Abstract
Objective
To examine functional performance differences using kinematic and kinetic analysis between participants with and without knee osteoarthritis (OA) to determine which outcomes best characterize persons with and without knee OA.
Methods
Participants with unilateral moderate knee OA (Kellgren–Lawrence grades 2 or 3) and controls without knee pain were matched for age, gender, and body mass index. Primary outcomes included temporal parameters, joint rotations and moments, and ground reaction forces assessed via 3D motion capture during walking and ascending/descending stairs. Secondary outcomes included timed functional activities (sit to stand; tying shoelaces), 48 hrs lower limb activity monitoring, and patient-reported outcome measures (Knee Injury and Osteoarthritis Outcome Score, Western Ontario and McMaster Universities Osteoarthritis Index, European Quality of Life–5 Dimensions).
Results
Eight matched pairs were analyzed. Compared with controls, OA participants exhibited significant reductions in peak frontal hip and sagittal knee moments, and decreased peak anterior ground reaction force with the affected limb while walking. Ascending stairs, OA participants had slower speed, fewer strides per minute, longer cycle and stance times, and increased trunk range of motion (ROM) in assessments of both limbs; longer swing time and reduced ankle ROM in the affected limb; and increased knee frontal ROM in the unaffected limb. Descending stairs, OA participants had fewer strides per minute and decreased trunk transverse ROM in assessments of both limbs; increased knee frontal ROM in the affected limb; and longer strides, shorter stance and cycle times, increased trunk sagittal and decreased knee transverse ROMs in the unaffected limbs vs controls. Compared with controls, OA participants had slower walking cadence (120–130 vs 100–110 steps/min, respectively), took significantly longer on timed functional measures, and had significantly worse scores in patient-reported outcomes.
Conclusion
Several objectives and patient-reported measures examined in this study could potentially be considered as outcomes in pharmacologic or physical therapy OA trials.
Keywords: osteoarthritis, knee, 3D motion analysis, biomechanics, kinetics, gait
Introduction
Knee osteoarthritis (OA), a major cause of disability worldwide,1 adversely affects health-related quality of life2 and causes significant morbidity.3 When conservative measures fail, patients often experience years of pain, affecting activities of daily living before proceeding, if eligible, to surgical intervention such as total knee arthroplasty.4
Numerous objective outcomes are used in OA studies, but it remains unclear which best characterize OA-related changes.5 A systematic review found evidence gaps with most measures; the 15.2-m fast-paced walk test, 30-s chair-stand test, and timed up-and-go test provided the most effective indicators of knee OA.6 An expert consensus report has recommended the 40-m fast-paced walk test, 30-s chair-stand test, stair-climb test, timed up-and-go test, and 6-min walk test.7
Furthermore, it remains unclear which outcome measures best differentiate persons with and without OA when age and body mass index (BMI) are matched, as knee OA incidence increases with age and BMI,8,9 and obesity is associated with increased OA progression.10
This study aimed to identify differences in physical function and performance of essential activities between patients with moderate knee OA and matched controls without knee symptoms. We hypothesized that participants with OA would demonstrate poorer results in all kinematic, kinetic, timed functional, and patient-reported measures compared with healthy controls, and that the results could potentially be used to guide pharmacologic and non-pharmacologic intervention studies for moderate knee OA.
Methods
Study design and procedures
This observational, non-interventional study, conducted at the School of Engineering, Cardiff University, Cardiff, Wales, UK, compared people with unilateral (medial or lateral compartment) moderate knee OA (Kellgren–Lawrence OA classification grade 2 or 3), with a control group without knee or lower limb symptoms matched for age, gender, and BMI. At visit 1, participants provided demographic and medical history information and an ActivPAL™ activity monitor (PALtechnologies Ltd, Glasgow, Scotland, UK) was attached to the right thigh (for consistency, as the ActivPAL is a measure of general activity) and worn for 48 hrs. ActivPAL registers activity utilizing acceleration and inclination logging technology and algorithms log walking speed and cadence.
They also completed validated subjective questionnaires: Knee Injury and Osteoarthritis Outcome Score (KOOS),11,12 European Quality of Life–5 Dimensions (EQ-5D),13–16 and Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index.17 The researchers checked that all questionnaires were completed appropriately.
Two days after visit 1, investigators phoned participants with OA to assess pain severity using a numeric pain rating scale from 0 (no pain) to 10 (maximum/extreme pain). Participants with ratings ≥4 and ≤7 returned to the laboratory that day for assessment. These thresholds were used to ensure that the assessments were made while patients were experiencing pain symptoms. A level of ≤7 was used as a maximum cut-off because participants with higher scores could potentially be adversely affected (ie, marked increase in pain) by walking and stair-climbing activities. Controls were assessed at the laboratory approximately 48 hrs after ActivPAL application.
At visit 2, participants were evaluated using 3D motion analysis with a full-body modified Helen Hayes marker set18 extended with thigh- and shank-fixed clusters, as well as additional foot and pelvis markers; iliac crest markers were used to better define the hip during stair trials. Nine Oqus infra-red cameras (Qualisys, Gothenburg, Sweden) captured 3D marker motion at 120 Hz. Force data were collected at 1080 Hz from four force plates (Bertec Corporation, Columbus, Ohio, USA) embedded in the laboratory floor. Participants were recorded while walking six times across the level laboratory floor (over the force plates) and while ascending and descending a 4-step staircase19 six times (three trials leading with each leg). Custom software (Visual3D, C-Motion, Inc, Germantown, Maryland, USA) was used to scale a model to the standing position for each participant and apply this model to the movement data. Reports containing temporal parameters, joint rotations, external joint moments (rotation forces acting around the joint), and ground reaction forces were produced for each participant. Participants also completed timed activities of daily living: sit to stand, stand to sit, tying shoelaces, and 10-m walk.
Study population
Patients with knee OA were recruited from Cardiff and Vale University Hospital Board Physiotherapy Department outpatient clinics, University Hospital of Wales, Cardiff, Wales, UK. Controls were recruited from Cardiff and the surrounding region via poster and electronic advertising forums for staff at Cardiff University. Volunteers contacted researchers by e-mail or telephone and were assessed for eligibility. Recruitment began January 2, 2015 and data collection ended on February 26, 2016. Recruitment continued through March 31, 2016 in an unsuccessful attempt to identify the final two matched control subjects.
