Abstract
Background
Arterial hypertension (HT) is common in the Russian adult population, with half of affected individuals inadequately controlled. Low adherence to medication seems likely to be a factor. We report a scoping review of studies on adherence to antihypertensive therapy (AHT) in Russia to determine the extent of research undertaken, the frequency of adherence among adults diagnosed with HT, methodologies used in the studies, and their ability to describe determinants of adherence.
Methods
A scoping review of published studies that have assessed adherence to AHT in Russian HT patients searched the main Russian and international electronic databases eLIBRARY.ru, Russian Medicine, Embase, MEDLINE for full-text reports published in the Russian language between 2000 and 2017. The last search was on November 28, 2017. Among 520 reports identified, 31 were included in the review.
Results
Eighteen studies assessed adherence using the 4-item Morisky Medication Adherence Scale (MMAS-4); others used bespoke questionnaires or pill counts. 25 studies assessed levels of adherence, 11 examined its determinants, and 18 examined intervention strategies. The proportion of “adherent” patients varied from 11 to 44% using the MMAS-4, from 23 to 74% when using bespoke questionnaires, and from 5 to 43% when using pill counts. Adherence was associated with sociodemographic factors, access to free drugs provided through the Medicine Assistance Scheme (MAS), use of home blood pressure (BP) monitoring, anxiety, and comorbidity. There was no evidence that adherence was associated with income or physical activity. Evidence of an association between MAS, grade of HT, or experience of hypertensive crisis was inconclusive. Various methods to improve adherence were studied including patient education (improved from 1.8 to 3.9 points, p = 0.0002 or 2.80 to 3.79 points, p < 0.0001 measured by the MMAS-4), telephone reminders (p < 0.0001), training in home BP monitoring (p < 0.05), and use of fixed-dose combinations (p < 0.05).
Conclusions
The main determinants of adherence to AHT are sociodemographic characteristics, the severity of HT, and presence of comorbidity. Patient education and use of fixed-dose combinations of drugs were identified as most important for improving adherence. Most studies assessing adherence use self-reported methods so there is a need for greater use of objective methods.
Trial registration
This scoping review has not been registered.
Electronic supplementary material
The online version of this article (10.1186/s13690-019-0366-9) contains supplementary material, which is available to authorized users.
Keywords: Hypertension, Medication adherence, Scoping review
Background
Russia has one of the highest mortality rates from circulatory diseases in the world. In 2015 the age-standardized death rate was 368.8 per 100.000 [1], 2.5–4 times higher than in West European countries [1]. Arterial hypertension (HT) is among the main risk factors [2], affecting an estimated 44% of the Russian adult population [3], with only 53% of those with HT being controlled [3].
One reason for poor blood pressure (BP) control is thought to be inadequate adherence to treatment, [4] a substantial problem everywhere. A review of 21 clinical studies conducted outside Russia found that adherence to antihypertensive therapy (AHT) falls with time from diagnosis, with about half of patients discontinuing treatment after one year [5]. This poor adherence is, as expected, associated with treatment failure and adverse cardiovascular events [6] while good adherence has been linked to fewer adverse cardiovascular outcomes [7].
The scale of the problem means that much research has been undertaken to identify factors associated with poor adherence and to develop measures to improve it. However, while some of the conclusions from this work are generalizable across countries, it is important to take account of context, as there may be differences in health beliefs (such as understanding of the importance of continuing treatment indefinitely for an asymptomatic condition), health systems (such as how medicines are paid for, and other circumstances). Moreover, context-specific evidence is more likely to be accepted by national policy makers.
Here we report a scoping review of all Russian language studies presented in full-text reports on the problem of adherence to antihypertensive medication in the Russian population, the factors associated with adherence, interventions to improve adherence to treatment, and their effectiveness. This makes two distinct contributions. First, it provides the most detailed and comprehensive overview of what is known from the published literature about this important issue in Russia, summarizing the often neglected corpus of work published in the Russian language. Second, we have summarized the results of research in the quantitative indicators and described of the revealed patterns.
As many readers will be unfamiliar with the Russian health system, we summarize the key elements of medicines supplies in Table 1.
Table 1.
Pharmaceuticals in the Russian health system
| State medical institutions in the Russian Federation provide free medical treatment to all in-patients but, after discharge, patients must pay the full cost unless they are in one of the groups entitled to free medications or at a 50% discount, as set out in a law from 1994. These include children in large families who are under a certain age (3 or 6 depending on family size), those receiving the minimum pension, invalids, veterans of the Great Patriotic War and other military operations, and those involved in the Chernobyl disaster. Entitlement extends to immediate family members. Since 2008, those in these categories can choose an alternative, whereby they receive monetary benefits instead. In practice, a growing number of the 19 million potential beneficiaries choose monetary benefits, leaving less than 4 million receiving. This can be explained by how free and subsidized medicines are available only in certain pharmacies in specific medical institutions and a widespread belief that essential drugs are often unavailable in these pharmacies. Those choosing monetary compensation can thus obtain their medicines from private pharmacies, albeit at additional cost. A recent study of medicines availability and affordability in state and private pharmacies in six Russian citizens did, however, find that common cardiovascular medicines were widely available and, in private pharmacies, reasonably affordable. However, where state pharmacies stocked generic versions, they did not also stock branded equivalents [8]. | |
| Information on prescribing for hypertension in Russia can be found in the RELIF III study. The most frequent classes of drugs were angiotensin-converting-enzyme inhibitors (78%), diuretics (40%), beta-blockers (36%), and calcium antagonists (19%). The authors reported that angiotensin-converting-enzyme inhibitors were more likely to be taken regularly, specifically Prestarium, Renitec, and Hartil [9]. |
Research questions
We address the following questions by means of a scoping review of the Russian language literature pertaining to studies of adherence to antihypertensive medications conducted in Russia:
What levels of adherence are found among adults diagnosed as hypertensive?
What sociodemographic and clinical factors are associated with adherence?
What robust evidence has been generated as to effective interventions used in Russia to increase adherence to treatment?
Methods
This scoping review was reported in accordance with the reporting guidance provided in the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement extension for Scoping Reviews (PRISMA-ScR) [10] (Additional file 1).
ScR protocol was not published.
Eligibility criteria
Reports were included in this scoping review if they met the following criteria:
Target population included the Russian adult population aged 18 years and over with a diagnosis of HT defined as BP ≥140/90 mmHg and/or taking regular antihypertensive medication;
Articles that report on adherence to AHT, regardless of how adherence is measured;
Types of study designs - randomized controlled trial studies, non-randomized trial studies, observational studies (cross-sectional, cohort studies), registers;
Reports published in the Russian language;
Full-text original reports;
Published in journals or proceedings of conferences;
Literature published from January 2000 to November 2017. The last search was conducted in November 28, 2017.
There was no restriction on duration of treatment or sample size. There were no restrictions on interventions, comparators, and outcomes.
Information sources
Searches of the main Russian and international electronic databases were complemented by iterative searches using Internet search engines, personal contact with Russian experts working on HT, and queries to authors of identified studies by phone or e-mail (also used where clarification was sought about survey methods, instruments (such as the name of the questionnaire) and duration of observation). Reference lists were also searched. This comprehensive approach was taken to reduce potential bias by including only easy-to-locate studies that may have larger effect sizes due to publication bias.
The Russian databases were:
eLIBRARY.ru - the largest Russian information portal (https://elibrary.ru/)
Central Scientific Medical Library “Russian Medicine” - second in size Russian medical information portal (http://www.scsml.rssi.ru/)
The international databases were:
Embase (https://www.elsevier.com/solutions/embase-biomedical-research)
MEDLINE (PubMed) (https://www.ncbi.nlm.nih.gov/pubmed)
The search for sources was conducted between October 2017 and November 2017 (last date searched).
For eLIBRARY.ru 333 publications were obtained by October 17, 2017.
