A–B). Furthermore, ChIP analysis using the p63α antibody followed by qRT-PCR analysis showed that COTI-2 significantly enhanced DNA binding of TAp63 to p21 and PUMA promoters consistent with this contention (Supplementary Fig. 13C).
Figure 4. COTI-2 restores the DNA binding properties and transcriptional activity of the p53 mutant protein in HNSCC cells.
An RNA-sequencing analysis of COTI-2 (1 μmol/L, 48 hours) and control treated PCI13-wtp53 and PCI13-G245D HNSCC cells comparing gene expression levels of 126 out of 310 p53 inducible genes (PIGs) was performed. A and B, cluster heatmap represents significant differentially expressed 82 and 94 p53 target genes as indicated with the color scale (fold change) for PCI13-wtp53 and PCI13-G245D cells respectively. Statistical significance was set based on the FDR cutoff of 0.05. C and D, qRT-PCR analyses to validate mRNA expression levels of selected p53 target genes presented in the heatmap, including p21, PUMA, NOXA, MDM2, PLK3, and ATF3 in these cells treated as indicated. E and F, chromatin immunoprecipitation (ChIP) assay. Lysates from PCI13-wtp53 and PCI13-G245D cells treated with COTI-2 for 24 hours were subjected to immunoprecipitation using anti-p53 antibody (DO-1) and followed by qRT-PCR of p53 recognition elements (p53REs) of p21, PUMA, NOXA, MDM2, PLK3, and ATF3. Results of functional DNA binding properties with COTI-2 treatment were presented as enrichment fold change. *p<0.05 and **p<0.001 compared to untreated control respectively.
COTI-2 leads to activation of AMPK and inhibition of the oncogenic mTOR pathways in HNSCC cells independent of p53 status.
Our results demonstrated that regardless of p53 status, HNSCC cells were similarly sensitive to COTI-2 in vitro. This suggests that a portion of the response to COTI-2 is p53 independent and further COTI-2 may have additional targets. Therefore, to identify other signaling pathways mediating the effects of COTI-2, proteomic profiling using a reverse phase protein array (RPPA) was performed in PCI13-wtp53, PCI13-pBabe (p53 null) and PCI13-G245D cells treated with COTI-2 for 8 and 36 hours as indicated. Significant changes in protein expression levels were selected based on adjusted P-value <0.05 for each comparison. The principal component analysis (PCA) for the normalized protein expression revealed that all samples were clustered by cell type, treatment type and time. Unlike the 36 hour time point, no significant differences between treatments versus control groups were observed at 8 hour time point in all cell lines tested. Therefore, we focused our attention on detecting differences in protein levels in samples from these cells treated with the drug for 36 hours. A total of 13, 64, and 76 out of 301 total proteins assessed by RPPA were significantly differentially expressed (adjusted P-value < 0.05) in wtp53, pBabe, and G245D expressing cells respectively (Supplementary Table S5–7). To focus our investigation, we limited our analysis to protein level changes of > 1.5 fold, with a false discovery rate of < 1% and P-value of < 0.05 (22). No significant differences in protein expression levels were observed in the wildtype p53 expressing cells among untreated and COTI-2 treated groups (Supplementary Table S8). However, we identified significant differences in protein expression levels in 13 out of 64 and 18 out of 76 proteins in cells null for p53 (pBabe) or expressing mutant p53 (G245D) respectively (Supplementary Table S9–10). The hierarchical cluster heatmap representing significantly differentially expressed proteins is shown in Figure 5A–B. Proteins involved in the AMPK and mTOR signaling pathways were among the top proteins that were significantly upregulated and downregulated respectively following COTI-2 treatment in both pBabe and G245D cells (Fig. 5A–B). Proteins involved in cell cycle regulation, apoptosis, gap junction and calcium regulation were also downregulated in pBabe and G245D cells (Fig. 5A–B). Differential expression of these proteins was further confirmed by western blotting (Fig. 5C–E). These results further suggest that COTI-2 activates apoptosis in HNSCC cells through reduction of the anti-apoptotic protein MCl-1 and induction of proapoptotic protein PDCD4 respectively. COTI-2 decreased expression of connexin-43, a transmembrane protein that regulates gap junction intercellular communication (23–26). Therefore, we examined if COTI-2-mediated decreased connexin-43 resulted in inhibition of gap junction-mediated cellular communication using the scrape loading and dye transfer technique (23–26). COTI-2 significantly decreased the number of cells labelled with the dye, indicating a possible involvement in regulation of cellular communication in HNSCC (Supplementary Fig. S14A–B). Taken together, the RPPA analysis suggests that COTI-2 acquires an additional non-p53 mechanism to inhibit tumor growth in HNSCC cells.
