Abstract
Background
To compare the cardiovascular event risk in type 2 diabetes patients newly receiving dapagliflozin vs. empagliflozin.
Methods
We conducted a retrospective cohort study by analyzing a multi-institutional electronic medical records database (Chang Gung Research Database) in Taiwan and included adult type 2 diabetes patients who were newly receiving sodium–glucose co-transporter 2 (SGLT2) inhibitors from 2016 to 2017. The primary outcome was a composite of cardiovascular death, myocardial infarction, ischemic stroke and heart failure. We followed up patients from initiation of SGLT2 inhibitors until the occurrence of cardiovascular events before December 31, 2018. We performed multivariable Cox proportional hazard modeling, adjusting for patients’ age, sex, laboratory data, co-morbidities, and concomitant medications.
Results
We identified 12,681 new SGLT2 inhibitor users with a mean age of 58.9 (SD 11.8) years, of whom 43.9% were female and 45.8% were new dapagliflozin users. A total of 10,442 person-years of dapagliflozin use and 12,096 person-years of empagliflozin use were included. Compared to empagliflozin users, new users of dapagliflozin were found to have similar risks for primary composite outcome (adjusted HR: 0.91; 95% CI 0.73–1.14), cardiovascular death (adjusted HR: 0.54; 95% CI 0.14–2.12), myocardial infarction (adjusted HR: 0.77, 95% CI 0.49–1.19) and ischemic stroke (adjusted HR: 1.15; 95% CI 0.80–1.65), but a lower risk of heart failure (adjusted HR: 0.68; 95% CI 0.49–0.95).
Conclusion
The risk of cardiovascular events was similar between dapagliflozin and empagliflozin new users, but dapagliflozin may have a better outcome in the reduction of heart failure in type 2 diabetes patients. Future prospective studies are required to confirm the findings.
Keywords: Sodium–glucose co-transporter 2 inhibitors, Cardiovascular disease, Heart failure, Real-world data
Background
Patients with type 2 diabetes suffer substantial morbidity and mortality from cardiovascular disease [1]. Current medication and lifestyle interventions may not be sufficient to reduce the risk of serious cardiovascular disease outcomes in the primary prevention cohorts of type 2 diabetes [2]. Although the largest absolute benefits of interventions for individual patients are achieved among those with established atherosclerotic cardiovascular disease, the large number of type 2 diabetes patients without a history of major cardiovascular disease makes knowledge about the effects of anti-diabetes medication on first events an additional priority [2].
Sodium–glucose co-transporter 2 (SGLT2) inhibitor which decreases glucose re-absorption in the kidney and increases excretion via the urine is the new drug class for type 2 diabetes management with favorable safety profiles [3, 4], and is recommended as an option for treatment intensification after the failure of metformin [5]. A meta-analysis of three large placebo-controlled cardiovascular outcome trials found that SGLT2 inhibitors reduced major adverse cardiovascular events by 11% (HR: 0.89, 95% CI 0.83–0.96). In addition, compared to incretin-based therapies, including glucagon-like-peptide 1 (GLP-1) agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors, SGLT2 inhibitors are associated with lower risks of all-cause and cardiovascular-specific mortality, and occurrence of heart failure and myocardial infarction [6]. Although the direct cardioprotective mechanisms are not fully understood, they may be related to hemodynamic and metabolic actions from SGLT2 inhibitors [7–9].
SGLT2 inhibitors, in addition to glycemic controls, also have a positive effect on the cardiometabolic markers, such as body weight, blood pressure and uric acid [10, 11], and as a result there has been increasing use of SGLT2 inhibitors for the management of type 2 diabetes in clinical practice [12]. However, not all SGLT2 inhibitors share the same pharmacokinetic properties; for example, dapagliflozin with its slower excretion by the kidney exerts its pharmacological effects longer, even after 18 h post-dose, whereas the effects of empagliflozin are markedly attenuated from 12 h post-dose [13]. Due to more stable and longer sodium excretion and osmotic diuresis effects when compared to empagliflozin, dapagliflozin has been reported to reduce the 24-h variability in systolic blood pressures and may be associated with a lower risk for cardiovascular diseases [14–17].
Current evidence has shown the beneficial effects of SGLT2 inhibitors on cardiovascular events, but the effects may differ between the individual SGLT2 inhibitors. Therefore, the purpose of this study was to determine the comparative cardiovascular event risk associated with dapagliflozin vs. empagliflozin in real-world type 2 diabetes patients.
Methods
Data source
This retrospective cohort study analyzed data from Chang Gung Research Database (CGRD), the largest multi-institutional electronic medical records database in Taiwan [18]. The CGRD was established for research purposes in 2016, and currently covers 1.3 million patients (6% of the population of Taiwan). The CGRD includes clinical data for all patients who had outpatient treatment or were hospitalized in one of the 7 Chang Gung Memorial Hospitals from northern to southern Taiwan. The CGRD identifies diseases based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) before 2016, and ICD-10-CM afterwards. Unlike administrative data, the CGRD contains laboratory data which can provide a valid estimate of association between outcomes and exposures. The structures of CGRD have been described in another publication [18], and the accuracy and validity of the diagnostic codes in CGRD are well established [19–21].
Study population
From the CGRD we identified type 2 diabetes patients newly treated with a SGLT2 inhibitor, either dapagliflozin or empagliflozin, during 2016–2017. The index date was defined as the date of SGLT2 inhibitor initiation. Because SGLT2 inhibitors were approved for type 2 diabetes management starting in 2016, all patients in this study could be considered as new users. We defined the baseline period as 1 year prior to the index date. Patients were excluded if they were less than 18 years old. Patients also needed to have had at least one clinical visit before the index date, in order to establish their baseline information. To ensure that cardiovascular events represented new cases, we excluded patients who had diagnoses of myocardial infarction, ischemic stroke and heart failure within 1 year prior to the index date. We excluded patients without laboratory examinations including body mass index (BMI), blood sugar controls (e.g., HbA1c), renal functions (e.g., estimated glomerular filtration rate; eGFR and urine albumin–creatinine ratio; UACR) or lipid profiles (e.g., low-density lipoprotein; LDL) before the index date, because these patients may not receive routine medical care in our hospitals. Finally, we also excluded patients who incurred cardiovascular events of interest within 30 days after the index date, considering that such a short-term exposure to SGLT2 inhibitors was unlikely to be the cause of cardiovascular events.
Outcomes definition and follow-up
The primary outcome was the composite event of cardiovascular mortality, myocardial infarction, ischemic stroke and heart failure in the diagnosis of hospitalization and outpatient data. As secondary outcomes we evaluated the risk of the aforementioned individual events. Although studies have indicated that these cardiovascular outcome diagnosis codes have high positive predictive values (91.5–97.9%) in Taiwan’s healthcare settings [20, 22, 23], we also reviewed the electronic medical records from 3 Chang Gung Memorial Hospitals to assess the validity of the diagnosis codes corresponding to the cardiovascular events of interest. Specifically, we confirmed the heart failure diagnoses by laboratory examination data (e.g., B-type Natriuretic Peptide, BNP), left ventricular ejection fraction (LVEF) from echocardiogram as well as the symptoms of heart failure (e.g., dyspnea). We reviewed the medical charts in 222 cardiovascular events, and found the positive predictive values of 75.0%, 95.8%, 91.9% and 89.8% for cardiovascular mortality, myocardial infarction, ischemic stroke and heart failure, respectively. We performed intention-to-treat analysis and followed patients until the occurrence of the cardiovascular outcomes, death or last date of clinical records in the CGRD or December 31, 2018.
