Abstract
Background
Terminal QRS distortion reflects advanced stage and large myocardial infarction predisposing the heart to adverse outcomes. Recent studies suggest that terminal QRS distortion is associated with morbidity and mortality in ST elevation myocardial infarction (STEMI). However, a systematic review and meta-analysis of the literature have not been done.
Objective
We assessed the association between terminal QRS distortion in patients with STEMI and mortality by a systematic review of the literature and a meta-analysis.
Methods
We comprehensively searched the databases of MEDLINE and EMBASE from inception to September 2017. Included studies were published prospective or retrospective cohort studies that compared all-cause mortality in subjects with STEMI with QRS distortion versus those without QRS distortion. Data from each study were combined using the random-effects, generic inverse variance method of DerSimonian and Laird to calculate risk ratios and 95% confidence intervals.
Results
Fifteen studies from January 1993 to May 2015 were included in this meta-analysis involving 7,479 subjects with STEMI (2,906 QRS distortion and 4,573 non-QRS distortion). QRS distortion was associated with increased mortality (pooled risk ratio = 1.81, 95% confidence interval: 1.37-2.40, p < 0.000, I2 = 41.6%). Considering the introduction of clopidogrel in 2004, we performed subgroup analyses before and after 2004, and the associated with higher mortality was still present (before 2004, RR 1.75, 95% CI 1.08-2.82, p = 0.022, I2 = 66.1%; after 2004, RR 1.96, 95% CI 1.44-2.65, p < 0.001, I2 = 0%).
Conclusions
Terminal QRS distortion increased all-cause mortality by 81%. Our study suggests that terminal QRS distortion is an important tool to assess the risk in patients with STEMI.
Keywords: Mortality, ST elevation, Terminal QRS distortion
INTRODUCTION
Electrocardiography (ECG) is essential in the diagnosis of ST-segment elevation myocardial infarction (STEMI), and it is also used as a prognostic tool for patients with STEMI.1 Sclarovsky and colleagues developed a grading system of pre-reperfusion ECG findings in STEMI called Sclarovsky-Birnbaum’s Grades of Ischemia Score (grade 1: tall peaked T waves in the involved leads, grade 2: abnormal T waves and ST elevation, grade 3: abnormal T waves, ST elevation, and distortion of the terminal portion of the QRS).2,3 Grade 3 ischemia, which is characterized by distortion of the terminal portion of the QRS complexes, has been shown to be a sign of more severe ischemia and poorer prognosis in STEMI.4,5 The presence of Grade 3 ischemia has been reported in 19 to 53% of STEMI patients.6 Terminal QRS distortion is defined as a decrease in S-wave amplitude in leads with a terminal S wave and an elevation of the J point > 50% of the height of the R wave amplitude in leads with qR configuration.7 Terminal QRS distortion is not observed in early repolarization, and thus it can be useful in distinguishing STEMI from early repolarization.8 The presence of terminal QRS distortion in STEMI has been associated with a larger infarct size, impaired myocardial salvage, lower epicardial reperfusion success, low left ventricular ejection fraction (LVEF), reperfusion injury, anterior location of myocardial infarction, higher risk of re-infarction, and mortality.6,9-11 Terminal QRS distortion has been associated with increased mortality in STEMI in several studies.9,10,12,13 However, most of these studies had small cohorts, and some of the results regarding mortality were conflicting.14-16 In this study, we aimed to assess the association between the presence of terminal QRS distortion in STEMI and mortality in a systematic review and meta-analysis.
METHODS
Search strategy
Two investigators (NL and CK) independently searched for published studies indexed in MEDLINE and EMBASE databases from inception to September 2017 using a search strategy (Figure 1) that included the terms "terminal QRS distortion", "grade 3 ischemia", "ST elevation", and "mortality". Only English language publications were included. A manual search for additional pertinent studies and review articles using references from the retrieved articles was also completed.
Figure 1.

Search methodology and selection process.
The eligibility criteria included
(1) Studies reporting the incidence of in-hospital mortality, major cardiovascular events (MACEs), and reinfarction in patients with STEMI with and without terminal QRS distortion.
(2) Relative risk, hazard ratio, odds ratio, incidence ratio, or standardized incidence ratio with 95% confidence interval or sufficient raw data for the calculation were provided.
(3) Patients with STEMI who did not have terminal QRS distortion were used as controls.
Study eligibility was independently determined by two investigators (NL and CK), and differences were resolved by mutual consensus. The Newcastle-Ottawa quality assessment scale was used to evaluate each study in three domains: recruitment and selection of the participants, similarity, and comparability between the groups, and ascertainment of the outcome of interest among cohort studies.17
Definitions
Terminal QRS distortion was defined according to QRS morphologies described in each study. STEMI was defined as typical chest pain with the presence of ST segment elevation of ≥ 2 mm in 2 contiguous leads or as defined in each study at the time of STEMI diagnosis. In-hospital mortality was defined as deaths within the follow-up time specified in each study. MACEs were defined as a combination of all-cause mortality readmissions for new congestive heart failure, recurrent infarction, or as defined in each study (Figure 2).
Figure 2.
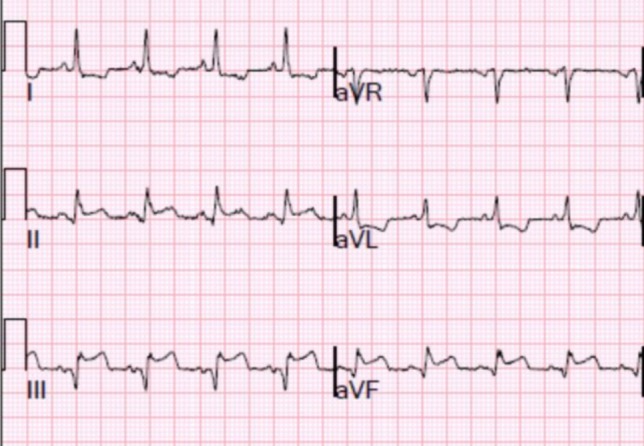
Electrocardiogram showing QRS distortion. An emergence of J point ≥ 50% of the R wave amplitude in a lead with qR configuration (III, aVF).