Figure 5. COTI-2 leads to activation of AMPK and inhibition of the oncogenic mTOR pathways in HNSCC cells independent of p53 status.
PCI13-pBabe and PCI13-G245D HNSCC cell lines treated with COTI-2 for 36 hours were subjected to reverse-phase protein array analysis (RPPA) as indicated. A and B, hierarchical cluster heatmap analyses of proteins and phospho-proteins from RPPA significantly different (protein level changes of > 1.5 fold, with an FDR ≥ 1% and P-value of < 0.05) between COTI-2 treatment and control in these cell lines (performed in triplicate). Red and green color scales indicate increased or decreased proteins or phosphoproteins levels, respectively. C-E, western blot analyses confirming differential expression of most of these proteins, including APMK and mTOR following the drug treatment.
COTI-2 displays single agent activity and enhances response to cisplatin and radiation in vivo in an orthotopic mouse model of oral tongue cancer.
Three- dimensional (3D) cell culture systems have been established as reliable models for monitoring cell growth, proliferation and drug testing due to their evident advantages in providing more physiologically relevant phenotypes similar to that in vivo (17,18). Therefore, the PCI13-G245D and HN31 cells were grown in 3D collagen culture and treated with COTI-2 as indicated previously. A significant effect on cell growth in 3D culture at day 5 was observed following treatment with COTI-2 (Fig. 6A). Additionally, TUNEL-positive staining was significantly increased in the 3D culture organoids treated with COTI-2, indicative of apoptosis (Fig. 6B–C), consistent with 2D in vitro data. Moreover, organoids established from the 3D culture of these mutant p53 cell lines and treated with COTI-2 for 5 days exhibited increased positive to negative ratios of nuclear immunostaining of p21 compared to untreated controls (Fig. 6D–E), consistent with the 2D in vitro data. The H&E staining of the 3D organoids is shown in Supplementary Figure S15I.
Figure 6. COTI-2 displays single agent activity and enhances response to cisplatin and radiation in vivo in an orthotopic mouse model of oral tongue cancer.
A, PCI13-G245D and HN31 cells grown in 3D collagen culture and monitored for survival following treatment with equivalent physiological dose of COTI-2 (2 μmol/L) as indicated for 1 and 5 days. B, TUNEL-positive staining determined at day 5 in the 3D culture organoids made from PCI13-G245D and HN31 cells following treatment with COTI-2. C, quantification of the positive TUNEL staining shown in B. D, positive nuclear immunostaining of p21 (brown) with addition of COTI-2 evaluated at day 5 compared to untreated controls in organoids made from these p53 mutant HNSCC cells. E, quantification of the positive staining of p21 shown in D. F and G, tumor growth curves in orthotopic mouse model oral tongues bearing HNSCC (PCI13-wtp53 and PCI13-G245D) cells following treatment with COTI-2 (75 mg/kg), cisplatin (4 mg/kg) either alone or in combination as described in methods. H and I, tumor growth curves in orthotopic mouse model oral tongues bearing these p53 mutant cells following treatment with COTI-2 (75 mg/kg), fractionated radiation dose (2 Gy)and in combination as described in methods. Each treatment group contains 6–12 mice.