For Russian Medicine 69 publications were obtained by October 20, 2017.
For Embase 87 publications were obtained by November 10, 2017.
For MEDLINE (PubMed) 31 publications were obtained by November 28, 2017.
The personal contacts with authors to identify additional sources were conducted from December 2017 to January 2018.
If more than one publication related to the same study, all were used to provide as much information as possible.
Full search strategies specific to the different databases are provided in Additional file 2.
Study selection
Having eliminated duplicates, titles and abstracts were reviewed by 2 researchers to assess eligibility, with differences resolved by discussion or, where necessary, by consultation with a third team member. Those not available electronically were obtained as hard copies. Those potentially eligible were read by each researcher to confirm eligibility and those retained were categorized to covering one or both of the following two areas:
Levels, patterns and determinants of adherence
Interventions to improve adherence, including evaluations of effectiveness
Each paper was read three times by a different team member who extracted the key findings.
Data charting process
Authors created a matrix (an Excel spreadsheet) to chart relevant information about all the sources reviewed. Specifically, the chart included details about the authors, year of publication, study setting, population/participant selection criteria, study design, sample size, age of participants, HT grade, nature of intervention, adherence measure, factor associated with adherence and main results (Table 2, Additional files 3, 4 and 5). Matrix was piloted with five papers and adapted in the light of this experience.
Table 2.
Characteristics of included studies on adherence to antihypertensive therapy in adult population in Russia from 2000 to 2017
| Reference | Year of publication | Study setting | Participant selection criteria | Design | Sample size | Age (years) | HT gradea | Critical appraisal/quality assessment of findings (a) Strengths (b) Weaknesses |
|---|---|---|---|---|---|---|---|---|
| Ageev et al. [11] | 2008 | Patients who visited outpatient department of the Russian Cardiology Scientific and Production Center |
Men or women older than 18, with SBP 140–179 mmHg, DBP 99–100 mmHg, high cardiovascular risk, not taking of ACE inhibitors and diuretics, without secondary HT, heart failure, renal and hepatic impairment, insulin-treated DM. Recruitment process not described |
Randomized non-blinded controlled intervention study | 60 | 62.5 ± 2.2 | 1–3 |
(a) prospective study, follow up period 6 mth; (b) small sample size, incorrect DBP level in inclusion criteria |
| Kobalava et al. [12, 13] | 2011 | Patients attending 240 cardiologists in 17 Regions | Men or women with uncontrolled HT, non-adherent, absence of contraindications to ACE inhibitors taking, no eligibility to receive MAS | Randomized non-blinded controlled intervention study | 906 | 56.2 ± 10.6 (female)/ 54.9 ± 10.9 (male) | Uncontrolled HTb |
(a) multicenter study, follow up period 12 mth, big sample size; (b) including only non-adherent patients |
| Sarycheva et al. [14] | 2017 |
Single outpatient clinic in Moscow Region. 300 patients have been examined before 150 patients included |
Men or women aged 40–65, with ineffective treatment of HT and dyslipidemia, SBP > 140 mmHg, DBP > 90 mmHg, without IHD, DM and other severe diseases | Randomized non-blinded controlled intervention study | 150 | 40-65y | HT patients with high cardiovascular risk |
(a) follow up period 12 mth; (b) there are no basic data of adherence |
| Fofanova et al. [15] | 2008 | Patients who visited outpatient department of the Russian Cardiology Scientific and Production Center | Men or women older than 18, with SBP 140–179 mmHg, DBP 99–100 mmHg, not taking of ACE inhibitors and diuretics, without secondary HT, heart failure, renal and hepatic impairment, insulin-treated DM. Recruitment process not described | Randomized non-blinded controlled intervention study | 60 |
61.2 ± 1.8 (female)/ 61.8 ± 2.1 (male) |
1–2 |
(a) patients with high and very high cardiovascular risk are included, for which adherence to therapy is particularly important, follow up period 6 mth; (b) small sample size, incorrect DBP level in inclusion criteria |
| Karpov et al. [16] | 2013 | Patients attending any of 700 cardiologists in 51 Regions, each recruiting 3 patients | Men or women older than 18, with uncontrolled HT on treatment. Recruitment process not described | Prospective observational intervention study | 2120 | 22–88 y | 2–3 |
(a) big sample size, multicenter study; (b) relatively short follow up period 3 mth and no control group |
| Glezer et al. [17] | 2016 | Patients attending 197 physicians in 48 Regions | Men or women aged 18–79, with essential HT, SBP ≥140 mmHg, DBP ≥90, but <110 mmHg | Prospective observational intervention study | 940 | 56.5 ± 11.5 | 1–2 |
(a) big sample size, multicenter study; (b) relatively short follow up period 3 mth, no control group |
| Glezer et al. [18] | 2015 | Patients attending 243 physicians in 51 Regions | Men or women older than 18, with HT taking 2 or more antihypertensive drugs who have not reached their BP target, SBP 140–179 mmHg, DBP 90–109 mmHg, without contraindications to ACE inhibitors and calcium channel blockers | Prospective observational intervention study |
1351 included, 1061 completed the protocol |
59.4 ± 11.1 | Essential HT |
(a) big sample size, multicenter study; (b) relatively short follow up period 3 mth, no control group |
| Glezer et al. [19] | 2016 | Patients attending 442 physicians in 29 cities | Men or women older than 18, with HT on treatment who have not reached their BP target | Prospective observational intervention study | 1969 | 60.1 ± 0.3 | No data |
(a) big sample size, multicenter study; (b) relatively short follow up period 3 mth, no control group |
| Kagramanyan [20] | 2015 |
Not stated The author is affiliation at Yaroslavl State Medical University |
Men or women aged 18–80, with grades 1–3 of HT, who visited the Municipal Clinical Hospital | Prospective observational intervention study | 50 |
64.06 ± 0.49 (female)/ 61.88 ± 1.28 (male) |
1–3 |
(a) studying of adherence in patients with 3 different socially significant nosologies - HT, asthma and alcohol abuse; (b) small sample size, large age range, the real number of HT patients is represented incorrectly |
| Kaskaeva et al. [21] | 2015 | Not stated | Male patients aged 20–64 with grades 1–3 of HT. Recruitment process not described | Non-randomized comparison of 3 groups | 250 | 20–64 y (male) | 1–3 |
(a) patients of employable age + relationship adherence to job; (b) described as randomized but groups selected on basis of employment: train drivers (112), other railway workers (50), non-railway workers (88) |
| Ushakova et al. [22] | 2005 | Regional cardiology clinic in Ivanovo city | Men or women with grade 2 of HT on treatment, without IHD and DM | Prospective observational intervention study | 52 | 50.08 ± 7.25 | 2 | (b) small sample size, no control group, patients with grade 2 of HT only included |
| Chazova et al. [23] | 2014 | Patients who visited outpatient department of the Russian Cardiology Scientific and Production Center | Recruitment process not described | Prospective observational intervention study | 193 | 60.3 ± 8.0 | No data |
(a) scope of sessions with patients, duration of sessions and number of the studying patients in group corresponded to the standards approved by the Ministry of Health, it is important for working at outpatient care settings; (b) the control group is formed from abandoning the patient education, the number of patients in the control group is 2 times less than in the intervention group (65:128), short follow up period 6 weeks |
| Fofanova et al. [24] | 2009 | Patients attending 185 cardiologists in 84 policlinics of Moscow | Men or women with SBP 140–179 or DBP 99–100 mmHg, not taking calcium channel blockers | Cross-sectional | 4816 | 62.2 ± 0.2 | 1–2 |