Data extraction
A standardized data collection form was used to obtain the following information from all studies: title of the study, name of the first author, year of study, year of publication, country of origin, number of participants, demographic data of the participants, method used to identify cases and controls, method used to diagnose the outcomes of interest (all-cause mortality), average duration of follow-up, adjusted and unadjusted risk ratios and their corresponding 95% confidence intervals, and a list of confounders that were adjusted for multivariate analysis.
To ensure accuracy, all investigators independently performed this data extraction process. Any data discrepancies were resolved by referring back to the original articles.
Statistical analysis
Meta-analysis of the included cohort was performed using a random-effect model with the generic inverse variance method of DerSimonian and Laird.18 The heterogeneity of effect size estimates across these studies was quantified using the I2 statistic and Q statistic. For the Q statistic, substantial heterogeneity was defined as p < 0.10. The I2 statistic ranges in value from 0 to 100% (I2 < 25%, low heterogeneity; I2 = 25%-50%, moderate heterogeneity; and I2 > 50%, substantial heterogeneity).19 Sensitivity analysis was performed to assess the influence of the individual studies on the overall results by omitting one study at a time. Publication bias was assessed using a funnel plot and Egger’s regression test20 (p < 0.05 was considered to be significant). All data analyses were performed using Stata SE 14.1 software from StataCorp LP.
RESULTS
Description of the included studies
Our search strategy yielded 28 potentially relevant articles (6 articles from EMBASE and 22 articles from MEDLINE). After excluding 2 duplicate articles, 26 articles underwent title and abstract review. Four were excluded at this stage since one was written in Turkish, one was an abstract presentation, and the other two were irrelevant topics, leaving 22 articles for full-length article review. Six studies were excluded because there was no outcome of interest, and one was excluded because the same population was used. Therefore, 7 retrospective and 8 prospective cohort studies with 2,906 patients with QRS distortion and 4,573 without QRS distortion were included in this meta-analysis. The clinical characteristics are described in Table 1.
Table 1. The clinical characteristics and summary of the included studies.
| First author | Country of origin | Year | Study type | Participant description | Exclusion criteria | Definition of terminal QRS distortion | Total population | Male (%) | Mean age (years) | In hospital mortality (n) | Median duration of follow up | Outcome definition | Conclusion by authors | NOS |
| Birnbaum | Israel | 1995 | Retrospective cohort study | Patients admitted within 6 hours from the onset of symptoms and had chest pain lasting ≥ 20 min in the GUSTO I Study. | LBBB, ventricular rhythm, negative T waves in the leads with ST elevation. | Distortion of the terminal portion of the QRS complex in two or more consecutive leads. | 2603 | 78.7 | 60.1 | 140 | N/A | In-hospital mortality | QRS distortion is an independent predictor of increased hospital. | 9 |
| Birnbaum | Israel | 1993 | Prospective cohort study | Patients admitted to the coronary care unit from y 1988 to 1991 who were in the early stages of an evolving first AMI involving the anterior wall. | History or evidence of a previous AMI. | Distortion of the terminal portion of the QRS complex in 2 or more adjacent leads. | 147 | N/A | 64 | 19 | N/A | In-hospital mortality | Categorizing the admission ECG according to the three patterns may aid the clinician in risk stratification. | 7 |
| Birnbaum | Israel | 2001 | Retrospective cohort study | Patients with acute myocardial infarction who were enrolled in the GUSTO IIb Angioplasty Substudy | IVCD, ventricular or paced rhythms, negative T waves in ≥ 2 adjacent leads, incomplete ECGs | Absence of a S wave below the TP-PR baseline in 2 leads or, ST J-point amplitude 50% of the R-wave amplitude measured in ≥ 2 all other leads. | 894 | 76.1 | 61 | 39 | N/A | In-hospital mortality | QRS distortion associated with higher in-hospital mortality and reinfarction and a trend toward a higher rate of 30-day mortality. | 9 |
| Birnbaum | USA | 1996 | Retrospective cohort study | The Thrombolysis in Myocardial infarction (TIMI) 4 trial | IVCD, or patients with no qualifying electrocardiograms available. | (1) In leads with initial QR configuration, emergence of the J point at > 50% of the R-wave amplitude; or (2) In leads without Q waves, absence of S waves. | 378 | 74.6 | 58.31 | 15 | N/A | In-hospital mortality | QRS distortion has a trend toward increase in the in-hospital mortality, reinfarction, HF, and LVEF < 40%, and higher rates of 1-year mortality in Anterior wall MI. | 9 |
| Mulay | India | 2013 | Prospective cohort study | STEMI patients who were eligible for thrombolysis, admitted in intensive coronary care unit. | LBBB, ventricular rhythm, ventricular pacing or negative T waves in leads demonstrating ST segment elevation. | Pattern A – J point elevation at a level above the lower half of the R in leads with qR configuration. Pattern B – Absence of S waves in leads with Rs configuration. | 160 | 76.7 | 53.4 | 13 | N/A | Hospital mortality | QRS distortion on admission ECG was the only variable found to be statistically significant. | 8 |
| Garcia-Rubira | Spain | 1995 | Prospective cohort study | Patients admitted to coronary care unit within the first 12 hour of an evolving first Q-wave myocardial infaction | Bundle branch block in the first ECG. | Distortion of terminal portion of the QRS complex in two or more adjacent leads. | 205 | 76.585366 | 61.9 | 23 | 13.1 days | In-hospital mortality | Terminal QRS distortion is a very strong predictor of poor outcome in acute myocardial infarction. | 8 |