Eligible participants were 45–65 years and able to comply with instructions and study procedures. OA participants had to have a 6-month history of moderate unilateral (medial or lateral) OA in either knee confirmed by radiographic evidence of joint space narrowing (grade 2 or 3 based on Kellgren–Lawrence OA classification) and pain intensity of ≥4 and ≤7 on a scale of 0 (no pain) to 10 (maximum/extreme pain). Verbal checks were made to ensure OA participants had no current soft tissue injuries to the lower limbs. Controls had to have good general health, normal strength, and full range of motion (ROM) of the lower extremities, with no history of knee OA, knee instability, major lower extremity joint surgery, or current soft tissue injuries to the lower limbs and no neurologic deficits.
The protocol initially required controls to be matched to OA participants within ±2 years of age and ±5 kg/m2 for BMI, as well as gender. Due to recruiting challenges (BMI was usually too low among controls who matched for age), criteria were subsequently changed to ±5 years for age and ±5 kg/m2 for BMI.
Key exclusion criteria for both groups included adhesive tape allergy; neurologic or balance disorder; recent (≤2 years) alcohol or other substance abuse; and history of cerebrovascular accident, head injury, or systemic inflammatory arthritis.
Participants had to discontinue oral or topical analgesics during the 48 hrs between visits 1 and 2. Up to 1000 mg paracetamol, four times daily, was permitted as rescue medication, in accordance with European League Against Rheumatism recommendations for knee, hip, and hand OA.20 However, participants had to have at least 2 consecutive days without pain medication before visit 2.
Outcomes
Primary endpoints were 3D kinematic and kinetic data from the motion analysis during level walking and stair ascent and descent, and included temporal-spatial parameters, joint rotations, joint moments, and ground reaction forces. Temporal-spatial measures consisted of walking speed (m/s), stride width/length (m), cycle time (s), step length (m), step time (s), stance time (s), swing time (s), and strides per minute. Kinematic joint ROM (in degrees) was determined at the hip, knee, ankle, pelvis, and trunk, and defined through transformation from tracking markers to the pose of each segment of the biomechanical model.
Cardan sequences of joint angles (X, Y, and Z, representing flexion/extension, abduction/adduction, and axial rotation, respectively, and equivalent to the “joint coordinate system”21,22) were used to compute ankle, knee, and hip kinematics, following International Society of Biomechanics recommendations.23 Trunk rotations were also calculated using Cardan sequence X-Y-Z, whereas a Z-Y-X (axial rotation, obliquity, tilt) description was used to compute pelvic rotations, following recommendations of Baker et al.24 Inverse dynamics analysis was applied to kinematics of the biomechanical model and location, magnitude, and direction of ground reaction forces acting on the foot. This process computed external moments acting about the ankle, knee, and hip joints, which were resolved to distal joint segments. 3D kinetic measures consisted of XYZ joint moments at stance phase for hip, knee, and ankle (percentage body weight ⨰ height) and XYZ power at stance phase for hip, knee, and ankle (w/kg). XYZ ground reaction forces (medial-lateral, anterior-posterior, and vertical) were normalized to body weight and XY center of pressure of ground reaction force during stance phase (M), normalized to foot width and length.
Secondary endpoints included KOOS,11,12 EQ-5D,13–16 and WOMAC Osteoarthritis Index17 scores, walking performance based on ActivPAL, and performance on the timed activities of daily living. ActivPAL measures were number of steps taken, cadence (steps/minute), time (minutes) spent lying/sitting and standing, number of stepping events, total number of upright events, and total number of seated events. Safety outcomes included adverse events (AEs), consisting of any untoward medical occurrence following data collection at the two visits, or reported in response to questioning via telephone between visits.
Statistics
No formal power calculation was performed for this exploratory study. The protocol called for 10 OA participants and 10 matched controls as this was deemed sufficient to answer the research question and objectives.
For the primary 3D motion analyses, only matched pairs of OA and control subjects were included. Kinematic and kinetic variables were calculated as mean ± standard deviation (SD) for each limb. After testing for normal distribution using histograms, data were deemed not to be normally distributed; therefore, the Wilcoxon signed-rank test, a paired non-parametric analysis, was used to analyze differences between OA patients and controls. For each matched pair, the affected leg of the OA participant was compared with the same leg (left/right) of the matched control; the same was done for the unaffected leg. Temporal-spatial measures were summarized by descriptive and inferential statistics. Descriptive statistics were used for patient-reported outcome questionnaires, timed functional tests, and ActivPAL results, and between-group differences (OA and controls) were analyzed by the Wilcoxon signed-rank test. Data that were missing due to technical errors were excluded from the analyses. All analyses were conducted using Statistics Package for Social Sciences (IBM, USA), Version 20.
Results
Participants
Of 65 OA participants screened, 11 were recruited; of 43 healthy participants screened, 8 controls were recruited. The eleventh OA subject was recruited as a replacement for a subject whose data were inadequate for processing because loose clothing had impaired marker visibility. Because we were unable to recruit two controls to match two OA patients with the highest BMIs, 3D motion analysis was performed on eight matched pairs. Other objective analyses included 10 OA participants and 8 controls. Of the 10 OA patients, 6 had OA in the right knee, and 4 had OA in the left knee. Baseline demographics were similar for OA participants and controls (Table 1).
Table 1.
Demographic characteristics
| OA Participants (n=10) | Controls (n=8) | P-valuea | |
|---|---|---|---|
| Age, mean (SD), years | 58.6 (6.02) | 59.62 (3.85) | 0.315 |
| Gender, n (%) | |||
| Female | 8 (80) | 6 (75) | – |
| Male | 2 (20) | 2 (25) | – |
| Height, mean (SD), m | 1.71 (0.089) | 1.70 (0.12) | 0.696 |
| Weight, mean (SD), kg | 91.52 (15.09) | 87.58 (16.01) | 0.573 |
| BMI, mean (SD), kg/m2 | 30.82 (3.14) | 29.80 (3.37) | 0.315 |
| Numeric pain rating, mean (SD)b | 5.22 (0.97) | Not applicable | – |
Notes: aWilcoxon signed-rank test. bNumeric pain rating scale from 0 (no pain) to 10 (highest pain).