(a) big sample size; (b) incorrect DBP level in inclusion criteria, only possible to extract baseline data |
| Donirova et al. [25] | 2012 | Ambulatory care facility | Men or women with HT on treatment | Cross-sectional | 74 | 18 y and older | No data | (b) small sample size (14 vs 60) |
| Loukianov et al. [26] | 2017 | Patients attending 185 physicians or cardiologists of the same from 3 randomly selected outpatient clinics of Ryazan and the Ryazan region in March–May 2012 (consecutive inclusion of all who applied from March 01 to May 27) | Patients older than 18, with combination of IHD, HT, chronic heart failure, permanent residence in the Ryazan and the Ryazan region | Register | 2303 |
70.3 ± 10.7 (ppl with history of MI), 69.9 ± 11.0 (ppl without history of MI) |
1–3 |
(a) collection of adherence data using MMAS-4 in a large outpatient register (b) all patients, irrespective of history of MI, had complex pathology of IHD, HT and chronic heart failure. Therefore it is impossible to estimate independent association between HT and adherence. |
| Fofanova et al. [27] | 2014 | Patients who visited outpatient department of the Russian Cardiology Scientific and Production Center | Men and women with HT and examined by psychiatrists | Cross-sectional | 161 |
19–75 (female)/ 53.4 ± 11.4 (male) |
1 |
(a) assessment of adherence and psychosomatic aspects; (b) groups selected on basis of adherence to treatment: low adh – 131 ppl, high adh – 30 ppl |
| Soboleva et al. [28] | 2012 | Regional clinical hospital and ambulatory care facility | Patients with grades 1–3 of HT and cardiovascular disease. Recruitment process not described. | Cross-sectional | 242 | 18 y and older | 1–3 | (b) only possible to extract baseline data |
| Oganov et al. [29] | 2007 | Patients attending 512 physicians in 20 cities | Men or women with HT and/or IHD | Cross-sectional | 2496 | 18 y and older | 1–3 |
(a) big sample size; (b) no prospective stage |
| Olejnikov et al. [30] | 2014 |
Not stated The authors are affiliation at Penza State Medical University |
Men or women older than 60, with grades 1–2 of HT. Recruitment process not described |
Cross-sectional | 75 | 66.6 ± 4.7 | 1–2 |
(a) studying adherence in the elderly; (b) non-standard way of MMAS-4 analyze, small sample size, only possible to extract baseline data |
| Smirnova et al. [31] | 2012 | Ambulatory care facility | Patients aged 45–75, with grades 1–2 of HT. Recruitment process not described | Randomized non-blinded controlled intervention study | 60 | Intervention group: 62 ± 9.4, control group: 63 ± 8.9 | 1–2 |
(a) complex intervention on adherence; (b) small sample size, relatively short follow up period – 3 mth |
| Vologdina et al. [32] | 2009 | Not stated | Men and women with IHD and grades 1–2 of HT. Recruitment process not described | Randomized non-blinded controlled by closed envelope method | 70 |
80.7 ± 2.7 (female)/ 80.3 ± 2.5 (male) |
1–2 |
(a) studying adherence in the elderly; (b) small sample size, relatively short follow up period – 3 mth |
| Sviryaev et al. [33] | 2006 | Ambulatory care facility | Men or women older than 18, with grades 1–2 of HT with irregular therapy | Prospective observational intervention study | 115 | 51.3 ± 9.6 | 1–2 |
(a) follow up period 6 mth; (b) no control group, numerical indicators of adherence level aren’t presented in the publication |
| Morozov et al. [34] | 2010 | The authors are affiliation at Russian military medical Academy, St. Petersburg | Patients with grades 1–2 of HT | Cross-sectional | 86 | 30–73 y (54 ± 4,8) | 1–2 | (b) only possible to extract baseline data, non-standard way of MMAS-4 analyze |
| Kotovskaya et al. [35] | 2015 | Patients attending 830 physicians in 113 cities | Men or women older than 18, with uncontrolled HT taking ACE inhibitors or angiotensin receptor blockers | Prospective observational intervention study | 2435 | 59.3 ± 11.2 | Uncontrolled HTb |
(a) big sample size, multicenter; (b) MMAS modified with 2 additional questions, no control group, relatively short follow up period – 3 mth |
| Panov et al. [36] | 2015 | Federal Medical Research Center, St. Petersburg | Patients with grades 1–2 of HT and IHD | Prospective observational intervention study | 60 | 57.65 ± 1.59 | 1–2 |
(a) follow up period - 12 mth; (b) small sample size |
| Oschepkova et al. [37] | 2004 | Patients who visited outpatient department of the Russian Cardiology Scientific and Production Center | Men and women aged 30–71, with grades 1–2 of HT, without MI, stroke, heart failure, heart arrhythmias. Recruitment process not described | Randomized non-blinded controlled intervention study | 30 | 54 ± 11 | 1–2 |
(a) home BP devices as a way to increase adherence; (b) described as randomized but main group – 19 ppl, control group − 11, small sample size |
| Kontsevaya et al. [38, 39] | 2015 | Patients who visited Outpatient Cardiology Clinic | Men or women with grades 1–3 of HT | Cross-sectional | 1419 | 61.94 ± 0.26 | 1–3 |
(a) big sample size, a large number of factors associated with adherence: sociodemographic, clinical, etc.; (b) no prospective stage |
| Kopnina et al. [40] | 2008 | Not stated | Patients with HT. Recruitment process not described | Cross-sectional | 30 | 51 ± 1.14 (female) | 2 | (b) small sample size, only women are included in the study |
| Sergeeva et al. [41] | 2012 | Patients of the cardiological and endocrinological department of the Regional Clinical Hospital |
Men and women with HT or HT + DM. Recruitment process not described |
Cross-sectional | 190 |
With HT: 47.6 ± 0.4, with HT + DM: 44.7 ± 0.2 |
1–3 |
(a) association of adherence with hypertensive crisis was shown; (b) no data on validation of bespoke questionnaire |
ACE inhibitors, angiotensin converting enzyme inhibitors, CVD cardiovascular diseases, DBP diastolic blood pressure, DM diabetes mellitus, HT arterial hypertension, IHD ischemic heart disease, MAS Medicine Assistance Scheme, MI myocardial infarction, MMAS-4 4-item Morisky Medication Adherence Scale, mth months, ppl people, SBP systolic blood pressure
a Definitions of office blood pressure levels (mmHg): grade 1 hypertension: 140–159 and/or 90–99; grade 2 hypertension: 160–179 and/or 100–109; grade 3 hypertension: ≥180 and/or ≥ 110
b Uncontrolled HT was defined with patients not taking a previously prescribed therapy, registered in the medical records or insufficiently effective therapy
Data items
A range of variables were extracted from reports of the studies.
Article details such as title, first author, date of publication, year of distribution, publication status, region(s) in which the study was conducted, and institutional setting.
Study design: randomized or non-randomized trial, cohort study, case-control study, register-based. If the design was a trial, we collected additional information on allocation concealment. Study objective, study duration, and sample sizes were also extracted.
Definitions of adherence: type of adherence measure: 4-item Morisky Medication Adherence Scale (MMAS-4), pill counts, bespoke questionnaire, etc.; indicators of adherence: MMAS-4 score points, percentage achieving a score of 4, pill counts compliance, and percentage of adherent people etc. (Additional file 3).
Determinants of adherence were extracted. These included baseline sociodemographic characteristics of HT patients: sex, age, education level, marital status, employment status, income, living in a city; disability; clinical characteristics of patients: HT grade, duration of HT, physical activity; associated clinical conditions (for example, ischemic heart disease, history of myocardial infarction, hypertensive crisis, etc.); concomitant diseases (for example, diabetes mellitus, panic attacks, subclinical depression, etc.); other data on instruments used in surveys; pharmacological therapy: therapeutic category, INN and commercial name, drug administration schedule, dose, dosage form; eligibility for the Medicine Assistance Scheme (MAS) which provides free drugs for certain categories of patients; home BP device availability; and frequency visits to the doctor (Additional file 4).