| Garcia-Rubira | Spain | 2007 | Prospective cohort study | Patients admitted with a STEMI in Coronary Care Unit between March, 2003 and May, 2007, with primary angioplasty. | LBBB, pacemaker rhythm, or cardiogenic shock at admission. | Patients with a J point height/R wave height > 0.5 in two or more adjacent leads. | 508 | 76.6 | 62.6 | 44 | N/A | In-hospital death or shock | Terminal QRS distortion is predictive for development of cardiogenic shock. | 9 |
| Hasdai | Israel | 1996 | Prospective cohort study | Patients admitted to coronary care unit with inferior wall MI) | Negative T waves in inferior leads, ST-segment depression in inferior leads, permanent pacemaker, valvular heart disease, cardiomyopathy. | Distortion of the terminal portion of the QRS complex in two or more inferior leads. | 213 | 53.52 | 66 | 36 | N/A | In-hospital death | Minimal ST-segment elevation in inferior leads and maximal ST-segment depression in left precortlial leads are high risk for in-hospital mortality. | 8 |
| Mcgehee | USA | 2007 | Retrospective cohort study | Patients who underwent primary PCT for STEMI between July 1999 and August 2004. | BBB, LVH, paced rhythm, ventricular rhythm, ECG with sign of reperfusion or more advanced stage of infarction, incomplete, data, isolated posterior MI. | (1) The absence of an S wave below the TP isoelectric line in more than 2 leads or (2) The ST J-point amplitude greater than 50% of the R wave amplitude in more than 2 of infarct related leads. | 155 | 72.9 | 57.7 | 4 | N/A | In-hospital death | QRS distortion associated with partial or poor ST segment resolution after primary PCI for STEMI. | 9 |
| Postma | Netherlands | 2011 | Prospective cohort study | Patients aged between 21 and 85 years with STEMI who underwent primary PCI in the Ongoing Tirofiban in Myocardial Evaluation 2 trial. | therapy-resistant cardiogenic shock, renal dysfunction, severe HT, contraindication to anticoagulation, increased bleeding risk, pregnancy or breastfeeding, life expectancy of less than 1 year, LBBB. | (1) Absence of an S wave below the TP-PR line in 2 or more leads or (2) ST J point amplitude of 50% or greater of the R-wave amplitude in 2 or more all other infarct-related leads. | 1308 | 76.1 | 61.69 | 39 | N/A | 30-day mortality | QRS distortion was associated with poor myocardial reperfusion and worse outcome. | 9 |
| Rommel | Germany | 2015 | Retrospective cohort study | Patients undergoing primary PCI who had pre-hospital 12-lead ECG in AIDA STEMI trial. | LBBB, negative T-waves in the leads with ST elevation. | (1) Complete loss of S waves in two adjacent leads with typical Rs configuration (i.e. V1-V3), or (2) ST-J point to R wave amplitude ratio .0.5 in other leads with qR configuration. | 572 | 77 | 61 | 14 | 12 months | Death within 12 months after infarction | QRS distortion associated with infarct size, myocardial salvage, microvascular obstruction, intramyocardial hemorrhage, as well as MACE. | 9 |
| Sejersten | Denmark | 2006 | Prospective cohort study | STEMI patients included in the Danish trial in Acute Myocardial Infarction-2 (DANAMI-2) study. | T wave inversion; missing randomisation ECG; BBB; incomplete ECG; no ST segment elevation; IVCD; LVH; ventricular rhythm; WPW syndrome; paced rhythm. | (1) Absence of an S wave below the TP-PR in > 2 leads in V1-3 or (2) ST J point amplitude > 50% of the R wave amplitude in > 2 of all other leads. | 1319 | 72.81 | 62.34 | 41 | N/A | 30-day mortality | Terminal QRS distortion is predictive for mortality among patients with STEMI. | 9 |
| Tanriverdi | Turkey | 2015 | Retrospective cohort study | Patients who were older than 18 years of age and were admitted to the hospital with the diagnosis of first acute STEMI. | Complete BBB, incomplete RBBB, and pacemaker rhythm. | (1) Emergence of the J point at ≥ 50% of the R wave, and/or (2) Disappearance of the S wave in leads V1-V3. | 248 | 76.209677 | 63.2 | 20 | N/A | In-hospital mortality | Presence of both fQRS and QRS distortion is associated with worse prognostic events and increased in-hospital mortality. | 8 |
| Wolak | USA | 2007 | Prospective cohort study | Patients admitted to coronary care unit with a first STEMI and referred for PCI between July 2004 and April 2005. | Previous AMI, > 12 hours of symptoms, LBBB, paced or ventricular rhythm, negative T waves in > 2 adjacent leads with maximal ST elevation, or incomplete ECG data. | (1) Absence of an S wave below the TP-PR in > 2 in leads V1-V3, or (2) ST J-point amplitude > 50% of the R-wave amplitude in > 2 all other infarct-related leads. | 100 | 77 | 59.5 | 5 | N/A | 30-day mortality | Distortion of the terminal QRS is a very strong independent predictor of failure to achieve myocardial reperfusion. | 9 |
| Zalenski | USA | 2000 | Retrospective cohort study | All patients coded by EDs as Acute myocardial infarction and transported by EMS. | LBBB | 2 or more consecutive leads with the emergence of the J point above the lower half of the R wave or the disappearance of the S wave in inferior AMI in leads V2-V4. | 174 | 58 | 66 | 37 | N/A | Cardiac mortality | Distorted QRS wave has no significant relationship to predicted mortality in the 2 year follow-up period. | 7 |
AIDA, abciximab intracoronary vs. intravenously drug application; AMI, acute myocardial infarction; BBB, bundle branch block; ECG, electrocardiogram; ED, emergency department; EMS, emergency medical service; GUSTO, Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; IVCD, intraventricular conduction delay; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NOS, Newcastle-Ottawa Quality Assessment; RBBB, right bundle branch block; PCI, percutaneous coronary intervention; STEMI, ST elevation myocardial infarction.