Abbreviations: BMI, body mass index; OA, osteoarthritis; SD, standard deviation.
Objective measures of function
Level walking
3D motion analysis during level walking noted that the OA-affected limb had significantly reduced maximum frontal (adduction) hip moment (P=0.036) and sagittal (flexion) knee moment (P=0.025) compared with the corresponding limb of controls (Table 2). A significant decrease in the anteriorly directed ground reaction force during the second half of stance was found for the OA-affected limb compared with the corresponding limb of controls (mean [SD] 0.16 [0.06] vs 0.22 [0.03] percent body weight [%BW]; P=0.025).
Table 2.
Level walking 3D motion analysis
| Mean (SD), Degrees | Mean (SD), Degrees | |||||
|---|---|---|---|---|---|---|
| OA-Affected limb (n=8) | Matched control limb (n=8) | P-valuea | OA-Unaffected limb (n=8) | Matched control limb (n=8) | P-valuea | |
| Hip moment | ||||||
| Max sagittal (flexion [+ve]) | 4.83 (1.72) | 4.94 (1.77) | 1.000 | 4.00 (1.62) | 4.65 (1.72) | 0.575 |
| Max frontal (adduction [+ve]) | 4.92 (1.32) | 6.20 (0.92) | 0.036b | 5.06 (1.64) | 5.51 (1.20) | 0.263 |
| Max transverse (internal rotation [+ve]) | 0.63 (0.39) | 0.67 (0.36) | 0.674 | 0.78 (0.34) | 0.52 (0.15) | 0.161 |
| Min sagittal (flexion [+ve]) | −5.02 (2.30) | −4.90 (1.09) | 1.000 | −5.61 (2.14) | −4.40 (1.30) | 0.123 |
| Min frontal (adduction [+ve]) | −1.23 (0.86) | −0.70 (0.23) | 0.069 | −1.46 (1.18) | −1.10 (0.34) | 0.327 |
| Min transverse (internal rotation [+ve]) | −0.64 (0.51) | −1.18 (0.25) | 0.069 | −0.72 (0.30) | −0.99 (0.48) | 0.327 |
| Knee moment | ||||||
| Max sagittal (flexion [+ve]) | 2.59 (1.20) | 5.00 (1.11) | 0.025b | 3.39 (0.77) | 4.36 (0.70) | 0.069 |
| Max frontal (adduction [+ve]) | 2.30 (1.39) | 2.75 (0.72) | 0.674 | 1.98 (0.95) | 2.11 (0.38) | 0.674 |
| Max transverse (internal rotation [+ve]) | 0.69 (0.40) | 0.82 (0.21) | 0.327 | 0.67 (0.32) | 0.63 (0.19) | 1.000 |
| Min sagittal (flexion [+ve]) | −2.45 (0.56) | −1.73 (0.98) | 0.123 | −2.33 (0.68) | −1.70 (0.82) | 0.208 |
| Min frontal (adduction [+ve]) | −0.68 (0.15) | −0.58 (0.26) | 0.327 | −0.73 (0.26) | −0.82 (0.26) | 0.575 |
| Min transverse (internal rotation [+ve]) | −0.15 (0.10) | −0.28 (0.16) | 0.327 | −0.16 (0.09) | −0.22 (0.08) | 0.484 |
| Ankle moment | ||||||
| Max sagittal (dorsiflexion [+ve]) | 7.96 (1.00) | 8.65 (1.23) | 0.161 | 8.41 (0.88) | 8.22 (0.95) | 0.674 |
| Max frontal (inversion [+ve]) | 0.23 (0.11) | 0.51 (0.30) | 0.069 | 0.33 (0.19) | 0.44 (0.29) | 0.484 |
| Max transverse (internal rotation [+ve]) | 1.47 (0.53) | 1.69 (0.42) | 0.401 | 1.45 (0.39) | 1.66 (0.52) | 0.484 |
| Min sagittal (dorsiflexion [+ve]) | −1.01 (0.21) | −0.99 (0.27) | 0.779 | −0.24 (0.67) | −0.24 (0.87) | 1.000 |
| Min frontal (inversion [+ve]) | −0.80 (0.25) | −0.52 (0.15) | 0.093 | −0.12 (0.28) | −0.12 (0.32) | 0.779 |
| Min transverse (internal rotation [+ve]) | −0.20 (0.12) | −0.22 (0.11) | 0.889 | 0.01 (0.20) | 0.02 (0.15) | 0.575 |
Notes: Joint moments (%BW*H) (sagittal, frontal, transverse planes) for affected and unaffected limbs of OA participants compared with participants without knee pathology are shown. aWilcoxon signed-rank test. bDenotes statistically significant difference (P<0.05).
Abbreviations: %BW*H, percent body weight x height; Min, minimum; Max, maximum; OA, osteoarthritis; SD, standard deviation.
Hip, knee, ankle, and trunk ROM were not significantly different between OA participants on the affected or unaffected limb and corresponding limbs of controls, nor were there significant differences in walking speed, stride width/length, cycle time, step length, step time, stance time, swing time, or strides per minute.
Ascending and descending stairs
There were no significant differences in ground reaction forces or joint moments of the hip, knee, and ankle in affected or unaffected limbs of OA participants and corresponding limbs of controls while ascending or descending stairs.
Table 3 shows temporal data from 3D motion analysis while ascending and descending stairs. During ascent, participants with OA had slower speed and fewer strides per minute with both limbs compared with controls. OA participants had longer cycle and stance times in both limbs, and longer swing time in the affected limb, compared with controls. During descent, OA participants took fewer strides per minute with both limbs vs controls. On measurements of unaffected limbs, while descending stairs, OA participants had longer stride length, and shorter stance and cycle time than controls.
Table 3.