Intervention characteristics
Examples of content extracted included specific strategies to address barriers to adherence: special packaging of medications (e.g. blister packs, pill boxes), amount of prescribed medications, eligibility for the MAS, interventions designed to improve communication with patients, including more frequent visits, motivational interviewing, patient education, home BP monitoring, and provision of written instructions etc. (Additional file 5); mode of delivery: face-to-face, telephone, internet etc.; professions involved: pharmacist, physician, etc.; duration and number of sessions/consultations; and any other types of interventions in the experimental group;
Other data recorded included the main results, BP dynamics, achievement of target BP levels, author’s conclusions, and any reason for excluding the article.
Study quality assessment
In assessing the quality of study, we considered use of validated questionnaires, objective methods for adherence assessing, study design, presence of randomization, blinding, sample size, and follow-up as appropriate.
Synthesis of results
We grouped the studies by study questions analyzed (adherence, determinants and interventions), and summarized the type of settings, populations and study designs for each question, along with the measures used and a summary of findings. The results of this scoping review were synthesized using both a numerical summary, outlining relevant data from the included studies, and a narrative synthesis interpreting the results.
Results
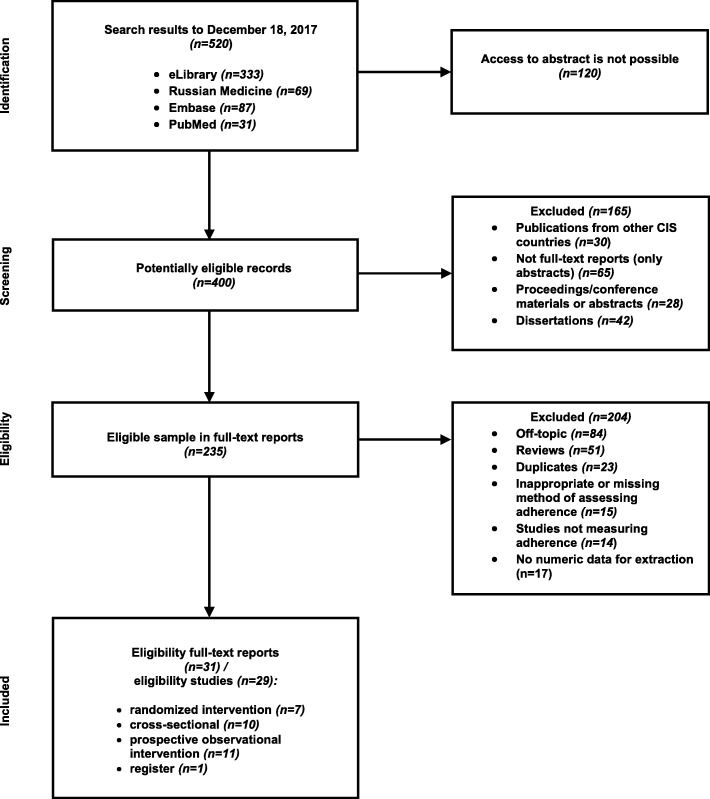
The initial search identified 520 references (Fig. 1). Of these 120 were excluded because it was not possible to obtain the abstract despite exhaustive searches. Most of these were published in regional journals unavailable in electronic format, with only tables of contents available online, printed in individual regions in small numbers.
Fig. 1.
Flow diagram indicating the study selection process on adherence to antihypertensive therapy in adult population in Russia from 2000 to 2017
The remaining 400 were screened against the inclusion criteria. The 235 potentially relevant reports were reviewed as full texts (eight were not available electronically, but three could be obtained as hard copies, leaving 5, from local journals that could not be obtained), leaving 31 eligible reports included in the review. The results from 2 studies were presented in 2 separate publications, resulting in us considering data from 29 individual studies.
The characteristics of the studies are presented in Table 2. Overall we included seven randomized intervention studies, ten cross-sectional studies, eleven prospective observational intervention studies (including six multicenter studies), and one report based on data from a register. None of the randomized studies were blinded. In four of the prospective observational intervention studies, it was only possible to extract baseline data as follow up data were not reported [24, 28, 30, 34]. Results from these studies were transformed into cross-sectional data.
The total number of patients in the studies selected for analysis was 23,127, with individual studies size ranging from 30 to 4816 participants.
The duration of follow-up varied from 6 weeks to 48 months.
Adherence measurement
Adherence to AHT was assessed using MMAS-4 in 18 of the 29 studies. Ten studies used a bespoke questionnaire or a single question about regularity of taking drugs that was included in a questionnaire on a wider range of issues, supplemented with 3 clarifying questions. The bespoke instruments included pill counts in 6 studies; the MMAS-4 but analyzed in a non-standard way in 1 study [34], and the Morisky-Green questionnaire, modified with 2 additional questions in one study [35]. Several studies used more than one method, for example MMAS-4 and pill counts.
No studies used objective methods of assessing adherence - measurement of drugs in biological fluids in blood, urine, or indirect methods - electronic dispensers. The bespoke questionnaires were only available in Russian and there was no information about whether they had been validated.
Adherence was assessed using data from cross-sectional surveys and from baseline data in prospective observational intervention studies. In studies using the MMAS-4 [11–19, 21, 23–27, 30, 33] adherence to AHT varied from 1.62 ± 0.27 [11] to 3.12 ± 0.86 points [35] out of a maximum of four (Additional file 3). Among these studies, the highest rates of adherence were found in post-marketing studies, all with large sample sizes. Scores were 2.8 [19], 2.78 [16], 2.95 [18] and 3.12 ± 0.86 points [35]. In the studies reporting baseline adherence, the highest rates were in those that included a high proportion of patients with concomitant diseases: ischemic heart disease, chronic kidney disease, stroke, transient ischemic attack, diabetes mellitus, etc. [29], who can be expected to have a strong motivation to adhere to medication. The lowest baseline adherence rates, with scores of 1.62 [11] and 1.7 [31] were observed in studies with a small number of patients who only had HT.
Eleven studies measured the proportion of “adherent” patients, i.e. reporting 4 points on the MMAS-4. Results varied, from 11.1% [15] to 44.2% [35]. The highest proportion of “adherent” patients was again noted in a study where many subjects had concomitant diseases [35].
Adherence, as measured by bespoke questionnaires was reported in eight out of nine papers [20, 22, 28, 29, 37–41], one of the studies being published in two papers [38, 39], three studies reporting baseline adherence [20, 22, 37]. In two studies, 38.5 and 74.9% of patients took AHT daily [22, 38], in another 56% fully complied with medical recommendations, including the use of antihypertensive drugs [28], “high adherent”, “sufficiently adherent” or “regularly taken” according to the criteria set by the researchers varied from 23.3 to 60.5% [20, 29, 37, 40, 41]. However, these findings are difficult to compare with those from other studies because of lack of information on the instruments used.
Adherence using pill counts was examined in five studies [31–34, 36] but baseline adherence rates were reported in only 3, ranging from 5 [31] to 43% [32]. This large difference may reflect the small sample sized (60 and 115).
Factors associated with adherence to antihypertensive therapy
12 studies examined associations between adherence and various socio-demographic, clinical and other variables (access to the MAS, home BP monitoring, frequency of visits to a doctor, etc.). Higher adherence was associated with female gender [29, 33, 35, 38, 41], age over 50 years [29, 33], not living alone [29, 38], employment [27, 33], higher education [33], and living in a city [41] (Additional file 4).
Higher adherence was associated with comorbidity, including: ischemic heart disease [24, 29, 38], history of myocardial infarction [26, 29], arrhythmias [27], diabetes mellitus [24, 29, 35], and psychiatric disorders [27]. High adherence was also associated with onset of HT at a young age [27] and use of fixed dose combination therapy [38].
The findings of studies of the association between adherence and grade of HT, experience of hypertensive crisis, and duration of HT were not consistent [27, 29, 33, 38–41].
There was no evidence that adherence was associated with income and physical activity [29].