QRS distortion in all studies was at the time of diagnosis.
Quality assessment of the included studies
The Newcastle-Ottawa scale (0 to 9) was used to evaluate the included studies on 3 domains: selection, comparability, and outcomes. Higher scores represent higher study quality. The score of each study ranged from 7 to 9, which reflected high quality of the included studies (Supplementary Table 1). Detailed evaluations of the studies are presented in Table 1.
Supplement Table 1. Newcastle-Ottawa quality assessment scale of included studies in meta-analysis.
| Study | Selection | Comparability | Outcome | Total score | |||||
| Represent-ativeness | Selection of the non-exposed cohort | Ascertainment of exposure | Endpoint not present at start | Comparability(Confounding) | Assessment of outcome | Follow-up duration | Adequacy follow-up | ||
| Birnbaum (1995) | * | * | * | * | ** | * | * | * | 9 |
| Birnbaum (1993) | * | * | * | * | * | * | * | 7 | |
| Birnbaum (2001) | * | * | * | * | ** | * | * | * | 9 |
| Birnbaum (1996) | * | * | * | * | ** | * | * | * | 9 |
| Mulay (2013) | * | * | * | * | * | * | * | * | 8 |
| Garcia-Rubira (1995) | * | * | * | * | * | * | * | * | 8 |
| Garcia-Rubira (2007) | * | * | * | * | ** | * | * | * | 9 |
| Hasdai (1996) | * | * | * | * | * | * | * | * | 8 |
| Mcgehee (2007) | * | * | * | * | ** | * | * | * | 9 |
| Postma (2011) | * | * | * | * | ** | * | * | * | 9 |
| Rommel (2015) | * | * | * | * | ** | * | * | * | 9 |
| Sejersten (2006) | * | * | * | * | ** | * | * | * | 9 |
| Tanriverdi (2015) | * | * | * | * | * | * | * | * | 8 |
| Wolak (2007) | * | * | * | * | ** | * | * | * | 9 |
| Zalenski (2000) | * | * | * | ** | * | * | 7 |
Notes: The Newcastle-Ottawa scale uses a star system (0 to 9) to evaluate included studies on 3 domains: selection, comparability, and outcomes. Star (*) = item presents. Maximum 1 star (*) for selection and outcome components and 2 stars (**) for comparability components. Higher scores represent higher study quality.
Meta-analysis results
A total of 15 studies (8 prospective,3,9,12-14,16,21,22 7 retrospective5,6,10,23-26) with 7,479 participants were included in the meta-analysis. The incidence of QRS distortion ranged from 19-52%. There was an association between terminal QRS distortion and all-cause mortality in the patients with STEMI [relative risk (RR) 1.81; 95% confidence interval (CI) 1.37-2.40, p < 0.001)] with moderate heterogeneity (I2 = 41.6%) (Figure 3A). We performed subgroup analysis for in-hospital mortality, which still showed a strong correlation with QRS distortion (RR 2.05; 95% CI 1.37-3.07, p = 0.001) with high heterogeneity (I2 = 55.0%) (Figure 3B). Further subgroup analysis of non-hospital mortality also demonstrated a substantial association with QRS distortion (RR 1.64; 95% CI 1.17-2.29, p = 0.004) with no heterogeneity (I2 = 0%) (Figure 3B). Furthermore, we performed subgroup analysis of patients with anterior infarction and inferior infarction, which showed that QRS distortion was significantly associated with all-cause mortality only in the anterior group (RR 2.84 95% CI 1.73-4.65, p < 0.001 with I2 = 42.7%) (Supplementary Figure 1). The inferior infarction group also showed increased all-cause mortality, but the difference was not significant (RR 2.10 95% CI 0.73-6.08, p = 0.169 with I2 = 83.3%) (Supplementary Figure 1).
Figure 3.

(A) Forest plot of studies comparing mortality rate in patients with and without QRS distortion. Horizontal lines represent the 95% CIs with marker size reflecting the statistical weight of the study using random-effects model. A diamond data marker represents the overall adjusted odds ratio (OR) and 95% CI for the outcome of interest. (B) Forest plot of studies comparing in-hospital and nonhospital mortality rate in patients with and without QRS distortion. Horizontal lines represent the 95% CIs with marker size reflecting the statistical weight of the study using random-effects model. A diamond data marker represents the overall adjusted odds ratio (OR) and 95% CI for the outcome of interest.
Supplementary Figure 1.

Forest plot of studies comparing anterior versus inferior infarction and overall mortality in patients with and without QRS distortion. Horizontal lines represent the 95% CIs with marker size reflecting the statistical weight of the study using random-effects model. A diamond data marker represents the overall adjusted odds ratio (OR) and 95% CI for the outcome of interest.
There have been advancements in treatment such as the introduction of clopidogrel and major changes in percutaneous coronary intervention (PCI) and stents from alternatives to first line from the ACC/AHA Guidelines in 2004.27 Since advances in these treatment methods may have affected outcomes, we conducted other subgroup analyses, which we subdivided by year (before or after 2004) and technique [thrombolysis or percutaneous transluminal coronary angioplasty (PTCA) and thrombolysis or stenting]. The results showed a substantial association between QRS distortion and mortality in both subgroups before 2004 (RR 1.75 CI 1.08-2.82, p = 0.022 with I2 = 66.1%) and after 2004 (RR 1.96 CI 1.44-2.65, p < 0.001 with I2 = 0%) (Supplementary Figure 2). There was also a significant association between QRS distortion and mortality in the thrombolysis (RR 2.94 CI 1.61-5.37, p < 0.001 with I2 = 53.4%) and stenting (RR 1.89 CI 1.39-2.57, p < 0.001 with I2 = 0.0%) groups. However, there was no difference between the PTCA and thrombolysis group (RR 1.06 95% CI 0.63-1.80, p = 0.820 with I2 = 39.0%) (Supplementary Figure 3).