Ascending and descending stairs, temporal data from 3D motion analysis
| Mean (SD) | Mean (SD) | |||||
|---|---|---|---|---|---|---|
| OA-Affected limb (n=8) | Matched control limb (n=8) | P-valuea | OA-Unaffected limb (n=8) | Matched control limb (n=8) | P-valuea | |
| Ascending stairs | ||||||
| Speed, m/s | 0.31 (0.06) | 0.33 (0.06) | 0.025b | 0.31 (0.06) | 0.33 (0.06) | 0.025b |
| Stride length, m | 0.45 (0.13) | 0.39 (0.02) | 0.069 | 0.52 (0.13) | 0.39 (0.02) | 0.067 |
| Cycle time, s | 1.60 (0.36) | 1.22 (0.18) | 0.025b | 1.65 (0.41) | 1.22 (0.18) | 0.049b |
| Stance time, s | 1.12 (0.25) | 0.84 (0.15) | 0.036b | 1.18 (0.38) | 0.84 (0.15) | 0.049b |
| Swing time, s | 0.48 (0.14) | 0.37 (0.05) | 0.036b | 0.47 (0.12) | 0.37 (0.05) | 0.207 |
| Strides per minute | 35.32 (5.34) | 44.43 (6.32) | 0.036b | 36.65 (6.30) | 43.06 (5.48) | 0.049b |
| Descending stairs | ||||||
| Speed, m/s | 0.31 (0.06) | 0.33 (0.07) | 0.208 | 0.31 (0.06) | 0.33 (0.07) | 0.208 |
| Stride length, m | 0.33 (0.06) | 0.45 (0.13) | 0.735 | 1.29 (0.12) | 0.52 (0.13) | 0.025b |
| Cycle time, s | 0.39 (0.02) | 1.60 (0.36) | 0.063 | 0.40 (0.04) | 1.64 (0.41) | 0.036b |
| Stance time, s | 1.22 (0.18) | 1.12 (0.25) | 0.063 | 1.21 (0.17) | 1.17 (0.38) | 0.036b |
| Swing time, s | 0.84 (0.15) | 0.48 (0.14) | 0.271 | 0.84 (0.17) | 0.47 (0.12) | 0.123 |
| Strides per minute | 39.40 (8.93) | 50.23 (6.98) | 0.043b | 38.80 (10.12) | 50.30 (6.73) | 0.036b |
Notes: aWilcoxon signed-rank test. bDenotes statistically significant difference (P<0.05).
Abbreviations: m, meters; m/s, meters per second; OA, osteoarthritis; s, seconds; SD, standard deviation.
Table 4 shows results for the ROM analysis while ascending and descending stairs. During ascent, knee ROM for OA-affected limbs was similar to corresponding limbs in controls, but OA participants had greater frontal knee ROM in the unaffected knee vs controls. Sagittal ankle ROM was decreased in OA-affected limbs vs controls, and sagittal and transverse trunk ROM were increased in the cycles of both limbs of OA participants compared with controls while ascending stairs. Hip ROM was not different for OA participants vs controls, for the affected or unaffected side.
Table 4.
Ascending and descending stairs, joint range of motion on 3D motion analysis
| Mean (SD), Degrees | Mean (SD), Degrees | |||||
|---|---|---|---|---|---|---|
| OA-Affected limb (n=8) | Matched control limb (n=8) | P-valuea | OA-Unaffected Limb (n=8) | Matched control limb (n=8) | P-valuea | |
| While ascending stairs | ||||||
| Hip ROM | ||||||
| Sagittal | 23.85 (4.73) | 21.85 (3.40) | 0.575 | 23.44 (4.28) | 21.19 (3.52) | 0.484 |
| Frontal | 14.52 (7.83) | 10.79 (3.70) | 0.093 | 15.13 (4.25) | 11.52 (3.01) | 0.889 |
| Transverse | 17.45 (5.39) | 13.51 (3.42) | 0.484 | 17.00 (4.51) | 12.07 (2.54) | 0.327 |
| Knee ROM | ||||||
| Sagittal | 68.70 (14.80) | 75.46 (4.90) | 0.401 | 77.40 (6.73) | 76.27 (5.67) | 0.327 |
| Frontal | 14.63 (5.23) | 8.49 (1.35) | 0.208 | 16.43 (5.29) | 8.18 (2.47) | 0.049b |
| Transverse | 14.19 (3.76) | 14.75 (2.36) | 0.263 | 17.21 (2.25) | 12.96 (3.35) | 0.263 |
| Ankle ROM | ||||||
| Sagittal | 50.65 (8.58) | 51.45 (6.97) | 0.049b | 52.53 (8.95) | 48.05 (5.65) | 0.069 |
| Frontal | 14.82 (4.62) | 14.90 (1.67) | 0.575 | 17.19 (5.90) | 15.07 (2.67) | 0.779 |
| Transverse | 13.60 (5.50) | 13.49 (5.99) | 0.401 | 13.16 (3.01) | 11.00 (3.00) | 0.327 |
| Trunk ROM | ||||||
| Sagittal | 5.69 (1.22) | 4.53 (1.00) | 0.017b | 6.38 (1.46) | 4.29 (0.95) | 0.036b |
| Frontal | 7.98 (3.60) | 5.15 (2.50) | 0.069 | 6.42 (2.71) | 5.69 (1.48) | 0.161 |
| Transverse | 20.26 (7.96) | 13.04 (1.86) | 0.036b | 22.26 (11.33) | 11.94 (2.60) | 0.017b |
| While descending stairs | ||||||
| Hip ROM | ||||||
| Sagittal | 13.50 (5.99) | 23.86 (4.73) | 0.327 | 21.20 (3.52) | 23.44 (4.28) | 0.069 |
| Frontal | 21.85 (3.40) | 14.53 (7.83) | 0.327 | 11.03 (3.00) | 15.13 (4.25) | 0.263 |
| Transverse | 10.80 (3.70) | 17.45 (5.39) | 0.161 | 11.52 (3.01) | 16.99 (4.51) | 0.123 |
| Knee ROM | ||||||
| Sagittal | 13.51 (3.42) | 68.70 (14.80) | 0.263 | 76.27 (5.67) | 77.39 (6.73) | 0.208 |
| Frontal | 75.47 (4.90) | 14.63 (5.23) | 0.025b | 12.08 (2.54) | 16.44 (5.03) | 0.123 |
| Transverse | 8.49 (1.35) | 14.20 (3.76) | 0.327 | 8.18 (2.47) | 17.21 (2.25) | 0.036b |
| Ankle ROM | ||||||
| Sagittal | 13.04 (1.86) | 50.66 (8.58) | 0.889 | 11.95 (2.60) | 52.53 (8.95) | 0.069 |
| Frontal | 51.46 (6.97) | 14.82 (4.62) | 0.889 | 48.05 (5.65) | 17.20 (5.90) | 0.263 |
| Transverse | 14.89 (1.67) | 13.65 (5.50) | 1.000 | 15.07 (2.67) | 13.16 (3.01) | 0.123 |
| Trunk ROM | ||||||
| Sagittal | 50.23 (6.98) | 5.69 (1.22) | 0.069 | 50.30 (6.74) | 6.38 (1.46) | 0.017b |
| Frontal | 4.53(1.00) | 7.98 (3.60) | 0.263 | 4.29 (0.95) | 6.42 (2.71) | 0.889 |
| Transverse | 5.15 (2.50) | 20.27 (7.96) | 0.025b | 5.70 (1.48) | 22.27 (11.33) | 0.049b |
Notes: aWilcoxon signed-rank test. bDenotes statistically significant difference (P<0.05).