Features of patient management in outpatient settings and adherence to therapy
Home BP monitoring [24, 29, 31] and more frequent visits to the doctor [34] were associated with better adherence. The association with eligibility for the MAS was also conflicting. In two studies [24, 39] it was associated with lower adherence [24] or failure to follow the recommended regimen [39] but in another, there was no association [38].
Taking multiple antihypertensive drugs (2 or more) was associated with decreased adherence [35, 39].
Assessment the effectiveness of interventions aimed at increasing adherence
Interventions to increase adherence were mainly either patient education (in various forms) in 6 studies [12, 13, 19–23] or optimization of the drug administration regimen in 11 studies [11–13, 15–19, 32, 33, 35, 36], among which 9 used a fixed-dose combination of drugs [11, 15, 16, 18, 19, 32, 33, 35, 36]. One study gave patients an electronic version of the SCORE scale, with the physician showing the patient how their risk would be reduced if they stopped smoking, controlled their BP and reduced their cholesterol [14]. In two studies [31, 37] patients were trained in home BP monitoring, intended to increase adherence.
Several studies used more than one method to increase adherence.
Randomized controlled intervention studies
In one study [12], a multi-faceted intervention, which included information within an educational program for patients, a free first package of antihypertensive drugs, regular visits to the doctor, and telephone reminders, was associated with a significant improvement in adherence. After 12 months, the proportion of adherent patients who achieved an MMAS-4 of 4 points in the intervention group was 71.7%, up from 52.2%, in the control group. The difference at follow up was highly significant (p < 0.0001) (Additional file 5). Demonstration of an electronic version of the SCORE scale to patients, highlighting benefits of reducing cardiovascular risk by smoking cessation, BP control, and reduced cholesterol, was associated with higher adherence than in a control group, with scores at 6 months of 2.75 and 1.88 points (p < 0.001), respectively, and 2.14 and 1.27 points (p < 0.001), respectively after 12 months [14].
Training patients in home BP monitoring was associated with significant improvement in adherence, with the effect persisting at 1 year [37].
Provision of an automatic BP monitor was associated with a significant increase in adherence to AHT, with a MMAS-4 score increasing from 1.7 ± 1.2 to 3.0 ± 1.1 points, p < 0.000 in the intervention group and from 5 to 96.4% (p < 0.001) in the index of compliance [31].
The use of fixed-dose combinations was associated with better adherence to therapy in several studies [11, 15, 16, 18, 19, 32, 33, 35, 36], including 3 randomized non-blinded controlled trials [11, 15, 32]. Two of these used the MMAS-4 [11, 15] and the other a compliance index [32].
Non-randomized intervention studies
Five studies evaluated so-called “patient education” or similar interventions [19–23]. Those using the MMAS-4 reported increases from 2.80 to 3.79 points (p < 0.0001) [19] or from 1.8 to 3.9 points (p = 0.0002) [23], while the proportion of patients with an MMAS-4 of 4 points increased from 38.6 to 57.9% (p = 0.04) [21]. Adherence, as measured by a bespoke questionnaire improved from 27 to 67% (p < 0.05) [20]. The proportion of patients who reported measuring their BP daily increased from 28.8 to 65.4% (p < 0.05) and taking antihypertensive drugs daily increased from 38.5 to 82.7% (p < 0.05) [22].
The use of fixed-dose combinations of antihypertensive drugs was evaluated in 7 non-randomized studies [16–19, 33, 35, 36]. As in the randomized trial studies, fixed-dose combinations were associated with significant increases in adherence, as measured by the MMAS-4 compared to baseline [16–19, 33, 35].
Using bespoke questionnaires, consistently high adherence was observed during the first year of treatment with a fixed-dose combination of an angiotensin converting enzyme inhibitor and calcium channel blocker, at 97 and 93% at 6 and 12 months respectively [36].
Critical appraisal/study quality assessment
Problems included the small number of randomized studies none of which were blinded, heterogeneity of patient groups in non-randomized studies, small sample size in some studies, heterogeneity of samples, presence of concomitant pathology, use of subjective methods of assessing adherence (questionnaires), and incomplete presentation or selective reporting of results [42].
Discussion
This is the first scoping review of Russian language studies on adherence to AHT. Our search strategy was designed to include as many primary publications as possible, although it was concerning that abstracts for a large number of studies could not be located. This highlights an issue that has not, to our knowledge, received adequate attention so far. There are a large number of regional medical journals, printed in small numbers, and while their tables of contents are available electronically, their content (including abstracts) is not. There is no central repository. While, in theory, it might be possible to obtain copies from publishers, the logistical barriers would be formidable and, given the methodological weaknesses reporting in many of the papers obtained, unlikely to be commensurate with the information that might be extracted from them.
In accordance with the questions and objectives in this scoping review, the key findings are as follows. Adherence was assessed using MMAS-4 in 18 studies and in other studies using bespoke questionnaires. In the Russian population, the baseline MMAS-4 scores varied from 1.62 ± 0.27 points [11] to 3.12 ± 0.86 points [35]. The proportion of patients with 4 points on the MMAS-4 varied from 11.1% [15] to 44.2% [35], while in studies using a bespoke questionnaire, the frequency of adherence varied from 23.3% [40] to 74.9% [38]. The latter were patients attending an outpatient cardiology clinic. Relatively low levels of adherence were observed in most studies [11, 20, 22, 23, 31, 32, 37, 40, 41]. These findings are consistent with a cross-sectional study by Cybulsky et al. on 1068 working-age men in Izhevsk, Russia, which reported 41% of patients taking antihypertensive drugs daily [43].
Many studies included quite large samples, from several hundred to several thousand people [12, 13, 16–19, 24, 26, 29, 35, 38, 39], but some were much smaller, from 30 to 75 people [11, 15, 20, 22, 25, 30–32, 36, 37, 40]. The follow up period varied from 6 weeks [23] to 12 months [12, 13, 36, 37]. One study lasted 4 years [16], but follow up data were unavailable for analysis.
Studies of determinants of adherence identified being female [29, 33, 35, 38, 41], age over 50 years [29, 33], not living alone [29, 38], employment [27, 33], higher education [33], and living in a city [41], comorbidity including: ischemic heart disease [24, 29, 38], history of myocardial infarction [26, 29], arrhythmias [27], and diabetes mellitus [24, 29, 35]. These are consistent with previous systematic review of studies from elsewhere [44]. Data of association with anxiety level, panic attacks and subclinical depression [19] differ from those in the publication [44].
Evidence for an association between eligibility for the MAS, grade of HT, and experience of hypertensive crisis was inconclusive [24, 27, 29, 33, 38–41]. In two [29, 40], patients with a higher BP were more adherent and in another two [27, 33] better adherence was observed in patients with a lower grade of HT. In one [41], patients who had experienced a hypertensive crisis had higher adherence but in another [39] adherence was lower. There were also conflicting findings on associations with duration of HT. In three [29, 38, 40] patients with a long history of HT were more adherent but the reverse was observed two [27, 39]. One reason for such discrepancies could be heterogeneity of patients included. In addition, sample sizes varied greatly, with 1419 [38, 39], n = 161 [27], n = 190 [41] and n = 30 [40]. There was no evidence that adherence was associated with income and physical activity [29].
Almost all interventions studies found significant results, possibly reflecting publication bias. They included optimization of the drug regimen [11, 15, 16, 18, 19, 32, 33, 35, 36], an educational program [12, 13, 19–23], provision of an automatic home BP monitor [31, 37], and an initiative to inform the patient of his or her risk [14].
Provision of an automatic BP monitor [31] and optimization of the drug regimen using fixed combinations [11, 15, 16, 18, 19, 32, 33, 35, 36] found to be effective elsewhere [45, 46], were associated with a significant increase in adherence to AHT. However, in several of the studies adherence also improved in control groups, most likely because both groups received a series of enhancements to treatment including self-monitoring diaries, and written recommendations on lifestyle changes, as well as intensive monitoring of both groups for the entire period of follow-up. Thus, two of the randomized controlled studies found no differences between intervention and control groups, in adherence or BP reduction [31, 32]. The authors of two studies [11, 15] concluded that the results support fixed-dose combinations, but this seemed difficult to justify from their findings.