Covariates
Approximately 40 potential confounding covariates based on previous studies and experts’ opinions were included in the regression models [24, 25]. We collected data on individual co-morbidities (i.e., hypertension and dyslipidemia, coronary heart diseases and microvascular complications) and computed composite scores of co-morbidities (i.e., Charlson comorbidity index [26]) at baseline. We also collected data of concomitant medications (i.e., statin, antiplatelet agents, anticoagulant agents, antihypertensive agents and antihyperglycemia agents) within 3 months before the index date. We included the most recent laboratory data before SGLT2 inhibitor initiation to evaluate the baseline cardiovascular risk. For the missing values of laboratory data, we performed multiple imputations using the Markov chain Monte Carlo method with a combination of 10 simulations [27, 28]. The details of co-morbidity and concomitant medications are described in Additional file 1: Table S1, Additional file 2: Table S2.
Negative control outcome
Negative control outcome could detect residual confounding and bias due to unobserved confounders [29, 30]. We examined the association of SGLT2 inhibitors with incident atrial fibrillation as a negative control outcome in this study population. Because there is currently no plausible mechanism by which SGLT2 inhibitors are associated with an altered risk of atrial fibrillation [25], any observed significant associations in this falsification test should be due to bias.
Statistical analysis
We defined the patients with empagliflozin as the reference group, and used multivariable Cox proportional hazards models to estimate the hazard ratios (HR) and 95% confidence intervals (CI) of outcomes. The variables used in the Cox proportional hazards models included age, sex, laboratory data, co-morbidities and concomitant medications (Table 1). All variables significantly related to outcomes from the prior uni-variate Cox regression model at alpha level of 0.1 were included by stepwise selection in the multivariate analyses. All analyses were two-tailed with a type I error level at 0.05, and were conducted using SAS Enterprise (Version 5.1; SAS Institute Inc., Cary, NC, USA).
Table 1.
Baseline patient characteristics
| Dapagliflozin | Empagliflozin | P value | |
|---|---|---|---|
| Patients, n (%) | 5812 (45.8) | 6869 (54.2) | |
| Age, n (%), years | < 0.001 | ||
| < 65 | 4041 (69.5) | 4497 (65.5) | |
| ≧ 65 | 1771 (30.5) | 2372 (34.5) | |
| Female, n (%) | 2647 (45.5) | 2920 (42.5) | < 0.001 |
| PDD/DDD, mean (SD)a | 0.9 (0.2) | 0.8 (0.4) | < 0.001 |
| Prescribed daily dose, n (SD)b | < 0.001 | ||
| Low dose, n (%) | 1451 (25.0) | 4071 (59.3) | |
| Full dose, n (%) | 4361 (75.0) | 2798 (40.7) | |
| BMI, n (%), kg/m2 | 0.576 | ||
| < 30 | 3905 (67.2) | 4583 (66.7) | |
| ≥ 30 | 1907 (32.8) | 2286 (33.3) | |
| HbA1c, n (%), % | < 0.001 | ||
| < 8.5 | 2541 (43.7) | 3308 (48.2) | |
| ≥ 8.5 | 3271 (56.3) | 3561 (51.8) | |
| eGFR, n (%), mL/min/1.73 m2 | < 0.001 | ||
| < 60 | 347 (6.0) | 930 (13.6) | |
| 60–90 | 2102 (36.2) | 2473 (36.0) | |
| ≥ 90 | 3363 (57.9) | 3466 (50.5) | |
| UACR, n (%), mg/g | < 0.001 | ||
| < 30 | 3007 (51.7) | 3336 (48.6) | |
| 30–300 | 1834 (31.6) | 2199 (32.0) | |
| ≥ 300 | 971 (16.7) | 1334 (19.4) | |
| LDL, n (%), mg/dL | 0.505 | ||
| ≥ 100 | 104 (1.8) | 134 (2.0) | |
| < 100 | 5708 (98.2) | 6735 (98.0) | |
| Index year, n (%) | < 0.001 | ||
| 2016 | 3326 (57.2) | 3624 (52.8) | |
| 2017 | 2486 (42.8) | 3245 (47.2) | |
| Hospital level, n (%) | < 0.001 | ||
| Medical centers | 3224 (55.5) | 3927 (57.2) | |
| Regional hospitals | 1540 (26.5) | 1533 (22.3) | |
| District hospitals | 1048 (18.0) | 1409 (20.5) | |
| Department, n (%) | < 0.001 | ||
| Metabolism and endocrinology | 3964 (68.2) | 4206 (61.2) | |
| Cardiology | 1181 (20.3) | 1792 (26.1) | |
| Others | 667 (11.5) | 871 (12.7) | |
| Comorbidity, n (%) | |||
| Hypertension | 3718 (64.0) | 4606 (67.1) | < 0.001 |
| Hyperlipidemia | 4274 (73.5) | 4974 (72.4) | 0.155 |
| Coronary heart diseasec | 832 (14.3) | 1207 (17.6) | < 0.001 |
| Atrial fibrillation | 113 (1.9) | 166 (2.4) | 0.071 |
| Peripheral artery disease | 67 (1.2) | 108 (1.6) | 0.044 |
| Diabetic retinopathy | 475 (8.2) | 577 (8.4) | 0.644 |
| Diabetic neuropathy | 583 (10.0) | 627 (9.1) | 0.085 |
| Diabetic nephropathy | 1510 (26.0) | 1893 (27.6) | 0.046 |
| Chronic obstructive pulmonary disease | 126 (2.2) | 177 (2.6) | 0.133 |
| Liver disease | 1052 (18.1) | 1278 (18.6) | 0.465 |
| Depression | 85 (1.5) | 102 (1.5) | 0.917 |
| Schizophrenia | 24 (0.4) | 19 (0.3) | 0.188 |
| Cancer | 366 (6.3) | 445 (6.5) | 0.678 |
| Charlson comorbidity index score, n (%) | 0.007 | ||
| < 2 | 2440 (42.0) | 2722 (39.6) | |
| ≥ 2 | 3372 (58.0) | 4147 (60.4) | |
| Previous hospitalization, n (%) | 554 (9.5) | 815 (11.9) | < 0.001 |
| Concomitant medications, n (%) | |||
| Anti-platelet agents | 1549 (26.7) | 2074 (30.2) | < 0.001 |
| Anti-coagulant agents | 100 (1.7) | 151 (2.2) | 0.054 |
| Beta blockers | 1249 (21.5) | 1690 (24.6) | < 0.001 |
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers | 3235 (55.7) | 4129 (60.1) | < 0.001 |
| Calcium channel blockers | 2056 (35.4) | 2775 (40.4) | < 0.001 |
| Loop diuretics | 153 (2.6) | 282 (4.1) | < 0.001 |
| Thiazides | 270 (4.7) | 342 (5.0) | 0.383 |