Abbreviations: OA, osteoarthritis; ROM, range of motion; SD, standard deviation.
While descending stairs, OA participants had greater knee frontal ROM in the affected limb, and less knee transverse ROM in the unaffected limb compared with controls. Trunk sagittal ROM was increased in cycles of the unaffected side of OA participants compared with the corresponding side of controls, and trunk transverse ROM was decreased on assessments of both sides of OA participants vs controls. Hip and ankle ROM did not differ during stair descent for either limb of OA participants compared with controls.
Time to complete functional tests
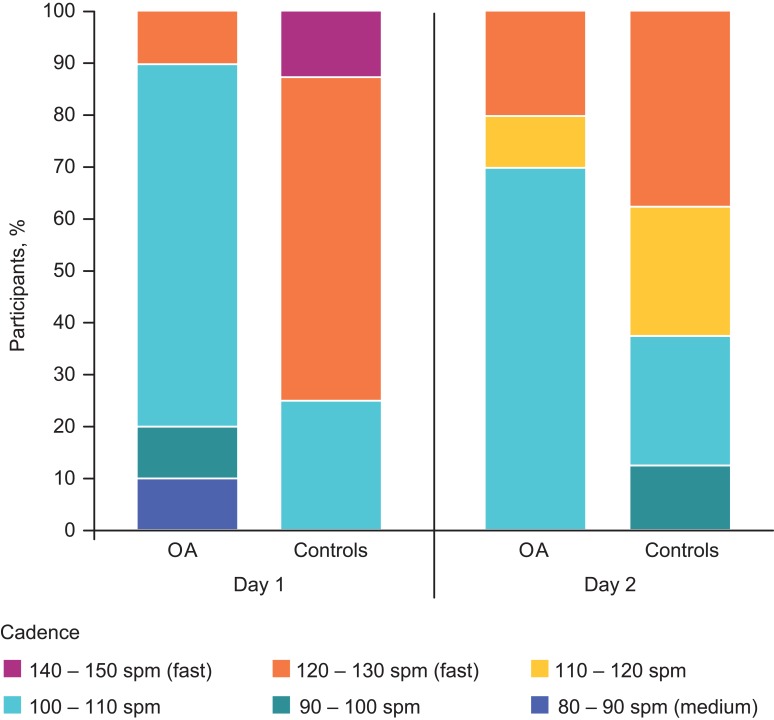
Based on ActivPAL, participants with OA had slower cadence while walking compared with controls. Descriptive data show that the most common cadence was 100–110 steps/minute for OA participants and 120–130 steps/minute for controls (Figure 1). Other ActivPAL measures were not significantly different between the two populations. OA participants took significantly longer than controls on all timed activities (Table 5).
Figure 1.
Cadence (steps per minute) measured by ActivPAL in OA participants (n=10) and controls (n=8). This figure graphically shows a categorical analysis of cadence (spm) gathered from the ActivPAL for each group (OA and controls) on days 1 and 2. The different colors indicate the proportion of subjects in each cadence category, spanning a range of approximately 11 spm; dark blue indicates the slowest spm and purple indicates the fastest.
Abbreviations: OA, osteoarthritis; spm, steps per minute.
Table 5.
Time to complete functional tests
| Functional test | Mean (SD) time to complete test, seconds | P-valuea | |
|---|---|---|---|
| OA Group (n=10) | Controls (n=8) | ||
| Timed 10-m walk | 9.63 (2.17) | 7.54 (1.01) | 0.034b |
| Tying shoelaces | |||
| Right leg | 17.32 (5.95) | 10.31 (4.00) | 0.009b |
| Left leg | 17.37 (7.56) | 10.71 (4.40) | 0.043b |
| Sit to stand | |||
| Right leg | 3.10 (2.07) | 1.57 (0.31) | 0.002b |
| Left leg | 2.91 (2.03) | 1.49 (0.29) | 0.001b |
| Stand to sit | |||
| Right leg | 2.95 (1.69) | 1.74 (0.44) | 0.027b |
| Left leg | 3.13 (2.13) | 1.67 (0.34) | 0.012b |
Notes: aWilcoxon signed-rank test. bDenotes statistically significant difference (P<0.05) for participants with OA vs healthy participants.
Abbreviations: OA, osteoarthritis; SD, standard deviation.
Subjective patient-reported outcomes
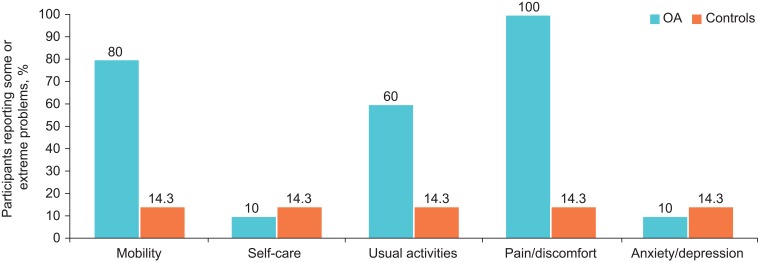
Patient-reported outcomes surveys (KOOS, WOMAC Osteoarthritis Index, and EQ-5D) were completed by all 10 OA subjects and 7 of the 8 controls. The OA participants had significantly lower scores than controls on all domains of the KOOS and WOMAC Osteoarthritis Index (Table 6). The EQ-5D asked participants to rate their current health state on a scale from 0 (worst imaginable) to 100 (best imaginable health). The mean (standard deviation [SD]) score was 81.3 (15.1) among OA participants and 96.9 (11.7) among controls (P=0.019). On other sections of the EQ-5D, a larger percentage of OA participants compared to controls reported having some or extreme problems with pain/discomfort, mobility, and usual activities (Figure 2).