Strengths and limitations
The randomized trials included, none of which were blinded, had a high risk of systemic error (bias), while using questionnaires that subjectively measure adherence and reporting disparate numbers of patients in the intervention and control groups [37], including only non-adherent patients [12, 13], and in two randomized non-blinded controlled trials no baseline adherence rates were reported (incomplete data presentation) [14, 37]. As a consequence, the risk of a systemic error in our review is close to critical.
In the vast majority of studies, the antihypertensive effect of adherence was measured as mean BP or probability of achieving target BP levels. Only one study measured 24-h BP [30] or used home BP monitoring [37].
Most studies used subjective methods of assessment, namely questionnaires, including some developed by the authors themselves, with no information on their testing or validation. The only objective method used for assessing adherence was indirect, using pill counts.
This situation is regrettable, because other methods for assessing adherence exist, differing in the degree of objectivity and information provided. The subjective methods include, first, various validated questionnaires, among which the MMAS-4 is most often used [47]. Subjective methods of assessing adherence, based on a patient’s self-assessment, should be used with caution. Adherence rates are overestimated by up to 20% compared with an objective assessment [47]. Objective indirect methods of assessing adherence include pill counts, as well as various electronic dispensers [48]. Electronic prescription and claims data on medication dispensed has also been used to evaluate an adherence, assessed by the proportion of days covered, although obviously it assumes that medicines dispensed are actually taken [49, 50]. Abroad, a way of controlling adherence is available, such as the analysis of electronic databases of pharmacy chains [51]. The “gold standard” for assessing adherence is measurement of drugs in biological fluids, for example, in blood or urine, with the latter preferred as it is less invasive [52].
Our findings suggest that interest in this issue is increasing among Russian researchers. There were 85% more publications in the period 2013 to 2017 than in the previous five-year period. However, the methodological quality of the papers has not improved. There is no obvious improvement in study design, with no more randomized trials and although the MMAS-4 questionnaire has been used more often, the recent increase in studies cannot be considered a major achievement.
Notwithstanding the many methodological weaknesses, these findings suggest a picture of unsatisfactory adherence to drug treatment in the Russian hypertensive population. However, the quality of studies of adherence to AHT is a serious problem not only in Russia, but also internationally. The 2014 Cochrane review of interventions to improve adherence [53] found only 13 studies on AHT that were suitable for inclusion. It noted the unsatisfactory (poor) overall level (quality) of such studies and emphasized the need to use objective indicators of adherence. For this purpose, Ascertaining Barriers for Compliance project was developed [54], as well as Emerge’s recommendations, that was published in order to solve the lack of standard methods to assess adherence [55].
There were the limitations of the scoping review process, e.g. last search dated was 2017, and reports published only in the Russian language.
Despite the limitations of most of the studies included this review makes a contribution in the following respects.
First, it is the first attempt to scope a comprehensive picture of the Russian literature on this topic. An important contribution of this paper is that it captures the full spectrum of research on adherence in Russia. It has identified some large studies, with prolonged follow up, using internationally accepted measures. Collectively, the small number of better quality studies does offer insights that can help inform the design of relevant policies, although further evidence is essential. Adherence to AHT in Russia is clearly a problem. Given the considerable economic burden that this creates, borne by both patients themselves and the health system as a whole, this is an issue that should be considered a high priority.
Second, the evaluative studies do point to some potentially promising measures. However, all should be subject to further evaluation and there is a clear need for much more research on interventions that have been found to be promising elsewhere [56–58].
Conclusions
The main determinants of adherence to AHT are sociodemographic factors, such as female gender, age over 50 years, not living alone, employment, higher education, and living in a city; comorbidity, including: ischemic heart disease, history of myocardial infarction, arrhythmias, diabetes mellitus, and psychiatric disorders; adherence was also associated with onset of HT at a young age and use of fixed dose combination therapy. The findings of studies of the association between adherence and grade of HT, experience of hypertensive crisis, and duration of HT were not consistent. Patient education, telephone reminders, home BP monitoring and fixed-dose combinations of drugs are most important for improving adherence. The interpretations of these findings are limited by unreliable measures of adherence. It is necessary to introduce objective methods for assessing of adherence. A central repository of studies published in regional medical journals should be created.
Additional files
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist. (DOCX 26 kb)
Full search strategies on adherence to antihypertensive therapy in adult population in Russia from 2000 to 2017. (DOCX 17 kb)
Levels of adherence in prevalence studies or baseline of interventions in adult population with hypertension in Russia from 2000 to 2017. (DOCX 22 kb)
Sociodemographic and clinical factors associated with adherence to antihypertensive therapy in adult population with hypertension in Russia from 2000 to 2017. (DOCX 30 kb)
Effectiveness of interventions aimed at increasing adherence in adult population with hypertension in Russia from 2000 to 2017. (DOCX 23 kb)
Acknowledgements
Not applicable.
Abbreviations
- AHT
Antihypertensive therapy
- BP
Blood pressure
- HT
Hypertension
- MAS
Medicine Assistance Scheme
- MMAS-4
4-item Morisky Medication Adherence Scale
- PRISMA-ScR
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews
- SCORE
Systematic Coronary Risk Evaluation scale
Authors’ contributions
EVB, EKB and IVK conducted literature search, selection of literature, data extraction, analysis of literature, wrote the first draft of the manuscript, review of manuscript. AVK, OMD, DL, MMK developed the study concept, was consulted to resolve any differences, revision of the first draft and subsequent revisions of the review. DL and MMK edited the manuscript and enhanced its intellectual content. All authors read and approved the final manuscript.
Funding
The work was conducted as part of the International Project on Cardiovascular Disease in Russia (IPCDR) which was supported in part by a Wellcome Trust Strategic Award [100217] and by grants from the Arctic University of Norway, UiT in Tromsø, the Norwegian Institute of Public Health and the Norwegian Ministry of Health and Social Affairs. Grant recipient – Professor David LEON.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its additional files.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elena Viktorovna Bochkareva, Email: ebochkareva@gnicpm.ru.
Ekaterina Kronidovna Butina, Email: ebutina@gnicpm.ru.
Irina Vitalievna Kim, Phone: +79265546830, Email: ivkimivkim@gmail.com, Email: ikim@gnicpm.ru.
Anna Vasilievna Kontsevaya, Email: koncanna@yandex.ru.
Oxana Mikhailovna Drapkina, Email: odrapkina@gnicpm.ru.
David Leon, Email: david.leon@lshtm.ac.uk.
Martin McKee, Email: martin.mckee@lshtm.ac.uk.
References
- 1.WHO Mortality Database. http://apps.who.int/healthinfo/statistics/mortality/whodpms/ Accessed 5 Sept 2018.