| Mineralocorticoid receptor antagonist | 59 (1.0) | 124 (1.8) | < 0.001 |
| Statin | 3662 (63.0) | 4538 (66.1) | < 0.001 |
| Fibrate | 535 (9.2) | 717 (10.4) | 0.020 |
| Ezetimibe | 646 (11.1) | 793 (11.5) | 0.447 |
| Metformin | 5364 (92.3) | 6124 (89.2) | < 0.001 |
| Sulfonylurea | 3604 (62.0) | 3751 (54.6) | < 0.001 |
| Dipeptidyl peptidase-4 inhibitors | 3616 (62.2) | 4437 (64.6) | 0.006 |
| Alpha-glucosidase inhibitors | 1002 (17.2) | 1240 (18.1) | 0.232 |
| Glinides | 94 (1.6) | 158 (2.3) | 0.006 |
| Thiazolidinediones | 1552 (26.7) | 1584 (23.1) | < 0.001 |
| Glucagon-like peptide-1 receptors antagonist | 75 (1.3) | 156 (2.3) | < 0.001 |
| Insulin | 1083 (18.6) | 1507 (21.9) | < 0.001 |
| Non-steroidal anti-inflammatory drugs | 475 (8.2) | 542 (7.9) | 0.560 |
BMI body mass index, DDD defined daily dose, eGFR estimated glomerular filtration rate, LDL low-density lipoprotein, PDD prescribed daily dose, UACR urine albumin–creatinine ratio
aThe ratio of prescribed daily dose (PDD)/defined daily dose (DDD) is defined by the WHO Collaborating Center (https://www.whocc.no/atc_ddd_index/) for drug comparisons [62]; that is, the PDD/DDD ratio of patients who used 10 mg dapagliflozin daily is equal to 17.5 mg empagliflozin (PDD/DDD ratio = 1)
bLow dose: dapagliflozin < 10 mg and empagliflozin < 25 mg; full dose: dapagliflozin 10 mg and empagliflozin 25 mg
cMyocardial infarction was not included because prevalent myocardial infarction cases were excluded before initiation of SGLT2 inhibitors in this study
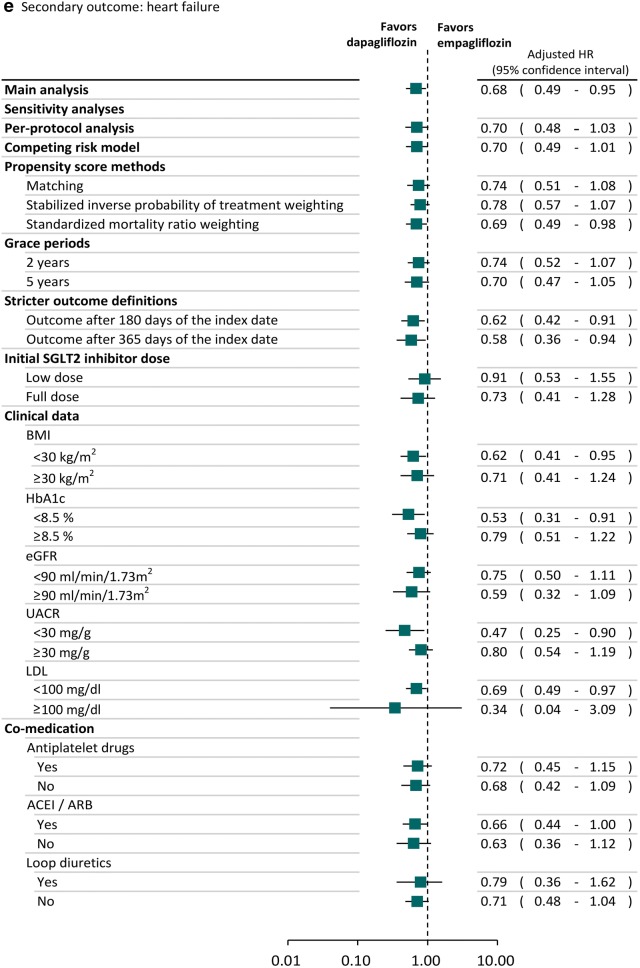
Sensitivity and subgroup analyses
We performed several sensitivity and subgroup analyses to create more homogenous groups for comparisons to test robustness of results from the main analyses. First, to evaluate the effects from non-adherence to the SGLT2 inhibitors, we performed per-protocol design and followed patients until the occurrence of cardiovascular outcome or discontinuation of the initial SGLT2 inhibitors (e.g., grace period over 90 days or switching to another SGLT2 inhibitor), whichever came first. Second, because patients who died from causes other than cardiovascular events (e.g., cancer) were no longer at risk of the study outcomes, this non-cardiovascular mortality was regarded as the major competing risk in the analyses. To address the possible competing risk events, we specifically compared rates of non-cardiovascular mortality between the empagliflozin and dapagliflozin groups. We found the rates of mortality were similar between the two groups (5.0 vs. 4.5 per 1000 person-years for empagliflozin vs. dapagliflozin, respectively), which implied that the effect of competing risk was minor. Nevertheless, we used the proportional subdistribution hazard models raised by Fine and Gray et al. [31] to manage possible competing risk of mortality. Third, we used three propensity score methods, including propensity score matching, stabilized inverse probability of treatment weighting (SIPTW) and standardized mortality ratio weighting (SMRW), to balance potential baseline differences between the empagliflozin and dapagliflozin groups. We describe patient characteristics before and after propensity score methods in Additional file 3: Table S3. Fourth, we increased washout periods up to 5 years for selection of patients without cardiovascular diseases to test the original analyses. Fifth, we excluded patients who incurred cardiovascular events of interest up to 1 year after the index date to avoid protopathic bias. Sixth, we repeated the analyses based on the specific SGLT2 inhibitor dose (i.e., low dose: dapagliflozin < 10 mg and empagliflozin < 25 mg; full dose: dapagliflozin 10 mg and empagliflozin 25 mg). Additionally, we conducted stratification analyses based on baseline risks for cardiovascular diseases, such as patients’ BMI levels (≥ 30 or < 30 kg/m2), blood sugar levels (HbA1c: ≥ 8.5 or < 8.5%), renal functions (eGFR: ≥ 90 or < 90 mL/min/1.73 m2; UACR: < 30 or ≥ 30 mg/g) and lipid profiles (LDL: ≥ 100 or < 100 mg/dL). Finally, we conducted additional analyses based on the specific concomitant medications which may modify the cardiovascular disease risk, including anti-platelet drugs, angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB) and loop diuretics.
Results
Study population
We included a total of 12,681 patients newly initiating SGLT2 inhibitors, of which 5812 received dapagliflozin and 6869 received empagliflozin (Fig. 1). Patients newly receiving empagliflozin had worse renal functions at baseline (e.g., eGFR: 93.0 ± 31.6 vs. 97.7 ± 28.6 mL/min/1.73 m2; UACR: 242.9 ± 723.3 vs. 171.5 ± 502.7 mg/g). The mean baseline HbA1c levels (8.9 ± 1.6 vs. 8.8 ± 1.7%) and LDL levels (81.8 ± 23.0 vs. 81.5 ± 23.0 mg/dL) were comparable between dapagliflozin and empagliflozin new users. There were similar rates of co-morbidity between the SGLT2 inhibitors, but patients with empagliflozin had higher rates of hypertension (67.1% vs. 64.0%) and coronary heart disease (17.6% vs. 14.3%) at baseline. Greater use of anti-platelet drugs (30.2% vs. 26.7%), ACEI/ARB (60.1% vs. 55.7%), beta-blockers (24.6% vs. 21.5%), calcium channel blockers (40.4% vs. 35.4%) and loop diuretics (4.1% vs. 2.6%) was found in patients initiating empagliflozin than in those initiating dapagliflozin (Table 1).
Fig. 1.