Supplementary Figure 2.
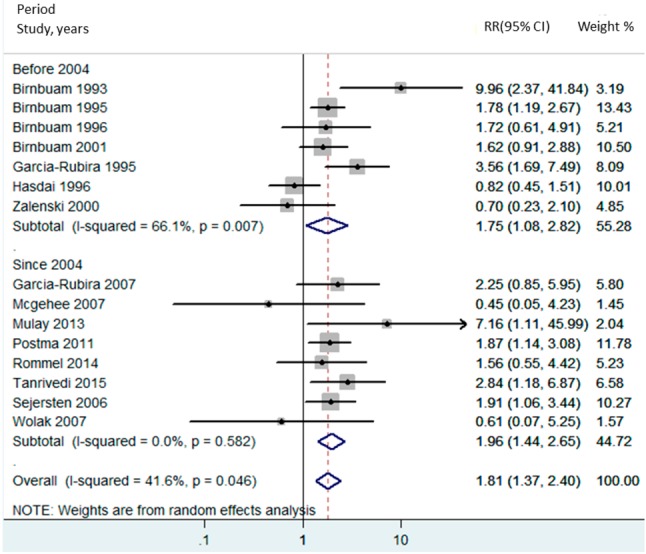
Forest plot of studies comparing period and overall mortality in patients with and without QRS distortion. Horizontal lines represent the 95% CIs with marker size reflecting the statistical weight of the study using random-effects model. A diamond data marker represents the overall adjusted odds ratio (OR) and 95% CI for the outcome of interest.
Supplementary Figure 3.

Forest plot of studies comparing technique and overall mortality in patients with and without QRS distortion. Horizontal lines represent the 95% CIs with marker size reflecting the statistical weight of the study using random-effects model. A diamond data marker represents the overall adjusted odds ratio (OR) and 95% CI for the outcome of interest.
We took into account other exploratory outcomes including MACEs, reinfarction, ventricular arrhythmia and shock. The analysis of the other outcomes demonstrated a statistically significant increased risk of MACEs (RR 1.69; 95% CI 1.31-2.18, p < 0.001) and reinfarction (RR 1.72; 95% CI 1.33-2.24, p < 0.001) (Supplementary Figure 4). There was also an increased rate of shock (RR 1.72; 95% CI 0.91-3.25, p = 0.062) (Supplementary Figure 5) and ventricular arrhythmia (RR 1.83 95% CI 0.77-4.63, p = 0.179) (Supplementary Figure 6) in the patients with QRS distortion. In particular, statistical heterogeneity was not found (I2 = 0%) in the secondary analyses of MACEs and reinfarction. Nevertheless, there was substantial heterogeneity in the analysis of shock (I2 = 55.4%) and ventricular arrhythmia (I2 = 85.9%). No publication bias was found in any of these analyses in either the funnel plot or Egger’s test (Figure 4). Sensitivity analysis to explore heterogeneity showed no significant change in our findings after omitting each study.
Supplementary Figure 4.

Forest plot of studies comparing reinfarction rate and MACE in patients with and without QRS distortion. Horizontal lines represent the 95% CIs with marker size reflecting the statistical weight of the study using random-effects model. A diamond data marker represents the overall adjusted odds ratio (OR) and 95% CI for the outcome of interest.
Supplementary Figure 5.

Forest plot of studies comparing shock rate in patients with and without QRS distortion. Horizontal lines represent the 95% CIs with marker size reflecting the statistical weight of the study using random-effects model. A diamond data marker represents the overall adjusted odds ratio (OR) and 95% CI for the outcome of interest.
Supplementary Figure 6.

Forest plot of studies comparing QRS distortion and fatal arrhythmia. Horizontal lines represent the 95% CIs with marker size reflecting the statistical weight of the study using random-effects model. A diamond data marker represents the overall adjusted odds ratio (OR) and 95% CI for the outcome of interest.
Figure 4.
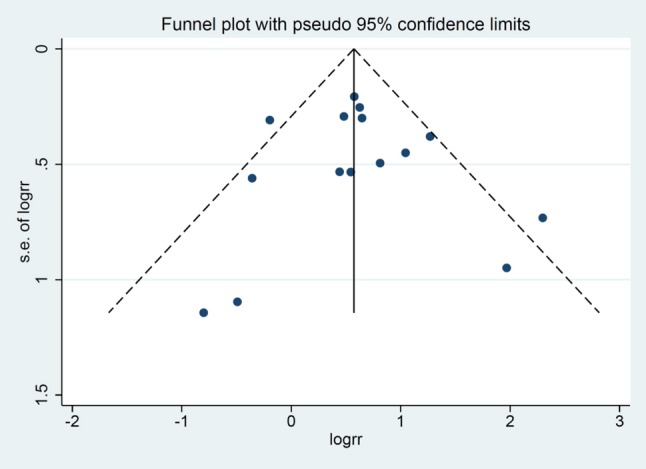
Funnel plot of QRS distortion and mortality. Circles represent published studies.
DISCUSSION
General
In this study, the incidence of QRS distortion ranged from 19-52% in the STEMI patients before undergoing either PCI or thrombolysis. Our meta-analysis demonstrated that the presence of terminal QRS distortion was associated with increased combined all-cause mortality, including in-hospital mortality, 30-day mortality, and mortality within 1 year. Additionally, our exploratory analysis suggested associations between the presence of QRS distortion and increased risks of reinfarction, MACEs, ventricular arrhythmia, and shock.
Regardless of the technique and changes in treatment according to the ACC/AHA Guidelines in 2004,27 the risk of all-cause mortality was still statistically significant among the patients with QRS distortion in both the stenting and thrombolysis groups. However, there was no difference in mortality between the PTCA and thrombolysis groups, which could be explained by the difference in treatment techniques.