- 2.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balanova YuA, Kontsevaya AV, Shalnova SA, Deev AD, Kapustina AV, Evstifeeva SE, et al. Life quality of persons with arterial hypertension in Russia - is there relation to treatment? (by data from populational study ESSE-RF). Russ J Cardiol. 2016;9(137):7–13. 10.15829/1560-4071-2016-9-7-13. [DOI]
- 4.Burnier M. Managing ‘resistance’: is adherence a target for treatment? Curr Opin Nephrol Hypertens. 2014;23:439–443. doi: 10.1097/MNH.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 5.Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336:1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolandaivelu K, Leiden BB, O’Gara PT, Bhatt DL. Non-adherence to cardiovascular medications. Eur Heart J. 2014;35:3267–3276. doi: 10.1093/eurheartj/ehu364. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34:2940–2948. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- 8.Imaeva AE, Balanova YA, Kontsevaya AV, Kapustina AV, Duplyakov DV, Malysheva OG, et al. Availability and affordability of medicines for the treatment of cardiovascular diseases in pharmacies in six regions of the Russian Federation. Ration Pharmacother Cardiol. 2018;14(6):804–815. doi: 10.20996/1819-6446-2018-14-6-804-815. [DOI] [Google Scholar]
- 9.Oganov RG, Porgosova GV, Koltunov IE, Belova YS, Vygodin VA. RELIPH - regular treatment and prevention - the key to improvement of situation with cardiovascular diseases in Russia: results of multicenter study. Part III Kardiologiia. 2008;4:46–53. [PubMed] [Google Scholar]
- 10.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 11.Ageev FT, Drobizhev MB, Smirnova MD, Fofanova TV, Plisyuk AG, Kadushina EB. Free or fixed combination of enalapril and hypothiazide in real ambulatory practice: what is better for a patient with arterial hypertension? Kardiologiia. 2008;5:10–15. [PubMed] [Google Scholar]
- 12.Kobalava ZD, Villevalde SV, Isikova KV. Impact of educational programs and rational use of perindopril on motivation and adherence in arterial hypertension. Results of the PRISMA study. Clin Pharmacol Ther. 2011;20(4):15–21. [Google Scholar]
- 13.Kobalava ZD, Villevalde SV, Isikova KV. Elevation of compliance and motivation to antihypertensive therapy in patients with arterial hypertension through educational programs and rational use of angiotensin converting enzyme perindopril. Results of the PRISMA study. Consilium Medicum. 2011;5:15–22. [PubMed] [Google Scholar]
- 14.Sarycheva A. А., Nebieridze D. V., Kamyshova T. V. IS IT POSSIBLE TO IMPROVE THE ADHERENCE TO TREATMENT OF HYPERTENSION AND DYSLIPIDEMIA IN PATIENTS WITHOUT CLINICAL MANIFESTATIONS OF ATHEROSCLEROSIS? Rational Pharmacotherapy in Cardiology. 2017;13(5):602–608. doi: 10.20996/1819-6446-2017-13-5-602-608. [DOI] [Google Scholar]
- 15.Fofanova TV, Plisyuk AG, Smirnova MD, Patrusheva IF, Kadushina EB, Ageev FT. Evaluation of treatment effectiveness and adherence to therapy of the combined Enziks preparation and a free combination of enalapril and indapamide in patients with arterial hypertension in the outpatient setting. Rus Heart J. 2008;5(7):298–302. [Google Scholar]
- 16.Karpov YuA on behalf of the FORTISSIMO program participants The FORTISSIMO program: advantages of fixed full dose combination of perindopril arginine and Indapamide in the treatment of poorly controlled arterial hypertension. Kardiologiia. 2013;3:37–43. [PubMed] [Google Scholar]
- 17.Glezer MG on behalf of the OPTIMUM program participants Evaluation of antihypertensive efficacy and patient adherence to treatment with the new formulation Perindopril arginine (orally disintegrating tablet) in general clinical practice: OPTIMUM Program. Kardiologiia. 2016;56(4):36–41. doi: 10.18565/cardio.2016.4.36-41. [DOI] [PubMed] [Google Scholar]
- 18.Glezer MG on behalf of program participants The use of fixed Perindopril A/Amlodipine combination provides high compliance to therapy, effective and safe arterial pressure lowering in patients with previous inefferctive therapy. The POTENTIAL Program. Kardiologiia. 2015;12:17–24. [PubMed] [Google Scholar]
- 19.Glezer MG, Deev AD on behalf of the participants of the program FORSAZH How to increase the effectiveness of antihypertensive therapy in clinical practice: results of the Russian observational program FORSAZH. Kardiologiia. 2016;1:18–24. doi: 10.18565/cardio.2016.1.13-24. [DOI] [PubMed] [Google Scholar]
- 20.Kagramanyan IN. The role of compliance in improving quality of medical care. Remedium. 2015;5:25–29. [Google Scholar]
- 21.Kaskaeva DS, Petrova MM, Krylova EJ, Tepper EA, Zorina EV. Assessment of compliance hypertensive patients at 6 months follow-up, dynamic railway workers. International journal of applied and fundamental research. 2015;4:50–52. [Google Scholar]
- 22.Ushakova SE, Kontsevaya AV, Knyazhevskaya OV, Kontsevaya TV, Nazarova OA, Kalinina AM. Therapeutic education and treatment compliance in hypertensive patients. Cardiovascular Therapy and Prevention. 2005;4(1):32–35. [Google Scholar]
- 23.Chazova IE, Ageev FT, Fofanova TV, Chikhladze NM, Kuznetsova MB, Smirnova MD, et al. Gerashhenko JuS. Education and self-education of the patients is an important step towards increasing patients acceptance of therapy. Systemic Hypertension. 2014;3:7–10. [Google Scholar]
- 24.Fofanova TV, Orlova YA, Patrusheva AF, Smirnova MD, Kuzmina AE, Deev AD, et al. Felodipine in outpatient practice: which can affect the effectiveness of treatment and adherence to the therapy of patients with arterial hypertension. Rossiiskii Meditsinskii Zhurnal. 2009;5(17):392–396. [Google Scholar]
- 25.Donirova OS, Rukosuyeva JA, Vampilova AR, Kazakova RG, Alekseeva NM, Osipova NI. The assesment of the reasons of low compliance in patients with arterial hypertension at an out-patient stage. Siberian Medical Journal (Irkutsk) 2012;114(7):111–113. [Google Scholar]
- 26.Loukianov MM, Kozminsky AN, Martsevich SY, Yakushin SS, Vorobyev AN, Zagrebelnyy AV, et al. Patients with combination of chronic heart failure, hypertension and history of myocardial infarction: clinical and anamnestic characteristics, administration of ACE inhibitors, angiotensin receptor blockers, β-blockers and adherence to the drug therapy (data of outpatient registry RECVASA) Ration Pharmacother Cardiol. 2017;13(2):207–212. doi: 10.20996/1819-6446-2017-13-2-207-212. [DOI] [Google Scholar]
- 27.Fofanova TV, Ageev FT, Kadushina EB, MYU D, Smirnova MD, Kuzmina AE. Psychosomatic aspects of low adherence to antihypertensive therapy in patients with arterial hypertension. Systemic Hypertension. 2014;3:11–16. doi: 10.26442/2075-082X_11.3.11-16. [DOI] [Google Scholar]
- 28.Soboleva M, Slobidenyuk E, Bukatova I, Kalagina Z. Compliance with the use of quality-of-life-improving medicines in hypertensive patients. Vrach. 2012;3:68–70. [Google Scholar]
- 29.Oganov RG, Porgosova GV, Koltunov IE, Belova YS, Vygodin VA, Spivak E. RELIPH - REgularnoye Letcheniye I ProPHylaktika (regular treatment and prevention) - the key to improvement of situation with cardiovascular diseases in Russia: results of multicenter study. Part II Kardiologiia. 2007;11:30–39. [PubMed] [Google Scholar]
- 30.Olejnikov VE, Eliseeva IV, Tomashevskaya YA, Borisova NA, Fadeeva SS. The efficacy of antihypertensive therapy in elderly patients and treatment compliance analysis. Ration Pharmacother Cardiol. 2014;10(4):391–396. doi: 10.20996/1819-6446-2014-10-4-391-396. [DOI] [Google Scholar]
- 31.Smirnova MD, Cygareishvili EV, Ageev FT, Svirida ON, Kuzmina AE, Fofanova TV. The availability of a home tonometer as a factor that increases therapy compliance in outpatients with arterial hypertension. Systemic Hypertension. 2012;4:44–49. [Google Scholar]