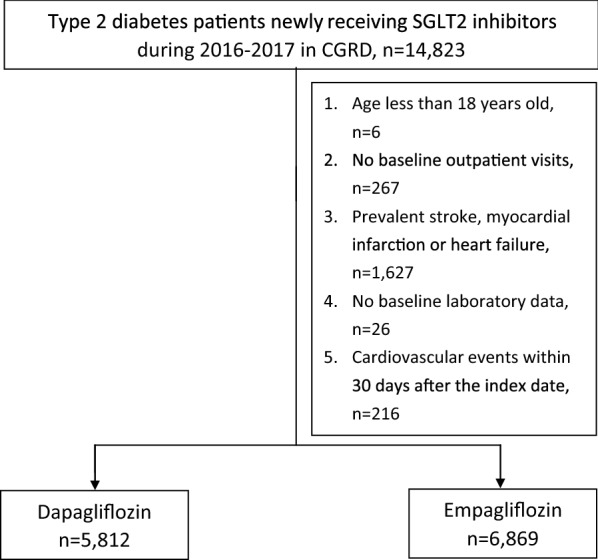
Patient selection flow chart. CGRD Chang Gung Research Database
SGLT2 inhibitors and composite CV outcomes
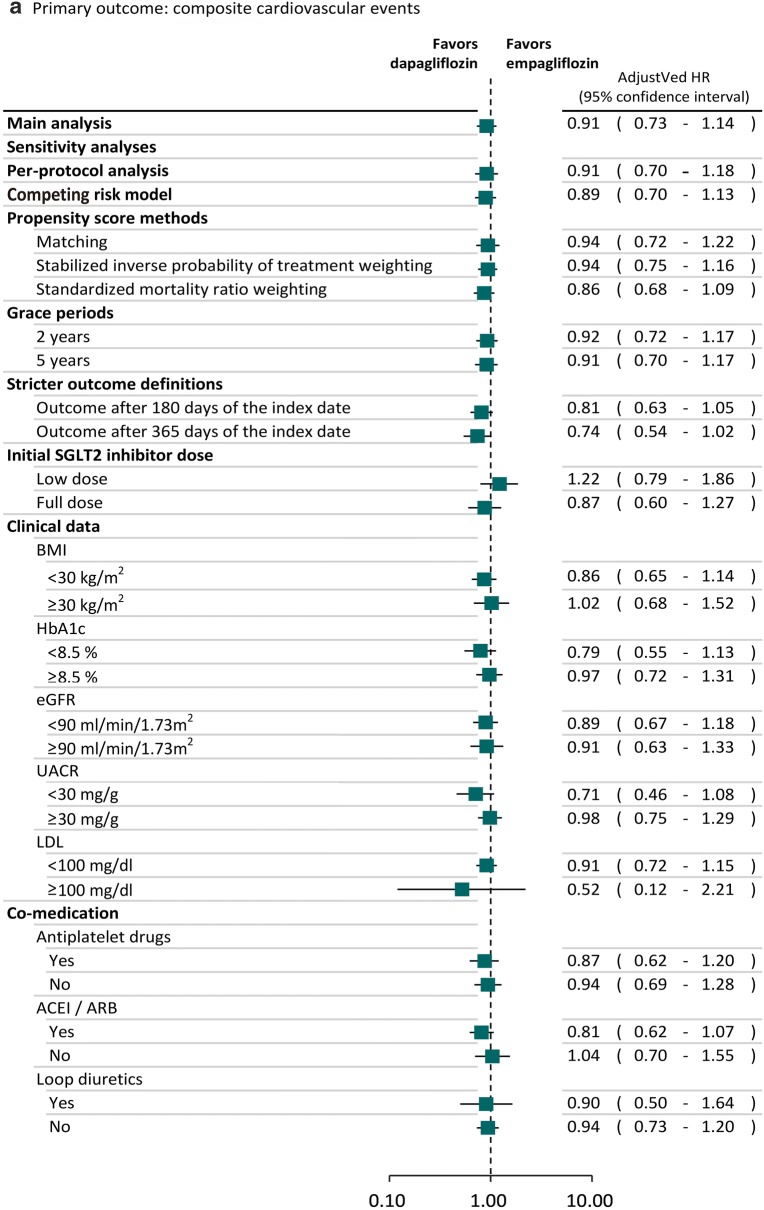
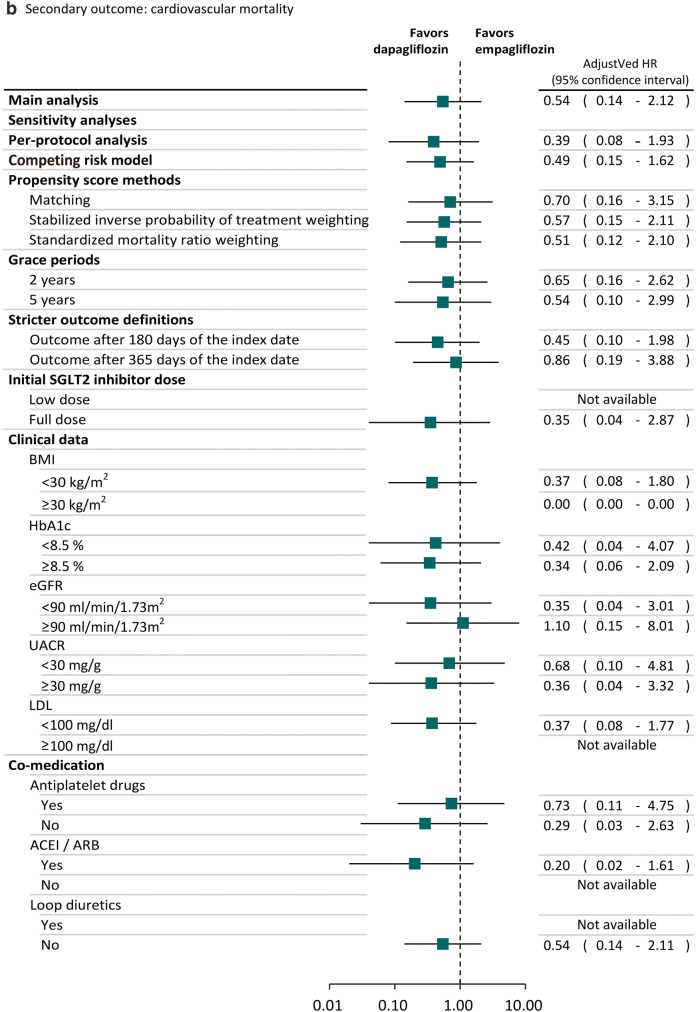
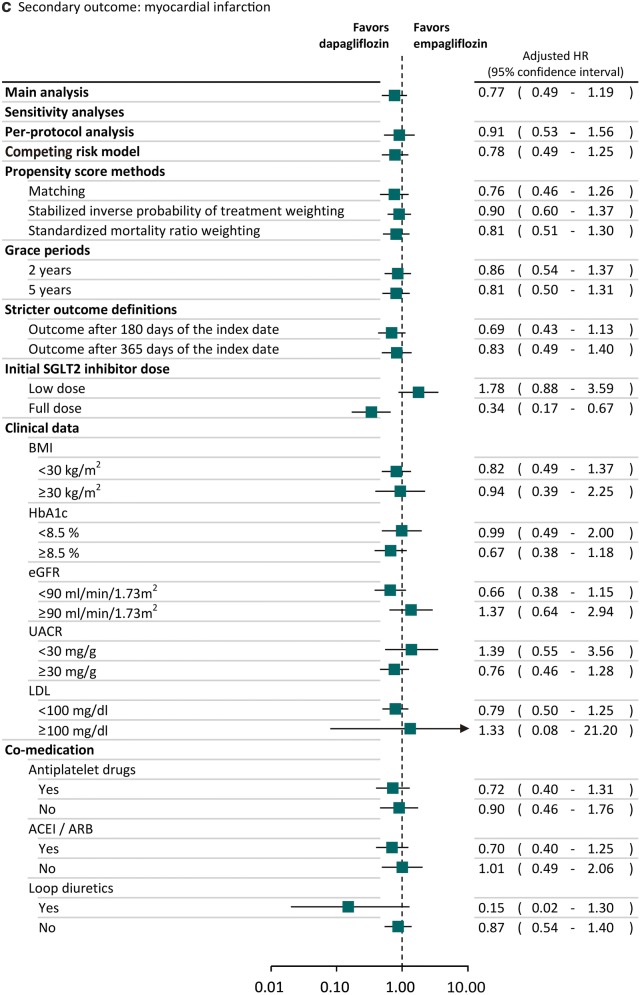
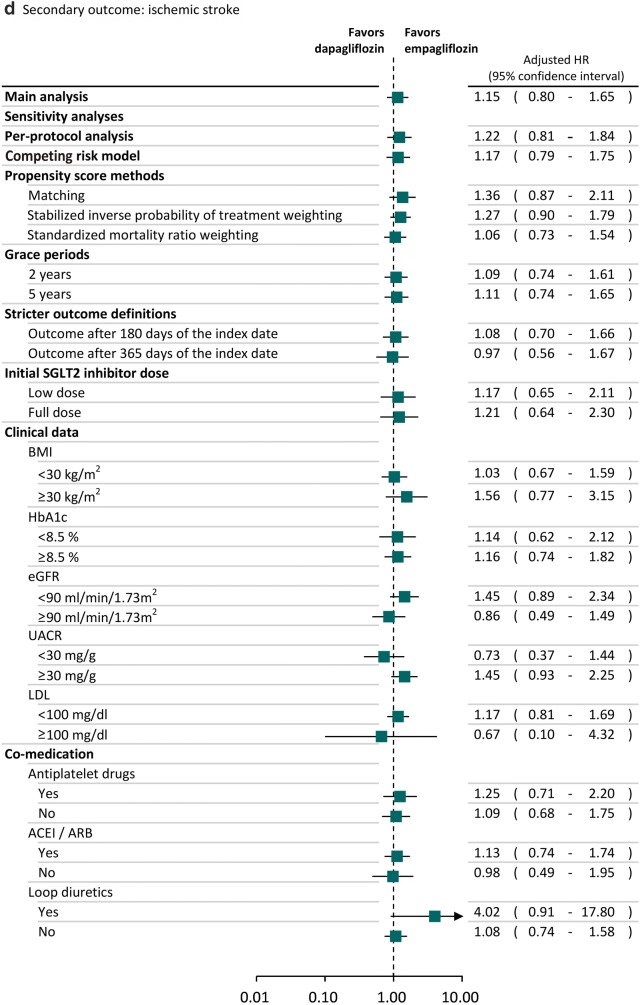
We followed a total of 10,442 and 12,096 person-years with mean follow-up time of 656 days and 643 days for the dapagliflozin and empagliflozin group, respectively. There were 128 and 197 composite cardiovascular events in the dapagliflozin (incidence rate: 12.3 per 1000 person-years) and empagliflozin (incidence rate: 16.3 per 1000 person-years) groups, respectively. We found a similar risk of composite cardiovascular outcome (adjusted HR: 0.91; 95% CI 0.73–1.14), cardiovascular mortality (adjusted HR: 0.54; 95% CI 0.14–2.12), myocardial infarction (adjusted HR: 0.77; 95% CI 0.49–1.19) and ischemic stroke (adjusted HR: 1.15; 95% CI 0.80–1.65) between dapagliflozin and empagliflozin (Table 2). However, dapagliflozin use was associated with a lower risk of heart failure than empagliflozin (adjusted HR: 0.68; 95% CI 0.49–0.95).
Table 2.
Comparative risk for cardiovascular events between dapagliflozin and empagliflozin (reference)
| Dapagliflozin, n (incidence rate)a | Empagliflozin, n (incidence rate)a | Crude HR | Adjusted HR | |
|---|---|---|---|---|
| Composite outcome | 128 (12.3) | 197 (16.3) | 0.75 (0.60–0.93) | 0.91 (0.73–1.14) |
| Specific outcome | ||||
| Cardiovascular mortality | 3 (0.3) | 7 (0.6) | 0.46 (0.12–1.76) | 0.54 (0.14–2.12) |
| Myocardial infarction | 33 (3.1) | 53 (4.3) | 0.70 (0.46–1.09) | 0.77 (0.49–1.19) |
| Ischemic stroke | 56 (5.3) | 63 (5.2) | 1.03 (0.72–1.48) | 1.15 (0.80–1.65) |
| Heart failure | 52 (4.9) | 109 (9.0) | 0.55 (0.39–0.76) | 0.68 (0.49–0.95) |
aIncidence rate was calculated by 1000 person-years
Negative control outcome
In falsification testing, there were neutral associations between SGLT2 inhibitors and incident atrial fibrillation (adjusted HR: 1.08; 95% CI 0.73–1.60) (Additional file 4: Table S4).
Sensitivity and subgroup analyses