Is QRS distortion still useful in the current era?
In addition to recent shifts in acute myocardial infarction treatment, many ECG parameters including terminal QRS distortion have been investigated for their prognostic ability. For example, Valle-Caballero et al.28 reported that QRS distortion was associated with a higher risk, extent and size of myocardial infarction. Likewise, Tanrivedi et al.29 demonstrated that a combination of QRS distortion and fragmented QRS was correlated with higher mortality, poor LVEF, high levels of cardiac biomarkers and low rate of ST resolution after PCI. We believe that these ECG parameters are still useful and applicable to any circumstance, especially in the setting of limited resources. In addition, our subgroup analyses showed that terminal QRS distortion was still associated with an equivalently increased risk of all-cause mortality both before and after 2004.
QRS distortion equivalency to other contemporary modalities
QRS distortion is thought to be an old-fashioned marker compared to other modern modalities including signal averaged ECG and cardiac imaging. However, it is still a useful tool to stratify the risk of morbidity and mortality among STEMI patients. Recently, signal averaged ECG has been recognized as a promising tool for prognostication. However, the predictive value compared to QRS distortion may not be as equivalent as it should. Bauer et al.30 demonstrated that ventricular late potentials (VLPs) were not predictive of long-term serious cardiac events. VLPs were also of limited use for very long-term risk stratification according to Shturman et al.31 Despite these unsatisfactory results, VLPs are particularly recommended to determine the risk of developing sustained ventricular tachycardia in patients recovering from myocardial infarction without bundle branch block.32
Regarding cardiovascular imaging, various modalities have been developed to help identify high-risk patients with STEMI. LVEF from echocardiography is currently the most important risk stratification tool, strongly recommended by the ACCF/AHA STEMI guidelines and ESC STEMI guidelines for further therapy including implantable cardioverter-defibrillator (ICD) implantation and biventricular pacing.33,34 This could be inferred that echocardiography is superior to ECG parameters. However, measurements during acute myocardial infarction can be misleading, since the LVEF may significantly improve after optimal revascularization. In addition, cardiac magnetic resonance imaging (MRI) has been shown to be a promising modality which may play a greater role in the cardiovascular field in the future. Eitel et al.35 conducted a prospective multicenter study to evaluate the prognostic impact of cardiac MRI, and found that only microvascular obstructions were associated with an up to 3-fold risk of MACEs. Another study36 suggested that infarct characteristics in cardiac MRI could provide additional prognostic value, especially mortality. Despite the high predictive value, more studies are required to verify the results since there were few participants, stringent inclusion and exclusion criteria, need of resourceful setting, and the generalizability of the results is still questionable. Taken together, ECG is still the best method given its convenience, simplicity and cost-effectiveness.
Pathophysiology
Many previous studies have demonstrated that the presence of QRS distortion or grade 3 ischemia is associated with increased morbidity and mortality.3,9,12,13,21,25 The mechanism of terminal QRS distortion has yet to be clearly elucidated. Myocardial ischemia has been shown to lead to delayed conduction in Purkinje fibers.37 This decreases the degree of cancellation, which causes an increase in R-wave and decrease in S-wave amplitudes on surface ECG.38 Since Purkinje cells are less sensitive to ischemia than contracting myocytes,39 an alteration at the terminal portion of the QRS complex, also known as terminal QRS distortion, will occur in the setting of profound ischemia.40 Based on these possible explanations, an association has also been reported between terminal QRS distortion and evidence of the significant extent of ischemia, supported by single-photon emission computed tomography (SPECT) findings,41 and less myocardial salvage as well as a rapid progression of necrosis.42 Previous studies have shown that terminal QRS distortion is associated with a more advanced stage of myocardial infarction, higher in-hospital mortality, higher risk of reinfarction, and poor prognosis of STEMI.43 With respect to ventricular arrhythmogenesis, the relationship between QRS distortion and ventricular arrhythmia is still unclear. Taken together, we hypothesize that QRS distortion is an indicator of poor ventricular substrate. Interestingly, our results showed that QRS distortion was only associated with higher overall mortality in the subjects with anterior infarction but not with inferior infarction, which is consistent with a previous study by Fakhei et al. that described the applicability of ECG indices of severity, and showed that acuteness of myocardial ischemia is only confined to anterior myocardial infarction.44
To the best of our knowledge, this is the first meta-analysis to examine the association between QRS distortion and increased mortality in STEMI patients undergoing either thrombolysis or PCI. We believe that QRS distortion may be integrated as part of a risk stratification tool in STEMI. Given our exploratory findings, further studies are warranted to determine the association between QRS distortion and MACEs, reinfarction and shock.
Limitations
There are several limitations to this meta-analysis. Studies with different methodologies and populations were included as well as variations in QRS definition in each study. The primary outcome of our study was a combination of all-cause mortality, which was defined differently in each study, from in-hospital mortality to one-year mortality. Our analysis only showed the outcome at 1 year at most from the beginning of each study. Therefore, we could not analyze the long-term outcomes. The proportion of patients with anterior infarction was different in each study, which could have impacted the incidence of mortality, shock, reinfarction, ventricular arrhythmia and MACEs in patients with QRS distortion. Nevertheless, we performed subgroup analysis between anterior and inferior infarction groups, which suggested that the location of infarction may have affected the outcome. Additionally, differences in follow-up time between studies may have resulted in a differences in the incidence of adverse outcomes. These factors may have introduced potential sources of moderate heterogeneity in our study. Nonetheless, we used sensitivity analysis methods in the random-effects model and found no difference in the imputed risk ratio and its 95% confidence interval.