Table 6.
Patient-reported outcome measures of pain and function
| Outcomes scale | OA Group (n=10) | Controls (n=7) | P-valuea |
|---|---|---|---|
| KOOS, mean (SD) scoreb | |||
| Pain | 54.18 (12.78) | 95.57 (11.7) | <0.005c |
| Symptoms | 52.85 (18.34) | 95.85 (6.69) | <0.005c |
| Activities of daily living | 63.40 (18.05) | 99.14 (2.26) | <0.005c |
| Sports/recreation | 32.63 (14.18) | 97.00 (5.74) | <0.005c |
| Quality of life | 31.89 (19.42) | 93.85 (13.78) | <0.005c |
| WOMAC, mean (SD) scored | |||
| Knee stiffness | 1.75 (0.754) | 0.214 (0.393) | <0.005c |
| Knee pain | 1.50 (0.707) | 0.075 (0.212) | <0.005c |
| Function | 1.49 (0.72) | 0.329 (0.086) | <0.005c |
| Total | 1.51 (0.71) | 0.486 (0.111) | <0.005c |
Notes: aWilcoxon signed-rank test. bIndividual items on the KOOS are each rated on a 5-point Likert scale; summed scores for each of the five dimensions of the scale are transformed to a percentage score from 0 (extreme knee problems) to 100 (no knee problems).11 cDenotes statistically significant difference (P<0.05) for participants with OA vs healthy participants. dIndividual items on the WOMAC are rated on a 5-point Likert scale (0=none to 4=extreme), and scores for each of the three dimensions are summed and then divided by the number of items in that dimension; the total score is obtained by summing the scores for all 26 items (7 for pain, 2 for stiffness, and 17 for physical function) and dividing by 26.17
Abbreviations: KOOS, Knee Injury and Osteoarthritis Outcome Score; OA, osteoarthritis; SD, standard deviation; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Figure 2.
Percentage of OA participants (n=10; blue) and controls (n=7; orange) reporting “Some Problems” or “Extreme Problems” (scores of 2 or 3) on the EQ-5D Questionnaire. The five domains of the EQ-5D are each scored as 1=no problems; 2=some problems; and 3=extreme problems.16
Abbreviations: EQ-5D, European Quality of Life–5 Dimensions; OA, osteoarthritis.
Safety
There were no AEs in this non-interventional study. OA participants all tolerated the study protocol without rescue analgesics.
Discussion
This study identified objective measures of functioning that differentiate people with and without knee OA. These results may help guide selection of outcomes for OA treatment intervention trials.
With 3D motion analysis, kinetic data demonstrated significantly decreased hip frontal (adduction) moments in OA-affected limbs during level walking, which has been seen in other studies of moderate or severe OA.25 The reduced knee sagittal (flexion) moment we observed in OA participants has also been seen with increasing OA severity25 and may be a compensatory mechanism of limiting knee flexion, and therefore compressive forces in the knee, during weight acceptance.
OA participants ascended stairs more slowly, spending longer time in stance and with greater swing time of the gait cycle. Reasons for this could include muscle weakness, apprehension of pain, or actual pain. However, increased stance time could indicate longer loading time, which may increase pain. Increased joint contact forces experienced during stair ascent, compared with level walking, may exacerbate pain.26 Compared with controls, OA participants had significantly decreased strides per minute on both limbs and increased stride length, and shorter cycle and stance time on the unaffected limb while descending stairs.
While ascending stairs, OA participants had increased sagittal and transverse trunk ROM in cycles assessing both limbs. While descending, sagittal trunk ROM was increased during assessments of the unaffected limb, and transverse trunk ROM was decreased during assessments of both limbs. Increased lateral trunk ROM has been observed in other studies of OA,27–29 and may be another compensatory mechanism. OA participants rotated their pelvis more during stair ascent, possibly because some chose to use the handrail, which was discouraged but available for safety. The only differences in hip, knee, and ankle kinematics during ascent were a decreased ankle sagittal ROM on the affected limb, and an increased frontal knee ROM on the unaffected limb. Knee frontal ROM was increased in the affected limb during descent, which could be due to a decrease in knee stability during the single support phase on the affected leg.
OA participants took fewer steps per minute while walking based on the ActivPAL and took longer to complete a timed 10-m walk, tie shoes, and sit or stand. It could be hypothesized that anticipation of pain, actual pain, and/or joint stiffness may have contributed to slower times to complete these tasks. Results of the ActivPAL cadence measure and timed 10-m walk contrast with the lack of difference in walking speed and walking cycle time on 3D motion capture. Reasons for this may be because on 3D motion capture, speed is calculated within the Visual3D (C-Motion, USA) software from heel strike to heel strike for each trial and then averaged. This represents a shorter distance with potentially more variable results, subject to a larger error margin compared with the longer 10-m walk, where differences are cumulative over the total number of gait cycles. It is also possible that cumulative fatigue and discomfort over the 1.5- to 2-hr assessment period contributed to slower walking on the timed 10-m walk, which was one of the last assessments performed. The ActivPAL data were collected over a 48-hr period of regular activity, whereas 3D motion analysis data were collected at a single time in the laboratory, making comparisons difficult. People may walk differently under laboratory conditions than when walking at their own pace, in their choice of footwear, in real-world settings. The longer period of data collection with the ActivPAL may also facilitate detection of differences.
Other studies have found walking speed and sitting/standing speed to be useful performance measures for persons with OA or other knee joint diseases.6,29–31 Consistent with our results, a previous study showed a slower sit-to-stand time in patients with knee OA compared with controls without OA.32 That study also found that OA patients lean forward, shifting their center of mass over their feet, while minimizing knee extension as they stand up, to improve stability as they leave the chair.32
Our study identified fewer differentiating outcomes between OA subjects and controls than previous 3D motion capture studies. One such study found moderate or severe OA to be associated with reduced speed, increased stance time and percentage, increased stride time, decreased stride length, reduced knee early stance flexion moments, increased knee mid-stance adduction moments, reduced peak hip adduction moments, and reduced late stance hip extension moments.25 Another study identified walking speed, stride length, hip and knee flexion, thorax obliquity, and knee adductor moments during early and terminal stance as objective parameters that differentiate persons with severe OA from persons without knee pathology.33 Our inclusion criteria for OA participants required that they have moderate OA (Kellgren–Lawrence grade 2 and 3), which may differ from other studies; also there may be differences in the type of data analyses performed and procedures used.