On the basis of the in vitro results, the impact of COTI-2 either alone or in combination with cisplatin and radiation was evaluated in an orthotopic nude mouse model implanted with isogenic PCI13-Wtp53 and PCI13-G245D cells in the tongue. COTI-2 (75 mg/kg) alone had significant effects on tumor growth in vivo at this tested optimal dose, independent of p53 status (Fig. 6F and G). While cisplatin treatment with physiological dose (4mg/kg) had little effect on tumor growth, addition of COTI-2 enhanced the response to cisplatin and significantly reduced oral tumor growth (P = 0.0358 and P =0.0006) in mice bearing PCI13-Wtp53 and PCI13-G245D cells respectively (Fig. 6F and G). The combination of COTI-2 and cisplatin showed a trend towards increase in tumor growth inhibition compared to COTI-2 alone as a result of its strong single agent activity (Fig. 6F and G). No survival benefits were observed with combination of cisplatin and COTI-2 at the dose tested (Supplementary Fig. S15A–B). We next determined the effect of COTI-2 on the response to radiation in vivo. A fractionated radiation (2 Gy) was delivered to the tumor-bearing tongue of mice expressing PCI13-G245D and HN31 mutant p53 cells, following treatment with COTI-2. Compared to untreated controls, significant tumor growth delay was observed with a combination of COTI-2 and radiation in mice bearing each cell lines (Fig. 6H and I). The combination of COTI-2 and radiation showed little tumor growth delay and survival benefits compared to radiation or COTI-2 alone (Fig 6H–I, and Supplementary Fig. S15C–D) in the models tested. Taken together, our results suggest that COTI-2 potentiates in vivo sensitivity of HNSCC mutant p53 cells to cisplatin and radiation. During the study, mice in the treatment arms showed no more than 10–20% body weight loss (Supplementary Fig. S15E–H).
Discussion
Several attempts have been made to design molecules that target mutant p53 in cancer cells (27). However, with the exception of PRIMA-1Met, all p53 reactivators showed unacceptable toxicities in mice precluding further clinical development (27). COTI-2, is a new small molecule, which has been proposed to restore a wildtype like-p53 function in mutant TP53 cancer cells (11,12). In this study, we show for the first time that COTI-2 is active as single agent against a variety of head and neck cancer cells irrespective of TP53 status, consistent with recent reports (11.12). COTI-2 significantly potentiates sensitivity to cisplatin and/or radiation in vitro and in vivo in HNSCC tumor cells that are inherently resistant to these treatments. The antitumor and apoptotic effects of COTI-2 are also evident in 3D-organotypic cultures of HNSCC. While COTI-2 induces apoptosis in HNSCC cells lacking TP53 or with mutant TP53, in wildtype TP53 cells, COTI-2 induces senescence rather than apoptosis. This could be due to the enhanced effect of COTI-2 on p21, a known inducer of senescence in these cells (19,28). Surprisingly, apoptosis was not apparent even though many apoptotic genes were upregulated in wildtype TP53 cells. It is likely that these proapoptotic genes were not induced with COTI-2 at the protein levels to cause significant apoptotic death in these cells. While it is not clear how COTI-2 potentiates the combination effect of cisplatin and /or radiation in wildtype TP53 cells, it is possible that this drug exerts anti-tumor activity through non-specific cytotoxic effects. Taken together, the different mechanisms of action in wildtype versus TP53 mutant cells suggests that this agent could have potential therapeutic roles in different settings.
COTI-2 belongs to the thiosemicarbazone family which can act as metal ion zinc [Zn2+] chelators to induce correct p53 protein folding and restore wildtype structure and function of mutant TP53 (10–12). In our study, COTI-2 showed no effects on intracellular zinc [Zn2+] levels in HNSCC mutant TP53 cells (Supplementary Fig. S16). These data suggest that COTI-2 either has low binding affinity for zinc or its action is independent of zinc chelation in HNSCC tumor cells. A recent study has shown that the thiosemicarbazone agent, ZMC1 (NSC319726) reactivates mutant TP53 (R175H) in cancer cells through modulation of zinc ions and redox changes (9). Redox changes often lead to generation of reactive oxygen species which play a pivotal role in triggering cellular senescence (28). Therefore, the senescence seen in wildtype TP53 expressing HNSCC cells following COTI-2 treatment could be mediated through ROS production. Additionally, our in vitro experiments guided by live cell imaging, demonstrate that, COTI-2 not only arrested cells at the G1 and G2/M cell cycle phases but also caused accumulation of fraction of cells in early S-phase as result of progressively slowed rates of DNA synthesis. These data further suggest that COTI-2-mediated cell death may involves stress-induced signals in HNSCC. Furthermore, addition of cisplatin resulted in significant replication stress and apoptotic death, perhaps in response to stalled and collapsed replication forks. Interestingly, COTI-2 treated cells showed increasing cytoplasmic vacuolization that persisted until apoptosis. Whether these vacuoles represent induction of autophagy or reflect altered metabolism remains to be determined.