CONCLUSIONS
In conclusion, our meta-analysis demonstrated an association between terminal QRS distortion and mortality in patients with STEMI undergoing PCI or thrombolysis. Terminal QRS distortion should be added as an additional tool for risk stratification in STEMI patients. Our findings suggest that further research is warranted to determine the utility of QRS distortion as an indicator of high-risk STEMI.
Acknowledgments
We would like to thank Elysse Tom, MD., for critical reading.
FINANCIAL SUPPORT
None.
CONFLICT OF INTEREST
None to declare.
AUTHOR CONTRIBUTION
Narut Prasitlumkum, conception design, data interpretation, draft manuscript, corresponding; Natee Sirinvaravong, data acquisition, draft manuscript; Nath Limpruttidham, data acquisition, data interpretation; Chanavuth Kanitsoraphan, data acquisition, draft manuscript; Elysse Tom, draft manuscript; Pattara Rattanawong, data acquisition, statistical analysis; Pakawat Chongsathidkiet, data acquisition; Thosaporn Boondarikpornpant, data acquisition.
REFERENCES
- 1.Bren GB, Wasserman AG, Ross AM. The electrocardiogram in patients undergoing thrombolysis for myocardial infarction. Circulation. 1987;76:II18–II24. [PubMed] [Google Scholar]
- 2.Sclarovsky S, Mager A, Kusniec J, et al. Electrocardiographic classification of acute myocardial ischemia. Isr J Med Sci. 1990;26:525–531. [PubMed] [Google Scholar]
- 3.Birnbaum Y, Sclarovsky S, Blum A, et al. Prognostic significance of the initial electrocardiographic pattern in a first acute anterior wall myocardial infarction. Chest. 1993;103:1681–1687. doi: 10.1378/chest.103.6.1681. [DOI] [PubMed] [Google Scholar]
- 4.Birnbaum Y, Sclarovsky S. The grades of ischemia on the presenting electrocardiogram of patients with ST elevation acute myocardial infarction. J Electrocardiol. 2001;34 Suppl:17–26. doi: 10.1054/jelc.2001.28819. [DOI] [PubMed] [Google Scholar]
- 5.Birnbaum Y, Kloner RA, Sclarovsky S, et al. Distortion of the terminal portion of the QRS on the admission electrocardiogram in acute myocardial infarction and correlation with infarct size and long-term prognosis (Thrombolysis in Myocardial Infarction 4 Trial). Am J Cardiol. 1996;78:396–403. doi: 10.1016/s0002-9149(96)00326-8. [DOI] [PubMed] [Google Scholar]
- 6.Rommel KP, Badarnih H, Desch S, et al. QRS complex distortion (Grade 3 ischaemia) as a predictor of myocardial damage assessed by cardiac magnetic resonance imaging and clinical prognosis in patients with ST-elevation myocardial infarction. Eur Heart J Cardiovasc Imaging. 2016;17:194–202. doi: 10.1093/ehjci/jev135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikus K, Birnbaum Y, Eskola M, et al. Updated electrocardiographic classification of acute coronary syndromes. Curr Cardiol Rev. 2014;10:229–236. doi: 10.2174/1573403X10666140514102754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DH, Walsh B, Smith SW. Terminal QRS distortion is present in anterior myocardial infarction but absent in early repolarization. Am J Emerg Med. 2016;34:2182–2185. doi: 10.1016/j.ajem.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 9.Mulay DV, Mukhedkar SM. Prognostic significance of the distortion of terminal portion of QRS complex on admission electrocardiogram in ST segment elevation myocardial infarction. Indian Heart J. 2013;65:671–677. doi: 10.1016/j.ihj.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnbaum Y, Herz I, Sclarovsky S, et al. Prognostic significance of the admission electrocardiogram in acute myocardial infarction. J Am Coll Cardiol. 1996;27:1128–1132. doi: 10.1016/0735-1097(96)00003-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee CW, Hong MK, Yang HS, et al. Determinants and prognostic implications of terminal QRS complex distortion in patients treated with primary angioplasty for acute myocardial infarction. Am J Cardiol. 2001;88:210–213. doi: 10.1016/s0002-9149(01)01627-7. [DOI] [PubMed] [Google Scholar]
- 12.Postma S, Heestermans T, Ten Berg JW, et al. Predictors and outcome of grade 3 ischemia in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Electrocardiol. 2011;44:516–522. doi: 10.1016/j.jelectrocard.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Sejersten M, Birnbaum Y, Ripa RS, et al. Influences of electrocardiographic ischaemia grades and symptom duration on outcomes in patients with acute myocardial infarction treated with thrombolysis versus primary percutaneous coronary intervention: results from the DANAMI-2 trial. Heart. 2006;92:1577–1582. doi: 10.1136/hrt.2005.085639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Rubira JC, Garcia-Borbolla R, Nunez-Gil I, et al. Distortion of the terminal portion of the QRS is predictor of shock after primary percutaneous coronary intervention for acute myocardial infarction. Int J Cardiol. 2008;130:241–245. doi: 10.1016/j.ijcard.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Rubira JC, Molano F, Trujillo F, et al. A new classification of lower infarcts with important prognostic significance. Rev Port Cardiol. 1996;15:793–797, 772. [PubMed] [Google Scholar]
- 16.Hasdai D, Sclarovsky S, Solodky A, et al. Prognostic significance of the initial electrocardiographic pattern in patients with inferior wall acute myocardial infarction. Clin Cardiol. 1996;19:31–36. doi: 10.1002/clc.4960190107. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Rubira JC, Perez-Leal I, Garcia-Martinez JT, et al. The initial electrocardiogram pattern is a strong predictor of outcome in acute myocardial infarction. Int J Cardiol. 1995;51:301–305. doi: 10.1016/0167-5273(95)02436-z. [DOI] [PubMed] [Google Scholar]