Differences in patient-reported outcomes were not unexpected given differences in inclusion/exclusion criteria for the two groups and the well-established effects of OA on these outcomes.2,25,34 The findings regarding patient-reported outcomes are also consistent with previous reports about the impact of OA on disability and quality of life.1,2,4 Of note, our participants with moderate OA were able to complete tasks of daily living, albeit with greater pain and impaired mobility than controls. Despite pain, it is beneficial for knee OA patients to continue to move and load their joints to ensure that essential synovial fluid receives adequate nutrition and continues to provide appropriate lubrication, and to maintain muscle strength and aid in joint stability. Effective analgesics may help restore function and quality of life for patients with knee OA and are considered appropriate according to Osteoarthritis Research Society International guidelines for non-surgical management of knee OA.35
Due to the small sample size, this exploratory study has limited power and precision. For the kinetic data, sample sizes were particularly small due to missing data and paired analysis. Given the small size of both groups, and the fact that controls were matched to OA participants for age and BMI, our study populations may not be representative of OA and general populations. In addition, we did not control for knee instability, which could be relevant as previous studies using motion analysis found that knee instability, as well as severity of OA, affected walking ability and gait.28,36 While we analyzed the primary 3D motion outcomes separately for the OA-affected and the non-affected knee, the timed functional activities were analyzed by left or right leg despite heterogeneity as to which knee was affected among OA subjects. In addition, no adjustments were made for multiple comparisons. The researchers and subjects were not blinded to the study groups (OA or control). Lack of blinding of the researchers who were involved in the data collection should, therefore, be regarded as a limitation of the study.
Due to the specificity of the inclusion criteria, we had to screen large numbers of people to recruit participants. A larger future study with greater statistical power would likely require recruitment from a wider geographical area and more extensive recruitment tools (eg, social media) to maintain these inclusion/exclusion criteria.
Conclusions
Measures that involve some aspect of speed of task execution (particularly ascending/descending stairs, tying shoes, sitting/standing), hip and knee moments while walking, trunk ROM, and strides per minute while ascending and descending stairs are useful in discriminating OA patients from participants without knee pathology and can be considered as outcomes in future OA intervention trials. If 3D motion analysis variables are used, ascending/descending stairs better distinguish OA patients from persons without knee pathology than does walking on a level surface. Timed functional tasks (10-m walk, time to tie shoelaces, time to sit and stand) and cadence (steps per minute) measured with the ActivPAL device are also representative of everyday tasks.
Acknowledgments
Writing support was provided by Peloton Advantage, an OPEN Health company, Parsippany, NJ, USA and was funded by GlaxoSmithKline Consumer Healthcare Pte, 150 Beach Road, #22-00 Gateway West, Singapore, 189720. This study was funded by GlaxoSmithKline Consumer Healthcare, 23 Rochester Park, Singapore, 139234. GlaxoSmithKline Consumer Healthcare reviewed the data report and publication, and provided funding for writing support.
Ethics approval and consent to participate
This study was approved by the National Institute for Social Care and Health Research (Research Ethics Committee) and Cardiff and Vale University Health Board. It was conducted in accordance with ethical principles delineated in the Declaration of Helsinki. All participants provided written informed consent prior to performing any study procedures.
Availability of data and materials
The dataset supporting the conclusions of this article can be requested for further research from www.clinicalstudydatarequest.com.
Abbreviations
AE, adverse event; BMI, body mass index; %BW, percent body weight; EQ-5D, European Quality of Life–5 Dimensions; EULAR, European League Against Rheumatism; KOOS, Knee Injury and Osteoarthritis Outcome Score; OA, osteoarthritis; ROM, range of motion; SD, standard deviation; WOMAC, Western Ontario and McMaster Universities.
Author contributions
All authors contributed to the design, acquisition, or data analysis; drafting and revising the article; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Dr Martina Hagen is an employee of GlaxoSmithKline Consumer Healthcare. Dr Gemma M Whatling reports grants from GlaxoSmithKline Consumer Healthcare during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–1330. doi: 10.1136/annrheumdis-2013-204763 [DOI] [PubMed] [Google Scholar]
- 2.Salaffi F, Carotti M, Stancati A, Grassi W. Health-related quality of life in older adults with symptomatic hip and knee osteoarthritis: a comparison with matched healthy controls. Aging Clin Exp Res. 2005;17(4):255–263. [DOI] [PubMed] [Google Scholar]
- 3.Dore AL, Golightly YM, Mercer VS, et al. Lower-extremity osteoarthritis and the risk of falls in a community-based longitudinal study of adults with and without osteoarthritis. Arthritis Care Res (Hoboken). 2015;67(5):633–639. doi: 10.1002/acr.22499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.London NJ, Miller LE, Block JE. Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med Hypotheses. 2011;76(6):887–892. doi: 10.1016/j.mehy.2011.02.044 [DOI] [PubMed] [Google Scholar]
- 5.Terwee CB, Mokkink LB, Steultjens MP, Dekker J. Performance-based methods for measuring the physical function of patients with osteoarthritis of the hip or knee: a systematic review of measurement properties. Rheumatology (Oxford). 2006;45(7):890–902. doi: 10.1093/rheumatology/kei267 [DOI] [PubMed] [Google Scholar]
- 6.Dobson F, Hinman RS, Hall M, Terwee CB, Roos EM, Bennell KL. Measurement properties of performance-based measures to assess physical function in hip and knee osteoarthritis: a systematic review. Osteoarthritis Cartilage. 2012;20(12):1548–1562. doi: 10.1016/j.joca.2012.08.015 [DOI] [PubMed] [Google Scholar]