- 32.Vologdina I, Poroshina E, Rozov A, Simonova O. Combined therapy in old patiens with coronary heart disease and arterial hypertension. Vrach. 2009;4:32–36. [Google Scholar]
- 33.Sviryaev YV, Zvartau NE, Rotari OP, Emelyanov IV, Avdonina NG, Conrady AO. New perspectives of improving of compliance - results of open study of adherence of patients to treatment with fixed in one blister combinations of enalapril and indapamide. Arterial hypertension. 2006;12(3):262–267. [Google Scholar]
- 34.Morozov SL, Smirnov SV, Kulikov AN, Shulenin KS, Chernyakhovskaya AA, Potapov EA, et al. Treatment adherence - reserve for efficiency of therapy of patients with essential hypertension. Bulletin of the Russian Military Medical Academy. 2010;2(30):42–46. [Google Scholar]
- 35.Kotovskaya Yu V, Villevalde SV, Tigai Zh G, Kobalava Zh D on behalf of physicians participating in the CONSTANTA program Hypertensive patients' adherence, motivation, and awareness during fixed-dose perindopril A and amlodipine combination treatment (results of the CONSTANTA trial) Ter Arkh. 2015;87(2):64–69. doi: 10.17116/terarkh201587264-69. [DOI] [PubMed] [Google Scholar]
- 36.Panov AV, Alugishvili MZ, Abesadze IT, Lohovinina NL, Korzenevskaya KV, Titenkov IV, et al. The antihypertensive effect of the fixed combination of Lisinopril and amlodipine in patients with coronary heart disease after coronary artery bypass grafting. Kardiologiia. 2015;6:27–33. doi: 10.18565/cardio.2015.6.27-33. [DOI] [PubMed] [Google Scholar]
- 37.Oschepkova EV, Cagareishvili EV, Rogoza AN. Self-monitoring of blood pressure by patients increases adherence to the treatment of arterial hypertension (1 year observation) Systemic Hypertension. 2004;2:32–37. [Google Scholar]
- 38.Kontsevaya AV, Romanenko TS, Vygodin VA, Fitilev SV. Evaluation of the regularity of antihypertensive drugs usage as a component of treatment adherence in outpatients of a specialized cardiology center. Ration Pharmacother Cardiol. 2015;11(3):238–246. doi: 10.20996/1819-6446-2015-11-3-238-246. [DOI] [Google Scholar]
- 39.Kontsevaya AV, Romanenko TS, Vygodin VA, Fitilev SV. Сhange of hypertension treatment scheme in outpatient care at specialized cardiologic institution, and factors associated with the change of antihypertension therapy. Russ J Cardiol. 2015;4(120):100–106. doi: 10.15829/1560-4071-2015-04-100-106. [DOI] [Google Scholar]
- 40.Kopnina EI, Buchina MM, Chernova MA, Buchina AV. Compliance with hypertensive patients. Bulletin of new medical technologies. 2008;15(1):150–151. [Google Scholar]
- 41.Sergeyeva VA. Analysis of antihypertensive therapy and its adherence in hypertensive patients with and without concomitant diabetes mellitus. Systemic Hypertension. 2012;4:35–39. [Google Scholar]
- 42.Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org.
- 43.Cybulsky M, Cook S, Kontsevaya AV, Vasiljev M, Leon DA. Pharmacological treatment of hypertension and hyperlipidemia in Izhevsk. Russia BMC Cardiovasc Disord. 2016;16:122. doi: 10.1186/s12872-016-0300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van der Laan DM, Elders PJM, Boons CCLM, Beckeringh JJ, Nijpels G, Hugtenburg JG. Factors associated with antihypertensive medication non-adherence: a systematic review. J Hum Hypertens. 2017;31:687–694. doi: 10.1038/jhh.2017.48. [DOI] [PubMed] [Google Scholar]
- 45.Mancia G, Rea F, Cuspidi C, Grassi G, Corrao G. Blood pressure control in hypertension. Pros and cons of available treatment strategies. J Hypertens. 2017;35(2):225–233. doi: 10.1097/HJH.0000000000001181. [DOI] [PubMed] [Google Scholar]
- 46.Karpov YA, Gorbunov VM, Deev AD. Effectiveness of fixed-dose perindopril/amlodipine on clinic, ambulatory and self-monitored blood pressure and blood pressure variability: an open-label, non comparative study in the general practice. High Blood Press Cardiovasc Prev. 2015;22:417–425. doi: 10.1007/s40292-015-0117-0. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen TM, Caze AL, Cottrellet N. What are validated self-report adherence scales really measuring?: a systematic review. Br J Clin Pharmacol. 2014;77:427–445. doi: 10.1111/bcp.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El Alili M, Vrijens B, Demonceau J, Evers SM, Hiligsmann M. A scoping review of studies comparing the medication event monitoring system [MEMS] with alternative methods for measuring medication adherence. Br J Clin Pharmacol. 2016;82:268–279. doi: 10.1111/bcp.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forbes CA, Deshpande S, Sorio-Vilela F, Kutikova L, Duffy S, Gouni-Bertholdet I, et al. A systematic literature review comparing methods for the measurement of patient persistence and adherence. Curr Med Res Opin. 2018;34(9):1613–1625. doi: 10.1080/03007995.2018.1477747. [DOI] [PubMed] [Google Scholar]
- 50.Despres F, Perreault S, Lalonde L, Forget A, Kettani FZ, Blais L. Impact of drug plans on adherence to and the cost of antihypertensive medications among patients covered by a universal drug insurance program. Can J Cardiol. 2014;30(5):560–567. doi: 10.1016/j.cjca.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 51.Corrao G, Zambon A, Parodi A, Poluzzi E, Baldi I, Merlino L, et al. Discontinuation of and changes in drug therapy for hypertension among newly-treated patients: a population-based study in Italy. J Hypertens. 2008;26:819–824. doi: 10.1097/HJH.0b013e3282f4edd7. [DOI] [PubMed] [Google Scholar]
- 52.Tomaszewski M, White C, Patel P, Masca N, Damani R, Hepworth J, et al. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart. 2014;100(11):855–861. doi: 10.1136/heartjnl-2013-305063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;11:CD000011. doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ascertaining Barriers for Compliance: policies for safe, effective and costeffective use of medicines in Europe. Final Report of the ABC progect. June 2012. http://abcproject.eu/img/ABC%20Final.pdf. Accessed 24 July 2019.
- 55.De Geest S, Zullig LL, Dunbar-Jacob J, Helmy R, Hughes DA, Wilson IB, et al. ESPACOMP medication adherence reporting guideline (EMERGE) Ann Intern Med. 2018;169(1):30–35. doi: 10.7326/M18-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conn VS, Ruppar TM, Chase JD, Enriquez M, Cooper PS. Interventions to improve medication adherence in hypertensive patients: systematic review and meta-analysis. Curr Hypertens Rep. 2015;17(12):94. doi: 10.1007/s11906-015-0606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conn VS, Ruppar TM. Medication adherence outcomes of 771 intervention trials: systematic review and meta-analysis. Prev Med. 2017;99:269–276. doi: 10.1016/j.ypmed.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu R, Xiea X, Lia S, Chena X, Wanga S, Huc C, et al. Interventions to improve medication adherence among Chinese patients with hypertension: a systematic review and meta-analysis of randomized controlled trails. Int J Pharm Pract. 2018;26(4):291–301. doi: 10.1111/ijpp.12452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist. (DOCX 26 kb)
Full search strategies on adherence to antihypertensive therapy in adult population in Russia from 2000 to 2017. (DOCX 17 kb)
Levels of adherence in prevalence studies or baseline of interventions in adult population with hypertension in Russia from 2000 to 2017. (DOCX 22 kb)
Sociodemographic and clinical factors associated with adherence to antihypertensive therapy in adult population with hypertension in Russia from 2000 to 2017. (DOCX 30 kb)
Effectiveness of interventions aimed at increasing adherence in adult population with hypertension in Russia from 2000 to 2017. (DOCX 23 kb)
Data Availability Statement
All data generated or analysed during this study are included in this published article and its additional files.