The trends of results from sensitivity and subgroup analyses were consistent with the main analysis (Fig. 2). Specifically, we found the lower risk of heart failure associated with dapagliflozin was also statistically significant in several sensitivity analyses. For example, the observed effects were still found after we used SMRW analyses (adjusted HR: 0.69; 95% CI 0.49–0.98). We found the results of sensitivity analyses in the subgroups of patients receiving low SGLT2 inhibitor dose (adjusted HR: 0.91; 95% CI 0.53–1.55) or full SGLT2 inhibitor dose (adjusted HR: 0.73; 95% CI 0.41–1.28) and different baseline cardiovascular risks based on the clinical data, such as BMI ≥ 30 kg/m2 (adjusted HR: 0.71; 95% CI 0.41–1.24) or BMI < 30 kg/m2 (adjusted HR: 0.62; 95% CI 0.41–0.95), were consistent with the main analysis, in that patients receiving dapagliflozin had lower risk of heart failure compared to empagliflozin. Dapagliflozin reduced the risk of heart failure compared to empagliflozin regardless of whether or not anti-platelet drugs, ACEI/ARB and loop diuretics were used concomitantly.
Fig. 2.
Sensitivity and subgroup analyses of comparative risk for cardiovascular events between SGLT2 inhibitors. BMI body mass index, CI confidence interval, eGFR estimated glomerular filtration rate, HR hazard ratio, LDL low-density lipoprotein, SIPTW stabilized inverse probability of treatment weighting, SMRW standardized mortality ratio weighting, UACR urine albumin–creatinine ratio
Discussion
Clinical trials have demonstrated the favorable cardiovascular effects of empagliflozin and dapagliflozin [32]. In the EMPA-REG OUTCOME trial, empagliflozin showed 14% reduction of composite cardiovascular events and 35% reduction of heart failure compared to placebo. In the DECLARE-TIMI 58 trials, dapagliflozin lowered the risk by 27% for heart failure compared to placebo [33, 34]. Notably, the protective effects in regard to heart failure remained robust after several post hoc analyses from the EMPA-REG OUTCOME and DECLARE-TIMI 58 trials [35–37]. However, differences in patient phenotypes in clinical trials and in drug properties between SGLT2 inhibitors may have produced varied cardiovascular effects [38], so this study could provide additional information about the comparative evaluations associated with dapagliflozin vs. empagliflozin.