- 22.Wolak A, Yaroslavtsev S, Amit G, et al. Grade 3 ischemia on the admission electrocardiogram predicts failure of ST resolution and of adequate flow restoration after primary percutaneous coronary intervention for acute myocardial infarction. Am Heart J. 2007;153:410–417. doi: 10.1016/j.ahj.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Birnbaum Y, Goodman S, Barr A, et al. Comparison of primary coronary angioplasty versus thrombolysis in patients with ST-segment elevation acute myocardial infarction and grade II and grade III myocardial ischemia on the enrollment electrocardiogram. Am J Cardiol. 2001;88:842–847. doi: 10.1016/s0002-9149(01)01889-6. [DOI] [PubMed] [Google Scholar]
- 24.McGehee JT, Rangasetty UC, Atar S, et al. Grade 3 ischemia on admission electrocardiogram and chest pain duration predict failure of ST-segment resolution after primary percutaneous coronary intervention for acute myocardial infarction. J Electrocardiol. 2007;40:26–33. doi: 10.1016/j.jelectrocard.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Tanriverdi Z, Dursun H, Simsek MA, et al. The predictive value of fragmented QRS and QRS distortion for high-risk patients with STEMI and for the reperfusion success. Ann Noninvasive Electrocardiol. 2015;20:578–585. doi: 10.1111/anec.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zalenski RJ, Grzybowski M, Ross MA, et al. ECG scores for a triage of patients with acute myocardial infarction transported by the emergency medical system. J Electrocardiol. 2000;33 Suppl:245–249. doi: 10.1054/jelc.2000.20298. [DOI] [PubMed] [Google Scholar]
- 27.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol. 2004;44:671–719. doi: 10.1016/j.jacc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Valle-Caballero MJ, Fernandez-Jimenez R, Diaz-Munoz R, et al. QRS distortion in pre-reperfusion electrocardiogram is a bedside predictor of large myocardium at risk and infarct size (a METOCARD-CNIC trial substudy). Int J Cardiol. 2016;202:666–673. doi: 10.1016/j.ijcard.2015.09.117. [DOI] [PubMed] [Google Scholar]
- 29.Tanriverdi Z, Colluoglu T, Unal B, et al. The prognostic value of the combined use of QRS distortion and fragmented QRS in patients with acute STEMI undergoing primary percutaneous coronary intervention. J Electrocardiol. 2018;51:210–217. doi: 10.1016/j.jelectrocard.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Bauer A, Guzik P, Barthel P, et al. Reduced prognostic power of ventricular late potentials in post-infarction patients of the reperfusion era. Eur Heart J. 2005;26:755–761. doi: 10.1093/eurheartj/ehi101. [DOI] [PubMed] [Google Scholar]
- 31.Shturman A, Vardi S, Bickel A, et al. Ventricular late potentials immediately post ST-elevation myocardial infarction, and very long-term mortality. Isr Med Assoc J. 2017;19:246–250. [PubMed] [Google Scholar]
- 32.Breithardt G, Cain ME, el-Sherif N, et al. Standards for analysis of ventricular late potentials using high-resolution or signal-averaged electrocardiography. A statement by a Task Force Committee of the European Society of Cardiology, the American Heart Association, and the American College of Cardiology. Circulation. 1991;83:1481–1488. doi: 10.1161/01.cir.83.4.1481. [DOI] [PubMed] [Google Scholar]
- 33.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–555. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 34.Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) Steg PG, James SK, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 35.Eitel I, de Waha S, Wohrle J, et al. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1217–1226. doi: 10.1016/j.jacc.2014.06.1194. [DOI] [PubMed] [Google Scholar]
- 36.Yan AT, Shayne AJ, Brown KA, et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 37.Lewis CT, Naumann DN, Crombie N, et al. Prehospital point-of-care lactate following trauma: a systematic review. J Trauma Acute Care Surg. 2016;81:748–755. doi: 10.1097/TA.0000000000001192. [DOI] [PubMed] [Google Scholar]
- 38.Holland RP, Brooks H. The QRS complex during myocardial ischemia. An experimental analysis in the porcine heart. J Clin Invest. 1976;57:541–550. doi: 10.1172/JCI108309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dehaan RL. Differentiation of the atrioventricular conducting system of the heart. Circulation. 1961;24:458–470. doi: 10.1161/01.cir.24.2.458. [DOI] [PubMed] [Google Scholar]
- 40.Spekhorst H, SippensGroenewegen A, David GK, et al. Body surface mapping during percutaneous transluminal coronary angioplasty. QRS changes indicating regional myocardial conduction delay. Circulation. 1990;81:840–849. doi: 10.1161/01.cir.81.3.840. [DOI] [PubMed] [Google Scholar]
- 41.Yang HS, Lee CW, Hong MK, et al. Terminal QRS complex distortion on the admission electrocardiogram in anterior acute myocardial infarction and association with residual flow and infarct size after primary angioplasty. Korean J Intern Med. 2005;20:21–25. doi: 10.3904/kjim.2005.20.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Billgren T, Maynard C, Christian TF, et al. Grade 3 ischemia on the admission electrocardiogram predicts rapid progression of necrosis over time and less myocardial salvage by primary angioplasty. J Electrocardiol. 2005;38:187–194. doi: 10.1016/j.jelectrocard.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Birnbaum Y, Herz I, Sclarovsky S, et al. Admission clinical and electrocardiographic characteristics predicting an increased risk for early reinfarction after thrombolytic therapy. Am Heart J. 1998;135:805–812. doi: 10.1016/s0002-8703(98)70038-9. [DOI] [PubMed] [Google Scholar]
- 44.Fakhri Y, Sejersten M, Schoos MM, et al. Electrocardiographic scores of severity and acuteness of myocardial ischemia predict myocardial salvage in patients with anterior ST-segment elevation myocardial infarction. J Electrocardiol. 2018;51:195–202. doi: 10.1016/j.jelectrocard.2017.11.002. [DOI] [PubMed] [Google Scholar]