One significant finding of our study is that COTI-2 restores site-specific-DNA binding properties of p53 mutant protein to the promoters of several p53 target genes determined by chromatin immunoprecipitation (ChIP). Moreover, COTI-2 produced a distinct p53 target gene signature that is remarkably different from untreated controls, confirming that the structural changes of the PCI13-G245D mutant TP53 resulted in a transcriptionally active protein mediating cell killing. These results are in agreement with study demonstrated that ZMC1 resulted in restoration of p53 transcriptional function in TOV112D cell lines (9). Additionally, our data suggest that the effect of COTI-2 in HNSCC lacking TP53 is mediated, at least in part, through activation of p53 target genes likely induced by the TAp63 gene family. Interestingly, COTI-2 increases expression of MDM2, a p53 interacting protein that represses p53 transcriptional activity. Inhibition of MDM2 with drugs such as Nutlin-3A or AMG-232 may increase p53 function and enhance cell killing in HNSCC tumors with mutant p53 following treatment with COTI-2.
Our proteomic profiling studies revealed that COTI-2 treatment affects other targets that are independent of p53 in HNSCC. Thus, COTI-2 treatment results in activation of the tumor suppressor, AMPK and inactivates the oncogene mTOR either directly or indirectly, possibly culminating in greater cell killing in HNSCC, consistent with published data (11). Activation of AMPKα is mainly initiated allosterically and by post-translational modification through phosphorylation of its threonine residue 172 (T172) by at least three kinases, namely liver kinase B1 (LKB1), calcium-/calmodulin-dependent kinase-kinase 2 (CaMKK2), and TGFβ-activated kinase 1 (TAK1) (29). Additionally, when energy is depleted, high levels of AMP and ADP bind to the AMPK γ-subunit and activates AMPK (29). Our data showed that COTI-2 treatment resulted in phosphorylation and activation of the CaMKK2 kinase isoforms at threonine 286 (T286). Therefore, it is highly likely that COTI-2 activates AMPK through calcium mediated activation of CaMKK2. It is also possible that in HNSCC cells, COTI-2 activates AMPK through modulation of energy metabolism involving ATP levels. One of the major downstream signaling pathways regulated by nutrient deprivation and AMPK is the mammalian target-of-rapamycin (mTOR pathway) (30). AMPK directly phosphorylates at least two proteins, mTORC1 and mTORC2 to induce rapid inhibition of mTOR activity (30). Thus, COTI-2 mediated activation of AMPK observed in our study may be responsible for inhibiting oncogenic mTOR in HNSCC cells. It is possible that AMPK activation and inhibition of mTOR pathways with COTI-2 leads to energy depletion and starvation resulting in the cell cycle changes and cell death observed in HNSCC. Our study also provided evidence that COTI-2 either directly or indirectly decreased cellular communication perhaps through downregulation of connexin-43.
In summary, we demonstrate that COTI-2 is highly effective in preclinical models of mutant TP53-bearing tumor cells that are inherently resistant to cisplatin and radiation. Furthermore, this drug appears to exert its action in a novel manner through a combination of effects including re-activation of a subset of mutant p53 and induction of DNA damage and replication stress responses leading to apoptotic cell death. Furthermore, unlike other thiosemicarbazones, our data indicate that COTI-2 is not a traditional zinc metallochaperone, and likely acts to increase apoptosis through both p53-dependent and p53-independent mechanisms in HNSCC. In our study, few mice died in the combination of COTI-2 and cisplatin, suggesting possible toxicity with the dose tested. However, an early results from an ongoing clinical trial conduced at our institution show that COTI-2 and in combination with cisplatin is safe and well tolerated in patients with solid tumors including HNSCC (privileged communications, Dr. Shannon Westin, MD Anderson Cancer Center). Further studies to identify optimal and effective combination dose of COTI-2 with cisplatin and/or radiation in vivo in HNSCC with different TP53 mutational status are warranted.
Supplementary Material
Translational Relevance.