The incidence rates of cardiovascular events in patients newly receiving dapagliflozin and empagliflozin were 12.3 and 16.3 per 1000 person-years in this real-world cohort. We found a higher incidence of cardiovascular events in empagliflozin than dapagliflozin in the present study as compared to that among clinical trials (13.4–15.8 per 1000 person-years) [32]. Possible reasons may involve the channeling of SGLT2 inhibitor use in clinical practice based on medical evidence. First, the cardiovascular benefits of empagliflozin were discovered earlier in clinical trials than those of dapagliflozin [33, 34], so clinical physicians might have prescribed empagliflozin to type 2 diabetes patients with high risk of cardiovascular diseases (e.g., more cardiovascular co-morbidity and concomitant cardiovascular mediation) at baseline. Second, empagliflozin could be initiated in patients with eGFR > 45 mL/min/1.73 m2, while dapagliflozin was only recommended in patients with eGFR > 60 mL/min/1.73 m2 [39]. It is possible that some empagliflozin users with impaired renal functions contributed to a higher baseline cardiovascular disease risk in this group [40]. After adjusting for these confounders by regression models, we found the initiation of dapagliflozin was associated with a similar risk of composite cardiovascular events as compared to empagliflozin.
An excess heart failure risk persists in type 2 diabetes patients despite optimal control of an array of conventional risk factors, including hyperglycaemia [41]. To date, only SGLT2 inhibitors have produced a robust and significant reduction of heart failure risk, which has remained of similar magnitude regardless of a history of heart failure or established cardiovascular diseases [24, 25, 42, 43]. In addition to the favorable effects on many co-morbidities related to heart failure, including diabetes, obesity and hypertension, the sodium excretion and osmotic diuresis associated with SGLT2 inhibitors could also contribute to a reduced risk of heart failure [44–46].
The incidence of heart failure in this study was 4.9 and 9.0 per 1000 patient-years in the dapagliflozin and empagliflozin groups, respectively. Based on the post hoc analysis from 97 heart failure cases followed by the initiations of the SGLT2 inhibitor treatment from 3 Chang Gung Memorial hospitals, we found 67.0% vs. 28.9% of them were heart failure with preserved ejection fraction (HFpEF, LVEF ≥ 40%) and heart failure with reduced ejection fraction (HFrEF, LVEF < 40%) respectively, and 4.1% were without a report of LVEF. We determined the outcome of heart failure by all hospital events from inpatient and outpatient records, because heart failure in real-world patients with type 2 diabetes often remains undiagnosed, and, if present, sharply increases mortality risk [25]. Our estimates were similar to the reports of heart failure with SGLT2 inhibitors in clinical trials (7.0–8.9 per 1000 patient-years) [32].
The possible explanations for the reduction of heart failure in dapagliflozin and empagliflozin have been addressed. For example, previous studies indicated dapagliflozin has beneficial effects on left ventricular diastolic functions, vascular remodeling and cardiometabolic markers [47–49]. Empagliflozin has also been reported with salutary changes in left ventricular mass and diastolic function in type 2 diabetes patients [50], which might decrease the risk of heart failure. Based on evidence from previous trials and our analyses, although both SGLT2 inhibitors seem to reduce the risk of heart failure, we consider that dapagliflozin may have greater effects on heart failure reduction compared to empagliflozin. A possible explanation of this finding may lie in the differences in the drugs’ pharmacokinetic properties and in SGLT2/SGLT1 receptor selectivity. First, dapagliflozin has longer lasting pharmacological effects [13], such as sodium excretion and osmotic dieresis [14, 15], and it may reduce blood pressure variability, which is beneficial with regard to heart failure [16, 17]. In addition, it has been reported that the ratio of SGLT2:SGLT1 receptor selectivity is lower in dapagliflozin (1200-fold) than in empagliflozin (2500-fold) [51]. Previous studies have indicated SGLT1 receptors are predominantly in the human intestine and the higher selectivity of SGLT1 receptors can lower the variations of postprandial blood glucose, which might help to reduce heart failure risk [52–54]. Although some reports indicated SGLT1 receptors are also expressed in cardiac myocytes which may reduce cardiac functions, the findings remain controversial because of conflicting conclusions from the studies [33, 47–49, 55]. Future studies are required to confirm the mechanisms addressed above to account for the difference in reduction of heart failure risks between SGLT2 inhibitors.
The risk of ischemic stroke in patients with diabetes mellitus is increased twofold compared with individuals without diabetes mellitus [1]. Therefore, it could be of great value to find out if different anti-diabetes medications have any protective or harmful effects regarding stroke, and compare them. Meta-analyses of randomized controlled trials showed no significant differences in stroke risk among different SGLT2 inhibitors [56]. Among SGLT2 inhibitors, we found a trend, though not significant, towards increased risk of ischemic stroke for dapagliflozin compared to empagliflozin in this study. It has been reported that the use of glucagon-like peptide-1 receptor agonists (GLP-RA) reduced the risk of stroke [57, 58], and therefore the significantly higher proportion of GLP1-RA comediation in the empagliflozin group might have contributed to the lower risk of stroke. Although the use of GLP1-RA has been considered in the multivariate Cox regression models, we also conducted a post hoc analysis to exclude patients receiving GLP1-RA and re-examined the risk of ischemic stroke. The result of this post hoc analysis (adjusted HR: 1.19; 95% CI 0.82–1.72) remained consistent with the main analysis. Concerns have been raised that elevated hematocrit and hypotension after the SGLT2 inhibitor treatment may be associated with an increased risk of stroke caused by sludging and hypoperfusion, respectively. Empagliflozin and dapagliflozin have been reported to increase hematocrit in clinical trials [59, 60], but little has been known about the comparative effects of hematocrit changes. However, several meta-analyses have shown no clinical difference in the reductions of systolic and diastolic blood pressures between empagliflozin and dapagliflozin [11, 61]. Considering that different patients’ characteristics in previous clinical trials based on the various inclusion and exclusion criteria could affect stroke risk, further head-to-head studies should confirm this finding.
Strengths of this study included the large real-world cohort to compare the cardiovascular risk in type 2 diabetes patients between dapagliflozin and empagliflozin. Additionally, this study included laboratory measurements which describe important contributing factors for cardiovascular diseases but which are usually lacking in most other administrative databases and which can allow a more precise estimate of cardiovascular risk. Finally, the consistent findings across sensitivity analyses support our internal validity.