- 7.Dobson F, Hinman RS, Roos EM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(8):1042–1052. doi: 10.1016/j.joca.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 8.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–199. doi: 10.1093/bmb/lds038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coggon D, Reading I, Croft P, McLaren M, Barrett D, Cooper C. Knee osteoarthritis and obesity. Int J Obes Relat Metab Disord. 2001;25(5):622–627. doi: 10.1038/sj.ijo.0801585 [DOI] [PubMed] [Google Scholar]
- 10.Holt HL, Katz JN, Reichmann WM, et al. Forecasting the burden of advanced knee osteoarthritis over a 10-year period in a cohort of 60-64 year-old US adults. Osteoarthritis Cartilage. 2011;19(1):44–50. doi: 10.1016/j.joca.2010.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)–development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88–96. doi: 10.2519/jospt.1998.28.2.88 [DOI] [PubMed] [Google Scholar]
- 12.Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS) – validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17. doi: 10.1186/1477-7525-1-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. [DOI] [PubMed] [Google Scholar]
- 14.Brazier J, Jones N, Kind P. Testing the validity of the Euroqol and comparing it with the SF-36 health survey questionnaire. Qual Life Res. 1993;2(3):169–180. [DOI] [PubMed] [Google Scholar]
- 15.Fransen M, Edmonds J. Reliability and validity of the EuroQol in patients with osteoarthritis of the knee. Rheumatology (Oxford). 1999;38(9):807–813. doi: 10.1093/rheumatology/38.9.807 [DOI] [PubMed] [Google Scholar]
- 16.Obradovic M, Lal A, Liedgens H. Validity and responsiveness of EuroQol-5 dimension (EQ-5D) versus Short Form-6 dimension (SF-6D) questionnaire in chronic pain. Health Qual Life Outcomes. 2013;11:110. doi: 10.1186/1477-7525-11-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 18.Kadaba MP, Ramakrishnan HK, Wootten ME. Measurement of lower extremity kinematics during level walking. J Orthop Res. 1990;8(3):383–392. doi: 10.1002/jor.1100080310 [DOI] [PubMed] [Google Scholar]
- 19.Whatling GM, Evans SL, Holt CA. Introducing a new staircase design to quantify healthy knee function during stair ascent and descent. Comput Methods Biomech Biomed Engin. 2010;13(3):371–378. doi: 10.1080/10255840903251296 [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Doherty M, Leeb BF, et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann Rheum Dis. 2009;68(1):8–17. doi: 10.1136/ard.2007.084772 [DOI] [PubMed] [Google Scholar]
- 21.Cole GK, Nigg BM, Ronsky JL, Yeadon MR. Application of the joint coordinate system to three-dimensional joint attitude and movement representation: a standardization proposal. J Biomech Eng. 1993;115(4a):344–349. doi: 10.1115/1.2895496 [DOI] [PubMed] [Google Scholar]
- 22.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105(2):136–144. doi: 10.1115/1.3138397 [DOI] [PubMed] [Google Scholar]
- 23.Wu G, Siegler S, Allard P, et al. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion–part I: ankle, hip, and spine. International Society of Biomechanics. J Biomech. 2002;35(4):543–548. [DOI] [PubMed] [Google Scholar]
- 24.Baker R. Pelvic angles: a mathematically rigorous definition which is consistent with a conventional clinical understanding of the terms. Gait Posture. 2001;13(1):1–6. [DOI] [PubMed] [Google Scholar]
- 25.Astephen JL, Deluzio KJ, Caldwell GE, Dunbar MJ. Biomechanical changes at the hip, knee, and ankle joints during gait are associated with knee osteoarthritis severity. J Orthop Res. 2008;26(3):332–341. doi: 10.1002/jor.20496 [DOI] [PubMed] [Google Scholar]
- 26.Costigan PA, Deluzio KJ, Wyss UP. Knee and hip kinetics during normal stair climbing. Gait Posture. 2002;16(1):31–37. [DOI] [PubMed] [Google Scholar]
- 27.van der Esch M, Steultjens MP, Harlaar J, van Den Noort JC, Knol DL, Dekker J. Lateral trunk motion and knee pain in osteoarthritis of the knee: a cross-sectional study. BMC Musculoskelet Disord. 2011;12:141. doi: 10.1186/1471-2474-12-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt MA, Wrigley TV, Hinman RS, Bennell KL. Individuals with severe knee osteoarthritis (OA) exhibit altered proximal walking mechanics compared with individuals with less severe OA and those without knee pain. Arthritis Care Res (Hoboken). 2010;62(10):1426–1432. doi: 10.1002/acr.20248 [DOI] [PubMed] [Google Scholar]
- 29.Turcot K, Armand S, Fritschy D, Hoffmeyer P, Suva D. Sit-to-stand alterations in advanced knee osteoarthritis. Gait Posture. 2012;36(1):68–72. doi: 10.1016/j.gaitpost.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 30.Kobsar D, Osis ST, Hettinga BA, Ferber R. Gait biomechanics and patient-reported function as predictors of response to a hip strengthening exercise intervention in patients with knee osteoarthritis. PLoS One. 2015;10(10):e0139923. doi: 10.1371/journal.pone.0139923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stauffer RN, Chao EY, Gyory AN. Biomechanical gait analysis of the diseased knee joint. Clin Orthop Relat Res. 1977;Jul-Aug(126):246–255. [PubMed] [Google Scholar]
- 32.Anan M, Shinkoda K, Suzuki K, Yagi M, Ibara T, Kito N. Do patients with knee osteoarthritis perform sit-to-stand motion efficiently? Gait Posture. 2015;41(2):488–492. doi: 10.1016/j.gaitpost.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 33.Sagawa Y Jr., Armand S, Lubbeke A, et al. Associations between gait and clinical parameters in patients with severe knee osteoarthritis: a multiple correspondence analysis. Clin Biomech (Bristol, Avon). 2013;28(3):299–305. doi: 10.1016/j.clinbiomech.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 34.Breedveld FC. Osteoarthritis–the impact of a serious disease. Rheumatology (Oxford). 2004;43(Suppl 1):i4–8. doi: 10.1093/rheumatology/keh102 [DOI] [PubMed] [Google Scholar]
- 35.McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–388. doi: 10.1016/j.joca.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 36.Farrokhi S, O’Connell M, Gil AB, Sparto PJ, Fitzgerald GK. Altered gait characteristics in individuals with knee osteoarthritis and self-reported knee instability. J Orthop Sports Phys Ther. 2015;45(5):351–359. doi: 10.2519/jospt.2015.5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article can be requested for further research from www.clinicalstudydatarequest.com.