TP53 mutations are highly prevalent in HPV-negative head and neck cancer and are often associated with aggressive disease and resistance to curative therapy with cisplatin and radiation. The majority of these TP53 mutations gain oncogenic properties which are independent of wild-type p53 functions. In this study, we have demonstrated that COTI-2, a novel p53 reactivator, has the ability to restore mutant p53 function, possesses single-agent activity and further potentiates responses to cisplatin and/or radiation in vitro and in vivo in pre-clinical models of HNSCC with different TP53 status. Our data provided a preclinical foundation for testing the safety and efficacy of COTI-2 in an ongoing phase I study of patients with refractory gynecologic and head and neck malignancies.
Funding:
This work was supported by the National Institute of Health/NIDCR R01DE024601 (A.A. Osman and J.N. Myers), Cotinga Pharmaceuticals (A.A. Osman and J.N. Myers), and The University of Texas MD Anderson Cancer Center-Oropharynx Cancer Program generously supported by Mr. and Mrs. Charles W. Stiefel awarded to A.A. Osman and J.N. Myers. This work used the services of MD Anderson’s Flow Cytometry and Cellular Imaging Core, Bioinformatics Shared Resource, and the Reverse Phase Protein Array (RPPA) Core, which are supported by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant (P30CA016672).
Conflicts of Interest Disclosure: Abdullah A. Osman and Jeffrey N. Myers have received research funding from Cotinga Pharmaceuticals. Other authors have no potential conflicts of interest to declare.
Abbreviations:
- HNSCC
head and neck squamous cell carcinoma
- COTI-2
Critical Outcome Technologies Inc.
- GOF
gain-of-function
- CDK1
cyclin dependent kinase 1
- AMPK
AMP-activated protein kinase
- mTOR
mammalian target of rapamycin
- MCl-1
myeloid leukemia cell differentiation protein
- PDCD4
programmed cell death protein-4
- EdU
5-ethynyl-2-deoxyuridine
- ChIP
chromatin immunoprecipitation
- RPPA
Reverse phase protein array
References
- 1.Adelstein DJ, Li Y, Adams GL, Wagner H Jr., Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003; 21:92–8. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011; 333(6046):1154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickering CR, Zhang J, Yoo SY, Bengtsson L, Moorthy S, Neskey DM, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013; 3(7):770–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Network*. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015; 517(7536):576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivlin N, Koifman G, Rotter V. p53 orchestrates between normal differentiation and cancer. Semin Cancer Biol. 2015; 32:10–7. [DOI] [PubMed] [Google Scholar]
- 6.Osman AA, Neskey DM, Katsonis P, et al. Evolutionary Action Score of TP53 Coding Variants Is Predictive of Platinum Response in Head and Neck Cancer Patients. Cancer Res. 2015; 75(7):1205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med 2002; (8):282–288. [DOI] [PubMed] [Google Scholar]
- 8.Bykov VJ, Issaeva N, Zache N, Shilov A, Hultcrantz M, Bergman J, Selivanova G, Wiman KG. Reactivation of mutant p53 and induction of apoptosis in human tumor cells by maleimide analogs. J Biol Chem 2005; 280:30384–30391. [DOI] [PubMed] [Google Scholar]
- 9.Yu X, Vazquez A, Levine AJ, Carpizo DR. Allele-Specific p53 Mutant Reactivation. Cancer Cell 2012; 21(5):614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X, Blanden AR, Narayanan S, Jayakumar L, Lubin D, Augeri D, Kimball SD, Loh SN, Carpizo DR. Small molecule restoration of wildtype structure and function of mutant p53 using a novel zinc-metallochaperone based mechanism. Oncotarget. 2014; 5(19):8879–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salim KY, Maleki Vareki S, Danter WR, Koropatnick J. COTI-2, a novel small molecule that is active against multiple human cancer cell lines in vitro and in vivo. Oncotarget. 2016; 7(27):41363–41379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maleki VS, Salim KY, Danter WR, Koropatnick J. Novel anti-cancer drug COTI-2 synergizes with therapeutic agents and does not induce resistance or exhibit cross-resistance in human cancer cell lines. PLoS One 2018; 13(1):e0191766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westin Shannon N., Wilberto Nieves-Neira Christian Lynam, Salim Kowthar Y., Silva Alison D., Ho Richard T., Mills Gordon B., Coleman Robert L., Janku Filip, Matei Daniela. Safety and early efficacy signals for COTI-2, an orally available small molecule targeting p53, in a phase I trial of recurrent gynecologic cancer [abstract] In: Proceedings of the American Association for Cancer Research Annual Meeting 2018; 2018 Apr 14–18; Chicago, IL: Philadelphia (PA): AACR; Cancer Res; 2018; 78(13 Suppl): Abstract nr CT033. [Google Scholar]