We acknowledge some potential limitations in this retrospective cohort study. First, our findings cannot be applied to the type 2 diabetes patients with major cardiovascular diseases at baseline because we focused on new cardiovascular events. Furthermore, our findings are derived from statistical inferences based on the use of several models to deal with baseline imbalances between groups. Future head-to-head prospective studies should confirm our findings. Third, information on some potential unmeasured confounders is not available in the CGRD, and these factors may have confounded the observed association between SGLT2 inhibitors and cardiovascular events. However, a neutral risk association of atrial fibrillation (negative control outcome) was observed between dapagliflozin and empagliflozin, which is indicative of a balanced profile for residual confounding and bias due to unobserved confounders at baseline. Fourth, while selection bias may be present in this study, we used several propensity score testing approaches to address observed potential confounders using as many as 40 covariates. Fifth, the competing risk of mortality may either hinder or modify the observation of cardiovascular events, and hence we used the Fine and Gray sub-distribution hazards model, which revealed similar risks as the main analyses. Sixth, we did not obtain data from outside the CGRD in Taiwan, which may have resulted in loss to follow-up. However, the findings from the per-protocol approach to minimize the effects of loss to follow-up were consistent with the main analyses. Seventh, we did not have information on diabetes duration, but we included patients’ laboratory data (e.g., glycemic and renal parameters) which are important determinants of cardiovascular risk. Finally, the comparisons associated with dapagliflozin vs. empagliflozin in these analyses apply only to cardiovascular outcomes, and no inference on side effects can be made from this study. We conducted a post hoc investigation of the urinary tract infection rate, which is the most commonly reported adverse effect of SGLT2 inhibitors, and found 7.7 and 7.5 per 1000 person-years in dapagliflozin and empagliflozin, respectively. We considered potential confounding factors in the regression models for adjustment and conducted a series of sensitivity and validation analyses to confirm the findings; however, some aforementioned limitations of the study remained unavoidable. Notwithstanding, the findings from this study may provide a clinical hypothesis for future prospective studies to confirm the differences in cardiovascular outcomes between SGLT2 inhibitors.
Conclusions
In this real-world study, we did not find a significant difference between dapagliflozin and empagliflozin in the risk of cardiovascular events among type 2 diabetes patients. The results showed that dapagliflozin users had a significantly lower risk of heart failure as compared to empagliflozin users, whereby the exact mechanisms of this difference require further studies.
Supplementary information
Additional file 1. Table S1. Diagnosis code for study outcome and co-morbidity.
Additional file 2. Table S2. Individual drug for study co-medication.
Additional file 3. Table S3. Baseline patient characteristics before and after propensity score methods.
Additional file 4. Table S4. Falsification analysis presenting hazard ratios for incident atrial fibrillation between SGLT2 inhibitors.
Acknowledgements
This study is based on data from the Chang Gung research Database provided by the Chang Gung Memorial Hospital. The interpretation and conclusions contained herein do not represent the presentation of Chang Gung Memorial Hospital. We would like to thank Dr. Swu-Jane Lin for suggestions on the manuscript revisions.
Abbreviations
- BMI
body mass index
- CGRD
Chang Gung Research Database
- CI
confidence intervals
- DPP-4
dipeptidyl peptidase 4
- eGFR
estimated glomerular filtration rate
- GLP-1
glucagon-like-peptide 1
- HR
hazard ratios
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- LDL
low-density lipoprotein
- SGLT2
sodium–glucose co-transporter 2
- SIPTW
stabilized inverse probability of treatment weighting
- SMRW
standardized mortality ratio weighting
- UACR
urine albumin–creatinine ratio
Authors’ contributions
Study concept and design: SCS and ECCL. Acquisition of subjects and/or data: YYC. Analysis and interpretation of data: SCS, KCC and ECCL. Preparation of manuscript: all authors. All authors read and approved the final manuscript.
Funding
This study received a grant from Chang Gung Medical Foundation (ID: CMRPG3H1551) and Ministry of Science and Technology of Taiwan (107-2320-B-006-070-MY3), which had no role in design, analysis, interpretation, reporting of results or the decision to develop this manuscript.
Availability of data and materials
Data sharing is not applicable to this study as data management and analysis were performed on a statistics server through remote access in Chang Gung Medical Foundation in Taiwan, for privacy and safety concerns.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board at Chang Gung Medical Foundation (ID: 201801493B0), and the need for informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shih-Chieh Shao, Email: s.c.shao@hotmail.com.
Kai-Cheng Chang, Email: thuope@hotmail.com.
Ming-Jui Hung, Email: hmj1447@cgmh.org.tw.
Ning-I Yang, Email: ningiy@gmail.com.
Yuk-Ying Chan, Email: yychan@cgmh.org.tw.
Yea-Huei Kao Yang, Email: yhkao@mail.ncku.edu.tw.
Edward Chia-Cheng Lai, Phone: +886-6-2353535, Email: edward_lai@mail.ncku.edu.tw.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12933-019-0919-9.
References
- 1.Emerging Risk Factors Collaboration. Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS Program (Canagliflozin cardiovascular assessment study) Circulation. 2018;137:323–334. doi: 10.1161/CIRCULATIONAHA.117.032038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Consoli A, Formoso G, Baldassarre MPA, et al. A comparative safety review between GLP-1 receptor agonists and SGLT2 inhibitors for diabetes treatment. Expert Opin Drug Saf. 2018;17:293–302. doi: 10.1080/14740338.2018.1428305. [DOI] [PubMed] [Google Scholar]
- 4.Donnan JR, Grandy CA, Chibrikov E, et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ Open. 2019;9:e022577. doi: 10.1136/bmjopen-2018-022577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes care. 2019;42(Suppl 1):S90–S102. doi: 10.2337/dc19-S009. [DOI] [PubMed] [Google Scholar]
- 6.Zheng SL, Roddick AJ, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580–1591. doi: 10.1001/jama.2018.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahnwong S, Chattipakorn SC, Chattipakorn N. Potential mechanisms responsible for cardioprotective effects of sodium-glucose co-transporter 2 inhibitors. Cardiovasc Diabetol. 2018;17:101. doi: 10.1186/s12933-018-0745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavaiola TS, Pettus J. Cardiovascular effects of sodium glucose cotransporter 2 inhibitors. Diabetes Metab Syndr Obes. 2018;11:133–148. doi: 10.2147/DMSO.S154602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallwitz B. The cardiovascular benefits associated with the use of sodium-glucose cotransporter 2 inhibitors—real-world data. Eur Endocrinol. 2018;14:17–23. doi: 10.17925/EE.2018.14.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Xu L, Tian D, et al. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2018;20:458–462. doi: 10.1111/dom.13101. [DOI] [PubMed] [Google Scholar]
- 11.Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab. 2016;18:783–794. doi: 10.1111/dom.12670. [DOI] [PubMed] [Google Scholar]
- 12.Heald AH, Fryer AA, Anderson SG, et al. Sodium-glucose co-transporter-2 inhibitors, the latest residents on the block: impact on glycaemic control at a general practice level in England. Diabetes Obes Metab. 2018;20:1659–1669. doi: 10.1111/dom.13281. [DOI] [PubMed] [Google Scholar]
- 13.Tahara A, Takasu T, Yokono M, Imamura M, Kurosaki E. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors in pharmacokinetics, pharmacodynamics, and pharmacologic effects. J Pharmacol Sci. 2016;130:159–169. doi: 10.1016/j.jphs.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4:211–220. doi: 10.1016/S2213-8587(15)00417-9. [DOI] [PubMed] [Google Scholar]