- 14.Osman AA, Monroe MM, Ortega Alves MV, Patel AA, Katsonis P, Fitzgerald AL, et al. Wee-1 kinase inhibition overcomes cisplatin resistance associated with high-risk TP53 mutations in head and neck cancer through mitotic arrest followed by senescence. Mol Cancer Ther 2015; 14:608–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006; 58:621–81. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka N, Patel AA, Silver NL, Lindemann A, Tang L, Jaksik R, et al. Synergistic antitumor activity of HDAC inhibitor vorinostat combined with WEE1 inhibitor AZD1775 in head and neck squamous cell carcinoma harboring high-risk TP53 mutation. Clin Cancer Res 2017; 23(21):6541–6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka N, Zhao M, Tang L, Patel AA, Xi Q, Van HT, Takahashi H, Osman AA, Zhang J, Wang J, Myers JN, Zhou G. Gain-of-function mutant p53 promotes the oncogenic potential of head and neck squamous cell carcinoma cells by targeting the transcription factors FOXO3a and FOXM1. Oncogene 2018; 37:1279–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka N, Osman AA, Takahashi Y, Lindemann A, Patel AA, Zhao M, Takahashi H, Myers JN. Head and neck cancer organoids established by modification of the CTOS method can be used to predict in vivo drug sensitivity. Oral Oncol 2018; 87:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skinner HD, Sandulache VC, Ow TJ, Meyn RE, Yordy JS, Beadle BM, et al. TP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation-induced senescence. Clin Cancer Res 2012; 18(1):290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang GS, Chen XA, Park B, Rhee HS, Li P, Han KH, et al. A Comprehensive and High Resolution Genome-wide Response of p53 to Stress. Cell Rep 2014; 8(2):514–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dohn M, Zhang S, Chen X. p63alpha and DeltaNp63alpha can induce cell cycle arrest and apoptosis and differentially regulate p53 target genes. Oncogene 2001; 20(25):3193–205. [DOI] [PubMed] [Google Scholar]
- 22.Byers LA, Wang J, Nilsson MB, Fujimoto J, Saintigny P, Yordy J, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov 2012; 2:798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Role of connexin 43 in different forms of intercellular communication–gap junctions, extracellular vesicles and tunnelling nanotubes. Ribeiro-Rodrigues TM, Martins-Marques T, Morel S, Kwak BR, Girao H. J of Cell Sci 2017;0, 1–12 doi: 10.1242/jcs.200667. [DOI] [PubMed] [Google Scholar]
- 24.El-Fouly MH, Trosko JE, Chang C-C. Scrape-Loading and Dye transfer, A Rapid and Simple Technique to Study Gap Junctional Intracellular Communication. Expt Cell Res 1987; 168:422–430. [DOI] [PubMed] [Google Scholar]
- 25.Babica P, Sovadinova I, Upham BL. Scrape Loading/Dye Transfer Assay. Methods Mol Biol. 2016; 1437:133–44. [DOI] [PubMed] [Google Scholar]
- 26.Azzam EI, De Toledo SM, Little JB. Direct evidence for the participation of gap junction mediated intercellular communication in the transmission of damage signals from a-particle irradiated to nonirradiated cells. PNAS 2001; 98(2):473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindemann A, Takahashi H, Patel AA, Osman AA, Myers JN. Targeting the DNA Damage Response in OSCC with TP53 Mutations. Journal of Dental Res 2018; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzgerald AL, Osman AA, Xie TX, Patel A, Skinner H, Sandulache V, Myers JN. Reactive oxygen species and p21Waf1/Cip1 are both essential for p52-mediated senescence of head and neck cancer cells. Cell Death Dis 2015:12; 6:e1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeon S-M. Regulation and function of AMPK in physiology and diseases. Exp Mol Med 2016; 48(7):e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017; 168(6):960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.