- 15.Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–428. doi: 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- 16.Muntner P, Whittle J, Lynch AI, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med. 2015;163:329–338. doi: 10.7326/M14-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Shi X, Ma C, et al. Visit-to-visit blood pressure variability is a risk factor for all-cause mortality and cardiovascular disease: a systematic review and meta-analysis. J Hypertens. 2017;35:10–17. doi: 10.1097/HJH.0000000000001159. [DOI] [PubMed] [Google Scholar]
- 18.Shao SC, Chan YY, Kao Yang YH, et al. The Chang Gung research database—a multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf. 2019;28:593–600. doi: 10.1002/pds.4713. [DOI] [PubMed] [Google Scholar]
- 19.Chao HY, Liu PH, Lin SC, et al. Association of In-hospital mortality and dysglycemia in septic patients. PLoS ONE. 2017;12:e0170408. doi: 10.1371/journal.pone.0170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin YS, Chen TH, Chi CC, et al. Different implications of heart failure, ischemic stroke, and mortality between nonvalvular atrial fibrillation and atrial flutter-a view from a national cohort study. J Am Heart Assoc. 2017;6:e006406. doi: 10.1161/JAHA.117.006406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang SL, Huang YL, Lee MC, et al. Association of varicose veins with incident venous thromboembolism and peripheral artery disease. JAMA. 2018;319:807–817. doi: 10.1001/jama.2018.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20:236–242. doi: 10.1002/pds.2087. [DOI] [PubMed] [Google Scholar]
- 23.Cheng CL, Lee CH, Chen PS, Li YH, Lin SJ, Yang YH. Validation of acute myocardial infarction cases in the national health insurance research database in taiwan. J Epidemiol. 2014;24:500–507. doi: 10.2188/jea.JE20140076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol. 2018;71:2628–2639. doi: 10.1016/j.jacc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Birkeland KI, Jorgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5:709–717. doi: 10.1016/S2213-8587(17)30258-9. [DOI] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 27.Harel O, Zhou XH. Multiple imputation: review of theory, implementation and software. Stat Med. 2007;26:3057–3077. doi: 10.1002/sim.2787. [DOI] [PubMed] [Google Scholar]
- 28.Ueda P, Svanstrom H, Melbye M, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ. 2018;363:k4365. doi: 10.1136/bmj.k4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasad V, Jena AB. Prespecified falsification end points: can they validate true observational associations? JAMA. 2013;309:241–242. doi: 10.1001/jama.2012.96867. [DOI] [PubMed] [Google Scholar]
- 30.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21:383–388. doi: 10.1097/EDE.0b013e3181d61eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 32.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 33.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 34.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 35.Furtado RHM, Bonaca MP, Raz I, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019;139:2516–2527. doi: 10.1161/CIRCULATIONAHA.119.039996. [DOI] [PubMed] [Google Scholar]
- 36.Fitchett D, Inzucchi SE, Cannon CP, et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation. 2019;139:1384–1395. doi: 10.1161/CIRCULATIONAHA.118.037778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zinman B, Inzucchi SE, Wanner C, et al. Empagliflozin in women with type 2 diabetes and cardiovascular disease—an analysis of EMPA-REG OUTCOME(R) Diabetologia. 2018;61:1522–1527. doi: 10.1007/s00125-018-4630-2. [DOI] [PubMed] [Google Scholar]
- 38.Clegg LE, Heerspink HJL, Penland RC, et al. Reduction of cardiovascular risk and improved estimated glomerular filtration rate by SGLT2 inhibitors, including dapagliflozin, is consistent across the class: an analysis of the placebo arm of EXSCEL. Diabetes Care. 2019;42:318–326. doi: 10.2337/dc18-1871. [DOI] [PubMed] [Google Scholar]
- 39.Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33–59. doi: 10.1007/s40265-014-0337-y. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Katzmarzyk PT, Horswell R, Zhao W, Johnson J, Hu G. Kidney function and the risk of cardiovascular disease in patients with type 2 diabetes. Kidney Int. 2014;85:1192–1199. doi: 10.1038/ki.2013.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giugliano D, Meier JJ, Esposito K. Heart failure and type 2 diabetes: from CVOTs, with hope. Diabetes Obes Metab. 2019;21:1081–1087. doi: 10.1111/dom.13629. [DOI] [PubMed] [Google Scholar]
- 42.Home P. Cardiovascular outcome trials of glucose-lowering medications: an update. Diabetologia. 2019;62:357–369. doi: 10.1007/s00125-018-4801-1. [DOI] [PubMed] [Google Scholar]
- 43.Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors) Circulation. 2017;136:249–259. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643–1658. doi: 10.1161/CIRCULATIONAHA.117.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 46.Karg MV, Bosch A, Kannenkeril D, et al. SGLT-2-inhibition with dapagliflozin reduces tissue sodium content: a randomised controlled trial. Cardiovasc Diabetol. 2018;17:5. doi: 10.1186/s12933-017-0654-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi T, Fukui T, Nakanishi N, et al. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetol. 2017;16:8. doi: 10.1186/s12933-016-0491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soga F, Tanaka H, Tatsumi K, et al. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol. 2018;17:132. doi: 10.1186/s12933-018-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ott C, Jumar A, Striepe K, et al. A randomised study of the impact of the SGLT2 inhibitor dapagliflozin on microvascular and macrovascular circulation. Cardiovasc Diabetol. 2017;16:26. doi: 10.1186/s12933-017-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verma S, Garg A, Yan AT, et al. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME trial? Diabetes Care. 2016;39:e212–e213. doi: 10.2337/dc16-1312. [DOI] [PubMed] [Google Scholar]
- 51.Anker SD, Butler J. Empagliflozin, calcium, and SGLT1/2 receptor affinity: another piece of the puzzle. ESC Heart Fail. 2018;5:549–551. doi: 10.1002/ehf2.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musso G, Gambino R, Cassader M, et al. Efficacy and safety of dual SGLT 1/2 inhibitor sotagliflozin in type 1 diabetes: meta-analysis of randomised controlled trials. BMJ. 2019;365:l1328. doi: 10.1136/bmj.l1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sha S, Polidori D, Farrell K, et al. Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover study. Diabetes Obes Metab. 2015;17:188–197. doi: 10.1111/dom.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yokota S, Tanaka H, Mochizuki Y, et al. Association of glycemic variability with left ventricular diastolic function in type 2 diabetes mellitus. J Am Coll Cardiol. 2019;73(9 Supplement 1):764. doi: 10.1016/S0735-1097(19)31372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanaka A, Node K. Exploration of the clinical benefits of sodium glucose co-transporter 2 inhibitors in diabetic patients with concomitant heart failure. Cardiovasc Diabetol. 2018;17:74. doi: 10.1186/s12933-018-0719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo M, Ding J, Li J, et al. SGLT2 inhibitors and risk of stroke in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018;20:1977–1982. doi: 10.1111/dom.13295. [DOI] [PubMed] [Google Scholar]
- 57.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 59.Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208–219. doi: 10.1016/S2213-8587(13)70084-6. [DOI] [PubMed] [Google Scholar]
- 60.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–657. doi: 10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baker WL, Buckley LF, Kelly MS, et al. Effects of sodium–glucose cotransporter 2 inhibitors on 24-hour ambulatory blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e005686. doi: 10.1161/JAHA.117.005686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.ATC/DDD Index 2019. WHO collaborating centre for drug statistics methodology. http://www.whocc.no/atc_ddd_index/. Accessed 1 July 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Diagnosis code for study outcome and co-morbidity.
Additional file 2. Table S2. Individual drug for study co-medication.
Additional file 3. Table S3. Baseline patient characteristics before and after propensity score methods.
Additional file 4. Table S4. Falsification analysis presenting hazard ratios for incident atrial fibrillation between SGLT2 inhibitors.
Data Availability Statement
Data sharing is not applicable to this study as data management and analysis were performed on a statistics server through remote access in Chang Gung Medical Foundation in Taiwan, for privacy and safety concerns.